Abstract
Plant microbiome engineering is a promising tool to unlock crop productivity potential and exceed the yield obtained with conventional chemical inputs. We studied the effect of Aspergillus niger inoculation on in-field lettuce (Lactuca sativa) growth in soils with limiting and non-limiting P concentrations. Lettuce plants originating from inoculated seeds showed increased plant diameter (6.9%), number of leaves (8.1%), fresh weight (23.9%), and chlorophyll content (3.8%) as compared to non-inoculated ones. Inoculation of the seedling substrate just before transplanting was equally efficient to seed inoculation, while application of a granular formulation at transplanting did not perform well. Plant response to P addition was observed only up to 150 kg P2O5 ha-1, but A. niger inoculation allowed further increments in all vegetative parameters. We also employed a high-throughput phenotyping method based on aerial images, which allowed us to detect changes in plants due to A. niger inoculation. The visible atmospherically resistant index (VARI) produced an accurate prediction model for chlorophyll content, suggesting this method might be used to large-scale surveys of croplands inoculated with beneficial microorganisms. Our findings demonstrate that A. niger inoculation surpasses the yield obtained with conventional chemical inputs, allowing productivity gains not reached by just increasing P doses.
Introduction
World population is forecast to reach 9.7 billion by 2050, which will demand doubling food production [1]. The sustainable way to increase food production is by raising crop yields on existing croplands, minimizing land clearance and greenhouse gas emissions [2, 3]. To reach a crop’s yield potential, a perfect combination of favourable climate, pest and disease control, and non-limiting nutrients and water is necessary. However, some estimations suggest that average crop yields plateau at 75–85% of the crop yield potential [4]. This gap between farm yields and the crop yield potential is attributed to the difficulty for farmers to achieve the perfect management and to the low response to additional inputs when the yield approaches the plateau, which discourage additional investments [4].
A commonly overlooked component of crop yield potential is the plant microbiota, which plays an important role in plant growth, health, and resilience to stress [5]. The benefits of plant microbiota for agriculture have gained substantial attention recently. Many studies reported diverse plant beneficial microorganisms (PBM) showing multiple mechanisms to promote plant growth and health, such as phytohormone production, facilitating nutrient acquisition, enhancing tolerance to salinity and drought, and disease suppression [5–9]. However, plant domestication may have undermined beneficial microbial associations in modern crop cultivars [10]. In this scenario, plant microbiome engineering has been proposed as a way to reinstate beneficial plant-microorganism associations in agroecosystems [10, 11]. Therefore, inoculation of PBM can raise crop yield and diminish the gap in the crop yield potential.
Aspergillus niger v. Tiegh is a PBM that has multiple mechanisms of plant growth promotion, such as phytohormone production [12, 13] and solubilization of P and K [14–16]. Inoculation of A. niger enhanced the growth of coffee (Coffea arabica L.) [17], maize (Zea mays L.) [13], and vegetable seedlings [18]. Moreover, A. niger can release P fixed to the soil [15] and, thus, may contribute to improve crop P use efficiency and access the soil legacy P, alleviating the pressure on world phosphate reserves [19]. We hypothesized that A. niger could enhance lettuce (Lactuca sativa L.) yield in soils with limiting and non-limiting concentrations of P. Lettuce is a worldwide distributed crop [20] in which inoculation can reinstate important microbial associations that may improve growth and yield [21, 22]. Thus, the aim of this research was to evaluate lettuce growth under different methods of A. niger inoculation and P availability levels.
Materials and methods
Experimental site
The experiment was carried out from April to July 2021 at the Vegetable Experimental Station of the Federal University of Uberlândia, located in Monte Carmelo, Minas Gerais state, Brazil (18°42’43.19” S, 47°29’55.8" W, 873 m altitude). Climate of the region is characterized according to Köppen as Aw-tropical, with hot and humid summer and cold and dry winter. Average daily temperature during the experiment period was 21.1°C, with an average minimum of 14.9 and a maximum of 27.3°C. Average relative humidity was 65.6%, with an average minimum of 44.9 and a maximum of 86.4%.
The soil presented clay texture (sand 25.5%, silt 10%, and clay 64.5%), pH (in H2O) 5.1, 23.8 mg P dm-3 (extracted by Mehlich-1), 113.4 mg K dm-3, 352.9 mg Ca dm-3, 40.1 mg Mg dm-3, 24.3 mg Al3+ dm-3, and organic matter 12 g kg-1.
Experimental setup
The experiment was arranged in a randomized block design in a 4 x 3 factorial scheme. The first factor consisted of four inoculation methods: seed inoculation (SI) at sowing, conidial suspension (CS) applied to seedling substrate just before transplanting, granular formulation (GR) applied to the planting hole at transplanting, and a non-inoculated (NI) control. All inoculated treatments received 102 conidia plant-1 (see details in the section Production and application of Aspergillus niger inoculants). The second factor consisted of three P doses: 0, 150, and 300 kg P2O5 ha-1. P was supplied as triple superphosphate (18.9% P). The experiment was carried out with four repetitions, adding up to 48 plots. Each plot consisted of 16 plants and the four central plants were considered for evaluation.
Lettuce seedlings were produced in polystyrene trays with 128 cells (40 cm3 cell-1) filled with a commercial coconut fiber substrate (Technes, São Paulo, SP, Brazil). Substrate presented pH 5.6, humidity 48%, density 260 kg m3, 0.8% fertilizer, and 0.2% corrective. Lettuce seeds of the UFU-197#2#1#1 genotype were obtained from the Biofortified Lettuce Genetic Improvement Program/UFU. Two seeds were sowed in each cell and after 10 days seedlings were thinned to one per cell. Seedlings were fertigated weekly with 13.4 mL per cell of a solution containing (total amount per cell): 5.02 μg N, 2.19 μg P, 5.55 μg K, 0.83 μg Mg, 0.083 μg Zn, 0.025 μg B, 0.0083 μg Fe, 0.083 μg Mn, and 0.92 μg S. Irrigation was performed daily according to plant needs. Trays were placed on 70-cm high benches in a greenhouse (5 x 6 m, height 3.5 m) covered with 150-μm clear plastic film and side curtains of anti-aphid white screen.
