Abstract
Background and Objectives
The association between patterns of physical/mental activity and dementia and how it is affected by disease susceptibility remains unknown. We aimed to examine the association between patterns of physical and mental activity and dementia and whether it can be modified by disease susceptibility to dementia.
Methods
In a prospective cohort study based on UK Biobank, 501,376 dementia-free participants were recruited in 2006–2010 and followed from 1 year after the recruitment date until the end of 2019 for ascertainment of dementia. Data on physical (i.e., physical activity at leisure time, housework-related activity, and transportation) and mental (i.e., intelligence, social contact, and use of electronic device) activity were collected using questionnaires at recruitment. Cox models were used to estimate the associations of physical and mental activity-related items, as well as major activity patterns identified by principal component analysis, with the risk of dementia, adjusted for multiple confounders. The modification role of disease susceptibility on such associations was assessed through stratified analyses by the polygenic risk score (PRS) of dementia generated based on summary statistics of independent genome-wide association studies, by the APOE genotype, and by the self-reported family history of dementia.
Results
The mean age at recruitment was 56.53, and 45.60% of the participants were male. During a mean follow-up of 10.66 years, 5,185 dementia cases were identified. When analyzed separately, multiple studied items related to physical and mental activity showed significant associations with the risk of dementia. The pattern analyses revealed that a higher level of adherence to activity patterns related to frequent vigorous and other exercises (hazard ratio 0.65, 95% CI 0.59–0.71), housework-related activity (0.79, 0.72–0.85), and friend/family visit (0.85, 0.75–0.96) was associated with a lower risk of dementia. We obtained comparable results for vascular dementia and Alzheimer disease as well as in the stratified analyses by the PRS for dementia, APOE genotype, or family history of dementia.
Discussion
Activity patterns more adherent to frequent vigorous and other exercises, housework-related activity, and friend/family visit were associated with a reduced risk of multiple types of dementia. Such associations are independent of disease susceptibility, highlighting the potential of these physical and mental activity patterns, as effective interventions, in the primary prevention of dementia.
Dementia is one of the leading causes for dependence and disability among the elderly, affecting approximately 50 million people worldwide with nearly 10 million newly diagnosed cases per year.1,2 Previous studies have identified several potential risk factors, including educational level, smoking, obesity, alcohol drinking, hypertension, hearing impairment, depression, and diabetes for dementia.2 Significant gaps remain, however, regarding the knowledge about modifiable risk factors and their potential role in the prevention of dementia.
A growing body of evidence supports the role of physical activity in maintaining cognitive capacity and preventing dementia.3,4 Meta-analyses of observational studies consistently indicated an association between exercise and reduced risk of dementia,5 especially in the case of Alzheimer disease (AD).6 However, because of the heterogeneity of physical activity measurements and methodologies (including sample size) of previous studies, knowledge about effective physical activity mode and intensity, in terms of dementia prevention, remains limited. Another concern is that multiple modes of physical activity are correlated and might interact with each other (i.e., persons who are physically active tend to have high levels of many types of physical activities). It is therefore necessary to consider such correlations and study overall patterns of physical activity. Similar attempts are also needed to elucidate the role of mental activity, such as visiting friends and playing computer games, on the risk of dementia, for which suggestive evidence has been provided in a few previous studies on cognition and brain health.7-9
Disease susceptibility plays an important role in the development of dementia.10,11 For instance, the ε4 allele of the APOE gene has been identified as the most well-established genetic risk factor for late-onset AD.12 In addition, numerous genome-wide association studies (GWASs) have recently uncovered more genetic variants associated with the risk of dementia,13,14 with an estimated disease heritability ranging from 13% to 80%.15 Thus, explorations on potential modifiable risk factors for dementia need to take into consideration an individual's inherent genetic susceptibility, with the aim of achieving better precision prevention.
In the present study, based on enriched phenotype information from the community-based UK Biobank cohort, together with individual-level genotype data and complete follow-up data on medical diagnoses, we aimed to assess the associations between physical and mental activity and the subsequent risk of dementia. We further explored whether such associations would differ for individuals with different susceptibility to dementia.
Methods
Data Source and Study Design
UK Biobank is an ongoing prospective cohort study that recruited over 500,000 participants aged 40–69 years during 2006–2010 from England, Scotland, and Wales.16 Information on sociodemographic characteristics, lifestyle factors (e.g., physical and mental activity), and medical and health conditions, as well as physical measures (e.g., anthropometry), was collected from all participants at recruitment. Data on medical diagnoses and survival status were derived by cross-linkage with multiple national health registers, including primary care, hospital inpatient, cancer, and death registers.17
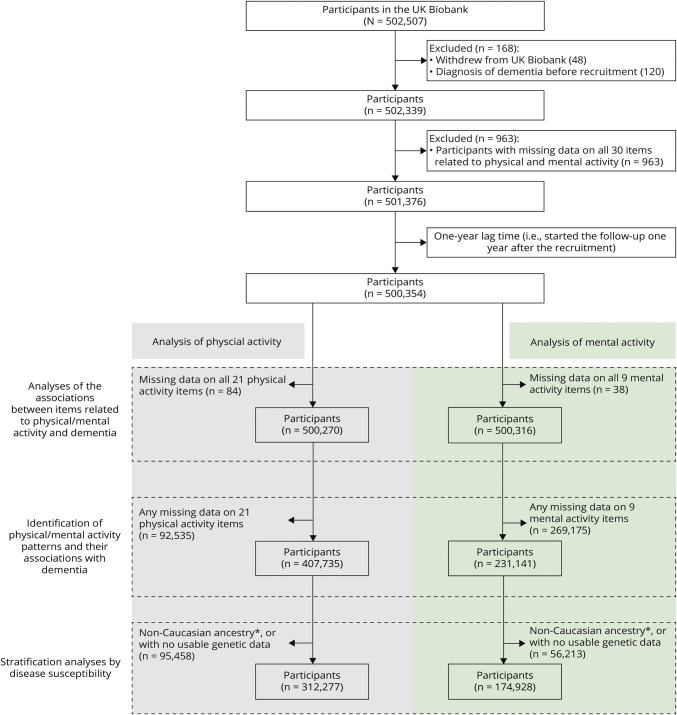
In the present study, among all 502,507 individuals in the UK Biobank, we excluded participants who withdrew their data (n = 48), were diagnosed with dementia before recruitment (n = 120), or had no available information on physical and mental activity (n = 963), leaving 501,376 participants for further analyses. (Figure 1). Separate cohorts were constructed to analyze 30 different items of physical and mental activity measured at baseline, where participants with a missing value for a specific item were removed from the analysis of that item. The remaining participants were then assigned to different groups according to their corresponding exposure levels (i.e., reference and exposed groups). Taking into consideration the potential diagnostic delay of dementia and thereby the risk of reverse causality (i.e., the self-reported physical and mental activities were influenced by preclinical symptoms of dementia),18 we started the follow-up for each participant from 1 year after the recruitment date (i.e., 1-year lag time) until a diagnosis of dementia, death, or the end of study (i.e., December 31, 2019), whichever occurred first.
