Abstract
Background:
London InterCommunity Health Centre (LIHC) launched a safer opioid supply (SOS) program in 2016, where clients are prescribed pharmaceutical opioids and provided with comprehensive health and social supports. We sought to evaluate the impact of this program on health services utilization and health care costs.
Methods:
We conducted an interrupted time series analysis of London, Ontario, residents who received a diagnosis of opioid use disorder (OUD) and who entered the SOS program between January 2016 and March 2019, and a comparison group of individuals matched on demographic and clinical characteristics who were not exposed to the program. Primary outcomes were emergency department (ED) visits, hospital admissions, admissions for infections and health care costs. We used autoregressive integrated moving average (ARIMA) models to evaluate the impact of SOS initiation and compared outcome rates in the year before and after cohort entry.
Results:
In the time series analysis, rates of ED visits (−14 visits/100, 95% confidence interval [CI] −26 to −2; p = 0.02), hospital admissions (−5 admissions/100, 95% CI −9 to −2; p = 0.005) and health care costs not related to primary care or outpatient medications (−$922/person, 95% CI −$1577 to −$268; p = 0.008) declined significantly after entry into the SOS program (n = 82), with no significant change in rates of infections (−1.6 infections/100, 95% CI −4.0 to 0.8; p = 0.2). In the year after cohort entry, the rate of ED visits (rate ratio [RR] 0.69, 95% CI 0.53 to 0.90), hospital admissions (RR 0.46, 95% CI 0.29 to 0.74), admissions for incident infections (RR 0.51, 95% CI 0.27 to 0.96) and total health care costs not related to primary care or outpatient medications ($15 635 v. $7310/person-year; p = 0.002) declined significantly among SOS clients compared with the year before. We observed no significant change in any of the primary outcomes among unexposed individuals (n = 303).
Interpretation:
Although additional research is needed, this preliminary evidence indicates that SOS programs can play an important role in the expansion of treatment and harm-reduction options available to assist people who use drugs and who are at high risk of drug poisoning.
The opioid overdose crisis is a major, continuing public health issue, with more than 29 000 opioid-related toxicity deaths occurring in Canada between January 2016 and December 2021.1 This crisis is driven primarily by contamination of the unregulated drug supply with illicitly derived fentanyl and fentanyl analogues, which directly contributed to 87% of opioid-related deaths in Ontario in 2020.2 In response, several interventions have been adapted or scaled up, including the distribution of naloxone to reverse opioid overdose,3 supervised consumption services and overdose prevention sites,4,5 opioid agonist therapy (OAT) and injectable OAT programs (iOAT).6–8 Evidence suggests that the expansion of these harm-reduction interventions across Canada since 2016 has averted some overdose-related deaths;9 however, slow scale-up and inequitable access to interventions across the country5,10,11 remain major impediments to a comprehensive response to the overdose crisis, which has worsened during the COVID-19 pandemic.2
Safer opioid supply (SOS) programs, in which individuals at high risk of overdose are prescribed pharmaceutical opioids as an alternative to a fentanyl-adulterated drug supply, have been integrated into the harm-reduction arsenal of several jurisdictions. 12–14 In these programs, the off-label prescription of pharmaceutical opioids — generally daily-dispensed, immediate-release hydromorphone provided as take-home doses — is often paired with long-acting opioid medications (primarily slow-release oral morphine and, less frequently, methadone) as well as additional interventions to promote engagement with care and management of co-existing conditions.15 This model was followed for Canada’s first formal SOS program, which began at the London InterCommunity Health Centre (LIHC) in 2016. In addition to providing pharmaceutical opioids, the LIHC program offers comprehensive health and social services, including primary care and management of infection with HIV and hepatitis C virus (HCV), alongside access to an interdisciplinary team that provides harm-reduction equipment, counselling, housing support and social services (details about the LIHC SOS program can be found in Appendix 1, eTable 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.220892/tab-related-content).16 Recent research examining pharmacy claims data found that safer supply prescribing has gradually increased in Ontario during this period, with 534 new initiations occurring between January 2016 and March 2020,15 and a peak in initiations in the second half of 2019, which corresponds to the publication of a safer supply guiding document for prescribers.12
More recently, practice and policy changes have supported the piloting and delivery of SOS programs across Canada. Specifically, in 2020, Health Canada announced funding for several pilot SOS programs,17 and there was cautious acknowledgement of safer supply prescribing by provincial regulators in Ontario.18 Furthermore, guidelines for risk mitigation prescribing (an adaptation of SOS to facilitate COVID-19–related isolation and physical distancing) were developed in 2 Canadian provinces (British Columbia and Quebec),19,20 and “prescribed safer supply” was integrated into a practice update on the treatment of opioid use disorder (OUD) in British Columbia.21 Despite the potential to reduce opioid-related deaths, concern has been raised about the possibility of patient harm with the provision of off-label opioids, including risks of invasive infections (hypothesized to occur from injecting crushed hydromorphone tablets) and overdose,22 and there is currently little published evidence describing the real-world impacts of SOS programs. We therefore conducted a study of health outcomes, health services utilization and health care costs among people exposed to the LIHC SOS program, to explore its relative effectiveness and safety. To test the specificity of our findings, we replicated our analyses in a comparison group of individuals with OUD who were not exposed to the LIHC program, living in the same region as LIHC clients and matched to LIHC clients on several demographic, health service and clinical variables.
Methods
Setting and design
We conducted an interrupted time series analysis among clients of the LIHC SOS program and unexposed residents of the Middlesex-London Public Health Unit (PHU) in Ontario who received a diagnosis of an OUD and were alive on Jan. 1, 2016, and who had a health care encounter related to a diagnosis of an OUD between Jan. 1, 2016, and Mar. 31, 2019.
