Abstract
Photoresponsive materials have been widely used in sensing, bioimaging, molecular switches, information storage, and encryption nowadays. Although a large amount of photoresponsive materials have been reported, the construction of these smart materials into precisely prescribed complex 3D geometries is rarely studied. Here we designed a novel photoresponsive material methyl methacrylate containing triphenylethylene (TrPEF2-MA) that can be directly used for digital light processing (DLP) 3D printing. Based on TrPEF2-MA, a series of photoresponsive 3D structures with reversible color switching under ultraviolet/visible light irradiations were fabricated. These complex photoresponsive 3D structures show high resolutions (50 μm), excellent repeatability (25 cycles without fatigue), and tunable saturate color degrees. Multicomponent DLP 3D printing processes were also carried out to demonstrate their great properties in information hiding and information-carrying properties. This design strategy for constructing photoresponsive 3D structures is attractive in the area of adaptive camouflage, information hiding, information storage, and flexible electronics.
1. Introduction
Photoresponsive materials are a type of smart materials that show obvious physical or chemical properties changes under light stimulus. Due to the advantages of light, such as nondirect contact, easy access, high-precision, wavelength-dependence, and no by-products [1–7], a growing number of light-responsive materials are developed and involved in the fields of anticounterfeiting detection [8, 9], information storage [10, 11], chemosensing [12, 13], bioimaging [14, 15], smart adsorbents [16, 17], and molecular machine [18–20]. As a typical photoresponsive phenomenon, photochromism displays reversible chemical reactions and obvious color changes upon the light irradiation (with a certain wavelength). A series of organic photochromic systems, such as dithienylethene [21–24], azobenzene [25–28], spiropyran [29–31], fulgides [32, 33], and their derivatives, have been designed and profoundly investigated. However, the applications of such molecular photochromic compounds were mainly demonstrated by doping or grating in polymer matrices to construct simple 2D films or three-dimensional (3D) shapes [34–37]. With the booming development of photochromic materials, the construction of stable macromolecular photochromic materials in precisely prescribed complex 3D geometries becomes an appealing topic.
3D printing, a technology that refers to the additive manufacturing process of building 3D solid objects from digital models, shows the capability of customizing complex 3D structures [38–42]. Along with recently rapid developments in 3D printing, a few attempts have been made to fabricate photoresponsive structures with complex geometries by 3D printing, which showed enormous potential in the applications of adaptive camouflage, information hiding, information storage, soft robotics, and flexible electronics [43–47]. For instance, the commercial 3D-printed powders (i.e., nylon 12) mixed with WO3 uncoated nanoparticles were used to create photoresponsive 3D structures by sintering-based 3D printing [48]. Hybrid organic-inorganic polyoxometalates were stabilized and solubilized by polymeric ionic liquid matrices, which could be further processed by 3D printing into photochromic 3D geometries [49]. Photoswitchable donor-acceptor Stenhouse adducts (DASAs) were postmodified on a two-photon laser processing complex microstructure [50]. However, due to the employed doping or postmodifying methods, the photoresponsive 3D structures suffered from unquantified and nonhomogeneous functional partials and poor solvent or thermal resistances. In addition, multicomponent 3D printing processes are hard to conduct with postmodify photochromic materials. Recently, triphenylethylene derivatives, which own admirable repeatability and simple chemical structures, are considered as promising photochromic materials [51–54]. Moreover, the easily modified chemical structure makes them promising candidates for preparing ultraviolet (UV) curable photochromic resins that are suitable for UV-based 3D printing technologies to directly establish macromolecular cross-linking network, such as digital light processing (DLP)-based 3D printing, a technology that is recognized as one of the most efficient 3D printing method to realize high-accuracy 3D structures with high speed [55–57].
Herein, we demonstrate a simple and novel photoresponsive material, methyl methacrylate containing triphenylethylene (TrPEF2-MA), which can be directly used for DLP 3D printing for the first time (Figure 1). The high-resolution 3D objects show great solvent/thermal resistance and precisely controllable photoresponsive properties. TrPEF2-MA with a simple chemical structure exhibits controllable photochromism and fast response. More importantly, the MA moiety in TrPEF2-MA endows the molecule with UV curable ability, which could be directly copolymerized during the DLP 3D printing process (Figure 2(a)). By adding molecule TrPEF2-MA and photoinitiator into poly(ethylene glycol) diacrylate (PEGDA), liquid resins for high-resolution DLP 3D printing were successfully prepared. A series of highly complex and precisive 3D structures with high resolution up to 50 μm were fabricated. Under the alternating UV and visible light irradiation, these printed stable macromolecular-based 3D structures featured reversible color switching between transparent and yellow (absorption at ca. 470 nm) with good repeatability. Besides, the saturate color degree of the photoresponsive 3D structures could be precisely controlled by adjusting the composition ratio of TrPEF2-MA in the resin. To further demonstrate the potential applications of the directly DLP 3D-printable photoresponsive material TrPEF2-MA, multicomponent 3D printing processes were carried out for the construction of the information (QR codes) hiding 3D structures, multicolor photoresponsive 3D structures, and information-carrying 3D structures. This study provides a novel strategy to directly design DLP 3D-printable photoresponsive material and reveals the potential applications of the areas of adaptive camouflage, information hiding, information storage, and flexible electronics.
Figure 1.
