Abstract
Background:
Orally administered water-soluble contrast (WSC) can track resolution of small-bowel obstruction (SBO), but no universal pathway for its use exists. We developed and implemented an evidence-based guideline for the use of WSC in the management of adhesive SBO, to be implemented across hospitals affiliated with the University of Toronto.
Methods:
We performed a systematic review and created a clinical practice guideline for WSC use in the management of adhesive SBO. The guideline was approved through consensus by an expert panel and implemented in 2018. We performed a prospective cohort study of guideline implementation at 1 pilot site (a large academic tertiary care centre), facilitated by the centre’s acute care general surgery service. Primary outcomes included compliance with the guideline and hospital length of stay (LOS). Secondary outcomes included rates of failure of nonoperative management, morbidity, mortality and readmission for recurrence of SBO within 1 year. Patients with adhesive SBO admitted in 2016 served as a control cohort.
Results:
We analyzed the data for 152 patients with adhesive SBO admitted to the centre, 65 in 2016 (historical cohort), 56 in January–June 2018 (transitional cohort) and 31 in July–December 2018 (implementation cohort). There was a significant increase in compliance with the WSC protocol in 2018, with the proportion of patients receiving WSC increasing from 45% (n = 25) in the transitional cohort to 71% (n = 22) in the implementation cohort (p < 0.001). The median LOS did not differ across the cohorts (p = 0.06). There was a significantly lower readmission rate in the transitional and implementation cohorts (13 [23%] and 9 [29%], respectively) than in the historical cohort (29 [45%]) (p = 0.04). Among patients assigned to nonoperative management initially, a significantly higher proportion of those who received WSC than those who did not receive WSC went on to undergo surgery (14.6% v. 3.6%, p = 0.01), with no difference in median time to surgery (p = 0.2).
Conclusion:
An evidence-based guideline for WSC use in SBO management was successfully developed and implemented; no difference in LOS or time to surgery was seen after implementation, but rates of immediate operation increased and readmission rates decreased. Our experience shows that implementation of an evidence-based clinical practice guideline is feasible through multidisciplinary efforts and coordination.
Abstract
Contexte:
La résolution de l’occlusion de l’intestin grêle (OIG) peut être surveillée par l’administration de produit de contraste hydrosoluble (PCH) par voie orale, mais il n’existe pas de protocole universel. Afin de pallier cette lacune, nous avons créé et mis en oeuvre une ligne directrice factuelle, destinée aux hôpitaux affiliés à l’Université de Toronto, pour l’administration de PCH dans la prise en charge de l’OIG sur adhérences.
Méthodes:
Nous avons réalisé une revue systématique puis élaboré un guide de pratique clinique, validé par consensus par un comité d’experts avant d’être mis en oeuvre en 2018. Une étude de cohorte prospective sur sa mise en oeuvre a été conduite au service de chirurgie générale d’urgence d’un établissement pilote (grand centre universitaire de soins tertiaires). Les indicateurs de résultats principaux étaient l’adhésion au guide et la durée d’hospitalisation; les secondaires comprenaient le taux d’échec de la prise en charge conservatrice, la morbidité, la mortalité et le taux de réadmission pour récidive d’OIG dans l’année. La cohorte témoin se composait de patients hospitalisés en 2016 pour une OIG sur adhérences.
Résultats:
Nous avons analysé les données de 152 patients atteints d’OIG sur adhérences admis au centre : 65 en 2016 (cohorte historique), 56 entre janvier et juin 2018 (cohorte de transition) et 31 entre juillet et décembre 2018 (cohorte d’application). Le respect du protocole d’administration de PCH a largement augmenté en 2018 : le pourcentage est passé de 45 % (n = 25) dans la cohorte de transition à 71 % (n = 22) dans la cohorte d’application (p < 0,001). La durée d’hospitalisation médiane est restée identique (p = 0,06), mais le taux de réadmission était beaucoup plus faible dans les cohortes de transition et d’application (13 [23 %] et 9 [29 %]) que dans la cohorte historique (29 [45 %]) (p = 0,04). L’administration de PCH dans le cadre de la prise en charge conservatrice de première intention est associée à une augmentation significative du taux de traitement chirurgical (14,6 % contre 3,6 % sans PCH; p = 0,01), sans variation du délai avant l’intervention (p = 0,2).
Conclusion:
Une ligne directrice factuelle sur l’administration de PCH dans la prise en charge de l’OIG a été conçue et mise en oeuvre. Aucune variation de la durée d’hospitalisation ou du délai avant l’intervention chirurgicale n’a été observée après l’application. Toutefois, le taux d’intervention chirurgicale à court terme a augmenté tandis que le taux de réadmission a diminué. Notre expérience montre que les efforts et la coordination pluridisciplinaires permettent d’appliquer un guide de pratique clinique fondé sur des données probantes.