Seedlings were transplanted to beds 48 days after sowing. Each experimental plot had 16 plants distributed in four rows with four plants each spaced 0.25 m apart. Spacing between plots and beds was 0.5 m. Nitrogen and potassium were supplied at 50 kg K ha-1 and 150 kg N ha-1 as urea and KCl, respectively [23]. Foliar applications of B, Ca, and K were carried out weekly using a commercial fertilizer formulation (3.8% B, 19% Ca, and 1% K2O) at 100 g ha-1. Irrigation was carried out daily. Cultural traits were performed according to plant needs.
Production and application of Aspergillus niger inoculants
The fungus A. niger FS1 was previously isolated from soil under native forest in Viçosa, Minas Gerais, Brazil [16]. Fungal conidia were produced in Petri dishes containing potato dextrose agar (PDA, Sigma-Aldrich, Saint Louis, MO, USA) incubated at 30°C for 10 days. Conidia were collected in a sterile Tween 80 0.01% (v/v) solution. The conidial suspension was vacuum filtered through a membrane with 0.45 μm pores and conidia retained on the membrane were dried in a desiccator with silica gel at room temperature (25°C) for 24 h. Dry conidia were collected and stored in microtubes at room temperature [17]. Dry conidia mass contained 4.5 x 107 conidia mg-1, as counted in a Neubauer chamber.
Conidia were inoculated to plants at a concentration of 102 conidia plant-1 [18] in three ways: seed inoculation (SI), application of a conidial suspension to seedling substrate just before transplanting (CS), and granular formulation applied to the planting hole at transplanting (GR).
Seed inoculation
Dry conidia of A. niger FS1 were suspended in sterile water at 0.005 mg conidia mL-1. To deliver 102 conidia per seed, 460 lettuce seeds were covered with 0.69 mL of this suspension two hours before sowing via pipetting and homogenized manually. Seeds were kept moist until sowing in trays.
Seedling inoculation with conidial suspension
Dry conidia of A. niger FS1 were suspended in sterile water at 0.002 mg conidia L-1. Each seedling received 3 mL of this conidial suspension 30 min before transplanting. The conidial suspension was added individually in each tray cell using a sterile micropipette.
Granular formulation applied to the planting hole at transplanting
The granular formulation was produced by mixing dry conidia with 26.5 g wheat flour, 3.8 g corn starch, 2.25 g granulated sugar, and 15 mL deionized water [17]. The amount of dry conidia added to the mixture was 0.013 mg to yield a concentration of 102 conidia per granule (average mass of each granule was 22.6 mg). The mixture was extruded into filaments of 2 mm diameter, which were cut to 2 mm in length and dried in an oven with forced air circulation at 50°C for 48 h [17]. The resulting granules had a cylindrical form with approximate 2 mm in height and a 2 mm diameter base. At transplanting, one granule was added to the planting hole just below the seedling root.
Analytical procedures
Vegetative growth parameters
Measurements of the plant diameter, stem diameter, fresh weight, number of commercial leaves (longer than 5 cm in length), and SPAD index were taken from the four central lettuce plants in each plot. The SPAD index was measured with a chlorophyl meter (Minolta SPAD-502CFL1030) in three leaves of each plant, taking reads from two opposed lateral leaves and from an intermediary one. All measurements were done at 33 days after transplanting for plant diameter and SPAD index and at day 34 for the rest of the variables.
Image-based phenotyping
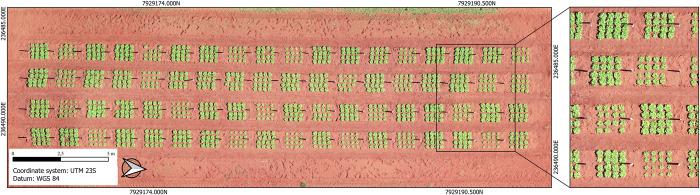
Aerial images of the lettuce canopy were taken 33 days after transplanting by a remotely piloted aircraft (Phantom 4 Pro drone) equipped with a RGB camera. The flight and photographing were controlled by the DJI and DroneDeploy software and performed at a height of 20 m, longitudinal and lateral overlap of 80%, direction at -93°, and speed of 3 m s-1. The images obtained were processed with the Pix4Dmapper software to generate an orthoimage with 1 cm of GSD (Ground Sample Distance). A sample of the orthoimage is shown in Fig 1 and the complete image is available as Supplementary Data (S1 Fig).
Fig 1. Orthoimage showing the aerial view of the experiment.
The zoomed image on the right highlights the spatial resolution of the orthoimage generated with a GSD of 1 cm.
The orthoimage was processed using the package FIELDimageR for R [24]. The histogram of the HUE vegetation index was generated and used to determine a cut-off value of 1.5 to segment the vegetation from the soil background. After segmentation, histograms of vegetation indices related to RGB bands were generated and vegetation indices were calculated according to equations shown in Table 1.
