Abstract
Introduction
Radiographic staging with bone scan or computed tomography is not indicated for men with low-risk prostate cancer. Physician compliance with these imaging recommendations has been widely variable, leading to inappropriate testing and increased costs. The purpose of this systematic review was to identify and learn from interventions associated with improved physician compliance to imaging guidelines for prostate cancer staging.
Methods
This systematic review followed Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines. PubMed was searched through January 2022 for the following medical subject headings (MeSH) terms: (‘practice patterns, physicians’ or ‘guideline adherence’ or ‘unnecessary procedures’ or ‘quality improvement’) and (‘prostatic neoplasms/diagnostic imaging’). Inclusion required discussion of an intervention for physician compliance to prostate cancer imaging guidelines and specific data describing associated outcomes. Publications focused on other malignancies or without this intervention, evaluation, or data were excluded.
Results
Of 82 papers screened, only five met inclusion criteria — representing 12 802 patients. Each focused on reducing unnecessary imaging and demonstrated statistically significant post-intervention improvement of physician compliance to imaging guidelines for staging prostate cancer. Four were multidimensional, with education, clinical champions, and performance feedback. One used the unidimensional intervention of an electronic medical record (EMR)-based clinical reminder order check (CROC). No studies used randomization or a control group.
Conclusions
Post-intervention improvement in physician compliance to imaging guidelines for staging prostate cancer has been associated with EMR-based CROC and combination interventions using clinical champions, education, and feedback. This has been observed at individual institutions and larger organizations spanning a region or state.
Introduction
Numerous studies demonstrate that guideline adherence increases efficient use of resources, subsequently reducing unnecessary imaging and its associated radiation exposures without increased risk to patients.1–9 In regard to prostate cancer, most guidelines agree that radiographic staging with bone scan or computed tomography (CT) is not indicated for men with low-risk disease and an exceedingly low probability of metastatic spread.10–13 For example, the American Urological Association (AUA) and European Association of Urology (EAU) both recommend that clinicians should not perform abdomino-pelvic CT or routine bone scans in the staging of low-risk patients.13,14 In fact, elimination of bone scan and CT imaging for staging of newly diagnosed, low-risk prostate cancer has been a recommendation selected for the Choosing Wisely campaign, with the aim to decreased wasteful testing and downstream benefit-lacking cost burden.15
Prior studies have shown, however, that physician compliance with imaging recommendations for prostate cancer staging has been widely variable,16–21 and that refining imaging practices for prostate cancer can reduce overtreatment and cost.22 For example, recommended staging imaging for high-risk prostate cancer has been underused,19,21 with as many as 51% of patients not receiving indicated CT/magnetic resonance imaging (MRI) imaging at a national level.19 Meanwhile, recent Surveillance, Epidemiology, and End Results (SEER)-Medicare data analysis showed non-recommended staging imaging was performed far more often in early prostate cancer cases than in early breast cancer — 41% vs. 14%, respectively.20
While there is evidence that physicians are the primary drivers in decision-making regarding staging imaging for prostate cancer,16 our understanding is incomplete as to “the mechanisms driving these decisions and the most effective physician-level strategies to address them.”20 The purpose of this systematic review is to address this literature gap by identifying, consolidating, and learning from studies exploring interventions intended to improve physician compliance to prostate cancer staging imaging guidelines.
Methods
This systematic review was conducted using Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines. Initial searches of the literature revealed key Medical Subject Headings (MeSH) terms of relevant studies. These MeSH terms were used to search PubMed through January 2022 and included ‘prostatic neoplasms/diagnostic imaging’ and either ‘practice patterns, physicians’; ‘guideline adherence’; ‘unnecessary procedures’; or ‘quality improvement’. This PubMed search resulted in 79 papers.
Other searches were conducted with generic non-MeSH terms, such as ‘intervention’ and ‘guideline’ and ‘imaging’ and ‘prostate cancer’, to capture additional related papers (e.g., those too recent to have MeSH terms applied). This yielded three additional studies.
The 82 papers were reviewed by two authors (SP, DM). Five were found to meet inclusion criteria by 1) relating to prostate cancer imaging; 2) relating to interventions to impact physician compliance with guidelines; and 3) having specific data describing the association between intervention and physician compliance. Papers about other malignancies or that had no intervention, evaluation, or data were excluded. Excluded papers were used for context in the background and discussion sections. The patient population, intervention, primary impact group, primary outcomes, methods, and limitations, were manually extracted from the relevant papers and summarized for comparison (Table 1).