Data sources
As part of the broader Health Outreach programming offered by LIHC, which provides support for people experiencing homelessness or who are heavily street involved in London, Ontario, the SOS program was initially offered to LIHC clients who were experiencing multiple, serious medical complications owing to their drug use and who were believed to be at high risk of imminent death as a result of unmanaged health conditions (such as recurrent infective endocarditis or untreated HIV). As the program developed, priority was also given to people whose needs were not being met by traditional health care, substance use or addiction treatment programs because of their drug use, homelessness or street-involvement (please see Appendix 1, eTable 1 for more information on eligibility criteria). We obtained client data from the LIHC SOS program, 23 which captured information on all individuals enrolled in this program over the study period. These data were deterministically (using client health card number) or probabilistically (using full name and date of birth) linked to Ontario’s administrative health databases at ICES, based on available client information. We used various health administrative databases to define the unexposed population and study outcomes, including the Narcotics Monitoring System, the Ontario Health Insurance Plan database, the Drug and Drug/Alcohol Related Death Database, the Ontario Drug Benefit database and the Canadian Institute for Health Information’s Discharge Abstract Database, National Ambulatory Care Reporting System and Ontario Mental Health Reporting System databases (see Appendix 1, eTable 2 for full list of databases and descriptions). We used these databases to identify individuals’ health care utilization and attached unit costs to service use.24 We excluded costs related to primary care physician services when calculating total health care costs because ICES databases do not include the costs from community health centres such as the LIHC. These data sets were linked using unique encoded identifiers and analyzed at ICES.
Study population
We defined the study population as all clients of the LIHC SOS program who entered the program during the study period, each of whom was matched with residents of the Middlesex-London PHU who were not exposed to the program. The unexposed group was defined as individuals within the PHU who were alive on Jan. 1, 2016; had a health care encounter or received treatment for an OUD between Jan. 1, 2014, and the cohort entry date; and had not received daily-dispensed, immediate-release hydromorphone in the 90 days before cohort entry, a restriction applied to exclude those who might have accessed SOS outside of the LIHC program. We defined OUD as receipt of OAT (methadone or buprenorphine-naloxone) or a health care encounter for OUD over this time period (Appendix 1, eTable 3). We excluded individuals with missing or invalid data on age or sex, those with inaccurate death information and those older than 105 years. The cohort entry date was the date of program entry for LIHC clients, and we assigned unexposed London residents the same cohort entry date as their matched LIHC-exposed counterpart. We matched each SOS-exposed individual with replacement with up to 4 unexposed London residents with OUD on age (within 3 years), sex, eligibility for public drug benefits, neighbourhood income quintile (based on postal code listed on health card), hospital visit for an opioid poisoning (past year), OAT (past year) and hospital admission for infective endocarditis (past year).
Clinical characteristics
Comorbidities measured included diagnosis of HIV or HCV infection, hospital admissions for mental health diagnoses (past year) and past-year hospital admissions for serious infections (osteomyelitis, septic arthritis, spinal infections, and skin and soft-tissue infections). We also measured prior health services utilization, including the number of physician visits, ED visits and inpatient hospital admissions in the previous year. Finally, we identified previous medication dispensing, including receipt of benzodiazepines (previous 30 d), OAT (previous 30 d, 1 yr and 5 yr), immediate-release and long-acting hydromorphone (previous 90 d), and any opioid prescription (previous 90 d).
Outcomes
Our primary outcomes were ED visits, inpatient hospital admissions, admissions for incident infections, and health care costs not related to primary care or outpatient medications. Secondary outcomes included mental health ED visits or admissions, opioid-related ED visits or admissions, ED visits or admissions related to any substance use disorder, and opioid-related deaths. Among individuals eligible for public drug benefits, we determined total costs of publicly funded medications and costs for hydromorphone and OAT. Details on outcome definitions can be found in Appendix 1, eTable 4. For interrupted time series analyses, we determined rates of each primary outcome in 30-day intervals in the 5 years before and 1 year (inclusive of month of cohort entry) after cohort entry. We determined outcome rates for each 30-day interval among individuals alive and eligible at the start of the interval. We also calculated annual rates of all primary and secondary outcomes in the twelve 30-day intervals before and after cohort entry, censoring individuals on death and program exit (exposed individuals only).
Statistical analysis
We summarized demographic and clinical characteristics using descriptive statistics and identified meaningful differences between groups using weighted standardized differences, where a value > 0.10 indicates a meaningful difference. 25 A difference-in-difference analysis was not appropriate as the parallel trends assumption necessary for drawing valid inferences from this type of analysis was violated. Therefore, we conducted an interrupted time series analysis using interventional autoregressive integrated moving average (ARIMA) models, to evaluate the safety and effectiveness of the LIHC SOS program for each of our primary outcomes over 30-day windows across the 6-year study period, with the unexposed group acting as a test of the specificity of the model findings. In all models we tested for change in the prevalence of outcomes using a step intervention function. We assessed stationarity using augmented Dickey–Fuller tests and differenced the time series as needed to produce stationary time series. We examined plots of the autocorrelation function, partial autocorrelation function and inverse correlation function to identify autoregressive and moving average components in each time series and corrected for autocorrelation remaining after differencing. We used residual plots and Ljung–Box χ2 tests to confirm that residuals from ARIMA models were a white noise process. We used negative binomial regression and Wilcoxon signed rank tests for comparisons of health care utilization and costs in the year before and after cohort entry. We performed analyses using SAS Enterprise Guide 7.1 (SAS Institute Inc., Cary, North Carolina).
Sensitivity analyses
We conducted several post hoc sensitivity analyses. First, we added matching criteria on HIV diagnosis at cohort entry to address ongoing observed imbalances in the primary analysis. Second, we compared the consistency of our findings using a ramp intervention function in ARIMA models. Finally, we replicated our pre- and post-comparisons to the subgroup of individuals with the highest expected health care utilization in the year before index, defined using the Johns Hopkins ACG System (Version 10) Resource Utilization Bands. Given the small sample size, we did not undertake ARIMA modelling for this subgroup.
