Outpatient cervical ripening with an osmotic cervical dilator significantly reduced hospital stay with no increase in adverse maternal or neonatal outcomes.
OBJECTIVE:
To assess whether outpatient cervical ripening with a synthetic osmotic dilator shortens the length of hospital stay in term pregnancies undergoing labor induction.
METHODS:
Pregnant participants scheduled for labor induction at term with unfavorable cervix (less than 3-cm dilated and less than 60% effaced) and not requiring inpatient maternal or fetal monitoring were consented, and synthetic osmotic dilator rods were inserted on the day of scheduled induction. After reassuring fetal heart tracing, patients randomized to the outpatient group were asked to return 12 hours after insertion or sooner if needed. Those randomized to the inpatient group remained in the hospital. After the first round of ripening, additional ripening, oxytocin, and labor management were left up to the clinical health care professionals. The primary outcome was the proportion of participants with hospital stays longer than 48 hours. We estimated that a sample size of 338 would provide 85% power to detect a 30% difference between groups.
RESULTS:
From November 2018 to November 2021, 339 participants were randomized (171 inpatient, 167 outpatient, one withdrawal). Four patients in the outpatient group were admitted before12 hours for suspected labor and rupture of membranes, and 19 in the inpatient group had the device removed before 12 hours. The proportion of participants with hospital stays longer than 48 hours was lower in the outpatient group compared with the inpatient group (89 [53%] vs 152 [89%], relative risk [RR] 0.60, 95% CI 0.52–0.70). Patients in the outpatient group had a shorter total length of stay and time from admission to active labor. They were more likely to have a vaginal delivery within 24 hours of admission and were less likely to receive analgesics during ripening. Route of delivery and other maternal and neonatal outcomes were not significantly different between groups.
CONCLUSION:
Outpatient cervical ripening with a cervical osmotic dilator decreased hospital stay compared with inpatient ripening, without significant adverse outcomes.
FUNDING SOURCE:
Medicem Technology s.r.o., Czech Republic.
CLINICAL TRIAL REGISTRATION:
ClinicalTrials.gov, NCT03665688.
Labor induction is generally undertaken when the risks of continuing pregnancy outweigh the benefits.1 Various ripening methods are available to maximize the success of labor induction in participants with an unfavorable cervix. These include pharmacologic agents, such as prostaglandins, and mechanical agents, such as the Foley balloon. Historically, mechanical methods were the first methods developed to ripen the cervix.2 Mechanical methods of cervical ripening are safe and cost-effective.3
Rates of labor induction in 2019 have risen to 29.4%, with a third requiring cervical ripening.4 In most U.S. centers, cervical ripening is performed as an inpatient procedure. Recent evidence with the use of mechanical dilators, bolstered by their limited effect on uterine contractility, supports the safety of allowing participants to go home after insertion.5 There have been several studies in outpatient cervical ripening,6–14 but none evaluated the role of synthetic osmotic dilators in the outpatient setting. Dilapan-S is a synthetic osmotic dilator (or rod) made of a patented hydrogel (AQUACRYL) that works by absorbing fluid from the cells of the cervical canal, resulting in reversible cell wall dehydration and softening. As the rod(s) volume increases endogenous prostaglandins are released from mechanical stretch resulting in cervical ripening. In a randomized clinical trial in the inpatient setting, the synthetic osmotic dilator was noninferior to the Foley balloon in terms of safety and efficacy with better patient satisfaction.15 Unlike the Foley balloon, however, this synthetic osmotic dilator is U.S. Food and Drug Administration–approved for cervical ripening and does not have any parts that protrude from the vagina, making it the ideal candidate for outpatient cervical ripening.
The usual practice with mechanical ripening methods is to insert the mechanical dilator in the hospital and then await cervical ripening, which can take up to 24 hours. Allowing the patient to return home after insertion is a promising strategy that lowers in-hospital health care costs and improves patient satisfaction. Our objective was to determine whether outpatient preinduction cervical ripening with a synthetic osmotic dilator shortens the length of hospital stay in pregnant patients scheduled for induction at term.
