Abstract
Bacterial infections in the respiratory tract are considered as one of the major challenges to the public health worldwide. Pulmonary delivery is an attractive approach in the management of bacterial respiratory infections with a few inhaled antibiotics approved. However, with the rapid emergence of antibiotic-resistant bacteria, it is necessary to develop new/alternative inhaled antibacterial agents in the post-antibiotic era. A pipeline of novel biological antibacterial agents, including antimicrobial peptides, RNAi therapeutics, and bacteriophages, has emerged to combat bacterial infections with excellent performance. In this review, the causal effects of bacterial infections on the related pulmonary infectious diseases will be firstly introduced. This is followed by an overview on the development of emerging antibacterial therapeutics for managing lung bacterial infections through nebulization/inhalation of dried powders. The obstacles and underlying proposals regarding their clinical transformation are also discussed to seek insights for further development. Research on inhaled therapy of these emerging antibacterials are still in the infancy, but the promising progress warrants further attention.
Keywords: antimicrobial peptides, bacteriophages, emerging antibacterials, pulmonary delivery, respiratory bacterial infections, RNAi
Introduction
Respiratory bacterial infections are one of the leading causes of death worldwide [1]. The colonized bacteria are either the major causative agents of pulmonary infectious diseases (e.g. tuberculosis and pneumonia) or linked with many lung illnesses complications (e.g. cystic fibrosis and lung cancer), which greatly threaten the public health and global economy [2]. Proper management of pathogenic bacteria relating to outrageous disorders is highly demanded. Inhalation therapies are attractive means for the treatment of respiratory infections because of a few recognizable advantages [3].
In comparison with other administration routes, pulmonary delivery can directly deliver the drug into the lung, and promptly allow for a high therapeutic concentration at the site of action with lower doses, minimizing undesirable systemic absorption and side effects [4]. Nonetheless, the options of antibacterial medicine that are formulated and approved for inhaled applications are limited [5]. Table I summarized the approved inhaled antibiotic therapies to target bacterial lung infections. While they are effective against lung infections caused by the antibiotic-sensitive strains, increasing therapeutic challenges arouse from the rapid emergence of antibiotic-resistant bacteria. Therefore, it is imperative to develop new/alternative inhaled antibacterial agents for clinical application in the post-antibiotic era.
Table I.
Current Inhaled Antibiotics Approved by the Food and Drug Administration
| Brand name | Active Pharmaceutical Ingredients | Formulation | Indication | Approved date |
|---|---|---|---|---|
| TOBI® | Tobramycin | Inhalation solution | The management of cystic fibrosis in adults and pediatric patients 6 years of age and older with P. aeruginosa | 1975 |
| Bethkis® | Tobramycin | Inhalation solution | The management of cystic fibrosis patients with P. aeruginosa | 1980 |
| Cayston® | Aztreonam | Inhalation solution | To improve respiratory symptoms in cystic fibrosis patients with P. aeruginosa | 1986 |
| TOBI® Podhaler™ | Tobramycin | Dry powder inhalation | The management of cystic fibrosis patients with P. aeruginosa | 2013 |
| Kitabis® Pak | Tobramycin | Inhalation solution | The management of cystic fibrosis in adults and pediatric patients 6 years of age and older with P. aeruginosa | 2014 |
| Arikayce® | Amikacin sulfate | Liposomal suspension | Adults who have limited or no alternative treatment options for treating Mycobacterium avium complex (MAC) lung disease | 2018 |
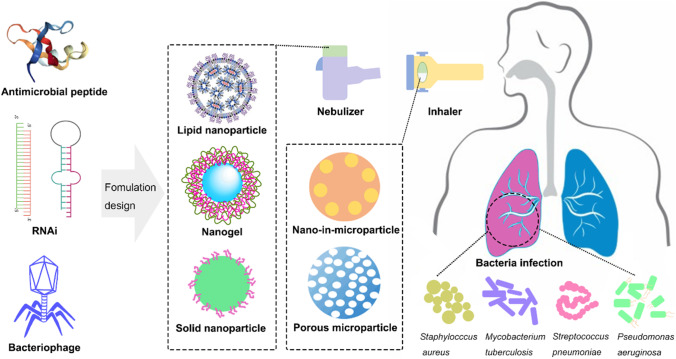
To date, a pipeline of new/non-conventional antibacterial therapeutics has emerged for the treatment of bacterial infections, especially those caused by multidrug-resistant (MDR) strains [6]. These emerging antibacterial agents are mostly biologicals, including antimicrobial peptides (AMPs), RNA interference (RNAi) therapeutics and bacteriophages (phages). They are designed to selectively interact with a specific target, such as the bacterial cell membrane, a cellular process, a gene, or a specific host pathogen [7]. These novel candidates exemplify promising features by providing a broad spectrum of action against both Gram-positive and Gram-negative bacteria and/or limiting drug resistance with excellent safety profiles [7–10]. Research and technology development also promotes the inhaled applications of these emerging alternatives (Fig. 1), which may result in better efficacy against bacterial lung infections.
Fig. 1.
Schematic diagram of different emerging antibacterials and their formulation strategies for pulmonary delivery to treat lung infections with bacteria.
In this review, the associations between bacterial infections and infectious respiratory diseases will be firstly introduced to provide some inspiration in the causal effects and the corresponding therapeutic strategies. Then, recent advances in the pulmonary delivery of the above mentioned emerging antibacterials for the treatment of respiratory bacterial infections is overviewed, with special focus on their advances in nebulization and dry powder formulations. Possible safety issues and challenges that need to be overcome for their clinical translation will also be covered.
Bacterial Lung Infections and Pulmonary Diseases
Bacteria are the dominant pathogens causing respiratory tract infections with different epidemiology and clinical presentations [11]. Bacterial infections of the lung can occupy all levels of the airway tree and are generally categorized into two main types by anatomical location: (i) upper respiratory tract infections, URTIs, and (ii) lower respiratory tract infections, LRTIs [12]. Between them, LRTIs are more difficult to handle and usually complicate a series of existing lung diseases [13]. In this respect, bacteria-associated LRTIs are briefly introduced and the currently-reported associations of bacterial infections with various pulmonary diseases are summarized here. A good understanding of the causal effects and underlying relationships can play vital roles in improving clinical infection management.
Bacterial-Caused Lung Diseases
Tuberculosis
Tuberculosis (TB) is the deadliest pulmonary infectious disease globally. According to the World Health Organization report, an estimated 10 million people contracted TB and 1.5 million people died from TB in 2021 [14]. TB is caused by the bacillus Mycobacterium tuberculosis (Mtb), which can persistently harbor within the alveolar macrophages by modifying host defense mechanisms. Even though the activation of macrophages by T cells and cytokines for bacterial control, the defense always remains incomplete. The balance between bacterial persistence and host immune response is prone to shift in a favor to bacterial survival and propagation [15].
Standard TB therapeutic regimens generally involve four kinds of antibiotics (rifampin, isoniazid, pyrazinamide and ethambutol) lasting for six to nine months, which greatly decrease patient compliance. The poor adherence with chemotherapy can cause a midway-withdrawal of drug administration, leading to treatment failure, relapse of symptoms and even emergence of MDR and extensive-drug resistant (XDR) strains. Currently, MDR-TB and XDR-TB account for more than 10% of TB-related deaths [16]. Genetic diversity can also contribute to drug resistance problem in TB therapy, arising limitations in clinical treatment options [17]. Moreover, conventional TB therapies via systemic or oral administration usually fail to deliver the therapeutic drugs to the infected lung tissues. A number of bio-barriers, such as macrophage cell membranes, lysosomes, and bacterial biofilms, are considered to be the obstacles for effective delivery and transportation of anti-TB drugs [11]. A lesson learned from the successfully-commercialized Arikayce (liposomal amikacin inhalation) revealed that liposome formulations could be used to handle the intracellular bacterial infections. With improved macrophage uptake due to the interaction between phospholipid membrane and cell membrane, this formulation design sheds light to new research directions in targeting intracellular TB infections in the lung.
Bacterial Pneumonia
Bacterial pneumonia, defined as bacteria-induced infectious diseases of the lower respiratory tract, is a leading cause of death among both children and adults worldwide [18]. Pneumonia can be classified into several types based on the origin, including community-acquired, nosocomial/hospital-acquired, and aspiration pneumonia [19]. The most likely aetiological agent producing community-acquired pneumonia (CAP) is Streptococcus pneumoniae, followed by Mycoplasma pneumoniae, Haemophilus influenzae, Chlamydia pneumoniae and Legionella pneumophila in descending order of frequency [19]. Hospital-acquired pneumonia (HAP) is reported to exhibit a higher mortality rate than any other hospital-acquired infections. It is mostly associated with some aerobic Gram-negative bacilli, like Pseudomonas aeruginosa and Enterobacteriaceae, and a few Gram-positive cocci, particularly Staphylococcus aureus, which has evolved into methicillin-resistant strains (MRSA) and provokes concerns for hospital-infection control [20].
Prompt treatment of pneumonia with antibiotics can relieve symptoms and lower mortality. However, the empirical use of broad-spectrum antibiotics is usually associated with resistant-bacterial infections, limiting the choice of antibiotic groups [21]. Furthermore, it is noteworthy that a variety of biological barriers in the lung hinder the efficient delivery of drugs to the target site in patients with pneumonia. Firstly, the overproduction and dehydration of mucus on the surface of lung epithelium, under pathological state, can greatly impact the performance of drug delivery systems administered through inhalation [11]. Meanwhile, increased inflammatory cells gathering could further block the deposition and retention of inhaled drugs in the lung [22]. Additionally, cellular barriers in acute pneumonia and bronchitis, where bacterial pathogen can invade and survive inside host cells, provide protections for bacteria from the antibacterial agents and the host immune system, constituting a major challenge for intracellular bacteria elimination [23]. Thus, these pathological conditions need to be carefully considered when designing rational inhalable delivery systems.
Bacterial Infections-Associated Lung Diseases
Obstructive Pulmonary Diseases
Chronic Obstructive Pulmonary Disease (COPD)
COPD is a chronic inflammatory disease of the lung. Bacterial infections are considered as a major complication of COPD, contributing to airway colonization, exacerbations of COPD and pneumonia [24]. The abundances of Streptococcus, Pseudomonas, Prevotella and Haemophilus are mostly detected with exacerbation of COPD [25]. Studies established that pathological bacteria caused up to 50% of exacerbated COPD while patients with stable COPD presented much lower burdens of related bacteria [26]. Inhaled corticosteroids, which are the routinely-used anti-inflammatory agents, are reported to be associated with lung microbiota disruption, resulting in the proliferation of S. pneumoniae [27]. The persistence of bacteria in the lower respiratory tract may abominably stimulate mucus hypersecretion and induce intense inflammatory reactions, which are prone to act as bio-barriers for inhaled drug delivery and efficacy [28]. Furthermore, infected bacteria may not only stay on the surface of epithelium, but also invade into epithelial cells. These intracellular-bacteria would escape from the bactericidal activity of antibiotics, leading to persistent lung infections. Therefore, there are callings for the development of intracellular-drug delivery systems [23].
