Abstract
Background and Objectives
Asymptomatic or persistent optic nerve enhancement in aquaporin-4 (AQP4)-immunoglobulin G (IgG)–positive neuromyelitis optica spectrum disorder (NMOSD) is thought to be rare. Improved understanding may have important implications for assessment of treatment efficacy in clinical trials and in clinical practice. Our objective was to characterize NMOSD interattack optic nerve enhancement.
Methods
This was a retrospective cohort study performed between 2000 and 2019 (median follow-up 5.5 [range 1–35] years) of patients with AQP4-IgG–positive optic neuritis (ON) evaluated at Mayo Clinic. MRI orbits were reviewed by a neuroradiologist, neuro-ophthalmologist, and neuroimmunologist blinded to the clinical history. Interattack optic nerve enhancement (>30 days after attack) was measured. The correlation between interattack enhancement and Snellen visual acuity (VA), converted to logarithm of the minimum angle of resolution (logMAR), at attack and at follow-up were assessed.
Results
A total of 198 MRI scans in 100 patients with AQP4-IgG+ NMOSD were identified, with 107 interattack MRIs from 78 unique patients reviewed. Seven scans were performed before any ON (median 61 days before attack [range 21–271 days]) and 100 after ON (median 400 days after attack [33–4,623 days]). Optic nerve enhancement was present on 18/107 (16.8%) interattack scans (median 192.5 days from attack [33–2,943]) of patients with preceding ON. On 15 scans, enhancement occurred at the site of prior attacks; the lesion location was unchanged, but the lesion length was shorter. Two scans (1.8%) demonstrated new asymptomatic lesions (prior scan demonstrated no enhancement). In a third patient with subjective blurry vision, MRI showed enhancement preceding detectable eye abnormalities on examination noted 15 days later. There was no difference in VA at preceding attack nadir (logMAR VA 1.7 vs 2.1; p = 0.79) or long-term VA (logMAR VA 0.4 vs 0.2, p = 0.56) between those with and without interattack optic nerve enhancement.
Discussion
Asymptomatic optic nerve enhancement occurred in 17% of patients with NMOSD predominantly at the site of prior ON attacks and may represent intermittent blood-brain barrier breakdown or subclinical ON. New asymptomatic enhancement was seen only in 2% of patients. Therapeutic clinical trials for NMOSD require blinded relapse adjudication when assessing treatment efficacy, and it is important to recognize that asymptomatic optic nerve enhancement can occur in patients with ON.
Aquaporin-4 (AQP4)-immunoglobulin G (IgG)–positive neuromyelitis optica spectrum disorder (NMOSD) is characterized by severe visual loss associated with frequent optic neuritis (ON).1 Asymptomatic myelitis and brain lesions can develop, but asymptomatic optic nerve enhancement has not been well characterized.2-4 Documentation of asymptomatic optic nerve enhancement and corresponding visual findings has important implications for clinical care and disease activity evaluation in clinical trials.5-7 Primary time-to-event analysis of clinical attacks requires careful clinical assessment to prevent misclassification of possible clinical attacks to avoid decreasing study power and drawing erroneous conclusions.8,9 In the inebilizumab trial, approximately 50% of clinician-reported attacks were thought to represent true relapses after adjudication committee review.6 Orbital MRI may potentially improve clinical sensitivity and specificity, but unanticipated asymptomatic findings necessitate careful adjudication.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the institutional review board of Mayo Clinic (Rochester, MN), and all patients consented to the use of their medical records for research purposes.
Design, Setting, and Participants
This was a retrospective cohort study in which patients evaluated at Mayo Clinic Rochester (MN), Jacksonville (FL), and Scottsdale (AZ) between January 1, 2000, and July 1, 2019, were identified. Inclusion criteria were patients diagnosed with ON and AQP4-IgG seropositive by cell-based assay or fluorescent-activated cell-sorting assay. Patients were identified by electronic medical records using the keywords “optic neuritis,” “aquaporin-4,” “AQP4,” “neuromyelitis optica,” or “NMO” with subsequent confirmation of aquaporin-4–IgG positivity using the Mayo Clinic Neuroimmunology Laboratory Database. Exclusion criteria were patients with incomplete or insufficient medical records, lack of research consent, and lack of high-quality (due to motion degradation or artifact) scans or lack of scans with adequate evaluation of the orbits.
