Abstract
Insertion sequences (ISs) and other transposable elements are associated with the mobilization of antibiotic resistance determinants and the modulation of pathogenic characteristics. In this work, we aimed to investigate the association between ISs and antibiotic resistance genes, and their role in the dissemination and modification of the antibiotic-resistant phenotype. To that end, we leveraged fully resolved Enterococcus faecium and Enterococcus faecalis genomes of isolates collected over 5 days from an inpatient with prolonged bacteraemia. Isolates from both species harboured similar IS family content but showed significant species-dependent differences in copy number and arrangements of ISs throughout their replicons. Here, we describe two inter-specific IS-mediated recombination events and IS-mediated excision events in plasmids of E. faecium isolates. We also characterize a novel arrangement of the ISs in a Tn1546-like transposon in E. faecalis isolates likely implicated in a vancomycin genotype–phenotype discrepancy. Furthermore, an extended analysis revealed a novel association between daptomycin resistance mutations in liaSR genes and a putative composite transposon in E. faecium , offering a new paradigm for the study of daptomycin resistance and novel insights into its dissemination. In conclusion, our study highlights the role ISs and other transposable elements play in the rapid adaptation and response to clinically relevant stresses such as aggressive antibiotic treatment in enterococci.
Keywords: antibiotic resistance gene, composite transposon, daptomycin, enterococcus, insertion sequences, Oxford nanopore sequencing, WGS
Data Summary
All supplementary tables are available on Zenodo: https://zenodo.org/record/6402290#.YkXJzSSZM-w under DOI:10.5281/zenodo.6402290. Figs S6, S7 and S9 are available on Zenodo: https://doi.org/10.5281/zenodo.6513079 under DOI: 10.5281/zenodo.6513079
Raw sequencing data, genome assemblies and functional annotations for the isolates described in this study are available under the BioProject accession number PRJNA735268 in the National Center for Biotechnology Information (NCBI) database.
Impact Statement.
Insertion sequences (ISs) and transposable elements are associated with the mobilization of antibiotic resistance determinants (ARDs) and the modulation of pathogenic characteristics; however, there is a lack of information on the association between ISs and ARDs and other aspects related to the nature, maintenance and transmission mechanisms of ISs in bacterial genomes. In this work, we present six isolates from two bacterial species, sharing the same niche (a patient with bacteraemia) and collected over a 4-day period. The isolates from both species showed a very similar IS family content, but a drastically different copy number and arrangement of ISs along their replicons. In this work we describe several IS-mediated rearrangements, deletions and two putative IS-mediated recombination events between Enterococcus faecalis and Enterococcus faecium species. We also describe a previously undescribed association between daptomycin resistance mutations in liaSR genes and a putative compound transposon in E. faecium genomes. Our results highlight the important role ISs and other transposable elements play in the rapid genomic adaptation and response of enterococci to clinically relevant stresses.
Introduction
Enterococcus spp. are ubiquitous bacteria that live as commensals in the gastrointestinal tract of humans and other mammals [1, 2]. Their genetic plasticity enables them to acquire genetic determinants [3] to thrive in modern hospital environments, where they often cause severe infectious diseases, especially in immunocompromised patients [2, 4]. Multidrug-resistant enterococci not only play an important role as nosocomial pathogens, but also are a reservoir of genes encoding antibiotic resistance that can be transferred to other commensal micro-organisms, increasing the risk of colonization and infection [4, 5].
In recent years, vancomycin-resistant Enterococcus (VRE) infection cases have increased rapidly around the world. Consequently, a number of studies have examined the epidemiology [6, 7], population dynamics [8–10] and evolutionary landscape [11–13] of Enterococcus . However, there is still a lack of knowledge about the level of influence that plasmids and mobile genetic elements have on the persistence and spread of VRE strains [14–16]. This is partly due to the presence of repetitive regions in genomes of Enterococcus that hinder full genome reconstruction [17, 18]. Some of these repetitive sequences, such as insertion sequences (ISs), have been considered to be pathogenicity enhancers [5, 19, 20]. Therefore, analysing these elements is of vital importance to determine the key characteristics of VRE strains, such as the level of antimicrobial resistance and virulence potential, or to identify pathogenic variants.
ISs are small autonomous transposable elements, generally flanked by short terminal inverted repeats, that usually carry at least one transposase (Tn) gene whose product mediates the transposition process (Fig. 1a) [21, 22]. ISs are not only able to move themselves to different locations within genomes through various mechanisms [23], but are also able to travel horizontally between genomes as part of other mobile genetic elements such as bacteriophages and plasmids [21, 24]. Transposases are mainly classified according to their transposition chemistry related to their catalytic domain topology and also based on their mode of transposition (replicative or non-replicative) [22–24]. Furthermore, DNA segments carrying several genes can move together as a single unit when flanked at both ends by two ISs in the same or a properly oriented direction [21, 22]. These larger transposable units are called compound or composite transposons (Fig. 1a). Well-known examples of composite transposons that carry antibiotic resistance determinants (ARDs) are Tn5 (kanamycin resistance) [25], Tn9 (chloramphenicol resistance) [26], Tn10 (tetracycline resistance) [27] and Tn4001 (gentamicin resistance) [28]. The important roles of ISs in the transmission, inactivation and modification of the resistant genotype and virulence of prokaryotic strains have been reviewed in previous studies [21, 24]. However, most of these studies were performed using contigs or scaffolds from genome assemblies that might be missing a number of repetitive ISs.
Fig. 1.
(a) Schematic representations of an insertion sequence and a composite transposon. The IS elements in the composite transposon are represented according to the orientation of their encoded transposase. Both IS elements can also be oriented inversely. (b, c) Distribution of IS occurrences in (b) 252 E. faecium complete genome sequences and (c) 79 E. faecalis using ISEScan. The y-axis indicates the density of each IS frequency, where density is approximately the number of occurrences for a given IS divided by the range of IS frequencies (210 for E. faecium, 69 for E. faecalis ). The sum of the density values of the plot is equal to 1. The x-axis represents the count of ISs present in all genomes of the corresponding species. The means of the number of ISs annotated in the three E. faecalis (b) and in the three E. faecium isolates (c) from this study are marked with a red dashed line. (d) Schematic representation of a novel transposable element found in E. faecalis isolates from this study. This figure was generated using BioRender.
It has been established that environmental stresses can induce elevated rates of IS transposition activities, also known as ‘transposition bursts’ [29]. However, a comprehensive analysis of the transposase activity among isolates that persist in the same stressful environment, such as high levels of antibiotic exposure, is lacking. In the same vein, it is not clear what role transposable elements play in large-scale genome variations due to IS-mediated genetic rearrangements and their association with ARDs in bacterial cells. This study was conducted using full genome assemblies from six isolates, collected from the blood of an inpatient with bloodstream co-infection of Enterococcus and diagnosed with acute lymphocytic leukaemia. We performed genome-wide computational analysis in order to provide insights into the landscape of transposable elements that could be involved in disseminating multidrug resistance. Our analyses were focused on the ISs found in the proximity of ARDs and on the genetic changes observed as a consequence of putative transposition activity at the species and inter-species level.
Methods
Sample collection and vancomycin susceptibility testing
The six blood samples were collected at different time points from an inpatient at the University of Arkansas for Medical Sciences (UAMS). This study involved blood samples which were sent to the Clinical Microbiology Laboratory for clinical use, not for this study. Therefore, there was no direct contact with the study participants. Cultures were grown on blood agar plates and processed on the BacT/ALERT 3D system (bioMérieux, Durham, NC, USA). The Accelerate Pheno system (Accelerate Diagnostics, Tucson, AZ, USA) was used for the identification and susceptibility testing of Enterococcus -positive blood cultures. For repeated positives, identification and susceptibility testing was confirmed using Vitek 2 MS and Vitek 2 systems. The vancomycin-resistant phenotype of the six isolates was obtained using E-tests (bioMérieux) and antimicrobial susceptibility tests. Results were interpreted using the M100 Clinical and Laboratory Standards Institute (CLSI) standards [30].
Whole-genome sequencing
Genomic DNA was extracted and sequenced as described elsewhere [31, 32]. Briefly, genomic DNA was extracted from VRE colonies using the Quick-DNA Fungal/Bacterial kit (Zymo Research, Irvine, CA, USA). A NanoDrop Spectrophotometer (Thermo Fisher, Waltham, MA, USA) was used to determine the purity of the extracted DNA by measuring the A260/280 and A260/230 ratios. The integrity and quantity of DNA were determined using an Agilent 2200 TapeStation (Agilent, Santa Clara, CA, USA) and Qubit 3.0 assay (Thermo Fisher), respectively. Purified DNA was aliquoted into two tubes for MinION (Oxford Nanopore Technologies, Oxford, UK) and Illumina (San Diego, CA, USA) sequencing. Oxford Nanopore Technologies (ONT) sequencing libraries were prepared using a PCR-free method of multiplexing samples with the Rapid Barcoding kit (SQK-RBK004, ONT), and sequencing of the barcoded DNA was performed on a single R9.4/FLO-MIN106 ONT flow cell on the MinION (version Mk1B, ONT) for 48 h. Illumina libraries for the six isolates were also sequenced using the NextSeq 550 platform at the UAMS Myeloma Center. Adapters were trimmed using fastp (version 0.19.5) [33], with default settings. Trimmomatic (version 0.38) [34] was used to remove poor-quality reads. The quality of pre- and post-processed reads was assessed with the FastQC tool (version 0.11.8) [35].