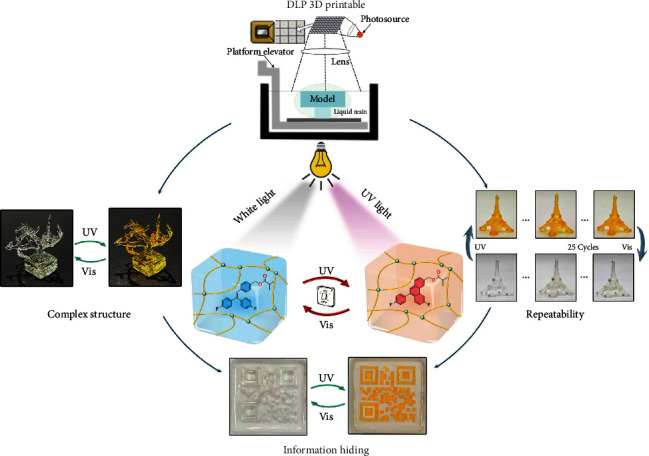
Comprehensive feature of 3D-printable photoresponsive materials.
Figure 2.
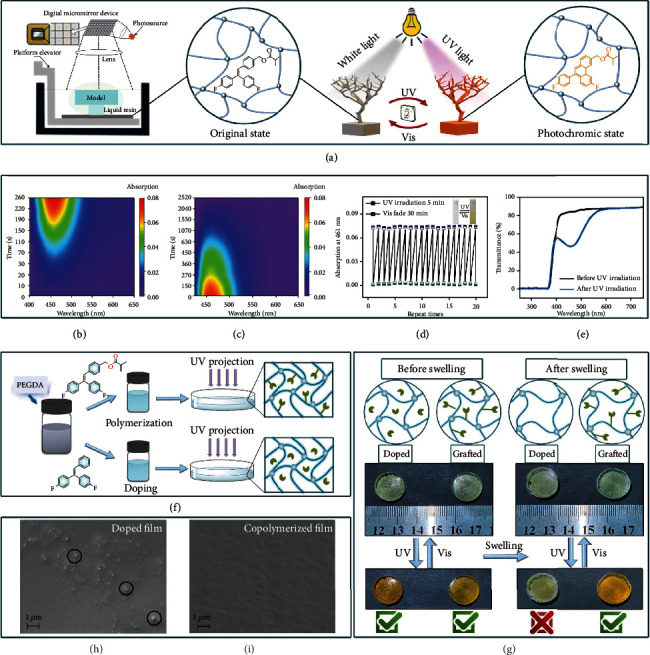
(a) Illustration of the photoresponsive material using the DLP 3D printing technology. (b) Time-dependent UV-Vis absorption spectra of TrPEF2-MA in degassed THF solution (1.0 × 10−1 M) during the irradiation process. (c) Time-dependent UV-Vis absorption spectra of TrPEF2-MA in degassed THF solution (1.0 × 10−1 M) during the bleaching process. (d) Recycling of the photochromic processes for TrPEF2-MA in solution state as a function of exposure to UV light (365 nm) and visible light for 5 minutes and 30 minutes, respectively. (e) The transmittance of the TrPEF2-MA polymer films with the thickness of ca. 2 mm before and after UV irradiation. (f) Different preparation processes of TrPEF2-MA containing doped and copolymerized films. (g) Comparison of photochromic properties between doped and grafted materials before and after swelling. SEM images of TrPEF2-MA containing doped (h) and copolymerized (i) films.
2. Results and Discussion
2.1. Synthesis and Photochromic Properties of TrPEF2-MA
The synthetic details of 4-(2,2-bis(4-fluorophenyl)vinyl) benzyl methacrylate (TrPEF2-MA) are described in the Supporting Information. All compounds were characterized with 1H NMR spectroscopy and high-resolution mass spectrometry. The 3D-printable photochromic material TrPEF2-MA was achieved with decent synthetic yields of 37.3% through a simple three-step synthetic route as shown in Scheme S1 in Supporting Information. The low-cost starting materials, simple synthetic route, and high synthetic yields provided the possibility of large-scale preparation of TrPEF2-MA.
Upon UV excitation (365 nm), noticeable photochromism of TrPEF2-MA was observed both in solution and thin film. The color changes of TrPEF2-MA was ascribed to the reversible ring-closure reaction of triphenylethylene group [51], as illustrated in Figure 2(a), according to previous literatures with similar structures. In THF solution, a low-energy absorption band with the maximum at 461 nm was observed, which gradually increased with the increasing irradiation time. After irradiation for ca. 260 seconds, enhancement of the absorption band stopped (Figure 2(b)). With consequent visible light irradiation, the color of TrPEF2-MA solution gradually faded to its original color in 2520 seconds (Figure 2(c)). Moreover, thermal-back reaction (ring-opening reaction in dark) of TrPEF2-MA in THF (1.0 × 10−1 mol/L) was also achieved according to the fitting curve of the absorbance band at 461 nm in the time-dependent absorption studies. The thermal-back reaction is relatively slow, and the half-life in THF solution is 597.5 seconds in dark at 303 K (Figure S6). In order to provide evidence for photochromic mechanism and identify the ring-closure structure of TrPEF2-MA. By exposing TrPEF2-MA under UV irradiation in oxygen atmosphere for 12 hours, dehydrogenated product TrPEF2-MA(O) was obtained. As shown in Scheme S2, it can be concluded from the chemical structure of TrPEF2-MA(O) that the photochromic mechanism is caused by the ring-closure reaction. In addition, compound TrPEF2-MA displayed an excellent reversibility during the photochromic process, which shows no obvious fatigue even after 20 photochromic and bleaching cycles (Figure 2(d)). The excellent recyclability and slow thermal-back reaction indicate its huge potential applications as rewritable and reversible photoresponsive materials.
