Abstract
Immune system responses are a vital defense mechanism against pathogens. Inflammatory mediators finely regulate complex inflammatory responses from initiation to resolution. However, in certain conditions, the inflammation is initiated and amplified, but not resolved. Understanding the biological mechanisms underlying the regulation of the immune response is critical for developing therapeutic alternatives, including pharmaceuticals and bioelectronic tools. The spleen is an important immune effector organ since it orchestrates innate and adaptive immune responses such as pathogen clearance, cytokine production, and differentiation of cells, therefore playing a modulatory role that balances pro- and anti-inflammatory responses. However, modulation of splenic immune activity is a largely unexplored potential therapeutic tool that could be used for the treatment of inflammatory and life-threatening conditions. This review discusses some of the mechanisms controlling neuroimmune communication and the brain-spleen axis.
Keywords: autonomic nervous system, brain, inflammation, inflammatory reflex, neuroimmune axis
INTRODUCTION
The spleen is a sentinel organ that continuously monitors the blood for pathogens, clears the blood of defective cells and microorganisms, stores platelets and immune cells, and initiates immune responses to antigens (1–3). Immune cells residing in the spleen are recruited to fight diseases and infections and a balance between proinflammatory and anti-inflammatory status in the spleen is crucial for the elimination of pathogens without harming the host. In this review, we highlight the neural mechanisms controlling the immunological function of the spleen and their clinical implications with a focus on autonomic regulation.
INNERVATION OF THE SPLEEN
The spleen is virtually devoid of sensory innervation, with the exception of rare afferent fibers from dorsal root ganglia (DRG) neurons that putatively innervate the splenic vasculature near the hilar region (4). The specific sensory stimuli detected by these rare afferent fibers and the functional role of this input are unknown. An alternative mechanism by which the spleen conveys signals to other organs is via a humoral route, i.e., by cell emigration, cytokines, and chemokines exiting the spleen through the venous and lymphatic circulation (1).
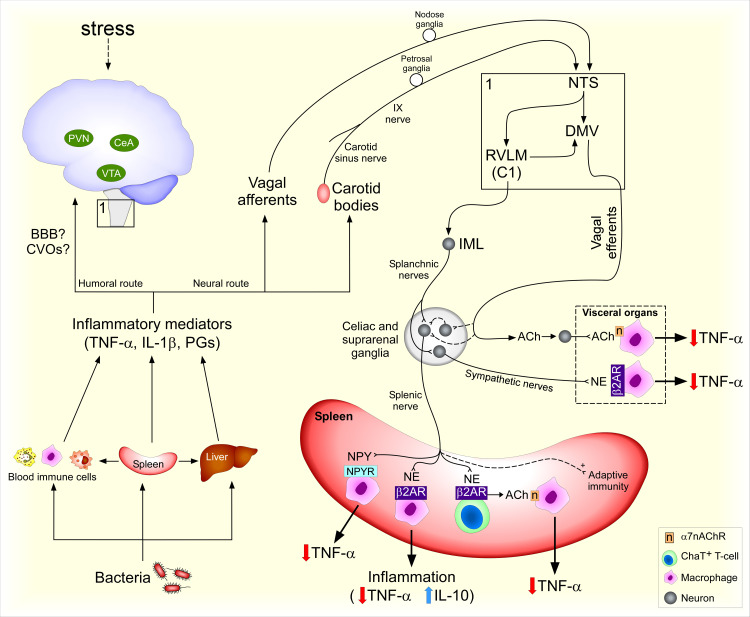
The spleen is innervated by the splenic nerve, which is composed of sympathetic noradrenergic fibers (5, 6) originating from the celiac ganglion (CG), suprarenal ganglia (SRG), and the thoracic sympathetic chain (4, 7, 8). Only sparse parasympathetic fibers are found in the spleen (9–12). Because there are technical experimental difficulties in specifically targeting vagal nerve pathways to the spleen, the functional implications of a direct parasympathetic innervation of the spleen are still debated (13, 14). Although it is generally accepted that the CG and SRG are innervated by sympathetic and parasympathetic fibers (8, 15–18), it is controversial whether splenic ganglion cells in the CG and SRG receive vagal inputs (8, 19) (Fig. 1). More studies are necessary to clarify whether there is a functional neural connection between parasympathetic nerve fibers and splenic sympathetic ganglion cells.
Figure 1.
Neural control of the spleen during endotoxemia. Bacterial antigens elicit a systemic inflammatory response. Cells and humoral factors emigrate from the spleen to the circulation and other organs. Inflammatory factors activate brain-mediated responses potentially via humoral (blood-brain-barrier, BBB, and circumventricular organs, CVOs) and neural routes (vagal and carotid sinus nerve afferents). Corticotropin-releasing hormone (CRH) neurons in central amygdala (CeA) and paraventricular nucleus (PVN) modulate adaptive immune responses to stress while ventral tegmental area (VTA) neurons modulate innate immune responses to bacteria. Peripheral afferents from the IX and X cranial nerves synapse with second-order neurons in the nucleus tractus solitarius (NTS), which can activate C1 neurons in the rostral ventrolateral medulla (RVLM) and neurons in the dorsal motor nucleus of the vagus (DMV). RVLM neurons synapse with preganglionic neurons in the intermediolateral column of the spinal cord (IML), including those giving rise to axons that form the splanchnic nerves. Axons from sympathetic ganglion cells in the celiac and suprarenal ganglia form the sympathetic splenic nerve, which nerve terminals release norepinephrine (NE) and neuropeptide Y (NPY). Choline acetyltransferase (ChAT)-expressing T-cells release acetylcholine (ACh) in response to β2-adrenergic receptor (AR) activation, leading to activation of α7 nicotinic receptor (α7nAChR) in macrophages. α7nAChR signaling blocks TNF-α release, thus reducing inflammation. β2-AR and NPY receptors (NPY-R) activation in immune cells reduces TNF-α production and β2-AR activation increases IL-10 release during inflammation. Afferent vagus nerve stimulation induces anti-inflammatory effects via increasing splenic nerve activity. Vagal efferent fibers send projections to sympathetic ganglia and viscera that, when stimulated, reduce inflammatory responses via β2-AR and α7nAChR. Dashed lines represent controversial or yet to be clarified pathways.
ROLES FOR NOREPINEPHRINE AND NEUROPEPTIDE Y IN SPLENIC FUNCTION
Catecholaminergic fibers innervate both the parenchymal cells and the blood vessels of the spleen, thereby contributing to the splenic influence on immune functions. Approximately 80% of catecholaminergic CG neurons coexpress neuropeptide Y (NPY) (17). Within the spleen, tyrosine hydroxylase (TH)+ nerve fibers that colocalize with NPY densely innervate the splenic vasculature and provide a minor innervation of the splenic parenchyma (17). Splenic nerve terminals can release norepinephrine (NE), NPY, and other neurotransmitters (20–23). Studies using rodents show that a reduction in blood flow initiated by the release of NE from nerve terminals surrounding splenic blood vessels contributes to local hypoxia in immune cells and blocks leukocyte interstitial migration, which suppresses immune responses to tumors and to viral and parasitic infections in an α1-adrenoreceptor (AR) and β2-AR-dependent fashion (21). However, during endotoxemia, endogenous interleukin (IL)-1β acting locally, in the spleen, increases splenic blood flow despite an increased splenic nerve activity (24, 25). The mechanisms by which IL-1β increases splenic blood flow in the presence of elevated splenic nerve activity are unknown.
Studies using confocal analysis and electron microscopy showed that TH nerve terminals within the spleen form synapse-like structures with B and T cells, which could induce and regulate immune responses (26–28), although release of NE from varicose axon terminals that are not in close proximity with immune cells may also play a role in modulating immune cell function in a paracrine fashion (29). In general, B and T cells express the β2-AR and innate immune cells, such as macrophages, express β2-AR, α1-AR, and α2-AR (30, 31). The β2-AR plays a critical role in the suppression of tumor necrosis factor (TNF)-α and increase of IL-10 secretion by immune cells (32–34), possibly via a noncanonical phosphatidylinositol 3-kinase (PI3K) intracellular pathway (35, 36). Many of these events are thought to occur in the spleen (37–40) (Fig. 1). In addition, splenic nerve activity and β2-AR activation protect against endotoxemia-induced hypotension and hypovolemia, improving survival (33, 34, 41). Overall, the sympathetic control of splenic function is important to modulate immune and cardiovascular functions, preventing cardiovascular collapse during endotoxemia.