METHODS
We conducted an open-label randomized control trial in two academic centers in the United States. University of Texas Medical Branch IRB approval was obtained, and ClinicalTrials.gov registration was completed before enrollment (clinical trial registration: ClinicalTrials.gov, NCT03665688). The trial followed CONSORT (Consolidated Standards of Reporting Trials) guidelines,16 and enrollment occurred between November 2018 and November 2021.
Pregnant patients receiving prenatal care in our maternal health care clinics and who were scheduled for labor induction at term were approached, and informed consent was obtained either in the clinic or in the labor and delivery unit.
Patients were eligible for inclusion if their age was at least 18 years, they were able to consent and had a singleton gestation at 37 weeks or more based according to the American College of Obstetricians and Gynecologists’ criteria.17 Additional inclusion criteria were a live fetus in cephalic presentation, intact membranes, and a cervix not more than 3-cm dilated and not more than 60% effaced. We excluded patients who had limited access to a telephone, lacked a support person (no adult available to accompany the patient during the outpatient cervical-ripening period), or declined placement in a hotel if they lived more than 60 minutes from our facility. Other exclusion criteria are listed in Box 1.
Box 1. Exclusion Criteria.
Active labor
Active genital herpes
Chorioamnionitis
Transfundal uterine or cervical surgery
Previous cesarean delivery
Nonreassuring fetal status
Need for continuous maternal or fetal monitoring during ripening
Contraindication for vaginal delivery
Active vaginal bleeding
Abnormal placental location or adherence (placenta previa or unresolved low-lying placenta)
Estimated fetal weight greater than 5,000 g (nondiabetic) or greater than 4,500 g (diabetic)
Intrauterine growth restriction (estimated fetal weight less than the 10th percentile)
Oligohydramnios (amniotic fluid index less than 5 cm or deep vertical pocket of less than 2 cm)
Fetal anomaly
Need for inpatient care (eg, hypertension, insulin-dependent diabetes)
Poor or no access to a telephone and cannot be placed in the hotel
Absence of support person (no adult accompanying the patient during outpatient cervical ripening period)
Randomization was performed using a computer-generated random list of numbers assigning patients to one of the two groups in the study. Randomization assignments were kept secure in opaque envelopes. A separate randomization sequence was generated for each site.
The synthetic osmotic cervical dilator was placed by trained medical personnel (residents, fellow, or faculty). Before the device placement, patients underwent continuous cardiotocography monitoring for 30 minutes. The cervix was then visualized with a sterile vaginal speculum and cleaned with iodine or chlorhexidine. As many synthetic osmotic dilator rods (Medicem Technology s.r.o.) as possible were inserted into the cervical canal under direct visualization. If necessary, a blunt ring forceps was used to grasp the anterior lip of the cervix for better visualization. The synthetic osmotic dilators were left for at least 12 hours and no longer than 24 hours. After placement, patients were monitored for at least 30 minutes. If no contraindications for outpatient management arose, such as active vaginal bleeding, rupture of membranes, nonreassuring fetal evaluation (defined as minimal or absent variability, abnormal baseline, or presence of decelerations), evidence of labor, or other severe medical conditions deemed by the clinical staff or the attending physician to preclude outpatient cervical ripening developed after insertion, the patient was randomized.
Patients randomized to the outpatient group were sent home if they could return to the hospital within 60 minutes if needed. Otherwise they were sent to a nearby hotel. Patients were allowed to ambulate, shower, and perform regular activities during that period. Instructions were given to patients to return to the labor and delivery unit 12 hours after insertion, or earlier if any excessive bleeding, rupture of membranes, pain, or other concerns (contractions, decreased fetal movement) occurred before 12 hours. A study leaflet was provided with detailed instructions, emergency contact information for appropriate study clinical staff, and space to document any oral medication taken for pain relief (acetaminophen). Patients were also instructed to enter the date and time of membrane rupture if applicable. After the designated 12 hours' time, or earlier if indicated, patients returned to be admitted to the labor and delivery unit for the standard protocol of labor induction. If the cervix remained unfavorable after extraction or spontaneous expulsion of the dilators (less than 3-cm dilated and less than 60% effaced), additional mechanical or pharmacologic cervical ripening was allowed at the health care professional's discretion.