The fact that TrPEF2-MA is an oily liquid at room temperature, the photochromic properties of TrPEF2-MA films added into polymer matrix were also investigated. 2-mm-thick photochromic films with the doping concentration of 10 wt% in polymer were prepared by photocuring the 3D-printable resin. The preparation details of the polymer films are described in the Supporting Information. Photophysical studies of TrPEF2-MA films were listed in Figure S1. The transparent flexible films showed good flexibility and photochromic properties. Upon UV light irradiation for ca. 50 seconds, the polymer films changed from colorless to yellow; simultaneously, the emission properties of the polymer films were quenched. Similar to the TrPEF2-MA in solution, a reverse process occurred when irradiated by visible light. The UV-Vis absorption spectra as a function of the photochromic bleaching time in films are shown in Figure S5. It can be clearly seen that the films faded within 2310 seconds upon visible light irradiation. Compared with the solution state (THF), the absorption bands of the doped film were slightly red-shifted to 482 nm, which is mainly due to the environmental constraints in the rigid polymer matrix [58]. The light transmittance of this film reached over 80% in daylight, while drastic decrement was observed in the range of 400 to 600 nm when exposed to UV light, as shown in Figure 2(e). The distinctive transmitting properties of the polymer films provide numerous potential applications, such as photoswitchable patterning and anticounterfeiting applications in solid surface and interface [59]. Moreover, the stability of the doped and copolymerized photoresponsive polymer samples was investigated as shown in Figure 2(f). The fabrication procedure of polymer films was described in details in the Supporting Information [51]. After soaking polymer films in THF solution overnight, the photochromic behavior of the doped polymer films disappeared, while the copolymerized films retained (Figure 2(g)). It reveals that the photoresponsive functional groups in doped polymer film are unstable when dissolved in organic solvent. In copolymerized films, the grafted triphenylethylene groups are much more stable under organic solvent corrosion. Moreover, the differential scanning calorimetry (DSC) and thermal gravimetric analyses (TGA) studies were carried out to study thermal properties of photoresponsive polymers (Figure S2 and S3). The copolymerized film showed a slightly higher degrading temperature (358°C) than that of the doped polymer film (271°C), indicating its better thermal stability. It could be ascribed to the strong covalent bonds between the cross-linking network. In addition, the scanning electron microscopy (SEM) studies of the two films were conducted as shown in Figures 2(h) and 2(i). It can be clearly seen that TrPEF2-MA can be more uniformly dispersed in the polymer matrix by copolymerization than direct doping. Hence, it could be concluded that the fabrication of photoresponsive 3D structures based on directly printable photoresponsive resins showed greater advantages both in thermal and chemical stabilities than those doped photoresponsive resins.
2.2. Constructions of Photoresponsive 3D Structures
To fabricate various photoresponsive 3D structures, the photochromic liquid resins were directly used in DLP 3D printing process as shown in Figure 3(a). By adding different ratios of the photoresponsive molecule TrPEF2-MA in PEGDA (cross-linker) [60, 61] and TPO (initiator) [62], four types of 3D-printable liquid resins, resin A (without TrPEF2-MA), resin B (with 5 wt% TrPEF2-MA), resin C (with 10 wt% TrPEF2-MA), and resin D (with 20 wt% TrPEF2-MA), were prepared, and the details were listed in Table S1.
Figure 3.
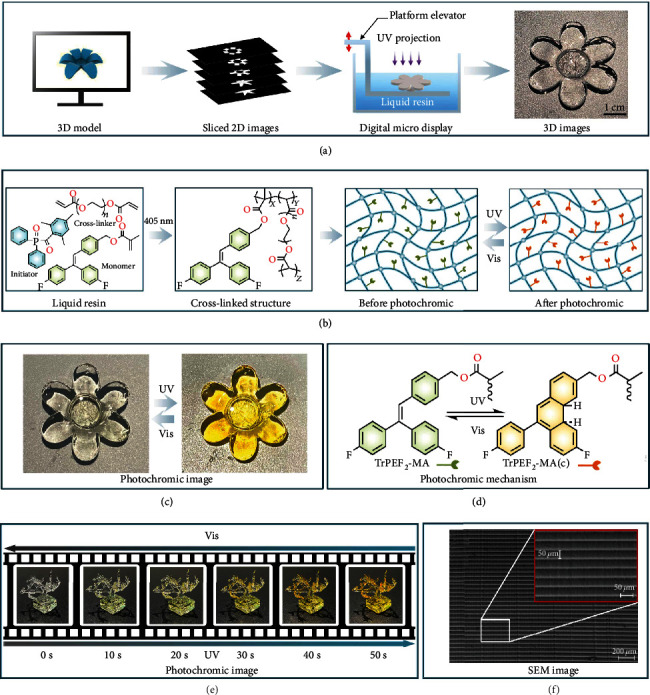
Constructions and the investigation of photoresponsive 3D structures. (a) Schematic of digital light processing-based 3D printing process. (b) Chemical structures of photopolymer inks (including monomer, cross-linker, and initiator) and formed cross-linked structure via photopolymerization and the general schemes of dynamic-based photochromic covalent bonds before and after UV/Vis irradiation. (c) Images of 3D-printed photoresponsive flower. (d) Schematic diagram of photochromic mechanism of triphenylethylene group. (e) Photochromic process of the 3D-printed tree based on TrPEF2-MA containing resin. (f) SEM images of the printed photoresponsive 3D structures.