Activation of ganglionic NPY neurons innervating the spleen attenuates the production of pro-inflammatory cytokines. Yu et al. (22) showed that NPY mRNA is expressed in both the SRG and CG neurons and that spleen-projecting neurons in SRG and CG express the Toll-like receptor (TLR)-4. Lipopolysaccharide (LPS), a surface membrane component of Gram-negative bacteria, is a TLR4 agonist. Direct application of LPS increases calcium influx in SRG-CG and peripheral administration of LPS upregulates NPY mRNA there (22). These data suggest that LPS activates NPY-expressing neurons that send inputs to the spleen. Neuronal NPY attenuates the production of proinflammatory cytokines in the spleen in response to LPS or in arthritis via NPY receptors (NPY-R) in the spleen and improves survival in septic mice (22). These data reveal a therapeutic potential for NPY-R agonists in modulating inflammation by acting on NPY-R on splenic macrophages and T-cells (22) (Fig. 1).
NEURAL REFLEXES MODULATING SPLENIC IMMUNE FUNCTION
The brain can control the production of cytokines in the spleen via the splenic nerves. The spleen is a substantial source of cytokines, such as TNF-α, in the circulation during systemic inflammation, as elucidated by studies in which surgical removal of the spleen largely reduces the initial increases in plasma TNF-α levels in response to systemic inflammation (38, 42, 43). Consequently, a blockade of TNF-α production in the spleen (as occurs with a central nervous system-driven increase in splenic nerve activity) causes a reduction in the release of TNF-α into the circulation.
Systemic inflammation activates a sympathetic neural pathway that reduces the production of inflammatory cytokines, including TNF-α, in the spleen (and other abdominal organs) during endotoxemia via the splanchnic nerves (44). Therefore, interruption of the sympathetic outflow to the spleen potentiates the inflammatory response to bacterial endotoxin (45). In agreement, loss of noradrenergic innervation to the spleen dysregulates splenic function and compartmentalization of immune cells in the spleen (26). Postmortem analyses showing the loss of noradrenergic nerve fibers in the spleen of septic patients are consistent with the interpretation that impaired neuroimmunomodulation of splenic function can predispose such patients to lethal immune responses (46).
In acute responses to endotoxemia, the endogenous pathways that restrain inflammatory responses do not require the parasympathetic nerves (Fig. 1). Cervical vagotomy, unlike transections of the splanchnic nerves, does not potentiate systemic inflammation or block increases in splanchnic or splenic sympathetic nerve activity during endotoxemia (45), which suggests that the vagus nerve is not required for restraining an endogenous immune response to acute systemic inflammation. Interestingly, vagus nerve stimulation has potent anti-inflammatory effects (discussed in therapeutic modulation of the immune function in the spleen below). It remains to be determined if, even in the absence of distal vagal fibers, inflammatory signals from the periphery can reach the cell bodies of vagal sensory neurons and thereby modulate autonomic reflexes regulating splenic function. It is important to point out that different immune challenges may elicit conserved and/or different body responses. Therefore, the findings from a particular animal model may not apply to all inflammatory conditions.
SENSORY PATHWAYS MODULATE INFLAMMATORY CYTOKINES LEVELS IN THE SPLEEN DURING SYSTEMIC INFLAMMATION
Both neural and humoral pathways are thought to convey information about the peripheral inflammatory status to the brain, and thereby modulate splenic immune functions via the splenic nerves. Here, we describe a few of the sensory neuroimmune pathways that may regulate the brain networks controlling immune function in the periphery.
Humoral Pathways
At least three humoral pathways may contribute to modulating the autonomic nervous system during an immune challenge. Circulating cytokines and bacterial endotoxins change blood-brain-barrier (BBB) permeability (47, 48) and can reach the brain parenchyma via the BBB (49–54). Brain areas lacking the BBB, such as the circumventricular organs (CVOs), are a potential target for inflammatory mediators to modulate the activity of neurons (55–59). In the absence of a breach in BBB, brain endothelial cells play a crucial role in the translation of inflammatory signals to neuronal activity, as occurs in bacterial fever (60, 61).
The increases in splenic nerve activity during endotoxemia are at least partially mediated by derivatives of arachidonic acid in the brain. Intracerebroventricular pretreatment with a cyclooxygenase inhibitor, which prevents the enzymatic conversion of arachidonic acid to functional derivatives, such as prostaglandin E2 (PGE2), significantly delays or abolishes the onset of LPS-evoked increases in splenic nerve activity (62). PGE2 also plays a critical role in bacterial fever (63). Pretreatment with an intracerebroventricular injection of PGE2 increases splenic sympathetic nerve activity and attenuates endotoxemia-induced splenic TNF-α expression (62, 64) (Fig. 1). Thus, PGE2 acts as a regulatory mediator serving to drive a fever response while also suppressing a splenic immune response. This balancing of the immune responses is important for ensuring the elimination of the invading pathogen while at the same time limiting damage to the host, and further has important implications for the use of pharmacological therapeutic approaches.
Nodose Ganglia Neurons and Vagus Nerve
Vagal sensory neurons located in the nodose ganglia are activated by inflammatory stimuli. Circulating inflammatory cytokines, such as IL-1β and TNF-α, lead to increases in afferent vagus nerve activity in rodent models (65–67). Another index of the vagal afferent nerve activation in response to cytokines is the expression of c-fos mRNA in neurons in the nodose ganglion following intravenous injection of IL-1β (66). However, cervical vagotomy does not prevent inflammation-induced production of c-Fos protein in the nucleus tractus solitarius (NTS) (68), where the primary vagal afferent fibers make synaptic contact with second-order neurons (69). NTS neurons also receive inputs from other sensory fibers, such as spinal afferents and glossopharyngeal afferent axons, which could be contributing to an elevated production of c-Fos independently of an excitatory vagal input. For instance, a putative role for renal afferents in the reflex regulation of immunity has been reviewed elsewhere (70). Considering that TLR4, IL-1 receptor, and PGE2 receptor (EP3) mRNAs are expressed in the nodose ganglia (66, 71, 72), inflammatory signals from the periphery may directly affect vagal sensory neurons at the level of the soma, independent of vagal afferent fiber activity. Supporting this notion, LPS induces production of calcitonin gene-related peptide (CGRP), a neurotransmitter produced by vagal sensory neurons, in nodose-jugular ganglia culture in a TLR4-dependent fashion, suggesting that vagal sensory neurons are directly responsive to inflammatory antigens (67, 71, 73). The role of vagal afferent fibers and nodose ganglia neurons in mediating an endogenous immune response via autonomic pathways still needs to be determined. In contrast, it has been shown that electrical stimulation of vagal afferents suppresses systemic inflammation likely via reflex activation of the splanchnic nerves (74). Activation of vagal afferents after high-fat enteral nutrition also produces anti-inflammatory effects during endotoxemia and hemorrhagic shock (75, 76).
Carotid Body: Is the Carotid Sinus Nerve an Afferent Pathway Regulating Splenic Immune Function?
The carotid bodies play a role in inflammatory responses. Cells in the carotid body express cytokine receptors, and inflammation increases carotid sinus nerve activity (77–80). In agreement, the chemoreflex response to potassium cyanide is potentiated by LPS, which may indicate that carotid sinus nerve activity is facilitated during systemic inflammation (81). It was shown recently that carotid body denervation increases mortality in septic rats and increases circulating cytokine levels in response to TNF-α (77, 82, 83). Thus, the carotid body is necessary for restraining inflammation induced by TNF-α, likely via a reflex activation of the splanchnic nerves (77) (Fig. 1).
Based on changes in splenic NE content, it was suggested that the spleen is one of the effector organs in the carotid body-splanchnic nerves reflex (77). However, changes in NE levels in the spleen do not only depend on splenic nerve activity but also on the turnover rate and perhaps on blood levels of NE since increased plasma NE levels are detected for at least 2 h after administration of TNF-α in subjects (84). Therefore, more studies are necessary to clarify if the spleen plays a role in the anti-inflammatory effects of the carotid body-splanchnic nerves reflex. Interestingly, electrical stimulation of the carotid sinus nerve reduces systemic inflammation via glucocorticoid receptors in immune cells, and at least part of this effect does not require the spleen (85). The interplay between the carotid sinus nerve and immune function is likely to be complex and requires further research to be more completely understood.
BRAIN AREAS CONTROLLING THE AUTONOMIC OUTFLOW TO THE SPLEEN
Anatomical studies in rodents using neuronal tracing showed several brain areas that are polysynaptically connected to the spleen (18, 86), presumably via the splenic sympathetic nerve. Little is known, however, about the functional aspects of the brain networks that control the immunological function of the spleen. Here, we highlight a few players in the brain-spleen axis (Fig. 1).