Table 1.
Summary of literature describing interventions to improve physician compliance to prostate cancer imaging guidelines and associated outcomes15,23–26
| Intervention | Study methods summary | Authors; year | n | Statistical significance | Baseline rate of low-risk scanning | Reduction in low-risk PCa scans | Absolute risk reduction | Number needed to treat | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Education, champion, feedback | Imaging patterns compared before & after multistep performance feedback & guideline review led by practice clinical champion | Ross I, et al; 201523 | 813 | p=0.03 (BS) p=0.17 (CT) |
3.7% (BS) 5.2% (CT) |
65% (BS) 38% (CT) |
2.4% (BS) 2% (CT) |
42 (BS) 50 (CT) |
100% of scans were negative; 19 practices in Michigan |
| Education, champion, feedback | Baseline imaging rates compared before & after year of champion-led education & performance feedback with site visits | Hurley P, et al; 201715 | 10,554 | p<0.0001 (BS and CT) | 11.0% (BS) 14.7% (CT) |
41% (BS) 48% (CT) |
4.5% (BS) 7% (CT) |
23 (BS) 15 (CT) |
98.4% (BS) and 99.7% (CT) of scans were negative. Increase in indicated imaging also observed (not statistically significant) |
| Education, champion, feedback | Baseline phase followed by 2 phases with champion-led comparative performance feedback & guideline education | Miller DC, et al; 201124 | 858 | p<0.01 (BS and CT) | 31% (BS) 28% (CT) |
48% (BS) 54% (CT) |
15% (BS) 15% (CT) |
7 (BS) 7 (CT) |
97.6% (BS) and 96.4% (CT) of scans were negative. Reduced practice variation & improved guideline adherence |
| Education, champion, feedback | Local champion led guideline education. Participating urologists staging practices audited & presented (deidentified) | Rutledge AB, et al; 201826 | 144 | p=0.84 (BS) p=0.01 (CT) |
21.4% (BS) 42.9% (CT) |
15% (BS) 100% (CT) |
3.2% (BS) 42.9% (CT) |
32 (BS) 3 (CT) |
Only low-risk patients reflected here; this Australian study also reported reduced scans among intermediate-risk patients |
| EMR CROC | Imaging rates per VA claims compared before & after CROC implementation | Ciprut SE, et al; 202025 | 433 | p=0.001 (BS and CT combined) | 35% (BS and CT combined) | 46% (BS and CT combined) | 16% (BS & CT combined) | 7 (BS & CT combined) | BS & CT data not delineated |
BS: bone scan; CROC: clinical reminder order check; CT: computed tomography; EMR: electronic medical record; PCa: prostate cancer; VA: Veterans Affairs.
Results
Of 82 papers screened, five studies met inclusion criteria (Figure 1), with a total of 12 802 patients.15,23–26 Findings are summarized in Table 1. Each of the included studies demonstrated statistically significant post-intervention improvement in physician compliance to imaging guidelines for staging low-risk prostate cancer.15,23–26 As outlined in Table 2, four of the five initiatives were multidimensional, with education (some form of learning made available for urologists and/or patients to inform management), clinical champions (leadership among urologists in implementing and promoting adherence to imaging guidelines), and some form of performance feedback (reporting to help urologists understand their level of compliance).15,23,24,26 The only unidimensional intervention studied was a clinical reminder order check (CROC) built into the electronic medical record (EMR).25 Four of the five studies were performed in the U.S., the other being performed in Australia.
Figure 1.
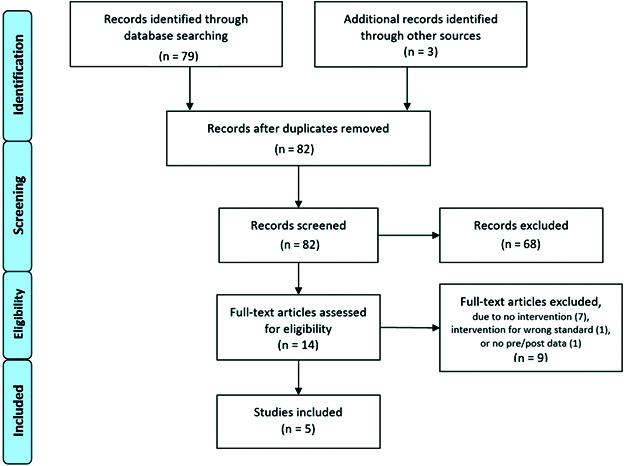
PRISMA flowchart of publication selection for inclusion.
