Abstract
Objective
To evaluate the dose-response relationship of 10, 20, and 40 mg ponesimod and long-term efficacy and safety of ponesimod 20 mg using an analysis of combined data from the phase 2 Core and Extension studies in patients with relapsing-remitting multiple sclerosis (RRMS).
Methods
In the Core study, 464 patients were randomized (1:1:1:1): placebo (n = 121), 10 mg (n = 108), 20 mg (n = 116), or 40 mg ponesimod (n = 119) once daily for 24 weeks. Patients who completed the Core study transitioned into the Extension study, which had treatment period 1 (TP1; up to 96 weeks) and TP2 and TP3 (up to 432 weeks). The 40 mg dose was discontinued due to low tolerability at the end of TP1, and the 10 mg dose was subsequently discontinued due to lower benefit-risk profile vs 20 mg at the end of TP2. All patients received 10 or 20 mg during TP2, followed by 20 mg in TP3. Annualized relapse rate (ARR), 6-month confirmed disability accumulation (CDA), time to first confirmed relapse, MRI outcomes, and safety were evaluated.
Results
A total of 435 patients received ≥1 dose of ponesimod (first randomized dose: 10 mg = 139, 20 mg = 145, and 40 mg = 151) at any time during the Core and/or the Extension study. As of March 31, 2019, 214 patients were still on ponesimod treatment. The median (range) of ponesimod exposure was 7.95 (0–9.36) years. Ponesimod 20 mg, from Core up to the end of TP3, was associated with sustained low clinical activity (ARR for confirmed relapses: 0.154; at week 432, Kaplan-Meier estimate for confirmed relapse was 43.9%, and 6-month CDA was 20.4%) and MRI disease activity, and over 64% of patients remained free of a confirmed relapse. Most common adverse events were nasopharyngitis (30%), headache (24%), and upper respiratory tract infection (21%).
Conclusion
The effects on multiple sclerosis disease control were maintained with ponesimod 20 mg for approximately 8 years with no new safety concerns identified.
Classification of Evidence
This study provides Class IV evidence that in individuals with RRMS, long-term treatment with ponesimod 20 mg was associated with a sustained low annualized confirmed relapse rate (0.154 at week 432), with 64% of patients remaining relapse-free.
Trial Registration Information
EudraCT Number 2008-006786-92 (Core study) and EudraCT Number 2009-011470-15 (Extension study).
Multiple sclerosis (MS) is a chronic, progressive, immune-mediated disease characterized by demyelination and recurrent inflammation in the CNS leading to substantial disability.1,2 Relapsing-remitting MS (RRMS) is the most common phenotype accounting for about 85% of all cases at the onset of disease.3,4 There are more than a dozen approved disease-modifying therapies with different mechanisms of actions, efficacy, safety, and tolerability profiles. In a chronic disease like MS, investigating the long-term outcomes is essential to inform treatment decisions.
Ponesimod is an orally active, selective S1P1 modulator that induces rapid, dose-dependent, and reversible reduction in the circulating lymphocyte count by preventing the lymphocyte egress from lymphoid organs.5 In a 24-week multicenter, double-blind, dose-finding, phase 2b Core study, ponesimod at 10, 20, and 40 mg once daily vs placebo showed a significant and dose-dependent reduction in inflammatory MRI activity and beneficial effect on clinical outcomes in patients with RRMS.6 A long-term ongoing Extension study is being conducted in patients who transitioned from the Core study. This report presents the results that led to the dose selection during the course of the study and long-term (approximately 8 years) interim efficacy and safety results of the continuous ponesimod 20 mg group based on the analysis of combined data from the Core and Extension studies in patients with RRMS. The data presented here cover the entire ponesimod treatment period of the Core and Extension studies, including a total of 435 patients who received at least 1 dose of ponesimod; the placebo period in the Core study was excluded.
Primary Research Question
To evaluate the dose-response relationship of 10, 20, and 40 mg ponesimod and long-term efficacy and safety of ponesimod 20 mg using an analysis of combined data from the phase 2 Core and Extension studies in patients with RRMS.
Methods
Patients
Inclusion and exclusion criteria for the Core study have been described previously.6 Patients who completed 24 weeks of treatment in the Core study were eligible to enter the prospective, multicenter, randomized, multiple-dose, parallel-group Extension study. Details of eligibility criteria are summarized in eAppendix 1, links.lww.com/WNL/C110.
