Keywords: guanylate cyclase, nitric oxide, pulmonary hypertension, transferrin receptor-1
Abstract
This study examines if heme biosynthesis-associated iron metabolism is regulated in pulmonary arteries by endothelin-1 (ET1) potentially through modulating cartilage oligomeric matrix protein (COMP) availability. Our studies in organoid-cultured endothelium-rubbed bovine pulmonary arteries (BPAs) observed COMP depletion by siRNA or hypoxia increases NOX2 and superoxide and depletes mitochondrial SOD2. ET1 also increases superoxide in a manner that potentially impairs mitochondrial heme biosynthesis. In this study, organoid culture of BPA with ET1 (10 nM) increases superoxide in the mitochondrial matrix and extramitochondrial regions associated with COMP depletion, and COMP (0.5 μM) inhibited these superoxide increases. As mitochondrial matrix superoxide could impair heme biosynthesis from protoporphyrin IX (PpIX) by decreasing Fe2+ availability and/or ferrochelatase (FECH), we studied ET1, COMP, and COMP siRNA effects on the expression of FECH, transferrin receptor-1 (TfR1, an indicator of iron availability) and soluble guanylate cyclase (sGC, a key heme-dependent protein), and on measurements of PpIX (HPLC) and heme content. ET1 decreased FECH, heme, and sGC, and increased TfR1 and iron. COMP reversed these effects of ET1, and COMP decreased PpIX and increased heme in the absence of ET1. COMP siRNA increased PpIX detection and TfR1 expression and decreased the expression of FECH and sGC. Nitric oxide (spermine NONOate) relaxation of BPA was inhibited by ET1, and this was attenuated by COMP during exposure to ET1. Thus, COMP depletion by ET1 or siRNA modulates pulmonary artery iron metabolism, which results in loss of heme biosynthesis and heme-dependent cGMP mechanisms.
INTRODUCTION
The extracellular matrix (ECM) protein cartilage oligomeric matrix protein (COMP) appears to have a role in maintaining the contractile phenotype of both pulmonary (1, 2) and systemic (3, 4) arterial smooth muscle potentially via its interactions with integrins and/or the type 2 BMP receptor (BMPR2). Our recent studies have detected evidence for hypoxia depleting COMP in cultured rat pulmonary arterial smooth muscle cells, organoid-cultured bovine pulmonary arteries (BPAs), and in lungs from mice and rats exposed to hypoxia (1, 2). In BPA, the depletion of COMP by hypoxia was associated with an increase in mitochondrial superoxide and decrease in the mitochondrial matrix superoxide dismutase, SOD2. As our recent studies have provided evidence for increased mitochondrial superoxide having roles in disrupting mitochondrial heme biosynthesis resulting from either decreased ferrochelatase (FECH) and/or availability of iron (Fe2+) needed for the conversion of protoporphyrin IX (PpIX) to heme (5, 6), we examined if COMP availability could alter aspects of iron metabolism in ways that could regulate heme biosynthesis in BPA.
Pulmonary hypertension (PH) is associated with a variety of evidence for aspects of iron deficiency and alterations in iron metabolism (7, 8). This included evidence for a lack of availability of iron for systemic heme biosynthesis in red blood cells in humans with pulmonary arterial hypertension (9). However, despite the wide array of evidence linking vascular dysfunction with PH, there is limited information on alterations in iron metabolism occurring in the pulmonary vasculature. Our previous study on organoid-cultured BPA exposed to endothelin-1 (ET1) provided evidence for an increase in mitochondrial superoxide based on HPLC detection of a superoxide-mediated oxidation product of MitoSox under conditions where mitochondrial matrix SOD2 was observed to be depleted and increased fluorescence potentially originating from an accumulation of PpIX could be detected (5). There is also evidence for the soluble form of guanylate cyclase (sGC) being depleted of its heme needed for activation by nitric oxide (NO) based on studies of the actions of a heme-independent activator of sGC in a neonatal pulmonary hypertension model (10). In addition, evidence exists for elevation of the transferrin receptor-1 (TfR1) being a factor contributing to the development of pulmonary hypertension (11). Thus, our study focuses on examining the role of COMP in regulating aspects of iron metabolism potentially contributing to a loss of heme biosynthesis and heme-dependent sGC that regulates pulmonary vascular function. In this study, we focused on probing the actions of COMP depletion by siRNA and the pulmonary hypertension mediator ET1 in organoid-cultured BPA, in the absence and presence of added COMP.
MATERIALS AND METHODS
Materials
All salts used for making physiological solutions were analyzed reagent grade from Baker Chemical. All gases were purchased from Airgas (Bronx, NY). Recombinant human cartilage oligomeric matrix protein (COMP) was obtained from Abcam (ab174082). Iron assay kit was purchased from Sigma-Aldrich (MAK025). Specific antibodies were purchased from the companies indicated: anti-COMP (Abcam, ab74524, 1:2,000), anti-SOD1 (Abcam, ab13498, 1:1,000), anti-SOD2 (Abcam, ab13533, 1:10,000), anti-SOD3 (Upstate Millipore, 07–704, 1:10,000), anti-NOX2 (Santa Cruz, sc-130543, 3:1,000), anti-NOX4 (Santa Cruz, sc-55142 1:200), anti-catalase (Abcam, ab16731 1:2,000), anti-ferrochelatase (Abcam, ab55965 1:1,000), anti-transferrin receptor 1 (Abcam, ab166929 1:200), anti-ferritin (Santa Cruz, sc-71102 1:200), anti-mitoferrin (Abcam, ab90170 1:200), anti-soluble guanylate cyclase β1 (Sigma, G4405 1:2,000), VDAC (Invitrogen, PA1-954A, 3:1,000), anti-GAPDH (Millipore Sigma, MAB374, 1:1,000), and anti-β actin (Sigma Chemical, A5441, 1:10,000). The ability of these antibodies to detect the proteins studied in the bovine species for the molecular weights indicated has been verified by the supplier and/or by multiple reports in the scientific literature, including data included in our previous studies (2, 6, 12). In addition, the COMP antibodies were also verified by siRNA data in this study and in our previous study (2). An inhibitor of soluble guanylate cyclase, ODQ (1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one) and nitric oxide donor Spermine NONOate were purchased from Cayman Chemical (Ann Arbor, MI), and PpIX was from Sigma. Superoxide detection probes were purchased from the companies indicated: MitoSox (Life Technologies, M36008) and dihydroethidium (Life Technologies, D11347).