Figure 1. Flowchart of the Study.
*Caucasian ancestry refers to a genetic ethnic group, involving individuals who self-identified as White British and have very similar genetic ancestry based on a principal components analysis of the genotypes.
Standard Protocol Approvals, Registrations, and Patients Consents
All UK Biobank participants signed informed consent before information collection and the study has full ethical approval from the NHS National Research Ethics Service (16/NW/0274). The present study (study protocol can be found in the supplement, links.lww.com/WNL/C140) was also approved by the biomedical research ethics committee of West China Hospital (reference number: 2019-1171).
Measurements of Physical and Mental Activity
Physical activity was assessed by 21 items from the well-validated short self-reported International Physical Activity Questionnaire19 at recruitment. There were 5 major categories, including physical activity at leisure time (i.e., strenuous sports, other exercise, walking for pleasure, and climbing a flight of stairs), housework-related activity (e.g., heavy DIY and light DIY), job-related activity (i.e., job involved standing or walking and job involved heavy manual or physical activity), transportation for work (i.e., by walk, cycle, car, and public transport), and transportation for others (i.e., by walk, cycle, car, and public transport). For specific physical activity within the first 2 categories, participants reported their attendance (yes or no), frequency (days per week), and duration (minutes per day). Additional information is available in eTable 1 (links.lww.com/WNL/C140).
We considered mental activity as conditions related to intelligence, social contact, and electronic device use, adherent to definitions used in previous studies.8,20 Specifically, in this study, items related to intelligence (i.e., educational level and attendance of adult education classes), social contact (i.e., attendance and frequency of friend/family visit, pub or social club visit, and religious and other group activity), and use of electronic device (i.e., frequency of playing computer games, watching television, and making or receiving calls on a mobile phone) were extracted from touchscreen questionnaires collected at baseline (eTable 1, links.lww.com/WNL/C140).
Ascertainment of Dementia
We identified cases of dementia from the UK Biobank inpatient data, according to corresponding codes of the International Classification of Diseases coding system (eTable 2, links.lww.com/WNL/C140). We also analyzed subtypes of dementia, including vascular dementia, AD, and other dementia (eTable 2). Diagnosis of dementia from UK Biobank inpatient data has previously been validated, showing a positive predictive value of 87.3% and 68.2% for any dementia and AD, respectively, compared with clinical expert adjudication of full-text medical records.21
Disease Susceptibility to Dementia
We assessed the disease susceptibility to dementia by the polygenic risk score (PRS) for dementia, APOE genotype, and family history of dementia. The PRS, representing an individual's load of common genetic variants to a specific trait, was estimated based on GWAS summary statistics of AD from independent populations.22 Specifically, based on the UK Biobank imputed genotype data, our analysis was restricted to the autosomal biallelic single nucleotide variations (SNVs [formerly SNPs]). Variants with a call rate <98%, a minor allele frequency <0.01, or deviation from Hardy-Weinberg equilibrium (p < 10−6) were removed from the analysis. We further excluded individuals with a genotyping rate <98%, outlier samples based on abnormal heterozygosity level, and up to the second related individuals (kinship coefficient ≥0.0884). The final analysis included 6,099,107 SNVs for 376,869 participants. We computed PRSs under 10 p value thresholds (i.e., 5 × 10−8, 1 × 10−6, 1 × 10−4, 1 × 10−3, 0.05, 0.1, 0.2, 0.3, 0.4, and 0.5), using the weighted method described previously.22 In a validated step, the PRS with the highest Nagelkerke squared (corresponding to a p threshold of 5 × 10−8 with 13 included SNVs) was selected for further analysis, which showed an association with risk of dementia in our data set (odds ratio 1.21, 95% CI 1.17–1.25 per 1 unit increase in the PRS).
Furthermore, because the ε4 allele of the APOE gene has been established as a genetic risk factor for late-onset AD, we determined the APOE genotype by APOE SNVs rs429358 and rs7412, where participants with 1 or 2 ε4 alleles were considered to be APOE ε4 carriers and the others as APOE ε4 noncarriers.23 In addition, family history of dementia was defined as dementia among any first-degree relative (father, mother, and siblings) according to self-report information at baseline. It was regarded as an index of a joint effect of genetic background and early upbringing environmental and lifestyle factors.
Covariates
Data on demographic factors (sex, age, and race and ethnicity), socioeconomic factors (income and education), and lifestyle (alcohol drinking and smoking status) were collected at baseline through questionnaires. The body mass index (BMI) was constructed from height and weight measured at the initial assessment center visit. The Townsend deprivation index (TDI) was calculated based on the postcode of family address, representing levels of area-based deprivation.24 The Charlson comorbidity index (CCI) was calculated, as a proxy of baseline somatic fitness, by summarizing the presence of 18 medical conditions (see details in the eTable 2, links.lww.com/WNL/C140)25 based on UK Biobank inpatient data. Because individuals with prevalent dementia were excluded from the analysis, the CCI calculation did not include dementia. In addition, we obtained information about the history of hypertension and hyperlipidemia based on medical diagnoses from primary care and inpatient hospital data (eTable 2). Cognitive function was evaluated by validated fully automated UK Biobank cognitive tests26 at recruitment. We used reaction time as an indicator of baseline cognitive function, with a higher score associated with poorer overall performance.
Statistical Analysis
Identification of Patterns of Physical and Mental Activity
We identified patterns of physical and mental activity, using principal component analysis (PCA). Briefly, PCA provides low-dimensional information extracted from numbers of variables with high correlations, which thereby enables the identification of distinct behavior patterns of major relevance, presented as significant principal components (PCs).27 The analysis was performed among 407,735 participants for physical activity, and 231,141 participants for mental activity, as individuals with any missing values were removed (Figure 1). We retained PCs with the highest eigenvalues and cumulatively explained variance over 50%. The PC score was calculated by integrating all included variables weighted by their factor loadings in this PC, with higher PC scores indicating greater adherence to the specific pattern. In the pattern analyses for each PC, the PC scores of all participants were ordered from the lowest to the highest and categorized into low, moderate, and high groups according to tertile distribution (low: <first tertile, moderate: first–second tertile, and high: >second tertile).