Study Design
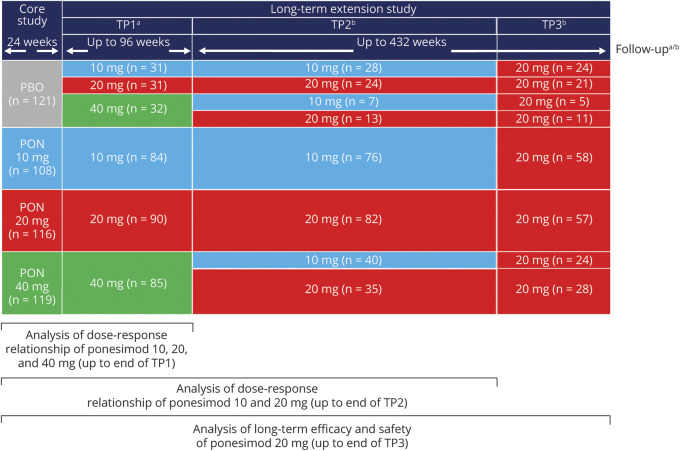
In the Core study, 464 patients were randomized (1:1:1:1), between October 2009 and November 2010, to 10, 20, 40 mg ponesimod, or placebo once daily for 24 weeks.6 Patients who completed the Core study transitioned into the Extension study between May 2010 and July 2011. The Extension study consisted of 3 treatment periods (TPs). During TP1, patients randomized to ponesimod during the Core study continued with the same dose, whereas patients who had received placebo during Core study were rerandomized (1:1:1) to receive 10, 20, or 40 mg ponesimod for up to 96 weeks. The 2-step uptitration scheme that was applied in the Core study was also followed during the initiation of treatment in the extension study. All patients were initiated on a 10 mg dose of ponesimod for the first 7 days of treatment. Patients in the 20 mg arm received the 20 mg dose from day 8 onward. Patients in the 40 mg arm received the 20 mg dose from days 8 to 14 and the 40 mg dose from day 15 onward. Mock uptitrations were performed to maintain the blind. Following a preliminary analysis of combined core and extension data in 2011, the sponsor decided to discontinue the ponesimod 40 mg dose due to poor tolerability at the end of TP1. At entry to TP2, patients who were on 40 mg during TP1 were rerandomized (1:1) to 10 or 20 mg, and patients who were on 10 or 20 mg continued with the same dose. Following a benefit-risk assessment of the 10 and 20 mg groups in 2016 and a corresponding recommendation from the Independent Data Monitoring Committee, it was decided to discontinue the 10 mg dose due to a lower benefit-risk profile vs 20 mg and switch all patients to 20 mg in TP3. The overall duration of TP2 and TP3 was up to 432 weeks. Following the final analysis of the Core study, the sponsor was unblinded to the Core treatment assignment and consequently TP1 assignment for patients who had received ponesimod in the Core study. Subsequently, the sponsor was unblinded to the TP1 assignment for the remaining ex-placebo patients and to the TP2 assignment for the ex–40 mg patients. The investigators and patients remained blinded to Core/TP1/TP2 treatment assignments. During TP3, blinding rules were not applicable as only the 20 mg dose was administered. Follow-up was performed at 7 and 30 days after the end of treatment (EOT) visit (for patients who discontinued during TP1 or who completed TP1 but chose not to enter TP2) or at 8, 30, and 90 days after the EOT visit (for patients who discontinued during TP2 or TP3 or who completed TP2 and chose not to enter TP3) (Figure 1). The study provides Class II evidence regarding the long-term efficacy, safety, and tolerability of ponesimod 10, 20, and 40 mg.
Figure 1. Study Design and Analysis Strategy.
aPatients who discontinued/completed during Core or TP1 had an EOT visit and follow-up visits at 7 and 30 days after the last study drug intake. bPatients who discontinued/completed during TP2 or TP3 had an EOT visit and follow-up visits at 8, 30, and 90 days after the last study drug intake. The placebo period was excluded from the analysis. Patients were grouped according to their first randomized ponesimod dose (10/20/40 mg). n is the number of patients randomized in each period. EOT = end of treatment; PBO = placebo; PON = ponesimod; TP = treatment period.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was conducted as per the International Conference on Harmonization, Guidelines for Good Clinical Practice, and the Declaration of Helsinki. The study protocol and all amendments were approved by the Independent Ethics Committee and/or Institutional Review Board at each participating study center. All patients provided written informed consent before entering the Core (EudraCT Number: 2008-006786-92) and Extension (EudraCT Number: 2009-011470-15) studies and at times of substantial protocol amendments.
Data Availability
The data sharing policy of the study sponsor, Janssen Pharmaceutical Companies of Johnson & Johnson, is available at janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA).
Study Outcomes
Efficacy
The primary efficacy exploratory end points were annualized (confirmed) relapse rate (ARR), time to first confirmed relapse, and time to 6-month confirmed disability accumulation (CDA). Relapse was defined as the occurrence of an acute episode of one or more new symptoms or a worsening of existing symptoms of MS, not associated with fever or infection, and lasting for at least 24 hours after a stable period of at least 30 days.
A confirmed relapse was a relapse accompanied by an increase from the previous clinically stable assessment (i.e., performed ≥30 days after the onset of any previous relapse) of ≥0.5 point in the Expanded Disability Status Scale (EDSS) score or 1 point in the score for ≥1 of the functional system (FS) scores (excluding the bowel and bladder and mental functional system). The confirmatory EDSS score assessment must have been performed ≤7 days of onset of a new symptom or worsening of an existing symptom of MS.
Time to 6-month CDA was based on a sustained increase from baseline in the EDSS scores over a continuous 6-month period. Disability accumulation was defined as an increase of ≥1 point in the EDSS score (if the baseline EDSS score was 1.0–5.0), an increase of ≥1.5 points (if the baseline EDSS score was 0), or an increase of ≥0.5 points (if the baseline EDSS score was ≥5.5). Other exploratory (MRI-related) end points included cumulative number of T1 gadolinium-enhancing (T1 Gd+) lesions, cumulative number of new/enlarging T2 lesions, and the cumulative number of combined unique active lesions (CUALs) and percentage change from baseline in brain volume over time. Clinical and MRI assessment schedules are described in eAppendix 1, links.lww.com/WNL/C110.
Safety
Safety outcomes included treatment-emergent adverse events (TEAEs), clinical laboratory tests (hematology, serum chemistry, urinalysis, and pregnancy tests), 12-lead ECGs, systolic and diastolic blood pressure (SBP and DBP), and pulmonary function tests (PFTs: forced expiratory volume in 1 second [FEV1], forced vital capacity [FVC], FEV1/FVC, and percent predicted FEV1 and FVC). Adverse events of special interest (AESI) included effects on heart rate and rhythm (including hypotension), hypertension, hepatobiliary disorders/liver enzyme abnormality, pulmonary function, macular edema, serious or severe infections, herpetic infections, skin malignancies, nonskin malignancies, and seizures. Safety assessment schedule is described in eAppendix 1, links.lww.com/WNL/C110.
