Abstract
Background and Objectives
Herpes zoster (HZ) is caused by reactivation of the neurotrophic varicella-zoster virus (VZV). Zoster may contribute to development of dementia through neuroinflammation, cerebral vasculopathy, or direct neural damage, but epidemiologic evidence is limited. We used data from linked nationwide Danish registries to conduct a cohort study of the association between zoster and dementia during 1997–2017. As secondary aims, we examined whether associations were more pronounced for zoster involving cranial nerves (mainly ophthalmic zoster) or the CNS and Alzheimer disease as an outcome.
Methods
We included people aged ≥40 years with zoster and a general population comparison cohort matched 5:1 by sex and birth year. We identified zoster and dementia in the registries using prescription records in the community and hospital diagnoses. We used Cox regression to compute confounder-adjusted hazard ratios (HRs) with 95% CIs for dementia associated with zoster during 0–1 year and 1–21 years of follow-up. We compared the cumulative incidence of dementia, inverse probability weighted for confounders.
Results
The study included 247,305 people with zoster and 1,235,890 matched general population comparators (median age 64 years; 61% female). The HR of all-cause dementia was 0.98 (95% CI 0.92–1.04) during the first year and 0.93 (95% CI 0.90–0.95) thereafter in people with zoster vs matched comparators. Dementia was diagnosed in 9.7% of patients with zoster and 10.3% of matched comparators by the end of follow-up. We observed no increased long-term risk of dementia in subgroup analyses, except possibly in people with CNS infection (HR 1.94; 95% CI 0.78–4.80). Analyses of Alzheimer disease as a separate outcome showed similar results.
Discussion
HZ is not associated with an increased risk of dementia, and contrary to expectation, we found a small decrease in the risk. The explanation for this finding is unclear, and systematic errors should be considered. Patients with CNS involvement had an almost 2-fold increased relative risk of dementia. The population attributable fraction of dementia due to this rare complication is estimated at 0.014%. Therefore, universal vaccination against VZV in the elderly is unlikely to reduce dementia risk.
Herpes zoster (HZ) is caused by reactivation of varicella-zoster virus (VZV)—a neurotrophic virus that lies latent in sensory ganglia following an episode of chickenpox.1 It has been postulated that latent viral infections, such as that with VZV, increase the risk of dementia.2,3 Thus, reactivation of the virus could promote neuroinflammation4 with generation of misfolded oligomers and the accumulation of amyloid plaques and neurofibrillary tangles composed of hyperphosphorylated tau protein.3,5-7 VZV may also infect astrocytes directly to promote production of intracellular amyloid and aggregation of amyloid fibrils in the extracellular environment.8 Another hypothesis is that HZ, in particular when involving cranial nerves, causes cerebral vasculopathy and stroke9,10 with neural damage as a result. Finally, severe HZ affecting the CNS1 could induce encephalopathy and ultimately dementia, similar to what is observed for HIV and syphilis.11
Epidemiologic evidence for the association between HZ and dementia is limited. A systematic review found that dementia was not associated with serologic evidence of past infection with VZV, nor with recent reactivation, as reflected by VZV nucleic acid in postmortem brain samples.2 Evidence from registry-based studies is more conflicting, showing up to 9% decreased12 or up to a 12% increased13,14 relative risk of dementia following diagnosis of HZ in East Asian populations. One study that focused specifically on HZ involving the ophthalmic branch of the trigeminal nerve (ophthalmic HZ) reported a hazard ratio (HR) for dementia of 2.97 (95% CI: 1.89–4.66).15
To help clarify these discrepant findings, we conducted a population-based matched cohort study to examine the hypothesis that reactivation of VZV, as measured by HZ diagnosis, is associated with an increased risk of dementia. As secondary aims, we examined whether associations were more pronounced for HZ with involvement of cranial nerves (including the ophthalmic nerve), HZ with CNS involvement, and for Alzheimer disease as a specific cause of dementia. It has been estimated that the lifetime prevalence of HZ is about 50% in people aged 85 years or older; however, the relative risk of HZ can be decreased by 96% for at least 3 years after vaccination with the recombinant VZV subunit zoster vaccine compared with placebo.16 HZ may therefore represent a potentially modifiable risk factor for dementia, a finding, if confirmed, that could be of importance, considering increasing population longevity and associated burden of both HZ and dementia.17
Methods
Setting
We conducted the study in Denmark using routinely collected health care data from linked nationwide registries. The study period was from January 1, 1997, to December 31, 2017, which ensured at least 2 years of recording in all registries. We identified primary and secondary (contributing) hospital diagnoses and hospital treatments through the Danish National Patient Registry18 and the Danish Psychiatric Central Registry,19 which include data on psychiatric admissions since 1970, medical admissions since 1978, and contacts with all hospital outpatient clinics and emergency departments since 1995. We used the Danish National Prescription Registry20 to identify prescriptions filled at Danish community pharmacies since January 1, 1995. We identified incident diagnoses of cancer, as recorded in the Danish Cancer Registry21 since 1943. We retrieved data on demographic characteristics, deaths, and emigrations from the Civil Registration System,22 which has provided a complete account of the entire Danish population on a day-to-day basis since 1968. The registry also assigns Danish residents with a unique personal identification number, which is used for registrations of health and social services in Denmark and thus permitted individual-level data linkage. eMethod 1, links.lww.com/WNL/C43, includes code lists for study variables and eMethod 2 our directed acyclic graph illustrating the study hypotheses and covariables.