Both the NTS and the rostral ventral lateral medulla (RVLM) contain neurons that are polysynaptically connected to the spleen (18). Neurons in the NTS receive and integrate sensory information from peripheral nerves (including vagi and carotid sinus nerve), as well as from other CNS sites, and densely innervate neurons in the RVLM. In turn, RVLM neurons send rostral and caudal projections that both regulate and contribute to the sympathetic premotor input to the spinal cord. Indeed, an NTS-RVLM-splanchnic nerve pathway appears to participate in a sympathetic reflex elicited by the carotid sinus nerves in response to circulating TNF-α (77), but the role of the spleen as an effector in this reflex remains to be elucidated. Optogenetic stimulation of C1 neurons, a population of sympathetic premotor neurons in the RVLM, reduces kidney damage in a renal ischemia-reperfusion model likely via increasing the sympathetic outflow to the spleen (87, 88). A putative NTS-RVLM (C1) pathway does not exclude other brain areas that could modulate splenic immune function during inflammation. In fact, supramedullary inputs provide a significant tonic excitatory input to NTS neurons and contribute to their spontaneous and vagus nerve-driven activity (89).
LPS increases the expression of inflammatory cytokines not only in the NTS but also in other brain areas that contribute to the control of autonomic functions such as the paraventricular nucleus (PVN), central amygdala (CeA), NTS, and dorsal motor nucleus of the vagus (DMV) (90). Stimulation of other brain areas that are polysynaptically connected to the spleen, such as the CeA and the PVN, as well as the ventral tegmental area (VTA), which may not be connected to the spleen (18, 86), leads to increased splenic immune cell activity. Stress modulates splenic adaptive immunological responses via differentially modulating corticotropin-releasing hormone (CRH)-expressing neurons in CeA or PVN. Optogenetic activation of CeACRH or PVNCRH neurons increases splenic sympathetic nerve activity and increases a splenic adaptive immune response, plasma cell formation (91). In addition, an intracerebroventricular injection of CRH increases the content of NE in the spleen and intracerebroventricular injection of a CRH antagonist reduces splenic NE in response to an intravenous injection of IL-1β (92). These findings show that CRH within the CNS contributes to the increase in sympathetic outflow to the spleen during systemic inflammation induced by IL-1β, thus participating in the regulation of splenic function. The precise pathways by which CRH neurons modulate splenic nerve activity remain unknown. The humoral component of the brain-spleen axis via the hypothalamus-pituitary-adrenal axis has been extensively reviewed elsewhere (93, 94). In addition, chemogenetic activation of VTA neurons increases immune cell activation and reduces bacteria survival (95), and these effects were prevented by chemical denervation of catecholaminergic splenic nerve fibers in rats (95). This indicates that a fine regulation between immune cell activation for bacterial clearance and inhibition of immune cell activity for preventing an exaggerated inflammatory response is likely to be mediated by the brain.
Only a few neurons located in the dorsal motor nucleus of the vagus (DMV) of some rats were transsynaptically labeled following pseudorabies virus (a transsynaptic retrograde tracer) injection in the spleen (18). Nonetheless, optogenetic stimulation of cholinergic neurons in the DMV increases splenic nerve activity and reduces circulating TNF-α levels during endotoxemia (19). The pathway comprising the cholinergic DMV neurons (vagal efferent fibers) that act to reduce systemic inflammation has been referred to as the “cholinergic anti-inflammatory pathway” and has been shown to be an efferent arm of a CNS circuit regulating the brain-spleen axis during systemic inflammation (19, 96).
Together, these studies show that activations of specific nuclei in the brain can increase splenic nerve activity and regulate immune function in the spleen. Interestingly, the splenic nerve-mediated effects can either decrease cytokine production by immune cells or recruit immune cells for the initiation of immune responses, suggesting that the brain can differentially regulate the splenic response. The specific conditions under which these diverse effects occur and the mechanisms underlying these effects are important areas for future studies.
A SPLEEN-TO-LIVER HUMORAL PATHWAY MODULATES INFLAMMATION
The splenic venous vasculature collects into the splenic vein that drains into the hepatic portal system (3, 97). This arrangement permits a unique hormonal communication axis between the spleen and liver that is particularly important during endotoxemia in which the number of neutrophils and macrophages in the liver increases (98). These cells are likely supplied by blood myeloid cells from the spleen since splenectomy reduces the number of macrophages in the liver and the levels of TNF-α in the portal vein and vena cava in response to LPS (98–100). These and other data validate the decades-old proposal that “splenic factors” are necessary for increasing Kupffer cell responsivity to endotoxin (101, 102).
Recently, leukotriene B4 from the spleen was demonstrated to sensitize Kupffer cells to secrete TNF-α in response to LPS (100). In addition, splenectomy reduces plasma TNF-α and Tnf gene expression in the liver while partial hepatectomy reduces plasma TNF-α without affecting Tnf gene expression in the spleen (100). These data reveal a unidirectional spleen-to-liver humoral axis influencing hepatic function in systemic inflammation (100) (Fig. 1). Indeed, leukotriene B4 receptor antagonists have emerged as therapeutic targets for the treatment of inflammatory diseases (103), but whether the anti-inflammatory effects of leukotriene B4 receptor are mediated by the spleen-liver axis is not known. Interactions of the spleen with other players such as the gut, gut microbiota, and higher cerebral functions have been reviewed recently elsewhere (104, 105).
THERAPEUTIC MODULATION OF THE IMMUNE FUNCTION IN THE SPLEEN
The noteworthy effects of the sympathetic (44) and parasympathetic (106) nervous systems in controlling immunological function and recent advances concerning the mechanisms regulating lethal inflammatory responses have opened new avenues for preclinical and clinical research focused on therapeutic approaches to inflammatory diseases, such as endotoxemia, sepsis, hemorrhagic shock, respiratory conditions, rheumatoid arthritis, obesity, and type 2 diabetes (106–109). Many of these inflammatory conditions can be ameliorated by using noninvasive techniques, such as ultrasound (110), acupuncture (111), laser acupuncture (112), electroacupuncture (113, 114), and pharmacotherapy, as well as by using invasive techniques, such as implantable stimulating electrodes surrounding nerves (115, 116). Here, we will discuss a few of these therapies and their mechanisms of action.
Anti-Inflammatory Effects of Vagus Nerve Stimulation
VNS ameliorates inflammatory diseases such as endotoxemia, sepsis, hemorrhagic shock, respiratory or intestinal inflammatory conditions, and, potentially, rheumatoid arthritis (109, 117–120). Selective stimulation of vagal afferent or efferent fibers induces anti-inflammatory effects by distinct neural pathways (40, 87). Interestingly, the anti-inflammatory effects of both afferent and efferent vagus nerve stimulation (VNS) require β2-AR activation (40). In contrast, only efferent VNS requires choline acetyltransferase (ChAT)-expressing T-cells (40). In this scenario, efferent, but not afferent, VNS activates acetylcholine (ACh) release from ChAT T-cells upon reflex adrenergic stimulation, then binding of ACh to α7 nicotinic receptors (α7nAChR) in splenic macrophages inhibits NF-κB phosphorylation, thus reducing TNF-α production (121–124). In addition, efferent VNS reduces intestinal inflammation via the enteric nervous system and resident macrophage α7nAChR in the gut independently of the spleen and T-cells (125, 126).
The anti-inflammatory effects of cervical afferent VNS are not prevented by subdiaphragmatic vagotomy or by blockade of muscarinic acetylcholine receptors (mAChRs) (87). Thus, afferent VNS does not require efferent vagus nerve fibers. Vagal afferent fibers can modulate immune function via the sympathetic nervous system, by reflexively activating the splanchnic and splenic nerves (8, 74, 87). The protective and anti-inflammatory effects of afferent VNS require C1 catecholaminergic neurons, located in the RVLM, whereas efferent VNS does not (87). A hypothesis for a neural circuit mediating the effects of afferent VNS on splenic sympathetic outflow is that vagal afferents activate NTS neurons, which project to C1 sympathetic premotor neurons to increase the sympathetic outflow to the spleen and other organs to induce anti-inflammatory effects. This hypothetical circuit does not exclude other brain areas that could modulate the immune function of the spleen.
Anti-Inflammatory Effects of Pharmaceuticals
Peripherally acting drugs can be beneficial for reducing splenic inflammatory responses. Based on the anti-inflammatory effects of NPY overexpression in SRG and CG neurons and the proinflammatory effects of NPY knockdown (22), activation of splenic NPY-R by administration of an NPY-R agonist is a potential therapeutical tool to reduce inflammation and sepsis mortality (22).
Altering the relevant brain circuits could also modulate splenic function. Centrally acting mAChR agonists and an acetylcholinesterase inhibitor, galantamine, reduce mortality and systemic inflammation in septic mice. The anti-inflammatory effect was absent in mice that were subjected to splenic neurectomy (127, 128), splenectomy (128, 129), or in α7nAChR knockout mice (128). Interestingly, galantamine reduced body weight in addition to reducing inflammatory cytokines in high-fat diet-fed mice (130). The brain networks underpinning the effects of centrally acting cholinergic drugs in inflammation and metabolism are not known.