ON was defined by the presence of ≥3 of the following clinical findings: decreased visual acuity (VA), pain with eye movement, visual field defect, relative afferent pupillary defect, changes in color vision, optic disc edema, or MRI abnormalities.10 MRIs were classified as attack scans if performed within 30 days of an ON, whereas MRIs >30 days after an ON and MRIs done prior to an ON, performed as part of routine monitoring without further decline in visual function (not meeting the ON criteria), were categorized as interattack scans or asymptomatic to avoid ascribing an underlying pathophysiology to the phenomenon.11,12 Persistence of enhancement at 60 and 90 days from attack was also investigated imputing the assumption that there was no new enhancement in those without follow-up scans after 60 or 90 days. MRIs with adequate orbital imaging on 1.5 or 3 T MRIs were independently reviewed by a neuroradiologist (P.M.), neurologist (S.S.S.), and neuro-ophthalmologist (J.J.C.), who were aware of NMOSD diagnosis but blinded to clinical details at imaging review. T1-weighted fat-saturated, MRI pre- and post-gadolinium administration was reviewed for optic nerve enhancement. Optic nerve enhancement on interattack scans was defined as new or increased enhancement from prior images not meeting the criteria for optic neuritis as has been defined above. Images were interpreted via consensus secondarily if interpretations were discordant. MRIs were reviewed for T2 hyperintensity of the optic nerves, optic nerve enlargement, and optic nerve atrophy. Optic nerve enlargement was qualitatively defined in relation to the unaffected adjacent nerve or in comparison to a prior scan if bilateral. Optic nerve atrophy was subjectively assessed in comparison to the unaffected adjacent nerve or in comparison to prior imaging if bilateral.
Clinical Evaluation
Medical records were reviewed for demographic information, attack information temporally closest to interattack scan (onset date, worst affected eye, and VA at nadir), total ON attacks, and last available VA. Medical record extraction was performed by author S.S.S. with validation of this data extraction performed by author J.J.C. Best-corrected VA was evaluated using Snellen VA charts and converted to logarithm of minimum angle of resolution (logMAR) values with values for nonnumeric values: count fingers = 1.7, hand motion = 2.0, light perception = 2.3, and no light perception = 3.0.
Statistical Analysis
Descriptive data were reported using medians, ranges, and percentiles, and continuous variables were analyzed using the Wilcoxon rank-sum test using available data, and analysis were performed without imputation of missing data. p Value was 2 sided and less than 0.05 was significant (BlueSky version 7.1).
Data Availability
Data not provided in the article because of space limitations may be anonymously shared at the request of any qualified investigator for purposes of replicating procedures and results.
Results
One hundred patients were identified, and a total 198 scans were included. Ninety-one scans were classified as attack scans, and 107 MRIs were classified as interattack scans (78 patients). Figure 1 highlights inclusion criteria.
Figure 1. Flowsheet of Inclusion Criteria.