Statistical Methods
Analyses were performed by the first randomized ponesimod dose in 3 periods (regardless of subsequent reassignment): (1) cumulative data from Core through Extension TP1 were analyzed for ponesimod 10, 20, and 40 mg; (2) cumulative data from Core through Extension TP2 were analyzed for the benefit-risk of ponesimod 10 and 20 mg; and (3) cumulative data up to the end of TP3 (entire ponesimod treatment period) were analyzed for the evaluation of long-term efficacy and safety of ponesimod 20 mg. The placebo period from the Core study was excluded from all analyses (Figure 1). The cutoff date for the analyses was March 31, 2019.
Baseline for the analysis was defined as the last assessment before the first dose of ponesimod. All data for all patients who received at least 1 dose of ponesimod at any time were included (Ponesimod Analysis Set [PAS]). Details of efficacy and safety analyses are described in eAppendix 1, links.lww.com/WNL/C110. An exploratory analysis was conducted to assess the ARR (confirmed relapse) in patients who discontinued prematurely or completed the treatment vs those with treatment ongoing in each respective analysis period. The study protocol and statistical analysis plan are available for further reference in eSAP 1 and eSAP 2.
Results
Patients
In total, 435 patients received ≥1 dose of ponesimod (first randomized dose: 10 mg, n = 139, 20 mg, n = 145, and 40 mg, n = 151) at any time during the Core and/or the Extension study and were included in the PAS.
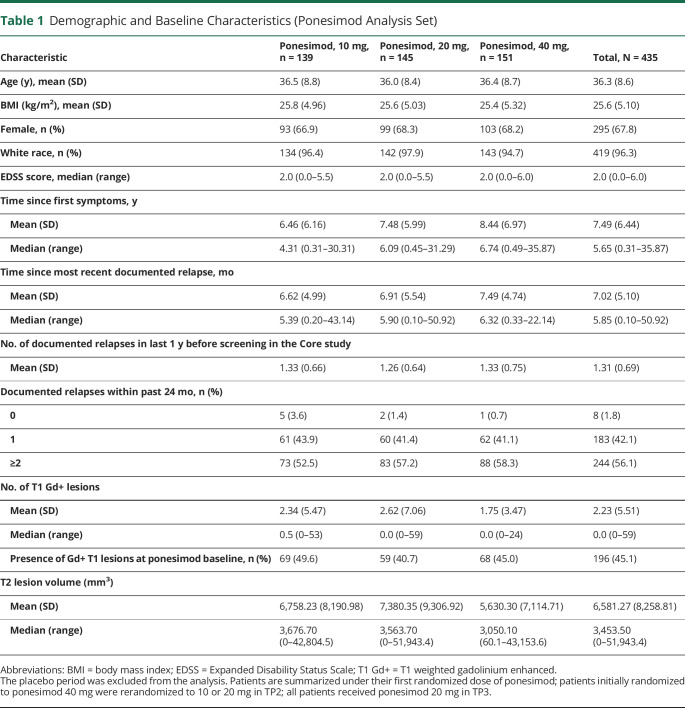
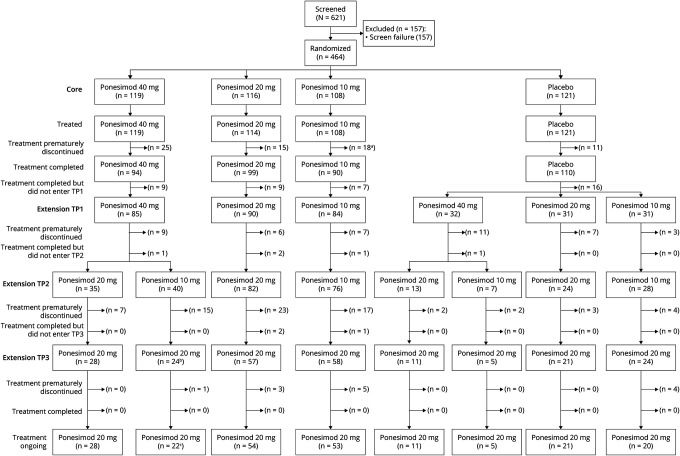
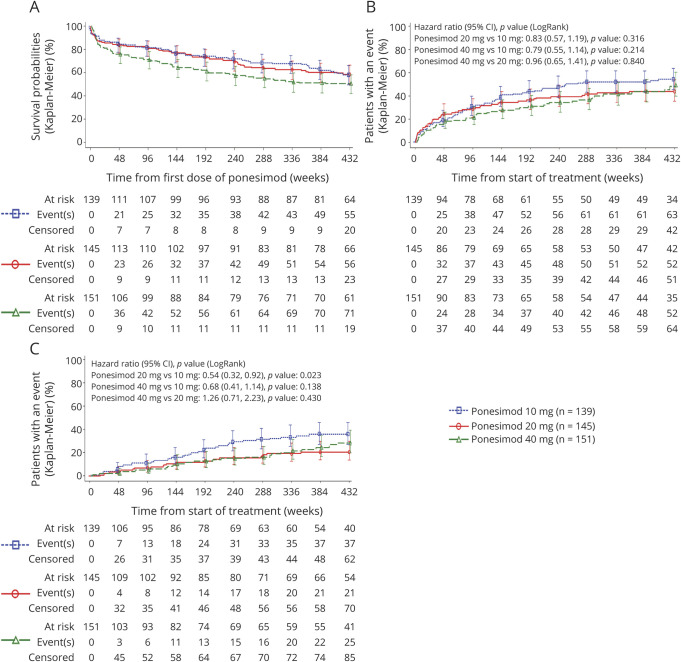
Demographics and baseline disease characteristics are shown in Table 1. Overall, baseline disease characteristics including both clinical and MRI findings were generally well balanced across treatment groups. As of March 31, 2019 (approximately 8 years of treatment), 214 patients in the PAS (49.2%) were still on ponesimod treatment and 186 (42.8%) had prematurely discontinued treatment, 2 (0.5%) had treatment interruption due to a planned pregnancy, and 33 (7.6%) had completed treatment as per protocol in one of the treatment periods of the Core or Extension studies (Figure 2). A higher rate of premature treatment discontinuation was observed in the 40 mg group (47.7%; n = 72) than in the 20 mg (39.3%; n = 57) and 10 mg (41.0%; n = 57) groups (Figure 3A). The most common reasons of premature treatment discontinuation were tolerability reasons/adverse events (AEs) (34.4%; 64/186) and patient decision (29.0%; 54/186).