Phenotypic Associations of Physical and Mental Activity With Subsequent Dementia
We assessed the associations between individual items of physical and mental activity, as well as patterns of physical and mental activity, and dementia using Cox regression models, represented as hazard ratios (HRs) and their 95% CIs. We first performed analysis for any dementia and then for vascular dementia, AD, and other dementia. In all models, we adjusted for age (as a continuous variable), sex (male or female), race and ethnicity (White, Asian, Black, mixed, or unknown), TDI (as a continuous variable), education (college/university degree: yes, no, or unknown), income (<£18,000, £18,000–£30,999, £31,000–£51,999, £52,000–£100,000, >£100,000, or unknown), smoking and alcohol status (never, previous, current, or unknown), BMI (<18.5, 18.5–24.9, 25.0–29.9, ≥30.0 kg/m2, or unknown), CCI (0 or ≥1), history of hypertension (yes or no), history of hyperlipidemia (yes or no), and family history of dementia (yes, no, or unknown). We also performed subgroup analysis by age group (≤65 years or >65 years), sex (male or female), and income level (<£18,000, £18,000-£51,999, or ≥£52,000).
The Modification Role of Disease Susceptibility to Dementia on the Phenotypic Associations
To explore whether the associations between patterns of physical and mental activity and dementia might be modified by disease susceptibility, we performed stratified analyses by the PRS for dementia (high: >second tertile or low: <first tertile), APOE genotype (APOE ε4 carrier or noncarrier), and family history of dementia (with or without).
Given that dementia patients who received other dementia as the initial clinical diagnosis were likely to be reclassified as other subtypes of dementia later, we reassessed the HRs for subtypes of dementia by allowing such a change in diagnosis during follow-up. Furthermore, using a sample of 496,667 participants with available results of reaction time from cognitive tests, we conducted sensitivity analyses by either additionally adjusting for or stratifying the analysis by different cognitive performance levels at baseline (i.e., using tertiles of reaction time: high <first tertile, moderate first–second tertile, or low >second tertile), considering that cognitive decline may be a prodromal phase of dementia.28 To deal with the possible correlation between patterns of physical and mental activity, we repeated the pattern analyses of physical activity by additionally adjusting for all meaningful PCs of mental activity, and vice versa, among participants with data on all physical and mental activity-related items at baseline (n = 199,045). In addition, to further test the robustness of our results to the concern of reverse causality,18 we applied 5-year and 10-year lag time (i.e., started the follow-up 5 or 10 years after the recruitment date), instead of 1-year lag time as used in the main analysis.
All data analyses were completed using R-4.0 software and Plink-1.9. A 2-tailed test with p < 0.05 was considered statistically significant.
Data Availability
Data from the UK Biobank (ukbiobank.ac.uk/) are available to all researchers on making an application.
Results
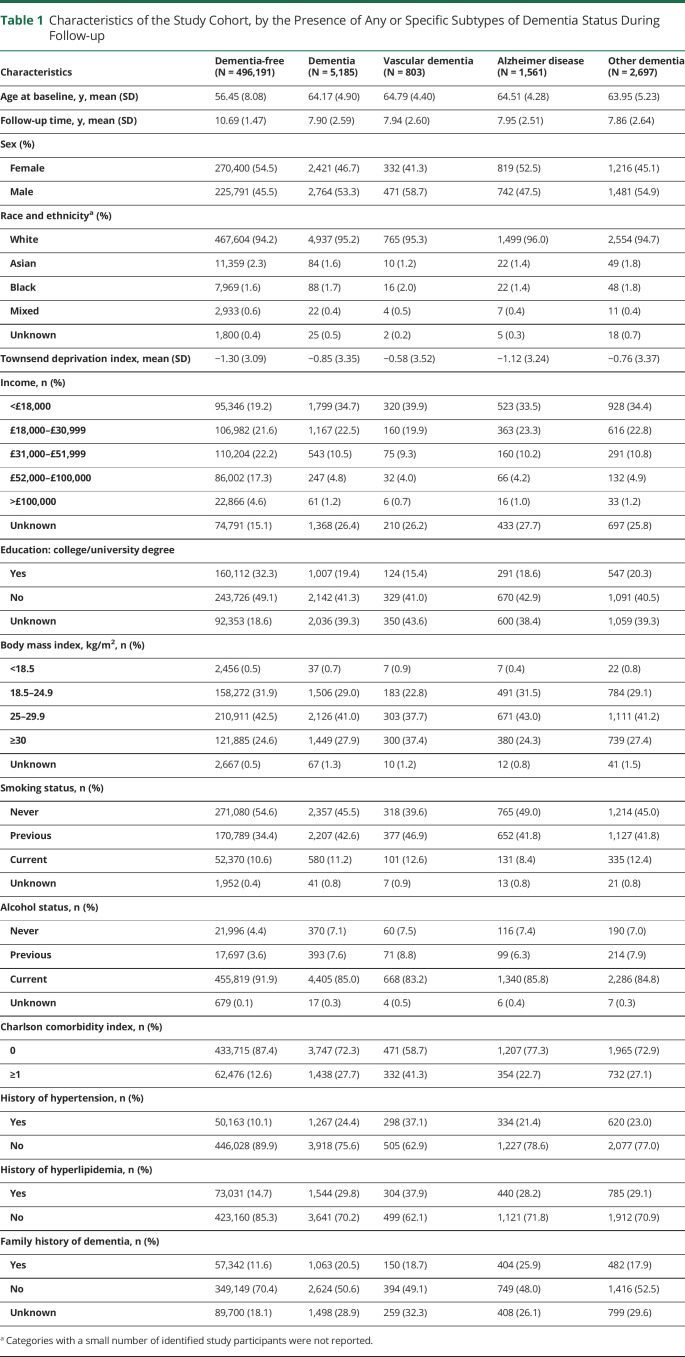
The mean age at recruitment was 56.53, and 45.60% (228,555/501,376) of the participants were male. During a mean follow-up of 10.66 years, 5,185 cases of dementia were identified, including 803 cases of vascular dementia, 1,561 cases of AD, and 2,697 cases of other dementia. Table 1 shows characteristics of participants with any or specific subtypes of dementia and those without dementia. Compared with other participants, the participants who developed dementia during follow-up were more likely to be older, male, have a history of hypertension or hyperlipidemia, and with lower socioeconomic status but a higher BMI and CCI at baseline. Few differences regarding the characteristics were noted between individuals who developed different subtypes of dementia.