Tissue Preparations
Bovine lungs and hearts (from predominantly male animals, sex not known) were obtained from a slaughterhouse in ice-cold phosphate buffer saline and prepared as previously described (12, 13). Left anterior descending bovine coronary arteries (BCAs) and the second or third main branches of bovine pulmonary arteries (BPAs) were used in experiments. BCAs and BPAs were cleaned of their connective tissue and then cut into rings of 2–3 mm in diameter and width. The endothelium was removed by rubbing the lumen. Organoid-cultured BCAs were used in experiments of Western blot protein analysis. BPA rings were used in experiments for superoxide measurements, Western blot protein analysis, assessments of heme biosynthesis, and studies on vascular function. As indicated in results, BPA rings were organoid cultured in the absence and presence of endothelin-1 (10 μM) with/without 0.5 μM COMP with Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% antibiotics (penicillin, streptomycin, and amphotericin B) for 48 h at 37°C with 5% CO2 (12, 14). Arteries were then either used for the studies described or frozen for protein analyses.
Cell Culture
Male Wistar rats (average weight of 200 g) were obtained from the Experimental Animal Center of Harbin Medical University. Protocols approved by the Chinese Laboratory Animal Institutes were used to obtain rat lung tissue for isolation of pulmonary arteries. Animal care and use conformed to the Guide for the Care and Use of Laboratory Animals and was conducted in compliance with the National Institutes of Health guidelines. The pulmonary arteries (PAs) of male rats were cut into small pieces after cleaned of their connective tissue and removed the endothelium by rubbing the lumen. Then PAs were dispersed by solution containing collagenase II (10 U/mL) at 37°C for 1 h. The dispersed vascular smooth muscle cells were then centrifuged for 10 min to get cell pellet. The cells were resuspended in DMEM with 20% serum and 1% penicillin-streptomycin and plated in T25 culture flask to culture. Cell viability (usually greater than 98%) was determined by Trypan Blue exclusion. SM22α fluorescence staining was used for pulmonary arterial smooth muscle cell (PASMC) identification. PASMCs from passages 2–5 were used for further experiments.
Detection of Changes in Mitochondrial and Extramitochondrial Superoxide
HPLC measurement of the superoxide-specific hydroxylated products of MitoSox and dihydroethidium (DHE) were used for quantifying changes in mitochondrial matrix and extramitochondrial matrix superoxide, using previously described methods (5). At the beginning of the experiment, a standard curve was generated by injecting increasing concentrations of Mito-2-hydroxyethidium or 2-hydroxyethidium in the column. After 48 h of organoid culture, BPA rings were incubated with either 5 μM MitoSox or DHE for 1 h in the dark to measure mitochondrial and extramitochondrial superoxide, respectively. They were washed several times with Krebs solution buffered with 10 mM HEPES-NaOH (pH 7.4) and then flash-frozen with liquid nitrogen. Tissues were first weighed and then pulverized in the presence of liquid nitrogen and dissolved in a solution of 100% acetonitrile (HPLC grade). These samples were incubated at −20°C for 1 h. After 1 h, samples were centrifuged, and the supernatant was used for HPLC analysis of the superoxide-specific hydroxylated product of MitoSox (Mito-2-hydroxyethidium) or of DHE (2-hydroxyethidium) using an HPLC system with a Jasco FP-1520 fluorescence detector and a Beckman ultrasphere reverse phase column (C18) (5 μ, 250 × 4.6 mm).
Heme Measurement
Heme content of BPA was measured in homogenates using a QuantiChrom heme assay kit purchased from BioAssay Systems, using a BIOTEK scanning microplate spectrophotometer to quantify changes in absorbance (14). Segments of BPA were homogenized in 20 mM MOPS + sucrose buffer to 5 mg BPA protein/mL. After centrifugation of homogenates at 2,000 g for 5 min, 50 μL of the supernatant obtained was used for heme quantification based on the manufacturer’s instructions, based on changes in absorbance at 400 nm. Supernatant protein content was measured using a Bio-Rad protein assay kit, for reporting tissue heme levels nmol/mg protein.
Measurement of Protoporphyrin IX
PpIX level from BPA rings was measured by adapting previously described HPLC methods (5). Protoporphyrin IX was extracted from 48-h cultured BPA rings treated with/without ET1 and COMP by using extraction buffer (ethyl acetate/acetic acid = 4/1). After centrifuging (10,000 g, 15 min, 4°C), the supernatant was transferred to a new tube for PpIX measurement and the sediment was collected for protein content assay. Mobile phase buffer (methanol/ammonium acid = 86/14) was added to the supernatant (1:1) and then 100 μL of the mixture was injected into an Agilent 1100 HPLC system using a normal phase a Phenomenex column (Luna 5 µ Silica-2 100 A, 250 × 4.6 mm) with methanol-ammonium acid (86:14) and 1 mL/min, as the mobile phase (5). PpIX level was measured using an excitation wavelength of 400 nm and emission of 630 nm. The HPLC data were normalized based on the protein content assay.
Iron Assay
Cells (2 × 106) were rapidly homogenized in 10 volumes of iron assay buffer, and then centrifuged at 16,000 g for 10 min at 4°C to remove insoluble material. Samples of 45 μL were brought to a final volume of 100 μL with iron assay buffer (from a Sigma-Aldrich MAK025 kit) in a 96-well plate and 5 μL of iron assay buffer was added to each sample. After mixing and incubating the reaction for 30 min at 25°C (protected from light), 100 μL of the iron probe reagent was added to each well containing standards or test samples; mixed samples were then incubated for 60 min at 25°C, and the absorbance at 593 nm (A593) was measured in a plate reader.