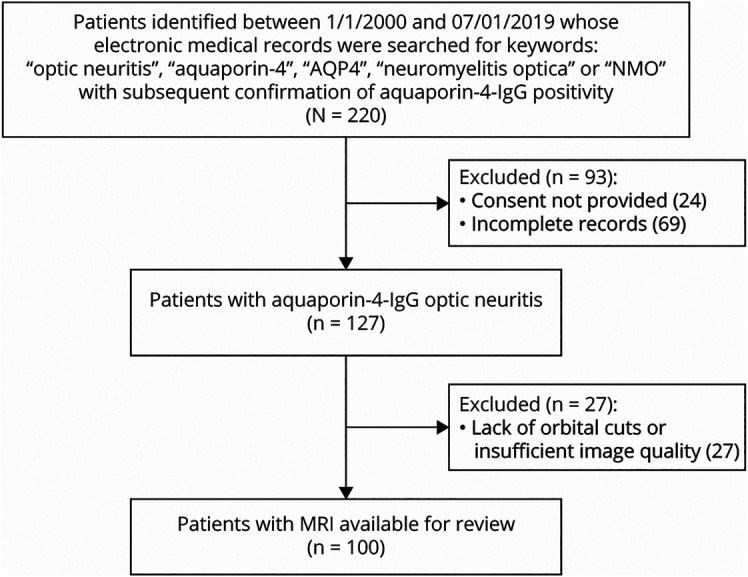
AQP = aquaporin‐4‐IgG; IgG = immunoglobulin G; NMO = neuromyelitis optica.
Demographic and radiographic characteristics are shown in Table. Optic nerve enhancement was present on 18/107 (17%) interattack scans (n = 7 preattack, median 61 days before attack [range 21–271 days]; n = 100 postattack, median 400 days after attack [range 33–4,623 days]) among 15/78 (19%) patients, with an overall agreement between 3 raters on 102 of 107 scans (95%). When considering only interattack MRIs performed >60 days or >90 days after the prior ON attack date, optic nerve enhancement remained present on 16/107(15%) and 13/107(12%) interattack scans, respectively, all of whom had prior ON at the site of enhancement.
Table.
Demographics, Clinical and Imaging Characteristics

Enhancement extending over more than 50% of the optic nerve was seen in 6/18 (33%) scans, with 4 patients having persistent optic chiasmal enhancement. Enhancement at the location of a prior ON was noted on 15 scans; qualitatively, 12 showed improved enhancement at the same location (intensity improved in all; lesion length improved in 7), and 3 did not have available prior MRIs to review, but the radiologic interpretation reported improved enhancement. Ongoing enhancement at the same location of enhancement as the preceding ON was documented up to several years after the prior attack in some cases (Figure 2, A–D). Two patients' scans demonstrated a new asymptomatic lesion (Figure 2, E and F), and another patient, a 73-year-old woman, had subjective orbital pain but no abnormalities in VA, visual fields, or optical coherence tomography until 15 days later (Figure 2, G and H). T2 hyperintense lesions on MRI were noted at the site of enhancement in 16 of 16 scans. Two interattack scans with optic nerve enhancement did not have corresponding T2 images of the orbit available for review.
Figure 2. Interattack Optic Nerve Enhancement.
An 11-year-old girl developed left ON with nadir VA 20/150 and final VA 20/50 after treatment. (A) Coronal and (B) axial T1-weighted MRI with gadolinium demonstrates an enlarged homogenously enhancing left optic nerve involving the chiasm at attack (arrow). The enhancement has improved but persists 20 months later in (C) coronal and (D) axial T1-weighting MRI (arrow). A 12-year-old girl with (E) axial MRI demonstrating asymptomatic left intraorbital optic nerve enhancement (arrow), significantly increased from an interattack scan performed 6 months prior (F, arrow). A 73-year-old woman with subjective orbital pain. (G) Coronal MRI demonstrates intraorbital optic nerve enhancement (arrow). Fifteen days later, she develops objective visual impairment. An interattack MRI performed 2 months prior showed no enhancement of the optic nerve (arrow, H). ON = optic neuritis; VA = visual acuity.
We investigated several potential confounders. There was no difference in the number of interattack scans performed in the group with and without interattack scan enhancement (median 1 [range 1–4] vs median 1 [range 1–4], p = 0.5). Enhancement was noted on 12/77 (15%) 1.5 T MRIs and on 6/30 (20%) 3 T MRIs without a statistically significant difference in detection rates between magnet strengths (p = 0.77). Before 2010, 15/20 (75%) of patients received IV steroids with or without plasmapheresis, whereas 44/58 (75%) of patients seen after 2010 received IV steroids with or without plasmapheresis; however, there was no significant difference (p = 0.95) in rates of immunotherapy administration before and after January 1, 2010, approximately the midpoint of our study. Similarly, when comparing patients who received plasmapheresis before (3/20 patients; 15%) and after (18/58 patients; 31%) January 1, 2010, there was no difference (p = 0.29) in administration rates of plasmapheresis. There was also no significant difference in the number of patients who had enhancing scans before (4/33 [12%]) and after January 1, 2010 (14/74 [18%]; p = 0.58).