Table 1.
Demographic and Baseline Characteristics (Ponesimod Analysis Set)
Figure 2. Patient Disposition.
aOne patient in the 10 mg dose group was recorded as discontinued from the Core study but continued to receive ponesimod 10 mg in the extension study. bOne patient did not transition to TP3 at the time of the cutoff for this interim analysis of March 31, 2019, due to interruption for a planned pregnancy between TP2 and TP3. cOne patient interrupted treatment for a planned pregnancy in TP3. TP = treatment period.
Figure 3. Time to Premature Treatment Discontinuation (A), Time to First Confirmed Relapse (B), and Time to First 6-Month Confirmed Disability Accumulation (C) Up to the End of TP3 (Ponesimod Analysis Set).
The placebo period was excluded from the analysis. Patients are summarized under their first randomized dose of ponesimod; patients initially randomized to ponesimod 40 mg were rerandomized to 10 or 20 mg in TP2; all patients received ponesimod 20 mg in TP3. TP = treatment period.
Analysis of Ponesimod 10, 20, and 40 mg: Combined Core/Extension TP1 Period
Efficacy
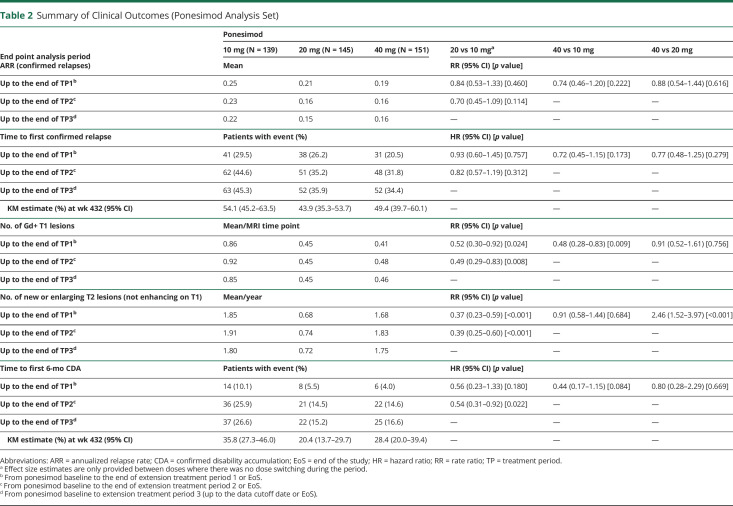
The median (range) exposure from Core to the end of Extension TP1 in 10, 20, and 40 mg was 2.0 (0–2.57), 2.1 (0–2.76), and 1.9 (0–2.59) years, respectively. A consistent dose-dependent reduction in disease activity was observed in the clinical parameters (ARR for confirmed relapse, time to first confirmed relapse, and 6-month CDA) with higher doses (40 and 20 mg ponesimod) than with the 10 mg dose (Table 2). Up to the end of TP1, confirmed relapses were reported in 29.5%, 26.2%, and 20.5% of patients in the 10, 20, and 40 mg groups, respectively.
Table 2.
Summary of Clinical Outcomes (Ponesimod Analysis Set)
A dose-dependent reduction in the mean number of T1 Gd+ lesions per patient per MRI time point was observed. As for new or enlarging T2 lesions and CUALs, an increased benefit with the 20 mg vs the 10 mg dose was observed, but no additional benefit was observed with 40 vs 20 mg. The mean number of new or enlarging T2 lesions per patient per year was 1.85, 0.68, and 1.68 in the 10, 20, and 40 mg dose groups, respectively (Table 2); the 40 mg group had an increase vs 20 mg due to extreme outliers.
Safety
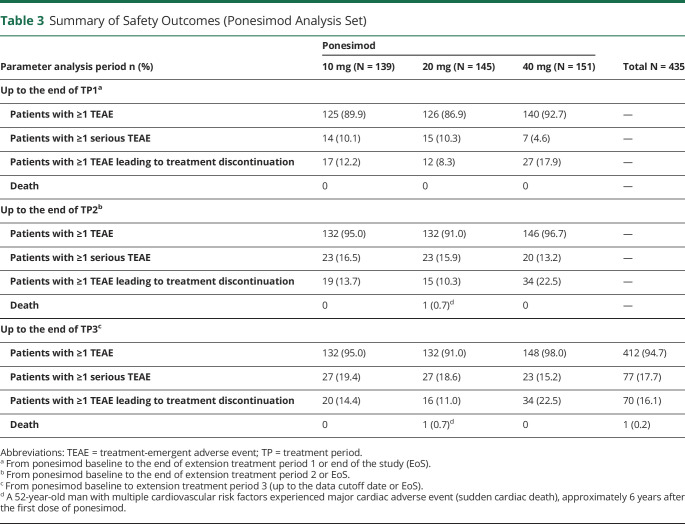
The most frequently reported TEAEs (≥10% of patients) in the 10, 20, or 40 mg dose groups were nasopharyngitis, headache, upper respiratory tract infection (URTI), alanine aminotransferase (ALT) increased, influenza, dyspnea, dizziness, cough, and peripheral edema (eTable 1, links.lww.com/WNL/C110). No deaths occurred up to TP1.