Western Blot Analysis
Frozen BPAs or BCAs were pulverized and then homogenized in lysis buffer containing protease and phosphatase inhibitors, as previously described (2). Bradford method was used to assay protein quantification, and samples were prepared for gel electrophoresis. Proteins were separated using a 10% SDS-polyacrylamide gel under reducing and denaturing conditions. Gels were transferred to polyvinylidene difluoride membranes, and the membranes were blocked with Tris-buffered saline with Tween 20 + 5% milk for 1 h. After this, the membranes were exposed to primary and secondary antibodies as per the manufacturer’s protocol. Protein bands were visualized with an enhanced chemiluminescence kit (Pierce, Rockford, IL) on X-OMAT autoradiography paper (Kodak, Rochester, NY) in a dark room. Protein levels were measured using densitometry analysis with the UN-SCAN-IT gel software by Silk Scientific (Orem, UT). Molecular mass (kDa) of different proteins are as follows: COMP, 72; SOD1, 18; SOD2, 25; SOD3, 45; NOX2, 61; NOX4, 70; sGC, 70; FECH, 50, TfR1, 85; ferritin, 20, mitoferrin, 39, GAPDH, 38 and β-actin 42. For detection of changes in the expression of mitochondrial proteins (SOD2, mitoferrin, and FECH), the mitochondrial protein VDAC (32 kDa) was used as a loading control. For detection of changes in the expression of COMP, SOD1, NOX2, NOX4, sGC, TfR1, and ferritin, β-actin was used as a loading control. GAPDH was used as a loading control for SOD3. The expression of these loading controls was not altered under the conditions examined in results.
Transfection of Small Interfering RNA
The methods and siRNA sequences used for knockdown of COMP in BPA were based on our previous documentation of their effectiveness in this vascular segment (2). The sequences used for the siRNA of COMP (siRNA-COMP) purchased from GenePharma Co., Ltd (Shanghai) were sense, 5′-AGAAACUUGAGCUGUGUUGAUGCC-3′, and anti-sense, 5′-GGCUAUCAAGACAGCUCAAGUUUCU-3′. A scramble stealth RNAi duplex served as a negative control. In vitro siRNA transfection (50 pM) of organoid-cultured BPA was performed using RNAi MAX (Invitrogen, CA). The transfection procedures followed the manufacturers’ instructions, and data in results confirm that the siRNA sequences used successfully knockdown the levels of COMP in BPA.
RNA Isolation and cDNA Reverse Transcription
PASMCs were made quiescent by incubation in serum-free media (SFM) for 24 h. Quiescent cells were exposed to ET1 for 48 h. Media was decanted and cells frozen to −80°C. RNAs were extracted using TRIzol from PASMCs. Quality of the RNA samples was assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). cDNA was generated using PrimeScript RT reagent Kit (TAKARA BIO. Cat. No.: RR037A).
Real-Time Quantitative Reverse Transcriptase-Polymerase Chain Reaction
Real-time quantitative polymerase chain reacted with SYBR Premix Ex TaqTM (TAKARA BIO. Cat. No.: RR420A). Cycle and threshold were obtained according to the manufacturer’s instructions. Changes in expression given are the average of three biological replicates. The primers for COMP are sense 5′- GTGACTTCGATGCTGACAAGGT-3′/antisense 5′- GTCTTGATAGCCGAAGATGAAGC-3′ and for β-actin are sense 5′ - GAGACCTTCAACACCCCAGCC-3′/antisense 5′- TCGGGGCATCGGAACCGCTCA-3′, and annealing temperature was 59°C.
Measurement of Vascular Reactivity in BPA
Endothelium-rubbed organoid-cultured BPA rings were mounted on Grass FT-03 or Coulborne Instruments force displacement transducers for recording isometric force development through a Powerlab data acquisition system obtained from ADInstruments (Colorado Springs, CO), as previously described (12, 13). Arterial rings were incubated at 37°C in Krebs-bicarbonate buffer (pH 7.4) containing 118 mM NaCl, 4.7 mM KCl, 1.5 mM CaCl2, 25 mM NaHCO3, 1.1 mM MgSO4, 1.2 mM KH2PO4, and 5.6 mM glucose under an atmosphere of 21% O2-5% CO2-74% N2 (pH 7.4) for 1 h under resting tension of 5 g. In all studies, arterial rings were depolarized with 123 mM KCl containing Krebs bicarbonate buffer, and the rings were then reequilibrated with Krebs-bicarbonate buffer for 30 min and subsequently contracted with Krebs bicarbonate containing 30 mM KCl. Arterial rings were then exposed to increasing cumulative concentrations of spermine-NONOate (10−8 M to 10−5 M) to examine the effects of the organoid culture treatment conditions used on relaxation to this nitric oxide-releasing agent.
Statistical Analysis
Statistical analysis was performed with GraphPad Prism 5 software. Animal experiments from bovine vascular tissue used ≥5 biological replicates from different animals; cell experiments used three independent experiments performed in duplicate or ≥5 biological replicates in cells cultured from different rats. All values are expressed as means ± SD. Statistical analyses between two groups were performed with paired Student’s t test, one-way ANOVA, and two-way ANOVA between multiple groups using nonadjacent or adjacent segments from the same bovine pulmonary or coronary artery, respectively. A value of P < 0.05 was used to establish statistical significance.
RESULTS
Endothelin-1 Decreased the Level of COMP in Pulmonary Arteries
To investigate the role of COMP in potential pulmonary hypertension-associated actions of ET1, we first examined the level of COMP in organoid-cultured bovine arteries. As shown in Fig. 1, A and C, the protein level of COMP significantly decreased (P < 0.01) in BPA and rat pulmonary arterial smooth muscle cells (P < 0.05) treated with ET1 (10 μM), and not in left anterior descending bovine coronary arterial segments studied (Fig. 1B). The mRNA level of COMP was not significantly altered in rat PASMCs treated with 10 μM of ET1 (Fig. 1D). These results provide evidence that COMP is selectively lowered by ET1 in pulmonary arteries, but not coronary arteries under the conditions studied. The mechanism of the reduced COMP by ET1 may be at posttranscriptional level.