Base calling and assembling
Base calling of Nanopore reads was performed using Guppy v4.4.2 [36], with --min_qscore 8. Libraries were demultiplexed using guppy_barcoder from Guppy v4.4.2. Porechop v0.2.3 (https://github.com/rrwick/Porechop) was used to remove adapters, and NanoFilt from NanoPack [37] was used for further quality filtering. Read quality was examined with NanoStat v1.2.0 from NanoPack [37]. Two different strategies were established to obtain fully resolved assemblies depending on the species. For E. faecium isolates, we used Unicycler v0.4.8 [38] hybrid assembler, with scaffolds generated by Canu v2.1.1 [39] and Illumina data as inputs. Fully resolved assemblies for E. faecalis isolates were obtained using Flye v2.8.2 assembler [40] with Oxford Nanopore reads, with two rounds of polishing using the Oxford Nanopore long-reads with Racon v1.4.20 [41] and Medaka v1.2.1 (https://github.com/nanoporetech/medaka). For both E. faecium and E. faecalis , final polishing with Illumina data was performed through two rounds of Pilon v1.23 [42].
Functional antimicrobial resistance and mobilome annotation
Functional annotation of the six isolates was performed using Prokka v1.14.6 [43] with the --rnammer option. Functional annotation of plasmid replicons and other chromosomal regions of interest was manually reannotated using PsiBlast and blastp from the National Center for Biotechnology Information (NCBI) and Enterococcus nr/nt databases. ARDs were annotated with Resistance Gene Identifier (RGI) software against the Comprehensive Antibiotic Resistance Database (CARD) [44]. ISEScan v1.7.2.1 [45] and the ISfinder database (https://isfinder.biotoul.fr/) [46] were used to identify transposable elements and their inverted repeat sequences. Plasmids were classified using PlasmidFinder2.0 [47]. CGview server (version beta) [48] was used to plot the functional and structural characteristics of the plasmids in this study.
Multilocus sequence typing (MLST)
MLST analysis was performed using mlst tool (T. Seemann, mlst Github https://github.com/tseemann/mlst) with public PubMLST typing schemes [49].
Comparative genome analysis
All replicons were divided into fragments of 5000 bp with an overlap of 3000 bp (2 000 step size) and compared via blastn v2.9.0 [50]. Bidirectional best hits between replicons from different isolates were identified using best bit score, e-value and percentage of sequence similarity. Mash v2.3 [51] with k=21 was used to determine genetic distances between isolates where sketch option with -s 10 000 was used to increase the sensitivity as described elsewhere [52]. Snippy (https://github.com/tseemann/snippy) was used to analyse the coreSNP of the six isolates. Figures were prepared using Clinker v0.0.20 [53], pyGenomeTracks [54] and BioRender (https://biorender.com/).
The genome sequences of the six isolates were aligned using minimap2 [55] with parameter settings of -x asm5 and then visualized using dotPlotly using the parameters -m 1000 -q 1000 s -t -l (https://github.com/tpoorten/dotPlotly). Artemis Comparison Tool (release 18.0.1) [56] was used to visualize the blastn alignments of the replicon sequences of the six isolates by species. Multiple genome alignments of the entire replicons was performed using Mugsy software v1.2.3 [57]. Multiple genome alignments of selected replicons were visualized using Gmaj software (https://globin.bx.psu.edu/dist/gmaj/).
All complete genomes available in GenBank for E. faecalis and E. faecium species were downloaded in December 2020.
Results
Fully resolved replicons in E. faecalis and E. faecium isolates obtained from hybrid de novo assembly
The six Enterococcus isolates from one patient with prolonged bacteraemia were collected from blood samples over a period of 4 days and identified as E. faecalis (isolates UAMS_EL53, UAMS_EL54 and UAMS_EL56) or E. faecium (UAMS_EF55, UAMS_EF57 and UAMS_EF58). Vancomycin susceptibility testing showed a susceptible phenotype for all E. faecalis isolates [minimum inhibitory concentration (MIC) ≤2 µg ml−1]. However, all E. faecium isolates were phenotypically resistant to vancomycin, with an MIC >16 µg ml−1 for the first two isolates and an MIC ≥64 µg ml−1 for the last collected isolate (UAMS_EF58) (Table S1). Two different de novo hybrid assembly strategies, depending on species (described in the Methods section) were used to generate fully resolved assemblies for all six isolates. Thus, complete circular replicons were obtained for all the chromosomes and for almost all of the plasmids for the six isolates in this study (Table 1).
Table 1.
Summary of de novo assembly results for E. faecalis and E. faecium isolates
|
Isolate |
UAMS_EL53 |
UAMS_EL54 |
UAMS_EL56 |
|---|---|---|---|
|
Collection order |
1 |
2 |
4 |
|
Species |
|||
|
Chromosome |
3 030005 c |
3 030422 c |
3 031832 c |
|
pUAMSEL1 |
119720 c |
119714 c |
119713 c |
|
pUAMSEL2 |
55301 c |
55304 c |
55288 c |
|
pUAMSEL3 |
53420 c |
53047 c |
53193 c |
|
PUAMSEL4 (vanA ) |
33652c×3 |
33656c×3 |
33659c×3 |
|
Isolate |
UAMS_EF55 |
UAMS_EF57 |
UAMS_EF58 |
|
Collection order |
3 |
5 |
6 |
|
Species |
|||
|
Chromosome |
2 841245 c |
2 841244 c |
2 841249 c |
|
pUAMSEF1(a,b) |
a.264192 c |
b.205436 c |
b.205407 c |
|
pUAMSEF2 |
79 782 |
79 769 |
79 679 |
|
pUAMSEF3 |
* |
57509 c |
57509 c |
|
pUAMSEF4 (vanA ) |
41819c×3 |
41819c×3 |
34693c×3 |
|
pUAMSEF5 |
4374 c |
4374 c |
4374 c |
c, circularized replicon according to assembler; ×3, inferred multiplicity based on normalized read depth per contig.
*, plasmid not detected.
Chromosome size for E. faecalis isolates [classified as ST6 by multilocus sequence typing (MLST)] were slightly larger (~3 Mb) than those in E. faecium isolates (~2.8 Mb) (classified as ST736 by MLST). Four to five complete plasmids were identified in all isolates (Table 1, Fig. S1). All six isolates harboured plasmids (pUAMSEF4 for E. faecium isolates and pUAMSEL4 for E. faecalis isolates) with a complete cluster of genes conferring vancomycin resistance (vanR, vanS, vanH, vanA, vanX, vanY and vanZ; phenotype vanA), revealing a genotype–phenotype discrepancy in E. faecalis isolates. It is worth noting that plasmids carrying vancomycin resistance genes presented greater differences in read depth compared to the other replicons in this study. The multiplicity of vanA replicons was inferred to be triplicated in all assemblies based on the normalized read depth (Table 1). Plasmid sequence comparison analyses showed that the vanA plasmid from E. faecalis is most similar to the vanA plasmid from E. faecium (Fig. S2A). Whole-genome distances obtained using Mash [51] on the six isolates of the study showed that the last two collected isolates for each species (UAMS_EL56, UAMS_EF58) had the lowest genetic similarity to the other isolates of their respective species (Fig. S2b). Mash distances between isolates from different species were ~0.18.
Arrangement, copy number and association of insertion sequences with antibiotic resistance genes differ in E. faecalis and E. faecium isolates
Detailed analysis of the ISs annotated by ISEScan [45] and ISfinder [46] in our 6 isolates identified 11 different IS families in E. faecalis isolates, including 1 putative novel IS family, and 12 IS families in E. faecium isolates (Tables 2, S2 and S3). The copy number of the IS families identified in both species varied considerably, with these elements being 5 times more abundant in E. faecium isolates (~210 ISs per isolate) than in E. faecalis isolates (~46 ISs per isolate). In E. faecalis isolates, the IS256 family (n=12 in UAMS_EL53 and UAMS_EL54 and n=13 in UAMS_EL56) was the most abundant IS, followed by ISs from the IS6 (n=9 in each isolate) and IS3 families (n=9 in each isolate). In E. faecium isolates, the ISL3 family (n=61) was the most abundant, with elements mainly distributed throughout the chromosome, followed by elements from families IS256 (n=35) and IS30 (n=27). Sequences from the IS66 and IS1380 families were found in all E. faecium isolates but not in E. faecalis . The IS elements identified in isolates from both species were distributed evenly along the chromosome (Table S2), but they were also found distributed among three of the four identified plasmids in E. faecalis isolates and in four of the five plasmids in E. faecium . The copy number of the annotated ISs was stable in all three E. faecalis isolates with the exception of one additional element from the IS256 family identified in the chromosome of UAMS_EL56. This IS256 element was located in an area surrounded by other ISs from IS256 and IS6 families, in which the only characterized gene was choloylglycine hydrolase family protein (Table S3)
Table 2.