Table 1.
Characteristics of the Study Cohort, by the Presence of Any or Specific Subtypes of Dementia Status During Follow-up
Associations Between Items Related to Physical/Mental Activity and Subsequent Dementia
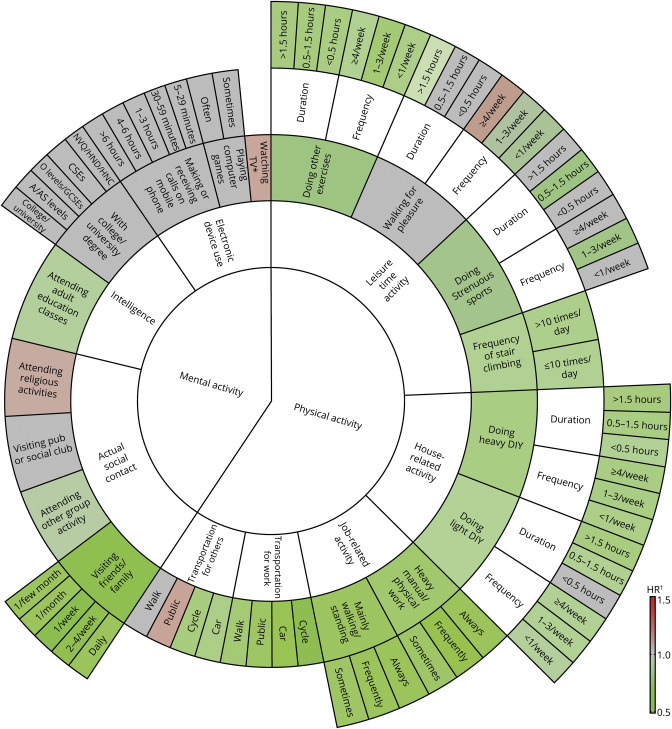
For physical activity, a higher level of the most studied items was associated with a lower risk for dementia, with the strongest (represented by a deeper degree of green color) association observed for items related to job-related activity and transportation (Figure 2 and eTable 3, links.lww.com/WNL/C140). For mental activity, while visiting pub or social club (HR 1.07, 95% CI 0.99–1.15) and watching TV (HR 1.07, 95% CI 1.05–1.09) seemed to be associated with a higher risk of dementia, the attendance of visiting friend/family (HR 0.66, 95% CI 0.56–0.77) and attending other group activity (HR 0.91, 95% CI 0.85–0.98) was associated with a lower risk of dementia (Figure 2 and eTable 4). The result pattern was largely similar for different subtypes of dementia (eTables 3 and 4).
Figure 2. HRs for the Associations Between All Items of Physical and Mental Activity and Dementia.
*Watching television was regarded as a continues variable. †HRs were derived from Cox regression models, adjusted for age, sex, race, and ethnicity, Townsend deprivation index, income, body mass index, smoking status, alcohol status, Charlson comorbidity index, history of hypertension, history of hyperlipidemia, and family history of dementia. HR = hazard ratio.
Associations of Identified Physical or Mental Activity Patterns With Subsequent Dementia
In the PCA, 5 PCs, accounting for 50.2% of the total variance, were identified for physical activity (see eFigure 1, links.lww.com/WNL/C140). We labeled each pattern by its contributing factors with top loading values, which was then named as pattern most related to vigorous and other exercise at leisure time, housework-related activity, transport-related activity, job-related activity, and walking for pleasure, respectively. Similarly, we recognized 4 PCs for mental activity (accounting for 52.5% of the total variance), named as patterns most related to pub or social club visit, watching TV, attending other group activity/adult education classes, and friend/family visit (eFigure 2).
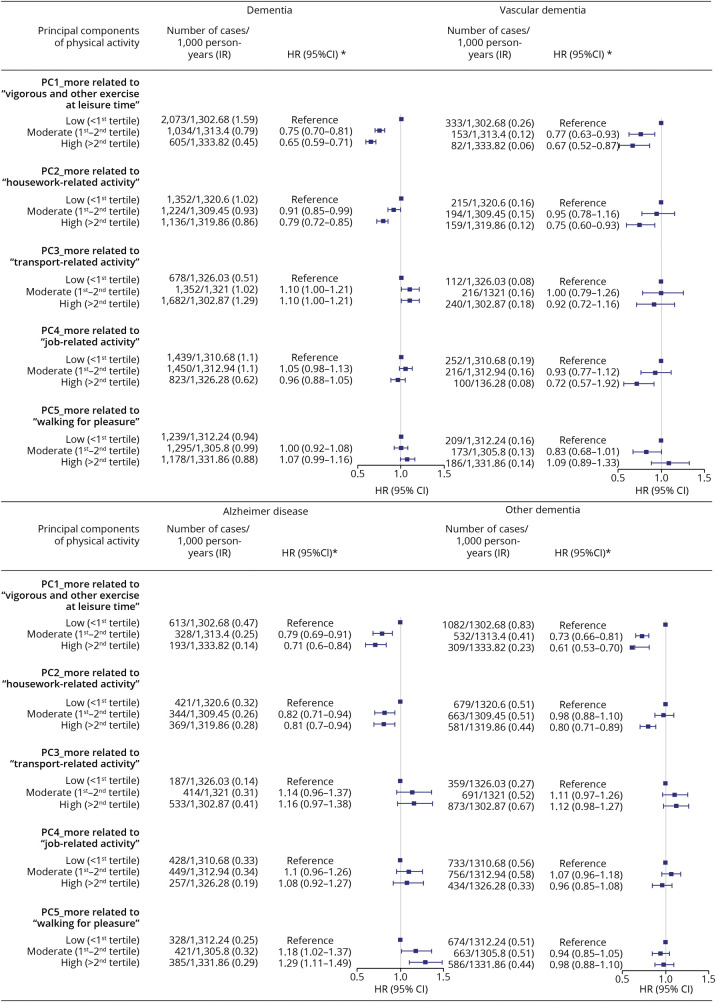
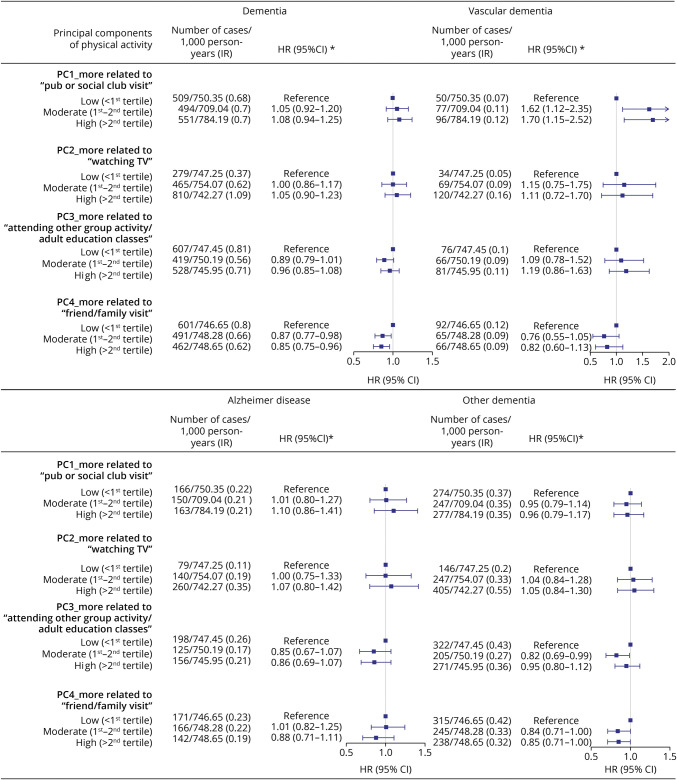