Patients randomized to inpatient management were admitted to the labor and delivery unit, and the standard clinical protocol was initiated for cervical ripening and labor induction. During the 12 hours of cervical ripening, the patient was placed on continuous fetal heart rate monitoring. During this period, clinical care was left to the health care professional's discretion (standard of care at the institution), including fetal heart monitoring or oral intake status. No other interventions were to occur during this period of 12 hours unless clinically indicated, including labor augmentation. Reasons for early removal of the dilators and management after 12 hours from insertion were identical to the outpatient group.
Our primary outcome was the rate of hospital stay longer than 48 hours (from admission to discharge). Secondary outcomes were defined a priori and included mode of delivery; vaginal deliveries within 24, 36, and 48 hours of hospital admission; time from hospital admission to reach the active stage of labor (defined as greater than 5-cm cervical dilation); change in Bishop score from insertion to extraction of the device; analgesia used during device placement and cervical ripening; adverse neonatal outcomes (composite and individual; 5-minute Apgar score less than 7, cord arterial pH less than 7.1, neonatal intensive care unit admission, transient tachypnea of the newborn, neonatal hypoglycemia, respiratory support, hyperbilirubinemia, and neonatal trauma); and total duration of maternal hospital stay. Each patient also completed a satisfaction survey regarding sleep, rest, pain, and activity.
Relevant medical history, obstetric history, demographic information (self-reported ethnicity, self-reported race) and intrapartum and postpartum events were abstracted from medical records into an online electronic database by qualified research staff. Collection of data on race and ethnicity was required by our IRB and funding source.
The sample size was estimated based on the determination of superiority. Based on our recent experience and data from an international registry trial,18 we assumed the proportion of participants with hospital stays longer than 48 hours in the inpatient group would be 54%. We estimated that a total sample size of 169 patients per group would provide 85% power to detect a 30% relative reduction in the primary outcome with a two-sided alpha of 0.05.
All analyses were by intention to treat. We did not plan nor perform any interim analyses. Wilcoxon rank-sum test, t test, Fisher exact test, or χ2 test were used as appropriate. For the patient satisfaction questionnaire, the Cochran-Armitage test for trend was used.19 Kaplan-Meier curves with censoring for CD were used to compare the time from admission to reaching active labor. Statistical computations were performed using TIBCO Spotfire S+ 8.2.
RESULTS
From November 2018 to November 2021, 5,394 participants were screened, 3,541 were ineligible, 1,220 declined participation, and 633 were preconsented but later became ineligible (Fig. 1). The remaining 339 participants were randomized (171 inpatient, 167 outpatient, one withdrawal).
Fig. 1. Flow diagram of eligible patients. FHR, fetal heart rate; BP, blood pressure; IUGR, intrauterine growth restriction; COVID-19, coronavirus disease 2019.
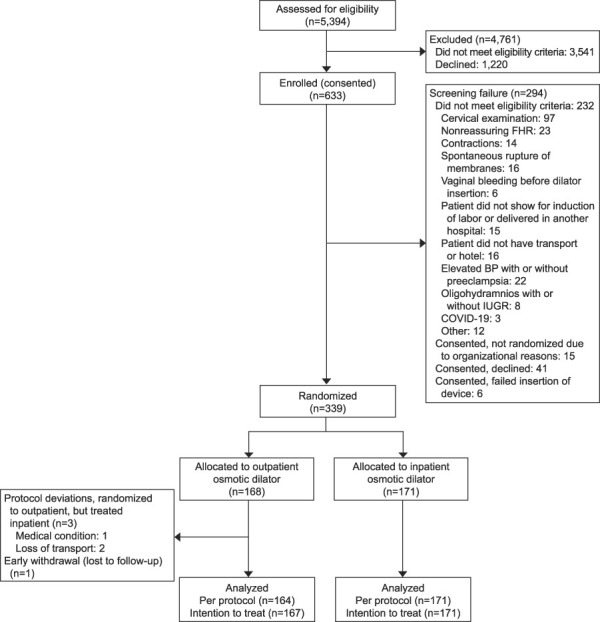
Saad. Outpatient Synthetic Osmotic Cervical Dilator. Obstet Gynecol 2022.