As illustrated in Figure 3(b), the solidified 3D structures were macromolecular-based networks, which were composed of cross-linked PEGDA and grafted TrPEF2-MA functional groups. The copolymerized chemical structures were further verified by the Fourier transform infrared (FTIR) spectra as shown in Figure S4. With 3D-printable liquid resin C, a simple “light-responsive flower” 3D structure was fabricated (Figures 3(a) and 3(c)). This transparent “light-responsive flower” could gradually turn to bright yellow upon UV irradiation (50 seconds) and recover to transparent by visible light irradiation (2310 seconds). According to previous results, the photochromic property of the 3D printing structure could be ascribed to the ring-closure reaction of the TrPEF2-MA functional groups as shown in Figure 3(d) [51]. Likewise, a more complex 3D structure “light-responsive tree” was also printed with the same resin as shown in Figure 3(e). The specific printing parameters and operational details are presented in Table S2. This “light-responsive tree” gradually turned from transparent to orangish yellow within 50 seconds under 365-nm UV light. Thus, the color saturations of the photoresponsive 3D structures could be precisely manipulated by adjusting the UV irradiation time. In addition, SEM studies of the printed 3D structures were performed as shown in Figure 3(f). All showed uniform layers with the thickness of ca. 50 μm, which is consistent with the preset slice thickness, indicating the high fidelity during the DLP 3D printing process.
One of the most important advances for DLP 3D printing technology is that it can achieve the manufacture of complex hollow structures. A series of high-resolution hollow structures were fabricated with these photoresponsive 3D-printable resins containing different mass fractions of TrPEF2-MA. These printed honeycomb structure (resin B), modified hollow cube (resin C), and porous hollow sphere (resin D) showed excellent printing resolutions and smooth surfaces, indicating the excellent performances for these resins in fabricating complex structures (Figure 4(a)). Under sufficient UV irradiations, these 3D hollow structures with different proportions of TrPEF2-MA turned to pale yellow (resin B), bright yellow (resin C), and orangish yellow (resin D), respectively, as shown in Figure 4(a).
Figure 4.
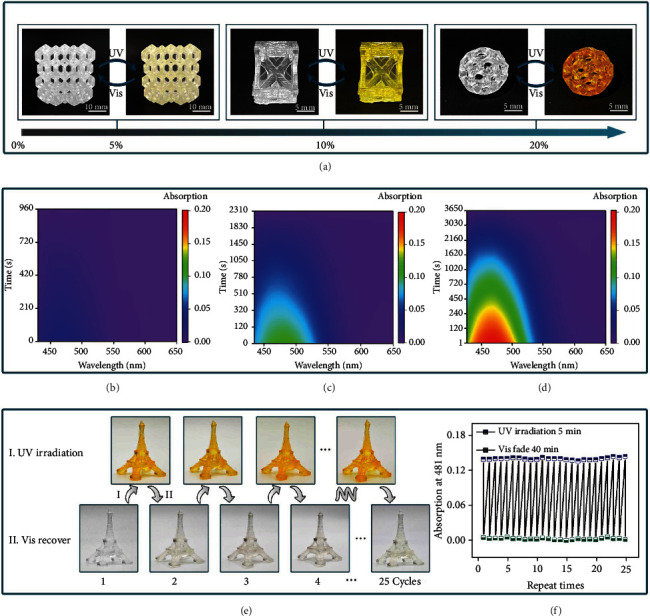
Photochromic properties of the printed 3D structures. (a) Photoresponsive pictures of the printed hollow 3D structures containing different mass fractions of TrPEF2-MA. (b) Time-dependent UV-Vis absorption spectra of the printed hollow 3D structures with resin B during the photochromic bleaching process. (c) Time-dependent UV-Vis absorption spectra of the printed hollow 3D structures with resin C during the photochromic bleaching process. (d) Time-dependent UV-Vis absorption spectra of the printed hollow 3D structures with resin D during the photochromic bleaching process. (e and f) Recycling of the photochromic process of 3D-printed Eiffel Tower as a function of exposure to UV light (365 nm) and visible light.
Moreover, UV/Vis reflectance spectra of these 3D hollow structures were collected. As shown in Figures 4(b)–4(d), the absorption maxima of resins B, C, and D are 468, 482, and 471 nm, respectively. It can be clearly seen that the absorption intensities significantly boosted with the increasing proportions of TrPEF2-MA, 0.032 in resin B, 0.108 in resin C, and 0.194 in resin D. Besides, the color maintaining times were prolonged from 960 seconds (resin B) to 3650 seconds (resin D), and the half-life was also extended from 261.5 seconds (resin B) to 866.4 seconds (resin D) as shown in Figure S7–S9. Moreover, through time-dependent UV-Vis absorption test at different temperatures, it was found the half-life of photochromism shortened with the increase of temperature as shown in Figure S10–S13. Thus, the color saturations and maintaining times of the photoresponsive 3D structures could be precisely controlled and easily adjusted by tuning the proportion of TrPEF2-MA in resins and temperature. In addition, all these complex hollow structures showed excellent printing resolutions and smooth surfaces, indicating the excellent stability for these resins during DLP 3D printing. Furthermore, the 3D hollow structures also displayed an excellent photochromic reversibility. After 25 photochromic and bleaching cycles, insignificant fatigue was detected (Figures 4(e)–4(f) and Movie S1). These superiorities of TrPEF2-MA indicate the potential applications in information hiding, information carrying, and others (Figure S14).