Table 2.
| Authors; ear | Educational intervention | Local clinical champion intervention | Feedback intervention |
|---|---|---|---|
| Ross I, et al; 201523 | Collaborative-wide meetings reviewed imaging guidelines & presented baseline de-identified practice-level data | Champions given practice- and physician-level data; reviewed with colleagues along with relevant guideline recommendations | General performance feedback given at collaborative-wide meetings; individual data given to local clinical champion |
| Hurley P, et al; 201715 | Educational toolkits with placards for posting in each practice’s clinics, and scripts to assists in patient education regarding guideline rational | Champions received collaborative-wide training, led local presentations with prepared slides, and were encouraged to engage high-intensity imaging users | Champions encouraged to periodically review and present the practice’s imaging performance data |
| Miller DC, et al; 201124 | Summary of AUA & NCCN guidelines included in data collection form; guidelines reviewed locally | Champions led local meetings and reviewed guidelines with partners | Three data-collection phases, with de-identified performance feedback provided between phases |
| Rutledge AB, et al; 201826 | Local meetings reviewed guidelines and underpinning evidence; written information distributed | Champion chaired educational meetings and presented local baseline staging practices data | De-identified baseline staging practices & internal metrics presented at regular meetings |
AUA: American Urological Association; BS: bone scan; CT: computed tomography; EAU: European Association of Urology; NCCN: National Comprehensive Cancer Network.
Regardless of intervention or country, each of the included studies demonstrated statistically significant post-intervention improvement in physician compliance to guidelines.15,23–26 Additionally, while the studies focused on reduction of unnecessary imaging, one also accounted for increasing indicated imaging.15 In this case, the metric improved after intervention, but without statistical significance. 15 Baseline rates of guideline-discordant imaging of low-risk prostate cancer was variable among these studies, ranging from 3.7% to 31% (bone scans), and 5.2% to 42.9% (CT), while post-intervention rates ranged from 1.3% to 18.2% (bone scans), and from 0% to 13% (CT).23,24,26
The four studies using a three-pronged approach of education, local clinical champion, and performance feedback had varied applications of each of these elements (Table 2). While all of these studies used educational meetings to review guidelines,15,23 one study also used educational written materials,26 and another included a guideline summary in the data collection forms.24 One study reinforced its educational intervention with educational materials and toolkits developed with placards to be posted in the clinics of each practice, and scripts provided to assist participating urologists in educating patients regarding the rationale for imaging recommendations.15 This study was also the only one to explicitly note specific encouragement of clinical champions to interact with colleagues who demonstrated more high intensity imaging use.15 In all of these studies, clinical champions universally led their local practice with performance feedback data at the practice and/or individual level.15,23,24,26
The studied EMR CROC intervention was different from the other studied interventions, as there was no champion or performance feedback component. Rather, an alert text window came up when a guideline-discordant bone scan, CT, or MRI was ordered, determined by the system if the patient had both a diagnosis of prostate cancer (ICD-9: 185; ICD-10: C61) and a prostate-specific antigen (PSA) reading <20 ng/mL in the preceding six months.25 The resulting alert sited guidelines from several professional guiding bodies and displayed the following text: “Imaging not recommended to stage men with PSA<10, Gleason<7, and clinical stage <T3. Imaging recommended for high-risk cancer. Excessive imaging may harm patients and waste resources.”25 Although results in this study were not separated out by type of scan, low-risk guideline-discordant imaging was reduced from 35% to 19% (p=0.001).25
Discussion
Prior studies have shown low compliance with imaging guidelines in low-risk prostate cancer patients. A retrospective study using SEER-Medicare data from 2004–2011 exemplified this, and seems to suggest this challenge is not universal among cancers, finding that non-recommended staging imaging was performed in 41% of early prostate cancer cases while a comparable measure for breast cancer was only 14%.20 While the mechanisms driving these decisions remain unclear, this study indicated that physician use of non-recommended services seemed to be attributable to consistent behavior more than to personalized patient care.20 Ironically, at the same time, recommended imaging for high-risk prostate cancer has been underused, with as much as 51% of patients not receiving indicated CT/ MRI imaging at a national level.19,21
Patients undergoing guideline-discordant imaging incur exposure not only to the radiation from the unnecessary imaging study, but also from followup imaging due to incidental findings. For example, one study reported that >8% of low-risk prostate cancer patients received followup radiographic imaging because of incidental findings in guideline-discordant imaging, none of which resulted in suspicious lesions requiring intervention or biopsy.27 The median exposure of a single, multiphase, abdominal and pelvis CT scan was reported to be 31 milli-sieverts (mSv), a dose estimated to cause cancer in one of every 660 exposed 60-year-old men.28 In addition to increased exposure and risk, guideline-discordant imaging costs the U.S. millions each year.21
It is important to understand if these trends are driven by local practices, individual physicians, or patients. Studies investigating questions like these have added depth and direction to large-scale efforts to improve cost-effective care. For example, a study exploring decision drivers leading to prostate cancer staging imaging in low-risk patients found evidence that physicians are the primary (and sometimes only) decision-makers, suggesting that interventions may most effectively reduce unnecessary scanning if physician behavior is targeted.16 Indeed, guideline-discordant scanning was reduced following such targeted interventions using either of two approaches, namely an EMR-based CROC and a multipronged approach that simultaneously included education, performance feedback, and clinical champions.