Ethics approval
The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, and therefore received an exemption from review by the Unity Health Toronto Research Ethics Board.
Results
Over the study period, 94 individuals entered the SOS program, of whom 2 (2.1%) could not be linked accurately to ICES databases. Among the linked individuals, we matched 82 (89.1%) to at least 1 unexposed individual, resulting in 303 matched unexposed individuals in the primary analysis (see Appendix 1, eTable 5 for characteristics of unmatched individuals). After matching, SOS-exposed and-unexposed individuals were similar with respect to many measured baseline characteristics, with an average age of 41 years, and 40.2% being male (based on sex listed on health card on index date) (Table 1). The majority (86.6%) were eligible for public drug benefits at cohort entry date, and 8.5% had had a hospital visit (ED or inpatient) related to an opioid toxicity in the previous year. However, clients in the SOS program were more likely to have a diagnosis of HIV (34.1% v. 7.6%; standardized difference [STD] 0.69) or HCV infection (69.5% v. 25.3%; STD 0.99) than unexposed individuals and were more likely to have a hospital visit (ED or inpatient) related to a substance use disorder (18.3% v. 9.5%; STD 0.26) or skin and soft-tissue infections (18.3% v. < 6.1%; STD > 0.10) in the year preceding cohort entry. Previous opioid use was generally similar between groups, with the exceptions of recent methadone use being more common among unexposed individuals (42.4% v. 30.5%; STD 0.25), and previous (nondaily) immediate-release hydromorphone (90 d) being more common among those starting the SOS program (34.1% v. 8.5%; STD 0.66).
Table 1:
Baseline characteristics of clients in safer opioid supply program and matched unexposed group
| Characteristic | No. (%)* of clients in SOS program at LIHC (n = 82) | No. (%)* of matched unexposed individuals (n = 303; weighted n = 82)† | Weighted standardized difference† |
|---|---|---|---|
| Age, yr; mean ± SD‡ | 40.8 ± 10.6 | 40.6 ± 5.5 | 0.01 |
| Male sex‡ | 33 (40.2) | 33.0 (40.2) | 0.00 |
| Income quintile‡ | |||
| 1 (lowest) | 57 (69.5) | 57.0 (69.5) | 0.00 |
| 2 | 12 (14.6) | 12.0 (14.6) | 0.00 |
| 3 | 9 (11.0) | 9.0 (11.0) | 0.00 |
| 4§ | ≤ 5 | ≤ 5 | 0.00 |
| 5 (highest)§ | ≤ 5 | ≤ 5 | 0.00 |
| Eligible for public drug benefits‡ | 71 (86.6) | 71 (86.6) | 0.00 |
| Diagnosis of HIV | 28 (34.1) | 6.3 (7.6) | 0.69 |
| Diagnosis of hepatitis C | 57 (69.5) | 20.8 (25.3) | 0.99 |
| Previous mental health–related or substance use hospital visit¶ (1 yr) | 20 (24.4) | 15.0 (18.3) | 0.15 |
| Anxiety disorders§ | ≤ 5 | ≤ 5 | < 0.10 |
| Deliberate self-harm§ | ≤ 5 | ≤ 5 | < 0.10 |
| Mood disorder§ | 0 (0.00) | ≤ 5 | > 0.10 |
| Schizophrenia and other psychotic disorders§ | ≤ 5 | ≤ 5 | < 0.10 |
| Substance use disorders | 15 (18.3) | 7.8 (9.5) | 0.26 |
| Other§ | ≤ 5 | ≤ 5 | < 0.10 |
| ED visit or hospital admission for opioid toxicity (1 yr)‡ | 7 (8.5) | 7.0 (8.5) | 0.00 |
| Inpatient hospital admission for any infection (1 yr) | 23 (28.0) | 9.3 (11.3) | 0.43 |
| Infective endocarditis§ | ≤ 5 | ≤ 5 | 0.00 |
| Skin and soft-tissue infection§ | 15 (18.3) | ≤ 5 | > 0.10 |
| Osteomyelitis, septic arthritis or spinal infection | 9 (11.0) | 7.3 (8.8) | 0.07 |
| Dispensed opioid agonist therapy (1 yr) | 50 (61.0) | 50.0 (61.0) | 0.00 |
| Dispensed methadone | |||
| Past 30 days | 25 (30.5) | 34.8 (42.4) | 0.25 |
| Past 365 days | 42 (51.2) | 41.3 (50.3) | 0.02 |
| Past 5 years | 60 (73.2) | 63.5 (77.4) | 0.10 |
| Dispensed buprenorphine-naloxone | |||
| Past 30 days | 6 (7.3) | 5.5 (6.7) | 0.02 |
| Past 365 days | 15 (18.3) | 10.3 (12.5) | 0.16 |
| Past 5 years | 23 (28.0) | 18.5 (22.6) | 0.13 |
| Dispensed immediate-release hydromorphone (90 d) | 28 (34.1) | 7.0 (8.5) | 0.66 |
| Dispensed long-acting hydromorphone (90 d)§ | 8 (9.8) | ≤ 5 | > 0.10 |
| Dispensed any opioid (90 d) | 52 (63.4) | 54.5 (66.5) | 0.06 |
| Dispensed benzodiazepines (30 d)§ | ≤ 5 | 7.0 (8.5) | > 0.10 |
| No. of physician visits (1 yr), mean ± SD | 24.50 ± 22.45 | 24.96 ± 10.15 | 0.02 |
| No. of emergency department visits (1 yr), mean ± SD | 3.28 ± 3.36 | 2.23 ± 2.54 | 0.25 |
| No. of inpatient hospital admissions (1 yr), mean ± SD | 0.95 ± 1.34 | 0.42 ± 0.57 | 0.44 |
Note: ED = emergency department, LIHC = London InterCommunity Health Centre, SD = standard deviation, SOS = safer opioid supply.