Asthma
Asthma is another long-term inflammatory disease of respiratory tract (especially in bronchi and bronchioles) with processive contractability of airway smooth muscles. Combinations of genetic and environmental factors are thought to affect the development and severity of asthma [29]. Specific types of bacteria are also reported to contribute to the exacerbations of asthma. Moraxella catarrhalis, H. influenza and S. pneumoniae are commonly-seen in hypopharynx of childhood asthma patients, while the level of Proteobacteria in the lower respiratory tract is related with the occurrence of asthma in adults [30, 31]. M. pneumoniae and C. pneumoniae infections are also reported to be associated with asthma chronicity, with 56% of the tested samples from asthmatic patients having positive indications of M. pneumoniae or C. pneumoniae [32]. Further study is still needed to assess whether the accumulation of bacteria is a temporary phenomenon or a direct participation into the pathogenesis of asthma.
Cystic Fibrosis (CF)
Cystic fibrosis (CF) is a progressive genetic disorder affecting mostly the lungs, which is caused by a genetic mutation in the Cystic Fibrosis Transmembrane-conductance Regulator (CFTR) protein. CFTR is responsible for mucus production. When CFTR is disordered, the mucus secretion would become much thicker, which may decrease the mucociliary clearance function thus resulting in bacterial colonization and infection [33]. This mucus built-up may further lead to the formation of bacterial micro-environment (known as biofilm), which is a compact bio-barrier for antibiotics and innate anti-infective agents to penetrate [34]. The continuous mucus secretions and persistent bacterial infections would greatly damage the lung, contributing to exacerbations of CF [35]. Bacterial pathogens, including S. aureus, H. influenzae, and P. aeruginosa, are the most common organisms inducing infections in CF patients [36]. Among these, P. aeruginosa infections are involved in most of the premature deaths of CF patients [36]. Inhaled antibiotics such as tobramycin, azithromycin and aztreonam are often administered for months to improve lung function by impeding the growth of pathogenic bacteria [11].
Lung Cancer
Lung cancer is a leading disease of cancer-related deaths among both men and women worldwide. According to GLOBOCAN 2020, lung cancer accounts for 11.4% of the total cancer cases and 18.0% of the total cancer deaths [37]. Following dietary factors and long-term tobacco smoking, infectious diseases are the third leading cause of lung cancer [38]. With increasing attention paid into the area of respiratory bacteriology, the interplays of lung bacterial microbiota dysbiosis with lung cancer development are now under investigation and discussion [38]. The effects of lung cancer management on the respiratory bacterial composition and infection have also been reported [39].
Investigating bacterial microbiota changes may also result in a better understanding of the role of bacteria in tumorigenesis. A significant relationship has been found between Mtb and lung cancer, possibly because the persistent infection by Mtb induces the production of tumor necrosis factor and leads to pulmonary inflammation, which promote the development of lung cancer [40]. However, the understanding of the underlying mechanisms linking the lung microbiome dynamics and lung cancer are still preliminary, and further studies are quite essential.
It is also noteworthy that bacterial infections not only contribute to cancer initiation and progression but also are the most commonly-encountered complications in lung cancer patients (with up to ~70% of concurrency rate), greatly affecting the survival of these patients [41]. S. aureus, S. pneumoniae, H. influenzae, P. aeruginosa, L. pneumophila, M. catarrhalis and E. coli are the most frequently isolated pathogens in lung cancer patients [42]. These infected bacteria are highly prone to inhabit in tumor tissues and are not easily removed from the tumor tissues [43]. Special infection management for lung cancer patients is thus highly needed.
Emerging Inhaled Antibacterials for Respiratory Bacterial Infection Treatment
Inhaled antibiotics have been proved to be effective management strategies against bacterial infections, particularly for CF patients. However, this therapeutic option also faces challenges with the emergence of drug-resistant bacteria, rendering their long-term usage questionable. Generally, mechanisms of antibiotic resistance in MDR bacteria can be classified into: i) direct inactivation of antibiotic molecules, ii) limiting uptake of antibiotics (e.g. by decreasing penetration, promoting expression of efflux pumps), iii) alteration of drug target, and iv) gaining adaptive resistance with global change in metabolic pathways [44]. These antibiotics-resistant mechanisms have been developed for millions of years of evolution, which are sophisticated and render further development of conventional antibiotics an ineffective strategy to address the MDR crisis. In this context, a few emerging biological antibacterials with great promise to target MDR bacteria are introduced in this section, including AMPs, RNAi therapeutics and phages, with specific focus on the innovative formulation strategies for their pulmonary delivery. Since the occurrence of bacterial resistance is often related to the concentration of localized antibacterial agents (e.g. antibiotics), the efficiency of pulmonary drug delivery should therefore be optimally guaranteed, in order to maximatily eradicate strains to minimize resistance development towards the corresponding drugs [45].
Inhalable products usually engage two components: One is the aerosol formulation, including liquids (solutions/suspensions/emulsions) or dry powders; and the other is a device for aerosol dispersion, such as nebulizers, metered dose inhalers (MDIs) and dry powder inhalers (DPIs). Due to the biological nature of most emerging antibacterials, their formulation strategies mostly focus on liquid formulations for nebulization. Nebulization is suitable for therapeutics requiring high doses with little patient coordination. Thus, they are typically applied in home or hospital stay, especially preferable for nosocomial pulmonary infection management. Comparing with liquid formulations, dry powder formulations can enhance the stability of biological antibacterials and DPIs are generally portable, easy to operate and the duration of administration is short, improving patient adherence [46]. Increasing attention have, therefore, devoted to developing novel engineering particles of emerging antibacterials for the treatment of respiratory infections.
Inhaled Antimicrobial Peptides
Therapeutic Principle of Antimicrobial Peptides (AMPs)
AMPs, also known as host defense peptides, are endogenous polypeptides produced by multicellular organisms as a first line of defense. They have been defined into different families according to the amino acid compositions/structures, where cecropin, defensin, magainin and cathelicidin are classified [47]. With the successful clinical translation of colistin and vancomycin, this class of antibacterial has continued to receive tremendous attention [48]. To date, there have been more than 2600 natural AMPs, which are the main basis for the design and synthesis of novel therapeutic peptide analogues [49]. Both the natural and synthetic peptides are potential candidates to either control bacterial infections by their direct bactericidal properties or treat chronic inflammatory diseases by modulating the host inflammatory responses [50].
AMPs usually appear non-receptor-mediated membrane-lytic bactericidal [51]. They are amphipathic cationic small peptides with 8–50 amino acids. The main target for these cationic peptides is the bacterial membranes because of the presence of negatively charged molecules. Upon attaching to the biological membranes, peptides shape from unstructured state into their configurations, with hydrophilic regions aligned on one side and hydrophobic residues on the opposite. By destabilizing the biological membranes of the bacteria, peptides can further translocate the membrane and interact with the intracellular components and subsequently, interfere cell processes to kill the bacteria [52]. A novel cathelicidin-related (CR) antimicrobial CR-163 showed excellent antibacterial activity against cystic fibrosis-related respiratory pathogens P. aeruginosa and S. aureus, and the intratracheal administration of CR-163 was found to be well tolerated in vivo [53].
In addition to direct microbial-killing abilities, AMPs also exhibit strong immunomodulatory functions, which can inhibit unwanted enhanced inflammations to achieve an immune homeostasis, beneficial for bacterial clearance [54]. The underlying mechanism is based on the activation of innate immune system, including recruitment of leukocytes, modulation of neutrophils and coordination of antigen presentation [47]. An in vivo study demonstrated LL-37 (cathelicidin family) promoted bacterial clearance by enhancing the neutrophil responses [55]. Defensins, such as HBD2 and HBD3, were found to induce dendritic cells to produce more interferon-α and consequently stimulate the initiation of T cell responses [56]. As for their anti-inflammatory function, a good example is that cathelicidin-deficient mice displayed more severe inflammatory responses compared with wild-type mice [57]. LL-37 was also reported to exhibit anti-inflammatory activity via modulation of toll-like receptor (TNR) signaling, like downregulating TLR2 and TLR4 [58, 59]. Collectively, the dual activities of these AMPs are especially vital in the management of bacterial lung infections accompanied with inflammation to protect the respiratory epithelium from the overwhelmed inflammatory reactions.
Formulation Development for Inhaled AMPs
In view of the limitations of direct lung administration of peptides, such as inactivation or arrest by lung barriers, the stability and efficacy of AMPs via pulmonary delivery can be improved through formulation strategies (Table II). To avoid their degradation by peptidases/proteases in lungs, nanocarriers are commonly-used with great advantages. Carriers can prevent the self-aggregation of AMPs, and allow the release of the incorporated AMPs in a time-controllable manner by sustaining the degradation of the carriers, thus improving both their chemical stability and efficacy [60]. It has been reported that the stability of LLKKK18 AMP (an LL-37 analog) was enhanced in the formulation of hyaluronic acid nanogels [61]. In an in vitro setting, macrophages incubated with the LLKKK18-loaded nanogels were found to exhibit lower intracellular levels of mycobacteria compared with those incubated with the free AMP. In a mycobacteria-infected mice model, intratracheal administration of the formulation with a low peptide dose showed significant reduction in lung bacteria loads, demonstrating its potential in managing TB [61]. A non-natural antimicrobial peptide, SET-M33, with branched structure demonstrated better resistance to degradation in biological fluids and great antibacterial capacity against 100 Gram-negative MDR clinical isolates [62]. It was successfully formed into a nanosystem (M33-NS) with a single-chain dextran nanoparticle. Intrapulmonary delivered M33-NS increased the lung residence time, resulting in better antibacterial efficacy in a P. aeruginosa associated mice pneumonia model via intratracheal delivery. Recently, an albumin-based nanodrug delivery system was developed for the cationic LL-37 AMP [63]. The nanoparticles showed sustained release of LL-37 for more than 48 h, prolonging the in vitro antimicrobial effects against P. aeruginosa. Its potential in alleviating bacterial lung infection was also confirmed in an acute P. aeruginosa lung infection mouse model. The intratracheally-delivered LL-37 loaded nanoparticles showed enhanced bacterial clearance compared with the free LL-37 [63]. Similarly, polyphenol-based capsules for AMP polymers were also reported to exhibit sustained release profile of encapsulated drugs and enhance intracellular delivery into alveolar macrophages in vitro [64]. The encapsulated peptide drugs could retain the high antimicrobial activity against E. coli in vitro, with desirable nebulization efficiency [64].
Table II.