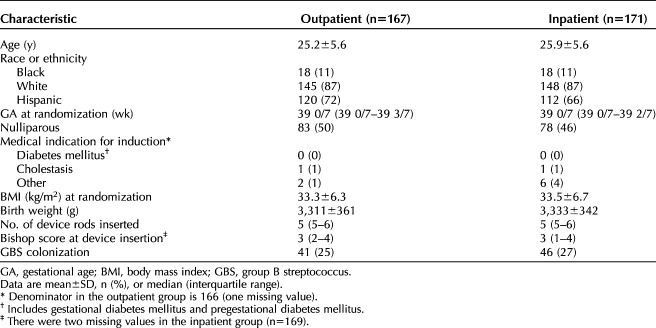
Baseline characteristics were similar between groups (Table 1). The median gestational age was 39 weeks, and the median number of synthetic osmotic dilator rods inserted was five in both groups. Nine (5.4%) patients in the outpatient group and 19 (11.1%) in the inpatient group had the device removed before 12 hours (P<.001).
Table 1.
Baseline Patient Characteristics by Group Allocation
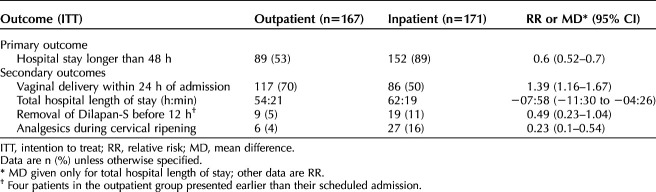
The proportion of participants with hospital stays longer than 48 hours was lower in the outpatient group compared with the inpatient group (89 [53.3%] vs 152 [88.9%], relative risk [RR] 0.60, 95% CI 0.52–0.70; Table 2). These results remained significant when the same analysis was done in the per-protocol population (Table 3).
Table 2.
Primary Outcome and Prespecified Secondary Outcomes
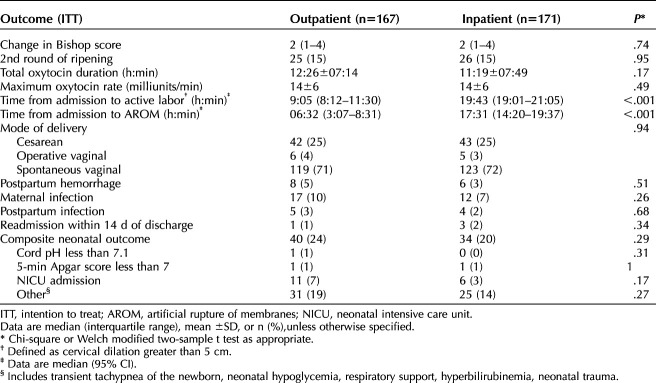
Table 3.
Maternal and Neonatal Outcomes
Compared with patients in the inpatient group, patients in the outpatient group had shorter total length of hospital stay (hours:min) (54:21 vs 62:19, mean difference −07:58, 95% CI −11:30 to −04:26) and were more likely to have a vaginal delivery within 24 hours of admission (117 [70.1%] vs 86 [50.3%], RR 1.39, 95% CI 1.16–1.67) and less likely to use analgesics during ripening (6 [3.6%] vs 27 [15.8%], RR 0.23, 95% CI 0.1–0.54). Time (hours:min) from admission to active labor (09:05 [08:12–11:30] vs 19:01 [19:01–21:05; (Appendix 2, available online at http://links.lww.com/AOG/C861) (Table 3) and from admission to artificial rupture of membranes (06:32 [3:07–8:31] vs 17:31 [14:20–19:37]) were shorter in the outpatient group (Table 3). The mean duration of oxytocin and maximum oxytocin rate were not significantly different between groups.