Unless otherwise indicated.
Counts and standardized differences weighted on number of unexposed individuals matched to each client in SOS program.
Matching criteria.
Censored according to privacy requirements for cell sizes smaller than 6. Accordingly, weighted standardized differences are suppressed to prevent residual disclosure.
Can be ED visit or inpatient stay in a mental health hospital or a mental health bed in an acute hospital.
Interrupted time series analysis
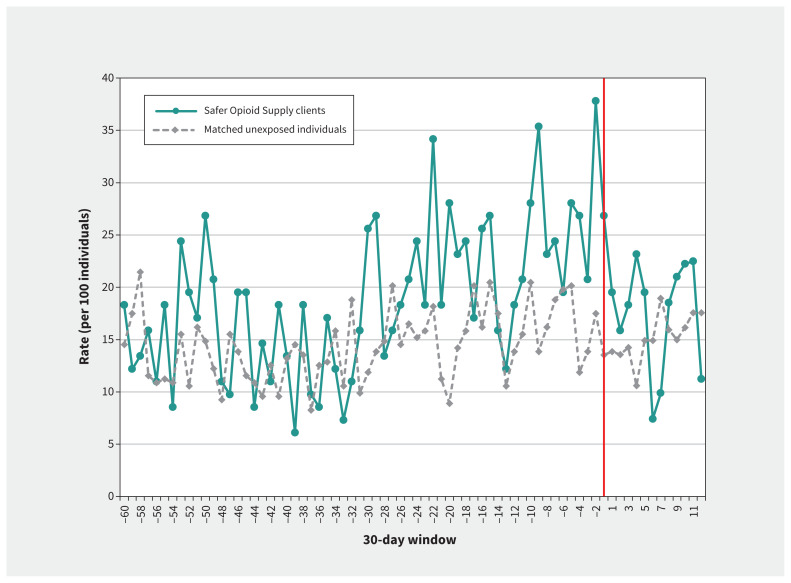
Among clients in the SOS program, ED visits (−13.9 visits per 100 individuals, 95% confidence interval [CI] −25.6 to −2.1; p = 0.02), inpatient hospital admissions (−5.2 admissions per 100 individuals, 95% CI −8.7 to −1.7; p = 0.005) and health care costs not related to primary care or outpatient medications (−$922 per person, 95% CI −$1577 to −$268; p = 0.008) all declined after entry into the SOS program, with no significant change in rates of admission for infection (p = 0.2) (Table 2; Figure 1; and Appendix 1, eFigures 1–6). Conversely, there was no significant change in any of the primary outcomes among matched unexposed individuals.
Table 2:
Results from ARIMA models using 6 years of data among clients in safer opioid supply program and the matched unexposed group
| Outcome | Group | Model* | Step estimate (95% CI) (rate)† | Step function (p value)‡ |
|---|---|---|---|---|
| Primary analysis | ||||
| Rate of ED visits | Safer supply | (0, 1 12, 1) | −13.9 (−25.6 to −2.1) | 0.02 |
| Matched unexposed | (0, 1 12, 1) | −2.0 (−6.3 to 2.3) | 0.4 | |
| Rate of hospital admissions | Safer supply | (0, 1 12, 1) | −5.2 (−8.7 to −1.7) | 0.005 |
| Matched unexposed | (0, 1 12, 1) | 0.6 (−1.1 to 2.4) | 0.5 | |
| Rate of admission for infection | Safer supply | (0, 1 12, 1) | −1.6 (−4.0 to 0.8) | 0.2 |
| Matched unexposed | (0, 1 12, 1) | 0.1 (−0.9 to 1.2) | 0.8 | |
| Health care costs§ | Safer supply | (0, 1 12, 2) | −922 (−1577 to −268) | 0.008 |
| Matched unexposed | (2, 1 12, 0) | −73 (−365 to 219) | 0.6 | |
| Sensitivity cohort matched on HIV diagnosis | ||||
| Rate of ED visits | Safer supply | (0, 1, 1) | −9.1 (−17.6 to −0.6) | 0.03 |
| Matched unexposed | (0, 1, 1) | −3.8 (−7.8 to 0.2) | 0.06 | |
| Rate of hospital admissions | Safer supply | (0, 1, 1) | −4.3 (−7.6 to −1.0) | 0.01 |
| Matched unexposed | (0, 1, 1) | −0.9 (−2.3 to 0.6) | 0.2 | |
| Rate of admission for infection | Safer supply | (0, 1, 1) | −1.1 (−2.7 to 0.6) | 0.2 |
| Matched unexposed | (2, 1, 0) | 0.4 (−0.7 to 1.4) | 0.5 | |
| Health care costs§ | Safer supply | (6, 1, 0) | −668 (−1209 to −126) | 0.02 |
| Matched unexposed | (4, 1, 0) | 29 (−309 to 367) | 0.9 | |
Note: ARIMA = autoregressive integrated moving average, CI = confidence interval, ED = emergency department.
Model specification represented as (p, d, q): p is the number of lags of the dependent variable, representing the autoregressive nature of the model; d represents the number of times the data have to be differenced to ensure stationarity, and “1 12” represents seasonal differencing; q is the number of lags for the error term, representing the moving average part of the model.
Rate reported per 100 individuals for all outcomes except health care costs, which are reported per person. Parameter estimate indicating the level change in the rate of each outcome as estimated by the ARIMA model. For example, a step estimate of −13.9 in the first row indicates a reduction in the monthly rate of ED visits of 13.9 visits per 100 individuals after entry into the safer opioid supply program.
p value corresponding to the parameter estimate indicating the level change in the rate of each outcome as estimated by the ARIMA model.
Excluding primary care costs.
Figure 1:
Rate of emergency department (ED) visits (per 100 individuals), stratified by exposure status. Rates of ED visits (per 100 individuals) are reported in 30-day intervals in the 5 years before index and 1 year after index among both clients of a safer opioid supply (SOS) program and the matched unexposed individuals. The vertical line indicates the index date (entry into SOS program among exposed individuals). Over the 1-year follow-up, 5 or fewer clients of the SOS program were censored, owing to program exit or death.