Adhesions after abdominal surgery are the most common cause of small-bowel obstruction (SBO).1–3 Surgical intervention for SBO is sometimes necessary, and delays in treatment can lead to illness and death. Determining which SBOs will resolve non-operatively is not standardized and relies on clinical acumen, which leads to variability in the management of stable adhesive SBO in patients with no signs of bowel ischemia.4
Traditional nonoperative SBO management involves nasogastric tube decompression, fluid resuscitation and serial clinical assessments until the obstruction resolves or the surgeon determines that the patient needs an operation. This could take several days of monitoring, with no standardized universal algorithm or timeline for surgeons to follow.5
Hyperosmolar water-soluble radiographic contrast (WSC) such as Gastrografin (diatrizoate meglumine and diatrizoate sodium solution, Bracco Diagnostics) has been used successfully in SBO, both diagnostically and therapeutically, by administration through a nasogastric tube.6 Diagnostically, WSC can be used to track the transit time of the contrast through the gastrointestinal tract via abdominal radiography in patients with SBO whose condition is stable. The general approach is to give WSC via the nasogastric tube and obtain serial abdominal radiographs, looking for the contrast to reach the colon as an indicator of SBO resolution.
A wide range of protocols for the use of WSC in SBO management have been described in the literature.6–8 The use of a WSC guideline as an adjunct to timely decisionmaking is an appealing concept. Existing reviews and meta-analyses have shown reduced hospital length of stay (LOS) for patients with SBO overall,6–8 and some have suggested that the use of WSC reduces the need for surgery for SBO.7 The argument for this is that the high osmolarity of WSC agents such as Gastrografin can promote shifting of intestinal wall edema at the obstruction point into the bowel lumen. This can increase the pressure gradient across the obstruction as well as bowel motility to help the obstruction resolve.6 This is theoretical, with limited supporting evidence, as the majority of reviews have not shown a reduction in the need for surgery.7
Our aim was to systematically review the current literature on the use of WSC in the management of adhesive SBO and develop a university-wide consensus guideline for WSC use in SBO. The university-wide guideline was validated by concurrent pilot implementation at one of our institutions.
Methods
Literature search
We performed an electronic database search on MEDLINE to evaluate recent literature and outcomes data on the use of WSC for SBO. Medical Subject Headings searched included “small bowel obstruction” OR “intestinal obstruction” OR “mechanical obstruction” OR “adhesive obstruction” AND “water soluble contrast” OR “water-soluble contrast” OR “Gastrografin” AND “management.” The search included English-language articles published between 1990 and 2018. Articles with a focus on SBO related to malignant disease, inflammatory bowel disease, ileus, pseudo-obstruction or other nonadhesive causes were excluded, as were articles from the obstetric or pediatric populations.
We assessed the quality of evidence in adherence to the Scottish Intercollegiate Guidelines Network (SIGN) recommendations (https://www.sign.ac.uk).9
Guideline creation and pilot implementation
Using the results of the literature review, we created a clinical practice guideline for the use of WSC in the management of adhesive SBO. The goal was to implement the guideline across hospitals affiliated with the University of Toronto, a network of 5 adult academic centres and several community training sites.
One of the large academic tertiary care centres served as a pilot site to assess outcomes after guideline implementation. This was facilitated by the centre’s acute care general surgery service. The pilot project was approved by the hospital’s research ethics board.
At the pilot centre, a prospectively collected database was created when the guideline was introduced, in 2018. Patients were included if they presented to the emergency department and had a general surgery consultation for a diagnosis of an adhesive SBO in 2018. Only patients admitted to the acute care surgery service were included.
The pathway information was available as of January 2018, and a standardized order set was available in July 2018. Therefore, patients were classified into a transitional cohort (January–June 2018) and an implementation cohort (July–December 2018) to reflect the full guideline implementation. We collected control data from a historical cohort of patients meeting the same inclusion criteria in 2016. Patients who presented multiple times throughout the study period were categorized based on their index admission; we reviewed subsequent causes for readmission and included these cases as recurrences if the patient was admitted for SBO. Patients were excluded if their obstruction was due to nonadhesive causes (malignant disease, inflammatory bowel disease, functional causes or hernia-related), had had abdominal surgery in the previous 6 weeks, were pregnant or were pediatric patients (age < 18 yr).
After successful implementation of the proposed guideline at the pilot site, the guideline was reviewed by an expert panel of surgeons and radiologists as part of the Best Practice in Surgery initiative at the University of Toronto (http://bestpracticeinsurgery.ca) in order to roll out the guideline university wide. The final guideline was approved by the expert panel.10
Outcomes
The primary outcomes were compliance with the WSC guideline and hospital LOS. Secondary outcomes included rates of failure of nonoperative management and subsequent need for surgery, in-hospital morbidity, mortality and readmission for recurrent SBO within 1 year after hospital discharge.
Statistical analysis
We compared the historical, transitional and implementation cohorts with regard to the primary and secondary outcomes through univariate analyses using the χ2 test, oneway analysis of variance and the Kruskal–Wallis rank test, as appropriate. We also performed a multiple linear regression analysis to analyze factors contributing to readmission. The regression incorporated age, gender, past medical history, initial management plan and use of WSC. We performed calculations using Stata 16 (StataCorp), with an α value of 0.05.