Interattack scans that had optic nerve enhancement were performed at a shorter interval to the preceding ON than those MRIs in which optic nerve enhancement was not detected (median 192.5 days from attack [33–2,943 days] vs median 480.5 days from attack [35–4,263 days], p = 0.04; Figure 3A). Patients with asymptomatic enhancement on interattack MRI were younger than those without persistent/new enhancement (27.5 years [interquartile range (IQR) 18.3–53; n = 89] vs 50 years [IQR 38–58; n = 16]; p = 0.04; Figure 3B), whereas sex did not differ (proportion female 12/14 [86%] vs 63/74 [85%]; p = 0.955). In addition, in the 10% (8 of 78) patients who were younger than 18 years, enhancement was noted on 4 of 9 interattack scan. There was no difference in frequency of attacks between interattack scans of patients with enhancement (median 2 attacks [range 1–9], n = 15) and without enhancement (median 2 attacks [range 1–9], n = 63; p = 0.6). There was no difference in the lesion length of the preceding ON between patients with subsequent interattack enhancement and those without (31 millimeters [range 15–70 mm, n = 18] vs 45 mm [range 21.5–71.5 mm, n = 80], p = 0.334). There was no difference in the preceding ON nadir of vision loss in those with asymptomatic enhancement and those without persistent/new enhancement (logMAR VA 1.7 vs 2.1; p = 0.79; Figure 3C). Long-term visual outcomes between patients with asymptomatic enhancement and those without were not different (logMAR VA 0.4 vs 0.2, p = 0.56; Figure 3D).
Figure 3. Boxplot Comparison of Patients With and Without Interattack Optic Nerve Enhancement on MRI.
(A) Comparison of days demonstrates a smaller time interval in those with persistent enhancement. (B) Comparison of age at the attack directly before interattack MRI shows a younger age in those with interattack scan enhancement. (C) Comparison of nadir visual acuity at optic neuritis attack directly preceding interattack scan demonstrates no significant difference between the 2 cohorts. (D) Comparison of visual acuity after attack recovery demonstrates no significant difference between the 2 cohorts.
Discussion
Asymptomatic optic nerve enhancement occurred in 17% of AQP4-IgG–positive NMOSD MRI scans 30 days after attack and remained persistent in 15% and 12% at 60 days and 90 days from attack, respectively. Optic nerve enhancement heralded a clinical attack in 1 patient and could be defined as presymptomatic. New asymptomatic enhancement was seen in 2 patients, both of whom had a remote history of ON in the affected eye and represented only 2% of interattack scans.
Given the long time frame over which the enhancement persisted at the site of prior enhancement without visual compromise, and because all patients with asymptomatic enhancement had a prior ON, intermittent or persistent blood-brain barrier breakdown may account for our findings rather than subclinical ON. Patients with asymptomatic interattack enhancement were younger. Younger patients with NMOSD are at a higher risk of ON, are more likely to have blindness over motor impairment, and may have a more robust autoimmunity at earlier stages of the disease, which may account for this radiographic finding, although in our small cohort, the VA did not differ at attack nadir or at recovery in these patients.13,14
Asymptomatic interattack brain abnormalities and myelitis have been reported in NMOSD and multiple sclerosis, but interattack optic nerve enhancement was previously thought to be uncommon.2-4,11 However, preliminary reports from the inebilizumab study in NMOSD found that approximately 50% of scans showed asymptomatic optic nerve enhancement at the time of an interattack surveillance MRI (in the absence of adjudicated attacks), which is more frequent than described in this study and in our clinical experience.15 This study defined interattack scans as MRIs performed beyond 30 days from the prior ON, which is the time frame in which gadolinium might be expected to resolve, but persistent enhancement appeared to be present beyond this period as seen by a clustering of patients with optic nerve enhancement at 60 and 90 days. Further studies are required to document the frequency and location of asymptomatic optic nerve enhancement and to clarify the significance of subclinical disease activity or intermittent blood-brain barrier and treatment-specific differences in outcomes.