Within-group changes in annual outcomes
In the 1 year before cohort entry, rates of most outcomes were higher among those initiating SOS than those who were not exposed (Table 3). In the year after cohort entry, rates of ED visits (3.09 v. 2.12 per person-year; rate ratio [RR] 0.69, 95% CI 0.53 to 0.90), inpatient hospital admissions (0.91 v. 0.42 per person-year; RR 0.46, 95% CI 0.29 to 0.74), admissions for incident infections (0.32 v. 0.16 per person-year; RR 0.51, 95% CI 0.27 to 0.96) and health care costs not related to primary care or outpatient medications ($15 635 v. $7310 per person-year; p = 0.002) declined significantly among clients in the SOS program compared with the year before SOS initiation. Medication costs among those eligible for public drug benefits (n = 71) rose from $12 840 to $21 119 per person-year (p < 0.001), which was partially attributable to costs related to increased hydromorphone and OAT use. There were no significant changes in rates of any other outcomes studied in this population (Table 3). Among unexposed individuals, we observed no change in any of the primary outcomes; significant decreases in secondary outcomes were observed in the number of mental health–related hospital visits (0.52 v. 0.31 per person-year; RR 0.60, 95% CI 0.42 to 0.87), substance use disorder–related hospital visits (0.29 v. 0.17 per person-year; RR 0.58, 95% CI 0.36 to 0.94) and costs for hydromorphone and OAT ($1719 v. $1626 per person-year; p = 0.02). All-cause mortality and opioid-related death were rare in both study populations, with no opioid-related deaths occurring among clients in the SOS program in the year after cohort entry.
Table 3:
Unadjusted comparisons of outcome rates in the 1 year before and after cohort entry*
| Health care utilization | Clients in SOS program (n = 82) | Matched unexposed individuals (n = 303) | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| 1 year before cohort entry | 1 year after cohort entry | Negative binomial RR (95% CI) | p value† | 1 year before cohort entry | 1 year after cohort entry | Negative binomial RR (95% CI) | p value† | |
|
|
|
|||||||
| N (rate per person-year) | N (rate per person-year) | |||||||
| Primary outcomes | ||||||||
|
| ||||||||
| No. of ED visits | 250 (3.09) | 170 (2.12) | 0.69 (0.53 to 0.90) | 0.007 | 591 (1.98) | 550 (1.86) | 0.94 (0.79 to 1.13) | 0.5 |
|
| ||||||||
| No. of hospital admissions | 74 (0.91) | 34 (0.42) | 0.46 (0.29 to 0.74) | 0.001 | 98 (0.33) | 95 (0.32) | 1.02 (0.75 to 1.38) | 0.9 |
|
| ||||||||
| No. of hospital admissions for any incident infection | 26 (0.32) | 13 (0.16) | 0.51 (0.27 to 0.96) | 0.04 | 30 (0.10) | 21 (0.07) | 0.72 (0.45 to 1.17) | 0.2 |
|
| ||||||||
| Incident infective endocarditis‡ | ≤ 5 | ≤ 5 | NE | NE | ≤ 5 | ≤ 5 | NE | NE |
|
| ||||||||
| Incident spinal infection, discitis, osteomyelitis or septic arthritis‡ | 10 (0.12) | ≤ 5 | –‡ | > 0.05 | 15 (0.05) | 9 (0.03) | 0.61 (0.35 to 1.06) | 0.08 |
|
| ||||||||
| Incident skin and soft-tissue infections | 14 (0.17) | 7 (0.09) | 0.51 (0.22 to 1.14) | 0.1 | 12 (0.04) | 11 (0.04) | 0.93 (0.44 to 1.96) | 0.8 |
|
| ||||||||
| Total health care costs not related to primary care or outpatient medications ($ per person)§ | 15 635 | 7310 | NE | 0.002 | 8316 | 6527 | NE | 0.7 |
|
| ||||||||
| Secondary outcomes | ||||||||
|
| ||||||||
| Mental health ED visits or admissions | 34 (0.42) | 28 (0.35) | 0.84 (0.45 to 1.54) | 0.6 | 155 (0.52) | 92 (0.31) | 0.60 (0.42 to 0.87) | 0.007 |
|
| ||||||||
| No. of opioid-related ED visits or admissions‡ | 10 (0.12) | ≤ 5 | –‡ | > 0.05 | 18 (0.06) | 21 (0.07) | 1.18 (0.52 to 2.68) | 0.7 |
|
| ||||||||
| No. of any substance use disorder-related ED visits or admissions | 20 (0.25) | 20 (0.25) | 1.02 (0.56 to 1.83) | 1.0 | 88 (0.29) | 50 (0.17) | 0.58 (0.36 to 0.94) | 0.03 |
|
| ||||||||
| All-cause mortality‡ | NA | ≤ 5 | NA | – | NA | 7 (0.02) | NA | – |
|
| ||||||||
| Opioid-related deaths‡ | NA | 0 | NA | – | NA | ≤ 5 | NA | – |
|
| ||||||||
| Total publicly funded medication costs ($ per person)¶ | 12 840 | 21 119 | NE | < 0.001 | 6162 | 6861 | NE | 1.0 |
|
| ||||||||
| Costs for hydromorphone and opioid agonist therapy ($ per person)¶ | 1080 | 3128 | NE | < 0.001 | 1719 | 1626 | NE | 0.02 |
Note: CI = confidence interval, ED = emergency department, NE = not estimable, NA = not applicable as all individuals were alive on their index date, RR = rate ratio, SOS = safer opioid supply.
Each year represents the 360 days before and after cohort entry to align with the 30-day windows.
p value from negative binomial model for all noncost outcomes. p value for cost-related outcomes from Wilcoxon signed rank test.