Modulation of the neurotransmitter and hormone serotonin is also a promising treatment for neural and peripheral inflammation. In the LPS model of bacterial infection, administration of an antidepressant drug (fluoxetine, a selective serotonin reuptake inhibitor), reduces plasma and splenic cytokine (TNF-α, IL-1β, and IL-6) levels and the expression of c-Fos and TNF-α mRNA in the PVN and the NTS (90). Chronic subdiaphragmatic vagotomy partially reverses the anti-inflammatory effects of fluoxetine in the periphery, but not in the brain (90). Our previous studies show that intracerebroventricular administration of serotonin reduces plasma TNF-α, IL-1β, and IL-6 and splenic TNF-α and IL-10 levels, prevents hypotension, and reduces corticosterone levels in endotoxemia (131, 132). These effects may be mediated by the splanchnic nerves since serotonin receptor agonists increase splanchnic nerve activity (133). As pointed out earlier, the splanchnic nerves control a sympathetic reflex with anti-inflammatory properties (134). Whether the anti-inflammatory effects of serotonin are mediated specifically by the splanchnic and/or splenic nerves and the neural circuits mediating these effects remain to be determined.
CONCLUSION
Elucidating the CNS neuroimmune pathways controlling splenic function is important for the development of anti-inflammatory therapies that act to modulate a splenic immune response or mimic sympathetic neural activity to the spleen. Neurochemical modulation of these central autonomic nervous system circuits could be of potential benefit to patients with inflammatory conditions such as arthritis, kidney injuries, sepsis, and endotoxemic shock.
GRANTS
This study was supported by the National Institutes of Health Grant DK112198 (to C.J.M.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.M.D.M. and C.J.M. conceived and designed research; C.M.D.M. prepared figure; C.M.D.M. drafted manuscript; C.M.D.M. and C.J.M. edited and revised manuscript; C.M.D.M. and C.J.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Shaun F. Morrison for the insightful discussions and comments on an earlier version of this manuscript.
REFERENCES
- 1. Lewis SM, Williams A, Eisenbarth SC. Structure and function of the immune system in the spleen. Sci Immunol 4: eaau6085, 2019. doi: 10.1126/sciimmunol.aau6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cesta MF. Normal structure, function, and histology of the spleen. Toxicol Pathol 34: 455–465, 2006. doi: 10.1080/01926230600867743. [DOI] [PubMed] [Google Scholar]
- 3. Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol 5: 606–616, 2005. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 4. Nance DM, Burns J. Innervation of the spleen in the rat: evidence for absence of afferent innervation. Brain Behav Immun 3: 281–290, 1989. doi: 10.1016/0889-1591(89)90028-7. [DOI] [PubMed] [Google Scholar]
- 5. Felten DL, Felten SY, Carlson SL, Olschowka JA, Livnat S. Noradrenergic and peptidergic innervation of lymphoid tissue. J Immunol 135, Suppl 2: 755s–765s, 1985. doi: 10.1016/S0145-305X(06)80005-4. [DOI] [PubMed] [Google Scholar]
- 6. Williams JM, Felten DL. Sympathetic innervation of murine thymus and spleen: a comparative histofluorescence study. Anat Rec 199: 531–542, 1981. doi: 10.1002/ar.1091990409. [DOI] [PubMed] [Google Scholar]
- 7. Bellinger DL, Felten SY, Lorton D, Felten DL. Origin of noradrenergic innervation of the spleen in rats. Brain Behav Immun 3: 291–311, 1989. doi: 10.1016/0889-1591(89)90029-9. [DOI] [PubMed] [Google Scholar]
- 8. Bratton BO, Martelli D, McKinley MJ, Trevaks D, Anderson CR, McAllen RM. Neural regulation of inflammation: no neural connection from the vagus to splenic sympathetic neurons. Exp Physiol 97: 1180–1185, 2012. doi: 10.1113/expphysiol.2011.061531. [DOI] [PubMed] [Google Scholar]
- 9. Gautron L, Rutkowski JM, Burton MD, Wei W, Wan Y, Elmquist JK. Neuronal and nonneuronal cholinergic structures in the mouse gastrointestinal tract and spleen. J Comp Neurol 521: 3741–3767, 2013. doi: 10.1002/cne.23376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buijs RM, van der Vliet J, Garidou ML, Huitinga I, Escobar C. Spleen vagal denervation inhibits the production of antibodies to circulating antigens. PLoS One 3: e3152, 2008. doi: 10.1371/journal.pone.0003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kooijman S, Meurs I, van Beek L, Khedoe PP, Giezekamp A, Pike-Overzet K, Cailotto C, van der Vliet J, van Harmelen V, Boeckxstaens G, Berbée JF, Rensen PC. Splenic autonomic denervation increases inflammatory status but does not aggravate atherosclerotic lesion development. Am J Physiol Heart Circ Physiol 309: H646–H654, 2015. doi: 10.1152/ajpheart.00787.2014. [DOI] [PubMed] [Google Scholar]
- 12. Guyot M, Simon T, Panzolini C, Ceppo F, Daoudlarian D, Murris E, Macia E, Abélanet S, Sridhar A, Vervoordeldonk MJ, Glaichenhaus N, Blancou P. Apical splenic nerve electrical stimulation discloses an anti-inflammatory pathway relying on adrenergic and nicotinic receptors in myeloid cells. Brain Behav Immun 80: 238–246, 2019. doi: 10.1016/j.bbi.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 13. Anderson C, McKinley M, Martelli D, McAllen R. Letter to the editor: parasympathetic innervation of the rodent spleen? Am J Physiol Heart Circ Physiol 309: H2158, 2015. doi: 10.1152/ajpheart.00766.2015. [DOI] [PubMed] [Google Scholar]
- 14. Kooijman S, de Jonge WJ, Rensen PC. Reply to “Letter to the editor: parasympathetic innervation of the rodent spleen?” Am J Physiol Heart Circ Physiol 309: H2159, 2015. doi: 10.1152/ajpheart.00805.2015. [DOI] [PubMed] [Google Scholar]
- 15. Berthoud HR, Powley TL. Characterization of vagal innervation to the rat celiac, suprarenal and mesenteric ganglia. J Auton Nerv Syst 42: 153–169, 1993. doi: 10.1016/0165-1838(93)90046-W. [DOI] [PubMed] [Google Scholar]
- 16. Taylor RB, Weaver LC. Spinal stimulation to locate preganglionic neurons controlling the kidney, spleen, or intestine. Am J Physiol Heart Circ Physiol 263: H1026–H1033, 1992. doi: 10.1152/ajpheart.1992.263.4.H1026. [DOI] [PubMed] [Google Scholar]
- 17. Kaestner CL, Smith EH, Peirce SG, Hoover DB. Immunohistochemical analysis of the mouse celiac ganglion: an integrative relay station of the peripheral nervous system. J Comp Neurol 527: 2742–2760, 2019. doi: 10.1002/cne.24705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cano G, Sved AF, Rinaman L, Rabin BS, Card JP. Characterization of the central nervous system innervation of the rat spleen using viral transneuronal tracing. J Comp Neurol 439: 1–18, 2001. doi: 10.1002/cne.1331. [DOI] [PubMed] [Google Scholar]
- 19. Kressel AM, Tsaava T, Levine YA, Chang EH, Addorisio ME, Chang Q, Burbach BJ, Carnevale D, Lembo G, Zador AM, Andersson U, Pavlov VA, Chavan SS, Tracey KJ. Identification of a brainstem locus that inhibits tumor necrosis factor. Proc Natl Acad Sci USA 117: 29803–29810, 2020. doi: 10.1073/pnas.2008213117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Romano TA, Felten SY, Felten DL, Olschowka JA. Neuropeptide-Y innervation of the rat spleen: another potential immunomodulatory neuropeptide. Brain Behav Immun 5: 116–131, 1991. doi: 10.1016/0889-1591(91)90011-x. [DOI] [PubMed] [Google Scholar]
- 21. Devi S, Alexandre YO, Loi JK, Gillis R, Ghazanfari N, Creed SJ, Holz LE, Shackleford D, Mackay LK, Heath WR, Sloan EK, Mueller SN. Adrenergic regulation of the vasculature impairs leukocyte interstitial migration and suppresses immune responses. Immunity 54: 1219–1230.e7, 2021. doi: 10.1016/j.immuni.2021.03.025. [DOI] [PubMed] [Google Scholar]