In addition, there is no consensus definition of clinical relapse of ON. All three recent trials used different criteria and adjudication procedures aimed at minimizing relapse misclassification and maximizing sensitivity and/or specificity.5-7,9 Between trials, procedures for relapse determination did not consistently require MRI optic nerve enhancement if characteristic clinical findings were identified. The adjudication of events consisting of eye pain without vision loss, isolated optic disc edema, and the utility of serial evaluations was not addressed. Routine performance of orbital MRI may minimize relapse misclassification; however, the significance in comparison to prior MRI findings to determine its role in defining ON relapse must be further clarified.
Limitations of the study include the retrospective case ascertainment resulting in incomplete availability of follow-up and attack scans for each patient included in the study, the inclusion of both 1.5 and 3 T images, subjective assessment of variables such as optic nerve enlargement and atrophy, and the 19-year time frame over which the MRIs were ascertained, resulting in variable scan quality and therefore potentially effecting scan interpretation. However, we investigated potential confounders and found that between patients with and without interattack enhancement, there was no significant difference in the number of interattack scans performed and no difference in detection with different magnet strengths. There was also no significant difference in the number of patients who received acute immunotherapy when comparing the first and second half of the enrollment period. Our study was limited by the small number of patients with MRIs before any attacks but reflects the real-world natural history of patients with NMOSD who develop ON, coinciding with those enrolled in NMOSD clinical trials.1 Future studies evaluating for optic nerve enhancement on MRI in patients without a history of ON could help further clarify subclinical disease activity in NMOSD as could inclusion of data from OCT, visual evoked potentials, and MRI neuroaxis.11,12
This study demonstrates that patients with AQP4-IgG–positive NMOSD can have asymptomatic optic nerve enhancement on MRI. Although our study suggests that this is from persistent or intermittent breakdown of the blood-brain, subclinical disease activity may be possible as well. This holds clinical and research-related implications and prompts several questions regarding outcome assessment, treatment efficacy, and adjudication of clinical ON attacks and subsequent treatment decisions in clinical trials.
Acknowledgment
The authors thank Jessica Sagen for her assistance in coordinating data, and Mary Curtis for her administrative assistance.
Glossary
- AQP4
aquaporin-4
- IgG
immunoglobulin G
- IQR
interquartile range
- logMAR
logarithm of minimum angle of resolution
- NMOSD
neuromyelitis optica spectrum disorder
- ON
optic neuritis
- VA
visual acuity
Appendix. Authors

Study Funding
No targeted funding reported.