Figure 1.
Endothelin-1 decreases the level of COMP in pulmonary arteries. Representative Western blot bands and quantitative analysis of COMP protein in organoid cultured BPA treated with ET1 (10 μM) for 48 h (n = 5; A); in left anterior descending BCA (n = 5) (B); in rat PASMCs treated with ET1 (10 μM) for 48 h (n = 5) (C); quantitative analysis of COMP mRNA in rat PASMCs treated with ET1 (10 μM) for 48 h (D). Results are from 5 independent experiments performed in duplicate; *P < 0.05 vs. control (paired t test, n = 5). BCA, bovine coronary artery; BPA, bovine pulmonary artery; COMP, cartilage oligomeric matrix protein; CTL, control; ET-1, endothelin-1.
COMP Attenuated the Increased Level of Superoxide Detected by MitoSox and DHE Induced by ET1 in BPA
We tried to identify aspects of relationships between COMP and changes in vascular smooth muscle superoxide levels induced by ET1 by using endothelium-denuded BPA. The pmol/mg tissue levels of superoxide-derived specific hydroxylated products of MitoSox and DHE were measured by HPLC. The data showed that the level of superoxide detected by MitoSox (P < 0.001) and DHE (P < 0.05) were elevated by ET1 in BPA after organoid culture for 48 h, and this was prevented by the presence of exogenous COMP (Fig. 2A). ET1 treatment for 48 h significantly decreased SOD2 (P < 0.05, Fig. 2B) and increased NOX2 (P < 0.05, Fig. 2C). The changes in SOD2 and NOX2 elicited by ET1 were prevented by exogenous COMP. Treatment of BPA with either ET1 or COMP did not cause significant changes in SOD1, SOD3 or mitochondrial content based on VDAC expression (Fig. 2B), and NOX4 or catalase (Fig. 2C) after culture of BPA with ET1 for 48 h. In conformation to observations reported in our previous study (2), knockdown of COMP with siRNA significantly increased the level of NOX2 (P < 0.01, Fig. 2D) and decreased the level of SOD2 (P < 0.05, Fig. 2D).
Figure 2.
Effects of ET1 and COMP on aspects of superoxide metabolism in BPA. A: HPLC quantified levels of mitochondrial matrix and extramitochondrial matrix superoxide-specific metabolites of MitoSox (n = 9) and DHE (n = 7). B and C: representative Western blot bands and summary data for Cu,Zn-SOD1 (n = 7), mitochondrial-SOD2 (n = 6), extracellular-SOD3 (n = 5), and NOX2 (n = 9), NOX4 (n = 10), and catalase (n = 8) in BPA treated with ET1 for 48 h in the absence or presence of COMP are shown. D: the effect of knockdown of COMP with siRNA on protein levels of COMP (n = 5), SOD2 (n = 9), and NOX2 (n = 7) detected by Western blotting. *P < 0.05, siRNA-COMP vs. scramble siRNA (analysis of two-way ANOVA with Sidak correction for multiple groups, paired t test for 2 groups) (C, treatment with 0.5 μM COMP for 48 h; ET+C, treatment with 10 μM ET1 and 0.5 μM COMP at the same time; scramble, scramble siRNA; COMPi, siRNA-COMP). BPA, bovine pulmonary artery; COMP, cartilage oligomeric matrix protein; CTL, control; DHE, dihydroethidium; ET-1, endothelin-1.
COMP Maintained the Level sGC Downregulated by ET1
Treatment of BPA with ET1 decreased sGCβ1 (Fig. 3A) expression under conditions where both mitochondrial and extramitochondrial superoxide were elevated. Treatment of BPA with COMP under organoid culture conditions did not significantly alter the expression of sGCβ1. However, cotreatment with ET1 together with COMP prevented depletion of sGCβ1 by ET1 (P = 0.0259). Treatment of BPA with siRNA targeting COMP also depleted sGCβ1 (Fig. 3B).
Figure 3.

Effects of ET1 and COMP on the expression of sGCβ1 and its potential impact on BPA relaxation to a nitric oxide-releasing agent spermine NONOate potentially influenced by both an acute scavenging of NO by superoxide and depletion of sGC during organoid culture with ET1. Effects of ET1 in presence or absence of COMP (n = 7) (A) and COMP siRNA (n = 7) (B) on sGCβ1 expression, detected by Western blot analysis. C: effects of ET1 in presence or absence of COMP during organ culture (n = 8, left) and acute treatments with the scavenger of superoxide Tempo (n = 6, right) on relaxation of BPA precontracted with 30 mM KCl to a nitric oxide-releasing agent spermine NONOate (n = 6). Data in the right panel also show elimination of this NO-mediated relaxation by acute treatment with 10 μM ODQ, an oxidant of the sGC heme. *P < 0.05 (other panel vs. control), #P < 0.05 (ET vs. ET+ COMP or ET vs. ET+ TEMPO) analysis by one-way ANOVA with Tukey’s multiple comparison tests, two-way ANOVA with Sidak correction for multiple groups, paired t test for 2 groups (C, treatment with 0.5 μM COMP for 48 h; ET+C, treatment with 10 μM ET1 and 0.5 μM COMP at the same time; scramble, scramble siRNA; COMPi, siRNA-COMP). BPA, bovine pulmonary artery; COMP, cartilage oligomeric matrix protein; CTL, control; ET-1, endothelin-1; sGC, soluble guanylate cyclase; ODQ, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one.