2.3. Multicomponent Photoresponsive 3D Structures
To clearly illustrate the potential applications of the photoresponsive 3D structures, multicomponent 3D printing processes were also performed with different resins to realize information hiding and information carrying in 3D structures. Firstly, a double-layered QR code structure was printed by automatically switching liquid resins during the printing process as shown in Figure 5(a) and Movie S2. The top layer (printed by liquid resin D) in the QR pattern was combined with a bottom plate (printed by liquid resin A). The printed double-layered QR code was transparent before UV irradiation, which could use for information hiding. Under UV light for 60 seconds, the top layer QR matrix was changed from transparent to yellow, which could be directly scanned with a cellphone to get the information “IFE.” The scannable QR code could be reversibly turned to transparent within 2310 seconds under visible light irradiation. In addition, a four-tier pyramid was printed with different resins containing TrPEF2-MA of 0, 5, 10, and 20 wt% from the bottom to the top, respectively (Figure 5(b)), as the color saturations of the photochromic 3D structure could be precisely tuned by manipulating the proportion of TrPEF2-MA in the resins. The photoreponsive four-tier pyramid altered to gradient-yellow upon UV light and recovered to transparent under visible light. The photoresponsive gradient-color 3D structures are quite striking in adaptive camouflage. As shown in Figure 5(c), a transparent framework was printed with resins A (0 wt% TrPEF2-MA) and C (10 wt% TrPEF2-MA) to realize information carrying and encryption. By rational arrangement of printing resins for the framework, information could be successfully hidden in the 3D structure. By irradiating the 3D framework with UV light, components printed with resin C turned to yellow, while the remaining component maintained transparent. Thus, four different numbers 6, 2, 9, and 0 appeared on each surface of the framework and disappeared under visible light irradiation. According to this demonstration, key information could be carried or hidden in the designed 3D structures. Therefore, the development of these new photoresponsive 3D-printable resins paves a new way to endow a wide variety of practical functions such as advanced anticounterfeiting, information hiding or carrying, and adaptive camouflage to traditional printed 3D structures.
Figure 5.
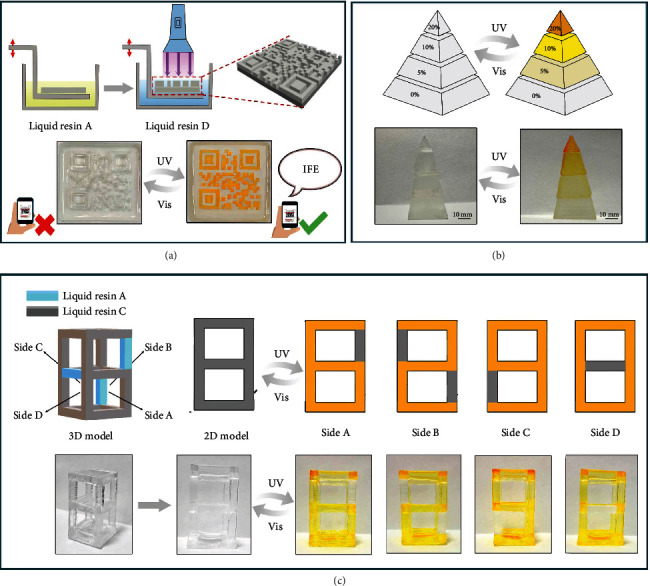
3D-printed multicomponent photoresponsive structures. (a) Schematic of 3D-printed multicomponent QR code with liquid resin A and resin D. (b) A printed photochromic four-tier pyramid with resins A-D. (c) Schematic of the 3D-printed multicomponent framework for information carrying and encryption.
3. Conclusion
In summary, we have proposed a rational strategy to construct photoresponsive prescribed complex 3D structures. By attaching the UV curable MA group to photochromic triphenylethylene, a photoresponsive material TrPEF2-MA that can be used for directly DLP 3D printing was realized. A series of photoresponsive 3D structures with high resolution (up to 50 μm) were fabricated. These 3D structures showed a striking color switching between transparent and yellow under alternating UV/visible light irradiation with good repeatability. To illustrate the information hiding and information-carrying properties, multicomponent DLP 3D printing processes were employed. Information hiding (QR codes), multicolor photoresponsive, and information-carrying multimaterial-based 3D structures were successfully printed. This work provides a novel and efficient strategy to construct photoresponsive 3D structures for information hiding and paves the way for more potential applications in adaptive camouflage, information storage, and flexible electronics.
Acknowledgments
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (51703253), the Fundamental Research Funds for the Central Universities, Key Research and Development Program of Shaanxi Province (2020GXLH-Z-010), the Chongqing Science and Technology Fund (cstc2020jcyj-msxmX0931), the Guangdong Basic and Applied Basic Research Foundation (2021A1515010633), the Ningbo Natural Science Foundation (202003N4060), the National Aerospace Science Foundation of China (2020Z073053007), the China Postdoctoral Science Foundation (2021M692624), the Natural Science Basic Research Program of Shaanxi Province (2022JQ-583), and the Henan Key Laboratory of Special Protective Materials (Grant No. SZKFKT202106).
Contributor Information
Biao Zhang, Email: iambzhang@nwpu.edu.cn.
Tao Yu, Email: iamtyu@nwpu.edu.cn.
Wei Huang, Email: iamdirector@fudan.edu.cn.
Data Availability
All relevant data that support the findings are available within this article and supporting information and are also available from authors upon reasonable request.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Authors' Contributions
Xiayu Zhang and Fukang Liu contributed equally to this work.
Supplementary Materials
Materials and methods, characterization data, emission spectra, TG analysis, DSC curves, absorption spectra of film, FT-IR spectra spectroscopy, and movies of 3D printing photoresponsive materials.