Inhalable AMPs Formulations for Treating Respiratory Bacterial Infections
| Form | AMP | Formulation | In vitro outcome | In vivo efficacy | Ref. |
|---|---|---|---|---|---|
| LLKKK18 | Hyaluronic acid nanogels | Lower levels of mycobacteria inside macrophages | Significant reduction of bacteria loads in the lungs of mice | [61] | |
| Liquid | SET-M33 | Dextran nanoparticles | Effective P. aeruginosa killing and acceptable cytotoxicity towards different animal cell lines | Improved lung residence after administered via aerosol in healthy rats; Efficient in pulmonary infection management in a BALB/c mouse model of pneumonia caused by P. aeruginosa | [62] |
| LL-37 | Albumin-based nanoparticles | Sustained antibacterial effect to P. aeruginosa | Enhanced bacterial clearance in a mouse model | [63] | |
| SNAPP | Polyphenol-based microcapsules | Enhanced intracellular delivery into alveolar macrophages | – | [64] | |
| Dry powder | Plectasin | Freeze-dried PLGA nanoparticles | Improved antibacterial efficacy in S. aureus-infected Calu-3 cells | – | [65] |
| D-LAK120-HP13/D-LAK120-A | Mannitol-incorporated powders | Preservation of secondary structures and desirable aerosolization performance | – | [66] |
With the acknowledged advantages of DPIs, increasing attention has gained in formulating AMP into inhalable dry powder formulations. However, it is a dual challenge to maintain the stability of AMPs upon processing and meanwhile achieve proper aerodynamic properties of the powders for inhalation. Plectasin, an antimicrobial defensin-class AMP, has been successfully encapsulated into poly(lactic-co-glycolic acid) (PLGA) nanoparticles and freeze-dried into powders [65]. The plectasin-loaded nanoparticles exhibited improved antibacterial efficacy in S. aureus infected bronchial epithelial Calu-3 cell monolayers as compared with the non-encapsulated plectasin. It was attributed to the improved cellular uptake of the encapsulated drug to target bacteria residing intracellularly [65]. Kwok et al prepared D-enantiomeric AMPs (D-LAK120-HP13 and D-LAK120-A) dry powders by spray drying (SD) with mannitol as a bulking agent. The spray-dried powders showed desirable aerosolization performance and the secondary structures of the AMPs were also preserved during the SD process, exhibiting good potential in treating tuberculosis by inhalation [66].
Great number of preclinical studies have demonstrated the effective antibacterial activity of AMPs in murine models, however, the inhalable application of AMPs in combating bacterial lung infections is still at an early stage. Although the cationic nature of AMPs could bring some benefits for bacterial elimination, it also increases the risk of toxicity to epithelium and radical inflammatory reactions in the lung, which may aggravate the original infective conditions. In this manner, specific strategies including incorporation of carriers in order for attenuating toxicity and better targeting while maintaining strong bactericidal ability is highly-desired. One good example is the design of nanofibers to manage skin injuries by Amariei et al [67]. By coating antimicrobial polypeptide ε-poly(l-lysine) with poly(acrylic acid) (PAA)/poly(vinyl alcohol) (PVA) electrospun nanofibers via electrostatic interactions, the functionalized formulations exhibited good biocompatibility. The peptide-loaded nanofibers also showed excellent durability, suppressing bacterial colonization for a duration of 14 days. As discussed above, while the nano-carrier strategies have been adopted to deliver AMPs via the pulmonary route, their biocompatibility in lung tissues was yet well-reported. Preclinical in vivo research to demonstrate the safety and effectiveness of the designed formulations are essential before applying the inhaled peptides into clinic.
Inhaled RNAi Therapeutics
Therapeutic Principle of Antibacterial RNAi
The pathogenesis of bacterial infections may be related to genetic dysregulation, such as the occurrence of attenuation in antimicrobial responses by the host cells [9]. In this regard, the interference with certain gene expression via RNA interference (RNAi) offers great potential to help address the unmet medical needs [68]. Small interfering RNAs (siRNAs) and microRNAs (miRNAs) are novel classes of therapeutic agents in gene regulation to treat a considerable range of disorders including lung infections [69]. Both siRNAs and miRNAs have great therapeutic advantages over traditional small drug molecules because they can virtually inhibit the expression of any genes and mRNA transcripts (especially for “non-druggable” targets) which are causally involved in the pathological development [70].
miRNAs play a key role in the management of bacterial lung infections by regulating the inflammatory responses as well as the host innate and adaptive immunity [71]. Several miRNAs, such as miR-29, miR-33, miR-146a, miR-155 and miR-302b, have been reported to exhibit efficient immune regulation properties in preclinical models [72, 73]. miR-302b was proved to inhibit the bacteria-triggered proinflammatory cytokines production by blocking the TLR signaling in a P. aeruginosa-infected mice model, thereby controlling the respiratory system homoeostasis. The immune modulation efficacy achieved by miR-302b could protect the infected mice from severe tissue injury caused by excessive inflammatory cytokines [72]. This was the first study implicating the role of a member from the miR-302 family in respiratory bacterial infections and related immune responses. The capability of suppressing the inflammatory responses to pulmonary infections via targeting the TLR signaling pathway was also demonstrated with miR-146a and miR-155 [73]. These findings unraveled the interplays between miRNAs and the innate immune reactions during pathogenic bacteria evasion. This may indicate a potential therapeutic auxiliary in treating lung bacterial infections, particularly for patients with pulmonary inflammation, such as those with COPD, asthma and CF.
siRNA-based antibacterial therapy is promising. Yanagihara et al investigated the silencing efficacy of siRNA on the expression of coagulase, an enzyme plays an important role in the pathogenesis of MRSA infections [74]. In vitro results demonstrated that siRNA could successfully inhibit both mRNA expression and the activity of MRSA coagulase. They also proved its in vivo efficacy in reducing the bacterial load in a pulmonary infective murine model [74]. Eliminating the inner membrane component (MexB) of P. aeruginosa by silencing MexB gene expression via siRNA was reported to be effective in reducing its pulmonary infectivity in a mouse model, with decreased viable bacterial counts and pathologic changes in the host lung [75]. Furthermore, the siRNA-mediated silencing of MexB in P. aeruginosa led to decreased neutrophil recruitment during the late stage of pulmonary infection, thus alleviating the inflammation-related damages to the lung tissues [75]. Pulmonary aerosolized delivery of siRNA targeting TGFβ1 cytokines gene silencing was found to effectively reduce the bacterial load in Mtb-infected mice. In addition, it was hypothesized that the ability of siRNA in immune-regulation to enhance the host antimicrobial capacity could be exploited together with the current chemotherapeutic regimens for TB treatment [76].
Formulation Development for Inhaled RNAi Therapeutics
To the best of the authors’ knowledge, no formulation studies have yet been performed with siRNA or miRNA specifically targeting bacterial lung infection. Considering that nucleic acid-based therapeutics have similar physicochemical properties and action sites, here the formulation strategies for pulmonary delivery of siRNA with other therapeutic effects are summarized. In general, nucleic acids are fragile and vulnerable to the high shear stress during nebulization. In addition, the degradation action due to the presence of nucleases in our body and the incapability of crossing the biological membrane limit the transport of RNAi therapeutics to the site of action [77, 78]. In order to obtain RNAi formulations with effective therapeutic and transfecting properties, options including lipid and polymer-based vectors are proposed [79, 80]. Cationic lipids are typical to form complexes with the negatively-charged RNAi via spontaneous electrostatic interaction, and therefore, have been incorporated into formulations for pulmonary RNAi delivery, such as DOTMA and DOTAP [77, 80]. Cationic polymers, such as polyethylenimine (PEI), polyamidoamine (PAMAM) dendrimers and chitosan, are also increasingly used for the delivery of RNAi therapeutics. These cationic polymers can form polyplexes spontaneously with the negatively-charged nucleic acids. Biodegradable and biocompatible polymers, such as PLGA, were also used to encapsulate RNAi drugs into nanoparticles, offering good protections to RNAi during pulmonary delivery and facilitating the cellular uptake [80]. Details on the delivery vectors for siRNA have been previously reviewed elsewhere [77, 81].
It is noteworthy that the airway inflammatory symptoms induced by bacterial infections usually need a relatively long time to treat, thus, a viable inhaled product of RNAi therapeutics should possess sufficient long-term storage stability for continuous usage [68]. Since powder formulations have been proved to be superior in many pharmaceutics, preparation of inhalable RNAi drug powders is also a hot research topic. Various particle engineering techniques (such as SD and spray-freeze drying (SFD)) have been utilized to prepare inhalable formulations of the encapsulated siRNA [82]. Among all, SD was commonly employed to prepare inhalable nanocomposite microparticles [83–85]. Jensen et al modified siRNA-loaded PLGA nanoparticles with a cationic lipid, dioleoyltrimethylammoniumpropane (DOTAP), and spray-dried into microparticles with mannitol [83]. Their results demonstrated that the powder production process had no negative impacts on the chemical stability and silencing capability of the siRNA nanoparticles. Bielski et al also confirmed the suitability of SD in engineering triphenylphosphonium (TPP) modified PAMAM dendrimers loaded with siRNA into inhalable microparticles with mannitol as the bulking agent. The formed microparticles could be formulated in pMDIs and DPIs with acceptable deep lung deposition (Fine Particle Fraction (FPF) of ca. 50% and 39%, respectively) [84]. The nano-in-micro approach was also used by Agnoletti et al to deliver siRNA-PAMAM dendrimer nanocomplexes prepared by a 3D-printed micromixer with different saccharides [85]. They found that the integrity and gene silencing efficiency of siRNAs were well preserved in the final powders, but the physical stability of powders depends on the excipients used, with trehalose and inulin provided the best preservation [85].
SFD is an emerging approach to produce inhalable porous particles. It was successfully used to incorporate siRNA-polyethyleneimine (PEI) nano-complex into porous mannitol particles [82]. The produced powder exhibited high aerosol performance and the intratracheally administered powder achieved specific and dose-dependent gene silencing activity in a mice model. Although the mechanisms of highly negatively-changed naked siRNA to cross the cellular membrane remained unclear, promising outcomes were noted with naked siRNA treatments in both animal models and human studies [77]. Lam and coworkers have employed both the SD and SFD techniques to produce inhalable powders of naked siRNA with mannitol as the major stabilizer and demonstrated reasonable aerosol performance [86–88]. The gene-silencing effect of the siRNA was also proved to be preserved in vitro [89].
Although positive results have been reported with the pulmonary delivery of RNAi therapeutics, the suitability of the above-mentioned formulation strategies to deliver RNAi drugs for respiratory antibacterial management is awaited to be confirmed in vitro and in vivo. It is also worth mentioning that the main target for RNAi molecules are immune cells/factors instead of the bacteria in most cases [9]. The presence of mucus layer on the lung surface, which is generally thicker under inflammation conditions, would present a major barrier for effective delivery of RNAi drugs to the underneath cells [90, 91]. Hence, formulating pulmonary RNAi therapeutics with good mucus penetration properties may prove to be beneficial. The natural polymer, chitosan, is recognized as a potential carrier for RNAi delivery to cross the mucus layer with its muco-permeable property [92], but strategies to improve the solubility and transfection efficiency for chitosan-based nanoparticles might be required. Alternatively, mucolytic agents to disintegrate the network of mucus or mucus inhibitors to inhibit mucus secretion could also be investigated when designing the formulations [77].