Table 1. Equations for calculation of vegetation indices from red (R), green (G), and blue (B) bands of images.
| Vegetation index | Equation | Reference |
|---|---|---|
| Overall Hue Index (HUE) | arctan(2*(B-G-R)/30.5*(G-R)) | [25] |
| Normalized Green Red Difference Index (NGRDI) | (G-R)(G+R) | [26] |
| Green Leaf Index (GLI) | (2G-R-B)/(2G+R+B) | [27] |
| Soil Color Index (SCI) | (R-G)/(R+G) | [28] |
| Brightness Index (BI) | √((R2+G2+B2)/3) | [29] |
| Visible Atmospherically Resistant Index (VARI) | (G-R)/(G+R-B) | [30] |
| Excess Green Index (ExG) | 2G-R-B | [31] |
| Modified Green Red Vegetation Index (MGVRI) | (G2-R2)/(G2+R2) | [32] |
A Pearson’s correlation matrix between the vegetation indices and the vegetative growth parameters was generated using the package Hmisc for R. Correlation coefficients were tested at p = 0.05. The vegetation index with the highest correlation coefficients with the measured vegetative growth parameters was chosen for fitting prediction models using the machine learning software Waikato Environment for Knowledge Analysis (WEKA, version 3.9.5). Linear regression models were fit using the percentage split option, where 90% of the dataset was considered for model training. The accuracy of fitted models was checked by the root mean squared error (RMSE) (Eq 1), and the normalized RMSE (NRMSE) (Eq 2).
| Eq 1 |
| Eq 2 |
where:
: estimated value
y: observed value
n: number of samples
Statistical analyses
Data from vegetative growth parameters were subjected to ANOVA and treatment means were compared by LSD test (p = 0.05) using the package ExpDes.pt for R [33]. Data were tested (p = 0.05) for normality and homoscedasticity by Shapiro-Wilk and O’Neil tests, respectively.
Multivariate analyses based on all vegetative growth parameters were performed by clustering and principal component methods. A dendrogram was constructed by the unweighted pair-group method with arithmetic mean (UPGMA) based on Euclidean distance. Validation of clustering was performed based on the cophenetic correlation coefficient [34] calculated in the software Genes [35]. The relative importance of variables on the dissimilarity between treatments was calculated as proposed by Singh [36]. Principal component analysis (PCA) was carried out based on the correlation matrix using the package stats for R.
Results
Vegetative growth parameters
Lettuce vegetative growth parameters were affected by A. niger inoculation and P dose, but these factors did not interact significantly (p > 0.05). Seed inoculation was superior to non-inoculated treatment for all vegetative growth parameters, except for stem diameter, increasing average plant diameter, number of leaves, fresh weight, and chlorophyll content (estimated by the SPAD index) by 6.9, 8.1, 23.9, and 3.8%, respectively (Fig 2A–2E). The inoculation with a conidial suspension applied to the seedling substrate just before transplanting produced similar results to seed inoculation for all vegetative growth parameters. On the other hand, plants inoculated with the granular formulation grew like non-inoculated ones, only showing higher values of the SPAD index (Fig 2E).
Fig 2. Vegetative growth parameters of lettuce as affected by Aspergillus niger FS1 inoculation (a-e) and P dose (f-j).
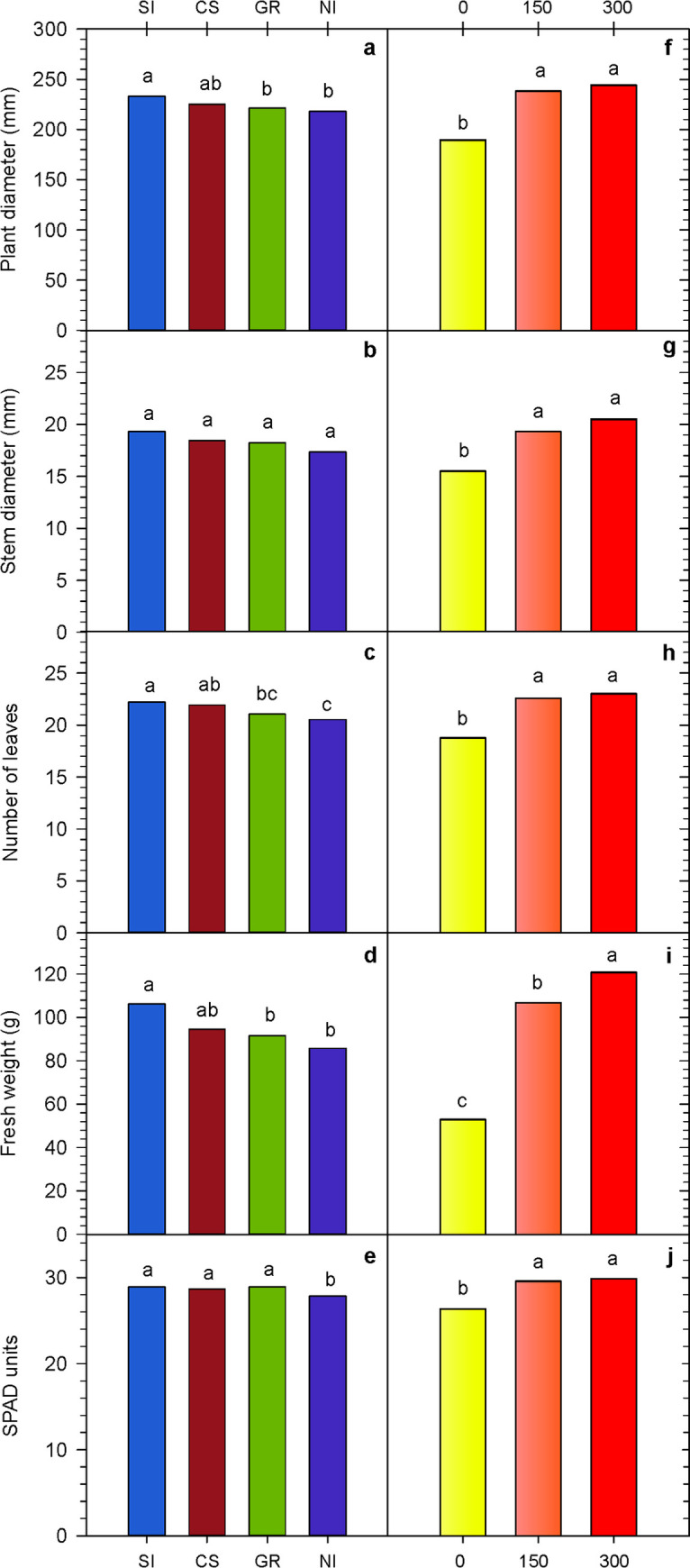
Interaction between factors inoculation and P dose was not significant (p > 0.05). For each factor, treatments labelled with distinct letters are significantly different (LSD test, p < 0.05). SI: Seed inoculation, CS: Conidial suspension, GR: Granular formulation, NI: Non-inoculated. P doses: 0, 150, and 300 kg P2O5 ha-1.