Study Population
We identified an exposed cohort of persons who during the study period had incident HZ defined as: (1) a first-ever inpatient, outpatient hospital clinic, or emergency department primary or secondary discharge diagnosis of HZ or (2) a first-time antiviral prescription likely to represent treatment for HZ (packages with 35 pills of 800 mg acyclovir or a 500-mg tablet dose of valacyclovir or famciclovir) during the study period.23,24 Antiviral prescriptions served as a surrogate for HZ diagnosed in general and private practice settings (e.g., dermatology and ophthalmology); diagnoses in these settings are not recorded in Danish registries. We restricted analyses to people aged 40 years or older who had no previous prescription of acyclovir, valacyclovir, or famciclovir at any dose, to decrease potential misclassification of herpes simplex, which typically affects younger patients and may require longer-term or episodic antiviral therapy.23 We have previously found a positive predictive value of 87% for this prescription-based algorithm.23 We considered the date of the first hospital contact (admission date or first outpatient appointment) with HZ or the date the prescription was filled to be the diagnosis date (index date). We excluded people with previous diagnoses of postherpetic neuralgia to ensure inclusion of current infections. In a secondary analysis, we restricted to those with a hospital-based diagnosis consistent with HZ involving (1) cranial nerves or (2) the CNS (defined by diagnoses of encephalitis, meningoencephalitis, meningitis, or myelitis) because more pronounced associations for these manifestations could support vasculopathy and encephalitis as potential mechanisms.
We used the Civil Registration System to sample (with replacement) a matched comparison cohort including up to 5 people from the general population who had the same sex and birth year as their matched patient with HZ and who were alive and living in Denmark on the diagnosis date. We assigned comparators with an index date identical to their matched patient with HZ. We excluded people with diagnoses of HZ or postherpetic neuralgia or a previous prescription for one of the antivirals at any dose before or on the index date. eFigure 1, links.lww.com/WNL/C43, shows the study flowchart.
Dementia
We defined dementia as a primary or secondary diagnosis of dementia from a hospital inpatient or outpatient clinic or as a prescription of an antidementia drug.25 We considered the date of the first hospital contact or prescription filled to be the diagnosis date. We excluded people with a diagnosis of dementia, mild cognitive impairment or an amnestic syndrome, or a prescription for antidementia drug before or on the index date. Our main outcome of interest was all-cause dementia; Alzheimer disease was a secondary outcome. We assumed that the indication for treatment with an antidementia drug was Alzheimer disease, although donepezil, rivastigmine, and galantamine are also approved for Lewy body disease (Parkinson disease dementia and dementia with Lewy bodies). We did not include vascular dementia as a specific outcome because of poor validity for its diagnosis code.26
Covariables
Based on our directed acyclic graph, we identified the following possible confounders on or before the index date: autoimmune disease, chronic kidney disease, chronic obstructive pulmonary disease, asthma, cancer, diabetes, use of glucocorticoids, HIV, lipid-lowering therapy, and traumatic head injury. When relevant, we supplemented diagnosis codes with prescription treatment (e.g., antidiabetic drugs for diabetes). As a potential mediator of the association between HZ and dementia, we included diagnoses of stroke as a time-updated variable with person-time attributed to the stroke category from time of stroke diagnosis (if any) or from start of follow-up if diagnosed before the index date. Although a transient ischemic attack may also signify vasculopathy, we did not include it as a mediator with stroke because of concern of misclassification of vague symptoms (e.g., dizziness, feeling fatigue, falling, and confusion).
Statistical Analysis
We followed cohorts from the day after the index date until the earliest of the following: first diagnosis of dementia, death, emigration, or end of the study (December 31, 2017). People in the comparison cohort who were diagnosed with HZ during follow-up were censored and included in the HZ cohort (with their own matched comparators) at the time of diagnosis. We characterized study cohorts by covariables and accumulated person-time. With time since the index date as time scale, we used Cox regression to compute unadjusted HRs with 95% CIs for the HZ cohort compared with the matched comparison cohort. We stratified the model by matched set to account for matching factors. We then additionally adjusted for the aforementioned potential confounders (confounder-adjusted model) and finally for both confounders and time-varying stroke (mediation model). We followed cohorts to first-ever diagnosis of dementia and performed analyses for all-cause dementia (main outcome) and for Alzheimer disease (secondary outcome). Because visual inspection of log-log plots showed that curves crossed or merged after 6–12 months of follow-up for most comparisons, we divided the follow-up period into 0–1 year and >1 year after the index date. Given the potential of selection (survival) bias inherent in the use of the HR27 and because we were interested in absolute risk estimates for the association, we computed crude and inverse probability-weighted (IPW) cumulative incidence curves for dementia (accounting for death as a competing risk) in patients with HZ and matched comparators. The IPW technique can be used for covariate adjustment of the cumulative risk function in competing risk analysis.28 The method requires no assumption about the form of the cumulative incidence functions, and the interpretation of the resulting cumulative incidence curves is straightforward. The inverse probability treatment weights are the inverse of a probability of being exposed, conditional on the observed covariates (in our case, variables from the confounder-adjusted model), and are estimated using a logistic regression model. As a result, a pseudopopulation is created in which the probability of being exposed does not depend on the measured covariates. The cumulative risk function is estimated for each type of event (i.e., dementia or death) from the weighted subpopulation of all exposed and unexposed individuals. Confidence bands are estimated using bootstrap, with newly calculated weights for each bootstrap replication.