Summary of the number of annotated insertion sequences per isolate using ISEScan
|
IS family |
UAMS_EL53 |
UAMS_EL54 |
UAMS_EL56 |
UAMS_EF55 |
UAMS_EF57 |
UAMS_EF58 |
|---|---|---|---|---|---|---|
|
IS3 |
9 |
9 |
9 |
26 |
26 |
26 |
|
IS6 |
9 |
9 |
9 |
20 |
19 |
19 |
|
IS21 |
4 |
4 |
4 |
2 |
2 |
2 |
|
IS30 |
3 |
3 |
3 |
27 |
27 |
27 |
|
IS66 |
0 |
0 |
0 |
7 |
7 |
7 |
|
IS110 |
1 |
1 |
1 |
12 |
12 |
12 |
|
IS200/IS605 |
1 |
1 |
1 |
5 |
5 |
5 |
|
IS256 |
12 |
12 |
13 |
35 |
35 |
35 |
|
IS982 |
3 |
3 |
3 |
8 |
8 |
8 |
|
IS1182 |
1 |
1 |
1 |
6 |
6 |
5 |
|
IS1380 |
0 |
0 |
0 |
2 |
2 |
2 |
|
ISL3 |
1 |
1 |
1 |
61 |
61 |
61 |
|
New |
2 |
2 |
2 |
0 |
0 |
0 |
|
Total no. |
46 |
46 |
47 |
211 |
210 |
209 |
Genetic variability can be useful to withstand stressful conditions [24, 58]. Rates of IS transposition can be increased by environmental factors, such as changes in the host environment and the presence of subinhibitory concentrations of antibiotics that serve as a signal to promote genetic variability [59]. To place the number of ISs found in our isolates in a global context, we compared the distribution of the number of annotated ISs in a set of complete E. faecalis and E. faecium genomes downloaded from the GenBank and our six isolates. Fig. 1b indicates the distribution of the number of ISs annotated in 252 complete E. faecium genome assemblies where the mean of the number of ISs annotated in our three E. faecium isolates is represented with a red line at the extreme right of the distribution (Fig. 1b). The same pattern was observed in E. faecalis , where only 3 out of 79 complete genomes had more ISs than our clinical isolates (Fig. 1c). Both species showed a bimodal distribution of annotated ISs. This bimodal distribution was accentuated in E. faecium strains, where most of the annotated genomes had a relatively small number of annotated ISs (n≤50) or over 150 annotated ISs (n≥190). However, we could not recover metadata related to the environmental source of most of the strains in this analysis, which prevented us from establishing correlations between the number of annotated ISs and the environmental source.
Considering a genetic neighbourhood of 10 upstream and downstream annotated open reading frames (ORFs), 14 of 21 annotated ARDs were found in the proximity of at least 1 IS in our 3 E. faecalis isolates (Table S3). Further analysis of the possible association between ISs and ARDs identified a previously unknown TE of ~5781 bp; this TE occurred twice at two different locations (with >99 % sequence similarity, 100 % coverage) in the chromosome of all our E. faecalis isolates (Table S2). The novel transposable element harboured genes involved in its integration and excision, including genes for integrase, excisionase, replicase, a helix–turn–helix transcriptional regulator, methylated-DNA–protein-cysteine methyltransferase (MGMT) and trimethoprim-resistant dihydrofolate reductase (dfrF), which is involved in the resistance to diaminopyrimidine antibiotics (Fig. 1d). The number of cargo genes and the length of this novel element suggest that it is a unit transposon rather than an IS element. Unit transposons are larger than IS elements, as they may carry several passenger genes, usually ARDs, bound by inverted repeats [22, 60]. Sequence similarity analysis of the entire region of 5781 bp against the NCBI database showed that this novel transposable element is 100 % conserved and exists in multiple copies in the genomes of other E. faecalis , including 28157_4, 27725_1 and 27688_1 strains. These E. faecalis genomes were sequenced using long and short reads and assembled following a hybrid de novo strategy (NCBI BioProject: PRJEB40976 from the University Medical Center of Utrecht). This transposable element could therefore be more widespread in E. faecalis , but its existence might have been concealed due to the lack of high-quality and complete assemblies for this species. According to CARD Prevalence v3.0.8 data, which were based on sequence data acquired from NCBI on 13 August 2020, the prevalence of the dfrF ARD in E. faecalis is low and found in only 4.03 % of all whole-genome sequences in this database (818 E. faecalis genomes). However, the prevalence of this gene in E. faecium species was 27.59 % (1468 E. faecium genomes). Indeed, E. faecium isolates from our study harboured several copies of dfrF. Searches of the novel transposable element family found in our E. faecalis isolates against the E. faecium isolates of our study resulted in dfrF–dfrF gene alignments with >99 % sequence similarity and 100 % coverage. But this was not the case for the other genes described in the transposable element from E. faecalis isolates. Sequence-based searches using the web resource for prokaryotic transposable elements, TnCentral [61] and tn_dna.seq database, yielded one significant alignment with a region of the Tn559 family (subclass Tn554) from Staphylococcus aureus ST398 [62]. This novel element was the only IS found in the genetic neighbourhood (defined as 10 upstream and downstream annotated ORFs) of ARDs in the chromosome of E. faecalis isolates.
We observed a different scenario when analysing the genetic neighbourhood of ARDs in our E. faecium isolates, where 20 out of 21 annotated ARDs had at least 1 IS in their proximity (±10 annotated ORFs). We also observed that IS elements in our E. faecium isolates were generally arranged as arrays. IS arrays consist of agglomerations of ISs in which several copies from the same IS family are close to each other, suggesting a high level of replicative transposition [63]. Regarding their relationship with ARDs, we observed that the MFS transporter permease gene efmA was the only ARD in E. faecium that did not have IS elements in its proximity.
Differences in IS copy number between our three E. faecium isolates (Table 2, Table S2, Table S3) were directly related to reorganizations in modular plasmids caused by IS-mediated deletions in the form of composite transposons (Fig. 1a, Fig. 2). The conjugative plasmid pUAMSEF1a is a RepA_N megaplasmid (>260 kb) with a strikingly high number of IS sequences (n=59) (Table 1, Table S2, Fig. 2a) that was only present in the E. faecium isolate UAMS_EF55. Despite the high number of IS families identified in this plasmid, only one ARD (aminoglycoside acetyltransferase, aacA-aphD) was found in pUAMSEF1a. However, there was a high number of efflux pumps, transporters and phosphotransferase systems, along with several genes related to plasmid maintenance, replication and transfer, such as several toprim genes; DNA primases, topoisomerases and resolvases; conjugal proteins; tra genes; and type IV secretion system components. Although pUAMSEF1a was only identified in the UAMS_EF55 isolate, UAMS_EF57 and UAMS_EF58 harboured two smaller plasmids (pUAMSEF1b and pUAMSEF3), which resulted from the excision of pUAMSEF1a (Figs 2a and S3, Table S3). The smaller plasmid product of pUAMSEF1a excision consisted of the segment bounded by two ISs in the same orientation, from the IS6 family in pUAMEF1a (specifically, two IS1297 elements, first described in Leuconostoc mesenteroides subsp . dextranicum NZDRI2218) [64]. This circularized section constituted in its entirety the replicon pUAMSEF3 present in the two other E. faecium isolates (UAMS_EF57 and UAMS_EF58) that were collected just 1 and 2 days later, respectively, showing the high genetic activity of E. faecium isolates in this background. One of the two flanking IS6 elements was preserved in pUAMSEF1b (Fig. S4) after excision. However, both flanking IS6 elements were lost in the smaller plasmid pUAMSEF3 after the IS-mediated excision event. The plasmid pUAMSEF1b, found in UAMS_EF57 and UAMS_EF58 isolates, consisted of a RepA_N replicon of ~205 400 bp, which is the largest circularized segment remaining after the IS-mediated excision of pUAMSEF3 (orange inner ring in Fig. 2a). It is worth noting that another putative IS-mediated excision event was identified in the vancomycin resistance plasmid pUAMSEF4 in our E. faecium isolates; this is described in the section dedicated to the vancomycin plasmids.
Fig. 2.
(a) Plasmid pUAMSEF1a from the first collected isolate of E. faecium , UAMS_EF55. (b) Plasmid pUAMSEF3, product of IS-mediated excision from pUAMSEF1a, present in UAMS_EF57 and UAMS_EF58 isolates.
Daptomycin resistance mutations in Liasr genes are associated with a putative composite transposon in E. faecium isolates
Among the ISs inferred in the previous section, some ISs in the vicinity of ARDs in E. faecium were identified as potential composite transposons. Note that experimental evidence for transposition of these IS-bounded structures has not been provided in this work.