P fertilizer application improved all vegetative growth parameters (Fig 2F–2J). However, plants fertilized with 150 and 300 kg P2O5 ha-1 grew similarly, except for fresh weight, in which plants fertilized with 300 kg P2O5 ha-1 showed higher values (Fig 2I).
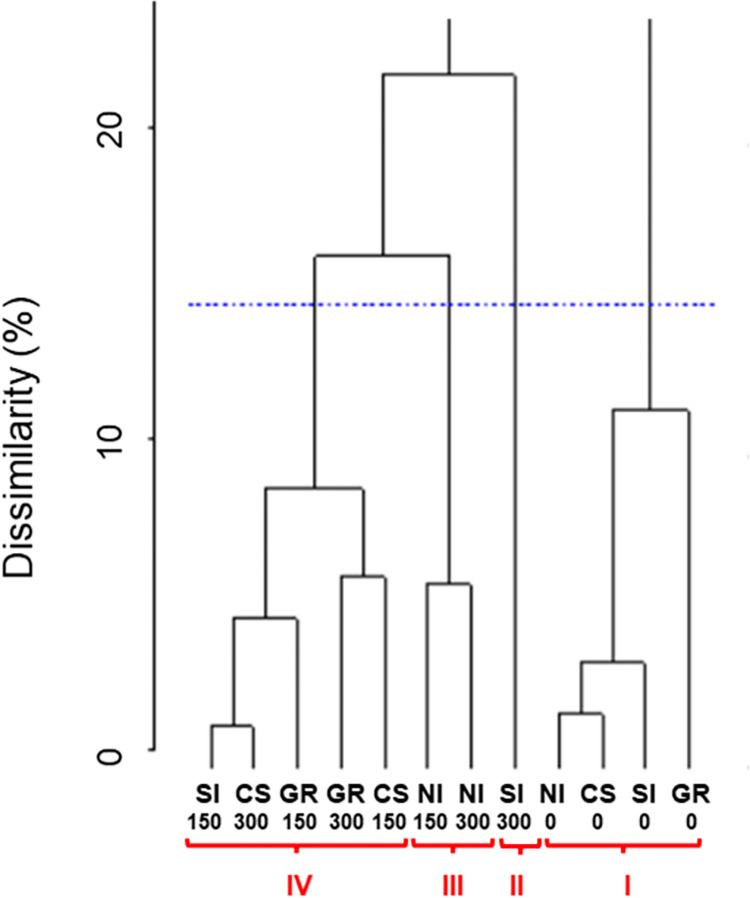
Cluster analysis by UPGMA divided the combinations of inoculation methods and P doses in four clusters (Fig 3) at a level of 15% of dissimilarity [37]. Cluster I included treatments without P addition, cluster II was formed by the combination of seed inoculation and 300 kg P2O5 ha-1, cluster III included non-inoculated treatments that received 150 and 300 kg P2O5 ha-1, and cluster IV included the rest of inoculated treatments fertilized with 150 or 300 kg P2O5 ha-1. Clusters formed by UPGMA had a cophenetic correlation coefficient of 0.874 (t test, p < 0.01), indicating that the dendrogram represents faithfully the matrix data. The SPAD index and fresh weight contributed the most for dissimilarity between treatments, representing 40.9 and 36.5% of the variability, respectively (Table 2).
Fig 3. Clustering of combinations of Aspergillus niger FS1 inoculation methods and P doses obtained by the unweighted pair-group method with arithmetic mean (UPGMA) based on Euclidean distance.
SI: Seed inoculation, CS: Conidial suspension, GR: Granular formulation, NI: Non-inoculated. P doses: 0, 150, and 300 kg P2O5 ha-1.
Table 2. Relative contribution of response variables for the dissimilarity between treatments calculated from Euclidean distance.
| Response variable | Relative contribution (%) |
|---|---|
| Plant diameter | 10.94 |
| SPAD index | 40.97 |
| Stem diameter | 7.64 |
| Fresh weight | 36.56 |
| Number of leaves | 3.87 |
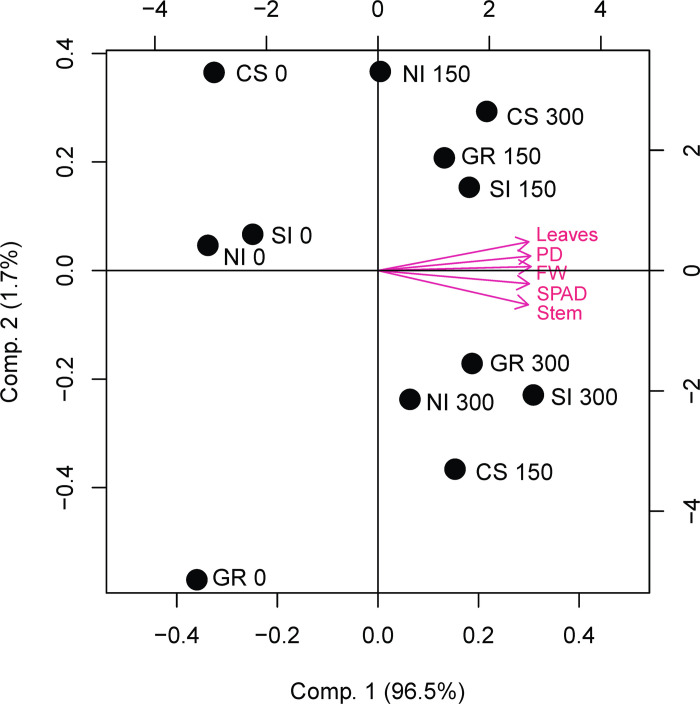
Principal component analysis showed that most of the variance (96.5%) was explained by component 1 (Fig 4), reflecting the high correlation between the plant variables. All variables showed positive loadings for this component so that the higher the score of the treatment the higher the lettuce growth parameters. Scores allowed to group the treatments in the same clusters obtained by UPGMA. The highest score was presented by the combination seed inoculation + 300 kg P2O5 ha-1 (2.9), followed by a group of treatments formed by inoculated plants fertilized with 150 or 300 kg P2O5 ha-1, which presented an average score of 1.6. Non-inoculated treatments that received 150 and 300 kg P2O5 ha-1 of the P dose presented a mean score of 0.3. Treatments without P addition presented the lowest scores, averaging -2.9.