Inhaled Phages
Therapeutic Principle of Phages
Phages are promising antibacterial options in treating respiratory bacterial infections [46]. As the natural viral predators of bacteria, phages can employ the host machinery for their propagation. The lytic activity of phages has been widely-used in phage therapy for the treatment of bacterial infections during the 1920s and 1930s. Although the “antibiotic era” hindered the pursuit of commercial phages development for more than 60 years, phage therapy has now re-emerged as an alternative of antibiotics [93]. Phage therapy exhibits multiple advantages over antibiotics treatment in combating bacterial lung infections. For example, phages can selectively target pathogenic bacteria without harming the beneficial commensal flora in the lung, thus avoiding complications such as antibiotic-induced dysbiosis or secondary pulmonary infections. Phages can also rapidly adjust to fight new mutant strains with great safety profiles. Meanwhile, with the simple composition of DNA and protein, the toxicity of phages to the body is quite low (even though certain immunogenicity concerns reporting in some findings), unlike the toxic molecules from antibiotics degradation [94]. The promise of inhaled phage therapy in animal models and human studies have been reviewed elsewhere [46, 95].
Formulation Development for Phages
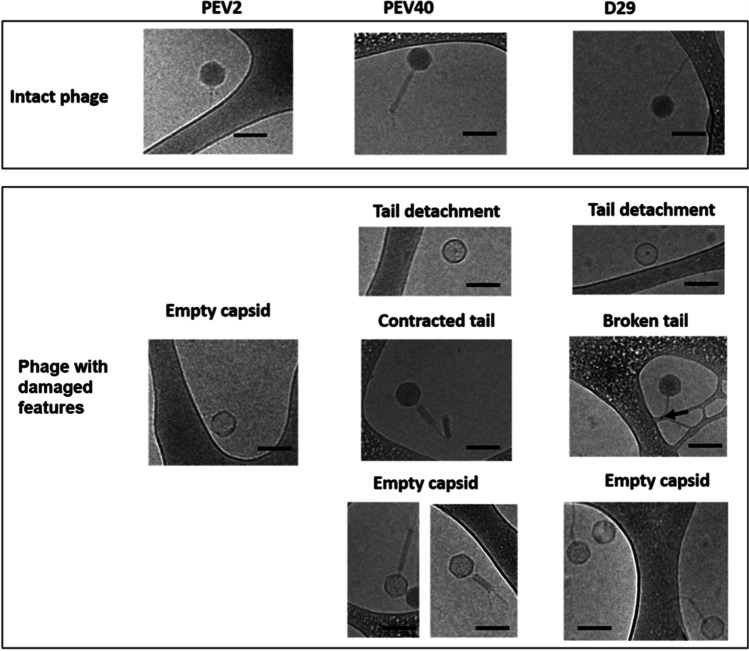
Pulmonary delivery of phage preparations has been carried out since 1960s, although these studies were restrictedly-performed in the clinic of only several countries [10]. Liquid formulations of phages are the most convenient dosage forms for either clinical or non-clinical phage therapy [46]. Liquid phage formulations are known to be easily prepared with acceptable stability [96], and can be nebulized with a large quantity and high efficiency. Phage D29 at a 6.9 ± 0.1 log10 PFU/mouse was delivered to mice via a vibrating mesh nebulizer prior to a challenge of Mtb infection and found to exhibit prophylactic effect by significantly decreasing bacterial burden in mouse lungs 24 h post-challenge [97]. The choice of a vibrating mesh nebulizer was based on the research group’s previous findings that it caused the minimum damage to D29 (< 0.5 log10 titer reduction) with reasonable delivered dose in a few minutes [98]. The effect of the jet nebulizer on the morphology changes of three tailed phages (PEV2, PEV40 and D29) was further investigated [99]. The structural damage of phages (as shown in Fig. 2) was found to be correlated with the loss of their infectivity. These findings suggested the importance of choosing a suitable device for nebulizing phage and provided guidance to the nebulizer choice for a recent clinical study on inhaled phage therapy [100]. Recently, a novel and portable hybrid surface and bulk acoustic wave platform was developed for the nebulization of phage K and a lytic enzyme (lysostaphin) to target S. aureus. The nebulizer was found to cause negligible damage to both the phage and lysostaphin, indicating the potential of the platform in multi-dose inhaled administration [101].
Fig. 2.
Structural damage to tailed phages caused by a jet-nebulizer. Reproduced with permission from [99], Elsevier, 2019.
The susceptibility of phages to environmental stresses (e.g., pH, temperature, ionic strength and humidity) is a concern for both the delivery and storage since varying degrees of susceptibility may have great impacts on their therapeutic effects [102]. The vulnerability of phage biologics can be ameliorated by encapsulating the therapeutic entities into nano-carriers [103]. Colloidal systems such as water-in-oil-in-water (W/O/W) emulsions were produced by integrating lipid nanodroplets with phage-encasing aqueous cores and were used in the treatment of P. aeruginosa-associated pulmonary infections by nebulization [104]. Bacteriophage D29 was encapsulated into 400-nm liposomes and the antibacterial action of free and liposomal phages against Mtb was assessed on the model of intracellular-infected RAW 264.7 macrophages. Data showed that the antibacterial effect of liposomal D29 phages was significantly higher than that of free phages and the penetration efficiency of liposomal D29 phages into infected macrophages was 6.7-fold enhanced compared with the free phages [105]. Despite the progress in the field of nanotechnology and nanoparticle systems, the application of inhaled phage-loaded nanoparticles is yet well-developed, which still requires further preclinical investigations to facilitate clinical translation.
Dry powder formulations for phage inhalation have attracted increasing interests, partly because of the advantages of enhanced stability and shelf-life of phages in dry states [106]. SD, freeze drying and SFD are the commonly-used technique to prepare phage-based dry powders. SFD has superiorities over conventional lyophilization or SD in terms of heat and mass transfer rates and control of the microstructure of the produced powders [107]. Regardless of the powder production technology, excipients play critical roles in stabilizing phages in the dry powder formulations upon production and storage. Amino acids, such as leucine and trileucine, are often used as dispersibility enhancers and moisture protectors, while sugars, including lactose, mannitol, trehalose and sucrose, are used as the stabilizers to preserve phages activities [106]. Table III summarizes the production methods and excipients used for phage powder production. The phages formulated in the carrier matrix were found to be biologically active without significant loss of viability and could achieve desirable aerosol performance. Since most sugar excipients, particularly trehalose and lactose, remained in the amorphous form to stabilize the incorporated phage, they are prone to recrystallization upon exposure to moisture in the environment. Handling and storing the phage powders at low humidity conditions are essential to ensure the phage stability within powders and their dispersibility [108]. Comparatively, the impacts of storage temperature on the phage powders seems to be less critical for phage powder. The excellent stability of phage powders at room temperature or by shipping delivery at 23 ± 7°C made cold-chain storage/infrastructure unnecessary.
Table III.
Examples of Processed Bacteriophage-Based Dry Powder Formulations
| Bacteriophages | Target bacteria | Production method | Excipients | Production loss | Storage loss | Ref. |
|---|---|---|---|---|---|---|
| KS4-M and FKZ | B. cepacian and P. aeruginosa | Lyophilization | Lactose/lactoferrin | 1–2 log10 | Stable and active after preservation at both 4 and 22°C for 3 months. | [109] |
| PEV2 | P. aeruginosa | SD | Trehalose, mannitol and leucine | ~0.75 log10 | Powder matrix with ≥40% trehalose showed good phage viability protection under 0 and 22% RH at 4 °C for 12 months |
[110] [111] [112] |
| SFD | ~2 log10 | |||||
| PEV1/PEV2/PEV20/PEV61 | SD | Lactose and leucine | <1 log10 | ~1.2 log10 titer reduction in formulations with <90% lactose; 1.7 log10 titer loss with <80% lactose | [113–117] | |
| 0.7 log10 after 12 months of storage at 25°C with <20% RH | [118] | |||||
| D29 | M. tuberculosis | Atmospheric SFD | Trehalose and mannitol | 0.6 log10 | – | [119] |
| CP30A | C. jejuni | SD | Trehalose and trileucine/pullulan | 1.0 ± 0.1 log10 | 0.6 ± 0.1 log10 at dry room temperature for 1 month | [120] |
| Trehalose and leucine | ~2 log10 | 0.1 ± 0.2 log10 by shipping at 23 ± 7°C | [121] | |||
| vB_AbaM-IME-AB406 | A. Baumannii | SD | Trehalose and leucine | < 0.5 log10 | < 1 log10 storage loss for formulation containing 40% trehalose 40% mannitol and 20% leucine | [108] |
Studies evaluating the in vivo behavior of pulmonary delivered phage powder formulations are rather limited. The antibacterial activity of phage PEV20 inhalable powders (spray dried with lactose and leucine) against P. aeruginosa was assessed in a mouse lung infection model. After 24 h of phage therapy, the bacterial load in the mice lungs was decreased by 5.3 log10 in the phage-treated group compared with the non-treated group, and additionally, phage titer in the lungs increased by 1 log10 after 24 h in the phage-treated group, demonstrating the efficacy of inhaled phage dry powder in treating lung infections [113]. Although this study showed the promising treatment efficacy of inhaled phage powders, further in vivo investigations, including pharmacokinetics, pharmacodynamics, and toxicity, are highly demanded for expediting the clinical translation.
As for the quality of the phage preparations, including both liquid and powder formulations, phage could maintain stable within 6-month of storage at room temperature or 4°C [96, 111, 115]. However, the level of bacterial endotoxin remained high even after purification, beyond the acceptable levels according to regulatory requirements for most preparations. The feasibility of wider application of phage therapy will be intricately linked to their manufacturability to yield highly purified and toxins-free products. Therefore, appropriate depyrogenation strategies are essential for phage preparations.
Future Perspectives and Conclusion
Respiratory bacterial infection evokes a big concern to public health around the world, causing huge burdens on economy and society. Due to the complexity of this disease, treatment options usually depend on the pathological features and concomitant complications. Efforts have been made to fight against this disorder, and the search for new/alternative antibacterial agents plays an important role. The emerging biological antibacterials discussed in the review, including antimicrobial peptides, RNAi therapeutics, and phages, have great promise to combat the MDR crisis. Great number of preclinical studies have also demonstrated their antibacterial and/or immunomodulatory potential in managing bacterial infections. To some extent, the inherent toxicity, immunogenicity, stability (in vivo and storage) and site-targeting issues associated with these biological antibacterials have limited their clinical translation [46, 77, 122]. Mitigating these problems with formulation approaches have been demonstrated to be feasible despite the limited-available work. More research to follow up on the promising findings will be essential for future clinical development of these inhaled biological antibacterials.