Geography may also influence these decisions, as suggested by SEER-Medicare imaging rates for the generally non-overlapping patient populations with prostate and breast cancers, despite their largely exclusive management providers.29 Compelling enough were these geographic associations that the study authors recommended that policy efforts for the Choosing Wisely campaign be focused on high-use geographic areas.29 Of the studies represented in our review, three were regional and/or statewide, within the state of Michigan.15,23,24 The remaining two studies, including a similar multidimensional approach and a CROC, were in Australia and New York, respectively.25,26 Positive outcomes in all five studies, regardless of location, size of targeted implementation group, or baseline use rates, is encouraging, suggesting significant ability to improve guideline adherence through similar interventional approaches regardless of geography, scale, or initial compliance levels. Yet unexplored is the cumulative effect of these varied interventions applied concurrently.
Given the limited number of studies and their diverse context of application, interventional differences, and varied rates of guideline adherence at baseline, it is difficult to clearly identify one interventional approach as superior to another. For example, while a larger absolute rate reduction (ARR) equates to a smaller number needed to treat and might suggest a more effective intervention, it is directly limited by the baseline rate of imaging, which ranges widely in these studies (3.7–42.9%).23,26 Thus, while the relatively large 16% ARR observed in one study represents only 46% from a 35% baseline imaging rate, the much smaller 2.4% ARR observed in another study represents a massive 65% reduction from its 3.7% baseline.23,25 As all five studies resulted in favorable outcomes with statistical significance, these metrics obfuscate the ranking of approaches in terms of superiority.
In considering how a program might track its response to an intervention, a systematic, automated method can optimize sensitivity and timely feedback. In the specific case of assessing imaging guideline adherence for newly diagnosed prostate cancer, the exponentially weighted moving average (EWMA) has been recommended, as it required statistically less time to signal a change in performance than other statistical process control methods.30
Regarding limitations, none of the five studies included a control group or randomization, thus causality was difficult to prove. Bias risk was only noted in two of the papers as a limitation; however, all four of the studies implementing education, performance feedback, and clinical champions inherently raised awareness of the initiatives by using these interventional approaches. This awareness introduces bias risk due to the Hawthorne effect, or the alteration of behavior by those being studied due to their awareness of being observed. In the case of the CROC study, it was noted that the study excluded a large percentage of cases, raising concern for selection bias. The authors of the study argued that this was unlikely to bias findings, however, as the pre- and post-intervention exclusion rates were similar (66% vs. 63%).31
Additionally, with only five published studies documenting any intervention to promote adherence to these guidelines, and no negative outcomes reported, we cannot conclude that these are the only two effective approaches to this challenge. There may be other untried and/or undocumented interventions that would be high-yield, and/or publication bias obscuring our knowledge of unsuccessful attempts. Further research is needed to assess other interventions that may be taken, and as institutions adopt similar or novel approaches to impacting their physicians’ compliance to these imaging guidelines, communication about these efforts and their outcomes should be encouraged to promote success among future implementing institutions.