Fig 4. Principal component analysis of combinations of Aspergillus niger FS1 inoculation methods and P doses.
Circles represent scores of treatments and arrows represent the loadings of plant characteristics on the components. PD: Plant diameter, FW: Fresh weight, SI: Seed inoculation, CS: Conidial suspension, GR: Granular formulation, NI: Non-inoculated. P doses: 0, 150, and 300 kg P2O5 ha-1.
Image-based phenotyping of lettuce
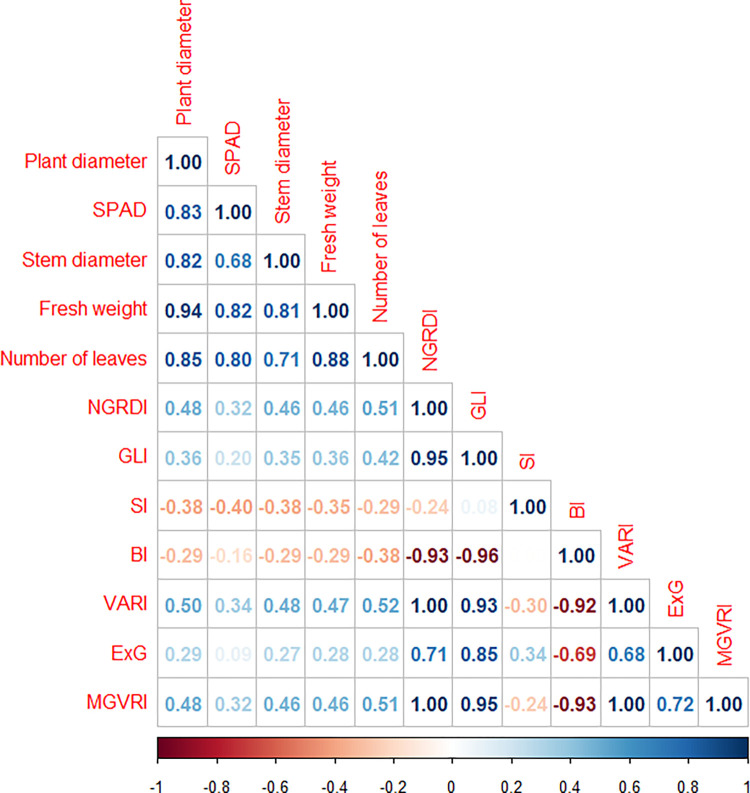
Vegetation indices obtained from aerial images of lettuce canopy showed significant correlation (p < 0.05) with vegetative growth parameters measured in lettuce plants (Fig 5). Positive correlations with vegetative parameters were observed for VARI, NGRDI, GLI, ExG, and MGVRI since these indices are related to chlorophyll content. Conversely, negative correlations were obtained for BI and SI, which are related to soil parameters. The highest positive correlations were observed for VARI and, thus, this index was chosen for fitting models to predict vegetative growth parameters.
Fig 5. Pearson’s correlation matrix between plant growth parameters and vegetation indices.
Positive and negative correlations are shown in blue and red tones, respectively. NGRDI: Normalized Green Red Difference Index, GLI: Green Leaf Index, SI: Soil Color Index, BI: Brightness Index, VARI: Visible Atmospherically Resistant Index, ExG: Excess Green Index (ExG), and MGVRI: Modified Green Red Vegetation Index.
The model for predicting SPAD index was the most accurate (NRMSE 4.87%), followed by models for number of leaves, plant diameter, and stem diameter (Table 3). On the other hand, fresh weight model presented high prediction error (RMSE 32.75%).
Table 3. Regression models for prediction of lettuce vegetative growth parameters based on the VARI (visible atmospherically resistant index) vegetation index obtained from aerial images of the lettuce canopy.