As most biologicals are prone for denaturation and degradation in the respiratory tracts, various nanocarrier systems, lipid-based and polymer-based, have been proposed and verified in improving the in vivo efficacy of reducing bacterial load in lungs for AMPs and phages or retaining the gene silencing efficiency for siRNA. As mentioned above, the nanocarriers could also facilitate the cellular uptake to reach the target action sites or the intracellular bacteria. However, most of the excipients used in the nano-formulations have not been approved for inhalation, thus the safety profiles of the nanocarriers would require detail investigation. This would be particularly important for patients with chronic conditions and required long-term antibacterial treatment, as accumulation of toxic or non/slowly-degradable carrier matrix is not desirable.
Free biological antibacterials have also been formulated into inhalable powders because of the superiority for drug handling and storage. Often, the biological and physical stability of powdered formulations are both dependent on the excipients used and the storage conditions. In most research, the choice of excipients is rather empirical. Further studies to investigate the stabilization mechanisms of the incorporated biologics in the powder form and its relationship with the excipients will be important for further development. In addition, most of the current researches focus on the formulation process in preparing inhalable powders, evaluation of the in vivo efficacy of the biologic powders are comparatively scarce, which will need further research effort.
The release profiles of encapsulated biologics from the formulations may deserve more attention and careful designs. It was reported that the most effective lipid nanoparticles could achieve only 1–4% of RNAi therapeutics release in the cytoplasm [123], limiting the transfection efficiency. Therefore, nanocarrier systems that are non-cytotoxic, degradable at reasonable rate and can offer good protection to the encapsulated biologics with controlled release profile are preferred. Since the reviewed biologics have specific target sites (cytoplasmic or specific organelles) within cells, designing delivery systems with active targeting capability can also be considered.
To commercially develop inhalable formulations of the emerging biological antibacterials, a reasonable aerosol performance is crucial, and the in vitro evaluation of aerodynamic behaviors for designed inhalable formulations is highly recommended. Factors, including the physical property of the aerosols and the anatomical/inhalation conditions of users, can impact the deposition efficiency of inhaled particles [45]. For patients, especially those with hyper-reactive airways or cough symptoms, inhalation parameters, such as inspiratory flow and breathing break at the end of inspiration, may show a big difference from the universally-defined standard [124]. A clinical study performed by Elkins et al reported that the mean inspiratory volume and peak inspiratory flow (PIF) of CF patients (aged ≥6 years old) across a high resistance RS01 DPI (with mean resistance of 0.036 kPa1/2/L min) were 1.83 ± 0.97 L and 75.5 ± 27.2 L/min, respective [125]. Tiddens et al also tested the inspiratory flow rates and volumes of CF patients with different disease degrees across four device resistances, providing a good reference for the development of inhalation preparations and devices for inhalation [126]. Therefore, adjusting the flow conditions for in vitro aerosol performance determination, such as regulating the simulated flowrates, to lean close to the actual pathological conditions of patients with respiratory tract diseases may be required. Further research to achieve better in vitro-in vivo correlation for the inhalable formulations of novel antibacterial biologics will prove to be valuable.
The nano-in-micro strategy has been generally considered as an effective approach to formulate nanodrugs into inhalable form of powders to be delivered with dry powder inhalers. This strategy is especially promising for pulmonary sustained drug delivery as drugs can be programmatically released along with the dissolution/erosion of carriers [127]. With the advantages of both micro- and nano-particles for inhalation, nano-in-micro particles could help with improving the therapeutic effect of drugs by promoting inhalation efficiency (micro) and targeting to site of action (nano) while reducing dose interval, increasing patients compliance to multicycle treatment [128]. In the development of this formulation form, there are, however, several obstacles to be overcome. One of the challenges is the establishment of drug release testing method for these particles because of their structural complexity. Microparticles must dissociate into primary nanoparticles prior to facilitate cell targeting, suggesting that the erosion rate of microparticle matrix should be accurately determined and controlled [129]. Drug release control of the nanoparticles should also be tested to achieve desirable therapeutic efficacy.
While some of the emerging biological antibacterials manage bacterial lung infections by acting with the host innate and adaptive immunity, others directly target the bacteria. Regardless of mode of actions, the biological nature of these antibacterials poise different levels of immunogenicity. Specifically, the positive charge of AMPs is a double-edged sword, which enables the lysis of bacterial membrane but also causes cytotoxicity and immunostimulation. For phage therapy, their immunogenicity could play a role in the antibacterial adjuvant but also stimulate the generation of anti-phage antibody, which resulted in ineffectiveness in multi-administration in clinical trials [130]. Therefore, a rational design of lung delivery system should take the pros and cons of immune responses into consideration. Effective modulation of the immune response arise from the drug administration is highly-desired for bacterial clearance in lungs, but only little is known. Further exploration in this aspect will be definitely needed. Meanwhile, given the successful example of mRNA-LNP vaccine during COVID-19 pandemic, developing RNAi-LNPs into inhalable vaccines for lung bacterial infections prevention, such as Mtb infections with high infectivity and mortality, seem to be promising and meaningful. To date, researches on inhaled mRNA vaccines for COVID-19 are booming [131, 132]. However, although present preclinical work on inhalable mRNA-LNP vaccine showed some advantage in stimulating antibody production, high mortality rate was observed, mainly due to massive inflammation in the lung brought by LNPs [133]. Modifications are needed for a further application. Overall, the development of inhaled therapy of biological antibacterials are still in the early stage. There are still many technical and regulatory challenges to be resolved on their way to clinical translation in mitigating bacterial lung infections, especially those caused by the MDR strains.
Acknowledgements
The work was supported by University Grants Committee Hong Kong (ref.24300619). The authors greatly appreciate the provision of graduate studentship to J Li.
Declarations
Conflict of Interest
The authors have declared no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1223–49. [DOI] [PMC free article] [PubMed]
- 2.Andrade F, Rafael D, Videira M, Ferreira D, Sosnik A, Sarmento B. Nanotechnology and pulmonary delivery to overcome resistance in infectious diseases. Adv Drug Deliv Rev. 2013;65(13–14):1816–1827. doi: 10.1016/j.addr.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Velkov T, Abdul Rahim N, Zhou Q, Chan H-K, Li J. Inhaled anti-infective chemotherapy for respiratory tract infections: successes, challenges and the road ahead. Adv Drug Deliv Rev. 2015;85:65–82. doi: 10.1016/j.addr.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Misra A, Hickey AJ, Rossi C, Borchard G, Terada H, Makino K, et al. Inhaled drug therapy for treatment of tuberculosis. Tuberculosis (Edinb). 2011;91(1):71–81. doi: 10.1016/j.tube.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Taccetti G, Francalanci M, Pizzamiglio G, Messore B, Carnovale V, Cimino G, et al. Cystic fibrosis: recent insights into inhaled antibiotic treatment and future perspectives. Antibiotics (Basel) 2021;10(3):338. doi: 10.3390/antibiotics10030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health O . 2020 antibacterial agents in clinical and preclinical development: an overview and analysis. Geneva: World Health Organization; 2021. [Google Scholar]
- 7.León-Buitimea A, Garza-Cárdenas CR, Garza-Cervantes JA, Lerma-Escalera JA, Morones-Ramírez JR. The demand for new antibiotics: antimicrobial peptides, nanoparticles, and combinatorial therapies as future strategies in antibacterial agent design. Front Microbiol. 2020;11:1669. doi: 10.3389/fmicb.2020.01669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zharkova MS, Orlov DS, Golubeva OY, Chakchir OB, Eliseev IE, Grinchuk TM, et al. Application of antimicrobial peptides of the innate immune system in combination with conventional antibiotics-a novel way to combat antibiotic resistance? Front Cell Infect Microbiol. 2019;9:128. doi: 10.3389/fcimb.2019.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Man DK, Chow MY, Casettari L, Gonzalez-Juarrero M, Lam JK. Potential and development of inhaled RNAi therapeutics for the treatment of pulmonary tuberculosis. Adv Drug Deliv Rev. 2016;102:21–32. doi: 10.1016/j.addr.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Düzgüneş N, Sessevmez M, Yildirim M. Bacteriophage therapy of bacterial infections: the rediscovered frontier. Pharmaceuticals (Basel) 2021;14(1):1–16. doi: 10.3390/ph14010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Z, Kłodzińska SN, Wan F, Nielsen HM. Nanoparticle-mediated pulmonary drug delivery: state of the art towards efficient treatment of recalcitrant respiratory tract bacterial infections. Drug Deliv Transl Res. 2021;11(4):1634–1654. doi: 10.1007/s13346-021-00954-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kradin RL, Digumarthy S. The pathology of pulmonary bacterial infection. Semin Diagn Pathol. 2017;34(6):498–509. doi: 10.1053/j.semdp.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1191–210. [DOI] [PMC free article] [PubMed]