Disclosure
D. Wingerchuk reports consulting for Alexion, VielaBio, Roche, Biogen, Genentech, TG Therapeutics, Reistone, and Mitsubishi Tanabe. B. Weinshenker receives royalties from RSR Ltd, Oxford University, Hospices Civil de Lyon, and MVZ Labor PD Dr. Volkmann und Kollegen GbR for a patent of NMO-IgG as a diagnostic test for neuromyelitis optica spectrum disorders, served on adjudication committee for clinical trials in neuromyelitis optica spectrum disorders being conducted by MedImmune/VielaBio and Alexion, and consulted for Chugai/Roche/Genentech and Mitsubishi-Tanabe regarding a clinical trial for neuromyelitis optica spectrum disorders. He has received honoraria for speaking at internal meetings of Genentech, Novartis, and external meetings for Roche. S.J. Pittock reports grants, personal fees, and nonfinancial support from Alexion Pharmaceuticals, Inc.; grants from Grifols, Autoimmune Encephalitis Alliance; grants, personal fees, nonfinancial support, and other from MedImmune, Inc. Dr. Pittock has a patent # 9,891,219 (Application #12-573942) “Methods for Treating Neuromyelitis Optica (NMO) by Administration of Eculizumab to an individual that is Aquaporin-4 (AQP4)-IgG Autoantibody positive.” E.P. Flanagan was a site principal investigator in a randomized placebo-controlled clinical trial of Inebilizumab (A CD19 inhibitor) in neuromyelitis optica spectrum disorders funded by MedImmune/Viela Bio. J.J. Chen reports consulting for Roche and UCB. The other authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flanagan EP, Weinshenker BG, Krecke KN, Pittock SJ. Asymptomatic myelitis in neuromyelitis optica and autoimmune aquaporin-4 channelopathy. Neurol Clin Pract. 2015;5(2):175-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee MY, Yong KP, Hyun JW, Kim SH, Lee SH, Kim HJ. Incidence of interattack asymptomatic brain lesions in NMO spectrum disorder. Neurology. 2020;95(23):e3124-e3128. [DOI] [PubMed] [Google Scholar]
- 4.Pittock SJ, Lennon VA, Krecke K, Wingerchuk DM, Lucchinetti CF, Weinshenker BG. Brain abnormalities in neuromyelitis optica. Arch Neurol. 2006;63(3):390-396. [DOI] [PubMed] [Google Scholar]
- 5.Yamamura T, Kleiter I, Fujihara K, et al. Trial of satralizumab in neuromyelitis optica spectrum disorder. N Engl J Med. 2019;381(22):2114-2124. [DOI] [PubMed] [Google Scholar]
- 6.Pittock SJ, Berthele A, Fujihara K, et al. Eculizumab in aquaporin-4-positive neuromyelitis optica spectrum disorder. N Engl J Med. 2019;381(7):614-625. [DOI] [PubMed] [Google Scholar]
- 7.Cree BAC, Bennett JL, Kim HJ, et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet. 2019;394(10206):1352-1363. [DOI] [PubMed] [Google Scholar]
- 8.Levy M, Fujihara K, Palace J. New therapies for neuromyelitis optica spectrum disorder. Lancet Neurol. 2021;20(1):60-67. [DOI] [PubMed] [Google Scholar]
- 9.Kahan BC, Feagan B, Jairath V. A comparison of approaches for adjudicating outcomes in clinical trials. Trials. 2017;18(1):266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassan MB, Stern C, Flanagan EP, et al. Population-based incidence of optic neuritis in the era of aquaporin-4 and myelin oligodendrocyte glycoprotein antibodies. Am J Ophthalmol. 2020;220:110-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Youl BD, Turano G, Miller DH, et al. The pathophysiology of acute optic neuritis. An association of gadolinium leakage with clinical and electrophysiological deficits. Brain. 1991;114(pt 6):2437-2450. [DOI] [PubMed] [Google Scholar]
- 12.Chen JJ, Flanagan EP, Jitprapaikulsan J, et al. Myelin oligodendrocyte glycoprotein antibody-positive optic neuritis: clinical characteristics, radiologic clues, and outcome. Am J Ophthalmol. 2018;195:8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palace J, Lin DY, Zeng D, et al. Outcome prediction models in AQP4-IgG positive neuromyelitis optica spectrum disorders. Brain. 2019;142(5):1310-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goronzy JJ, Weyand CM. Immune aging and autoimmunity. Cell Mol Life Sci. 2012;69(10):1615-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedemann P, Wingerchuk D, Weinshenker BG, et al. Quiescent MRI activity in neuromyelitis optica spectrum disorder: results from the N-MOmentum randomized placebo-controlled trial. Presented at MSVirtual 2020 – 8th Joint ACTRIMS-ECTRIMS Meeting, September 11-13, 2020. https://vielabio.com/wp-content/uploads/MRI-outcomes_ECTRIMS-ACTRIMS-2020-poster_09Sept20.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data not provided in the article because of space limitations may be anonymously shared at the request of any qualified investigator for purposes of replicating procedures and results.