- 22. Yu J, Xiao K, Chen X, Deng L, Zhang L, Li Y, Gao A, Gao J, Wu C, Yang X, Zhou Q, Yang J, Bao C, Jiao J, Cheng S, Guo Z, Xu W, Cao X, Guo Z, Dai J, Hu J, Fu Z, Cao G. Neuron-derived neuropeptide Y fine-tunes the splenic immune responses. Neuron 110: 1327–1339.e6, 2022. doi: 10.1016/j.neuron.2022.01.010. [DOI] [PubMed] [Google Scholar]
- 23. Fried G, Terenius L, Brodin E, Efendic S, Dockray G, Fahrenkrug J, Goldstein M, Hökfelt T. Neuropeptide Y, enkephalin and noradrenaline coexist in sympathetic neurons innervating the bovine spleen. Biochemical and immunohistochemical evidence. Cell Tissue Res 243: 495–508, 1986. doi: 10.1007/BF00218056. [DOI] [PubMed] [Google Scholar]
- 24. Rogausch H, Böck T, Voigt KH, Besedovsky H. The sympathetic control of blood supply is different in the spleen and lymph nodes. Neuroimmunomodulation 11: 58–64, 2004. doi: 10.1159/000072970. [DOI] [PubMed] [Google Scholar]
- 25. Rogausch H, del Rey A, Kabiersch A, Reschke W, Ortel J, Besedovsky H. Endotoxin impedes vasoconstriction in the spleen: role of endogenous interleukin-1 and sympathetic innervation. Am J Physiol Regul Integr Comp Physiol 272: R2048–R2054, 1997. doi: 10.1152/ajpregu.1997.272.6.R2048. [DOI] [PubMed] [Google Scholar]
- 26. Murray K, Barboza M, Rude KM, Brust-Mascher I, Reardon C. Functional circuitry of neuro-immune communication in the mesenteric lymph node and spleen. Brain Behav Immun 82: 214–223, 2019. doi: 10.1016/j.bbi.2019.08.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Felten SY, Olschowka J. Noradrenergic sympathetic innervation of the spleen: II. Tyrosine hydroxylase (TH)-positive nerve terminals form synapticlike contacts on lymphocytes in the splenic white pulp. J Neurosci Res 18: 37–48, 1987. doi: 10.1002/jnr.490180108. [DOI] [PubMed] [Google Scholar]
- 28. Noble BT, Brennan FH, Popovich PG. The spleen as a neuroimmune interface after spinal cord injury. J Neuroimmunol 321: 1–11, 2018. doi: 10.1016/j.jneuroim.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 29. Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve–an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev 52: 595–638, 2000. [PubMed] [Google Scholar]
- 30. Grisanti LA, Perez DM, Porter JE. Modulation of immune cell function by α1-adrenergic receptor activation. Curr Top Membr 67: 113–138, 2011. doi: 10.1016/B978-0-12-384921-2.00006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007). Brain Behav Immun 21: 736–745, 2007. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ignatowski TA, Gallant S, Spengler RN. Temporal regulation by adrenergic receptor stimulation of macrophage (MΦ)-derived tumor necrosis factor (TNF) production post-LPS challenge. J Neuroimmunol 65: 107–117, 1996. doi: 10.1016/0165-5728(96)00004-5. [DOI] [PubMed] [Google Scholar]
- 33. Ağaç D, Estrada LD, Maples R, Hooper LV, Farrar JD. The β2-adrenergic receptor controls inflammation by driving rapid IL-10 secretion. Brain Behav Immun 74: 176–185, 2018. doi: 10.1016/j.bbi.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Estrada LD, Ağaç D, Farrar JD. Sympathetic neural signaling via the β2-adrenergic receptor suppresses T-cell receptor-mediated human and mouse CD8+ T-cell effector function. Eur J Immunol 46: 1948–1958, 2016. doi: 10.1002/eji.201646395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grailer JJ, Haggadone MD, Sarma JV, Zetoune FS, Ward PA. Induction of M2 regulatory macrophages through the β2-adrenergic receptor with protection during endotoxemia and acute lung injury. J Innate Immun 6: 607–618, 2014. doi: 10.1159/000358524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Körner A, Schlegel M, Kaussen T, Gudernatsch V, Hansmann G, Schumacher T, Giera M, Mirakaj V. Sympathetic nervous system controls resolution of inflammation via regulation of repulsive guidance molecule A. Nat Commun 10: 633, 2019. doi: 10.1038/s41467-019-08328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, Chavan S, Tracey KJ. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci USA 105: 11008–11013, 2008. doi: 10.1073/pnas.0803237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325: 612–616, 2009. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vida G, Peña G, Kanashiro A, Thompson-Bonilla Mdel R, Palange D, Deitch EA, Ulloa L. β2-Adrenoreceptors of regulatory lymphocytes are essential for vagal neuromodulation of the innate immune system. FASEB J 25: 4476–4485, 2011. doi: 10.1096/fj.11-191007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Murray K, Rude KM, Sladek J, Reardon C. Divergence of neuroimmune circuits activated by afferent and efferent vagal nerve stimulation in the regulation of inflammation. J Physiol 599: 2075–2084, 2021. doi: 10.1113/JP281189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Andrew PS, Kaufman S. Splenic denervation worsens lipopolysaccharide-induced hypotension, hemoconcentration, and hypovolemia. Am J Physiol Regul Integr Comp Physiol 280: R1564–R1572, 2001. doi: 10.1152/ajpregu.2001.280.5.R1564. [DOI] [PubMed] [Google Scholar]
- 42. Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, Tracey KJ, Ulloa L. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med 203: 1623–1628, 2006. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Semaeva E, Tenstad O, Skavland J, Enger M, Iversen PO, Gjertsen BT, Wiig H. Access to the spleen microenvironment through lymph shows local cytokine production, increased cell flux, and altered signaling of immune cells during lipopolysaccharide-induced acute inflammation. J Immunol 184: 4547–4556, 2010. doi: 10.4049/jimmunol.0902049. [DOI] [PubMed] [Google Scholar]
- 44. McAllen RM, McKinley MJ, Martelli D. Reflex regulation of systemic inflammation by the autonomic nervous system. Auton Neurosci 237: 102926, 2022. doi: 10.1016/j.autneu.2021.102926. [DOI] [PubMed] [Google Scholar]
- 45. Martelli D, Yao ST, McKinley MJ, McAllen RM. Reflex control of inflammation by sympathetic nerves, not the vagus. J Physiol 592: 1677–1686, 2014. doi: 10.1113/jphysiol.2013.268573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hoover DB, Brown TC, Miller MK, Schweitzer JB, Williams DL. Loss of sympathetic nerves in spleens from patients with end stage sepsis. Front Immunol 8: 1712, 2017. doi: 10.3389/fimmu.2017.01712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Skinner RA, Gibson RM, Rothwell NJ, Pinteaux E, Penny JI. Transport of interleukin-1 across cerebromicrovascular endothelial cells. Br J Pharmacol 156: 1115–1123, 2009. doi: 10.1111/j.1476-5381.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Blamire AM, Anthony DC, Rajagopalan B, Sibson NR, Perry VH, Styles P. Interleukin-1β -induced changes in blood-brain barrier permeability, apparent diffusion coefficient, and cerebral blood volume in the rat brain: a magnetic resonance study. J Neurosci 20: 8153–8159, 2000. doi: 10.1523/JNEUROSCI.20-21-08153.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Erickson MA, Banks WA. Neuroimmune axes of the blood-brain barriers and blood-brain interfaces: bases for physiological regulation, disease states, and pharmacological interventions. Pharmacol Rev 70: 278–314, 2018. doi: 10.1124/pr.117.014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Banks WA, Ortiz L, Plotkin SR, Kastin AJ. Human interleukin (IL) 1 alpha, murine IL-1 alpha and murine IL-1 beta are transported from blood to brain in the mouse by a shared saturable mechanism. J Pharmacol Exp Ther 259: 988–996, 1991. [PubMed] [Google Scholar]
- 51. McLay RN, Kastin AJ, Zadina JE. Passage of interleukin-1-beta across the blood-brain barrier is reduced in aged mice: a possible mechanism for diminished fever in aging. Neuroimmunomodulation 8: 148–153, 2000. doi: 10.1159/000054275. [DOI] [PubMed] [Google Scholar]
- 52. Argaw AT, Zhang Y, Snyder BJ, Zhao ML, Kopp N, Lee SC, Raine CS, Brosnan CF, John GR. IL-1β regulates blood-brain barrier permeability via reactivation of the hypoxia-angiogenesis program. J Immunol 177: 5574–5584, 2006. doi: 10.4049/jimmunol.177.8.5574. [DOI] [PubMed] [Google Scholar]