- 14.Butler MS, Gigante V, Sati H, Paulin S, Al-Sulaiman L, Rex JH, et al. Analysis of the clinical pipeline of treatments for drug-resistant bacterial infections: despite progress, more action is needed. Antimicrob Agents Chemother. 2022;66(3):199–121. doi: 10.1128/aac.01991-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaufmann SHE, Dorhoi A, Hotchkiss RS, Bartenschlager R. Host-directed therapies for bacterial and viral infections. Nat Rev Drug Discov. 2018;17(1):35–56. doi: 10.1038/nrd.2017.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eldholm V, Balloux F. Antimicrobial resistance in mycobacterium tuberculosis: the odd one out. Trends Microbiol. 2016;24(8):637–648. doi: 10.1016/j.tim.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Bloemberg GV, Keller PM, Stucki D, Trauner A, Borrell S, Latshang T, et al. Acquired resistance to Bedaquiline and Delamanid in therapy for tuberculosis. N Engl J Med. 2015;373(20):1986–1988. doi: 10.1056/NEJMc1505196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds JH, McDonald G, Alton H, Gordon SB. Pneumonia in the immunocompetent patient. Br J Radiol. 2010;83(996):998–1009. doi: 10.1259/bjr/31200593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garg M, Prabhakar N, Gulati A, Agarwal R, Dhooria S. Spectrum of imaging findings in pulmonary infections. Part 1: bacterial and viral. Pol J Radiol. 2019;84:205–213. doi: 10.5114/pjr.2019.85812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillet Y, Issartel B, Vanhems P, Fournet JC, Lina G, Bes M, et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002;359(9308):753–759. doi: 10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- 21.Niederman MS, Mandell LA, Anzueto A, Bass JB, Broughton WA, Campbell GD, et al. Guidelines for the management of adults with community-acquired pneumonia. Am J Respir Crit Care Med. 2001;163(7):1730–1754. doi: 10.1164/ajrccm.163.7.at1010. [DOI] [PubMed] [Google Scholar]
- 22.Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56(6):588–599. doi: 10.1046/j.1365-2125.2003.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ladavière C, Gref R. Toward an optimized treatment of intracellular bacterial infections: input of nanoparticulate drug delivery systems. Nanomedicine (Lond) 2015;10(19):3033–3055. doi: 10.2217/nnm.15.128. [DOI] [PubMed] [Google Scholar]
- 24.D'Anna SE, Maniscalco M, Cappello F, Carone M, Motta A, Balbi B, et al. Bacterial and viral infections and related inflammatory responses in chronic obstructive pulmonary disease. Ann Med. 2021;53(1):135–150. doi: 10.1080/07853890.2020.1831050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sethi S. Infection as a comorbidity of COPD. Eur Respir J. 2010;35(6):1209–1215. doi: 10.1183/09031936.00081409. [DOI] [PubMed] [Google Scholar]
- 26.Sethi S, Murphy TF. Bacterial infection in chronic obstructive pulmonary disease in 2000: a state-of-the-art review. Clin Microbiol Rev. 2001;14(2):336–363. doi: 10.1128/CMR.14.2.336-363.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singanayagam A, Glanville N, Cuthbertson L, Bartlett NW, Finney LJ, Turek E, et al. Inhaled corticosteroid suppression of cathelicidin drives dysbiosis and bacterial infection in chronic obstructive pulmonary disease. Sci Transl Med. 2019;11(507):38–79. doi: 10.1126/scitranslmed.aav3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toews GB. Impact of bacterial infections on airway diseases. Eur Respir Rev. 2005;14(95):62–68. doi: 10.1183/09059180.05.00009504. [DOI] [Google Scholar]
- 29.Taylor SL, Leong LEX, Choo JM, Wesselingh S, Yang IA, Upham JW, et al. Inflammatory phenotypes in patients with severe asthma are associated with distinct airway microbiology. J Allergy Clin Immunol. 2018;141(1):94. doi: 10.1016/j.jaci.2017.03.044. [DOI] [PubMed] [Google Scholar]
- 30.Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17(5):704–715. doi: 10.1016/j.chom.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang YJ, Nariya S, Harris JM, Lynch SV, Choy DF, Arron JR, et al. The airway microbiome in patients with severe asthma: associations with disease features and severity. J Allergy Clin Immunol. 2015;136(4):874–884. doi: 10.1016/j.jaci.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin RJ, Kraft M, Chu HW, Berns EA, Cassell GH. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001;107(4):595–601. doi: 10.1067/mai.2001.113563. [DOI] [PubMed] [Google Scholar]
- 33.Chmiel JF, Aksamit TR, Chotirmall SH, Dasenbrook EC, Elborn JS, LiPuma JJ, et al. Antibiotic management of lung infections in cystic fibrosis. II. Nontuberculous mycobacteria, anaerobic bacteria, and fungi. Ann Am Thorac Soc. 2014;11(8):1298–1306. doi: 10.1513/AnnalsATS.201405-203AS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ciofu O, Tolker-Nielsen T, Jensen P, Wang H, Høiby N. Antimicrobial resistance, respiratory tract infections and role of biofilms in lung infections in cystic fibrosis patients. Adv Drug Deliv Rev. 2015;85:7–23. doi: 10.1016/j.addr.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 35.Carmody LA, Zhao J, Schloss PD, Petrosino JF, Murray S, Young VB, et al. Changes in cystic fibrosis airway microbiota at pulmonary exacerbation. Ann Am Thorac Soc. 2013;10(3):179–187. doi: 10.1513/AnnalsATS.201211-107OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klinger-Strobel M, Lautenschläger C, Fischer D, Mainz JG, Bruns T, Tuchscherr L, et al. Aspects of pulmonary drug delivery strategies for infections in cystic fibrosis--where do we stand? Expert Opin Drug Deliv. 2015;12(8):1351–1374. doi: 10.1517/17425247.2015.1007949. [DOI] [PubMed] [Google Scholar]
- 37.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 38.Mao Q, Jiang F, Yin R, Wang J, Xia W, Dong G, et al. Interplay between the lung microbiome and lung cancer. Cancer Lett. 2018;415:40–48. doi: 10.1016/j.canlet.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 39.Ganzel C, Silverman B, Chemtob D, Ben Shoham A, Wiener-Well Y. The risk of tuberculosis in cancer patients is greatest in lymphoma and myelodysplastic syndrome/myeloproliferative neoplasm: a large population-based cohort study. Leuk Lymphoma. 2019;60(3):720–725. doi: 10.1080/10428194.2018.1499904. [DOI] [PubMed] [Google Scholar]
- 40.Pilaniya V, Gera K, Kunal S, Shah A. Pulmonary tuberculosis masquerading as metastatic lung disease. Eur Respir Rev. 2016;25(139):97–98. doi: 10.1183/16000617.00002315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akinosoglou KS, Karkoulias K, Marangos M. Infectious complications in patients with lung cancer. Eur Rev Med Pharmacol Sci. 2013;17(1):8–18. [PubMed] [Google Scholar]
- 42.Halley A, Leonetti A, Gregori A, Tiseo M, Deng DM, Giovannetti E, et al. The role of the microbiome in cancer and therapy efficacy: focus on lung cancer. Anticancer Res. 2020;40(9):4807–4818. doi: 10.21873/anticanres.14484. [DOI] [PubMed] [Google Scholar]
- 43.Zhang M, Li M, Du L, Zeng J, Yao T, Jin Y. Paclitaxel-in-liposome-in-bacteria for inhalation treatment of primary lung cancer. Int J Pharm. 2020;578:119–127. doi: 10.1016/j.ijpharm.2020.119177. [DOI] [PubMed] [Google Scholar]
- 44.Munita JM, Arias CA. Mechanisms of antibiotic resistance. Microbiol Spectr. 2016;4(2):1–37. doi: 10.1128/microbiolspec.VMBF-0016-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Douafer H, Andrieu V, Brunel JM. Scope and limitations on aerosol drug delivery for the treatment of infectious respiratory diseases. J Control Release. 2020;325:276–292. doi: 10.1016/j.jconrel.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 46.Chang RYK, Wallin M, Lin Y, Leung SSY, Wang H, Morales S, et al. Phage therapy for respiratory infections. Adv Drug Deliv Rev. 2018;133:76–86. doi: 10.1016/j.addr.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mookherjee N, Anderson MA, Haagsman HP, Davidson DJ. Antimicrobial host defence peptides: functions and clinical potential. Nat Rev Drug Discov. 2020;19(5):311–332. doi: 10.1038/s41573-019-0058-8. [DOI] [PubMed] [Google Scholar]
- 48.Divyashree M, Mani MK, Reddy D, Kumavath R, Ghosh P, Azevedo V, et al. Clinical applications of antimicrobial peptides (AMPs): where do we stand now? Protein Pept Lett. 2020;27(2):120–134. doi: 10.2174/0929866526666190925152957. [DOI] [PubMed] [Google Scholar]
- 49.Li W, Separovic F, O'Brien-Simpson NM, Wade JD. Chemically modified and conjugated antimicrobial peptides against superbugs. Chem Soc Rev. 2021;50(8):4932–4973. doi: 10.1039/D0CS01026J. [DOI] [PubMed] [Google Scholar]
- 50.Fjell CD, Hiss JA, Hancock RE, Schneider G. Designing antimicrobial peptides: form follows function. Nat Rev Drug Discov. 2011;11(1):37–51. doi: 10.1038/nrd3591. [DOI] [PubMed] [Google Scholar]
- 51.Kumar P, Kizhakkedathu JN, Straus SK. Antimicrobial peptides: diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules. 2018;8(1):1–24. doi: 10.3390/biom8010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Graf M, Mardirossian M, Nguyen F, Seefeldt AC, Guichard G, Scocchi M, et al. Proline-rich antimicrobial peptides targeting protein synthesis. Nat Prod Rep. 2017;34(7):702–711. doi: 10.1039/C7NP00020K. [DOI] [PubMed] [Google Scholar]
- 53.Van Eijk M, van Dijk A, van der Ent CK, Arets HGM, Breukink E, van Os N, et al. PepBiotics, novel cathelicidin-inspired antimicrobials to fight pulmonary bacterial infections. Biochim Biophys Acta Gen Subj. 1865;2021(9):1–11. doi: 10.1016/j.bbagen.2021.129951. [DOI] [PubMed] [Google Scholar]
- 54.Hancock RE, Haney EF, Gill EE. The immunology of host defence peptides: beyond antimicrobial activity. Nat Rev Immunol. 2016;16(5):321–334. doi: 10.1038/nri.2016.29. [DOI] [PubMed] [Google Scholar]
- 55.Beaumont PE, McHugh B, Gwyer Findlay E, Mackellar A, Mackenzie KJ, Gallo RL, et al. Cathelicidin host defence peptide augments clearance of pulmonary Pseudomonas aeruginosa infection by its influence on neutrophil function in vivo. PLoS One. 2014;9(6):1–12. doi: 10.1371/journal.pone.0099029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tewary P, de la Rosa G, Sharma N, Rodriguez LG, Tarasov SG, Howard OM, et al. β-Defensin 2 and 3 promote the uptake of self or CpG DNA, enhance IFN-α production by human plasmacytoid dendritic cells, and promote inflammation. J Immunol. 2013;191(2):865–874. doi: 10.4049/jimmunol.1201648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Severino P, Ariga SK, Barbeiro HV, de Lima TM, de Paula SE, Barbeiro DF, et al. Cathelicidin-deficient mice exhibit increased survival and upregulation of key inflammatory response genes following cecal ligation and puncture. J Mol Med (Berl) 2017;95(9):995–1003. doi: 10.1007/s00109-017-1555-z. [DOI] [PubMed] [Google Scholar]