Censored according to privacy requirements for cell sizes smaller than 6. p value and RR also suppressed to avoid residual disclosure.
Excludes costs for primary care and costs of medications dispensed from community pharmacies.
Among public drug beneficiaries only (n = 71 clients of SOS program, and n = 262 matched unexposed individuals).
Sensitivity analyses
In the analysis of individuals also matched on previous diagnosis of HIV infection (n = 71 clients in the SOS program matched to 232 unexposed individuals; Table 2 and Appendix 1, eTables 6 and 7), the results were generally consistent with our primary findings. We also found similar results in our analysis of individuals in the highest resource utilization bands, with the exception of a statistically significant decline in health care costs not related to primary care or outpatient medications among the unexposed group. Although not a direct comparison, the magnitude of change among the unexposed group (32% reduction in costs; p = 0.04) was smaller than that of clients in the SOS program (57% reduction in costs; p = 0.0001; Appendix 1, eTable 8). Finally, findings from ARIMA models using a ramp intervention function were consistent with those of our main analysis (Appendix 1, eTable 9).
Interpretation
Safer opioid supply prescribing is a relatively new intervention in Canada, with little published evidence on patient outcomes. This analysis of 82 clients enrolled in an SOS program in London, Ontario, suggests that the program led to important declines in ED visits, inpatient hospital admissions, admissions for incident infections, and health care costs not related to primary care or outpatient medications in the year after program initiation, with no corresponding change observed in a matched group of unexposed individuals residing in London who did not access this program. All-cause mortality and opioid-related death were rare in both study groups, with no opioid-related deaths occurring among clients in the SOS program in the year after cohort entry, and 5 or fewer such deaths occurring among unexposed individuals. Given that concerns have previously been raised regarding the potential for increased risk of infectious complications, overdose and death for SOS program participants,22 the significant decline in health services utilization among clients in the SOS program alongside no change in infection rates, opioid-related deaths or all-cause mortality found in this study provides a measure of reassurance regarding the safety of these programs. This is particularly notable as this has occurred during a period of substantially increasing rates of hospital admissions for infectious complications and opioid-related deaths in Ontario.26,27
The high baseline prevalence of HIV (34.1%), HCV (69.5%) and infectious complications (28.0%) among LIHC clients in the year before cohort entry suggests that the SOS program is reaching a high-acuity group of people who are experiencing serious medical complications from their drug use. This is also reflective of policy at the LIHC SOS program, which prioritized program admission to people believed to be at high risk of imminent death owing to unmanaged health conditions such as infective endocarditis and untreated HIV.16 We also found a high degree of OAT access in the year before cohort entry among those in both study populations, with slightly lower OAT use in the previous 30 days among people starting SOS compared with unexposed individuals. This suggests that the LIHC SOS program is reaching a group of people with previous experience on OAT and who may be currently uninterested in, or have not had success with, this form of treatment in the past. Clients in the SOS program also had a higher rate of immediate-release hydromorphone use in the 90 days before cohort entry. This finding is likely driven by the high rates of inpatient hospital admissions before initiating SOS, as hydromorphone “weaning” prescriptions are commonly provided at the time of discharge after inpatient hospital admissions.16 Taken together, these findings suggest that the LIHC SOS program is reaching a population of people at high risk of fatal overdose and complications from unregulated drug use who may not have derived benefit from existing treatment options. Therefore, the observed reductions in health services utilization and rising medication costs in this population after enrolment suggest that SOS programs can play an important role as part of a multifaceted response needed to the overdose crisis, particularly among medically and socially complex populations who would benefit most from the ancillary services typically embedded in these programs, including housing support and engagement with and retention in HIV and HCV care.
In concert with reduced health services utilization, we observed substantial reductions in health care costs not related to primary care or outpatient medications in the first year after SOS enrolment, suggesting that SOS programs may result in considerable savings to the health care system. Although we found that medication costs increased among clients in the SOS program, annual medication costs for prescribed opioids were relatively modest ($3128 of $21 119; 14.8%). Therefore, the increase in medication costs likely represents improved access to treatment for conditions such as HIV and HCV, a finding that corresponds with preliminary program evaluation findings.16 Moreover, increased medication costs in the SOS-exposed cohort may represent an overall positive benefit to the health of individual clients, which may translate into long-term cost savings to the health care system.
Given that the SOS program at LIHC provides comprehensive primary care and social supports to clients, it is difficult to separate the relative impact of safer supply prescribing from the impact of the wrap-around supports provided. Emerging qualitative and program evaluation research highlights how clients in SOS programs attribute access to safer supply medications as being responsible for stabilizing their patterns of drug use and improving their health by reducing their use of drugs from the unregulated street supply (thereby reducing overdose risk) and, in some cases, reducing drug use overall or ceasing the use of drugs by injection.16,28 There is likely strong benefit to the provision of comprehensive programming that includes SOS prescribing alongside comprehensive health and social supports to a high-risk population, and the relative contribution of different program elements to client outcomes is an important topic for future research.
Limitations
Although this study provides important preliminary data on health-related outcomes among clients in SOS programs, several limitations warrant discussion.
First, we were restricted to data from 1 program in Ontario, limiting the generalizability of our findings.
Second, because clients of LIHC differed from the unexposed group in determinants of health that could not be accounted for using administrative data, we were unable to conduct a matched study comparing health service use between these groups. However, this was not our main intent, which was to explore the relative safety and effectiveness of the SOS program using within-group comparisons to minimize the potential for unobserved confounding, with the unexposed group acting as a test of the specificity of our findings. Because we conducted multiple comparisons in this study, we cannot rule out the possibility of spurious findings of significance for some outcomes. Further, we cannot definitively exclude the possibility that reduced health services utilization among clients in the SOS program reflects in part regression to the mean, although our use of ARIMA models with 5 years of baseline data mitigates this concern.