The proportion of patients with a TEAE leading to treatment discontinuation was higher in the 40 mg group (17.9%; n = 27) vs 10 mg and 20 mg groups (12.2%; n = 17 and 8.3%; n = 12, respectively), with the most frequent TEAEs (by system organ class [SOC]) in the 40 mg group being respiratory, thoracic, and mediastinal disorders (6.0%; n = 9) and investigations (5.3%; n = 8). Dose-related patterns were observed in transient liver effects and pulmonary effects. Reporting of AEs of infection was balanced across dose groups up to TP1 (59.7%; n = 83, 56.6%; n = 82, and 60.3%; n = 91 in the 10, 20, and 40 mg dose groups, respectively); however, a dose-related trend was observed in the reporting of serious and/or severe infections (AESI infection; 0%, 1.4%; n = 2, and 6.0%; n = 9, respectively). Ponesimod treatment was associated with transient first-dose effects on heart rate, conduction, and blood pressure following the first ponesimod dose (10 mg); however, the effects observed on repeated administration of ponesimod and following uptitration to 20 and 40 mg on days 8 and 15 were smaller than on day 1.
Benefit-Risk Analysis of Ponesimod 10 and 20 mg: Combined Core/Extension TP1/TP2 Period
Efficacy
The median (range) exposure from Core to the end of Extension TP2 in 10 and 20 mg was 6.9 (0–8.12) and 6.7 (0–8.07) years, respectively. Ponesimod 20 mg was associated with a consistent reduction of disease activity across all analyzed clinical and MRI end points vs 10 mg. The mean estimated number of T1 Gd+ lesions per patient per scan was reduced by 51% and that of new or enlarging T2 lesions per patient was reduced by 61% in the 20 mg group vs the 10 mg group. In addition, the ARR was reduced by 30%, and the risk of 6-month CDA was estimated to be 46% lower in the 20 mg vs the 10 mg group (Table 2).
Safety
No new safety signals emerged during the combined Core/Extension TP2 (Table 3). Most common TEAEs (≥10% of patients) in the 10 or 20 mg groups were nasopharyngitis, headache, URTI, urinary tract infection, fatigue, bronchitis, back pain, influenza, ALT increased, dizziness, cough, dyspnea, rhinitis, or peripheral edema (eTable 2, links.lww.com/WNL/C110). The most commonly reported serious TEAEs (by SOC and preferred term) in the 10 vs 20 mg groups were neoplasms (2.2% [n = 3; one each of basal cell carcinoma, breast cancer, and adenocarcinoma of the cervix] vs 2.8% [n = 4; one each of invasive ductal breast carcinoma, breast cancer, benign hydatidiform mole, and uterine leiomyoma]) and nervous system disorders (2.9% [n = 4; one each of seizure, epilepsy, somnolence, and tension headache] vs 1.4% [n = 2; one each of cervical radiculopathy and TIA]), respectively. One death in the 20 mg group was reported during the study; a 52-year-old man with multiple cardiovascular risk factors (hypertension, dyslipidemia, and smoking), history of peripheral vascular disease (axillary artery thrombosis), and vascular surgery died suddenly of unknown causes approximately 6 years after the first dose of ponesimod. The investigator considered the death to be unrelated to study treatment. The proportion of patients with a TEAE leading to treatment discontinuation was higher in the 10 mg (19 [13.7%]) vs the 20 mg group (15 [10.3%]); the most commonly reported (by SOC) were cardiac disorders (4.3% vs 2.1%), investigations (2.2% vs 2.8%), and nervous system disorders (3.6% vs 0.7%), respectively. A lower proportion of patients in the 10 mg vs 20 mg group had ALT elevations ≥3× ULN (6.5% [9/139] vs 9.1% [13/143]) and a >20% decrease in FEV1 (26.1% [36/138] vs 44.8% [64/143]), respectively.
Table 3.
Summary of Safety Outcomes (Ponesimod Analysis Set)
Long-term Efficacy and Safety of Ponesimod 20 mg: Combined Core/Extension TP1/TP2/TP3 Period
Efficacy
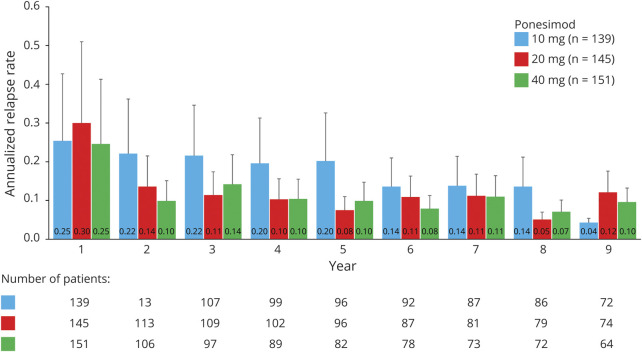
The cumulative exposure to ponesimod from Core up to the end of TP3 (cutoff: March 31, 2019) across all doses was 2,372.5 patient-years. In the 20 mg group, the cumulative exposure was 1,230.8 patient-years, and the median (range) exposure was 8.0 (0–9.4) years. Ponesimod 20 mg over long-term treatment of approximately 8 years showed sustained low levels of MS disease activity across all analyzed clinical (ARR, time to first confirmed relapse, and 6-month CDA) and MRI end points (Table 2 and Figure 3, B and C). The ARR for confirmed relapses showed a decrease between years 1 and 2 in the 20 mg group, at which point the ARR stabilized (Figure 4). The ARR for confirmed relapses up to the end of TP3 in the 20 mg group was 0.15, wherein the ARR for patients who discontinued prematurely or completed the treatment early was 0.28 and in patients with treatment ongoing was 0.10 (eTable 3, links.lww.com/WNL/C110). The percentage of patients in the 20 mg group who had experienced a 6-month CDA by the end of TP3 was 15.2%.