- 58.Coorens M, Schneider VAF, de Groot AM, van Dijk A, Meijerink M, Wells JM, et al. Cathelicidins inhibit Escherichia coli-induced TLR2 and TLR4 activation in a viability-dependent manner. J Immunol. 2017;199(4):1418–1428. doi: 10.4049/jimmunol.1602164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kahlenberg JM, Kaplan MJ. Little peptide, big effects: the role of LL-37 in inflammation and autoimmune disease. J Immunol (Baltimore, Md : 1950). 2013;191(10):4895–901. [DOI] [PMC free article] [PubMed]
- 60.Song X, Liu P, Liu X, Wang Y, Wei H, Zhang J, et al. Dealing with MDR bacteria and biofilm in the post-antibiotic era: application of antimicrobial peptides-based nano-formulation. Mater Sci Eng C Mater Biol Appl. 2021;128:112–318. doi: 10.1016/j.msec.2021.112318. [DOI] [PubMed] [Google Scholar]
- 61.Silva JP, Gonçalves C, Costa C, Sousa J, Silva-Gomes R, Castro AG, et al. Delivery of LLKKK18 loaded into self-assembling hyaluronic acid nanogel for tuberculosis treatment. J Control Release. 2016;235:112–124. doi: 10.1016/j.jconrel.2016.05.064. [DOI] [PubMed] [Google Scholar]
- 62.Falciani C, Zevolini F, Brunetti J, Riolo G, Gracia R, Marradi M, et al. Antimicrobial peptide-loaded nanoparticles as inhalation therapy for Pseudomonas aeruginosa infections. Int J Nanomedicine. 2020;15:1117–1128. doi: 10.2147/IJN.S218966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang L, Liu Y, Wang N, Wang H, Wang K, Luo XL, et al. Albumin-based LL37 peptide nanoparticles as a sustained release system against Pseudomonas aeruginosa lung infection. ACS Biomater Sci Eng. 2021;7(5):1817–1826. doi: 10.1021/acsbiomaterials.0c01084. [DOI] [PubMed] [Google Scholar]
- 64.Song J, Cortez-Jugo C, Shirbin SJ, Lin Z, Pan S, Qiao GG, et al. Immobilization and intracellular delivery of structurally nanoengineered antimicrobial peptide polymers using polyphenol-based capsules. Adv Funct Mater. 2022;32(6):1–12. doi: 10.1002/adfm.202107341. [DOI] [Google Scholar]
- 65.Water JJ, Smart S, Franzyk H, Foged C, Nielsen HM. Nanoparticle-mediated delivery of the antimicrobial peptide plectasin against Staphylococcus aureus in infected epithelial cells. Eur J Pharm Biopharm. 2015;92:65–73. doi: 10.1016/j.ejpb.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 66.Kwok PC, Grabarek A, Chow MY, Lan Y, Li JC, Casettari L, et al. Inhalable spray-dried formulation of D-LAK antimicrobial peptides targeting tuberculosis. Int J Pharm. 2015;491(1–2):367–374. doi: 10.1016/j.ijpharm.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 67.Amariei G, Kokol V, Vivod V, Boltes K, Letón P, Rosal R. Biocompatible antimicrobial electrospun nanofibers functionalized with ε-poly-l-lysine. Int J Pharm. 2018;553(1–2):141–148. doi: 10.1016/j.ijpharm.2018.10.037. [DOI] [PubMed] [Google Scholar]
- 68.Kandil R, Merkel OM. Pulmonary delivery of siRNA as a novel treatment for lung diseases. Ther Deliv. 2019;10(4):203–206. doi: 10.4155/tde-2019-0009. [DOI] [PubMed] [Google Scholar]
- 69.Dyawanapelly S, Ghodke SB, Vishwanathan R, Dandekar P, Jain R. RNA interference-based therapeutics: molecular platforms for infectious diseases. J Biomed Nanotechnol. 2014;10(9):1998–2037. doi: 10.1166/jbn.2014.1929. [DOI] [PubMed] [Google Scholar]
- 70.Lam JK, Chow MY, Zhang Y, Leung SW. siRNA versus miRNA as therapeutics for gene silencing. Mol Ther Nucleic Acids. 2015;4(9):1–20. doi: 10.1038/mtna.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou X, Li X, Wu M. miRNAs reshape immunity and inflammatory responses in bacterial infection. Signal Transduct Target Ther. 2018;3:1–13. doi: 10.1038/s41392-018-0006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou X, Li X, Ye Y, Zhao K, Zhuang Y, Li Y, et al. MicroRNA-302b augments host defense to bacteria by regulating inflammatory responses via feedback to TLR/IRAK4 circuits. Nat Commun. 2014;5:1–13. doi: 10.1038/ncomms4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vergadi E, Vaporidi K, Theodorakis EE, Doxaki C, Lagoudaki E, Ieronymaki E, et al. Akt2 deficiency protects from acute lung injury via alternative macrophage activation and miR-146a induction in mice. J Immunol. 2014;192(1):394–406. doi: 10.4049/jimmunol.1300959. [DOI] [PubMed] [Google Scholar]
- 74.Yanagihara K, Tashiro M, Fukuda Y, Ohno H, Higashiyama Y, Miyazaki Y, et al. Effects of short interfering RNA against methicillin-resistant Staphylococcus aureus coagulase in vitro and in vivo. J Antimicrob Chemother. 2006;57(1):122–126. doi: 10.1093/jac/dki416. [DOI] [PubMed] [Google Scholar]
- 75.Gong FY, Zhang DY, Zhang JG, Wang LL, Zhan WL, Qi JY, et al. siRNA-mediated gene silencing of MexB from the MexA-MexB-OprM efflux pump in Pseudomonas aeruginosa. BMB Rep. 2014;47(4):203–208. doi: 10.5483/BMBRep.2014.47.4.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rosas-Taraco AG, Higgins DM, Sánchez-Campillo J, Lee EJ, Orme IM, González-Juarrero M. Local pulmonary immunotherapy with siRNA targeting TGFβ1 enhances antimicrobial capacity in mycobacterium tuberculosis infected mice. Tuberculosis (Edinb) 2011;91(1):98–106. doi: 10.1016/j.tube.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lam JK, Liang W, Chan HK. Pulmonary delivery of therapeutic siRNA. Adv Drug Deliv Rev. 2012;64(1):1–15. doi: 10.1016/j.addr.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fujita Y, Takeshita F, Kuwano K, Ochiya T. RNAi therapeutic platforms for lung diseases. Pharmaceuticals (Basel) 2013;6(2):223–250. doi: 10.3390/ph6020223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mottais A, Le Gall T, Sibiril Y, Ravel J, Laurent V, d'Arbonneau F, et al. Enhancement of lung gene delivery after aerosol: a new strategy using non-viral complexes with antibacterial properties. Biosci Rep. 2017;37(6):1–17. doi: 10.1042/BSR20160618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chow MYT, Qiu Y, Lam JKW. Inhaled RNA therapy: from promise to reality. Trends Pharmacol Sci. 2020;41(10):715–729. doi: 10.1016/j.tips.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bardoliwala D, Patel V, Javia A, Ghosh S, Patel A, Misra A. Nanocarriers in effective pulmonary delivery of siRNA: current approaches and challenges. Ther Deliv. 2019;10(5):311–332. doi: 10.4155/tde-2019-0012. [DOI] [PubMed] [Google Scholar]
- 82.Okuda T, Morishita M, Mizutani K, Shibayama A, Okazaki M, Okamoto H. Development of spray-freeze-dried siRNA/PEI powder for inhalation with high aerosol performance and strong pulmonary gene silencing activity. J Control Release. 2018;279:99–113. doi: 10.1016/j.jconrel.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 83.Jensen DK, Jensen LB, Koocheki S, Bengtson L, Cun D, Nielsen HM, et al. Design of an inhalable dry powder formulation of DOTAP-modified PLGA nanoparticles loaded with siRNA. J Control Release. 2012;157(1):141–148. doi: 10.1016/j.jconrel.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 84.Bielski E, Zhong Q, Mirza H, Brown M, Molla A, Carvajal T, et al. TPP-dendrimer nanocarriers for siRNA delivery to the pulmonary epithelium and their dry powder and metered-dose inhaler formulations. Int J Pharm. 2017;527(1–2):171–183. doi: 10.1016/j.ijpharm.2017.05.046. [DOI] [PubMed] [Google Scholar]
- 85.Agnoletti M, Bohr A, Thanki K, Wan F, Zeng X, Boetker JP, et al. Inhalable siRNA-loaded nano-embedded microparticles engineered using microfluidics and spray drying. Eur J Pharm Biopharm. 2017;120:9–21. doi: 10.1016/j.ejpb.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 86.Chow MYT, Qiu Y, Lo FFK, Lin HHS, Chan HK, Kwok PCL, et al. Inhaled powder formulation of naked siRNA using spray drying technology with l-leucine as dispersion enhancer. Int J Pharm. 2017;530(1–2):40–52. doi: 10.1016/j.ijpharm.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 87.Liang W, Chan AYL, Chow MYT, Lo FFK, Qiu Y, Kwok PCL, et al. Spray freeze drying of small nucleic acids as inhaled powder for pulmonary delivery. Asian J Pharm Sci. 2018;13(2):163–172. doi: 10.1016/j.ajps.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liang W, Chow MYT, Lau PN, Zhou QT, Kwok PCL, Leung GPH, et al. Inhalable dry powder formulations of siRNA and pH-responsive peptides with antiviral activity against H1N1 influenza virus. Mol Pharm. 2015;12(3):910–921. doi: 10.1021/mp500745v. [DOI] [PubMed] [Google Scholar]
- 89.Liang W, Chow MYT, Chow SF, Chan HK, Kwok PCL, Lam JKW. Using two-fluid nozzle for spray freeze drying to produce porous powder formulation of naked siRNA for inhalation. Int J Pharm. 2018;552(1–2):67–75. doi: 10.1016/j.ijpharm.2018.09.045. [DOI] [PubMed] [Google Scholar]
- 90.Qiu Y, Lam JK, Leung SW, Liang W. Delivery of RNAi therapeutics to the airways-from bench to bedside. Molecules. 2016;21(9):1–32. doi: 10.3390/molecules21091249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kandil R, Merkel M. Therapeutic delivery of RNA effectors: diseases affecting the respiratory system. Pharmazie. 2016;71(1):21–26. [PubMed] [Google Scholar]
- 92.Li J, Cai C, Li J, Li J, Li J, Sun T, et al. Chitosan-based nanomaterials for drug delivery. Molecules. 2018;23(10):1–26. doi: 10.3390/molecules23102661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kannen V, Parry L, Martin FL. Phages enter the fight against colorectal cancer. Trends Cancer. 2019;5(10):577–579. doi: 10.1016/j.trecan.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 94.Manohar P, Loh B, Athira S, Nachimuthu R, Hua X, Welburn SC, et al. Secondary bacterial infections during pulmonary viral disease: phage therapeutics as alternatives to antibiotics? Front Microbiol. 2020;11:14–34. doi: 10.3389/fmicb.2020.01434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Abedon ST, Kuhl SJ, Blasdel BG, Kutter EM. Phage treatment of human infections. Bacteriophage. 2011;1(2):66–85. doi: 10.4161/bact.1.2.15845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cooper CJ, Denyer SP, Maillard JY. Stability and purity of a bacteriophage cocktail preparation for nebulizer delivery. Lett Appl Microbiol. 2014;58(2):118–122. doi: 10.1111/lam.12161. [DOI] [PubMed] [Google Scholar]