Figures 3 and 4 show the HRs and 95% CIs for any and different subtypes of dementia by different levels of adherence to each physical/mental activity pattern, using the low PC score group as the reference. For physical activity, we observed a lower risk of dementia in relation to a greater adherence to patterns related to frequent vigorous and other exercise (high vs low, HR 0.65, 95% CI 0.59–0.71) and housework-related activity (high vs low, HR 0.79, 95% CI 0.72–0.85), whereas null results were found for other identified patterns. Similar findings were noted for all subtypes of dementia. For mental activity, we observed a lower risk of dementia in relation to a greater adherence to the pattern of friend/family visit, with an HR of 0.85 (95% CI 0.75–0.96) when comparing individuals with high vs low PC scores. In the analysis of subtypes, a statistically significant association was noted only for other dementia (high vs low, HR 0.85, 95% CI 0.71–1.00). We obtained similar results across different groups of age at recruitment (eTable 5, links.lww.com/WNL/C140), sex (eTable 6), and income level (eTable 7).
Figure 3. Associations Between Physical Activity Patterns and Any or Specific Subtypes of Dementia.
*HRs and 95% CIs were derived from Cox regression models, adjusted for age, sex, race, and ethnicity, Townsend deprivation index, education, income, body mass index, smoking status, alcohol status, Charlson comorbidity index, history of hypertension, history of hyperlipidemia, and family history of dementia. HR = hazard ratio; IR = incidence rate; PC = principal component; Ref = reference.
Figure 4. Associations Between Mental Activity Patterns and Any or Specific Subtypes of Dementia.
*HRs and 95% CIs were derived from Cox regression models, adjusted for age, sex, race, and ethnicity, Townsend deprivation index, education, income, body mass index, smoking status, alcohol status, Charlson comorbidity index, history of hypertension, history of hyperlipidemia, and family history of dementia. HR = hazard ratio; IR = incidence rate; PC = principal component; Ref = reference.
The Modification Role of Disease Susceptibility to Dementia on the Observed Associations
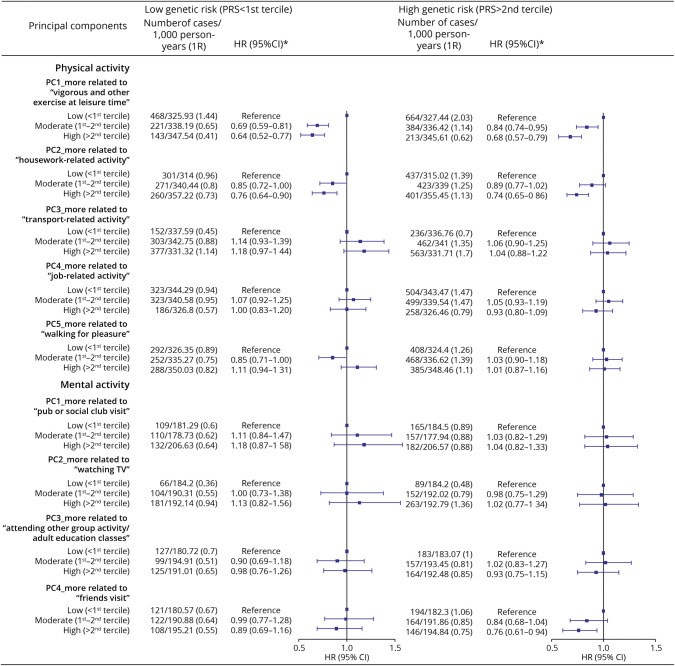
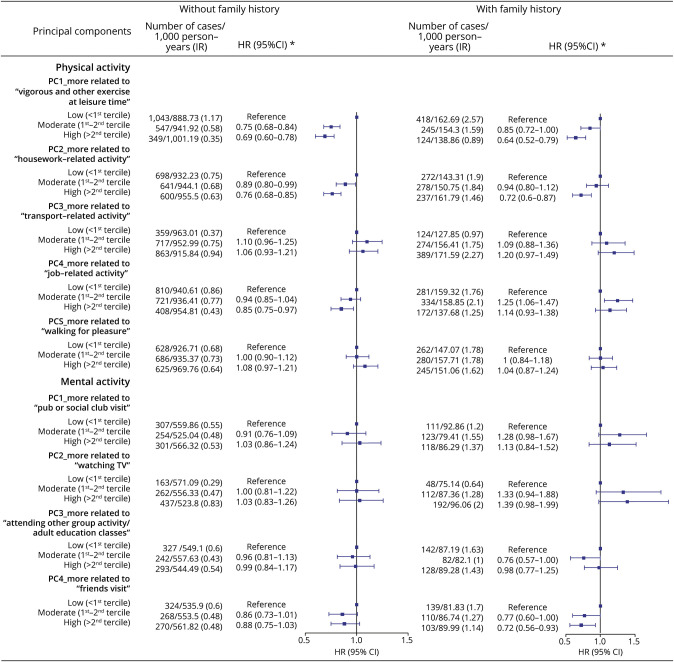
The associations were largely comparable for individuals with high and low genetic risk of dementia, measured by the PRS (Figure 5) or APOE genotype (eTable 8). For instance, the HR was 0.64 (95% CI 0.52–0.77) and 0.68 (95% CI 0.57–0.79) for a greater adherence to vigorous and other exercise pattern among individuals with a low and high genetic risk for dementia, respectively. The corresponding HRs were 0.63 (95% CI 0.54–0.72) and 0.68 (95% CI 0.59–0.78) among APOE ε4 carriers and noncarriers, respectively. Similar results were also noted among participants with and without a family history of dementia (e.g., for vigorous and other exercise pattern, HR 0.64 [95% CI 0.52–0.79] for individuals with a family history of dementia and HR 0.69 [95% CI 0.60–0.78] for individuals without, Figure 6).