Spermine-NONOate was used in this study as an NO donor, under conditions where NO-mediated relaxation functions primarily through a Fe2+ heme-dependent stimulation of sGC. As shown in Fig. 3C, the organoid culture of ET1 significantly reduced relaxation to NONOate at doses of 10−7 M, 10−6 M, and 10−5 M. Organoid culture of ET1 together with COMP (Fig. 3C, left) cotreatment significantly attenuated the inhibitory effects of ET1, under conditions where the COMP treatment in the absence of ET1 did not alter relaxation to NONOate. Acute treatment of BPA with the superoxide scavenger Tempo during the assessment of vascular function after organoid culture with ET1 partially reversed the inhibitory effect of ET1 on relaxation to NONOate (Fig. 3C, right), suggesting that increased superoxide was potentially inhibiting relaxation by scavenging NO. However, the relaxation to NONOate remained significantly inhibited by the organoid culture with ET1 in the presence of the acute Tempo treatment, which is an observation consistent with detection of a potential role for sGC depletion in the remaining loss of NO-mediated relaxation. Since acute treatment with the sGC heme oxidant, 10 μM ODQ, essentially eliminated detection of NONOate-induced relaxation (Fig. 3C, right) in control organoid-cultured BPA rings, under conditions where this agent eliminates cGMP increased in BPA to spermine NONOate (13), the response to NONOate in these experiments is one that is mediated by NO stimulation of sGC vasodilation. None of these treatments had a significant effect on the force generated by 30 mM KCl used to examine relaxation responses to NONOate under any of the conditions examined in these studies (not shown). Thus, the actions of ET1 appeared to attenuate cGMP-associated relaxation to a NO donor. These data suggest that a combination of the acute scavenging of NO by increased superoxide and partial depletion of sGC contributes to the observed loss of NO-stimulated cGMP-mediated BPA relaxation on exposure to ET1 under the organoid-cultured conditions examined. In addition, the presence of increased COMP potentially functioned to attenuate the roles for both increased superoxide and sGC depletion attenuating the actions of NO.
Effects of Organoid Culture of BPA with ET1 and COMP on FECH Expression, Protoporphyrin IX, and Heme Levels
As our previous studies (5, 6) suggest that increased mitochondrial superoxide could inhibit the heme biosynthetic function of FECH and/or deplete detection of this enzyme by Western blot analysis, the effects of organoid culture of BPA with ET1 and COMP on FECH expression, protoporphyrin IX, and heme levels were examined under the conditions (shown in Fig. 2A) where mitochondrial superoxide was elevated (see Fig. 4). Treatment of BPAs with ET1 decreased FECH expression detected by Western blot analysis (P < 0.001, Fig. 4A), and exogenous COMP prevented this effect of ET1 (P < 0.05, Fig. 4A). Knockdown COMP with siRNA decreased FECH (P < 0.001, Fig. 4B). PpIX was examined to detect if ET1 influences BPA levels of PpIX in the absence and presence of COMP. Although ET1 or knockdown COMP expression with siRNA significantly increased the levels of PpIX (P < 0.01, Fig. 4C), exogenous COMP directly decreased PpIX levels in 48-h organoid-cultured BPAs (P < 0.01, Fig. 4C). ET1 reduced the level of heme in 48-h organoid-cultured BPAs (P < 0.05, Fig. 4D), and the presence of exogenous COMP appeared to prevent detection of heme depletion by ET1. Heme levels were also observed to be increased by exogenous COMP in BPA compared with control (P < 0.05, Fig. 4D).
Figure 4.
Effects of organoid culture of BPA with ET1 and modulation of COMP on FECH expression, protoporphyrin IX, and heme levels. Effects of ET1 in presence or absence of COMP (n = 4) (A) and COMP siRNA (n = 7) (B) on FECH expression detected by Western blot analysis. C: influence of ET1 (n = 5), COMP (n = 7), and COMP siRNA (n = 5) on BPA protoporphyrin IX (PpIX) levels, detected by HPLC. D: influence of ET1 in presence or absence of COMP (n = 7–8) on BPA heme levels, *P < 0.05 vs. control (analysis of one-way ANOVA with Tukey’s multiple comparison test). The mitochondrial protein VDAC as loading control for FECH (C, treatment with 0.5 μM COMP for 48 h; ET+C, treatment with 10 μM ET1 and 0.5 μM COMP at the same time; scramble, scramble siRNA; COMPi, siRNA-COMP). BPA, bovine pulmonary artery; COMP, cartilage oligomeric matrix protein; CTL, control; ET-1, endothelin-1; FECH, ferrochelatase.
Effects of Organoid Culture of BPA with ET1 and/or COMP Modulation on Additional Aspects of Iron Metabolism
Although the Fig. 4A data provide evidence for ET1 decreasing the levels of FECH and heme, it is not known if other aspects of cellular iron metabolism could be influenced by the actions of ET1 and/or by COMP modulation. As expression of TfR1 is stimulated by a cellular iron deficiency to promote import of circulating iron (15), the effects of TfR1 modulation by ET1 and COMP siRNA were examined. Data show that ET1 increased TfR1 expression in organoid culture of BPA (P < 0.001, Fig. 5A) and ferrous iron in rat PASMCs (P < 0.05, Fig. 5C), and that COMP significantly lowered the level of TfR1 (P < 0.001, Fig. 5A) and ferrous iron (P < 0.05, Fig. 5C) in the presence of ET1. In contrast, treatment of BPA with ET1 did not detectably alter the levels of the cellular exporting transport protein for iron ferroportin or the iron storage protein ferritin, however, it decreased expression of the mitochondrial iron transport protein mitoferrin (P < 0.05, Fig. 5A). Treatment of BPA with siRNA for COMP also increased the expression of TfR1 (P < 0.05), it lowered the expression of ferritin (P < 0.05), and it did not alter the expression of mitoferrin (Fig. 5B). Although treatment of BPA with COMP in the absence or presence of ET1 showed a trend toward increasing ferritin, this effect was not statistically significant (Fig. 5A).
Figure 5.