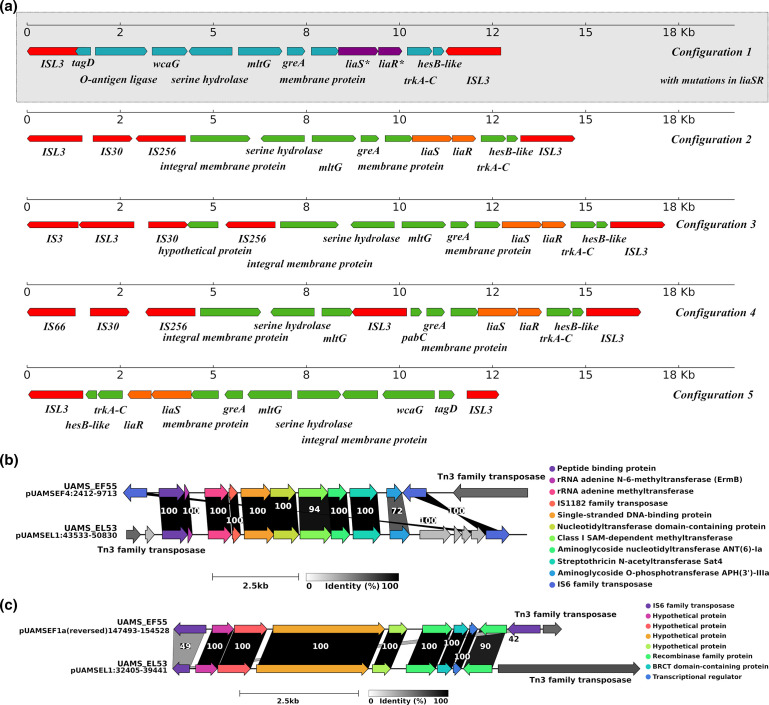
A noteworthy example of a putative composite transposon is the one found in the three E. faecium isolates formed by two ISL3 elements identically oriented surrounding liaSR genes. LiaFSR is a three-component regulatory system involved in cell envelope homeostasis. As previously reported in multiple studies [65, 66], amino acid substitutions in LiaR (W73C) and LiaS (T120A) are associated with daptomycin (DAP)-resistant phenotype in E. faecium . Notably, all of our E. faecium isolates carried the LiaRW73C and LiaST120A mutations. To test the hypothesis that there is an association between this ISL3-bounded structure and the presence of mutations associated with DAP resistance in liaSR, we extended our investigation to all complete, publicly accessible E. faecium genomes. Among 249 complete E. faecium genomes, we observed that 33 genomes carried both LiaRW73C and LiaST120A mutations. Consistently, all 33 genomes also contained the same ISL3-bounded structure found in our E. faecium isolates (Fig. 3a). In detail, this putative composite transposon is composed of 2 ISL3 sequences identically oriented surrounding 11 other genes in almost all cases (Figure S5, Table S4). The genes flanked by the ISL3 elements were identified as tagD, encoding a glycerol-3-phosphate cytidylyltransferase, which is central to the synthesis of teichoic acids in the cell wall of Gram-positive bacteria [67]; an O-antigen ligase family protein gene involved in lipopolysaccharide biosynthesis [68]; wcaG, encoding an epimerase; a serine hydrolase gene; mltG, encoding an inner membrane endolytic transglycosylase capable of cleaving at internal positions within a glycan polymer [66]; greA, encoding a transcription elongation factor involved in bacterial invasion in other genera [69]; a cell wall-active antibiotic-response protein gene; liaS and liaR; trkA-C, encoding a potassium-uptake protein; and hesB, encoding an iron–sulfur cluster biosynthesis protein.
Fig. 3.
(a) IS configurations surrounding liaSR genes in 252 complete E. faecium genomes. Configuration 1, highlighted in grey, was only found in E. faecium with mutations in liaSR genes (LiaST120A and LiaRW73C). Mutated liaSR genes are marked with (*) in the figure. (b) A putative IS-mediated recombination event between E. faecalis and E. faecium isolates with emrB genes and linkage genes aad [6]-sat4-aph(3’)-IIIa. (c) A putative IS-mediated recombination event between pUAMSEF1a and pUAMSEL1 from E. faecalis and E. faecium isolates.
As observed previously [32, 70], the presence of LiaRW73C and LiaST120A mutations does not always result in DAP-resistant phenotype, but it has been directly related to poor bactericidal activity and further development of DAP-resistant phenotype when DAP therapy is continued [32, 71, 72]. To confirm that the arrangement of the putative composite transposition could be associated with the presence of LiaRW73C and LiaST120A substitutions in E. faecium genomes, we surveyed a region of ~15000 bp that included liaSR genes in the other 216 complete E. faecium genomes that did not carry liaSR mutations. Most of these genomes (n=116) did not harbour any ISL3 in the proximity of liaSR genes. Among the strains with at least two ISs in the area surrounding liaSR genes (n=39), we identified four main IS-associated gene arrangements (Fig. 3a). The most prevalent arrangement was configuration 2, which was present in 26 out of 249 surveyed genomes, followed by configuration 3, found in 5 of the genomes. Most of the configurations contained variations in the copy number of some of the annotated ISs (mainly of IS256 in configuration 2, which was duplicated in some strains). However, none of the configurations exhibited the pseudo-compound transposon-like structure found in our isolates with mutations in liaSR genes, flanked by identically oriented IS elements from the ISL3 family.
E. faecalis and E. faecium isolates harbour two regions with putative IS-mediated recombination events
Sequence similarity analysis of all ISs found in the six isolates showed that the DNA sequence of some ISs (n=21) was highly conserved between the six isolates of this study, and those conserved ISs were found in several copies in different genomic regions [>99 % sequence similarity, 100 % coverage including IRs, transposase(s), and cargo gene(s) when applicable]. These highly conserved interspecific ISs (IS3, IS6, IS256, IS982 and IS1182 families – elements IS1485, IS1216, IS256, ISEfa13, ISEfm1 and IS1182, respectively) belong to 5 families out of the 11 and 12 families found in E. faecalis and E. faecium , respectively. To investigate whether any of these highly conserved ISs had been transferred between the two different species through IS-mediated homologous recombination events, we extended the sequence similarity analysis to the regions flanking these highly preserved ISs by up to 4000 bp upstream and downstream from matched ISs between species. Our analysis identified two ~7000 bp regions sharing >99.9 % sequence similarity with 100 % coverage between replicons from different species and bounded by IS elements (Fig. 3b, c). However, these two putative recombination events could have occurred between the two species at any time in their recent history and not necessarily during the specific co-infection described here, as these two species are regular inhabitants of the gastrointestinal tract.
The putative IS-mediated recombination event depicted in Fig. 3b was localized in pUMASEL1 and pUAMSEF4 plasmids from E. faecalis and E. faecium isolates, respectively. Further investigation of this putative recombination event showed a high resemblance, in terms of gene content, to a Tn5405-like element, mainly in E. faecium isolates. The Tn5405 element is a ~12 kb composite transposon that was previously identified in methicillin-resistant staphylococci [73] and later described in E. faecium species [74]. The gene clusters identified in our isolates contained the inducible by erythromycin ermB gene, two aminoglycoside ARDs (aad(6) and aph(3′)-IIIa), and the streptothricin resistance determinant gene sat-4 (previously described linkage aad(6)-sat4-aph(3′)-IIIa) [72]. The genes in the Tn5405 element are usually flanked by inverted repeats of two IS1182 elements. In our isolates, only one IS1182 was preserved inside the region, while the entire area was bounded by IS6 family elements (specifically IS1216) on one side and by Tn3 family transposases (TnBth1 in case of E. faecalis and Tn4430 for E. faecium isolates) on the other side, which did not share sequence similarity (Fig. 3b).
Immediately downstream of the region described above in pUAMSEL1 we found a second region of 7032 bp that was shared by all six isolates and was located in the pUAMSEF1a plasmid in E. faecium strains (Fig. 3c). No ARDs were identified in this region, which mainly harboured a set of genes encoding hypothetical proteins along with a recombinase, a BRCT domain-containing protein (usually belonging to proteins involved in cell cycle checkpoint functions that respond to DNA damage), and a transcriptional regulator. These regions were bounded in both species by exactly the same elements as those found in the previously described event: two identically oriented IS6 family elements (IS1216) and a Tn3 family transposase in one of the flanks in E. faecium isolates and one IS6 family (IS1216) and a Tn3 family transposase in the case of E. faecalis isolates. Analyses of these 2 regions using blastn with the NCBI nr/nt database (keeping only those genomes that harboured the entire region with identical flanking sequences and up to 70 internal mismatches) showed that these 2 regions are more prevalent among strains from E. faecium species, although they were found in a small number of E. faecalis strains (n=12 in the case of the first described region and n=16 in case of the second described region, without considering the isolates from this study) (Table S5). Multiple genome alignments of all the replicons in which these two regions were found indicate that only the regions described in our isolates (shown in Fig. 3b, c and bounded by ISs) were preserved in all replicons analysed (Figs S6 and S7).
Novel arrangement of IS elements in Tn1546-like transposon in E. faecalis isolates likely implicated in a genotype–phenotype discrepancy
All of our E. faecalis isolates presented vancomycin MIC values of approximately 2 µg ml−1, indicating that they were vancomycin-sensitive (Table S1). However, all of these isolates also contained plasmids with complete vanA gene clusters known to confer vancomycin resistance, indicating a genotype–phenotype discrepancy (Fig. 4). Restoration of the vancomycin-resistant phenotype in enterococcal strains harbouring a ‘silent’ copy of vancomycin resistance genes through different molecular mechanisms has been described before [75].
Fig. 4.
(a) Representation of the circularized vancomycin plasmid pUAMSEL4 from E. faecalis . The orange inner ring represents blastn alignment against pUAMSEF4 from E. faecium UAMS_EF55. The width of the orange bar represents the percentage of sequence similarity (full width implies values closer to 100 % sequence similarity). Genes annotated as hypothetical proteins are coloured in grey. (b) Representation of the circularized vancomycin plasmid pUAMSEF4 from E. faecium . The orange inner ring represents blastn alignment against pUAMSEL4 from E. faecalis . The red inner ring represents blastn alignment against pUAMSEF4 from UAMS_EF58 isolate. (c) Representation of vanA clusters and arrangements of ISs in E. faecalis and E. faecium isolates. Panels (a) and (b) were created using CGview server (version beta) and (c) was created using BioRender.