References
- 1.Qu D. H., Wang Q. C., Zhang Q. W., Ma X., Tian H. Photoresponsive host-guest functional systems. Chemical Reviews . 2015;115(15):7543–7588. doi: 10.1021/cr5006342. [DOI] [PubMed] [Google Scholar]
- 2.Jerca F. A., Jerca V. V., Hoogenboom R. Photoresponsive polymers on the move. Chem . 2017;3(4):533–536. doi: 10.1016/j.chempr.2017.09.010. [DOI] [Google Scholar]
- 3.Stuart M. A. C., Huck W. T., Genzer J., et al. Emerging applications of stimuli-responsive polymer materials. Nature Materials . 2010;9(2):101–113. doi: 10.1038/nmat2614. [DOI] [PubMed] [Google Scholar]
- 4.Bisoyi H. K., Li Q. Light-driven liquid crystalline materials: from photo-induced phase transitions and property modulations to applications. Chemical Reviews . 2016;116(24):15089–15166. doi: 10.1021/acs.chemrev.6b00415. [DOI] [PubMed] [Google Scholar]
- 5.Gu Y., Alt E. A., Wang H., Li X., Willard A. P., Johnson J. A. Photoswitching topology in polymer networks with metal-organic cages as crosslinks. Nature . 2018;560(7716):65–69. doi: 10.1038/s41586-018-0339-0. [DOI] [PubMed] [Google Scholar]
- 6.Kitamura I., Kato K., Berk R. B., et al. Photo-triggered large mass transport driven only by a photoresponsive surface skin layer. Scientific Reports . 2020;10(1):1–10. doi: 10.1038/s41598-020-69605-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lv J., Liu Y., Wei J., Chen E., Qin L., Yu Y. Photocontrol of fluid slugs in liquid crystal polymer microactuators. Nature . 2016;537(7619):179–184. doi: 10.1038/nature19344. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Z., Wang W., Jin P., et al. A building-block design for enhanced visible-light switching of diarylethenes. Nature Communications . 2019;10(1):1–9. doi: 10.1038/s41467-019-12302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z., Liu X., Wang G., et al. Photoresponsive supramolecular coordination polyelectrolyte as smart anticounterfeiting inks. Nature Communications . 2021;12(1):1–8. doi: 10.1038/s41467-021-21677-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corredor C. C., Huang Z. L., Belfield K. D. Two-photon 3D optical data storage via fluorescence modulation of an efficient fluorene dye by a photochromic diarylethene. Advanced Materials . 2006;18(21):2910–2914. doi: 10.1002/adma.200600826. [DOI] [Google Scholar]
- 11.Kaspar C., Ravoo B., van der Wiel W. G., Wegner S., Pernice W. The rise of intelligent matter. Nature . 2021;594(7863):345–355. doi: 10.1038/s41586-021-03453-y. [DOI] [PubMed] [Google Scholar]
- 12.Roeffaers M. B., Sels B. F., Uji-i H., et al. Spatially resolved observation of crystal-face-dependent catalysis by single turnover counting. Nature . 2006;439(7076):572–575. doi: 10.1038/nature04502. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L., Liang H., Jacob J., Naumov P. Erratum: photogated humidity-driven motility. Nature Communications . 2015;6(1):1–12. doi: 10.1038/ncomms8862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rust M. J., Bates M., Zhuang X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM) Nature Methods . 2006;3(10):793–796. doi: 10.1038/nmeth929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welleman I. M., Hoorens M. W., Feringa B. L., Boersma H. H., Szymański W. Photoresponsive molecular tools for emerging applications of light in medicine. Chemical Science . 2020;11(43):11672–11691. doi: 10.1039/D0SC04187D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang Y., Tan P., Qi S. C., et al. Metal–organic frameworks with target-specific active sites switched by photoresponsive motifs: efficient adsorbents for tailorable CO2 Capture. Angewandte Chemie International Edition . 2019;58(20):6600–6604. doi: 10.1002/anie.201900141. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Y., Tan P., Liu X. Q., Sun L. B. Process-oriented smart adsorbents: tailoring the properties dynamically as demanded by adsorption/desorption. Accounts of Chemical Research . 2022;55(1):75–86. doi: 10.1021/acs.accounts.1c00555. [DOI] [PubMed] [Google Scholar]
- 18.Tian H., Yang S. Recent progresses on diarylethene based photochromic switches. Chemical Society Reviews . 2004;33(2):85–97. doi: 10.1039/b302356g. [DOI] [PubMed] [Google Scholar]
- 19.Wezenberg S. J., Feringa B. L. Supramolecularly directed rotary motion in a photoresponsive receptor. Nature Communications . 2018;9(1):1–7. doi: 10.1038/s41467-018-04249-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iamsaard S., Villemin E., Lancia F., Abhoff S. J., Fletcher S. P., Katsonis N. Preparation of biomimetic photoresponsive polymer springs. Nature Protocols . 2016;11(10):1788–1797. doi: 10.1038/nprot.2016.087. [DOI] [PubMed] [Google Scholar]
- 21.Poon C. T., Lam W. H., Yam V. W. W. Gated photochromism in triarylborane-containing dithienylethenes: a new approach to a “lock-unlock” system. Journal of the American Chemical Society . 2011;133(49):19622–19625. doi: 10.1021/ja208097a. [DOI] [PubMed] [Google Scholar]
- 22.Chan J. C. H., Lam W. H., Yam V. W. W. A highly efficient silole-containing dithienylethene with excellent thermal stability and fatigue resistance: a promising candidate for optical memory storage materials. Journal of the American Chemical Society . 2014;136(49):16994–16997. doi: 10.1021/ja5101855. [DOI] [PubMed] [Google Scholar]