Figure 4. ARR by Year for Confirmed Relapses Up to the End of TP3 (Ponesimod Analysis Set).
The placebo period was excluded from the analysis. Patients are summarized under their first randomized dose of ponesimod; patients initially randomized to ponesimod 40 mg were rerandomized to 10 or 20 mg in TP2; all patients received ponesimod 20 mg in TP3. ARR = annualized relapse rate (confirmed relapses per year); TP = treatment period.
To the end of TP3, 30.0% of patients in the 20 mg group had no CUALs. A gradual mean decrease from the ponesimod baseline value in brain volume was observed in the 20 mg group up to the end of TP3. At week 408, the mean (SD) percent change from the ponesimod baseline value in brain volume was −2.4% (2.56) in the 20 mg dose group (eTable 4 and eFigure 1, links.lww.com/WNL/C110).
Safety
Overall, TEAEs were consistent with those observed from the Core study through TP1 and TP2 (Table 3). Most TEAEs were of mild or moderate severity. The most frequently reported TEAEs (≥10% of patients in the total ponesimod group) were nasopharyngitis, headache, URTI, bronchitis, back pain, urinary tract infection, ALT increased, fatigue, influenza, cough, dizziness, and dyspnea (eTable 5, links.lww.com/WNL/C110). Serious TEAEs were observed in 18.6% (n = 27) of patients in the 20 mg and in 17.7% (n = 77) of the total ponesimod group. No serious TEAE was reported at an incidence of >1% of the total ponesimod group. A total of 16 (11.0%) patients in the 20 mg and 70 (16.1%) in the total ponesimod groups experienced ≥1 TEAE that led to treatment discontinuation, with the most common being dyspnea (7 patients), followed by ALT increased (5 patients).
The most commonly reported AESI are summarized in eTable 6, links.lww.com/WNL/C110. The majority of pulmonary AEs were nonserious and resolved with or without discontinuation of treatment. Liver enzyme abnormalities included ALT ≥3× ULN (15/143 [10.5%] patients in the 20 mg and 45/432 [10.4%] in the total ponesimod groups). One patient in the 20 mg group had an ALT and aspartate transaminase ≥3× ULN and total bilirubin ≥2× ULN concomitantly with a serious TEAE of chronic hepatitis C infection (PCR confirmed), leading to treatment discontinuation. The overall exposure-adjusted incidence rates of infection AESI in the 20 mg and total ponesimod groups were 0.6 and 1.1 per 100 patient-years, respectively. Two patients (0.5%) in the total ponesimod group experienced an infection AESI leading to treatment discontinuation. One TEAE (genital herpes simplex, 0.7%) in the 20 mg group led to treatment discontinuation. No opportunistic infections were reported. The AESI category of macular edema was reported in 4 (2.8%) patients in the 20 mg and in 8 (1.8%) in the total ponesimod groups; of the 8 cases, only 2 were confirmed by the Ophthalmology Safety Board.
Nonskin malignancies were reported in 8 (1.8%) patients in the total ponesimod group: invasive ductal breast carcinoma (n = 3), breast cancer (n = 2), B-cell lymphoma, adenocarcinoma of the cervix (n = 1), and esophageal adenocarcinoma (n = 1; the patient had Barrett esophagus). Skin malignancies were reported in 6 (1.4%) patients in the total ponesimod group: basal cell carcinoma (n = 5), keratoacanthoma (n = 1), and neoplasm skin (n = 1). Hypertension AESI was observed in 15 (10.3%) patients in the 20 mg and 50 (11.5%) patients in the total ponesimod group (eTable 6, links.lww.com/WNL/C110). None of the hypertension AEs events were severe, serious, or led to discontinuation of study treatment.
After an initial dose-related decrease, the lymphocyte count remained stable over time, suggesting the absence of progressive decline in the lymphocyte count and late-onset lymphopenia. The lymphocyte count returned to near-baseline values at follow-up day 7 and day 30 (eFigure 2 and eTable 7, links.lww.com/WNL/C110). A lymphocyte count <0.2 × 109/L was reported in 13 (9.1%) patients in the 20 mg and 36 (8.3%) in the total ponesimod group. Following a small on-treatment absolute mean increases from baseline in SBP and DBP, mean values for all treatment groups mostly returned to near-baseline levels at follow-up visit day 30 (eFigure 3 and eTables 8 and 9, links.lww.com/WNL/C110). After a dose-dependent decrease in mean %predicted FEV1 and FVC during ponesimod treatment, a partial recovery was observed in all groups at follow-up day 7, and values remained relatively stable through the last follow-up visit (eFigure 4 and eTables 10 and 11, links.lww.com/WNL/C110).
Classification of Evidence
This study provides Class IV evidence that in individuals with RRMS, long-term treatment with ponesimod 20 mg was associated with a sustained low annualized confirmed relapse rate (0.154 at week 432), with 64% of patients remaining relapse-free.
Discussion
To support the study objectives and to support the decisions made during the study regarding the selection of the optimal ponesimod doses, analyses were performed in 3 different periods from the Core study through Extension TP1, TP2, and TP3, respectively. Based on the findings of the analysis up to TP1, a dose-response trend was observed for most of the efficacy end points, suggesting an increased benefit with the 20 and 40 mg doses vs the 10 mg dose. However, the ponesimod 40 mg dose was associated with reduced tolerability compared with the lower doses. The overall benefit/risk profile of the 20 mg dose was favorable compared with 40 mg, which supported the decision to stop the development of the 40 mg dose and rerandomize patients receiving the 40 mg dose to 10 mg or 20 mg at the entry of TP2.