A comparison of the vancomycin plasmids (pUAMSEL4 and pUAMSEF4) between the isolates from the two species from this study showed that vanA-containing plasmids differed in size, genetic content and type of plasmid replication protein (rep) gene. However, the genetic sequences of all seven genes in the vanA cluster (vanR, vanS, vanH, vanA, vanX, vanY, and vanZ) were highly conserved when aligned between isolates from both species (inner orange ring, Fig. 4a, b).
Plasmid pUAMSEL4 (carrying vanA gene cluster) from E. faecalis belongs to the incompatibility (Inc) 18-like family [76], which is a broad host range of conjugative plasmids that, under laboratory conditions, have been transferred successfully to a wide variety of microbes, such as lactococci [77], streptococci [78] and staphylococci [79, 80]. On the other hand, the E. faecium vanA plasmid pUAMSEF4 was classified as belonging to the RepA_N family, which has a narrow host range [81]. All E. faecalis vanA replicons were nearly identical, but we observed a significant difference, in terms of content and size, in the vanA plasmid from the last collected E. faecium isolate (UAMS_EF58) (Figs 4 and S2A). The decrease in size of the UAMS_EF58 vanA plasmid was due to an IS-mediated deletion of a region located between the two identically oriented IS6 elements (specifically, IS1216) located in the first quarter of pUAMSEF4 (red inner ring in Figs 4b and S8). Thus, pUAMSEF4 from UAMS_EF58 consisted of a replicon ~7000 bp shorter than the vanA plasmids found in previously collected E. faecium isolates UAMS_EF55 and UAMS_EF57. In this excision event, both identically oriented IS6 elements were retained in the van plasmid from UAMS_EF58, but none of the genes bounded by them were retained (Figs 4b and S8). The cluster of genes absent in pUAMSEF4 from UAMS_EF58 contained several ARDs that code for resistance to aminoglycosides (aadE, aphA), nucleosides (sat-4), and macrolides, lincosamides and streptogramins (ermAB) (Fig. 4b, Table S3).
Although the sequence of genes in the vanA cluster was highly preserved among our six isolates, vanA gene clusters from both species presented different organizations of the Tn1546-like element. The Tn1546-like transposon in pUAMSEF4 from E. faecium isolates (Fig. 4c) showed a genetic organization that is highly preserved among hospital-acquired vancomycin-resistant E. faecium isolates [82]. However, we observed an unusual genetic organization of the Tn1546-like transposon in our E. faecalis isolates; this peculiar organization of ISs in the vanA cluster was not found in any other strains when the entire region was queried in the NCBI public database (Fig. S9).
Discussion
Enterococci are widely distributed across many different niches, but their primary habitat is the gastrointestinal tract [83]. Among enterococci, E. faecalis is the most common cause of opportunistic infection, but E. faecium is intrinsically more resistant to antibiotics. More than half of the nosocomial E. faecium isolates in the USA have expressed resistance to ampicillin and vancomycin and high-level resistance to aminoglycosides [84].
While the important role that ISs and other mobile genetic elements have in the persistence and dissemination of ARDs has been already established in previous works on enterococci [85, 86], there is a lack of information on the association between ISs and ARDs and other aspects regarding the nature, maintenance and mechanisms of transmission of ISs in bacterial genomes. In this work, we present two bacterial species, sharing the same environment (patient with bacteraemia), with a very similar content of IS families but drastically different copy numbers and arrangements of ISs throughout their replicons. E. faecium genomes harboured a strikingly high number of ISs (Fig. 1b) and showed a higher level of IS-mediated activity (4 days passed between the first and last sample being collected). Considering the first isolate collected for each species as the reference (UAMS_EL53 for E. faecalis and UAMS_EF55 for E. faecium ), the only significant intraspecific genomic differences found between the isolates of the study were the IS-mediated events described in this paper, highlighting the significant role ISs and other transposable elements play as elements that generate rapid genomic adaptations (Fig. S1) [24].
We identified 11 different IS families in the E. faecalis isolates that were also present in the E. faecium isolates. An additional IS family was identified in the E. faecium isolates. The presence of a high number of shared IS families with highly conserved DNA sequences between strains from both species suggests that these elements could have been spread through horizontal gene transfer. Most importantly, IS propagation generates multiple homologous sequences scattered throughout the bacterial genomes, which are substrates for homologous recombination [63], paving the way for a successful transmission of the antibiotic resistance determinants and virulence factors that frequently accompany these elements in E. faecalis and E. faecium genomes. In agreement with previous reports, our six isolates showed a strong correlation between the IS elements identified and the annotated ARDs, in the sense that the majority of ARDs had at least one IS element in their surrounding regions, suggesting that IS elements might have a role in the transfer of these ARDs and other virulence factors [22, 60].
Although mutations produced by ISs are generally deleterious [29, 58] and bacterial genomes possess mechanisms that suppress transposition [29, 87, 88], ISs can promote adaptive evolution by gene inactivation, triggering genomic deletions and modulating gene expression, which can be beneficial in the transition between free-living to parasitic lifestyles [24, 89]. Thus, a high level of transposition activity has been understood as the mechanism of adaptation to stressful and dynamic environmental conditions that can generate sufficient genetic variation to increase fitness [89]. Therefore, the strikingly high number of ISs found in the E. faecium isolates from this study in comparison with those from the set of complete E. faecium obtained from public repositories (Fig. 1) could be a consequence of the high rates of replicative transposition in response to the stressful and competitive conditions of the bloodstream environment, which is not the natural habitat of enterococci. However, it is worth mentioning that the high number of ISs found in our isolates compared to previous reports [90] could be partially due to our use of high-quality, complete genomes, considering that draft assemblies are typically highly fragmented and their contigs are usually broken at IS locations [91] due to the repetitive nature of IS elements [22, 60, 63].
It has been documented that elements from the IS6 family with flanking ISs in direct repeat are able to form transposons that resemble compound transposons through a mechanism that involves the formation of cointegrates, also known as pseudo-compound transposons [92–94], and also via circular translocatable units [94, 95]. Potential mechanisms of circular molecular transposition (translocatable units) mediated by elements from IS6 and IS26 families have been described [94, 96]. Transposon-derived circular forms have been documented in Gram-negative bacteria such as Acinetobacter baumannii [97] and Escherichia coli [98]. In this work, we provide computational evidence of the formation of a complete conjugative plasmid (pUAMSEF3) with all of the necessary machinery and functional elements for its own replication and transference (Fig. 2b); this plasmid was the product of IS-mediated excision from a larger plasmid (pUAMSEF1a) (Fig. 2a), supporting the key role of translocatable units in disseminating genetic material as autonomous entities.
We also observed multiple putative pseudo-compound transposons when analysing the genomes of our three E. faecium isolates (Figs 1a and 3a). However, this type of structure had never been described before, to the best of our knowledge, for members of the ISL3 family. The structure observed in our E. faecium isolates is composed of two directly oriented ISL3 elements surrounding liaSR with DAP-resistant associated mutations and surrounding genes. Further analysis including all complete, publicly available E. faecium genomes confirmed that the novel structure is specific to E. faecium genomes with DAP resistance mutations in liaSR genes. Our findings suggest a new paradigm for the investigation of the mechanisms of DAP resistance and their dissemination in E. faecium species, in the sense that the development of a DAP-resistant phenotype might not be produced by sequential co-mutations in liaSR genes but through IS-mediated transmission of the entire set of genes already carrying these mutations. However, we found no duplication of this cluster in our E. faecium isolates or in the other 33 complete E. faecium genomes harbouring liaSR mutations. In the same vein, the upstream and downstream genetic neighbourhood of the ISL3 bounded structure was shared between all complete isolates queried, therefore homologous recombination might be a more plausible mechanism of propagation for these mutations in liaSR genes than IS-mediated transposition. Homologous recombination mediated by ISs between related strains has been previously documented in other works [99–101]. Further detailed examination of the IS duplications at target sites and experimental assays should be carried out to determine whether this ISL3 bounded structure, observed in all E. faecium genomes carrying amino acid substitutions in LiaR (W73C) and LiaS (T120A), is crucial in their dissemination.
Our E. faecalis isolates harboured a complete vanA cluster but presented MIC values ≤2 for vancomycin, indicating a genotype–phenotype discrepancy. It has previously been reported that the vanA cluster (vanRSHAXYZ) is prone to IS-mediated alterations that can affect the resistance phenotype of a strain, even in the case of a vanA-positive genotype [102, 103]. Among the most widely documented effects of IS transposition is gene inactivation [24, 104], and the novel arrangement of ISs found in the vanA cluster of our three E. faecalis isolates might explain the observed genotype–phenotype discrepancy. Similar events have been documented in other species [102, 103]. A particularly interesting case was described by Siversten and colleagues [103] in which initially vancomycin-susceptible E. faecium isolates were able to survive for several days during subclinical breakpoint exposure to glycopeptides until they developed the resistant phenotype after the excision of one IS element present in the vanA gene cluster. We speculate that this novel arrangement of IS elements in the Tn1546-like transposon in E. faecalis isolates with a vanA-positive genotype from our study might be the cause of the ‘silenced’ phenotype observed when these isolates were collected.