Results
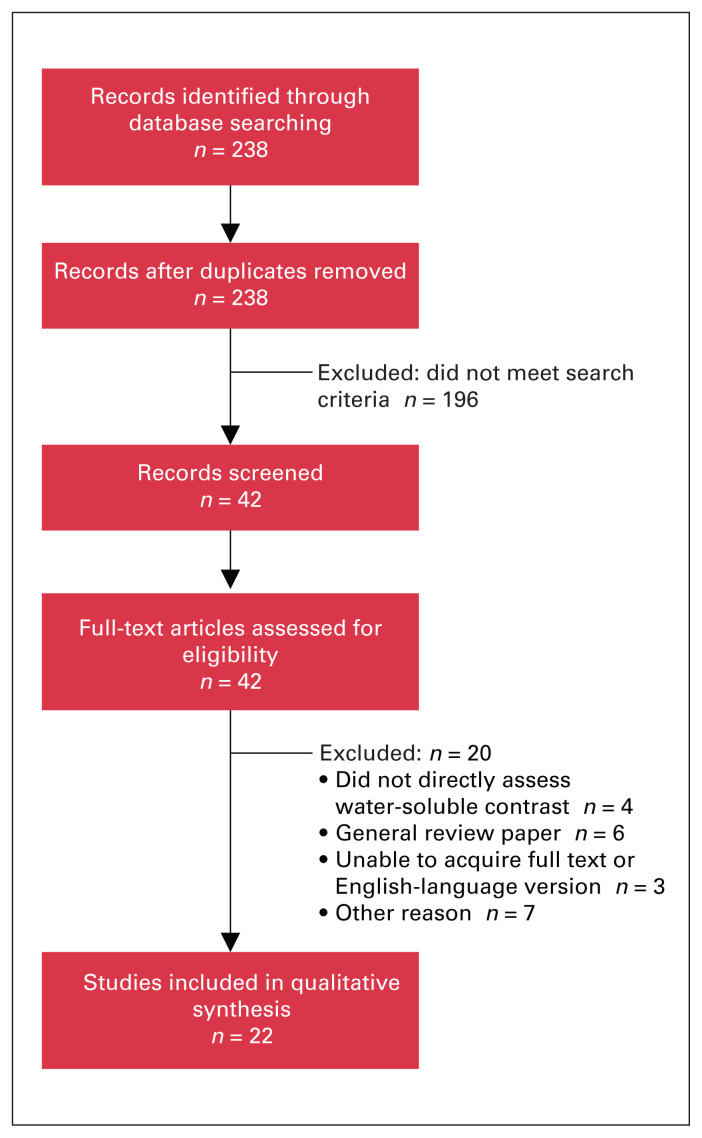
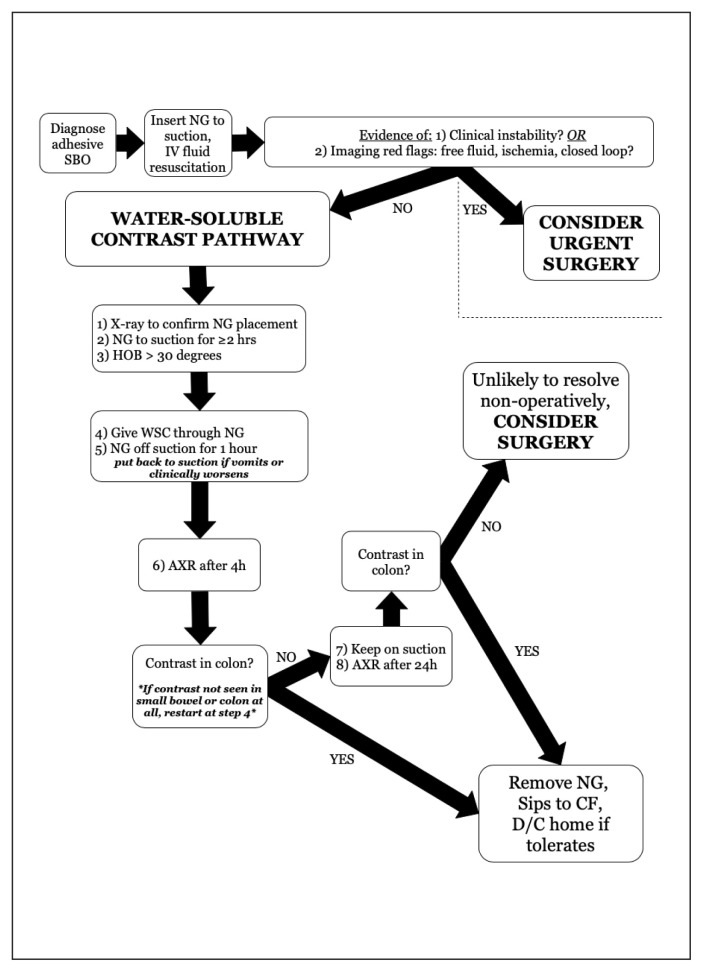
The literature search identified 238 articles, of which 196 were excluded because they did not meet the search criteria. The remaining 42 studies were reviewed, and another 20 studies were excluded (Figure 1). The remaining 22 studies (3 systematic reviews, 9 randomized controlled trials and 10 observational studies) (Table 1) were used to create the WSC guideline (Figure 2).
Fig. 1.
Flow diagram showing study selection.
Table 1.