Only 4 of 167 outpatient patients presented before their scheduled time of admission. The main reasons for early presentation included contractions, membrane rupture, and no medical indication. Maternal complications such as postpartum infection, hemorrhage, and maternal infections did not differ among both groups. Cesarean delivery rates were not significantly different between groups, with failure of labor progress being the most common indication. Neonatal outcomes were not significantly different between groups (Table 3).
Patient satisfaction surveys are provided in Appendix 3, available online at http://links.lww.com/AOG/C861. Patients in the outpatient group were more able to walk, eat, and shower than those in the inpatient group. They felt that outpatient cervical ripening was beneficial and would choose the same approach for their subsequent pregnancy.
DISCUSSION
We found that outpatient cervical ripening with a synthetic osmotic dilator decreased hospital stay for more than 48 hours without increasing adverse events. Eighty-five percent of patients did not require a second round of cervical ripening, and participants in the outpatient group used analgesia less frequently than those in the inpatient group. Although secondary outcomes such as maternal infection, chorioamnionitis, cesarean delivery rates, and neonatal outcomes were not statistically different, our trial was not powered to detect such differences.
There are at least three randomized trials regarding inpatient cervical ripening using this synthetic osmotic dilator for term pregnancies.15,20,21 Saad et al15 compared this synthetic osmotic dilator to the Foley balloon. The former was noninferior to the Foley method regarding safety and efficacy with better patient satisfaction. Gavara et al20 compared this synthetic osmotic dilator to low-dose oral misoprostol. They found that the dilator was noninferior regarding vaginal delivery within 36 hours, with better patient satisfaction. A recently published randomized clinical trial study showed similar cesarean delivery rates in the synthetic mechanical dilator (37%) compared with dinoprostone vaginal insert (34%) in a mainly nulliparous population. Maternal and neonatal adverse events were similar in both inpatient interventions.21
The available evidence for outpatient cervical ripening, primarily focused on the Foley balloon, shows a decrease in labor and delivery time. A recent meta-analysis, which included eight trials (740 patients), showed that outpatient cervical ripening with a Foley balloon led to significantly less time in labor and delivery unit (mean difference −7.24 hours, 95% CI −11.03 to −3.34) and to lower rates of cesarean delivery RR 0.76 (95% CI 0.59–0.98). The review mentions only one unpublished study that had hospital length of stay as a primary outcome. The authors did not report any differences in other maternal or neonatal outcomes.5
The evidence supports the benefits of outpatient mechanical cervical ripening. An advantage of synthetic osmotic dilators over Foley balloon is that the former does not protrude through the introitus, is U.S. Food and Drug Administration–approved, and does not require tension, readjustment, or interventions, making it more amenable to outpatient management. Patients with Foley balloons may also need to return to the hospital earlier than expected because of expulsion and pain. In their randomized trial comparing inpatient with outpatient Foley balloon, Ausbeck et al13 found that 22% (14/63) of the patients randomized to outpatient returned before their scheduled admission for the following reasons: contractions (n=6), active labor (n=3), device-related (pain related to the Foley balloon (n=2), Foley balloon expulsion (n=2), and motor vehicle accident (n=1). The overall hospital stay did not differ between both interventions (3.3±0.9 days vs 3.5±0.9 days, P=.27) This contrasts with our study, in which only 2% (4/167) returned and the total hospital length of stay was shorter by approximately 8 hours.