- 53. Banks WA, Kastin AJ, Gutierrez EG. Interleukin-1α in blood has direct access to cortical brain cells. Neurosci Lett 163: 41–44, 1993. doi: 10.1016/0304-3940(93)90224-9. [DOI] [PubMed] [Google Scholar]
- 54. Coceani F, Lees J, Dinarello CA. Occurrence of interleukin-1 in cerebrospinal fluid of the conscious cat. Brain Res 446: 245–250, 1988. doi: 10.1016/0006-8993(88)90883-9. [DOI] [PubMed] [Google Scholar]
- 55. Vitkovic L, Konsman JP, Bockaert J, Dantzer R, Homburger V, Jacque C. Cytokine signals propagate through the brain. Mol Psychiatry 5: 604–615, 2000. [Erratum in Mol Psychiatry 6: 249, 2001]. doi: 10.1038/sj.mp.4000813. [DOI] [PubMed] [Google Scholar]
- 56. Quan N, Whiteside M, Herkenham M. Time course and localization patterns of interleukin-1β messenger RNA expression in brain and pituitary after peripheral administration of lipopolysaccharide. Neuroscience 83: 281–293, 1998. doi: 10.1016/s0306-4522(97)00350-3. [DOI] [PubMed] [Google Scholar]
- 57. Broadwell RD, Sofroniew MV. Serum proteins bypass the blood-brain fluid barriers for extracellular entry to the central nervous system. Exp Neurol 120: 245–263, 1993. doi: 10.1006/exnr.1993.1059. [DOI] [PubMed] [Google Scholar]
- 58. Hashimoto M, Ishikawa Y, Yokota S, Goto F, Bando T, Sakakibara Y, Iriki M. Action site of circulating interleukin-1 on the rabbit brain. Brain Res 540: 217–223, 1991. doi: 10.1016/0006-8993(91)90510-3. [DOI] [PubMed] [Google Scholar]
- 59. Maness LM, Kastin AJ, Banks WA. Relative contributions of a CVO and the microvascular bed to delivery of blood-borne IL-1α to the brain. Am J Physiol Endocrinol Physiol 275: E207–E212, 1998. doi: 10.1152/ajpendo.1998.275.2.E207. [DOI] [PubMed] [Google Scholar]
- 60. Ching S, Zhang H, Belevych N, He L, Lai W, Pu XA, Jaeger LB, Chen Q, Quan N. Endothelial-specific knockdown of interleukin-1 (IL-1) type 1 receptor differentially alters CNS responses to IL-1 depending on its route of administration. J Neurosci 27: 10476–10486, 2007. doi: 10.1523/JNEUROSCI.3357-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Knoll JG, Krasnow SM, Marks DL. Interleukin-1β signaling in fenestrated capillaries is sufficient to trigger sickness responses in mice. J Neuroinflammation 14: 219, 2017. doi: 10.1186/s12974-017-0990-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. MacNeil BJ, Jansen AH, Janz LJ, Greenberg AH, Nance DM. Peripheral endotoxin increases splenic sympathetic nerve activity via central prostaglandin synthesis. Am J Physiol Regul Integr Comp Physiol 273: R609–R614, 1997. doi: 10.1152/ajpregu.1997.273.2.R609. [DOI] [PubMed] [Google Scholar]
- 63. Madden CJ, Morrison SF. Central nervous system circuits that control body temperature. Neurosci Lett 696: 225–232, 2019. doi: 10.1016/j.neulet.2018.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nance DM, Macneil BJ. Immunoregulation by the sympathetic nervous system. Neuroimmune Biol 1: 121–139, 2001. doi: 10.1016/S1567-7443(01)80013-2. [DOI] [Google Scholar]
- 65. Niijima A. The afferent discharges from sensors for interleukin 1β in the hepatoportal system in the anesthetized rat. J Auton Nerv Syst 61: 287–291, 1996. doi: 10.1016/S0165-1838(96)00098-7. [DOI] [PubMed] [Google Scholar]
- 66. Ek M, Kurosawa M, Lundeberg T, Ericsson A. Activation of vagal afferents after intravenous injection of interleukin-1beta: role of endogenous prostaglandins. J Neurosci 18: 9471–9479, 1998. doi: 10.1523/JNEUROSCI.18-22-09471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zanos TP, Silverman HA, Levy T, Tsaava T, Battinelli E, Lorraine PW, Ashe JM, Chavan SS, Tracey KJ, Bouton CE. Identification of cytokine-specific sensory neural signals by decoding murine vagus nerve activity. Proc Natl Acad Sci USA 115: E4843–E4852, 2018. doi: 10.1073/pnas.1719083115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hermann GE, Emch GS, Tovar CA, Rogers RC. c-Fos generation in the dorsal vagal complex after systemic endotoxin is not dependent on the vagus nerve. Am J Physiol Regul Integr Comp Physiol 280: R289–R299, 2001. doi: 10.1152/ajpregu.2001.280.1.R289. [DOI] [PubMed] [Google Scholar]
- 69. Fawley JA, Hegarty DM, Aicher SA, Beaumont E, Andresen MC. Dedicated C-fiber vagal sensory afferent pathways to the paraventricular nucleus of the hypothalamus. Brain Res 1769: 147625, 2021. doi: 10.1016/j.brainres.2021.147625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Okusa MD, Rosin DL, Tracey KJ. Targeting neural reflex circuits in immunity to treat kidney disease. Nat Rev Nephrol 13: 669–680, 2017. doi: 10.1038/nrneph.2017.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jia L, Lee S, Tierney JA, Elmquist JK, Burton MD, Gautron L. TLR4 signaling selectively and directly promotes CGRP release from vagal afferents in the mouse. eNeuro 8: ENEURO.0254-20.2020, 2021. doi: 10.1523/ENEURO.0254-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hosoi T, Okuma Y, Matsuda T, Nomura Y. Novel pathway for LPS-induced afferent vagus nerve activation: possible role of nodose ganglion. Auton Neurosci 120: 104–107, 2005. doi: 10.1016/j.autneu.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 73. Steinberg BE, Silverman HA, Robbiati S, Gunasekaran MK, Tsaava T, Battinelli E, Stiegler A, Bouton CE, Chavan SS, Tracey KJ, Huerta PT. Cytokine-specific neurograms in the sensory vagus nerve. Bioelectron Med 3: 7–17, 2016. doi: 10.15424/bioelectronmed.2016.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Komegae EN, Farmer DGS, Brooks VL, McKinley MJ, McAllen RM, Martelli D. Vagal afferent activation suppresses systemic inflammation via the splanchnic anti-inflammatory pathway. Brain Behav Immun 73: 441–449, 2018. doi: 10.1016/j.bbi.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lubbers T, De Haan JJ, Hadfoune M, Zhang Y, Luyer MD, Grundy D, Buurman WA, Greve JW. Lipid-enriched enteral nutrition controls the inflammatory response in murine Gram-negative sepsis. Crit Care Med 38: 1996–2002, 1996. doi: 10.1097/CCM.0b013e3181eb90d7. [DOI] [PubMed] [Google Scholar]
- 76. Luyer MD, Greve JW, Hadfoune M, Jacobs JA, Dejong CH, Buurman WA. Nutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerve. J Exp Med 202: 1023–1029, 2005. doi: 10.1084/jem.20042397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Katayama PL, Leirão IP, Kanashiro A, Luiz JPM, Cunha FQ, Navegantes LCC, Menani JV, Zoccal DB, Colombari DSA, Colombari E. The carotid body detects circulating tumor necrosis factor-alpha to activate a sympathetic anti-inflammatory reflex. Brain Behav Immun 102: 370–386, 2022. doi: 10.1016/j.bbi.2022.03.014. [DOI] [PubMed] [Google Scholar]
- 78. Fernández R, Nardocci G, Simon F, Martin A, Becerra A, Rodríguez-Tirado C, Maisey KR, Acuña-Castillo C, Cortes PP. Lipopolysaccharide signaling in the carotid chemoreceptor pathway of rats with sepsis syndrome. Respir Physiol Neurobiol 175: 336–348, 2011. doi: 10.1016/j.resp.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 79. Wang X, Zhang XJ, Xu Z, Li X, Li GL, Ju G, Wang BR. Morphological evidence for existence of IL-6 receptor alpha in the glomus cells of rat carotid body. Anat Rec A Discov Mol Cell Evol Biol 288: 292–296, 2006. doi: 10.1002/ar.a.20310. [DOI] [PubMed] [Google Scholar]
- 80. Wang X, Wang BR, Duan XL, Zhang P, Ding YQ, Jia Y, Jiao XY, Ju G. Strong expression of interleukin-1 receptor type I in the rat carotid body. J Histochem Cytochem 50: 1677–1684, 2002. doi: 10.1177/002215540205001213. [DOI] [PubMed] [Google Scholar]