Figure 5. Associations of Physical and Mental Activity Patterns With Dementia Among Individuals With Different Levels of Disease Susceptibility.
*HRs and 95% CIs were derived from Cox regression models, adjusted for age, sex, race, and ethnicity, Townsend deprivation index, education, income, body mass index, smoking status, alcohol status, Charlson comorbidity index, history of hypertension, and history of hyperlipidemia. HR = hazard ratio; IR = incidence rate; PC = principal component; PRS = polygenic risk score; Ref = reference.
Figure 6. Associations of Physical and Mental Activity Patterns With Dementia Among Individuals With and Without a Self-Reported Family History of Dementia.
*HRs and 95% CIs were derived from Cox regression models, adjusted for age, sex, race, and ethnicity, Townsend deprivation index, education, income, body mass index, smoking status, alcohol status, Charlson comorbidity index, history of hypertension, and history of hyperlipidemia. HR = hazard ratio; IR = incidence rate; PC = principal component; Ref = reference.
In sensitivity analyses, the observed estimates for subtypes of dementia remained identical after allowing a change in the initial other dementia diagnosis (28.7% [763/2,660] of other dementia cases experienced a changed diagnosis, see eTable 9). Further adjustment for or stratification by level of cognitive function at baseline did not change the results greatly (eTables 10 and 11, links.lww.com/WNL/C140). Likewise, reassessing the HRs for PCs of physical activity by additionally adjusting for significant PCs of mental activity, and vice versa, led to largely similar results (eTable 12). We further obtained similar point estimates when 5- or 10-year lag time was used in the analyses of physical activity patterns. However, with the reduced number of cases in these new lag time analyses, no statistically significant association was noted in the analysis of mental activity patterns (eTable 13).
Discussion
Based on a large cohort of 501,376 UK Biobank participants, our study indicated that both physical and mental activity could influence the risk of dementia among the elderly. Particularly, we used PCA to identify distinct patterns of physical and mental activity based on correlated individual activity items and found that individuals with a greater adherence to activity patterns related to frequent vigorous and other exercise at leisure time, housework-related activity, and friend/family visit had a reduced risk of multiple types of dementia. Furthermore, our findings based on the stratified analysis by the PRS for dementia, APOE genotype, and family history of dementia consistently revealed that the protective role of these physical and mental activity patterns was present for all study participants, irrespective of different disease susceptibilities. This novel finding highlights the potential of physical and mental activity interventions in the primary prevention of dementia for the general population.
Our finding of a decreased risk of dementia among individuals with a higher level of physical activity is consistent with previous studies. For instance, individuals with the highest level of physical activity were noticed to have a 28% decreased risk for dementia (45% for AD), compared with those with the lowest level, in a meta-analysis of 163,797 individuals from 16 studies.29 Similarly, another meta-analysis of 15 prospective studies involving 33,816 individuals concluded that physical activity could protect individuals without dementia against cognitive decline during a follow-up of 1–12 years.30 However, null findings have also been reported.31 A few randomized controlled trials (RCTs) failed to indicate cognitive benefit from aerobic exercise for cognitively healthy participants.32 Besides the heterogeneity in the measurement or definition of physical activity exposures (e.g., type, frequency, and duration), the limited sample size and follow-up period, particularly in RCTs, and the incomplete consideration of other important factors, such as other lifestyle factors and disease susceptibility, might have contributed to the inconsistent results. Moreover, every individual has multiple types of physical activity in their daily life, which closely correlate and interact. Although attempts have been made to explore the most beneficial types of physical activity,33 limited attention has been given to the interactions between physical activity-related factors. In the present study, we applied cluster analysis to identify patterns of physical activity, instead of using individual items separately, for association analyses. Indeed, despite significant results observed for most studied items of physical activity, only vigorous and other exercise at leisure time and housework-related activity were associated with a reduced risk of dementia, after considering the overall physical activity pattern (i.e., the exposure level of other physical activities of the same individual). In addition, it is worthwhile to note that although physical activity might be a possible preventive measure in the predementia stage, the role of vigorous activity among patients clinically diagnosed with dementia remains less clear.34,35 For example, a recent multicenter randomized clinical trial of 494 patients with dementia indicated potentially greater cognitive impairment among dementia patients who received a 12-month aerobic and strength exercise program, compared with the controls, although the difference between the intervention and control groups was relatively small and not statistically significant.35
A similar phenomenon was noted in the analysis of mental activity. Multiple items related to mental activity were associated with decreased dementia risk when analyzed separately (e.g., high educational attainment as noted in previous reports36), whereas only the pattern featured by frequent friend/family visit was linked to reduced risk of dementia in the analysis of mental activity pattern. Together with our novel finding that the protective effect of these physical and mental activity patterns did not differ by disease susceptibility to dementia, these results highlight the importance of leisure time physical exercise, housework-related activity, and active social contact in the prevention of dementia for elderly population.
