To the Editor: Clinical trials and real-world data generally suggest that biologics do not increase susceptibility to COVID-19.1 However, it remains unknown whether these therapies may confer a protective effect against contracting COVID-19. Therefore, we sought to assess the risk of COVID-19 infection in patients with psoriasis compared with the general population and in patients receiving systemic and topical therapies. This study used the Symphony Health dataset, a large repository of pharmacy data, inpatient and outpatient medical claims, and remittance data (over 300 million patients, 7 million COVID-19 cases, and payer information: Medicaid/Medicare/commercial/cash).
Patients with at least 2 recorded International Classification of Diseases-10 diagnosis codes for psoriasis (L40.x) (n = 167,027) and controls without International Classification of Diseases-10 codes for psoriasis (n = 1,002,162) were randomly sampled in a 1:6 ratio between May 1, 2019, and January 1, 2020. Two recorded diagnosis codes for psoriasis were required to increase the positive predictive value, a strategy employed by prior studies.2 , 3 Each patient was assigned to 1 of 9 mutually exclusive cohorts based on the last prescription dispense (biologic: Tumor necrosis factor [TNF]-α inhibitor, ustekinumab, interleukin [IL] 17 inhibitor, and IL-23 inhibitor; oral: acitretin, cyclosporine, methotrexate, and apremilast cohorts; topical: none of the above medications). Follow-up began on January 1, 2020, and ended with the first occurrence of any of the following: (1) COVID-19 diagnosis code or (2) November 11, 2020 (the end of the study). Vaccination status was unable to be ascertained from the database because Emergency Use Authorization vaccine approved by the Food and Drug Administration did not occur until December 2020.
Demographics were summarized by frequency (percentage) and mean (SD) (Table I ). Logistic regression models were constructed with psoriasis status as the independent variable, COVID-19 International Classification of Diseases-10 diagnosis code as the dependent variable, and the following covariates: age, sex, race, congestive heart failure (I50.X), chronic obstructive pulmonary disease (J41/J43/J44), type-2 diabetes mellitus (E11.x/E13.x), and obesity (E66.0-E66.2/E66.8-E66.9/Z68.3-Z68.5).
Table I.
Cohort characteristics
| Demographics | Psoriasis (n = 167,027) | No psoriasis (n = 1,002,162) | Total (n = 1,169,189) |
|---|---|---|---|
| Male No. (%) | 77,725 (46.5) | 444,472 (44.3) | 522,197 (44.7) |
| Age, mean (SD), y | 58.1 (13.6) | 57.7 (16.1) | 57.7 (15.7) |
| Race No. (%) | |||
| Caucasian | 132,036 (79.1) | 748,490 (74.7) | 880,526 (75.3) |
| Hispanic | 15,568 (9.3) | 90,413 (9.0) | 105,981 (9.1) |
| African American | 13,848 (8.3) | 130,392 (13.0) | 144,240 (12.3) |
| Asian | 2894 (1.7) | 17,171 (1.7) | 20,065 (1.7) |
| Other | 2681 (1.6) | 15,696 (1.6) | 18,377 (1.6) |
| High-risk factors (ICD-10) for COVID-19, No. (%) | |||
| Congestive heart failure | 10,354 (6.2) | 48,025 (4.8) | 58,379 (5.0) |
| Type 1 diabetes mellitus | 37,975 (22.7) | 158,987 (15.9) | 196,962 (16.9) |
| Obesity | 44,557 (26.7) | 145,347 (14.5) | 189,904 (16.2) |
| Chronic obstructive pulmonary disease | 16,514 (9.9) | 64,145 (6.4) | 80,659 (6.9) |
| Psoriasis treatment cohorts∗ | |||
| Topical | 99,395 (59.5) | NA | NA |
| Systemic treatments | Oral systemic cohort, n = 31,468 (18.8) | Biologic cohort†, n = 36,164 (21.7) | Total systemic treatments received, n = 67,632 |
|---|---|---|---|
| Oral systemics No. (%) | |||
| Methotrexate | 21,478 (68.3) | 230 (0.6) | 21,708 (32.1) |
| Apremilast | 7398 (23.5) | 99 (0.3) | 7497 (11.1) |
| Cyclosporine | 1573 (5.0) | 7 (0.02) | 1580 (2.3) |
| Acitretin | 1072 (3.4) | 5 (0.01) | 1077 (1.6) |
| Biologics No. (%) | |||
| TNF-α inhibitors | |||
| Adalimumab | 0 | 9553 (26.4) | 9553 (14.1) |
| Infliximab | 0 | 3366 (9.3) | 3366 (5.0) |
| Etanercept | 0 | 4201 (11.6) | 4201 (6.2) |
| Certolizumab | 0 | 1438 (4.0) | 1438 (2.1) |
| IL-12/23 inhibitor | |||
| Ustekinumab | 0 | 5085 (14.1) | 5085 (7.5) |
| IL-17 inhibitors | |||
| Secukinumab | 0 | 6266 (17.3) | 6266 (9.3) |
| Ixekizumab | 0 | 3135 (8.7) | 3135 (4.6) |
| Brodalumab | 0 | 142 (0.4) | 142 (0.2) |
| IL-23 inhibitors | |||
| Guselkumab | 0 | 1687 (4.7) | 1687 (2.5) |
| Risankizumab | 0 | 1021 (2.8) | 1021 (1.5) |
| Tildrakizumab | 0 | 312 (0.9) | 312 (0.5) |
ICD, International Classification of Diseases; IL, interleukin; NA, not available; TNF, tumor necrosis factor.