- 81. Amorim MR, de Deus JL, Cazuza RA, Mota CMD, da Silva LEV, Borges GS, Batalhão ME, Cárnio EC, Branco LGS. Neuroinflammation in the NTS is associated with changes in cardiovascular reflexes during systemic inflammation. J Neuroinflammation 16: 125, 2019. doi: 10.1186/s12974-019-1512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Reyes EP, Abarzúa S, Martin A, Rodríguez J, Cortés PP, Fernández R. LPS-induced c-Fos activation in NTS neurons and plasmatic cortisol increases in septic rats are suppressed by bilateral carotid chemodenervation. Adv Exp Med Biol 758: 185–190, 2012. doi: 10.1007/978-94-007-4584-1_26. [DOI] [PubMed] [Google Scholar]
- 83. Nardocci G, Martin A, Abarzúa S, Rodríguez J, Simon F, Reyes EP, Acuña-Castillo C, Navarro C, Cortés PP, Fernández R. Sepsis progression to multiple organ dysfunction in carotid chemo/baro-denervated rats treated with lipopolysaccharide. J Neuroimmunol 278: 44–52, 2015. doi: 10.1016/j.jneuroim.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 84. Van der Poll T, Romijn JA, Endert E, Borm JJ, Büller HR, Sauerwein HP. Tumor necrosis factor mimics the metabolic response to acute infection in healthy humans. Am J Physiol Endocrinol Physiol 261: E457–E465, 1991. doi: 10.1152/ajpendo.1991.261.4.E457. [DOI] [PubMed] [Google Scholar]
- 85. Falvey A, Duprat F, Simon T, Hugues-Ascery S, Conde SV, Glaichenhaus N, Blancou P. Electrostimulation of the carotid sinus nerve in mice attenuates inflammation via glucocorticoid receptor on myeloid immune cells. J Neuroinflammation 17: 368, 2020. doi: 10.1186/s12974-020-02016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ueno M, Ueno-Nakamura Y, Niehaus J, Popovich PG, Yoshida Y. Silencing spinal interneurons inhibits immune suppressive autonomic reflexes caused by spinal cord injury. Nat Neurosci 19: 784–787, 2016. doi: 10.1038/nn.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tanaka S, Abe C, Abbott SBG, Zheng S, Yamaoka Y, Lipsey JE, Skrypnyk NI, Yao J, Inoue T, Nash WT, Stornetta DS, Rosin DL, Stornetta RL, Guyenet PG, Okusa MD. Vagus nerve stimulation activates two distinct neuroimmune circuits converging in the spleen to protect mice from kidney injury. Proc Natl Acad Sci USA 118: e2021758118, 2021. doi: 10.1073/pnas.2021758118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Abe C, Inoue T, Inglis MA, Viar KE, Huang L, Ye H, Rosin DL, Stornetta RL, Okusa MD, Guyenet PG. C1 neurons mediate a stress-induced anti-inflammatory reflex in mice. Nat Neurosci 20: 700–707, 2017. doi: 10.1038/nn.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Cooper CM, Farrand AQ, Andresen MC, Beaumont E. Vagus nerve stimulation activates nucleus of solitary tract neurons via supramedullary pathways. J Physiol 599: 5261–5279, 2021. doi: 10.1113/JP282064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ondicova K, Tillinger A, Pecenak J, Mravec B. The vagus nerve role in antidepressants action: Efferent vagal pathways participate in peripheral anti-inflammatory effect of fluoxetine. Neurochem Int 125: 47–56, 2019. doi: 10.1016/j.neuint.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 91. Zhang X, Lei B, Yuan Y, Zhang L, Hu L, Jin S, Kang B, Liao X, Sun W, Xu F, Zhong Y, Hu J, Qi H. Brain control of humoral immune responses amenable to behavioural modulation. Nature 581: 204–208, 2020. doi: 10.1038/s41586-020-2235-7. [DOI] [PubMed] [Google Scholar]
- 92. Shimizu N, Hori T, Nakane H. An interleukin-1 beta-induced noradrenaline release in the spleen is mediated by brain corticotropin-releasing factor: an in vivo microdialysis study in conscious rats. Brain Behav Immun 8: 14–23, 1994. doi: 10.1006/brbi.1994.1002. [DOI] [PubMed] [Google Scholar]
- 93. Zhang B, Zhong J, Gao Z. A brain-spleen axis regulates humoral immunity. Neurosci Bull 37: 427–429, 2021. doi: 10.1007/s12264-020-00610-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mueller B, Figueroa A, Robinson-Papp J. Structural and functional connections between the autonomic nervous system, hypothalamic-pituitary-adrenal axis, and the immune system: a context and time dependent stress response network. Neurol Sci 43: 951–960, 2022. doi: 10.1007/s10072-021-05810-1. [DOI] [PubMed] [Google Scholar]
- 95. Ben-Shaanan TL, Azulay-Debby H, Dubovik T, Starosvetsky E, Korin B, Schiller M, Green NL, Admon Y, Hakim F, Shen-Orr SS, Rolls A. Activation of the reward system boosts innate and adaptive immunity. Nat Med 22: 940–944, 2016. doi: 10.1038/nm.4133. [DOI] [PubMed] [Google Scholar]
- 96. Kelly MJ, Breathnach C, Tracey KJ, Donnelly SC. Manipulation of the inflammatory reflex as a therapeutic strategy. Cell Rep Med 3: 100696, 2022. doi: 10.1016/j.xcrm.2022.100696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Schmidt EE, MacDonald IC, Groom AC. Comparative aspects of splenic microcirculatory pathways in mammals: the region bordering the white pulp. Scanning Microsc 7: 613–628, 1993. [PubMed] [Google Scholar]
- 98. Mizukami T, Yokoyama H, Okamura Y, Ohgo H, Fukuda M, Kamegaya Y, Kato S, Ishii H. Splenectomy attenuates superoxide anion release into the hepatic sinusoids after lipopolysaccharide challenge. J Hepatol 31: 235–241, 1999. doi: 10.1016/s0168-8278(99)80219-0. [DOI] [PubMed] [Google Scholar]
- 99. Wacker HH, Radzun HJ, Parwaresch MR. Kinetics of Kupffer cells as shown by parabiosis and combined autoradiographic/immunohistochemical analysis. Virchows Arch B Cell Pathol Incl Mol Pathol 51: 71–78, 1986. doi: 10.1007/BF02899017. [DOI] [PubMed] [Google Scholar]
- 100. Fonseca MT, Moretti EH, Marques LMM, Machado BF, Brito CF, Guedes JT, Komegae EN, Vieira TS, Festuccia WT, Lopes NP, Steiner AA. A leukotriene-dependent spleen-liver axis drives TNF production in systemic inflammation. Sci Signal 14: eabb0969, 2021. doi: 10.1126/scisignal.abb0969. [DOI] [PubMed] [Google Scholar]
- 101. Billiar TR, Maddaus MA, West MA, Curran RD, Wells CA, Simmons RL. Intestinal gram-negative bacterial overgrowth in vivo augments the in vitro response of Kupffer cells to endotoxin. Ann Surg 208: 532–540, 1988. doi: 10.1097/00000658-198810000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Billiar TR, West MA, Hyland BJ, Simmons RL. Splenectomy alters Kupffer cell response to endotoxin. Arch Surg 123: 327–332, 1988. doi: 10.1001/archsurg.1988.01400270061009. [DOI] [PubMed] [Google Scholar]
- 103. Hicks A, Monkarsh SP, Hoffman AF, Goodnow R Jr.. Leukotriene B4 receptor antagonists as therapeutics for inflammatory disease: preclinical and clinical developments. Expert Opin Investig Drugs 16: 1909–1920, 2007. doi: 10.1517/13543784.16.12.1909. [DOI] [PubMed] [Google Scholar]
- 104. Wei Y, Wang T, Liao L, Fan X, Chang L, Hashimoto K. Brain-spleen axis in health and diseases: A review and future perspective. Brain Res Bull 182: 130–140, 2022. doi: 10.1016/j.brainresbull.2022.02.008. [DOI] [PubMed] [Google Scholar]
- 105. Buchmann Godinho D, da Silva Fiorin F, Schneider Oliveira M, Furian AF, Rechia Fighera M, Freire Royes LF. The immunological influence of physical exercise on TBI-induced pathophysiology: Crosstalk between the spleen, gut, and brain. Neurosci Biobehav Rev 130: 15–30, 2021. doi: 10.1016/j.neubiorev.2021.08.006. [DOI] [PubMed] [Google Scholar]
- 106. Pavlov VA, Chavan SS, Tracey KJ. Molecular and functional neuroscience in immunity. Annu Rev Immunol 36: 783–812, 2018. doi: 10.1146/annurev-immunol-042617-053158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. van Maanen MA, Vervoordeldonk MJ, Tak PP. The cholinergic anti-inflammatory pathway: towards innovative treatment of rheumatoid arthritis. Nat Rev Rheumatol 5: 229–232, 2009. doi: 10.1038/nrrheum.2009.31. [DOI] [PubMed] [Google Scholar]