| Vegetative growth parameter | Equation | RMSE | NRMSE (%) |
|---|---|---|---|
| Plant diameter | 685.2348 VARI + 128.4624 | 21.68 | 9.67 |
| SPAD index | 31.6518 VARI + 24.1401 | 1.39 | 4.87 |
| Stem diameter | 72.6749 VARI + 8.1902 | 2.05 | 11.17 |
| Fresh weight | 763.8214 VARI—12.281 | 32.75 | 34.64 |
| Number of leaves | 57.0566 VARI + 13.4589 | 1.95 | 9.10 |
RMSE: Root-mean-square error, NRMSE: Normalized root-mean-square error
Discussion
Microbial inoculants are emerging as pivotal tools to unlock crop productivity potential and exceed the yield obtained with conventional chemical inputs. In this research, we demonstrate that lettuce plants inoculated with A. niger FS1 grew more than uninoculated ones even when plants had no P limitation. While the response to P addition was observed only up to 150 kg P2O5 ha-1, A. niger inoculation allowed further increments in all vegetative parameters. Microbial plant growth promotion is a multifactorial process resulting from different mechanisms that enhance plant fitness under diverse environmental conditions [9, 38]. Moreover, different microbial mechanisms can be recruited by plants according to their needs. Plants cultivated in poor nutrient soils recruited preferentially phosphate solubilizers while microorganisms capable of phytohormone production predominated in rich nutrient soils [39]. Aspergillus niger strains have shown the capacity to produce phytohormones such as indoleacetic acid and gibberellin [12, 13] and solubilize P and K from minerals [14–16]. Therefore, under nutrient scarcity, this species might release organic acids to make nutrients like P available to plants or, on the other hand, produce phytohormones to stimulate root and shoot development when nutrients are not limiting plant growth [40]. This would allow the fungus to switch mechanisms according to the plant’s needs, representing a smart choice for inoculant development. Our results indicate that well-nourished lettuce plants still benefit from inoculation with A. niger, suggesting that metabolic alterations due to fungal metabolites like phytohormones were taking place in plants. Indeed, we observed an increased chlorophyll content (SPAD index) in inoculated plants, which could be linked [41] with these fungal-promoted metabolic alterations.
The best ways to deliver A. niger conidia to plants were seed inoculation and application of a conidial suspension to the seedling substrate before transplanting. Both methods can be carried out in nurseries, allowing the introduction of A. niger as a core microorganism that may directly benefit the plant after transplanting to the soil as well as help to establish a network with beneficial indigenous microorganisms [11]. Moreover, a preliminary study showed that lettuce seedlings emerged from seeds inoculated with A. niger grew more and presented a well-developed root than uninoculated ones [18]. Additionally, once established in the seedling rhizosphere, A. niger would have a competitive advantage over native microbiota in the field, which probably was the advantage of these inoculation methods over in-furrow application of the granular formulation.
Although there is evidence that A. niger can release P fixed to soil [15], this mechanism was not observed in this study. The factors A. niger inoculation and P dose did not interact, demonstrating that the effect of A. niger on lettuce growth is independent of the P availability in the soil. The soil used in the experiment had 23.8 mg extractable P dm-3. This amount was not enough to meet the plant’s requirements once the addition of phosphate fertilizer increased plant growth. At this limiting P concentration, A. niger inoculation did not improve plant growth as would be expected. Microbial phosphate solubilization in the soil is probably lower than in vitro conditions since carbon and energy sources in the rhizosphere are disputed resources by the microbiota. This hampers the production of high concentrations of organic acids necessary for phosphate solubilization [16, 42, 43].
We used a high-throughput phenotyping method based on aerial images to evaluate changes in plants due to A. niger inoculation. The visible atmospherically resistant index (VARI) produced good prediction models for vegetative growth parameters, especially the SPAD index. The VARI is a vegetation index for quantitative estimation of vegetation fraction with the visible range of the spectrum [30]. Therefore, leaf pigments like chlorophyll and carotenoids have a major influence on this index, which explains the good adjustment (RMSE 1.39) of the prediction model for the SPAD index [44]. Image-based phenotyping has been successfully employed to detect infestation with plant pathogens, but changes in plant microbiota are still difficult to detect [45]. Our result is particularly interesting since it enables large-scale surveys of croplands inoculated with beneficial microorganisms, allowing detection of positive effects in the plant-microorganism interaction. Moreover, the VARI was strongly associated with chlorophyll content, allowing the efficient and quick selection of croplands inoculated with beneficial microorganisms in an indirect and non-destructible way. This image-based phenotyping method represent an alternative to time-consuming traditional SPAD measurements, enabling the use of images taken using a low-cost remotely piloted aircraft (RPA) equipped with a RGB camera to monitor microbial inoculations.
This research provides evidence of field growth promotion of lettuce by A. niger FS1. We demonstrated that even well-nourished plants benefited from A. niger inoculation. Therefore, inoculation with A. niger surpasses the yield obtained with conventional chemical inputs, allowing productivity gains not reached by just increasing P doses. Moreover, plant phenotyping by traditional measurement of growth parameters and aerial imaging allowed the detection of higher growth and chlorophyll content in inoculated plants. Thus, this research demonstrates the potential of high-throughput phenotyping methods based on aerial images for large-scale cropland surveys to determine the effect of microorganism inoculation on plant growth.
Supporting information
(TIF)
Acknowledgments
The authors thank the Laboratory of Botany/Federal University of Uberlândia for kindly lending the SPAD meter.
Data Availability
The datasets generated during the current study are available in the Mendeley Data repository, DOI: 10.17632/82c548svpy.1.
Funding Statement
This work was supported by the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), grant number APQ-01842-17 to GOM, the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), grant number 407793/2021-6 to GOM, and NOOA Ciência e Tecnologia Agrícola Ltda, grant to GOM and PVS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Alexandratos N, Bruinsma J. World agriculture towards 2030/2050: the 2012 revision. Rome; 2012. Report No.: 12–03.