In summary, while our computational results are all biological observations that need to be explored experimentally, our results substantiate that IS-mediated events differ between the two species, E. faecium and E. faecalis , from the same environment under complex selective forces occasioned by intensive antibiotic treatment. In addition, some of these IS-mediated reorganizations are noticeable even between isolates collected from the same subject in a short period of time. Our results highlight the major role of ISs and other transposable elements in the rapid genomic adaptation and response of enterococci to clinically relevant stresses.
Supplementary Data
Funding information
This work was supported by the University of Arkansas for Medical Sciences (UAMS) College of Medicine Barton Pilot Grant FY19 programme (AWD00052801); the UAMS Translational Research Institute (UL1 TR003107) through the NIH National Center for Advancing Translational Sciences; the National Science Foundation under award no. OIA-1946391; and the National Institute of Allergy and Infectious Disease (NIAID) under award no. R21AI169138. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Acknowledgements
Editorial support was provided by the Science Communication Group at the University of Arkansas for Medical Sciences. Data processing and analysis was performed with the GRACE cluster, provided by the University of Arkansas for Medical Sciences (UAMS) and managed by the Department of Biomedical Informatics.
Authors and contributors
Conceptualization: S.J. and A.K. Resources: S.J. and A.K. Funding acquisition: S.J. and A.K. Supervision: S.J. Methodology: S.J. and Z.U. Formal analysis: Z.U. and K.Z.A. Investigation: Z.U. Visualization and writing – original draft: Z.U. Writing – review and editing and validation: Z.U., K.Z.A. and S.J.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical statement
A waiver of informed consent was approved because this research involves no more than minimal risk to the subjects. This study was approved by the Institutional Review Board of the University of Arkansas for Medical Sciences (IRB no. 228137).
Footnotes
Abbreviations: ARD, antibiotic resistance determinant; DAP, daptomycin; E. faecalis, Enterococcus faecalis; E. faecium, Enterococcus faecium; IS, insertion sequence; MLST, multilocus sequencing typing; Tn, transposase; VRE, vancomycin-resistant Enterococcus.
All supporting data, code and protocols have been provided within the article or through supplementary data files. Nine supplementary figures and five supplementary tables are available with the online version of this article.
References
- 1.Lewis K, Caboni M. The making of a pathogen. Cell Host Microbe. 2017;21:653–654. doi: 10.1016/j.chom.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Ch’ng J-H, Chong KKL, Lam LN, Wong JJ, Kline KA. Biofilm-associated infection by enterococci. Nat Rev Microbiol. 2019;17:82–94. doi: 10.1038/s41579-018-0107-z. [DOI] [PubMed] [Google Scholar]
- 3.Price VJ, McBride SW, Hullahalli K, Chatterjee A, Duerkop BA, et al. Enterococcus faecalis CRISPR-Cas is a robust barrier to conjugative antibiotic resistance dissemination in the murine intestine. mSphere. 2019;4:e00464-19. doi: 10.1128/mSphere.00464-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiore E, Van Tyne D, Gilmore MS, Fischetti VA, Novick RP. Pathogenicity of Enterococci . Microbiol Spectr. 2019;7 doi: 10.1128/microbiolspec.GPP3-0053-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molina L, Udaondo Z, Duque E, Fernández M, Bernal P, et al. Specific gene loci of clinical Pseudomonas putida isolates. PLOS ONE. 2016;11:e0147478. doi: 10.1371/journal.pone.0147478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faron ML, Ledeboer NA, Buchan BW. Resistance mechanisms, epidemiology, and approaches to screening for vancomycin-resistant Enterococcus in the health care setting. J Clin Microbiol. 2016;54:2436–2447. doi: 10.1128/JCM.00211-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rios R, Reyes J, Carvajal LP, Rincon S, Panesso D, et al. Genomic epidemiology of vancomycin-resistant Enterococcus faecium (VREfm) in Latin America: revisiting the global VRE population structure. Sci Rep. 2020;10:5636. doi: 10.1038/s41598-020-62371-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Hal SJ, Ip CLC, Ansari MA, Wilson DJ, Espedido BA, et al. Evolutionary dynamics of Enterococcus faecium reveals complex genomic relationships between isolates with independent emergence of vancomycin resistance. Microb Genom. 2016;2 doi: 10.1099/mgen.0.000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Hal SJ, Willems RJL, Gouliouris T, Ballard SA, Coque TM, et al. The global dissemination of hospital clones of Enterococcus faecium . Genome Med. 2021;13:52. doi: 10.1186/s13073-021-00868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Hal SJ, Willems RJL, Gouliouris T, Ballard SA, Coque TM, et al. The interplay between community and hospital Enterococcus faecium clones within health-care settings: a genomic analysis. Lancet Microbe. 2022;3:e133–e141. doi: 10.1016/S2666-5247(21)00236-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lebreton F, Manson AL, Saavedra JT, Straub TJ, Earl AM, et al. Tracing the Enterococci from paleozoic origins to the Hospital. Cell. 2017;169:849–861. doi: 10.1016/j.cell.2017.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pöntinen AK, Top J, Arredondo-Alonso S, Tonkin-Hill G, Freitas AR, et al. Apparent nosocomial adaptation of Enterococcus faecalis predates the modern hospital era. Nat Commun. 2021;12:1523. doi: 10.1038/s41467-021-21749-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Top J, Arredondo-Alonso S, Schürch AC, Puranen S, Pesonen M, et al. Genomic rearrangements uncovered by genome-wide co-evolution analysis of a major nosocomial pathogen, Enterococcus faecium . Microb Genom. 2020;6:12. doi: 10.1099/mgen.0.000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Igbinosa EO, Beshiru A. Antimicrobial resistance, virulence determinants, and biofilm formation of Enterococcus species from ready-to-eat seafood. Front Microbiol. 2019;10:728. doi: 10.3389/fmicb.2019.00728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arredondo-Alonso S, Top J, McNally A, Puranen S, Pesonen M, et al. Plasmids shaped the recent emergence of the major nosocomial pathogen Enterococcus faecium . mBio. 2020;11:e03284-19. doi: 10.1128/mBio.03284-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bayjanov JR, Baan J, Rogers MRC, Troelstra A, Willems RJL, et al. Enterococcus faecium genome dynamics during long-term asymptomatic patient gut colonization. Microb Genom. 2019;5 doi: 10.1099/mgen.0.000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freitas AR, Tedim AP, Francia MV, Jensen LB, Novais C, et al. Multilevel population genetic analysis of vanA and vanB Enterococcus faecium causing nosocomial outbreaks in 27 countries (1986-2012) J Antimicrob Chemother. 2016;71:3351–3366. doi: 10.1093/jac/dkw312. [DOI] [PubMed] [Google Scholar]
- 18.Orlek A, Stoesser N, Anjum MF, Doumith M, Ellington MJ, et al. Plasmid classification in an Era of whole-genome sequencing: application in studies of antibiotic resistance epidemiology. Front Microbiol. 2017;8:182. doi: 10.3389/fmicb.2017.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao MD, Nguyen SH, Ganesamoorthy D, Elliott AG, Cooper MA, et al. Scaffolding and completing genome assemblies in real-time with nanopore sequencing. Nat Commun. 2017;8:14515. doi: 10.1038/ncomms14515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rezaei Javan R, Ramos-Sevillano E, Akter A, Brown J, Brueggemann AB. Prophages and satellite prophages are widespread in Streptococcus and may play a role in Pneumococcal pathogenesis . Nat Commun. 2019;10:4852. doi: 10.1038/s41467-019-12825-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siguier P, Gourbeyre E, Chandler M. Bacterial insertion sequences: their genomic impact and diversity. FEMS Microbiol Rev. 2014;38:865–891. doi: 10.1111/1574-6976.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Partridge SR, Kwong SM, Firth N, Jensen SO. Mobile genetic elements associated with antimicrobial resistance. Clin Microbiol Rev. 2018;31:e00088-17. doi: 10.1128/CMR.00088-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hickman AB, Dyda F. John Wiley & Sons, Ltd; 2015. [ March 10; 2021 ]. Mechanisms of DNA Transposition. In: Mobile DNA III.https://onlinelibrary.wiley.com/doi/abs/10.1128/9781555819217.ch25 accessed. [Google Scholar]
- 24.Vandecraen J, Chandler M, Aertsen A, Van Houdt R. The impact of insertion sequences on bacterial genome plasticity and adaptability. Crit Rev Microbiol. 2017;43:709–730. doi: 10.1080/1040841X.2017.1303661. [DOI] [PubMed] [Google Scholar]
- 25.Berg DE, Berg CM, Sasakawa C. Bacterial transposon Tn5: evolutionary inferences. Mol Biol Evol. 1984;1:411–422. doi: 10.1093/oxfordjournals.molbev.a040324. [DOI] [PubMed] [Google Scholar]
- 26.Alton NK, Vapnek D. Nucleotide sequence analysis of the chloramphenicol resistance transposon Tn9. Nature. 1979;282:864–869. doi: 10.1038/282864a0. [DOI] [PubMed] [Google Scholar]
- 27.Foster TJ, Davis MA, Roberts DE, Takeshita K, Kleckner N. Genetic organization of transposon Tn10. Cell. 1981;23:201–213. doi: 10.1016/0092-8674(81)90285-3. [DOI] [PubMed] [Google Scholar]
- 28.Prudhomme M, Turlan C, Claverys J-P, Chandler M. Diversity of Tn4001 transposition products: the flanking IS256 elements can form tandem dimers and IS circles. J Bacteriol. 2002;184:433–443. doi: 10.1128/JB.184.2.433-443.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Y, Aandahl RZ, Tanaka MM. Dynamics of bacterial insertion sequences: can transposition bursts help the elements persist? BMC Evol Biol. 2015;15:288. doi: 10.1186/s12862-015-0560-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing. 29th ed. Clinical and Laboratory Standards Institute, Wayne, PA: CLSI supplement M100; 2019. [Google Scholar]
- 31.Udaondo Z, Wongsurawat T, Jenjaroenpun P, Anderson C, Lopez J, et al. Draft genome sequences of 48 vancomycin-Resistant Enterococcus faecium strains isolated from inpatients with bacteremia and urinary tract infection. Microbiol Resour Announc. 2019;8:e00222-19. doi: 10.1128/MRA.00222-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Udaondo Z, Jenjaroenpun P, Wongsurawat T, Meyers E, Anderson C, et al. Two cases of vancomycin-resistant Enterococcus faecium bacteremia with development of daptomycin-resistant phenotype and its detection using Oxford nanopore equencing. Open Forum Infect Dis. 2020;7:faa180. doi: 10.1093/ofid/ofaa180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen S, Zhou Y, Chen Y, Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrews S. FASTQC A quality control tool for high throughput sequence data. Internet. 2010. [ February 22; 2019 ]. https://www.bibsonomy.org/bibtex/f230a919c34360709aa298734d63dca3 accessed.