Third, we were unable to identify patients with overdoses treated in the community who were not transported to hospital, meaning that our overdose rates are likely an underestimate.
Fourth, we do not have access to costs of primary care provided through community health centres and are therefore unable to estimate changing primary care costs among clients in the SOS program. Although we anticipate that primary care costs would likely increase among clients in an SOS program, these increases would represent improved engagement with care, usually considered a positive outcome.
Fifth, we defined OUD on the basis of previous treatment with OAT or hospital visits with diagnoses suggesting an opioid dependence. Although this definition has not been validated, it uses highly specific codes with a low potential for misclassification and has been used in previous publications.26,29,30
Finally, we were unable to measure potential diversion of opioids dispensed through SOS and the associated risks to clients not in an SOS program in the community. Although this is a potential concern, it must be balanced against the benefits of increasing access to a safer alternative to the unpredictable unregulated drug supply. Further research is needed to understand the degree of diversion that occurs, and any associated risks or benefits to the community.
Conclusion
In Ontario, SOS is currently reaching a limited number of people, 15 and although the province of British Columbia has released a policy for the expansion of “prescribed safer supply,” 31 other provinces have been more reticent. Additional research is needed to deepen our understanding of the benefits and potential risks of different models of SOS, but our findings provide preliminary evidence that SOS programs can play an important role in the expansion of treatment and harm reduction options available to people who use drugs at high risk of drug poisoning.
Supplementary Material
Acknowledgements
This study was conducted by the Ontario Drug Policy Research Network (ODPRN), a province-wide network of researchers who respond to policy-makers’ needs for relevant research to guide and inform their decisions, using the administrative claims databases housed at ICES. The ODPRN is funded by grants from the Ontario Ministry of Health. The authors thank IQVIA Solutions Canada Inc. for use of its Drug Information File. This document used data adapted from the Statistics Canada Postal Code Conversion File, which is based on data licensed from ©Canada Post Corporation, and/or data adapted from the Ontario Ministry of Health Postal CodeOM Conversion File, which contains data copied under license from Canada Post Corporation and Statistics Canada. The authors thank Tonya Campbell for her insight on early versions of the project protocol.
Footnotes
Competing interests: Tara Gomes reports receiving grants paid to support the research program from the Ontario Ministry of Health, a grant paid to support the conduct of the study from the Canadian Institutes of Health Research (CIHR) and Canada Research Chair funding for salary support. Andrea Sereda is the medical lead in the Safer Opioid Supply program based out of the London InterCommunity Health Centre (LIHC). Dr. Sereda has also received support for attending meetings or travel from the LIHC (as an employee). Gillian Kolla is supported by a Banting postdoctoral fellowship from the CIHR and a postdoctoral fellowship from the Canadian Network on Hepatitis C. Separate from this study, Dr. Kolla received funding from LIHC’s Substance Use and Addictions Program grant to conduct an independent preliminary evaluation of the Safer Opioid Supply program. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Andrea Sereda, Gillian Kolla, Tara Gomes and Tony Antoniou contributed to the conception of the work. All authors designed the study. Daniel McCormack contributed to the acquisition and analysis of the data, and all authors interpreted the data. Gillian Kolla and Tara Gomes drafted the manuscript. All of the authors revised the manuscript critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work. Tara Gomes and Gillian Kolla are co–first authors.
Funding: This study was funded by grants from the Ontario Ministry of Health (grant #0691) and the Canadian Institutes of Health Research (grant #153070).
Data sharing: The data set from this study is held securely in coded form at ICES. Although data-sharing agreements prohibit ICES from making the data set publicly available, access may be granted to those who meet prespecified criteria for confidential access, available at https://www.ices.on.ca/DAS. The full data set creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.
Disclaimer: This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Ministry of Long-Term Care. Parts of this material are based on data and information compiled and provided by the Ontario Ministry of Health and the Canadian Institute of Health Information (CIHI). The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES, CIHI or the Ontario Ministries of Health or Long-Term Care is intended or should be inferred.
References
- 1.Special Advisory Committee on the Epidemic of Opioid Overdoses. Opioid- and stimulant-related harms in Canada. Ottawa: Public Health Agency of Canada; 2022. Available: https://health-infobase.canada.ca/substance-related-harms/opioids-stimulants (accessed 2022 July 27). [Google Scholar]
- 2.Gomes T, Murray R, Kolla G, et al. Changing circumstances surrounding opioid-related deaths in Ontario during the COVID-19 pandemic. Toronto: Ontario Drug Policy Research Network, Office of the Chief Coroner for Ontario and Ontario Agency for Health Protection and Promotion (Public Health Ontario); 2021. Available: https://odprn.ca/research/publications/opioid-related-deaths-in-ontario-during-covid/ (accessed 2022 Apr. 21). [Google Scholar]
- 3.Antoniou T, Martins D, Campbell T, et al. Impact of policy changes on the provision of naloxone by pharmacies in Ontario, Canada: a population-based time-series analysis. Addiction 2021;116:1514–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kerr T, Mitra S, Kennedy MC, et al. Supervised injection facilities in Canada: past, present, and future. Harm Reduct J 2017;14:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strike C, Watson TM. Losing the uphill battle? Emergent harm reduction interventions and barriers during the opioid overdose crisis in Canada. Int J Drug Policy 2019;71:178–82. [DOI] [PubMed] [Google Scholar]
- 6.Fairbairn N, Ross J, Trew M, et al. Injectable opioid agonist treatment for opioid use disorder: a national clinical guideline. CMAJ 2019;191:E1049–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oviedo-Joekes E, Brissette S, Marsh DC, et al. Diacetylmorphine versus methadone for the treatment of opioid addiction. N Engl J Med 2009;361:777–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oviedo-Joekes E, Guh D, Brissette S, et al. Hydromorphone compared with diacetylmorphine for long-term opioid dependence: a randomized clinical trial. JAMA Psychiatry 2016;73:447–55. [DOI] [PubMed] [Google Scholar]
- 9.Irvine MA, Kuo M, Buxton JA, et al. Modelling the combined impact of interventions in averting deaths during a synthetic-opioid overdose epidemic. Addiction 2019;114:1602–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eydt E, Glegg S, Sutherland C, et al. Service delivery models for injectable opioid agonist treatment in Canada: 2 sequential environmental scans. CMAJ Open 2021;9:E115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bardwell G, Lappalainen L. The need to prioritize research, policy, and practice to address the overdose epidemic in smaller settings in Canada. Can J Public Health 2021;112:733–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hales J, Kolla G, Man T, et al. Safer opioid supply programs (SOS): a harm reduction informed guiding document for primary care teams; updated 2021. Available: https://bit.ly/3dR3b8m (accessed 2021 Jan. 29).