- 2.Withers PJA, Rodrigues M, Soltangheisi A, De Carvalho TS, Guilherme LRG, Benites VDM, et al. Transitions to sustainable management of phosphorus in Brazilian agriculture. Sci Rep. 2018;8: 1–13. doi: 10.1038/s41598-018-20887-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tilman D, Balzer C, Hill J, Befort BL. Global food demand and the sustainable intensification of agriculture. Proc Natl Acad Sci. 2011;108: 20260–20264. doi: 10.1073/pnas.1116437108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Wart J, Kersebaum KC, Peng S, Milner M, Cassman KG. Estimating crop yield potential at regional to national scales. F Crop Res. 2013;143: 34–43. doi: 10.1016/j.fcr.2012.11.018 [DOI] [Google Scholar]
- 5.Chialva M, Lanfranco L, Bonfante P. The plant microbiota: composition, functions, and engineering. Curr Opin Biotechnol. 2022;73: 135–142. doi: 10.1016/j.copbio.2021.07.003 [DOI] [PubMed] [Google Scholar]
- 6.Olanrewaju OS, Glick BR, Babalola OO. Mechanisms of action of plant growth promoting bacteria. World J Microbiol Biotechnol. 2017;33: 1–16. doi: 10.1007/s11274-017-2364-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vessey JK. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil. 2003;255: 571–586. doi: 10.1023/A:1026037216893 [DOI] [Google Scholar]
- 8.Vassilev N, Vassileva M, Martos V, Galvez A, Flor-Peregrin E, Garcia del Moral LF. Phosphate sources, microorganisms, and P plant nutrition: challenges and future trends. Arch Crop Sci. 2019;3: 61–63. doi: 10.36959/718/603 [DOI] [Google Scholar]
- 9.Trivedi P, Leach JE, Tringe SG, Sa T, Singh BK. Plant–microbiome interactions: from community assembly to plant health. Nat Rev Microbiol. 2020;18: 607–621. doi: 10.1038/s41579-020-0412-1 [DOI] [PubMed] [Google Scholar]
- 10.Pérez-Jaramillo JE, Mendes R, Raaijmakers JM. Impact of plant domestication on rhizosphere microbiome assembly and functions. Plant Mol Biol. 2016;90: 635–644. doi: 10.1007/s11103-015-0337-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toju H, Peay KG, Yamamichi M, Narisawa K, Hiruma K, Naito K, et al. Core microbiomes for sustainable agroecosystems. Nat Plants. 2018;4: 247–257. doi: 10.1038/s41477-018-0139-4 [DOI] [PubMed] [Google Scholar]
- 12.Galeano RMS, Franco DG, Chaves PO, Giannesi GC, Masui DC, Ruller R, et al. Plant growth promoting potential of endophytic Aspergillus niger 9-p isolated from native forage grass in Pantanal of Nhecolândia region, Brazil. Rhizosphere. 2021;18: 100332. doi: 10.1016/j.rhisph.2021.100332 [DOI] [Google Scholar]
- 13.Lubna, Asaf S, Hamayun M, Gul H, Lee I-J, Hussain A. Aspergillus niger CSR3 regulates plant endogenous hormones and secondary metabolites by producing gibberellins and indoleacetic acid. J Plant Interact. 2018;13: 100–111. doi: 10.1080/17429145.2018.1436199 [DOI] [Google Scholar]
- 14.Lopes-Assad ML, Avansini SH, Rosa MM, de Carvalho JRP, Ceccato-Antonini SR, Carvalho JRP, et al. The solubilization of potassium-bearing rock powder by Aspergillus niger in small-scale batch fermentations. Can J Microbiol. 2010;56: 598–605. doi: 10.1139/W10-044 [DOI] [PubMed] [Google Scholar]
- 15.Nascimento JM do, Vieira Netto JAF, Valadares RV, Mendes G de O, Silva IR da, Vergütz L, et al. Aspergillus niger as a key to unlock fixed phosphorus in highly weathered soils. Soil Biol Biochem. 2021;156: 108190. doi: 10.1016/j.soilbio.2021.108190 [DOI] [Google Scholar]
- 16.Mendes GO, Freitas ALM, Pereira OL, Silva IR, Vassilev NB, Costa MD. Mechanisms of phosphate solubilization by fungal isolates when exposed to different P sources. Ann Microbiol. 2014;64: 239–249. doi: 10.1007/s13213-013-0656-3 [DOI] [Google Scholar]
- 17.Araújo VC, Rossati KF, Xavier LV, Oliveira VA de, Carmo GJ dos S, Assis GA de, et al. Enhanced growth in nursery of coffee seedlings inoculated with the rhizosphere fungus Aspergillus niger for field transplantation. Rhizosphere. 2020;15: 100236. doi: 10.1016/j.rhisph.2020.100236 [DOI] [Google Scholar]
- 18.Mundim G de SM, Maciel GM, Mendes G de O. Aspergillus niger as a biological input for improving vegetable seedling production. Microorganisms. 2022;10: 674. doi: 10.3390/microorganisms10040674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pavinato PS, Cherubin MR, Soltangheisi A, Rocha GC, Chadwick DR, Jones DL. Revealing soil legacy phosphorus to promote sustainable agriculture in Brazil. Sci Rep. 2020;10: 15615. doi: 10.1038/s41598-020-72302-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Filgueira FAR. Novo manual de olericultura -Agrotecnologia moderna na produção e comercialização de hortaliças. 3rd ed. Viçosa: Editora UFV; 2008. [Google Scholar]
- 21.Filho APM, de Medeiros E V, Barbosa JG, Barbosa JMP, Kuklinsky-Sobral Ú, Souza-Motta C. Combined effect of Pseudomonas sp. and Trichoderma aureoviride on lettuce growth promotion. Biosci J. 2019;35: 419–430. doi: 10.14393/BJ-v35n2a20198-41892 [DOI] [Google Scholar]
- 22.Wonglom P, Ito S ichi, Sunpapao A. Volatile organic compounds emitted from endophytic fungus Trichoderma asperellum T1 mediate antifungal activity, defense response and promote plant growth in lettuce (Lactuca sativa). Fungal Ecol. 2020;43: 100867. doi: 10.1016/j.funeco.2019.100867 [DOI] [Google Scholar]