Summary of evidence for the use of water-soluble contrast in the management of adhesive small-bowel obstruction
| Study | Study design/no. of patients | Supports WSC use | SIGN grade9 | Findings |
|---|---|---|---|---|
| Ceresoli et al.,6 2016 | Meta-analysis of 21 studies, 947 diagnostic, some data on therapeutic role | Yes | 1+ |
|
| Branco et al.,7 2010 | Meta-analysis of 14 studies (10 RCT, 4 observational), 508 diagnostic, 765 therapeutic | Yes | 1+ |
|
| Abbas et al.,8 2007 | Meta-analysis of 10 studies (6 RCT, 4 cohort) | Yes | 1+ |
|
| Scotté et al.,11 2017 | RCT, 242 (121 WSC v. 121 no WSC) | No | 1− |
|
| Farid et al.,12 2010 | RCT, 110 (55 WSC v. 55 no WSC) | Yes | 1− |
|
| Kumar et al.,13 2009 | RCT, 41 (21 WSC v. 20 no WSC) | Yes | 1− |
|
| Di Saverio et al.,14 2008 | RCT, 76 (38 WSC v. 38 no WSC) | Yes | 1− |
|
| Burge et al.,15 2005 | RCT, 35 (18 WSC v. 17 no WSC) | Yes | 1− |
|
| Biondo et al.,16 2003 | RCT, 90 (44 WSC v. 46 no WSC) | Yes | 1− |
|
| Choi et al.,17 2002 | RCT, 124 initially managed nonoperatively or operatively; those with SBO after 48 h received WSC (19) or operation (16) | Yes | 1− |
|
| Fevang et al.,18 2000 | RCT, 98 (48 WSC v. 50 no WSC) | No | 1− |
|
| Feigin et al.,19 1996 | RCT, 50 (25 WSC v. 25 no WSC) | No | 1− |
|
| Miquel et al.,20 2017 | Observational, 174 (all WSC) | Yes | 2− |
|
| Kuehn et al.,21 2017 | Observational, 105 (all WSC) | Yes | 2− |
|
| Zielinski et al.,22 2017 | Observational, 316 (173 WSC v. 143 no WSC) | Yes | 2+ |
|
| Bueno-Lledo et al.,23 2016 | Observational, 235 (all WSC) | Yes | 2− |
|
| Galardi et al.,24 2013 | Observational, 103 (72 WSC v. 31 no WSC) | Yes | 2+ |
|
| Atahan et al.,25 2010 | Observational, 37 (all WSC) | Yes | 2− |
|
| Kapoor et al.,26 2006 | Observational, 62 initially managed conservatively; those with SBO after 48 h received WSC (24) | Yes | 2− |
|
| Yagci et al.,27 2005 | Observational, 317 (199 WSC v. 118 no WSC) | Yes | 2+ |
|
| Aulin et al.,28 2005 | Observational, 126 (all WSC); if WSC reached colon within 8 h, trial result was considered negative | Yes | 2+ |
|
| Choi et al.,29 2005 | Observational, 245 (WSC after 48 h); if contrast did not appear in large bowel within subsequent 24 h, patient underwent surgery | Yes | 2− |
|
LOS = length of stay; RCT = randomized controlled trial; SBO = small-bowel obstruction; SIGN = Scottish Intercollegiate Guidelines Network; WSC = water-soluble contrast.
Fig. 2.
Pathway for the use of water-soluble contrast (WSC) in the management of adhesive small-bowel obstruction (SBO) (University of Toronto Best Practice in Surgery program10). AXR = abdominal radiography; CF = clear fluid; D/C = discharge; HOB = head of bed; IV = intravenous; NG = nasogastric tube.
Protocols and time intervals for radiography after WSC administration were found to vary, but most studies suggest allowing 2–36 hours for the contrast to reach the colon, after which the obstruction is very unlikely to resolve on its own.6 The reviewed studies showed high sensitivity and specificity for successful WSC passage to the colon to predict SBO resolution, and suggested decreased LOS with the use of WSC.6–8 Our choice of timing of radiography after WSC was informed by a comparative analysis performed by Ceresoli and colleagues6 showing equivalence of various timing protocols.
Pilot study results
A total of 297 patients were admitted with SBO in the studied time frames (2016 and 2018). Of the 297, 152 had adhesive SBOs (65 in the historical cohort, 56 in the transitional cohort and 31 in the implementation cohort). The patients’ baseline characteristics are summarized in Table 2. There were no significant differences in age, noncardiac comorbidities or surgical history among the 3 cohorts. There was a significantly higher prevalence of cardiac comorbidities in the implementation group than in the 2 other cohorts (p < 0.001).
Table 2.
Baseline characteristics of patients admitted for adhesive small-bowel obstruction
| Characteristic | Cohort; no. (%) of patients* | p value† | ||
|---|---|---|---|---|
| Historical n = 65 |
Transitional n = 56 |
Implementation n = 31 |
||
| Age, mean ± SD, yr | 61.4 ± 17.5 | 69.4 ± 17.5 | 68.0 ± 18.8 | 0.06‡ |
| Female gender | 31 (48) | 30 (54) | 24 (77) | 0.02§ |
| Comorbidities | ||||
| Cardiac | 9 (14) | 18 (32) | 16 (52) | < 0.001§ |
| Respiratory | 5 (8) | 6 (11) | 4 (13) | 0.6§ |
| Renal | 2 (3.) | 4 (7) | 3 (10) | 0.4§ |
| Diabetes | 7 (11) | 13 (23) | 3 (10) | 0.1§ |
| History of cancer | 30 (46) | 20 (36) | 14 (45) | 0.5§ |
| Past surgical history | 59 (89) | 55 (95) | 30 (97) | 0.4§ |
SD = standard deviation.