In a secondary analysis, we restricted to patients with HZ (and their comparators) with a hospital inpatient or outpatient clinic diagnosis consistent with HZ in dermatomes of the cranial nerves or with CNS involvement. As a subgroup analysis, we stratified results for all-cause dementia by age group at the index date, sex, and mode of HZ identification (by hospital diagnosis or antiviral prescription), and stroke. Patients with an HZ hospital diagnosis and prescription on the same day were classified as identified in the hospital setting. The analysis by stroke included persons in a matched set who had concordant stroke status (present or absent) at baseline.
To examine the robustness of our findings, we performed several sensitivity analyses where we repeated the main analysis after (1) dividing follow-up into shorter periods (0–1 year, 0–5 years, 0–10 years, 0–15 years, and entire follow-up) because we expected any potential ascertainment bias to be most pronounced in early follow-up periods; (2) restricting to those aged 60 years or older on the index date given higher validity of HZ identification for this age group23 and because the event rate for dementia is expected to be low among younger people; (3) restricting to those with the index date before September 2014 when the live-attenuated zoster vaccine was marketed; (4) excluding prescriptions for antidementia drugs from the outcome definition (except for excluding prevalent dementia at baseline) because the drugs could potentially be used for mild cognitive impairment off-label and against recommendations; (5) changing the censoring criteria so that people in the comparison cohort who joined the HZ-exposed cohort during follow-up continued to be followed in the comparison cohort as well (a more conservative intention-to-treat approach); (6) changing the definition of Alzheimer disease to include also codes for unspecified dementia, which often represent Alzheimer disease26; and (7) excluding those with a hospital diagnosis or prescription record suggesting the presence of Parkinson disease or Lewy body dementia at baseline (competing indications for antidementia drugs) and censoring people if the codes appeared during follow-up (relevant for Alzheimer disease only). In a post hoc analysis, we additionally adjusted for selected cardiovascular diseases (acute myocardial infarction, angina pectoris, heart failure, and hypertension) and liver disease at baseline. We performed all analyses using SAS version 9.4.
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the Data Protection Agency (record number: 1-16-02-370-14). Approval by an ethical review board or informed consent was not required.
Data Availability
No additional unpublished data are available because this study used existing data from the Danish nationwide registries, which are accessible to researchers who fulfill local requirements following approval by the Danish Data Protection Agency and the Danish Health Data Authority.
Results
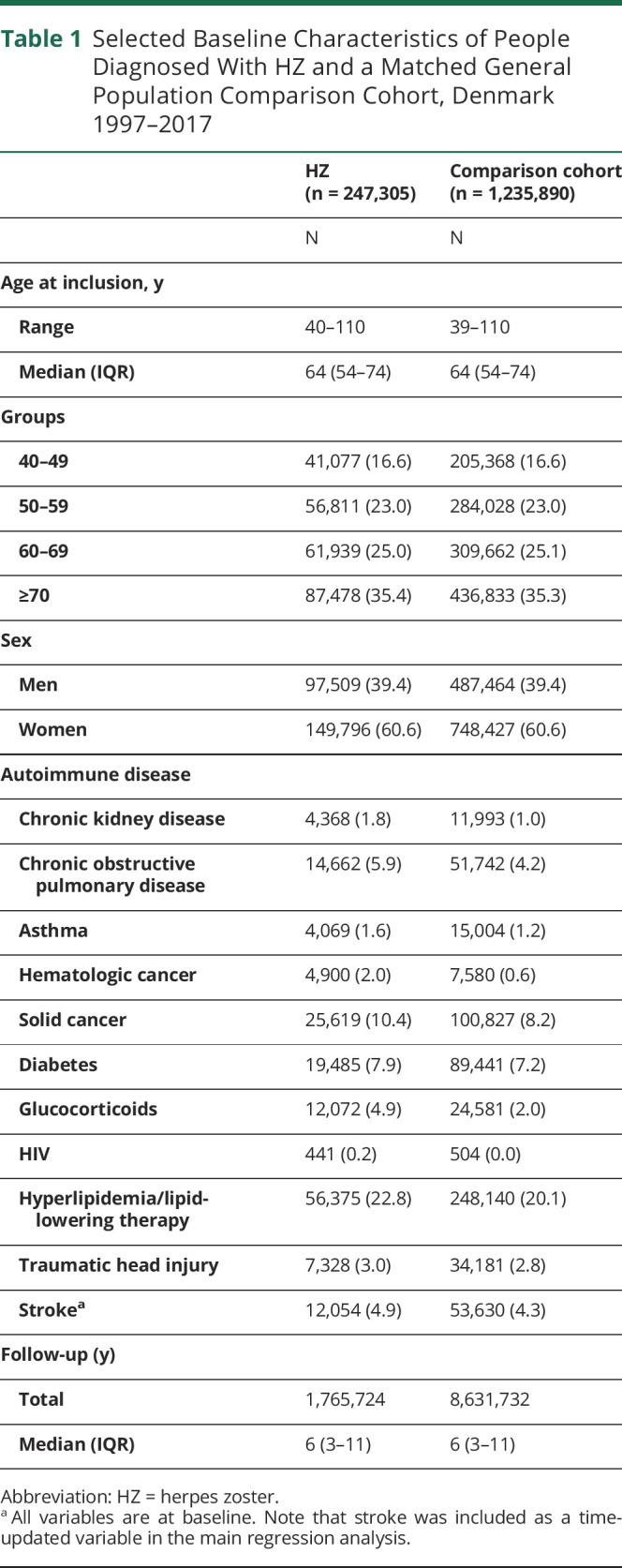
We identified 247,305 people with HZ and 1,235,890 matched comparators (eFigure 1, links.lww.com/WNL/C43). The median age was 64 years (interquartile range: 54–74 years; total range 40–110 years), and 61% were women (Table 1). HIV infection, hematologic cancer, use of oral glucocorticoids, and chronic kidney disease were more prevalent in patients with HZ than matched comparators.