- 36.Wick RR, Judd LM, Holt KE. Performance of neural network basecalling tools for Oxford Nanopore sequencing. Genome Biol. 2019;20:129. doi: 10.1186/s13059-019-1727-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Coster W, D’Hert S, Schultz DT, Cruts M, Van Broeckhoven C. NanoPack: visualizing and processing long-read sequencing data. Bioinformatics. 2018;34:2666–2669. doi: 10.1093/bioinformatics/bty149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wick RR, Judd LM, Gorrie CL, Holt KE. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLOS Comput Biol. 2017;13:e1005595. doi: 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koren S, Walenz BP, Berlin K, Miller JR, Bergman NH, et al. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 2017;27:722–736. doi: 10.1101/gr.215087.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolmogorov M, Yuan J, Lin Y, Pevzner PA. Assembly of long, error-prone reads using repeat graphs. Nat Biotechnol. 2019;37:540–546. doi: 10.1038/s41587-019-0072-8. [DOI] [PubMed] [Google Scholar]
- 41.Vaser R, Sović I, Nagarajan N, Šikić M. Fast and accurate de novo genome assembly from long uncorrected reads. Genome Res. 2017;27:737–746. doi: 10.1101/gr.214270.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLOS ONE. 2014;9:e112963. doi: 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 44.Alcock BP, Raphenya AR, Lau TTY, Tsang KK, Bouchard M, et al. CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2020;48:D517–D525. doi: 10.1093/nar/gkz935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie Z, Tang H. ISEScan: automated identification of insertion sequence elements in prokaryotic genomes. Bioinformatics. 2017;33:3340–3347. doi: 10.1093/bioinformatics/btx433. [DOI] [PubMed] [Google Scholar]
- 46.Siguier P, Perochon J, Lestrade L, Mahillon J, Chandler M. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res. 2006;34:D32–6. doi: 10.1093/nar/gkj014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carattoli A, Hasman H. Plasmidfinder and in silico pMLST: identification and typing of plasmid replicons in whole-genome sequencing (WGS) Methods Mol Biol. 2020;2075:285–294. doi: 10.1007/978-1-4939-9877-7_20. [DOI] [PubMed] [Google Scholar]
- 48.Grant JR, Stothard P. The CGView Server: a comparative genomics tool for circular genomes. Nucleic Acids Res. 2008;36:W181–4. doi: 10.1093/nar/gkn179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jolley KA, Maiden MCJ. BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics. 2010;11:595. doi: 10.1186/1471-2105-11-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 51.Ondov BD, Treangen TJ, Melsted P, Mallonee AB, Bergman NH, et al. Mash: fast genome and metagenome distance estimation using MinHash. Genome Biol. 2016;17:132. doi: 10.1186/s13059-016-0997-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abram K, Udaondo Z, Bleker C, Wanchai V, Wassenaar TM, et al. Mash-based analyses of Escherichia coli genomes reveal 14 distinct phylogroups. Commun Biol. 2021;4:117. doi: 10.1038/s42003-020-01626-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gilchrist CLM, Chooi Y-H. Clinker & clustermap.js: automatic generation of gene cluster comparison figures. Bioinformatics. 2021:btab007. doi: 10.1093/bioinformatics/btab007. [DOI] [PubMed] [Google Scholar]
- 54.Lopez-Delisle L, Rabbani L, Wolff J, Bhardwaj V, Backofen R, et al. pyGenomeTracks: reproducible plots for multivariate genomic datasets. Bioinformatics. 2021;37:422–423. doi: 10.1093/bioinformatics/btaa692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics. 2018;34:3094–3100. doi: 10.1093/bioinformatics/bty191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carver TJ, Rutherford KM, Berriman M, Rajandream M-A, Barrell BG, et al. ACT: the artemis comparison tool. Bioinformatics. 2005;21:3422–3423. doi: 10.1093/bioinformatics/bti553. [DOI] [PubMed] [Google Scholar]
- 57.Angiuoli SV, Salzberg SL. Mugsy: fast multiple alignment of closely related whole genomes. Bioinformatics. 2011;27:334–342. doi: 10.1093/bioinformatics/btq665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nzabarushimana E, Tang H. Insertion sequence elements-mediated structural variations in bacterial genomes. Mob DNA. 2018;9:29. doi: 10.1186/s13100-018-0134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blázquez J, Couce A, Rodríguez-Beltrán J, Rodríguez-Rojas A. Antimicrobials as promoters of genetic variation. Curr Opin Microbiol. 2012;15:561–569. doi: 10.1016/j.mib.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 60.Razavi M, Kristiansson E, Flach C-F, Larsson DGJ. The association between insertion sequences and antibiotic resistance genes. mSphere. 2020;5:e00418-20. doi: 10.1128/mSphere.00418-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ross K, Varani AM, Snesrud E, Huang H, Alvarenga DO, et al. TnCentral: a prokaryotic transposable element database and web portal for transposon analysis. mBio. 2021;12:e02060–21. doi: 10.1128/mBio.02060-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kadlec K, Schwarz S. Identification of the novel dfrK-carrying transposon Tn559 in a porcine methicillin-susceptible Staphylococcus aureus ST398 strain. Antimicrob Agents Chemother. 2010;54:3475–3477. doi: 10.1128/AAC.00464-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee H, Doak TG, Popodi E, Foster PL, Tang H. Insertion sequence-caused large-scale rearrangements in the genome of Escherichia coli . Nucleic Acids Res. 2016;44:7109–7119. doi: 10.1093/nar/gkw647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ward LJH, Brown JCS, Davey GP. Identification and sequence analysis of IS1297, an ISS1-like insertion sequence in a Leuconostoc strain . Gene. 1996;174:259–263. doi: 10.1016/0378-1119(96)00091-1. [DOI] [PubMed] [Google Scholar]
- 65.Arias CA, Panesso D, McGrath DM, Qin X, Mojica MF, et al. Genetic basis for in vivo daptomycin resistance in enterococci. N Engl J Med. 2011;365:892–900. doi: 10.1056/NEJMoa1011138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Campeau SA, Schuetz AN, Kohner P, Arias CA, Hemarajata P, et al. Variability of daptomycin MIC values for Enterococcus faecium when measured by reference broth microdilution and gradient diffusion tests. Antimicrob Agents Chemother. 2018;62:e00745-18. doi: 10.1128/AAC.00745-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mericl AN, Friesen JA. Comparative kinetic analysis of glycerol 3-phosphate cytidylyltransferase from Enterococcus faecalis and Listeria monocytogenes . Med Sci Monit. 2012;18:BR427–34. doi: 10.12659/msm.883535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Han W, Wu B, Li L, Zhao G, Woodward R, et al. Defining function of lipopolysaccharide O-antigen ligase WaaL using chemoenzymatically synthesized substrates. J Biol Chem. 2012;287:5357–5365. doi: 10.1074/jbc.M111.308486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cui G, Wang J, Qi X, Su J. Transcription elongation factor GreA plays a key role in cellular nvasion and virulence of Francisella tularensis subsp. novicida. Sci Rep. 2018;8:6895. doi: 10.1038/s41598-018-25271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Munita JM, Mishra NN, Alvarez D, Tran TT, Diaz L, et al. Failure of high-dose daptomycin for bacteremia caused by daptomycin-susceptible Enterococcus faecium harboring LiaSR substitutions. Clin Infect Dis. 2014;59:1277–1280. doi: 10.1093/cid/ciu642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol. 2012;10:266–278. doi: 10.1038/nrmicro2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kebriaei R, Rice SA, Singh KV, Stamper KC, Dinh AQ, et al. Influence of inoculum effect on the efficacy of daptomycin monotherapy and in combination with β-Lactams against daptomycin-susceptible Enterococcus faecium harboring LiaSR Substitutions. Antimicrob Agents Chemother. 2018;62:e00315-18. doi: 10.1128/AAC.00315-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Derbise A, de Cespedes G, el Solh N. Nucleotide sequence of the Staphylococcus aureus transposon, Tn5405, carrying aminoglycosides resistance genes. J Basic Microbiol. 1997;37:379–384. doi: 10.1002/jobm.3620370511. [DOI] [PubMed] [Google Scholar]