- 108. Pavlov VA, Tracey KJ. The vagus nerve and the inflammatory reflex–linking immunity and metabolism. Nat Rev Endocrinol 8: 743–754, 2012. doi: 10.1038/nrendo.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Li S, Qi D, Li JN, Deng XY, Wang DX. Vagus nerve stimulation enhances the cholinergic anti-inflammatory pathway to reduce lung injury in acute respiratory distress syndrome via STAT3. Cell Death Discov 7: 63, 2021. doi: 10.1038/s41420-021-00431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gigliotti JC, Huang L, Ye H, Bajwa A, Chattrabhuti K, Lee S, Klibanov AL, Kalantari K, Rosin DL, Okusa MD. Ultrasound prevents renal ischemia-reperfusion injury by stimulating the splenic cholinergic anti-inflammatory pathway. J Am Soc Nephrol 24: 1451–1460, 2013. doi: 10.1681/ASN.2013010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Huang CL, Tsai PS, Wang TY, Yan LP, Xu HZ, Huang CJ. Acupuncture stimulation of ST36 (Zusanli) attenuates acute renal but not hepatic injury in lipopolysaccharide-stimulated rats. Anesth Analg 104: 646–654, 2007. doi: 10.1213/01.ane.0000255288.68199.eb. [DOI] [PubMed] [Google Scholar]
- 112. Erthal V, Maria-Ferreira D, Werner MF, Baggio CH, Nohama P. Anti-inflammatory effect of laser acupuncture in ST36 (Zusanli) acupoint in mouse paw edema. Lasers Med Sci 31: 315–322, 2016. doi: 10.1007/s10103-015-1845-z. [DOI] [PubMed] [Google Scholar]
- 113. Torres-Rosas R, Yehia G, Peña G, Mishra P, del Rocio Thompson-Bonilla M, Moreno-Eutimio MA, Arriaga-Pizano LA, Isibasi A, Ulloa L. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat Med 20: 291–295, 2014. doi: 10.1038/nm.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hu S, Du M-H, Luo H-M, Wang H, Lv Y, Ma L, Lin Z-L, Shi X, Gaischek I, Wang L, Litscher G. Electroacupuncture at Zusanli (ST36) prevents intestinal barrier and remote organ dysfunction following gut ischemia through activating the cholinergic anti-inflammatory-dependent mechanism. Evid Based Complement Alternat Med 2013: 592127, 2013. doi: 10.1155/2013/592127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Falvey A, Metz CN, Tracey KJ, Pavlov VA. Peripheral nerve stimulation and immunity: the expanding opportunities for providing mechanistic insight and therapeutic intervention. Int Immunol 34: 107–118, 2022. doi: 10.1093/intimm/dxab068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Pavlov VA, Chavan SS, Tracey KJ. Bioelectronic medicine: from preclinical studies on the inflammatory reflex to new approaches in disease diagnosis and treatment. Cold Spring Harb Perspect Med 10: a034140, 2020. doi: 10.1101/cshperspect.a034140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405: 458–462, 2000. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 118. de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud HR, Uematsu S, Akira S, van den Wijngaard RM, Boeckxstaens GE. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol 6: 844–851, 2005. [Erratum in Nat Immunol 6: 954, 2005]. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 119. Guarini S, Altavilla D, Cainazzo MM, Giuliani D, Bigiani A, Marini H, Squadrito G, Minutoli L, Bertolini A, Marini R, Adamo EB, Venuti FS, Squadrito F. Efferent vagal fibre stimulation blunts nuclear factor-κB activation and protects against hypovolemic hemorrhagic shock. Circulation 107: 1189–1194, 2003. doi: 10.1161/01.cir.0000050627.90734.ed. [DOI] [PubMed] [Google Scholar]
- 120. Drewes AM, Brock C, Rasmussen SE, Møller HJ, Brock B, Deleuran BW, Farmer AD, Pfeiffer-Jensen M. Short-term transcutaneous non-invasive vagus nerve stimulation may reduce disease activity and pro-inflammatory cytokines in rheumatoid arthritis: results of a pilot study. Scand J Rheumatol 50: 20–27, 2021. doi: 10.1080/03009742.2020.1764617. [DOI] [PubMed] [Google Scholar]
- 121. Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421: 384–388, 2003. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 122. Rosas-Ballina M, Olofsson PS, Ochani M, Valdés-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, Mak TW, Tracey KJ. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334: 98–101, 2011. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Parrish WR, Rosas-Ballina M, Gallowitsch-Puerta M, Ochani M, Ochani K, Yang LH, Hudson L, Lin X, Patel N, Johnson SM, Chavan S, Goldstein RS, Czura CJ, Miller EJ, Al-Abed Y, Tracey KJ, Pavlov VA. Modulation of TNF release by choline requires α7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol Med 14: 567–574, 2008. doi: 10.2119/2008-00079.Parrish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Peña G, Cai B, Liu J, van der Zanden EP, Deitch EA, de Jonge WJ, Ulloa L. Unphosphorylated STAT3 modulates alpha 7 nicotinic receptor signaling and cytokine production in sepsis. Eur J Immunol 40: 2580–2589, 2010. doi: 10.1002/eji.201040540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Matteoli G, Gomez-Pinilla PJ, Nemethova A, Di Giovangiulio M, Cailotto C, van Bree SH, Michel K, Tracey KJ, Schemann M, Boesmans W, Vanden Berghe P, Boeckxstaens GE. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut 63: 938–948, 2014. doi: 10.1136/gutjnl-2013-304676. [DOI] [PubMed] [Google Scholar]
- 126. Zhou H, Liang H, Li ZF, Xiang H, Liu W, Li JG. Vagus nerve stimulation attenuates intestinal epithelial tight junctions disruption in endotoxemic mice through α7 nicotinic acetylcholine receptors. Shock 40: 144–151, 2013. doi: 10.1097/SHK.0b013e318299e9c0. [DOI] [PubMed] [Google Scholar]
- 127. Rosas-Ballina M, Valdés-Ferrer SI, Dancho ME, Ochani M, Katz D, Cheng KF, Olofsson PS, Chavan SS, Al-Abed Y, Tracey KJ, Pavlov VA. Xanomeline suppresses excessive pro-inflammatory cytokine responses through neural signal-mediated pathways and improves survival in lethal inflammation. Brain Behav Immun 44: 19–27, 2015. doi: 10.1016/j.bbi.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Pavlov VA, Parrish WR, Rosas-Ballina M, Ochani M, Puerta M, Ochani K, Chavan S, Al-Abed Y, Tracey KJ. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav Immun 23: 41–45, 2009. doi: 10.1016/j.bbi.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Munyaka P, Rabbi MF, Pavlov VA, Tracey KJ, Khafipour E, Ghia JE. Central muscarinic cholinergic activation alters interaction between splenic dendritic cell and CD4+CD25- T cells in experimental colitis. PLoS One 9: e109272, 2014. doi: 10.1371/journal.pone.0109272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Satapathy SK, Ochani M, Dancho M, Hudson LK, Rosas-Ballina M, Valdes-Ferrer SI, Olofsson PS, Harris YT, Roth J, Chavan S, Tracey KJ, Pavlov VA. Galantamine alleviates inflammation and other obesity-associated complications in high-fat diet-fed mice. Mol Med 17: 599–606, 2011. doi: 10.2119/molmed.2011.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Mota CMD, Borges GS, Amorim MR, Carolino ROG, Batalhao ME, Anselmo-Franci JA, Carnio EC, Branco LGS. Central serotonin prevents hypotension and hypothermia and reduces plasma and spleen cytokine levels during systemic inflammation. Brain Behav Immun 80: 255–265, 2019. doi: 10.1016/j.bbi.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 132. Mota CMD, Rodrigues-Santos C, Fernández RAR, Carolino ROG, Antunes-Rodrigues J, Anselmo-Franci JA, Branco LGS. Central serotonin attenuates LPS-induced systemic inflammation. Brain Behav Immun 66: 372–381, 2017. doi: 10.1016/j.bbi.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 133. Anderson IK, Martin GR, Ramage AG. Evidence that activation of 5-HT2 receptors in the forebrain of anaesthetized cats causes sympathoexcitation. Br J Pharmacol 116: 1751–1756, 1995. doi: 10.1111/j.1476-5381.1995.tb16658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Martelli D, Farmer DGS, McKinley MJ, Yao ST, McAllen RM. Anti-inflammatory reflex action of splanchnic sympathetic nerves is distributed across abdominal organs. Am J Physiol Regul Integr Comp Physiol 316: R235–R242, 2019. doi: 10.1152/ajpregu.00298.2018. [DOI] [PubMed] [Google Scholar]