Table 1.
Selected Baseline Characteristics of People Diagnosed With HZ and a Matched General Population Comparison Cohort, Denmark 1997–2017
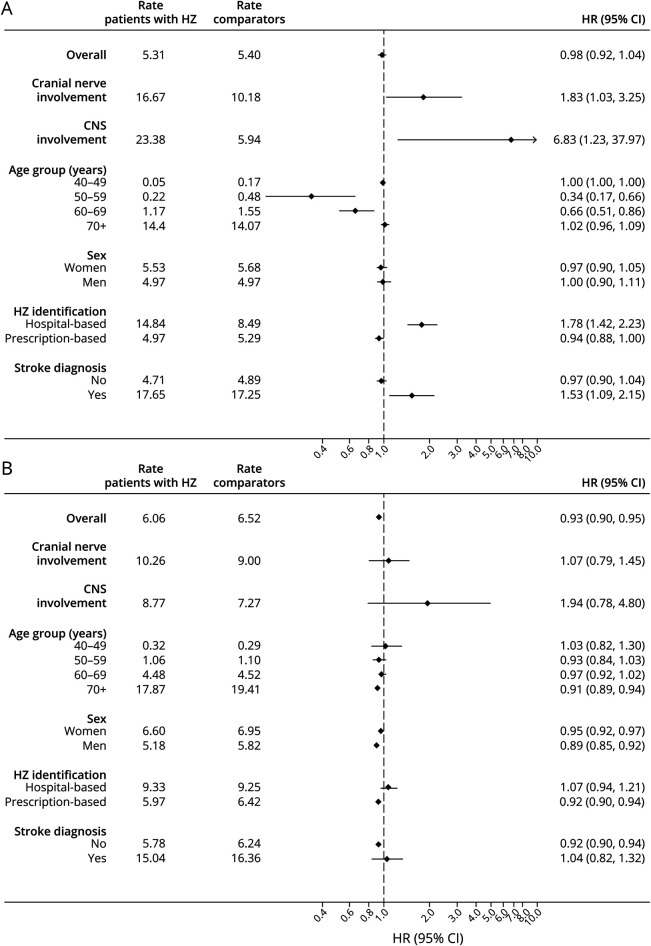
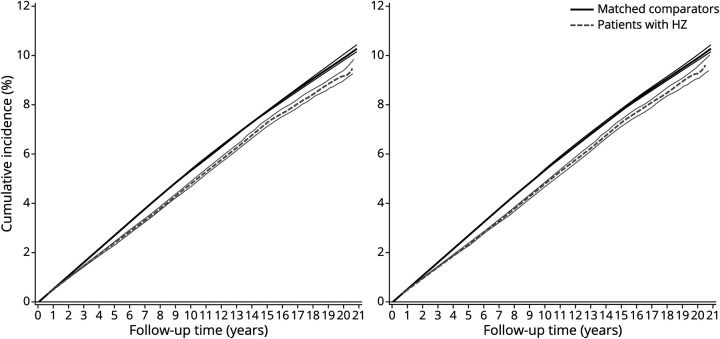
Rates and confounder-adjusted HRs of all-cause dementia associated with HZ are presented in Figure 1. eTable 1, links.lww.com/WNL/C43, shows the number of people, events, person-years at risk, and results from the different regression models. We observed no substantial differences between estimates from unadjusted, confounder-adjusted, and mediator-adjusted models. The confounder-adjusted HR of all-cause dementia was 0.98 (95% CI 0.92–1.04) during the first year and 0.93 (95% CI 0.90–0.95) thereafter in people with HZ compared with matched people from the general population. The IPW cumulative incidence of dementia by the end of follow-up was 9.7% (95% CI 9.4%–10.0%) in patients with HZ vs 10.3% (95% CI 10.1%–10.4%) in matched comparators (Figure 2).
Figure 1. Rate (per 1,000 Person-Years) and HRsa With 95% CIs of Dementia Associated With HZ Within 0–1 year (A) and 1–21 years (B) of Follow-up, Denmark 1997–2017.
HR = hazard ratio; HZ = herpes zoster. aCalculated with Cox proportional hazards regression accounting for matching factors (age and sex) and adjusted for autoimmune disease, chronic kidney disease, chronic obstructive pulmonary disease, asthma, hematologic cancer, solid cancer, diabetes, glucocorticoids, HIV, lipid-lowering therapy, and traumatic head injury.