Effects of organoid culture of BPA with ET1 and modulation of COMP on the expression of proteins influencing iron metabolism. Effects of ET1 and COMP on TfR1 (n = 8), ferritin (n = 5), and ET1 on mitoferrin (n = 8), ferroportin (n = 10) (A); COMP siRNA on TfR1 (n = 5), ferritin (n = 9), and mitoferrin (n = 5) (B) in organoid cultured BPA for 48 h, detected by Western blotting. C: ferrous iron in PASMCs was measured by iron assay kit (results were from 3 independent experiments performed in duplicate). Analysis of two-way ANOVA with Sidak correction for multiple groups, paired t-test for 2 groups (C, treatment with 0.5 μM COMP for 48 h; ET+C, treatment with 10 μM ET1 and 0.5 μM COMP at the same time; scramble, scramble siRNA; COMPi, siRNA-COMP). BPA, bovine pulmonary artery; COMP, cartilage oligomeric matrix protein; CTL, control; ET-1, endothelin-1; TfR1, transferrin receptor-1.
DISCUSSION
The present study provides new evidence for how mitochondrial superoxide-mediated heme biosynthesis inhibitory actions of ET1 influence heme-dependent regulation of sGC by NO in endothelium-denuded BPA as a result of ET1 controlling the depletion of COMP. These regulatory actions of COMP are based on their inhibition by increasing COMP levels and by the observed actions of siRNA depleting COMP promoting the effects elicited by ET1. The study builds on our recent observations showing that hypoxia and siRNA depletion of COMP increases mitochondrial matrix superoxide associated with depletion of SOD2 and extramitochondrial matrix superoxide through systems including increased NOX2, by documenting that these responses are associated with ET1 depleting COMP and treatment with COMP attenuating these actions of ET1. Thus, COMP depletion by hypoxia or ET1 has similar actions on these superoxide-regulating systems. Interactions of ET1 and COMP modulation of aspects of iron metabolism and heme biosynthesis detected in the present study that are shown in the model in Fig. 6 may also provide insight into the origins of poorly understood alterations in iron metabolism and roles for heme biosynthesis regulation in controlling vascular function through their impact on systems including sGC regulation by NO.
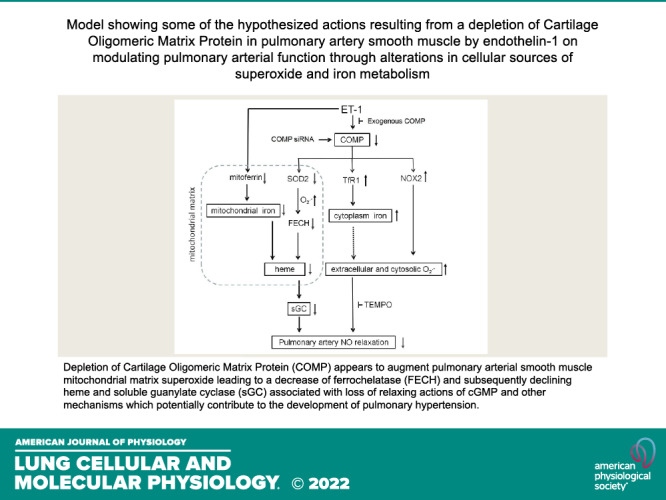
Figure 6.
Model showing some of the hypothesized actions resulting from a depletion of COMP in bovine pulmonary smooth muscle elicited by exposure to endothelin-1 for 48 h. The absence of COMP decreases SOD2 and increases TfR1, ferrous iron, and NOX2, associated with increases in mitochondrial matrix and extramitochondrial matrix sources of superoxide. The augmented superoxide in mitochondria leads to the decrease of FECH and subsequently declining heme and sGC. The decreasing sGC in cytoplasm, loss of relaxing actions of cGMP and other iron-promoted oxidant mechanisms potentially contribute to the constriction of pulmonary smooth muscle and other processes associated with the development of pulmonary hypertension. COMP, cartilage oligomeric matrix protein; FECH, ferrochelatase; TfR1, transferrin receptor-1.
The actions of ET1 on BPA reported in the present study have similarities with actions of angiotensin II (ANG II) previously observed (6) in bovine coronary arteries in that both agents increased mitochondrial matrix and extramitochondrial matrix/cytosolic superoxide, depleted FECH, and suppressed heme biosynthesis in a manner associated with sGC depletion, under conditions where the scavenging of extramitochondrial matrix superoxide with Tempo during vascular responses detected evidence for both a superoxide-dependent and superoxide-independent loss of relaxation to a NONOate nitric oxide donor. The similarities in these responses may originate from exposure during organoid culture to increased mitochondrial matrix superoxide disrupting the iron-sulfur center of FECH and cytosolic superoxide contributing to oxidizing the Fe2+ or ferrous heme form of sGC (6). This oxidation is the process that generates the Fe3+ or ferric heme form of sGC and/or heme-free sGC, which has been linked to promoting increased sGC depletion by proteolysis (16). Although the present study with ET1 and a previous study with 48 h of exposure to hypoxia (1) provide evidence that COMP is not depleted in BCA under conditions where it is depleted in BPA, further studies beyond those included in the present study are needed to define differences in the regulation and roles for changes in COMP expression between BPA and BCA. As siRNA depletion of COMP promotes increases in mitochondrial matrix and extramitochondrial superoxide together with depletion of FECH and sGC, and since increased COMP attenuates the influence of ET1 on these systems, COMP depletion appears to be a key factor in action of ET1 that contributes to impairing both mitochondrial heme biosynthesis and heme-dependent regulation of sGC.
Moreover, during hypoxia, mitochondria from vascular cells release superoxide (O2•−) from complex III to the intermembrane space, where it is converted to hydrogen peroxide (H2O2) by SOD1 (17, 18). The H2O2 then enters the cytosol, where it activates multiple responses contributing to smooth muscle contraction and remodeling. Our data showed that ET1 significantly increased mitochondrial matrix superoxide and decreased SOD2 via COMP-dependent pathway, which might result in actions that increase the release of mitochondria-derived H2O2 from SOD1 and mediate cellular responses. There are reports that COMP can translocate to the mitochondria matrix to maintain mitochondrial hemostasis of vascular smooth muscle cells (19). Our previous study provided evidence that COMP was mainly expressed in PASMCs of PA and COMP plays important role in PASMC through stabilizing BMPR2 (1). Here we found that ET1 reduced COMP in protein levels in PASMCs that potentially led to a COMP deficiency in the extracellular matrix because it was the addition of extracellular COMP that regulated the mitochondrial function.