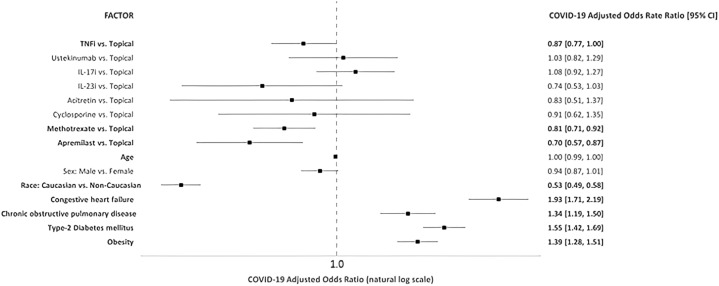
Psoriasis was associated with 18% higher odds of incident COVID-19 (adjusted odd ratio [aOR], 1.18; 95% CI, 1.13-1.23) compared with controls (Supplementary Fig 1, available via Mendeley at https://data.mendeley.com/datasets/68fht87h68/1). In contrast to data from Northeast Italian cohorts, our results appear to align with recent findings from a global registry-based study suggesting that patients receiving no systemic therapy were estimated to have an increased risk of COVID-19 hospitalization compared with patients on biologics.4 , 5 In analyses of psoriasis patients (Fig 1 ), TNF inhibitor (aOR, 0.87; 95% CI, 0.77-1.00), methotrexate (aOR, 0.81; 95% CI, 0.71-0.92), and apremilast (aOR, 0.70; 95% CI, 0.57-0.87) use had decreased odds of incident COVID-19 compared with patients on topical therapy. Odds ratios remained unchanged after excluding patients on concomitant biologic and oral therapy. Among the limitations, first, we cannot differentiate between the impact of psoriasis severity and systemic therapy on the risk of COVID-19, because disease severity was defined based on treatment history. Second, smoking status and other cardiovascular comorbidities were not adjusted in the logistic regression model. Nonetheless, the protective role exerted by TNF-inhibitor and methotrexate is supported by the mechanistic plausibility of proinflammatory cytokine inhibition, particularly of TNF-α, IL-6, and IL-1. Our findings suggest that these drug classes do not increase the risk of acquiring COVID-19 and, thus, are safe options for continuing psoriasis treatment during the COVID-19 pandemic.
Fig 1.
Multivariable logistic regression assessing factors (International Classification of Diseases-10) associated with COVID-19 infection comparing systemic versus topical therapy.∗
∗Multivariable logistic regression models were constructed with COVID-19 as the dependent variable, the treatment cohort as the independent variable with the topical cohort as the reference group. The following covariates were specified in the model: age (linear), sex (male vs female), race (Caucasian vs non-Caucasian), congestive heart failure, chronic obstructive pulmonary disease, type 2 diabetes mellitus, and obesity. Adjusted odds ratios were computed for all treatment comparisons with the topical cohort. P value of <.05 was considered significant. CI, Confidence interval; IL, interleukin; TNFi, tumor necrosis factor inhibitor.
Conflicts of interest
Dr Wu is or has been an investigator, consultant, or speaker for AbbVie, Almirall, Amgen, Arcutis, Aristea Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Dermavant, Dr Reddy's Laboratories, Eli Lilly, Galderma, Janssen, LEO Pharma, Mindera, Novartis, Regeneron, Sanofi Genzyme, Solius, Sun Pharmaceutical, UCB, Valeant Pharmaceuticals North America LLC, and Zerigo Health. With no relation to the present work, Dr Egeberg has received research funding from Pfizer, Eli Lilly, Novartis, Bristol-Myers Squibb, AbbVie, Janssen Pharmaceuticals, the Danish National Psoriasis Foundation, the Simon Spies Foundation, and the Kgl Hofbundtmager Aage Bang Foundation, and honoraria as a consultant and/or speaker from AbbVie, Almirall, Leo Pharma, Galápagos NV, Sun Pharmaceuticals, Samsung Bioepis Co, Ltd, Pfizer, Eli Lilly and Company, Novartis, Galderma, Dermavant, UCB, Mylan, Bristol-Myers Squibb, and Janssen Pharmaceuticals. Authors Liu, Thatiparthi, and Martin have no conflicts of interest to declare.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Jones M.E., Kohn A.H., Pourali S.P., et al. The use of biologics during the COVID-19 pandemic. Dermatol Clin. 2021;39(4):545–553. doi: 10.1016/j.det.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Icen M., Crowson C.S., McEvoy M.T., Gabriel S.E., Maradit Kremers H. Potential misclassification of patients with psoriasis in electronic databases. J Am Acad Dermatol. 2008;59(6):981–985. doi: 10.1016/j.jaad.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takeshita J., Gelfand J.M., Li P., et al. Psoriasis in the US Medicare population: prevalence, treatment, and factors associated with biologic use. J Invest Dermatol. 2015;135(12):2955–2963. doi: 10.1038/jid.2015.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piaserico S., Gisondi P., Cazzaniga S., Naldi L. Lack of evidence for an increased risk of severe COVID-19 in psoriasis patients on biologics: a cohort study from Northeast Italy. Am J Clin Dermatol. 2020;21(5):749–751. doi: 10.1007/s40257-020-00552-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahil S.K., Dand N., Mason K.J., et al. Factors associated with adverse COVID-19 outcomes in patients with psoriasis-insights from a global registry-based study. J Allergy Clin Immunol. 2021;147(1):60–71. doi: 10.1016/j.jaci.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]