Figure 2. Crude (Left Panel) and Inverse Probability-Weighteda (Right Panel) Cumulative Incidence Curves With 95% CIs for Dementia in People With Herpes Zoster (HZ) and a Matched General Population Comparison Cohort, Denmark 1997–2017, Accounting for Death as a Competing Risk.
aWeighted for autoimmune disease, chronic kidney disease, chronic obstructive pulmonary disease, asthma, hematologic cancer, solid cancer, diabetes, glucocorticoids, HIV, lipid-lowering therapy, and traumatic head injury.
Ophthalmic HZ was the most common form of cranial nerve involvement (83%; 990 of 1,190). Although estimates were imprecise, we found an increased HR of dementia within the first year for those with HZ involving cranial nerves (HR 1.83; 95% CI 1.03–3.25) or the CNS (HR 6.83; 95% CI 1.23–37.97). Stratified analyses showed decreasing HRs with decreasing age, but no effect modification by sex. Within the first year, the HR was increased among those who had a hospital record of HZ compared with those without any previous hospital or prescription record of HZ (HR 1.78; 95% CI 1.42–2.23) and among patients with HZ diagnosed with stroke after the index date compared with those with neither HZ nor stroke at baseline (HR 1.53; 95% CI 1.09–2.15). After the first year of follow-up, the HR remained increased only for HZ with CNS involvement (HR 1.94; 95% CI 0.78–4.80). For the remaining subgroups, HZ was unassociated with a risk or associated with a slightly decreased risk of dementia. The lowest HR was found in men (HR 0.89; 95% CI 0.85–0.92 compared with 0.95; 95% CI 0.92–0.97 in women). In a post hoc analysis restricted to ophthalmic HZ, the confounder-adjusted HR was 1.70 (0.91–3.17) in the first year and 1.23 (0.89–1.70) thereafter (eTable 2, links.lww.com/WNL/C43).
The analysis of Alzheimer disease as an outcome revealed similar associations as for all-cause dementia: the confounder-adjusted HR was 0.91 (95% CI 0.82–1.01) for the first year of follow-up and 0.93 (95% CI 0.90–0.97) thereafter (eTable 3, links.lww.com/WNL/C43). Furthermore, all sensitivity analyses supported the main findings (eTables 4–6).
Discussion
Contrary to our prediction, we found that HZ was associated with a small (7%) decreased relative risk of dementia in a cohort followed for up to 21 years. However, the overall difference in the absolute risk was small (below 1%). In the small subgroup of patients with HZ infection involving the CNS, there was a 2-fold increase in the relative risk of dementia after 1 year of follow-up and a higher risk during the first year of follow-up, but estimates were imprecise. Furthermore, the population attributable fraction for dementia associated with HZ infection involving the CNS would be only 0.014% for a disease prevalence of 0.03% (based on a complication rate of 0.10% in our study population and an assumed prevalence of HZ of 30% in the population).29 Findings were similar when Alzheimer disease was the outcome.
Previous population-based studies report both decreased12 and increased13-15 risks of dementia after HZ infection. A case-control study from the National Health Insurance Service–National Sample Cohort in South Korea found lower odds of prior HZ (OR 0.91; 95% CI 0.84–0.97) among 11,445 people aged 60 years or older with dementia compared with 45,780 matched controls without dementia.12 This result contrasts with findings from 2 earlier cohort studies in people 50 years or older: one based on the same National Sample Cohort13 and the other derived from the National Health Insurance Research Database in Taiwan.14 The South Korean study followed 34,505 people with HZ and 195,089 people without HZ for up to 11 years (HR 1.12; 95% CI 1.05–1.19).13 The Taiwanese study followed 39,205 people with HZ and an equal number of matched comparators for up to 17 years (HR 1.11; 95% CI 1.04–1.78).14
We found that dementia risk was almost doubled in the first year after cranial nerve HZ, of which most cases involved the ophthalmic nerve. This result is in accordance with an increased risk of dementia in a separate Taiwanese report where 846 people with ophthalmic HZ aged 40 years or older were followed for 5 years and compared with 2,538 people with no HZ diagnosis in the year before cohort entry (HR 2.97; 95% CI 1.89–4.66).15 Our results suggest that the association is limited to the first year after HZ, which may represent detection bias (see below).
In addition to being conducted in different populations, prior studies12-15 may have been affected by selection bias, although the magnitude or direction of potential bias is unknown. In the cohort studies, follow-up began at the start of the study period or on the diagnosis date for people with HZ, whereas comparators were selected from a population without HZ at any time during the study period. This sampling technique, which conditions on future information on the absence of HZ, may have selected people who were disproportionally healthy (and therefore less likely to develop HZ) or who died during the study period (and therefore had less chance to develop HZ), both of which would affect dementia risk. A similar sampling technique was applied to identify controls without dementia in the Korean case-control study.