- 23.Ribeiro AC, Guimarães PTG, Alvarez VH V. Recomendações para o uso de corretivos e fertilizantes em Minas Gerais—5° Aproximação. 5o Edição. Viçosa—MG: Editora UFV; 1999. [Google Scholar]
- 24.Matias FI, Caraza‐Harter M V, Endelman JB. FIELDimageR: An R package to analyze orthomosaic images from agricultural field trials. Plant Phenome J. 2020;3. doi: 10.1002/ppj2.20005 [DOI] [Google Scholar]
- 25.Escadafal R, Belghith A, Bem MH. Indices spectraux pour la télédétection de la dégradation des milieux naturels en Tunisie aride. Mes Phys Signatures spectrales en Télédétection. 1994; 17–21. [Google Scholar]
- 26.Tucker CJ. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens Environ. 1979;8: 127–150. doi: 10.1016/0034-4257(79)90013-0 [DOI] [Google Scholar]
- 27.Louhaichi M, Borman MM, Johnson DE. Spatially Located Platform and Aerial Photography for Documentation of Grazing Impacts on Wheat. Geocarto Int. 2001;16: 65–70. doi: 10.1080/10106040108542184 [DOI] [Google Scholar]
- 28.Mathieu R, Pouget M, Cervelle B, Escadafal R. Relationships between Satellite-Based Radiometric Indices Simulated Using Laboratory Reflectance Data and Typic Soil Color of an Arid Environment. Remote Sens Environ. 1998;66: 17–28. doi: 10.1016/S0034-4257(98)00030-3 [DOI] [Google Scholar]
- 29.Richardson AJ., Wiegand CL. Distinguishing vegetation from soil background information. Photogramm Eng Remote Sensing. 1977;43: 1541–1552. [Google Scholar]
- 30.Gitelson AA, Kaufman YJ, Stark R, Rundquist D. Novel algorithms for remote estimation of vegetation fraction. Remote Sens Environ. 2002;80: 76–87. doi: 10.1016/S0034-4257(01)00289-9 [DOI] [Google Scholar]
- 31.Woebbecke DM, Meyer GE, Bargen K Von, Mortensen DA. Color Indices for Weed Identification Under Various Soil, Residue, and Lighting Conditions. Trans ASAE. 1995;38: 259–269. doi: 10.13031/2013.27838 [DOI] [Google Scholar]
- 32.Bendig J, Yu K, Aasen H, Bolten A, Bennertz S, Broscheit J, et al. Combining UAV-based plant height from crop surface models, visible, and near infrared vegetation indices for biomass monitoring in barley. Int J Appl Earth Obs Geoinf. 2015;39: 79–87. doi: 10.1016/j.jag.2015.02.012 [DOI] [Google Scholar]
- 33.Ferreira EB, Cavalcanti PP, Nogueira DA. ExpDes: an R package for ANOVA and experimental designs. Appl Math. 2014;05: 2952–2958. doi: 10.4236/am.2014.519280 [DOI] [Google Scholar]
- 34.Mantel N. The detection of disease clustering and a generalized regression approach. Cancer Res. 1967;27: 209–220. [PubMed] [Google Scholar]
- 35.Cruz CD. Genes: a software package for analysis in experimental statistics and quantitative genetics. Acta Sci Agron. 2013;35: 271–276. [Google Scholar]
- 36.Singh D. The relative importance of characters affecting genetic divergence. Indian J Genet Plant Breed. 1981;41: 237–245. [Google Scholar]
- 37.Cruz CD, Regazzi AJ, Carneiro PCS. Modelos biométrico aplicados ao melhoramento genético. Viçosa: UFV; 2012. [Google Scholar]
- 38.Bano S, Wu X, Zhang X. Towards sustainable agriculture: rhizosphere microbiome engineering. Appl Microbiol Biotechnol. 2021;105: 7141–7160. doi: 10.1007/s00253-021-11555-w [DOI] [PubMed] [Google Scholar]
- 39.da Costa PB, van Elsas JD, Mallon C, Borges LG dos A, Passaglia LMP. Efficiency of probiotic traits in plant inoculation is determined by environmental constrains. Soil Biol Biochem. 2020;148: 107893. doi: 10.1016/j.soilbio.2020.107893 [DOI] [Google Scholar]
- 40.Vassileva M, Mendes GDO, Deriu MA, Benedetto G di, Flor-Peregrin E, Mocali S, et al. Fungi, P-solubilization, and plant nutrition. Microorganisms. 2022;10: 1716. doi: 10.3390/microorganisms10091716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu X, Li Y, Zhong S. Interplay between light and plant hormones in the control of Arabidopsis seedling chlorophyll biosynthesis. Front Plant Sci. 2017;8: 1433. doi: 10.3389/fpls.2017.01433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mendes GO, Bahri-Esfahani J, Csetenyi L, Hillier S, George TS, Gadd GM. Chemical and physical mechanisms of fungal bioweathering of rock phosphate. Geomicrobiol J. 2021;38: 384–394. doi: 10.1080/01490451.2020.1863525 [DOI] [Google Scholar]
- 43.Mendes G de O, Dyer T, Csetenyi L, Gadd GM. Rock phosphate solubilization by abiotic and fungal‐produced oxalic acid: reaction parameters and bioleaching potential. Microb Biotechnol. 2022;15: 1189–1202. doi: 10.1111/1751-7915.13792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cassetari LS, Gomes MS, Santos DC, Santiago WD, Andrade J, Guimarães AC, et al. β-carotene and chlorophyll levels in cultivars and breeding lines of lettuce. Acta Hortic. 2015;1083: 469–473. doi: 10.17660/ActaHortic.2015.1083.60 [DOI] [Google Scholar]
- 45.Carvalho S, van der Putten WH, Hol WHG. The potential of hyperspectral patterns of winter wheat to detect changes in soil microbial community composition. Front Plant Sci. 2016;7: 759. doi: 10.3389/fpls.2016.00759 [DOI] [PMC free article] [PubMed] [Google Scholar]