- 23.Wu N. M. W., Ng M., Yam V. W. W. Photochromic benzo[b]phosphole alkynylgold(I) complexes with mechanochromic property to serve as multistimuli-responsive materials. Angewandte Chemie International Edition . 2019;58(10):3027–3031. doi: 10.1002/anie.201806272. [DOI] [PubMed] [Google Scholar]
- 24.Wu N. M. W., Ng M., Lam W. H., Wong H. L., Yam V. W. W. Photochromic heterocycle-fused thieno [3, 2-b] phosphole oxides as visible light switches without sacrificing photoswitching efficiency. Journal of the American Chemical Society . 2017;139(42):15142–15150. doi: 10.1021/jacs.7b08333. [DOI] [PubMed] [Google Scholar]
- 25.Bandara H. D., Burdette S. C. Photoisomerization in different classes of azobenzene. Chemical Society Reviews . 2012;41(5):1809–1825. doi: 10.1039/C1CS15179G. [DOI] [PubMed] [Google Scholar]
- 26.Gelebart A. H., Jan Mulder D., Varga M., et al. Making waves in a photoactive polymer film. Nature . 2017;546(7660):632–636. doi: 10.1038/nature22987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou H., Xue C., Weis P., et al. Photoswitching of glass transition temperatures of azobenzene-containing polymers induces reversible solid-to-liquid transitions. Nature Chemistry . 2017;9(2):145–151. doi: 10.1038/nchem.2625. [DOI] [PubMed] [Google Scholar]
- 28.Baroncini M., d'Agostino S., Bergamini G., et al. Photoinduced reversible switching of porosity in molecular crystals based on star-shaped azobenzene tetramers. Nature Chemistry . 2015;7(8):634–640. doi: 10.1038/nchem.2304. [DOI] [PubMed] [Google Scholar]
- 29.Qi Q., Li C., Liu X., et al. Solid-state photoinduced luminescence switch for advanced anticounterfeiting and super-resolution imaging applications. Journal of the American Chemical Society . 2017;139(45):16036–16039. doi: 10.1021/jacs.7b07738. [DOI] [PubMed] [Google Scholar]
- 30.Kortekaas L., Browne W. R. The evolution of spiropyran: fundamentals and progress of an extraordinarily versatile photochrome. Chemical Society Reviews . 2019;48(12):3406–3424. doi: 10.1039/C9CS00203K. [DOI] [PubMed] [Google Scholar]
- 31.Kundu P. K., Samanta D., Leizrowice R., et al. Light-controlled self-assembly of non-photoresponsive nanoparticles. Nature Chemistry . 2015;7(8):646–652. doi: 10.1038/nchem.2303. [DOI] [PubMed] [Google Scholar]
- 32.Bahajaj A. A., Asiri A. M. Photochromic properties of (E)-dicyclopropyl- methylene-(2,5-dimethyl-3-furylethylidene)-succinic anhydride doped in PMMA polymer film. Optical Materials . 2006;28(8-9):1064–1067. doi: 10.1016/j.optmat.2005.01.030. [DOI] [Google Scholar]
- 33.Yokoyama Y. Fulgides for memories and switches. Chemical Reviews . 2000;100(5):1717–1740. doi: 10.1021/cr980070c. [DOI] [PubMed] [Google Scholar]
- 34.Torres-Pierna H., Ruiz-Molina D., Roscini C. Highly transparent photochromic films with a tunable and fast solution-like response. Materials Horizons . 2020;7(10):2749–2759. doi: 10.1039/D0MH01073A. [DOI] [Google Scholar]
- 35.Müller L. A., Zimmermann T., Nyström G., Burgert I., Siqueira G. Mechanical properties tailoring of 3D printed photoresponsive nanocellulose composites. Advanced Functional Materials . 2020;30(35):p. 2002914. doi: 10.1002/adfm.202002914. [DOI] [Google Scholar]
- 36.Tannouri P., Arafeh K. M., Krahn J. M., Beaupré S. L., Menon C., Branda N. R. A photoresponsive biomimetic dry adhesive based on doped PDMS microstructures. Chemistry of Materials . 2014;26(15):4330–4333. doi: 10.1021/cm502222c. [DOI] [Google Scholar]
- 37.Wen X., Zhang B., Wang W., et al. 3D-printed silica with nanoscale resolution. Nature Materials . 2021;20(11):1506–1511. doi: 10.1038/s41563-021-01111-2. [DOI] [PubMed] [Google Scholar]
- 38.Walker D. A., Hedrick J. L., Mirkin C. A. Rapid, large-volume, thermally controlled 3D printing using a mobile liquid interface. Science . 2019;366(6463):360–364. doi: 10.1126/science.aax1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ligon S. C., Liska R., Stampfl J., Gurr M., Mülhaupt R. Polymers for 3D printing and customized additive manufacturing. Chemical Reviews . 2017;117(15):10212–10290. doi: 10.1021/acs.chemrev.7b00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuk H., Lu B., Lin S., et al. 3D printing of conducting polymers. Nature Communications . 2020;11(1):1–8. doi: 10.1038/s41467-020-15316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang B., Kowsari K., Serjouei A., Dunn M. L., Ge Q. Reprocessable thermosets for sustainable three-dimensional printing. Nature Communications . 2018;9(1):1–7. doi: 10.1038/s41467-018-04292-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Truby R. L., Lewis J. A. Printing soft matter in three dimensions. Nature . 2016;540(7633):371–378. doi: 10.1038/nature21003. [DOI] [PubMed] [Google Scholar]
- 43.Yang H., Leow W. R., Wang T., et al. 3D printed photoresponsive devices based on shape memory composites. Advanced Materials . 2017;29(33) doi: 10.1002/adma.201701627. [DOI] [PubMed] [Google Scholar]