Conclusions
Improved physician compliance to imaging guidelines for staging prostate cancer has been associated with both EMR-based CROC and combination interventions using clinical champions, education, and feedback. Post-intervention improvement in compliance has been observed at individual institutions and larger organizations spanning a region or state. No studies lacking associated improvement in compliance after intervention were identified, perhaps due to publication bias, and perhaps suggesting additional unexplored and effective solutions to this challenge. We recommend that institutions apply similar or new interventions to seek decreased inefficient variations in care on a larger scale. As they do so, documentation and publication of these efforts to communicate both successful and unsuccessful strategies may help further clarify optimal conditions to promote physician compliance to guidelines.
Footnotes
Competing interests: The authors do not report any competing personal or financial interests related to this work.
This paper has been peer-reviewed.
References
- 1.Larson SM. Better use of bone scans in prostate cancer. Nat Rev Urol. 2015;12:190–1. doi: 10.1038/nrurol.2014.305. [DOI] [PubMed] [Google Scholar]
- 2.Lu YM, Chien TM, Ke HL, et al. The most suitable guidelines for performing bone scans in prostate cancer staging – one southern Taiwan medical center’s results. Urol Sci. 2016;27:208–11. doi: 10.1016/j.urols.2015.06.287. [DOI] [Google Scholar]
- 3.Kanda Swamy GV, Bennett A, Narahari K, et al. Establishing the pathways and indications for performing isotope bone scans in newly diagnosed intermediate risk localized prostate cancer – results from a large contemporaneous cohort. BJU Int. 2017;120:E59–63. doi: 10.1111/bju.13850. [DOI] [PubMed] [Google Scholar]
- 4.Preisser F, Mazzone E, Nazzani S, et al. North American population-based validation of the National Comprehensive Cancer Network practice guideline recommendations for locoregional lymph node and bone imaging in prostate cancer patients. Br J Cancer. 2018;119:1552–6. doi: 10.1038/s41416-018-0323-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanaka N, Fujimoto K, Shinkai T, et al. Bone scan can be spared in asymptomatic prostate cancer patients with PSA of ≤20 ng/ml and Gleason score of ≤6 at the initial stage of diagnosis. Jap J Clin Oncol. 2011;41:1209–13. doi: 10.1093/jjco/hyr118. [DOI] [PubMed] [Google Scholar]
- 6.Hirobe M, Takahashi A, Hisasue S, et al. Bone scanning – who needs it among patients with newly diagnosed prostate cancer? Jap J Clin Oncol. 2007;37:788–92. doi: 10.1093/jjco/hym097. [DOI] [PubMed] [Google Scholar]
- 7.Mcarthur C, Mclaughlin G, Meddings RN. Changing the referral criteria for bone scan in newly diagnosed prostate cancer patients. Br J Radiol. 2012;85:390–4. doi: 10.1259/bjr/79184355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Ghazo MA, Ghalayini IF, Al-Azab RS, et al. Do all patients with newly diagnosed prostate cancer need staging radionuclide bone scan? a retrospective study. Int Braz J Urol. 2010;36:685–92. doi: 10.1590/S1677-55382010000600006. [DOI] [PubMed] [Google Scholar]
- 9.Lee SH, Chung MS, Park KK, et al. Is it suitable to eliminate bone scan for prostate cancer patients with PSA ≤20 ng/mL? World J Urol. 2012;30:265–9. doi: 10.1007/s00345-011-0728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohler JL, Antonarakis ES, Armstrong AJ, et al. Prostate Cancer, Version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2019;17:479–505. doi: 10.6004/jnccn.2019.0023. [DOI] [PubMed] [Google Scholar]
- 11.Ma MW, Gao XS, Lyu F, et al. Development of a nomogram predicting metastatic disease and the assessment of NCCN, AUA, and EAU guideline recommendations for bone imaging in prostate cancer patients. World J Urol. 2021;39:1815–23. doi: 10.1007/s00345-020-03363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer 2020v4. [Accessed March 26, 2021]. Available at: https://uroweb.org/wp-content/uploads/EAU-EANM-ESTRO-ESUR-SIOG-Guidelines-on-Prostate-Cancer-2020v4.pdf.