The strengths of our study include the randomization and its appropriate sample size. Our primary outcome of length of hospital stay less than 48 hours may be unusual and may be related to the approximately 12 hours of outpatient cervical ripening but has some advantages. We did not use cesarean delivery as the primary outcome because we did not expect outpatient ripening with the same method to decrease cesarean delivery rates compared with inpatients. However, because patients with cesarean delivery usually have hospital stays longer than 48 hours, this primary outcome would have accounted for a higher cesarean delivery rate and complications, even in those who delivered vaginally. On a health-system level, length of stay is typically accounted for by days, particularly when considering that many payers bundle postpartum stay, up to a specified number of days, within the payment for delivery. Limitations to our study include generalizability, because it included low-risk patients with stringent eligibility criteria. Being an open-label trial, bias may have been introduced and led to the observed results. Our analyses involving the secondary outcomes should be interpreted with caution as we were not powered to identify slight differences.
In conclusion, outpatient cervical ripening with a synthetic osmotic dilator hat reduced hospital stay compared with inpatient ripening with better patient satisfaction and pain control. Future studies and analyses are needed to assess cost benefits, neonatal safety, maternal infection, and mode of delivery.
Authors' Data Sharing Statement
Will individual participant data be available (including data dictionaries)? No
What data, in particular, will be shared? Not available
What other documents will be available? Not available
When will data be available (start and end dates)? Not applicable
By what access criteria will data be shared (including with whom, for what types of analyses, and by what mechanism)? Not applicable
Footnotes
Supported by Medicem Technology s.r.o., Czech Republic. The funder did not have any role in the conduct of the trial, analysis of the data, or drafting of this manuscript. An independent third party performed all statistical analyses.
Financial Disclosure Antonio Saad and George Saade have acted as expert consultants to the sponsor. The other authors did not report any potential conflicts of interest.
Presented at the RCOG World Congress 2022, June 13–15, 2022, London, United Kingdom.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews are available at http://links.lww.com/AOG/C862.
REFERENCES
- 1.Grobman WA, Rice MM, Reddy UM, Tita ATN, Silver RM, Mallett G, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med 2018;379:513–23. doi: 10.1056/NEJMoa1800566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thiery M, De Boever J, Merchiers E, Martens G. Hormones and cervical ripening. Am J Obstet Gynecol 1989;160:1251–3. doi: 10.1016/0002-9378(89)90207-x [DOI] [PubMed] [Google Scholar]
- 3.Gelber S, Sciscione A. Mechanical methods of cervical ripening and labor induction. Clin Obstet Gynecol 2006;49:642–57. doi: 10.1097/00003081-200609000-00022 [DOI] [PubMed] [Google Scholar]
- 4.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK. Births: final data for 2018. Natl Vital Stat Rep 2019;68:1–47. [PubMed] [Google Scholar]
- 5.Pierce-Williams R, Lesser H, Saccone G, Harper L, Chen V, Sciscione A, et al. Outpatient cervical ripening with balloon catheters: a systematic review and meta-analysis. Obstet Gynecol 2022;139:255–68. doi: 10.1097/AOG.0000000000004644 [DOI] [PubMed] [Google Scholar]
- 6.Wilkinson C, Bryce R, Adelson P, Turnbull D. A randomised controlled trial of outpatient compared with inpatient cervical ripening with prostaglandin E2 (OPRA study). BJOG 2015;122:94–104. doi: 10.1111/1471-0528.12846 [DOI] [PubMed] [Google Scholar]