Except where noted otherwise.
Historical cohort versus transitional cohort versus implementation cohort.
One-way analysis of variance.
χ2 test.
Initial planned management (operative v. nonoperative) varied among the cohorts: a significantly higher proportion of patients in the transitional and implementation groups (13 [23%] and 4 [13%], respectively) than in the historical group (4 [6%]) were scheduled for immediate operative intervention (p = 0.03). The remaining patients were managed nonoperatively, and there was no difference between cohorts in the proportion who required an operation after failure of initial nonoperative management (p = 0.2) (Table 3). There was no significant difference in nasogastric tube use across the groups. Among patients who had surgery, there was a significantly lower rate of open operation in the transitional cohort than in the historical and implementation cohorts (3 [17%] v. 3 [60%] and 4 [57%], respectively, p = 0.001). There was also a significantly higher rate of bowel resection in the transitional cohort (7 [12%] v. 1 [2%] and 3 [10%], respectively, p = 0.04).
Table 3.
Management
| Variable | Cohort; no. (%) of patients | p value* | ||
|---|---|---|---|---|
| Historical | Transitional | Implementation | ||
| Planned management | ||||
| Initial nonoperative | 61 (94) | 43 (77) | 27 (87) | 0.03 |
| Initial operative | 4 (6) | 13 (23) | 4 (13) | |
| Nasogastric tube use | 52 (80) | 48 (86) | 29 (94) | 0.2 |
| Received WSC | 1 (2) | 25 (45) | 22 (71) | < 0.001 |
| Operative approach† | ||||
| Open | 3 (60) | 3 (17) | 4 (57) | 0.001 |
| Laparoscopic | 2 (40) | 15 (83) | 3 (43) | |
| Bowel resection | 1 (2) | 7 (12) | 3 (10) | 0.04 |
| Failed nonoperative intent, required surgery | 2 (3) | 4 (7) | 4 (13) | 0.2 |
WSC = water-soluble contrast.
Historical cohort versus transitional cohort versus implementation cohort; χ2 test.
Denominator is the number of patients in each cohort who proceeded to surgery (initially or after failed nonoperative trial).
Outcomes
There was a significant increase in compliance with the WSC protocol in 2018, with the proportion of patients receiving WSC increasing from 45% (n = 25) in the transitional cohort to 71% (n = 22) in the implementation cohort (p < 0.001).
There was no significant difference between the cohorts in median LOS (3 d, 4 d and 2 d in the historical, transitional and implementation cohorts, respectively, p = 0.06) (Table 4). The differences in LOS remained nonsignificant even after we stratified by initial management intent (p = 0.2).
Table 4.
Outcomes
| Outcome | Cohort; no. (%) of patients* | p value† | ||
|---|---|---|---|---|
| Historical | Transitional | Implementation | ||
| Length of stay, median, d | ||||
| All patients | 3 | 4 | 2 | 0.06‡ |
| Patients with initial nonperative intent | 3 | 3 | 2 | 0.2‡ |
| 1-yr readmission for recurrence | 29 (45) | 13 (23) | 9 (29) | 0.04§ |
| No. of recurrences, mean ± SD | ||||
| All patients | 0.95 ± 1.11 | 0.43 ± 0.92 | 0.42 ± 0.67 | 0.003‡ |
| Patients with previous surgery | 1.00 ± 1.11 | 0.00 ± 0.94 | 0.43 ± 0.69 | 0.002‡ |
| Operation for recurrence | 8 (28) | 2 (15) | 2 (22) | 0.8§ |
| Time to follow-up, median, d | 33.0 | 31.5 | 29.0 | 0.3‡ |
| Complications | ||||
| Any | 2 (3) | 3 (5) | 3 (10) | 0.5§ |
| Clavien–Dindo grade > III | 1 (2) | 2 (4) | 0 (0) | 0.5§ |
| Died | 0 (0) | 4 (7) | 1 (3) | 0.07§ |
SD = standard deviation.
Except where noted otherwise.
Historical cohort versus transitional cohort versus implementation cohort.
Kruskal–Wallis test.
χ2 test.
There was a significantly lower readmission rate in the transitional and implementation cohorts (13 [23%] and 9 [29%], respectively) than in the historical cohort (29 [45%]) (p = 0.04) (Table 4). The median time to follow-up in clinic was similar across cohorts (33.0 d, 31.5 d and 29.0 d in the historical, transitional and implementation cohorts, respectively, p = 0.3). Among readmitted patients, there was no significant difference between the cohorts in the number of those who required an operation (p = 0.8), or in overall morbidity (p = 0.5) or mortality (p = 0.07).
No complications secondary to aspiration or aspiration pneumonitis related to oral contrast administration were observed in any of the cohorts. There were 5 deaths across the cohorts, 4 (7%) in the transitional group, 1 (1%) in the implementation group and 0 in the historical group (p = 0.07) (Table 4). The causes of death were postoperative sepsis and multiorgan failure in 1 patient in the transitional group, and worsening obstruction leading to bowel compromise in 4 patients, all of whom were older patients who opted for palliative care. Three of the older patients had received WSC before clinical deterioration.