The increased dementia risk observed in patients with HZ with CNS involvement is in accordance with studies that specifically considered CNS infection with VZV.30-34 A Danish study of 517 people with VZV DNA detected in the CSF by PCR found that the HR of dementia was 3.4 (95% CI 1.5–7.7) within 0–1 years and 2.4 (95% CI 1.4–4.1) within 1–12 years of follow-up, compared with 9,823 age- and sex-matched people from the general population.30 Similarly, a Swedish study found cognitive impairment in 14 patients with CNS infection with VZV 3 years earlier compared with 28 age- and sex-matched controls.31 Three small case series also support memory impairment up to 7 years after acute infection,32-34 but one of the studies found no alterations in concentrations of biomarkers (e.g., neurofilament light chain, amyloid-β, and total-tau) in the CSF.34 Finally, a population-based cohort study from the United Kingdom found an increased risk of diagnosis with cognitive problems (rate ratio 3.07; 95% CI 2.54–3.71) or dementia (rate ratio 2.66; 95% CI 1.94–3.65) in 2,460 people with encephalitis of unspecified cause compared with 47,914 people without encephalitis.35
The association between HZ involving the CNS and dementia may be mediated by neuroinflammation and direct cerebral damage. Although vasculopathy could also play a role, inclusion of stroke diagnoses in the mediation model did not attenuate HRs. Stroke is therefore not part of the explanation, although we cannot exclude the possibility of microinfarction, which does not cause clinical stroke. The particularly strong association in the first year may represent acute or subacute effects of HZ, misclassification of reversible cognitive change due to the acute infection, or diagnostic bias stemming from extensive clinical examination in the acute phase. The latter may explain the short-term increase in the risk of dementia among those with stroke as a complication and among those with hospital-based HZ diagnoses, including ophthalmic HZ. Although our prescription-based algorithm likely identified most cases of ophthalmic HZ treated in private specialty practice, these patients could not be singled out for subgroup analyses and may represent milder and less comorbid patients than those treated by hospital ophthalmology departments.
The slightly decreased long-term risk of dementia, including Alzheimer disease, was unexpected. Milder symptoms of HZ may be overlooked, neglected, or wrongly diagnosed in people with early undiagnosed dementia or milder degrees of cognitive impairment, leading to misclassification and an apparent noncausal decreased risk of dementia in those with HZ.
We were not able to examine whether antiviral treatment modifies the association between HZ and dementia. Our cohort was mainly identified based on antiviral prescriptions at HZ-specific doses; we had no information on treatments dispensed at hospitals but assume that most patients received antiviral drugs in this setting. Our findings may therefore not generalize to patients with HZ who were not treated and whose symptoms may have been milder. In a randomized controlled trial including patients with herpes simplex encephalitis treated with an initial standard course of intravenous acyclovir, extendend treatment with valacyclovir 2 g thrice daily for 90 days was associated with a possible 19% relative reduction in cognitive sequela at 12 months compared with placebo (adjusted OR 0.81; 95% CI 0.24 -2.77); however, results were imprecise and compatible with both a substantial decreased and increase in risk.36 A multicenter observational cohort study in Wales, Germany, Scotland, and Denmark also found a slightly decreased relative risk of dementia in elderly treated with antivirals for herpes infections; however, results were heterogeneous among countries, and there was no variation by the number of treatment courses, type of dementia, or herpes subtype in settings where this information was available.37 We found almost identical estimates in our cohort, focusing specifically on HZ using a validated algorithm in primary care and hospital-diagnosed patients. Two other East Asian studies have examined the role of antivirals for HZ specifically, showing that any treatment with antivirals after start of follow-up was associated with up to a 45% decrease in the relative risk of dementia.13,14 Considering that HZ is usually treated for a short period of 7–10 days, confounding from factors that predict treatment with antivirals (e.g., health-seeking), could explain the decreased risk of dementia in ours and these previous studies. Our interpretation is supported by a strong association between prescribed treatment and reduced mortality in one of the studies (HR 0.61; 95% CI 0.55–0.68).13 Similar selection mechanisms are likely to explain the decreased risk of dementia in people vaccinated against HZ in recent studies.38-40
Some HZ-exposed patients in our study may have had herpes simplex, which has also been implicated in dementia pathogenesis.2 However, the association was similar when we restricted analyses to people aged 60 years or older, where validity for our prescription-based algorithm is particularly high.23 Depression is sometimes misdiagnosed as dementia and has been associated with HZ. However, we do not expect that misclassification of depression as dementia depends on the history of HZ. Misclassification among dementia types is possible,26,41 but our main outcome was all-cause dementia for which the positive predictive value of hospital discharge diagnoses is estimated at 86%. The positive predictive value for an Alzheimer disease hospital diagnosis is also high, and results were robust in our sensitivity analysis that classified people with unspecified dementia as having Alzheimer disease. We also expect high validity of antidementia drugs for identifying dementia because they are not approved for other conditions and results were robust in the sensitivity analysis relying on diagnoses only.
Major confounding from lifestyle (smoking, alcohol, obesity, and physical exercise) is unlikely because our previous research found no evidence of strong associations with HZ.42 The study also suggested that socioeconomic factors are not associated with HZ in Denmark. Furthermore, the aforementioned multicenter study on antiviral treatment and the risk of dementia found no effect of adjusting for education level and civil status.37 Other studies have reported a possible association between socioeconomic measures and an increased risk of zoster.43,44 If this were the case, the true HR for dementia would be slightly lower than observed in our study (i.e., further below the null) because of an increased dementia risk in those who are socially deprived.37 Confounding by high-level of perceived psychological stress may have a similar effect, given its potential association with an increased risk of HZ45 and dementia.46 Finally, we lacked information on potentially relevant biomarkers, including serial measurements of virus or antibodies in blood or CSF, brain scan results, and markers of genetic susceptibility, such as apolipoprotein genotype, which may modify Alzheimer disease risk after exposure to herpes simplex.47 Also, follow-up may have been too short to detect an association in the oldest participants of our study, limiting generalizability of our findings to the oldest age groups.