Our previous studies (5, 6) have provided evidence to hypothesize that both depletion of FECH activity and/or an impaired availability of iron in the mitochondrial matrix could be factors in decreasing mitochondrial heme biosynthesis. Data from experiments in the present study indicate that increased ET1 and/or COMP depletion results in a loss of heme in BPA associated with increased PpIX and decreased FECH expression. Since, in the absence of ET1, increased COMP promoted increased heme, and decreased PpIX, without altering FECH expression, COMP could potentially have actions that either promote increased availability of mitochondrial matrix ferrous iron for heme biosynthesis and/or stimulatory actions on FECH activity which remain to be defined. COMP also decreased the expression of TfR1 in the absence of ET1, which could be an indication of decreased cytosolic iron availability suppressing iron-regulatory protein (IRP) binding to iron-response element (IRE) that functions to increase TfR1 expression (15). Under the organoid culture conditions of the present study, ET1 did not alter the levels of ferroportin in BPA, a protein that exports iron to the extracellular environment (15). Changes in ferroportin that have been detected in pulmonary hypertension have generally been associated with the in vivo actions of changes in circulating hepcidin that are associated with the progression of this disease (20). In contrast, ET1 suppressed the levels of mitoferrin (Fig. 5A), a protein used to transport iron into the mitochondria for iron-sulfur cluster assembly and heme biosynthesis (15). As siRNA depletion of COMP did not deplete mitoferrin (Fig. 5B), the depletion of this protein by ET1 may be the result of an action of ET1 that is independent of COMP depletion. It is difficult to interpret observations related to the actions of ET1 and changes in COMP expression on ferritin expression shown in Fig. 5, A and B, and their impact on subcellular iron metabolism, because there are likely to be differences in several systems influencing these processes such as TfR1 expression, superoxide levels, and levels of iron-associated reactive oxidant species under the various conditions examined in the present study (15). Overall, observations in the present study provide additional support for both depletion of FECH activity and/or impaired alterations in availability of iron in different subcellular regions as being factors potentially contributing to impairing mitochondrial heme biosynthesis and other aspects of altered iron metabolism in pulmonary arterial smooth muscle that could be factors in pathophysiological actions of ET1 and/or COMP depletion.
Observations reported in this study have detected a novel prominent role for COMP depletion in several key initial pathophysiological actions of ET1 on pulmonary arterial smooth muscle linked to the promotion of oxidant processes, subcellular alterations in iron metabolism associated with disruption of mitochondrial heme biosynthesis, and its impact on heme-dependent sGC regulation, a key process in the beneficial physiological and therapeutic actions of NO. Although a loss of cGMP signaling could remove its inhibitory effects on many processes associated with pulmonary hypertension development such as increased Rho kinase (21, 22), additional oxidant-iron interactions could then promote alternative cGMP-independent pathophysiological mechanisms such as Rho kinase activation by Rho A carbonylation (23). Thus, COMP appears to suppress oxidant processes driving pulmonary vascular dysfunction and loss of the pulmonary artery contractile phenotype (1, 2), and, as shown in this study, to also preserve key mechanisms preventing against these changes including maintaining mitochondrial heme biosynthesis and NO regulation of vascular function through heme-dependent stimulation of sGC and beneficial actions of cGMP regulation.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
This work and the investigators reported in this paper were supported by NIH Grants HL115124, HL12979, and HL151187 and grants from the Natural Science Foundation of the P. R. China (31400989), the Natural Science (C2015069) and Postdoctoral Science (LBHQ15087) Foundation of Heilongjiang Province.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.Y., N.A., and M.S.W. conceived and designed research; H.Y., N.A., M.R.K., B.Z., A.L., and Y.W. performed experiments; H.Y., N.A., B.Z., A.L., Y.W., D.S., and M.S.W. analyzed data; H.Y., N.A., D.S., and M.S.W. interpreted results of experiments; H.Y. and N.A. prepared figures; H.Y. and M.S.W. drafted manuscript; H.Y., N.A., and M.S.W. edited and revised manuscript; H.Y., N.A., M.R.K., B.Z., A.L., Y.W., D.S., and M.S.W. approved final version of manuscript.