The underlying mechanisms linking physical activity with a decreased risk of dementia remain unknown. Several possible explanations have been proposed, including the neuroprotective effect of physical exercise possibly through promoting release of brain-derived neurotrophic factors.37,38 Also, regular aerobic exercise may improve cerebral blood flow and thus reduce the development of age-associated decline.39 In addition, exercise has been proven to have antioxidant effects40 and may consequently delay the oxidative damage in brain, which is the hallmark of dementia pathogenesis.41 Furthermore, physical activity can indirectly influence other modifiable factors for cognitive function, including obesity, hypertension, insulin resistance, depression, and cardiovascular fitness.42,43 Finally, our results of mental activity pattern reinforce the benefit of social contact (e.g., friend/family visit), as social isolation might directly result in cognitive inactivity or faster cognitive decline44 and indirectly influence functions of brain though increasing the risk of cardiovascular disease45 and depression.46
The major merit of our study is the use of the UK Biobank data, a database with a community-based cohort design, detailed data on physical and mental activity and other lifestyle factors, and a complete follow-up of more than 10 years. The large sample size enables the performance of important subgroup analyses. For instance, although the study cohort was relatively young, the age-specific analysis showed similar results between individuals at 65 years or younger and those above 65 years at baseline, suggesting that the associations noted in the present study are likely applicable to both early- and late-onset dementia. The collection of physical and mental activity data and dementia was conducted independently, minimizing the risk of differential information bias. In addition, besides a screening on the associations for all relevant items of physical and mental activity, we used PCA to detect the specific patterns of physical and mental activity in the study population, which allowed the consideration of multiple activity-related items simultaneously. Moreover, we were able to consider disease susceptibility to dementia by the level of the PRS or APOE genotype (based on individual-level genotype data) and family history of dementia (self-reported) that demonstrated a universal importance of physical and mental activity patterns on dementia among individuals with different disease susceptibility. Finally, with the availability of enriched information from the comprehensive questionnaire surveys, we were able to control for a wide range of important factors, including different cognitive performance levels at baseline.
Notable limitations include the measurement of physical and mental activity based on self-reported questionnaire at recruitment, leading to the concern of information bias due to recall or lack of repeated measurements. Further studies, with objective and repeated surveillance on physical and mental activity, are warranted. Second, because diagnostic delay is common for dementia, the observed associations were subject to reverse causality, although we applied 1-year lag time in the main analyses. However, such concern should have been partially released by the similar results obtained in the sensitivity analyses using 5- or 10-year lag time. Furthermore, the accuracy of dementia subtype diagnosis is likely unsatisfactory, which might have led to a concern of misclassification between subtypes of dementia, particularly between vascular and other dementias. In our data, we found that approximately 30% of other dementia cases received a diagnosis of another dementia subtype during follow-up. This, however, does not seem to influence our results. Anyway, future studies with accurate classification of dementia subtypes are still warranted. Finally, the UK Biobank participants are not representative of the general UK population, given that its baseline survey has a low response rate (6% of the invited individuals participated in the study).16,47 However, despite such a bias, the reproducibility of findings from UK Biobank-based risk factor studies, such as those focusing on cardiovascular disease and cancer outcomes, has been shown as satisfactory.48 However, the generalizability of our findings to the whole UK or other populations needs to be tested.
In conclusion, based on the longitudinal cohort of UK Biobank, a greater adherence to a physical activity pattern of frequent vigorous and other exercise or housework-related activity, as well as a greater adherence to a mental activity pattern featured by frequent friend/family visit, are associated with a lower risk of multiple types of dementia. Moreover, because the suggested protective effects of these activity patterns were consistently observed among individuals with different disease susceptibilities, such findings further underscore the potential of these identified physical and mental activity patterns, as effective interventive strategies, for the primary prevention of dementia among the general population.
Acknowledgment
This research was conducted using the UK Biobank Resource under Application 54803. The authors thank the team members and colleagues involved in West China Biomedical Big Data Center-UK Biobank project for their support.
Glossary
- AD
Alzheimer disease
- BMI
body mass index
- CCI
Charlson comorbidity index
- GWAS
genome-wide association study
- HR
hazard ratio
- PC
principal component
- PCA
principal component analysis
- PRS
polygenic risk score
- RCT
randomized controlled trial
- SNVs
single nucleotide variations
- TDI
Townsend deprivation index
Appendix. Authors
Footnotes
CME Course NPub.org/cmelist
Study Funding
This work was supported by the National Natural Science Foundation of China (No. 81971262 to H.S.), 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (No. ZYYC21005 to H.S. and No. ZYGD20005 to L.Y.), and National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (No. Z20201013 to H.S.).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Larson EB, Yaffe K, Langa KM. New insights into the dementia epidemic. N Engl J Med. 2013;369(24):2275-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2008(3):Cd005381. [DOI] [PubMed] [Google Scholar]
- 4.Walsh EI, Smith L, Northey J, Rattray B, Cherbuin N. Towards an understanding of the physical activity-BDNF-cognition triumvirate: a review of associations and dosage. Ageing Res Rev. 2020;60:101044. [DOI] [PubMed] [Google Scholar]
- 5.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673-2734. [DOI] [PubMed] [Google Scholar]
- 6.Hersi M, Irvine B, Gupta P, Gomes J, Birkett N, Krewski D. Risk factors associated with the onset and progression of Alzheimer's disease: a systematic review of the evidence. Neurotoxicology. 2017;61:143-187. [DOI] [PubMed] [Google Scholar]
- 7.West GL, Zendel BR, Konishi K, et al. Playing Super Mario 64 increases hippocampal grey matter in older adults. PLoS One. 2017;12(12):e0187779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sommerlad A, Sabia S, Singh-Manoux A, Lewis G, Livingston G. Association of social contact with dementia and cognition: 28-year follow-up of the Whitehall II cohort study. PLoS Med. 2019;16(8):e1002862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan D, Shafto M, Kievit R, et al. Lifestyle activities in mid-life contribute to cognitive reserve in late-life, independent of education, occupation, and late-life activities. Neurobiol Aging. 2018;70:180-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80(19):1778-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hebert LE, Bienias JL, Aggarwal NT, et al. Change in risk of Alzheimer disease over time. Neurology. 2010;75(9):786-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer's disease. Neuron. 2009;63(3):287-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreno-Grau S, de Rojas I, Hernandez I, et al. Genome-wide association analysis of dementia and its clinical endophenotypes reveal novel loci associated with Alzheimer's disease and three causality networks: the GR@ACE project. Alzheimers Dement. 2019;15(10):1333-1347. [DOI] [PubMed] [Google Scholar]