The analysis up to the end of TP2 suggested that the ponesimod 20 mg group had lower disease activity compared with the 10 mg group, with clinically important and consistent reduction of disease activity across all relevant clinical and MRI parameters. Ponesimod 10 and 20 mg groups, however, continued to show a similar and favorable safety and tolerability profile. The benefit/risk analysis supported the decision to discontinue the 10 mg dose and switch all patients to the 20 mg dose.
The results observed during the entire ponesimod treatment period for the clinical and MRI parameters suggest that the effects on the MS disease control were maintained with ponesimod 20 mg over long-term treatment of approximately 8 years. The ARR and number of T1 Gd+ lesions, new or enlarging T2 lesions, and CUALs remained consistently low throughout the study.
The ARR in the ponesimod 20 mg group declined between years 1 and 2 and remained low in subsequent years. Patients treated over the long term in the ponesimod 20 mg group experienced a confirmed relapse, on an average, every 6.5 years. The treatment effects of ponesimod were generally comparable to those of other S1P receptor modulators such as fingolimod and siponimod.7,8
Considering the long-term duration of the study, the treatment effect in patients who discontinued or completed treatment vs patients with ongoing treatment was evaluated. The on-study disease activity among patients who discontinued the study tended to be higher than in those who did not discontinue. Based on a small number of confirmed relapses observed during the posttreatment follow-up period of up to 90 days in patients who stopped ponesimod treatment, there was no evidence of rebound disease activity. An analysis of posttreatment disease activity in patients who discontinued ponesimod will be the subject of a separate publication.
Evidence suggests that brain atrophy occurs in the early stages of MS and at a faster rate in patients with MS than in healthy participants. A decrease in brain volume of 0.5%–1.0% per year for patients with MS compared with 0.1%–0.3% per year in healthy individuals has been estimated, which seems to correlate with measures of disability.9-11 After approximately 8 years of treatment (i.e., at week 408), brain volume loss (BVL) was 2.4% in the ponesimod 20 mg group (i.e., an average of 0.3% per year), suggesting that with long-term treatment, the annual rate of BVL was in line with upper estimates in healthy individuals. Furthermore, the disability accumulation with ponesimod treatment was also low over time. Over a period of approximately 8 years of treatment (at week 432), only 20% of patients in the 20 mg group had experienced 6-month CDA.
Long-term treatment with ponesimod 20 mg showed an acceptable safety and tolerability profile with no new or unexpected safety concerns in patients with RRMS. The overall pattern of TEAEs, serious TEAEs, or AESI with long-term ponesimod treatment was comparable to that reported in the Core study.6 Treatment with ponesimod was associated with a low discontinuation rate due to tolerability reasons or AEs (15%), suggesting favorable long-term safety and tolerability. The discontinuation rate of ponesimod was comparable to that of fingolimod and siponimod in respective phase 2 extension studies.7,8 The most common TEAEs observed with ponesimod treatment were nasopharyngitis, headache, and URTI. One case of sudden cardiac death (assessed as unrelated to study treatment by the investigator) was reported approximately 6 years after the first dose of ponesimod. For any treatment that affects the immune system, long-term observation for serious infections and malignancies is necessary; the incidence of malignancies was comparable to that of the general population, and no opportunistic infection was observed with ponesimod treatment of approximately 8 years.
Consistent with the pharmacodynamic effects of ponesimod, the lymphocyte count was reduced early during treatment, and the effect was stable over time and rapidly reversible (within 7–30 days) following discontinuation of treatment, although the sample size was relatively small at follow-up. Similarly, increases in SBP and DBP observed during ponesimod treatment were reversible following treatment discontinuation. The PFT parameters, FEV1 and FVC, had declined after ponesimod treatment, and a partial recovery in PFT parameters was observed in all groups after discontinuation of treatment. In line with the findings of the Core study and with other S1P receptor modulators,7,8 treatment with ponesimod was associated with transient first-dose effects on heart rate, conduction, and BP following the first ponesimod dose (10 mg). These effects diminished on repeated administration and following uptitration to 20 and 40 mg on days 8 and 15, respectively, with no dose-related trends observed, showing desensitization. To further mitigate the first-dose effects, a gradual uptitration regimen, starting with ponesimod 2 mg, was implemented in phase 3 studies.
One of the potential limitations of the study was the necessary unblinding of the sponsor to treatment assignment in TP1 for patients receiving ponesimod in the Core study at the time of the Core study final analysis and subsequently to the TP1 assignment for the remaining ex-placebo patients and the TP2 assignment for the ex–40 mg patients. However, blinding of the patient and investigators to all treatment assignments was maintained. Also, as the analysis of the MRI scans performed by a blinded central reading facility showed results consistent with the clinical parameters (ARR and CDA), the risk of bias is considered minimal and without consequence for the validity and integrity of the trial results. The results of the long-term analyses may be influenced by selection bias, as patients who responded to and tolerated the treatment are more likely to remain in the study long term. Furthermore, the timing of EDSS score assessments was not consistent throughout the period and varied from 12 to 48 weeks. Lack of placebo or active control group must also be considered.
In conclusion, ponesimod 20 mg appeared to be the optimal dose based on the favorable benefit/risk maintained over long-term treatment. The results observed in clinical and MRI parameters suggest that the effects on MS disease control were maintained with ponesimod 20 mg over long-term treatment of approximately 8 years. No new safety concerns were identified with treatment with ponesimod 20 mg during the combined study periods. With sustained benefits on disease activity and favorable safety profile over a long-term period, ponesimod 20 mg was evaluated in patients with relapsing multiple sclerosis in phase 3 studies.12 Based on the positive long-term efficacy and safety and data from a phase 3 study, showing the superiority of ponesimod over teriflunomide 14 mg, ponesimod was approved by the US Food and Drug Administration for the treatment of adults with relapsing multiple sclerosis.