REFERENCES
- 1. Yu H, Jia Q, Feng X, Chen H, Wang L, Ni X, Kong W. Hypoxia decrease expression of cartilage oligomeric matrix protein to promote phenotype switching of pulmonary arterial smooth muscle cells. Int J Biochem Cell Biol 91: 37–44, 2017. doi: 10.1016/j.biocel.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 2. Yu H, Alruwaili N, Hu B, Kelly MR, Zhang B, Sun D, Wolin MS. Potential role of cartilage oligomeric matrix protein in the modulation of pulmonary arterial smooth muscle superoxide by hypoxia. Am J Physiol Lung Cell Mol Physiol 317: L569–L577, 2019. doi: 10.1152/ajplung.00080.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang L, Zheng J, Du Y, Huang Y, Li J, Liu B, Liu CJ, Zhu Y, Gao Y, Xu Q, Kong W, Wang X. Cartilage oligomeric matrix protein maintains the contractile phenotype of vascular smooth muscle cells by interacting with alpha(7)beta(1) integrin. Circ Res 106: 514–525, 2010. doi: 10.1161/CIRCRESAHA.109.202762. [DOI] [PubMed] [Google Scholar]
- 4. Du Y, Wang Y, Wang L, Liu B, Tian Q, Liu C-J, Zhang T, Xu Q, Zhu Y, Ake O, Qi Y, Tang C, Kong W, Wang X. Cartilage oligomeric matrix protein inhibits vascular smooth muscle calcification by interacting with bone morphogenetic protein-2. Circ Res 108: 917–928, 2011. doi: 10.1161/CIRCRESAHA.110.234328. [DOI] [PubMed] [Google Scholar]
- 5. Alhawaj R, Patel D, Kelly MR, Sun D, Wolin MS. Heme biosynthesis modulation via δ-aminolevulinic acid administration attenuates chronic hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 308: L719–L728, 2015. doi: 10.1152/ajplung.00155.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patel D, Alhawaj R, Kelly MR, Accarino JJ, Lakhkar A, Gupte SA, Sun D, Wolin MS. Potential role of mitochondrial superoxide decreasing ferrochelatase and heme in coronary artery soluble guanylate cyclase depletion by angiotensin II. Am J Physiol Heart Circ Physiol 310: H1439–H1447, 2016. doi: 10.1152/ajpheart.00859.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ghio S, Fortuni F, Capettini AC, Scelsi L, Greco A, Vullo E, Raineri C, Guida S, Turco A, Gargiulo C, Oltrona Visconti L. Iron deficiency in pulmonary arterial hypertension: prevalence and potential usefulness of oral supplementation. Acta Cardiol 76: 162–167, 2021. doi: 10.1080/00015385.2019.1694760. [DOI] [PubMed] [Google Scholar]
- 8. Quatredeniers M, Montani D, Cohen-Solal A, Perros F. Iron deficiency in pulmonary arterial hypertension: perspectives. Pulm Circ 11: 20458940211021301, 2021. doi: 10.1177/20458940211021301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Decker I, Ghosh S, Comhair SA, Farha S, Tang WH, Park M, Wang S, Lichtin AE, Erzurum SC. High levels of zinc-protoporphyrin identify iron metabolic abnormalities in pulmonary arterial hypertension. Clin Transl Sci 4: 253–258, 2011. doi: 10.1111/j.1752-8062.2011.00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chester M, Seedorf G, Tourneux P, Gien J, Tseng N, Grover T, Wright J, Stasch JP, Abman SH. Cinaciguat, a soluble guanylate cyclase activator, augments cGMP after oxidative stress and causes pulmonary vasodilation in neonatal pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 301: L755–L764, 2011. doi: 10.1152/ajplung.00138.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Naito Y, Hosokawa M, Sawada H, Oboshi M, Hirotani S, Iwasaku T, Okuhara Y, Morisawa D, Eguchi A, Nishimura K, Soyama Y, Fujii K, Mano T, Ishihara M, Tsujino T, Masuyama T. Transferrin receptor 1 in chronic hypoxia-induced pulmonary vascular remodeling. Am J Hypertens 29: 713–718, 2016. doi: 10.1093/ajh/hpv163. [DOI] [PubMed] [Google Scholar]
- 12. Neo BH, Kandhi S, Wolin MS. Roles for redox mechanisms controlling protein kinase G in pulmonary and coronary artery responses to hypoxia. Am J Physiol Heart Circ Physiol 301: H2295–H2304, 2011. doi: 10.1152/ajpheart.00624.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mingone CJ, Gupte SA, Ali N, Oeckler RA, Wolin MS. Thiol oxidation inhibits nitric oxide-mediated pulmonary artery relaxation and guanylate cyclase stimulation. Am J Physiol Lung Cell Mol Physiol 290: L549–L557, 2006. doi: 10.1152/ajplung.00331.2005. [DOI] [PubMed] [Google Scholar]
- 14. Mingone CJ, Gupte SA, Chow JL, Ahmad M, Abraham NG, Wolin MS. Protoporphyrin IX generation from delta-aminolevulinic acid elicits pulmonary artery relaxation and soluble guanylate cyclase activation. Am J Physiol Lung Cell Mol Physiol 291: L337–L344, 2006. doi: 10.1152/ajplung.00482.2005. [DOI] [PubMed] [Google Scholar]
- 15. Wang J, Pantopoulos K. Regulation of cellular iron metabolism. Biochem J 434: 365–381, 2011. doi: 10.1042/BJ20101825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stasch J-P, Schmidt PM, Nedvetsky PI, Nedvetskaya TY, HS AK, Meurer S, Deile M, Taye A, Knorr A, Lapp H, Müller H, Turgay Y, Rothkegel C, Tersteegen A, Kemp-Harper B, Müller-Esterl W, Schmidt HHHW. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest 116: 2552–2561, 2006. doi: 10.1172/JCI28371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thompson CB. Into thin air: how we sense and respond to hypoxia. Cell 167: 9–11, 2016. doi: 10.1016/j.cell.2016.08.036. [DOI] [PubMed] [Google Scholar]
- 18. Waypa GB, Smith KA, Schumacker PT. O2 sensing, mitochondria and ROS signaling: the fog is lifting. Mol Aspects Med 47–48: 76–89, 2016. doi: 10.1016/j.mam.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jia Y, Wang M, Mao C, Yu F, Wang Y, Xiao R, Jiang C, Zheng L, Xu Q, Zheng M, Fu Y, Hu Q, Kong W. COMP-prohibitin 2 interaction maintains mitochondrial homeostasis and controls smooth muscle cell identity. Cell Death Dis 9: 676, 2018. doi: 10.1038/s41419-018-0703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ramakrishnan L, Pedersen SL, Toe QK, Quinlan GJ, Wort SJ. Pulmonary arterial hypertension: iron matters. Front Physiol 9: 641, 2018. doi: 10.3389/fphys.2018.00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oka M, Fagan KA, Jones PL, McMurtry IF. Therapeutic potential of RhoA/Rho kinase inhibitors in pulmonary hypertension. Br J Pharmacol 155: 444–454, 2008. doi: 10.1038/bjp.2008.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jernigan NL, Naik JS, Weise-Cross L, Detweiler ND, Herbert LM, Yellowhair TR, Resta TC. Contribution of reactive oxygen species to the pathogenesis of pulmonary arterial hypertension. PLoS One 12: e0180455, 2017. doi: 10.1371/journal.pone.0180455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wong C-M, Preston IR, Hill NS, Suzuki YJ. Iron chelation inhibits the development of pulmonary vascular remodeling. Free Radic Biol Med 53: 1738–1747, 2012. doi: 10.1016/j.freeradbiomed.2012.08.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.