In conclusion, we found evidence that people with HZ have a slightly reduced long-term relative risk of dementia compared with people in the general population without HZ. This finding could be explained by missed HZ diagnoses in people with undiagnosed dementia or possibly by a protective role of antiviral medications. Our analyses could not address putative effects of antiviral treatment, and this topic merits further research. Although we found that patients with CNS involvement had an almost two-fold increased relative risk of dementia, the population attributable fraction of dementia due to this rare complication is low, suggesting that universal vaccination against VZV in the elderly has limited potential to reduce dementia risk.
Acknowledgment
S.A.J.S. reports grants from the Edel and Wilhelm Daubenmerkls Charitable Foundation during the conduct of the study. The Department of Clinical Epidemiology, Aarhus University Hospital, receives funding for other studies from companies in the form of research grants to (and administered by) Aarhus University. None of these studies has relation to the present study. VWH was supported by NIH grant P30 AG066515.
Glossary
- HR
hazard ratio
- HZ
herpes zoster
- IPW
inverse probability weighted
- VZV
varicella-zoster virus
Appendix. Authors
Footnotes
Infographic links.lww.com/WNL/C244
Study Funding
This work was supported by the Edel and Wilhelm Daubenmerkls Charitable Foundation (record number 100060 to S.A.J.S).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Gershon AA, Gershon MD, Breuer J, et al. Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J Clin Virol. 2010;48(suppl 1):S2-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warren-Gash C, Forbes HJ, Williamson E, et al. Human herpesvirus infections and dementia or mild cognitive impairment: a systematic review and meta-analysis. Sci Rep. 2019;9(1):4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li H, Liu C-C, Zheng H, Huang TY. Amyloid, tau, pathogen infection and antimicrobial protection in Alzheimer's disease -conformist, nonconformist, and realistic prospects for AD pathogenesis. Transl Neurodegener. 2018;7(1):34-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bronzuoli MR, Iacomino A, Steardo L, Scuderi C. Targeting neuroinflammation in Alzheimer's disease. J Inflamm Res. 2016;9:199-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kagan BL, Jang H, Capone R, et al. Antimicrobial properties of amyloid peptides. Mol Pharm. 2012;9(4):708-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Álvarez G, Aldudo J, Alonso M, et al. Herpes simplex virus type 1 induces nuclear accumulation of hyperphosphorylated tau in neuronal cells. J Neurosci Res. 2012;90(5):1020-1029. [DOI] [PubMed] [Google Scholar]
- 7.Eimer WA, Vijaya Kumar DK, Navalpur Shanmugam NK, et al. Alzheimer's disease-associated β-amyloid is rapidly seeded by herpesviridae to protect against brain infection. Neuron. 2018;99(1):56-63.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bubak AN, Como CN, Coughlan CM, et al. Varicella zoster virus infection of primary human spinal astrocytes produces intracellular amylin, amyloid-beta, and an amyloidogenic extracellular environment. J Infect Dis. 2019;221(7):1088-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol. 2009;8(8):731-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forbes HJ, Williamson E, Benjamin L, et al. Association of herpesviruses and stroke: systematic review and meta-analysis. PLoS One. 2018;13(11):e0206163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almeida OP, Lautenschlager NT. Dementia associated with infectious diseases. Int Psychogeriatr. 2005;17(suppl 1):S65-S77. [DOI] [PubMed] [Google Scholar]
- 12.Choi HG, Park BJ, Lim JS, et al. Herpes zoster does not increase the risk of neurodegenerative dementia: a case-control study. Am J Alzheimers Dis Other Demen. 2021;36:15333175211006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bae S, Yun S-C, Kim M-C, et al. Association of herpes zoster with dementia and effect of antiviral therapy on dementia: a population-based cohort study. Eur Arch Psychiatry Clin Neurosci. 2021;271(5):987-997. [DOI] [PubMed] [Google Scholar]
- 14.Chen VC-H, Wu S-I, Huang K-Y, et al. Herpes zoster and dementia: a nationwide population-based cohort study. J Clin Psychiatry. 2018;79(1):16m11312. [DOI] [PubMed] [Google Scholar]
- 15.Tsai M-C, Cheng W-L, Sheu J-J, et al. Increased risk of dementia following herpes zoster ophthalmicus. PLoS One. 2017;12(11):e0188490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gagliardi AM, Andriolo BN, Torloni MR, et al. Vaccines for preventing herpes zoster in older adults. Cochrane Database Syst Rev. 2019;11:CD00858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt M, Schmidt SAJ, Sandegaard JL, et al. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mors O, Perto GP, Mortensen PB. The Danish psychiatric central research register. Scand J Public Health. 2011;39(7 suppl):54-57. [DOI] [PubMed] [Google Scholar]
- 20.Pottegård A, Schmidt SAJ, Wallach-Kildemoes H, et al. Data resource profile: the Danish national prescription registry. Int J Epidemiol. 2017;46(3):798-798f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gjerstorff ML. The Danish cancer registry. Scand J Public Health. 2011;39(7 suppl):42-45. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt M, Pedersen L, Sørensen HT. The Danish civil registration system as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541-549. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt SAJ, Vestergaard M, Baggesen LM, et al. Prevaccination epidemiology of herpes zoster in Denmark: quantification of occurrence and risk factors. Vaccine. 2017;35(42):5589-5596. [DOI] [PubMed] [Google Scholar]