- 74.Werner G, Hildebrandt B, Witte W. Linkage of erm(B) and aadE-sat4-aphA-3 in multiple-resistant Enterococcus faecium isolates of different ecological origins. Microb Drug Resist. 2003;9 Suppl 1:S9–16. doi: 10.1089/107662903322541847. [DOI] [PubMed] [Google Scholar]
- 75.Thaker MN, Kalan L, Waglechner N, Eshaghi A, Patel SN, et al. Vancomycin-variable enterococci can give rise to constitutive resistance during antibiotic therapy. Antimicrob Agents Chemother. 2015;59:1405–1410. doi: 10.1128/AAC.04490-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kohler V, Vaishampayan A, Grohmann E. Broad-host-range Inc18 plasmids: Occurrence, spread and transfer mechanisms. Plasmid. 2018;99:11–21. doi: 10.1016/j.plasmid.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 77.Langella P, Chopin A. Conjugal transfer of plasmid pIP501 from Lactococcus lactis to Lactobacillus delbrückii subsp. bulgaricus and Lactobacillus helveticus . FEMS Microbiol Lett. 1989;51:149–152. doi: 10.1016/0378-1097(89)90498-9. [DOI] [PubMed] [Google Scholar]
- 78.Thompson JK, Collins MA. Evidence for the conjugal transfer of the broad host range plasmid pIP501 into strains of Lactobacillus helveticus . J Appl Bacteriol. 1988;65:309–319. doi: 10.1111/j.1365-2672.1988.tb01897.x. [DOI] [PubMed] [Google Scholar]
- 79.Schaberg DR, Clewell DB, Glatzer L. Conjugative transfer of R-plasmids from Streptococcus faecalis to Staphylococcus aureus . Antimicrob Agents Chemother. 1982;22:204–207. doi: 10.1128/AAC.22.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu W, Clark N, Patel JB. pSK41-like plasmid is necessary for Inc18-like vanA plasmid transfer from Enterococcus faecalis to Staphylococcus aureus in vitro . Antimicrob Agents Chemother. 2013;57:212–219. doi: 10.1128/AAC.01587-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mikalsen T, Pedersen T, Willems R, Coque TM, Werner G, et al. Investigating the mobilome in clinically important lineages of Enterococcus faecium and Enterococcus faecalis . BMC Genomics. 2015;16:282. doi: 10.1186/s12864-015-1407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang G, Yu F, Lin H, Murugesan K, Huang W, et al. Evolution and mutations predisposing to daptomycin resistance in vancomycin-resistant Enterococcus faecium ST736 strains. PLoS ONE. 2018;13:e0209785. doi: 10.1371/journal.pone.0209785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.He Q, Hou Q, Wang Y, Li J, Li W, et al. Comparative genomic analysis of Enterococcus faecalis: insights into their environmental adaptations. BMC Genomics. 2018;19:527. doi: 10.1186/s12864-018-4887-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.O’Driscoll T, Crank CW. Vancomycin-resistant Enterococcal infections: epidemiology, clinical manifestations, and optimal management. Infect Drug Resist. 2015;8:217–230. doi: 10.2147/IDR.S54125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hegstad K, Mikalsen T, Coque TM, Werner G, Sundsfjord A. Mobile genetic elements and their contribution to the emergence of antimicrobial resistant Enterococcus faecalis and Enterococcus faecium . Clin Microbiol Infect. 2010;16:541–554. doi: 10.1111/j.1469-0691.2010.03226.x. [DOI] [PubMed] [Google Scholar]
- 86.Shankar N, Baghdayan AS, Gilmore MS. Modulation of virulence within a pathogenicity island in vancomycin-resistant Enterococcus faecalis . Nature. 2002;417:746–750. doi: 10.1038/nature00802. [DOI] [PubMed] [Google Scholar]
- 87.Foster PL. Stress-induced mutagenesis in bacteria. Crit Rev Biochem Mol Biol. 2007;42:373–397. doi: 10.1080/10409230701648494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hua-Van A, Le Rouzic A, Boutin TS, Filée J, Capy P. The struggle for life of the genome’s selfish architects. Biol Direct. 2011;6:19. doi: 10.1186/1745-6150-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Consuegra J, Gaffé J, Lenski RE, Hindré T, Barrick JE, et al. Insertion-sequence-mediated mutations both promote and constrain evolvability during a long-term experiment with bacteria. Nat Commun. 2021;12:980. doi: 10.1038/s41467-021-21210-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Clewell DB, Weaver KE, Dunny GM, Coque TM, Francia MV, et al. Enterococci: From Commensals to Leading Causes of Drug Resistant Infection. Boston: Massachusetts Eye and Ear Infirmary; 2014. [PubMed] [Google Scholar]
- 91.Sohn J, Nam J-W. The present and future of de novo whole-genome assembly. Brief Bioinformatics. 2018:23–40. doi: 10.1093/bib/bbw096. [DOI] [PubMed] [Google Scholar]
- 92.Harmer CJ, Pong CH, Hall RM. Structures bounded by directly-oriented members of the IS26 family are pseudo-compound transposons. Plasmid. 2020;111:102530. doi: 10.1016/j.plasmid.2020.102530. [DOI] [PubMed] [Google Scholar]
- 93.Galas DJ, Chandler M, Berg DE, Howe MM. Mob DNA. 1989 [Google Scholar]
- 94.Varani A, He S, Siguier P, Ross K, Chandler M. The IS6 family, a clinically important group of insertion sequences including IS26. Mob DNA. 2021;12:11. doi: 10.1186/s13100-021-00239-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Harmer CJ, Moran RA, Hall RM. Movement of IS26-associated antibiotic resistance genes occurs via a translocatable unit that includes a single IS26 and preferentially inserts adjacent to another IS26. mBio. 2014;5:e01801–14. doi: 10.1128/mBio.01801-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Harmer CJ, Hall RM. IS26-Mediated formation of transposons carrying antibiotic resistance genes. mSphere. 2016;1:e00038-16. doi: 10.1128/mSphere.00038-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Karah N, Dwibedi CK, Sjöström K, Edquist P, Johansson A, et al. Novel aminoglycoside resistance transposons and transposon-derived circular forms detected in carbapenem-resistant acinetobacter baumannii clinical solates. Antimicrob Agents Chemother. 2016;60:1801–1818. doi: 10.1128/AAC.02143-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ghosh H, Doijad S, Bunk B, Falgenhauer L, Yao Y, et al. Detection of translocatable units in a blaCTX-M-15 extended-spectrum β-lactamase-producing ST131 Escherichia coli isolate using a hybrid sequencing approach. Int J Antimicrob Agents. 2016;47:245–247. doi: 10.1016/j.ijantimicag.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 99.Daveran-Mingot M-L, Campo N, Ritzenthaler P, Le Bourgeois P. A natural large chromosomal inversion in Lactococcus lactis is mediated by homologous recombination between two insertion sequences. J Bacteriol. 1998;180:4834–4842. doi: 10.1128/JB.180.18.4834-4842.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zong Z, Partridge SR, Iredell JR. ISEcp1-mediated transposition and homologous recombination can explain the context of bla(CTX-M-62) linked to qnrB2. Antimicrob Agents Chemother. 2010;54:3039–3042. doi: 10.1128/AAC.00041-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang Z, Fu Y, Du X-D, Jiang H, Wang Y. Potential transferability of mcr-3 via IS26-mediated homologous recombination in Escherichia coli . Emerg Microbes Infect. 2018;7:55. doi: 10.1038/s41426-018-0057-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gagnon S, Lévesque S, Lefebvre B, Bourgault A-M, Labbé A-C, et al. vanA-containing Enterococcus faecium susceptible to vancomycin and teicoplanin because of major nucleotide deletions in Tn1546. J Antimicrob Chemother. 2011;66:2758–2762. doi: 10.1093/jac/dkr379. [DOI] [PubMed] [Google Scholar]
- 103.Sivertsen A, Pedersen T, Larssen KW, Bergh K, Rønning TG, et al. A silenced vanA gene cluster on a transferable plasmid caused an outbreak of vancomycin-variable Enterococci . Antimicrob Agents Chemother. 2016;60:4119–4127. doi: 10.1128/AAC.00286-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rojo-Bezares B, Estepa V, Cebollada R, de Toro M, Somalo S, et al. Carbapenem-resistant Pseudomonas aeruginosa strains from a Spanish hospital: characterization of metallo-beta-lactamases, porin OprD and integrons. Int J Med Microbiol. 2014;304:405–414. doi: 10.1016/j.ijmm.2014.01.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.