In a subgroup analysis among patients assigned to nonoperative management initially, a significantly higher proportion of those who received WSC than those who did not receive WSC went on to undergo surgery (7/48 [14.6%] v. 3/83 [3.6%], p = 0.01) (Table 5). The median time to surgery was 1 day in both groups. Among the 41 patients who received WSC and did not proceed to surgery, a significantly higher proportion had SBO resolution at 4 hours than at 24 hours (24 [58.5%] v. 12 [29.3%], p = 0.04) (Table 5); none required surgery after the SBO was deemed to be resolved.
Table 5.
Subgroup analysis by water-soluble contrast status
| Variable | No. (%) of patients* | p value | |
|---|---|---|---|
| No WSC n = 104 |
WSC n = 48 |
||
| Planned initial management | |||
| Nonoperative | 83 (79.8) | 48 (100.0) | < 0.001§¶ |
| Operative | 21 (20.2) | 0 (0.0) | |
| Nasogastric tube use | 81 (77.9) | 48 (100.0) | < 0.001§¶ |
| Failed nonoperative intent, required surgery | 3 (3.6) | 7 (14.6) | 0.01§¶ |
| Operative approach† | |||
| Open | 17 (73.9) | 4 (57.1) | 0.7§¶ |
| Laparoscopic | 5 (21.7) | 3 (42.9) | |
| Bowel resection | 9 (8.6) | 2 (4.2) | 0.5§¶ |
| Timing of radiographic resolution after WSC administration, h‡ | |||
| 4 | — | 24 (58.5) | 0.04¶** |
| 24 | — | 12 (29.3) | |
| Time to surgery, median, d | 1 | 1 | 0.2§†† |
WSC = water-soluble contrast.
Except where noted otherwise.
Denominator is the number of patients in each cohort who proceeded to surgery (initially or after failed nonoperative trial).
In 12 patients the SBO resolved after 24 hours, at which point no radiograph would have been obtained.
Comparing WSC versus no WSC.
χ2 test.
Comparing resolution at 4 hours versus 24 hours.
Wilcoxon rank-sum test.
On multiple regression analysis, only age was found to have a significant effect on time to readmission, with older patients having earlier recurrences (p = 0.03).
Discussion
Our study showed that, compared to traditional management approaches, the use of a WSC guideline in the management of adhesive SBO was associated with lower rates of readmission for recurrence, with no change in morbidity or mortality rates. There was a significant increase in compliance with the guideline over the study period, which indicates the feasibility of a newly developed and implemented guideline.
Although other authors have suggested that use of a WSC protocol reduces LOS,7,8,14–16 our pilot study showed no significant change in LOS after implementation of the WSC guideline. This may have been due to the early adaptation to and challenges of implementing a new clinical practice in a real-world setting. With ongoing use and familiarity with the guideline, this may change. The rate of compliance with the guideline reached more than 70% in the 6 months after implementation at our pilot centre.
We found that, among patients assigned to nonoperative management initially, a significantly higher proportion of those who received WSC than those who were managed in the traditional nonoperative manner without WSC went on to undergo surgery (15% v. 4%, respectively). These findings are contrary to the hypothesis that the use of WSC reduces the need for surgery.6 After implementation of the WSC protocol, we saw a significantly lower 1-year readmission rate compared to the historical group (29% v. 45%), with a similar median time to follow-up.
A potential explanation for the higher surgery rates among patients who received WSC and were initially assigned to nonoperative management may be a temporal shift in the management of SBO at our institution. The WSC pathway allows for earlier operation instead of the traditional approach of prolonged waiting for nonoperative resolution, leading to more operations overall. This is supported by recent evidence from our centre. Behman and colleagues30 reported that operative management of the first episode of adhesive SBO was associated with reduced risk of recurrence, and another study by the same group showed improved long-term survival, a lower rate of complications and better outcomes with earlier operation.31 Given these and other data,3 a practice change for SBO management may be warranted. Some surgeons are opting to operate sooner rather than later, even in patients with no signs of bowel ischemia whose condition is stable.30,31
In the patients in whom the WSC trial failed (i.e., who did not have contrast in the colon past the obstruction point at 24 h), the SBO may have resolved after 24 hours if given the chance and managed in the same manner as the traditional approach group. With the limits of a retrospective review, it is challenging to determine this. However, our approach of performing radiography at 4 hours and then 24 hours, then proceeding to plan an operation if the SBO is not resolved at 24 hours, may still be warranted given the data showing reduced recurrence and morbidity with operative management.30,31
The majority of patients who received WSC and were successfully managed nonoperatively had SBO resolution on the 4-hour radiograph. This implies that a shorter interval may be needed to assess the success of WSC, thus accelerating clinical decision-making. For these patients, the reasons behind the delay between resolution at 4 hours and discharge are unclear. It may have been due to potential delays in receiving radiograph reports or in nasogastric tube removal and diet advancement; however, these data were not collected as part of this study.