- 44.Li J., Pumera M. 3D printing of functional microrobots. Chemical Society Reviews . 2021;50(4):2794–2838. doi: 10.1039/D0CS01062F. [DOI] [PubMed] [Google Scholar]
- 45.Gliozzi A. S., Miniaci M., Chiappone A., Bergamini A., Morin B., Descrovi E. Tunable photo-responsive elastic metamaterials. Nature Communications . 2020;11(1):1–8. doi: 10.1038/s41467-020-16272-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nocentini S., Martella D., Parmeggiani C., Wiersma D. S. 3D printed photoresponsive materials for photonics. Advanced Optical Materials . 2019;7(16):p. 1900156. doi: 10.1002/adom.201900156. [DOI] [Google Scholar]
- 47.Ruskowitz E. R., DeForest C. A. Photoresponsive biomaterials for targeted drug delivery and 4D cell culture. Nature Reviews Materials . 2018;3(2):1–17. doi: 10.1038/natrevmats.2017.87. [DOI] [Google Scholar]
- 48.Powell A. W., Stavrinadis A., Christodoulou S., Quidant R., Konstantatos G. On-demand activation of photochromic nanoheaters for high color purity 3D printing. Nano Letters . 2020;20(5):3485–3491. doi: 10.1021/acs.nanolett.0c00414. [DOI] [PubMed] [Google Scholar]
- 49.Wales D. J., Cao Q., Kastner K., Karjalainen E., Newton G. N., Sans V. 3D-printable photochromic molecular materials for reversible information storage. Advanced Materials . 2018;30(26) doi: 10.1002/adma.201800159. [DOI] [PubMed] [Google Scholar]
- 50.Ulrich S., Wang X., Rottmar M., et al. Nano-3D-printed photochromic micro-objects. Nano-3D-Printed Photochromic Micro‐Objects. Small . 2021;17(26):p. 2101337. doi: 10.1002/smll.202101337. [DOI] [PubMed] [Google Scholar]
- 51.Ou D., Yu T., Yang Z., et al. Combined aggregation induced emission (AIE), photochromism and photoresponsive wettability in simple dichloro-substituted triphenylethylene derivatives. Chemical Science . 2016;7(8):5302–5306. doi: 10.1039/C6SC01205A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu T., Ou D., Wang L., et al. A new approach to switchable photochromic materials by combining photochromism and piezochromism together in an AIE-active molecule. Materials Chemistry Frontiers . 2017;1(9):1900–1904. doi: 10.1039/C7QM00160F. [DOI] [Google Scholar]
- 53.Wang L., Yu T., Xie Z., et al. Design, synthesis and photochromism studies of thienyl containing triarylethylene derivatives and their applications in real-time photoresponsive surfaces. Journal of Materials Chemistry C . 2018;6(32):8832–8838. doi: 10.1039/C8TC02698J. [DOI] [Google Scholar]
- 54.Zhang X., Yu T., Huang C., et al. Switching excitons between the emissive and photochromic pathways in the triphenylethylene system. Journal of Materials Chemistry C . 2021;9(34):11126–11131. doi: 10.1039/D1TC02393D. [DOI] [Google Scholar]
- 55.Kuang X., Wu J., Chen K., et al. Grayscale digital light processing 3D printing for highly functionally graded materials. Science advances . 2019;5(5):p. eaav5790. doi: 10.1126/sciadv.aav5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aubert T., Huang J. Y., Ma K., Hanrath T., Wiesner U. Porous cage-derived nanomaterial inks for direct and internal three- dimensional printing. Nature Communications . 2020;11(1):1–9. doi: 10.1038/s41467-020-18495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang C., Boorugu M., Dopp A., et al. 4D printing reconfigurable, deployable and mechanically tunable metamaterials. Materials Horizons . 2019;6(6):1244–1250. doi: 10.1039/C9MH00302A. [DOI] [Google Scholar]
- 58.Kim S., Yoon S. J., Park S. Y. Highly fluorescent chameleon nanoparticles and polymer films: multicomponent organic systems that combine FRET and photochromic switching. Journal of the American Chemical Society . 2012;134(29):12091–12097. doi: 10.1021/ja3027295. [DOI] [PubMed] [Google Scholar]
- 59.Li N., Cao X., Chang T., Long S., Jin P. Selective photochromism in a self-coated WO3/WO3-x homojunction: enhanced solar modulation efficiency, high luminous transmittance and fast self-bleaching rate. Nanotechnology . 2019;30(25):p. 255703. doi: 10.1088/1361-6528/ab0778. [DOI] [PubMed] [Google Scholar]
- 60.Ge Q., Chen Z., Cheng J., et al. 3D printing of highly stretchable hydrogel with diverse UV curable polymers. Science Advances . 2021;7(2, article eaba4261) doi: 10.1126/sciadv.aba4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee J., Bisso P. W., Srinivas R. L., Kim J. J., Swiston A. J., Doyle P. S. Universal process-inert encoding architecture for polymer microparticles. Nature Materials . 2014;13(5):524–529. doi: 10.1038/nmat3938. [DOI] [PubMed] [Google Scholar]
- 62.Pawar A. A., Saada G., Cooperstein I., et al. High-performance 3D printing of hydrogels by water-dispersible photoinitiator nanoparticles. Science Advances . 2016;2(4, article e1501381) doi: 10.1126/sciadv.1501381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Materials and methods, characterization data, emission spectra, TG analysis, DSC curves, absorption spectra of film, FT-IR spectra spectroscopy, and movies of 3D printing photoresponsive materials.
Data Availability Statement
All relevant data that support the findings are available within this article and supporting information and are also available from authors upon reasonable request.