- 24.Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44(suppl 1):S1-S26. [DOI] [PubMed] [Google Scholar]
- 25.Taudorf L, Nørgaard A, Islamoska S, et al. Declining incidence of dementia: a national registry-based study over 20 years. Alzheimers Dement. 2019;15(11):1383-1391. [DOI] [PubMed] [Google Scholar]
- 26.Phung TKT, Andersen BB, Høgh P, et al. Validity of dementia diagnoses in the Danish hospital registers. Dement Geriatr Cogn Disord. 2006;24(3):220-228. [DOI] [PubMed] [Google Scholar]
- 27.Hernán MA. The hazards of hazard ratios. Epidemiology. 2010;21(1):13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neumann A, Billionnet C. Covariate adjustment of cumulative incidence functions for competing risks data using inverse probability of treatment weighting. Comput Methods Programs Biomed. 2016;129:63-70. [DOI] [PubMed] [Google Scholar]
- 29.Mansournia MA, Altman DG. Population attributable fraction. BMJ. 2018;360:k757. [DOI] [PubMed] [Google Scholar]
- 30.Omland LH, Vestergaard HT, Dessau RB, et al. Characteristics and long-term prognosis of Danish patients with varicella zoster virus detected in the cerebrospinal fluid, compared with the background population. J Infect Dis. 2021;224(5):850-859. [DOI] [PubMed] [Google Scholar]
- 31.Grahn A, Nilsson S, Nordlund A, et al. Cognitive impairment 3 years after neurological Varicella-zoster virus infection: a long-term case control study. J Neurol. 2013;260(11):2761-2769. [DOI] [PubMed] [Google Scholar]
- 32.Mailles A, De Broucker T, Costanzo P, et al. Long-term outcome of patients presenting with acute infectious encephalitis of various causes in France. Clin Infect Dis. 2012;54(10):1455-1464. [DOI] [PubMed] [Google Scholar]
- 33.Hokkanen L, Launes J, Poutiainen E, et al. Subcortical type cognitive impairment in herpes zoster encephalitis. J Neurol. 1997;244(4):239-245. [DOI] [PubMed] [Google Scholar]
- 34.Eckerström M, Nilsson S, Zetterberg H, et al. Cognitive impairment without altered levels of cerebrospinal fluid biomarkers in patients with encephalitis caused by varicella-zoster virus: a pilot study. Sci Rep. 2020;10(1):22400-22409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Granerod J, Davies NWS, Ramanuj PP, et al. Increased rates of sequelae post-encephalitis in individuals attending primary care practices in the United Kingdom: a population-based retrospective cohort study. J Neurol. 2017;264(2):407-415. [DOI] [PubMed] [Google Scholar]
- 36.Gnann JW, Sköldenberg B, Hart J, et al. Herpes simplex encephalitis: lack of clinical benefit of long-term valacyclovir therapy. Clin Infect Dis. 2015;61(5):683-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schnier C, Janbek J, Williams L, et al. Antiherpetic medication and incident dementia: observational cohort studies in four countries. Eur J Neurol. 2021;28(6):1840-1848. [DOI] [PubMed] [Google Scholar]
- 38.Lophatananon A, Mekli K, Cant R, et al. Shingles, Zostavax vaccination and risk of developing dementia: a nested case-control study-results from the UK Biobank cohort. BMJ Open. 2021;11(10):e045871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lehrer S, Rheinstein PH. Herpes zoster vaccination reduces risk of dementia. Vivo. 2021;35(6):3271-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scherrer JF, Salas J, Wiemken TL, et al. Impact of herpes zoster vaccination on incident dementia: a retrospective study in two patient cohorts. PLoS One. 2021;16(11):e0257405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salem LC, Andersen BB, Nielsen TR, et al. Overdiagnosis of dementia in young patients - a nationwide register-based study. Dement Geriatr Cogn Disord. 2011;34(5-6):292-299. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt SAJ, Sørensen HT, Langan SM, Vestergaard M. Associations of lifestyle and anthropometric factors with the risk of herpes zoster: a nationwide population-based cohort study. Am J Epidemiol. 2021;190(6):1064-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jain A, van Hoek AJ, Walker JL, et al. Inequalities in zoster disease burden: a population‐based cohort study to identify social determinants using linked data from the U.K. Clinical Practice Research Datalink. Br J Dermatol. 2018;178(6):1324-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Esteban-Vasallo MD, Domínguez-Berjón MF, Gil-Prieto R, et al. Sociodemographic characteristics and chronic medical conditions as risk factors for herpes zoster: a population-based study from primary care in Madrid (Spain). Hum Vaccin Immunother. 2014;10(6):1650-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt SAJ, Sørensen HT, Langan SM, Vestergaard M. Perceived psychological stress and risk of herpes zoster: a nationwide population-based cohort study. Br J Dermatol. 2021;185(1):130-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gradus JL, Horvath-Puho E, Lash TL, et al. Stress disorders and dementia in the Danish population. Am J Epidemiol. 2019;188(3):493-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin WR, Shang D, Itzhaki RF. Neurotropic viruses and Alzheimer disease. Interaction of herpes simplex type 1 virus and apolipoprotein E in the etiology of the disease. Mol Chem Neuropathol. 1996;28(1-3):135-141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional unpublished data are available because this study used existing data from the Danish nationwide registries, which are accessible to researchers who fulfill local requirements following approval by the Danish Data Protection Agency and the Danish Health Data Authority.