- 97.Carrigy NB, Larsen SE, Reese V, Pecor T, Harrison M, Kuehl PJ, et al. Prophylaxis of mycobacterium tuberculosis H37Rv infection in a preclinical mouse model via inhalation of nebulized bacteriophage D29. Antimicrob Agents Chemother. 2019;63(12):1–13. doi: 10.1128/AAC.00871-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Carrigy NB, Chang RY, Leung SSY, Harrison M, Petrova Z, Pope WH, et al. Anti-tuberculosis bacteriophage D29 delivery with a vibrating mesh nebulizer, jet nebulizer, and soft mist inhaler. Pharm Res. 2017;34(10):2084–2096. doi: 10.1007/s11095-017-2213-4. [DOI] [PubMed] [Google Scholar]
- 99.Leung SSY, Carrigy NB, Vehring R, Finlay WH, Morales S, Carter EA, et al. Jet nebulization of bacteriophages with different tail morphologies - structural effects. Int J Pharm. 2019;554:322–326. doi: 10.1016/j.ijpharm.2018.11.026. [DOI] [PubMed] [Google Scholar]
- 100.Lebeaux D, Merabishvili M, Caudron E, Lannoy D, Van Simaey L, Duyvejonck H, et al. A case of phage therapy against Pandrug-resistant Achromobacter xylosoxidans in a 12-year-old lung-transplanted cystic fibrosis patient. Viruses. 2021;13(1):1–10. doi: 10.3390/v13010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Marqus S, Lee L, Istivan T, Kyung Chang RY, Dekiwadia C, Chan HK, et al. High frequency acoustic nebulization for pulmonary delivery of antibiotic alternatives against Staphylococcus aureus. Eur J Pharm Biopharm. 2020;151:181–188. doi: 10.1016/j.ejpb.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 102.Jończyk E, Kłak M, Międzybrodzki R, Górski A. The influence of external factors on bacteriophages--review. Folia Microbiol (Praha) 2011;56(3):191–200. doi: 10.1007/s12223-011-0039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cooper CJ, Koonjan S, Nilsson AS. Enhancing whole phage therapy and their derived antimicrobial enzymes through complex formulation. Pharmaceuticals (Basel) 2018;11(2):1–25. doi: 10.3390/ph11020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rios AC, Vila MMDC, Lima R, Del Fiol FS, Tubino M, Teixeira JA, et al. Structural and functional stabilization of bacteriophage particles within the aqueous core of a W/O/W multiple emulsion: a potential biotherapeutic system for the inhalational treatment of bacterial pneumonia. Process Biochem. 2018;64:177–192. doi: 10.1016/j.procbio.2017.09.022. [DOI] [Google Scholar]
- 105.Lapenkova MB, Alyapkina YS, Vladimirsky MA. Bactericidal activity of liposomal form of lytic mycobacteriophage D29 in cell models of tuberculosis infection in vitro. Bull Exp Biol Med. 2020;169(3):361–364. doi: 10.1007/s10517-020-04887-6. [DOI] [PubMed] [Google Scholar]
- 106.Wang X, Xie Z, Zhao J, Zhu Z, Yang C, Liu Y. Prospects of inhaled phage therapy for combatting pulmonary infections. Front Cell Infect Microbiol. 2021;11:1–20. doi: 10.3389/fcimb.2021.758392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Malik DJ, Resch G. Editorial: manufacturing, formulation and delivery issues for phage therapy to become a reality. Front Microbiol. 2020;11:1–3. doi: 10.3389/fmicb.2020.584137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yan W, He R, Tang X, Tian B, Liu Y, Tong Y, et al. The influence of formulation components and environmental humidity on spray-dried phage powders for treatment of respiratory infections caused by Acinetobacter baumannii. Pharmaceutics. 2021;13(8):1–17. doi: 10.3390/pharmaceutics13081162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Golshahi L, Lynch KH, Dennis JJ, Finlay WH. In vitro lung delivery of bacteriophages KS4-M and ΦKZ using dry powder inhalers for treatment of Burkholderia cepacia complex and Pseudomonas aeruginosa infections in cystic fibrosis. J Appl Microbiol. 2011;110(1):106–117. doi: 10.1111/j.1365-2672.2010.04863.x. [DOI] [PubMed] [Google Scholar]
- 110.Leung SS, Parumasivam T, Gao FG, Carrigy NB, Vehring R, Finlay WH, et al. Production of inhalation phage powders using spray freeze drying and spray drying techniques for treatment of respiratory infections. Pharm Res. 2016;33(6):1486–1496. doi: 10.1007/s11095-016-1892-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Leung SSY, Parumasivam T, Gao FG, Carter EA, Carrigy NB, Vehring R, et al. Effects of storage conditions on the stability of spray dried, inhalable bacteriophage powders. Int J Pharm. 2017;521(1–2):141–149. doi: 10.1016/j.ijpharm.2017.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Leung SSY, Parumasivam T, Nguyen A, Gengenbach T, Carter EA, Carrigy NB, et al. Effect of storage temperature on the stability of spray dried bacteriophage powders. Eur J Pharm Biopharm. 2018;127:213–222. doi: 10.1016/j.ejpb.2018.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chang RYK, Chen K, Wang J, Wallin M, Britton W, Morales S, et al. Proof-of-principle study in a murine lung infection model of antipseudomonal activity of phage PEV20 in a dry-powder formulation. Antimicrob Agents Chemother. 2018;62(2):1714–1717. doi: 10.1128/AAC.01714-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chang RY, Wong J, Mathai A, Morales S, Kutter E, Britton W, et al. Production of highly stable spray dried phage formulations for treatment of Pseudomonas aeruginosa lung infection. Eur J Pharm Biopharm. 2017;121:1–13. doi: 10.1016/j.ejpb.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chang RYK, Wallin M, Kutter E, Morales S, Britton W, Li J, et al. Storage stability of inhalable phage powders containing lactose at ambient conditions. Int J Pharm. 2019;560:1–8. doi: 10.1016/j.ijpharm.2019.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li M, Chang RYK, Lin Y, Morales S, Kutter E, Chan HK. Phage cocktail powder for Pseudomonas aeruginosa respiratory infections. Int J Pharm. 2021;596:1–8. doi: 10.1016/j.ijpharm.2021.120200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lin Y, Chang RYK, Britton WJ, Morales S, Kutter E, Li J, et al. Inhalable combination powder formulations of phage and ciprofloxacin for P. aeruginosa respiratory infections. Eur J Pharm Biopharm. 2019;142:543–552. doi: 10.1016/j.ejpb.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lin Y, Yoon Kyung Chang R, Britton WJ, Morales S, Kutter E, Li J, et al. Storage stability of phage-ciprofloxacin combination powders against Pseudomonas aeruginosa respiratory infections. Int J Pharm. 2020;591:1–7. doi: 10.1016/j.ijpharm.2020.119952. [DOI] [PubMed] [Google Scholar]
- 119.Ly A, Carrigy NB, Wang H, Harrison M, Sauvageau D, Martin AR, et al. Atmospheric spray freeze drying of sugar solution with phage D29. Front Microbiol. 2019;10:1–11. doi: 10.3389/fmicb.2019.00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Carrigy NB, Liang L, Wang H, Kariuki S, Nagel TE, Connerton IF, et al. Trileucine and pullulan improve anti-Campylobacter bacteriophage stability in engineered spray-dried microparticles. Ann Biomed Eng. 2020;48(4):1169–1180. doi: 10.1007/s10439-019-02435-6. [DOI] [PubMed] [Google Scholar]
- 121.Carrigy NB, Liang L, Wang H, Kariuki S, Nagel TE, Connerton IF, et al. Spray-dried anti-Campylobacter bacteriophage CP30A powder suitable for global distribution without cold chain infrastructure. Int J Pharm. 2019;569:1–9. doi: 10.1016/j.ijpharm.2019.118601. [DOI] [PubMed] [Google Scholar]
- 122.Ersoy SC, Heithoff DM, Barnes L, Tripp GK, House JK, Marth JD, et al. Correcting a fundamental flaw in the paradigm for antimicrobial susceptibility testing. EBioMedicine. 2017;20:173–181. doi: 10.1016/j.ebiom.2017.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Meng N, Grimm D. Membrane-destabilizing ionizable phospholipids: novel components for organ-selective mRNA delivery and CRISPR-Cas gene editing. Signal Transduct Target Ther. 2021;6(1):1–3. doi: 10.1038/s41392-021-00642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Depreter F, Pilcer G, Amighi K. Inhaled proteins: challenges and perspectives. Int J Pharm. 2013;447(1–2):251–280. doi: 10.1016/j.ijpharm.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 125.Elkins MR, Robinson P, Anderson SD, Perry CP, Daviskas E, Charlton B. Inspiratory flows and volumes in subjects with cystic fibrosis using a new dry powder inhaler device. Open Respir Med J. 2014;8:1–7. doi: 10.2174/1874306401408010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tiddens HA, Geller DE, Challoner P, Speirs RJ, Kesser KC, Overbeek SE, et al. Effect of dry powder inhaler resistance on the inspiratory flow rates and volumes of cystic fibrosis patients of six years and older. J Aerosol Med. 2006;19(4):456–465. doi: 10.1089/jam.2006.19.456. [DOI] [PubMed] [Google Scholar]
- 127.Silva AS, Tavares MT, Aguiar-Ricardo A. Sustainable strategies for nano-in-micro particle engineering for pulmonary delivery. J Nanopart Res. 2014;16(11):1–17. doi: 10.1007/s11051-014-2602-0. [DOI] [Google Scholar]
- 128.Loira-Pastoriza C, Todoroff J, Vanbever R. Delivery strategies for sustained drug release in the lungs. Adv Drug Deliv Rev. 2014;75:81–91. doi: 10.1016/j.addr.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 129.Li YZ, Sun X, Gong T, Liu J, Zuo J, Zhang ZR. Inhalable microparticles as carriers for pulmonary delivery of thymopentin-loaded solid lipid nanoparticles. Pharm Res. 2010;27(9):1977–1986. doi: 10.1007/s11095-010-0201-z. [DOI] [PubMed] [Google Scholar]
- 130.Łusiak-Szelachowska M, Międzybrodzki R, Fortuna W, Borysowski J, Górski A. Anti-phage serum antibody responses and the outcome of phage therapy. Folia Microbiol (Praha). 2021;66(1):127–131. doi: 10.1007/s12223-020-00835-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Popowski KD, Moatti A, Scull G, Silkstone D, Lutz H, López de Juan Abad B, et al. Inhalable dry powder mRNA vaccines based on extracellular vesicles. Matter. 2022;5:1–15. doi: 10.1016/j.matt.2022.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Roh EH, Fromen CA, Sullivan MO. Inhalable mRNA vaccines for respiratory diseases: a roadmap. Curr Opin Biotechnol. 2022;74:104–109. doi: 10.1016/j.copbio.2021.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ndeupen S, Qin Z, Jacobsen S, Bouteau A, Estanbouli H, Igyártó BZ. The mRNA-LNP platform's lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience. 2021;24(12):1–15. doi: 10.1016/j.isci.2021.103479. [DOI] [PMC free article] [PubMed] [Google Scholar]