- 13.American Urological Association. [Accessed September 8, 2021];Prostate cancer: Clinically localized guideline. Available at: https://www.auanet.org/guidelines/guidelines/prostate-cancer-clinically-localized-guideline. [Google Scholar]
- 14. [Accessed September 8, 2021];EAU-ESUR-ESTRO-SIOG guidelines on prostate cancer. Available at: https://uroweb.org/wp-content/uploads/EAU-ESUR-ESTRO-SIOG-Guidelines-on-Prostate-Cancer-large-text-V2.pdf. [Google Scholar]
- 15.Hurley P, Dhir A, Gao Y, et al. A statewide intervention improves appropriate imaging in localized prostate cancer. J Urology. 2017;197:1222–8. doi: 10.1016/j.juro.2016.11.098. [DOI] [PubMed] [Google Scholar]
- 16.Makarov DV, Sedlander E, Braithwaite RS, et al. A qualitative study to understand guideline-discordant use of imaging to stage incident prostate cancer. Implement Sci. 2016;11:118. doi: 10.1186/s13012-016-0484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palvolgyi R, Daskivich TJ, Chamie K, et al. Bone scan overuse in staging of prostate cancer: An analysis of a veterans affairs cohort. Urology. 2011;77:1330–6. doi: 10.1016/j.urology.2010.12.083. [DOI] [PubMed] [Google Scholar]
- 18.Makarov DV, Hu EYC, Walter D, et al. Appropriateness of prostate cancer imaging among veterans in a delivery system without incentives for overutilization. Health Services Res. 2016;51:1021–51. doi: 10.1111/1475-6773.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falchook AD, Hendrix LH, Chen RC. Guideline: Discordant use of imaging during workup of newly diagnosed prostate cancer. JOP. 2015;11:e239–46. doi: 10.1200/JOP.2014.001818. [DOI] [PubMed] [Google Scholar]
- 20.Lipitz-Snyderman A, Sima CS, Atoria CL, et al. Physician-driven variation in non-recommended services among older adults diagnosed with cancer. JAMA Intern Med. 2016;176:1541. doi: 10.1001/jamainternmed.2016.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falchook AD, Salloum RG, Hendrix LH, et al. Use of bone scan during initial prostate cancer workup, downstream procedures, and associated Medicare costs. Int J Radiat Oncol Biol Phys. 2014;89:243–8. doi: 10.1016/j.ijrobp.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winn AN, Kelly M, Ciprut S, et al. The cost, survival, and quality-of-life implications of guideline-discordant imaging for prostate cancer. Cancer Reports. 2022;5:e1468. doi: 10.1002/cnr2.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross I, Womble P, Ye J, et al. MUSIC: Patterns of care in the radiographic staging of men with newly diagnosed low risk prostate cancer. J Urol. 2015;193:1159–62. doi: 10.1016/j.juro.2014.10.102. [DOI] [PubMed] [Google Scholar]
- 24.Miller DC, Murtagh DS, Suh RS, et al. Regional collaboration to improve radiographic staging practices among men with early stage prostate cancer. J Urol. 2011;186:844–9. doi: 10.1016/j.juro.2011.04.078. [DOI] [PubMed] [Google Scholar]
- 25.Ciprut SE, Kelly MD, Walter D, et al. A clinical reminder order check (CROC) intervention to improve guideline-concordant imaging practices for men with prostate cancer: A pilot study. Urology. 2020;145:113–9. doi: 10.1016/j.urology.2020.05.101. [DOI] [PubMed] [Google Scholar]
- 26.Rutledge AB, McLeod N, Mehan N, et al. A clinician-centered program for behavior change in the optimal use of staging investigations for newly diagnosed prostate cancer. BJU Int. 2018;121:22–7. doi: 10.1111/bju.14144. [DOI] [PubMed] [Google Scholar]
- 27.Lavery HJ, Brajtbord JS, Levinson AW, et al. Unnecessary imaging for the staging of low-risk prostate cancer is common. Urology. 2011;77:274–8. doi: 10.1016/j.urology.2010.07.491. [DOI] [PubMed] [Google Scholar]
- 28.Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169:2078–86. doi: 10.1001/archinternmed.2009.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makarov D, Sen S, Soulos P, et al. Mp11-06 regional-level inappropriate imaging rates for prostate and breast cancers are correlated: Potential lessons for the Choosing Wisely campaign. J Urol. 2014;191:e97–e97. doi: 10.1016/j.juro.2014.02.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inadomi M, Singh K, Qi J, et al. Prospective monitoring of imaging guideline adherence by physicians in a surgical collaborative: Comparison of statistical process control methods for detecting outlying performance. BMC Med Inform Decis Mak. 2020;20:89. doi: 10.1186/s12911-020-1126-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ciprut SE, Kelly MD, Walter D, et al. Author reply. Urology. 2020;145:119. doi: 10.1016/j.urology.2020.05.103. [DOI] [PubMed] [Google Scholar]