Successful implementation of a hospital-wide evidence-based guideline was enabled by the early involvement of multiple key stakeholders, including surgeons, radiologists, nursing staff and radiology technologists. The guideline is continually being reviewed and refined, and we recommend the following quality-improvement strategies, which have been used to allow ongoing improvement and sustainability at our institution:
Create a local implementation team. Ensure the engagement of members of the radiology department along with their staff and imaging technicians. As well, invite nurses from the emergency department and wards to assist with implementation. Having a local resident on the implementation team is strongly recommended, as this allows input from a front-line trainee’s perspective.
Make the recommendations part of a dedicated electronic/standardized patient order set.
Provide education to nursing staff, specifically in the emergency department and on surgical wards, on the protocol and their roles in nasogastric tube management, contrast administration and timing of radiography.
If not already available, institute a system of rapid outpatient follow-up after discharge (< 2 wk) to ensure complete resolution of the SBO and no further concerns. The acute care surgery model at many hospitals already has this clinic resource for follow-up in place. If rapid follow-up is not available, ensure that there are clear discharge instructions as to when the patient should contact the surgeon or return to the emergency department if symptoms recur.
Limitations
The strengths of our study include its prospective data collection, relatively high short-term compliance rates and the literature review used to create a universal guideline for the use of WSC in the management of adhesive SBO. The study’s limitations are its retrospective nature and the limited sample sizes included in each cohort, most prominently in the implementation cohort.
Conclusion
A guideline for the use of WSC in the management of adhesive SBO was successfully developed and implemented on an acute care surgery service. Although LOS did not change after implementation of the guideline, the number of patients undergoing immediate surgery increased and the number of readmissions decreased. This may have been due to early operative decision-making as a result of the guideline, or it may represent a change in management practices over time. Our experience shows that the implementation of an evidence-based clinical practice guideline is feasible through multidisciplinary efforts and coordination.
Footnotes
Presented in part at the Canadian Surgery Forum, Sept. 5–7, 2019, Montréal, Que., and the 14th Annual Academic Surgical Congress, Feb. 5–7, 2019, Houston, Tex.
Competing interests: Ashlie Nadler reports an Academic enrichment fund grant from her surgical group practice, Division of General Surgery, Sunnybrook Health Sciences Centre. No other competing interests were declared.
Contributors: B. Elsolh, M.-A. Nguyen, F. Berger, E. Pearsall, D. Naidu and A. Nadler designed the study. B. Elsolh and M.-A. Nguyen acquired the data, which B. Elsolh, C. Patel, R. McLeod and A. Nadler analyzed. B. Elsolh wrote the manuscript, which M.-A. Nguyen, F. Berger, C. Patel, E. Pearsall, R. McLeod, D. Naidu and A. Nadler critically revised. All authors gave final approval of the article to be published.
References
- 1.Ten Broek RPG, Krielen P, Di Saverio S, et al. Bologna guidelines for diagnosis and management of adhesive small bowel obstruction (ASBO): 2017 update of the evidence-based guidelines from the World Society of Emergency Surgery ASBO working group. World J Emerg Surg 2018;13:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ten Broek RPG, Issa Y, Van Santbrink EJP, et al. Burden of adhesions in abdominal and pelvic surgery: systematic review and meta-analysis. BMJ 2013;347:f5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Steensel S, van den Hil LCL, Schreinemacher MHF, et al. Adhesion awareness in 2016: an update of the national survey of surgeons. PLoS One 2018;13:e0202418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foster NM, McGory ML, Zingmond DS, et al. Small bowel obstruction: a population-based appraisal. J Am Coll Surg 2006;203: 170–6. [DOI] [PubMed] [Google Scholar]
- 5.Azagury D, Liu RC, Morgan A, et al. Small bowel obstruction: a practical step-by-step evidence-based approach to evaluation, decision making, and management. J Trauma Acute Care Surg 2015;79: 661–8. [DOI] [PubMed] [Google Scholar]
- 6.Ceresoli M, Coccolini F, Catena F, et al. Water-soluble contrast agent in adhesive small bowel obstruction: a systematic review and meta-analysis of diagnostic and therapeutic value. Am J Surg 2016; 211:1114–25. [DOI] [PubMed] [Google Scholar]
- 7.Branco BC, Barmparas G, Schnüriger B, et al. Systematic review and meta-analysis of the diagnostic and therapeutic role of water-soluble contrast agent in adhesive small bowel obstruction. Br J Surg 2010; 97:470–8. [DOI] [PubMed] [Google Scholar]