- 14.Jansen IE, Savage JE, Watanabe K, et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer's disease risk. Nat Genet. 2019;51(3):404-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brainstorm C, Anttila V, Bulik-Sullivan B, et al. Analysis of shared heritability in common disorders of the brain. Science. 2018;360(395):eaap8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sudlow C, Gallacher J, Allen N, et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bycroft C, Freeman C, Petkova D, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Power MC, Weuve J, Sharrett AR, Blacker D, Gottesman RF. Statins, cognition, and dementia—systematic review and methodological commentary. Nat Rev Neurol. 2015;11:220-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon SJ, Suh SY, Lee YJ, et al. Prospective validation of objective prognostic score for advanced cancer inpatients in South Korea: a multicenter study. J Palliat Med. 2017;20(1):65-68. [DOI] [PubMed] [Google Scholar]
- 20.Najar J, Östling S, Gudmundsson P, et al. Cognitive and physical activity and dementia: a 44-year longitudinal population study of women. Neurology. 2019;92(12):e1322-e1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilkinson T, Schnier C, Bush K, et al. Identifying dementia outcomes in UK Biobank: a validation study of primary care, hospital admissions and mortality data. Eur J Epidemiol. 2019;34(6):557-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambert JC, Ibrahim-Verbaas CA, Harold D, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat Genet. 2013;45(12):1452-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seripa D, D'Onofrio G, Panza F, Cascavilla L, Masullo C, Pilotto A. The genetics of the human APOE polymorphism. Rejuvenation Res. 2011;14(5):491-500. [DOI] [PubMed] [Google Scholar]
- 24.Townsend P, Phillimore P, Beattie A. Health and Deprivation: Inequality and the North. Routledge; 1988. [Google Scholar]
- 25.Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sørensen HT. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol. 2011;11:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fawns-Ritchie C, Deary IJ. Reliability and validity of the UK Biobank cognitive tests. PLoS One. 2020;15(4):e0231627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jannasch F, Riordan F, Andersen LF, Schulze MB. Exploratory dietary patterns: a systematic review of methods applied in pan-European studies and of validation studies. Br J Nutr. 2018;120(6):601-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weuve J, Proust-Lima C, Power MC, et al. Guidelines for reporting methodological challenges and evaluating potential bias in dementia research. Alzheimers Dement. 2015;11(9):1098-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol Med. 2009;39(1):3-11. [DOI] [PubMed] [Google Scholar]
- 30.Sofi F, Valecchi D, Bacci D, et al. Physical activity and risk of cognitive decline: a meta-analysis of prospective studies. J Intern Med. 2011;269(1):107-117. [DOI] [PubMed] [Google Scholar]
- 31.Sabia S, Dugravot A, Dartigues JF, et al. Physical activity, cognitive decline, and risk of dementia: 28 year follow-up of Whitehall II cohort study. BMJ. 2017;357:j2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young J, Angevaren M, Rusted J, Tabet N. Aerobic exercise to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2015(4):CD005381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oja P, Kelly P, Pedisic Z, et al. Associations of specific types of sports and exercise with all-cause and cardiovascular-disease mortality: a cohort study of 80 306 British adults. Br J Sports Med. 2017;51(10):812-817. [DOI] [PubMed] [Google Scholar]
- 34.Christofoletti G, Oliani MM, Gobbi S, Stella F, Bucken Gobbi LT, Renato Canineu P. A controlled clinical trial on the effects of motor intervention on balance and cognition in institutionalized elderly patients with dementia. Clin Rehabil. 2008;22(7):618-626. [DOI] [PubMed] [Google Scholar]
- 35.Lamb SE, Sheehan B, Atherton N, et al. Dementia And Physical Activity (DAPA) trial of moderate to high intensity exercise training for people with dementia: randomised controlled trial. BMJ. 2018;361:k1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caamano-Isorna F, Corral M, Montes-Martinez A, Takkouche B. Education and dementia: a meta-analytic study. Neuroepidemiology. 2006;26:226-232. [DOI] [PubMed] [Google Scholar]
- 37.Loprinzi PD, Frith E. A brief primer on the mediational role of BDNF in the exercise-memory link. Clin Physiol Funct Imaging. 2019;39(1):9-14. [DOI] [PubMed] [Google Scholar]
- 38.Nascimento CM, Pereira JR, de Andrade LP, et al. Physical exercise in MCI elderly promotes reduction of pro-inflammatory cytokines and improvements on cognition and BDNF peripheral levels. Curr Alzheimer Res. 2014;11(8):799-805. [DOI] [PubMed] [Google Scholar]
- 39.Ainslie PN, Cotter JD, George KP, et al. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J Physiol. 2008;586(16):4005-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Sousa CV, Sales MM, Rosa TS, Lewis JE, de Andrade RV, Simoes HG. The antioxidant effect of exercise: a systematic review and meta-analysis. Sports Med. 2017;47:277-293. [DOI] [PubMed] [Google Scholar]
- 41.Rottkamp CA, Nunomura A, Raina AK, Sayre LM, Perry G, Smith MA. Oxidative stress, antioxidants, and Alzheimer disease. Alzheimer Dis Assoc Disord. 2000;14(suppl 1):S62-S66. [DOI] [PubMed] [Google Scholar]
- 42.Jensen CS, Hasselbalch SG, Waldemar G, Simonsen AH. Biochemical markers of physical exercise on mild cognitive impairment and dementia: systematic review and perspectives. Front Neurol. 2015;6:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown BM, Peiffer JJ, Martins RN. Multiple effects of physical activity on molecular and cognitive signs of brain aging: can exercise slow neurodegeneration and delay Alzheimer's disease? Mol Psychiatry. 2013;18:864-874. [DOI] [PubMed] [Google Scholar]
- 44.Kuiper JS, Zuidersma M, Oude Voshaar RC, et al. Social relationships and risk of dementia: a systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev. 2015;22:39-57. [DOI] [PubMed] [Google Scholar]
- 45.Hemingway H, Marmot M. Evidence based cardiology: psychosocial factors in the aetiology and prognosis of coronary heart disease. Systematic review of prospective cohort studies. BMJ. 1999;318(7196):1460-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santini ZI, Koyanagi A, Tyrovolas S, Mason C, Haro JM. The association between social relationships and depression: a systematic review. J Affect Disord. 2015;175:53-65. [DOI] [PubMed] [Google Scholar]
- 47.Fry A, Littlejohns TJ, Sudlow C, et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol. 2017;186(9):1026-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Batty GD, Gale CR, Kivimäki M, Deary IJ, Bell S. Comparison of risk factor associations in UK Biobank against representative, general population based studies with conventional response rates: prospective cohort study and individual participant meta-analysis. BMJ. 2020;368:m131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from the UK Biobank (ukbiobank.ac.uk/) are available to all researchers on making an application.