- 7.Wilkinson C, Adelson P, Turnbull D. A comparison of inpatient with outpatient balloon catheter cervical ripening: a pilot randomized controlled trial. BMC Pregnancy Childbirth 2015;15:126. doi: 10.1186/s12884-015-0550-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henry A, Madan A, Reid R, Tracy SK, Austin K, Welsh A, et al. Outpatient Foley catheter versus inpatient prostaglandin E2 gel for induction of labour: a randomised trial. BMC Pregnancy Childbirth 2013;13:25. doi: 10.1186/1471-2393-13-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kruit H, Heikinheimo O, Ulander VM, Aitokallio-Tallberg A, Nupponen I, Paavonen J, et al. Foley catheter induction of labor as an outpatient procedure. J Perinatol 2016;36:618–22. doi: 10.1038/jp.2016.62 [DOI] [PubMed] [Google Scholar]
- 10.Sciscione AC, Muench M, Pollock M, Jenkins TM, Tildon-Burton J, Colmorgen GH. Transcervical Foley catheter for preinduction cervical ripening in an outpatient versus inpatient setting. Obstet Gynecol 2001;98:751–6. doi: 10.1016/s0029-7844(01)01579-4 [DOI] [PubMed] [Google Scholar]
- 11.Policiano C, Pimenta M, Martins D, Clode N. Outpatient versus inpatient cervix priming with Foley catheter: a randomized trial. Eur J Obstet Gynecol Reprod Biol 2017;210:1–6. doi: 10.1016/j.ejogrb.2016.11.026 [DOI] [PubMed] [Google Scholar]
- 12.Kuper SG, Jauk VC, George DM, Edwards RK, Szychowski JM, Mazzoni SE, et al. Outpatient Foley catheter for induction of labor in parous women: a randomized controlled trial. Obstet Gynecol 2018;132:94–101. doi: 10.1097/AOG.0000000000002678 [DOI] [PubMed] [Google Scholar]
- 13.Ausbeck EB, Jauk VC, Xue Y, Files P, Kuper SG, Subramaniam A, et al. Outpatient Foley catheter for induction of labor in nulliparous women: a randomized controlled trial. Obstet Gynecol 2020;136:597–606. doi: 10.1097/AOG.0000000000004041 [DOI] [PubMed] [Google Scholar]
- 14.Haavisto H, Polo-Kantola P, Anttila E, Kolari T, Ojala E, Rinne K. Experiences of induction of labor with a catheter - a prospective randomized controlled trial comparing the outpatient and inpatient setting. Acta Obstet Gynecol Scand 2021;100:410–7. doi: 10.1111/aogs.14037 [DOI] [PubMed] [Google Scholar]
- 15.Saad AF, Villarreal J, Eid J, Spencer N, Ellis V, Hankins GD, et al. A randomized controlled trial of Dilapan-S vs Foley balloon for preinduction cervical ripening (DILAFOL trial). Am J Obstet Gynecol 2019;220:275.e1–9. doi: 10.1016/j.ajog.2019.01.008 [DOI] [PubMed] [Google Scholar]
- 16.Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Obstet Gynecol 2010;115:1063–70. doi: 10.1097/AOG.0b013e3181d9d421 [DOI] [PubMed] [Google Scholar]
- 17.Methods for estimating the due date. Committee Opinion No. 700. American College of Obstetricians and Gynecologists. Obstet Gynecol 2017;129:e150–4. doi: 10.1097/AOG.0000000000002046 [DOI] [PubMed] [Google Scholar]
- 18.Gupta J, Chodankar R, Baev O, Bahlmann F, Brega E, Gala A, et al. Synthetic osmotic dilators in the induction of labour-an international multicentre observational study. Eur J Obstet Gynecol Reprod Biol 2018;229:70–5. doi: 10.1016/j.ejogrb.2018.08.004 [DOI] [PubMed] [Google Scholar]
- 19.Buonaccorsi JP, Laake P, Veierød MB. On the power of the Cochran-Armitage test for trend in the presence of misclassification. Stat Methods Med Res 2014;23:218–43. doi: 10.1177/0962280211406424 [DOI] [PubMed] [Google Scholar]
- 20.Gavara R, Saad AF, Wapner RJ, Saade G, Fu A, Barrow R, et al. Cervical ripening efficacy of synthetic osmotic cervical dilator compared with oral misoprostol at term: a randomized controlled trial. Obstet Gynecol 2022;139:1083–91. doi: 10.1097/aog.0000000000004799 [DOI] [PubMed] [Google Scholar]
- 21.Gupta JK, Maher MA, Stubbs MC, Brocklehurst P, Daniels JP, Hardy P, et al. A randomized trial of synthetic osmotic cervical dilator for induction of labor versus dinoprostone vaginal insert. Am J Obstet Gynecol MFM 2022 Aug 9. doi: 10.1016/j.ajogmf.2022.100628 [DOI] [PubMed] [Google Scholar]