- 8.Abbas S, Bissett IP, Parry BR. Oral water soluble contrast for the management of adhesive small bowel obstruction. Cochrane Database Syst Rev 2007;(3):CD004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baird AG, Lawrence JR. Guidelines: Is bigger better? A review of SIGN guidelines. BMJ Open 2014;4:e004278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The adhesive small bowel obstruction water-soluble contrast guideline. A clinical practice guideline developed by the University of Toronto’s Best Practice in Surgery group. Toronto: University of Toronto Best Practice in Surgery program; 2019. Available: http://bestpracticeinsurgery.ca/guidelines/sbo/ (accessed 2020 Mar. 30). [Google Scholar]
- 11.Scotté M, Mauvais F, Bubenheim M, et al. Use of water-soluble contrast medium (Gastrografin) does not decrease the need for operative intervention nor the duration of hospital stay in uncomplicated acute adhesive small bowel obstruction? A multicenter, randomized, clinical trial (Adhesive Small Bowel Obstruction Study) and systematic review. Surgery 2017;161:1315–25. [DOI] [PubMed] [Google Scholar]
- 12.Farid M, Fikry A, El Nakeeb A, et al. Clinical impacts of oral Gastrografin follow-through in adhesive small bowel obstruction (SBO). J Surg Res 2010;162:170–6. [DOI] [PubMed] [Google Scholar]
- 13.Kumar P, Kaman L, Singh G, et al. Therapeutic role of oral water soluble iodinated contrast agent in postoperative small bowel obstruction. Singapore Med J 2009;50:360–4. [PubMed] [Google Scholar]
- 14.Di Saverio S, Catena F, Ansaloni L, et al. Water-soluble contrast medium (Gastrografin) value in adhesive small intestine obstruction (ASIO): a prospective, randomized, controlled, clinical trial. World J Surg 2008;32:2293–304. [DOI] [PubMed] [Google Scholar]
- 15.Burge J, Abbas SM, Roadley G, et al. Randomized controlled trial of Gastrografin in adhesive small bowel obstruction. ANZ J Surg 2005; 75:672–4. [DOI] [PubMed] [Google Scholar]
- 16.Biondo S, Parés D, Mora L, et al. Randomized clinical study of Gastrografin® administration in patients with adhesive small bowel obstruction. Br J Surg 2003;90:542–6. [DOI] [PubMed] [Google Scholar]
- 17.Choi HK, Chu KW, Law WL. Therapeutic value of Gastrografin in adhesive small bowel obstruction after unsuccessful conservative treatment: a prospective randomized trial. Ann Surg 2002;236:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fevang BT, Jensen D, Fevang J, et al. Upper gastrointestinal contrast study in the management of small bowel obstruction — a prospective randomised study. Eur J Surg 2000;166:39–43. [DOI] [PubMed] [Google Scholar]
- 19.Feigin E, Seror D, Szold A, et al. Water-soluble contrast material has no therapeutic effect on postoperative small-bowel obstruction: results of a prospective, randomized clinical trial. Am J Surg 1996; 171:227–9. [DOI] [PubMed] [Google Scholar]
- 20.Miquel J, Biondo S, Kreisler E, et al. Failure of conservative treatment with Gastrografin® for adhesive small bowel obstruction after colorectal surgery. Int J Colorectal Dis 2017;32:1051–5. [DOI] [PubMed] [Google Scholar]
- 21.Kuehn F, Weinrich M, Ehmann S, et al. Defining the need for surgery in small-bowel obstruction. J Gastrointest Surg 2017; 21:1136–41. [DOI] [PubMed] [Google Scholar]
- 22.Zielinski MD, Haddad NN, Cullinane DC, et al. Multi-institutional, prospective, observational study comparing the Gastrografin challenge versus standard treatment in adhesive small bowel obstruction. J Trauma Acute Care Surg 2017;83:47–54. [DOI] [PubMed] [Google Scholar]
- 23.Bueno-Lledó J, Barber S, Vaqué J, et al. Adhesive small bowel obstruction: predictive factors of lack of response in conservative management with Gastrografin. Dig Surg 2016;33:26–32. [DOI] [PubMed] [Google Scholar]
- 24.Galardi N, Collins J, Friend K. Use of early Gastrografin small bowel follow-through in small bowel obstruction management. Am Surg 2013;79:794–6. [PubMed] [Google Scholar]
- 25.Atahan K, Aladagli I, Cökmez A, et al. Hyperosmolar water-soluble contrast medium in the management of adhesive small-intestine obstruction. J Int Med Res 2010;38:2126–34. [DOI] [PubMed] [Google Scholar]
- 26.Kapoor S, Jain G, Sewkani A, et al. Prospective evaluation of oral Gastrografin in postoperative small bowel obstruction. J Surg Res 2006;131:256–60. [DOI] [PubMed] [Google Scholar]
- 27.Yagci G, Kaymakcioglu N, Can MF, et al. Comparison of Urografin versus standard therapy in postoperative small bowel obstruction. J Invest Surg 2005;18:315–20. [DOI] [PubMed] [Google Scholar]
- 28.Aulin A, Sales JP, Bachar S, et al. Telebrix Gastro in the management of adhesive small bowel obstruction. Gastroenterol Clin Biol 2005;29: 501–4. [DOI] [PubMed] [Google Scholar]
- 29.Choi HK, Law WL, Ho JWC, et al. Value of Gastrografin in adhesive small bowel obstruction after unsuccessful conservative treatment: a prospective evaluation. World J Gastroenterol 2005; 11:3742–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Behman R, Nathens AB, Mason S, et al. Association of surgical intervention for adhesive small-bowel obstruction with the risk of recurrence. JAMA Surg 2019;154:413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Behman R, Nathens AB, Haas B, et al. Surgery for adhesive small-bowel obstruction is associated with improved long-term survival mediated through recurrence prevention: a population-based, propensity-matched analysis. J Trauma Acute Care Surg 2019;87: 636–44. [DOI] [PubMed] [Google Scholar]