- 13.Ivsins A, Boyd J, Beletsky L, et al. Tackling the overdose crisis: the role of safe supply. Int J Drug Policy 2020;80:102769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonn M, Palayew A, Bartlett S, et al. Addressing the syndemic of HIV, hepatitis C, overdose, and COVID-19 among people who use drugs: the potential roles for decriminalization and safe supply. J Stud Alcohol Drugs 2020;81:556–60. [PubMed] [Google Scholar]
- 15.Young S, Kolla G, McCormack D, et al. Characterizing safer supply prescribing of immediate release hydromorphone for individuals with opioid use disorder across Ontario, Canada. Int J Drug Policy 2022;102:103601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolla G, Long C, Perri M, et al. Safer opioid supply program: preliminary report. London (ON): London InterCommunity Health Centre; 2021. Available: https://lihc.on.ca/wp-content/uploads/2022/01/2021-SOS-Evaluation-Full.pdf (accessed 2022 Jan. 29). [Google Scholar]
- 17.Government of Canada highlights support for safer drug supply projects in Ontario [press release]. Ottawa: Health Canada; 2020. Sept. 18. Available: https://www.canada.ca/en/health-canada/news/2020/09/government-of-canada-highlights-support-for-safer-drug-supply-projects-in-ontario.html (accessed 2022 Jan. 29). [Google Scholar]
- 18.Advice to the profession: prescribing drugs. Toronto: College of Physicians and Surgeons of Ontario; 2021. Available: https://www.cpso.on.ca/Physicians/Policies-Guidance/Policies/Prescribing-Drugs/Advice-to-the-Profession-Prescribing-Drugs (accessed 2021 Oct. 28). [Google Scholar]
- 19.Risk mitigation in the context of dual public health emergencies — interim clinical guidance update. Vancouver: British Columbia Centre on Substance Use; updated 2022. Available: https://www.bccsu.ca/wp-content/uploads/2022/02/Risk-Mitigation-Guidance-Update-February-2022.pdf (accessed 2022 Apr. 21). [Google Scholar]
- 20.Goyer M-E, Hudon K, Plessis-Bélair M-C, et al. Substance replacement therapy in the context of the COVID-19 pandemic in Québec: clinical guidance for prescribers. Montréal: Institut universitaire sur les dépendances; 2020. Available: http://dependanceitinerance.ca/wp-content/uploads/2020/10/Guide-Pharmaco-COVID_ANG-VF.19.10.20.pdf (accessed 2021 Oct. 28). [Google Scholar]
- 21.Opioid use disorder. Practice update. Vancouver: British Columbia Centre on Substance Use; 2022. Available: https://www.bccsu.ca/wp-content/uploads/2022/02/Opioid-Use-Disorder-Practice-Update-February-2022.pdf (accessed 2022 Apr. 21). [Google Scholar]
- 22.Bromley LA. Problems with hydromorphone prescribing as a response to the opioid crisis. CMAJ 2020;192:E219–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Safer Opioid Supply program. London (ON): Intercommunity Health Centre; 2020. Available: https://lihc.on.ca/programs/safer-opioid-supply-program/ (accessed 2022 Mar. 1). [Google Scholar]
- 24.Wodchis WP, Bushmeneva K, Nikitovic M, et al. Guidelines on person-level costing using administrative databases in Ontario. Toronto: Health System Performance Research Network; 2013. [Google Scholar]
- 25.Mamdani M, Sykora K, Li P, et al. Reader’s guide to critical appraisal of cohort studies: 2. Assessing potential for confounding. BMJ 2005;330:960–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomes T, Kitchen SA, Tailor L, et al. Trends in hospitalizations for serious infections among people with opioid use disorder in Ontario, Canada. J Addict Med 2022;16:433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomes T, Juurlink DN. Understanding the implications of a shifting opioid landscape in Ontario. Healthc Q 2019;22:6–11. [DOI] [PubMed] [Google Scholar]
- 28.McNeil R, Fleming T, Mayer S, et al. Implementation of safe supply alternatives during intersecting COVID-19 and overdose health emergencies in British Columbia, Canada, 2021. Am J Public Health 2022;112:S151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choremis B, Campbell T, Tadrous M, et al. The uptake of the pharmacy-dispensed naloxone kit program in Ontario: a population-based study. PLoS One 2019;14:e0223589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomes T, Murray R, Kolla G, et al. Patterns of medication and healthcare use among people who died of an opioid-related toxicity during the COVID-19 pandemic in Ontario. Toronto: The Ontario Drug Policy Research Network; 2022. Available: https://odprn.ca/research/publications/opioid-related-deaths-and-healthcare-use/ (accessed 2022 Mar. 1). [Google Scholar]
- 31.Access to prescribed safer supply in British Columbia: policy direction. Vancouver: British Columbia Ministry of Mental Health and Addictions, British Columbia Ministry of Health; 2021. Available: https://www2.gov.bc.ca/assets/gov/overdose-awareness/prescribed_safer_supply_in_bc.pdf (accessed 2022 Apr. 21). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.