Acknowledgment
Ramji Narayanan (SIRO Clinpharm Pvt. Ltd., India) provided writing assistance, funded by Janssen Global Services, LLC, and Bradford Challis and Robert Achenbach (Janssen Global Services, LLC, NJ) provided additional editorial support for the development of this manuscript.
Glossary
- ALT
alanine aminotransferase
- ARR
annualized relapse rate
- CDA
confirmed disability accumulation
- CUALs
combined unique active lesions
- DBP
diastolic blood pressure
- EDSS
Expanded Disability Status Scale
- EOT
end of treatment
- FEV1
forced expiratory volume in 1 second
- FS
functional system
- FVC
forced vital capacity
- MS
multiple sclerosis
- PAS
Ponesimod Analysis Set
- RRMS
relapsing-remitting multiple sclerosis
- SBP
systolic blood pressure
- SOC
system organ class
- TEAE
treatment-emergent adverse event
- TP
treatment period
- URTI
upper respiratory tract infection
Appendix 1. Authors
Appendix 2. Coinvestigators

Footnotes
Class of Evidence NPub.org/coe
Study Funding
Funding was provided by Janssen Research & Development, LLC, and the study was supported by Actelion Pharmaceuticals, Part of the Janssen Pharmaceutical Companies of Johnson & Johnson, Allschwil, Switzerland.
Disclosure
M. Freedman has received honoraria or consulting fees from Actelion, Bayer HealthCare, Biogen, Chugai, EMD Canada, Genzyme, Hoffmann-La Roche, Merck Serono, Novartis, Sanofi-Aventis, and Teva Canada Innovation; has served on advisory boards for Actelion, Bayer HealthCare, Biogen, Hoffmann-La Roche, Merck Serono, Novartis, Opexa, and Sanofi-Aventis; and has participated in a speakers' bureau for Genzyme. C. Pozzilli has served on scientific advisory boards for Novartis, Merck, Biogen, Almirall, Hoffmann-La Roche, and Actelion and received funding for travel and speaker honoraria from Biogen, Sanofi Genzyme, Actelion, and Novartis and research support from Biogen, Merck, Novartis, and Hoffmann-La Roche. E.K. Havrdova has received honoraria or consulting fees from Actelion, Biogen, Celgene, Merck, Novartis, Sanofi Genzyme, and Teva and has been supported by the Czech Ministry of Education, research project PROGRES Q27/LF1. A. Lemle, M. Burcklen, A. Larbalestier, B. Hennessy, T. Sidorenko, and A. Vaclavkova are employees of Actelion, Part of the Janssen Pharmaceutical companies of Johnson & Johnson, and hold stock in Johnson & Johnson; T. Olsson has received advisory board and/or lecture honoraria and/or unrestricted MS research grants from Biogen, Novartis, Merck, Genzyme, Almirall, AstraZeneca, and Roche. Go to Neurology.org/N for full disclosures.
References
- 1.Dobson R, Giovannoni G. Multiple sclerosis–a review. Eur J Neurol. 2019;26(1):27-40. [DOI] [PubMed] [Google Scholar]
- 2.Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple sclerosis. Lancet. 2018;391:1622-1636. [DOI] [PubMed] [Google Scholar]
- 3.Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Degenhardt A, Ramagopalan SV, Scalfari A, Ebers GC. Clinical prognostic factors in multiple sclerosis: a natural history review. Nat Rev Neurol. 2009;5(12):672-682. [DOI] [PubMed] [Google Scholar]
- 5.D'Ambrosio D, Freedman MS, Prinz J. Ponesimod, a selective S1P1 receptor modulator: a potential treatment for multiple sclerosis and other immune-mediated diseases. Ther Adv Chronic Dis. 2016;7:18-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olsson T, Boster A, Fernández Ó, et al. Oral ponesimod in relapsing-remitting multiple sclerosis: a randomised phase II trial. J Neurol Neurosurg Psychiatry. 2014;85(11):1198-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montalban X, Comi G, Antel J, et al. Long-term results from a phase 2 extension study of fingolimod at high and approved dose in relapsing multiple sclerosis. J Neurol. 2015;262(12):2627-2634. [DOI] [PubMed] [Google Scholar]
- 8.Kappos L, Li DK, Stüve O, et al. Safety and efficacy of siponimod (BAF312) in patients with relapsing-remitting multiple sclerosis: dose-blinded, randomized extension of the phase 2 BOLD study. JAMA Neurol. 2016;73:1089-1098. [DOI] [PubMed] [Google Scholar]
- 9.Simon JH. Brain atrophy in multiple sclerosis: what we know and would like to know. Mult Scler. 2006;12:679-687. [DOI] [PubMed] [Google Scholar]
- 10.Zivadinov R, Sepcic J, Nasuelli D, et al. A longitudinal study of brain atrophy and cognitive disturbances in the early phase of relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry. 2001;70(6):773-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eshaghi A, Prados F, Brownlee WJ, et al. Deep gray matter volume loss drives disability worsening in multiple sclerosis. Ann Neurol. 2018;83(2):210-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kappos L, Fox RJ, Burcklen M, et al. Ponesimod compared with teriflunomide in patients with relapsing multiple sclerosis in the active-comparator phase 3 OPTIMUM study: a randomized clinical trial. JAMA Neurol. 2021;78(5):558-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sharing policy of the study sponsor, Janssen Pharmaceutical Companies of Johnson & Johnson, is available at janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA).