Abstract
Purpose
Cat scratch disease (CSD) frequently has ophthalmologic manifestations. The ophthalmologist's approach to treating neuroretinitis is familiar, but few eye care providers are comfortable answering the next question of “what do I do with my cat?” Published guidelines are often vague in answering the complexities of real-life conundrums that can lead patients and their doctors to believe that risk mitigation should involve removal of the animal. Here, we present demonstrative scenarios informed by clinical practice and provide updated recommendations.
Observations
A 10-year-old boy presented with reduced vision in the right eye. Funduscopic examination identified optic nerve head edema with subretinal fluid, and a macular star developed one week later, consistent with the diagnosis of neuroretinitis. Serology confirmed Bartonella henselae antibodies and a diagnosis of CSD. The father disclosed that the family has recently adopted three kittens, who have scratched the boy and the patient's younger sister. The physician and patient's family find themselves at a loss regarding best practices for what should be done with the kittens.
Conclusions and Importance
B. henselae has been detected in a variety of mammals and can be transmitted via vectors such as fleas. Even well-appearing animals can transmit the bacteria, months to years after their initial infection. Symptoms, clinical and laboratory findings will depend on bacterial load and strain virulence, as well as the physiological/immunological status of the host, with people at the extremes of age and the immunocompromised being at greater disease risk. Flea control is crucial to minimize transmission risk. Our veterinary expert (EBB) recommends testing (with serology and PCR) and treating infected animals (with doxycycline and a quinolone). Patients should be counseled to speak with their pets’ veterinarian. When addressing the concerns of our CSD patients in clinical practice, ophthalmologists should be aware of the strategies for minimizing Bartonella transmission risk, and cognizant of the One Health approach for managing zoonoses.
Keywords: Cat-scratch disease, Neuroretinitis, Bartonella, One health, Bartonellosis
1. Introduction
Cat scratch disease (CSD) is a zoonotic illness caused by Bartonella species of bacteria, most frequently Bartonella henselae. It is encountered in ophthalmology practice most commonly in the forms of Parinaud's oculoglandular disease and neuroretinitis, which can cause long-term visual sequelae. Although in recent years much has been learned regarding Bartonella species, virulence, modes of transmission, and associated diseases in humans and in animals, clinical research has not kept pace. When humans have CSD, the recommendations of the Centers for Disease Control (CDC),1 American Association of Feline Practitioners (AAFP),2 and the Advisory Board on Cat Disease (ABCD),3 focus on prevention of transmission instead of providing specific guidelines regarding the management of the pet. Classic recommendations include 1) eliminate the vector, fleas, 2) avoid being wounded by animals, and 3) disinfect wounds. The incidence of CSD has been recently estimated at 4.7 per 100,000 in the United States,4 with ophthalmic manifestations expected in 5% of cases. In patients with neuroretinitis, 73% manifest systemic symptoms.5 Although a constant feature in our history-taking in ophthalmology, once the question of animal exposure(s) is affirmed by the patient, the discussion is usually terminated.3 When the patient asks how to manage their pet, ophthalmologists are usually at a loss.6 Published guidelines1,2 can lead patients to believe that they can attempt to remove fleas, or to remove the animal, and physicians caring for CSD patients have attested to giving this unfortunate advice.7 Here we present an illustrative case of Bartonella-induced neuroretinitis acquired from the family pet, along with questions posed by the family, with the aim of discussing how to deal with such cases in clinical practice. We posed these questions with a few veterinarians and CDC experts and reviewed current guidelines for clinical practice when diagnosing CSD, to suggest an approach to dealing with scenarios in which these guidelines fall short.
2. Case report
A previously healthy, 10-year-old boy presented with painless visual loss with black spots in the visual field of the right eye 1 week prior. He had headaches for the previous month but no history of flu-like illness or fever. On further questioning his father disclosed that the family had recently adopted 3 kittens and the boy recalled being scratched multiple times. He did not wash or treat the scratches.
On ophthalmologic examination, he had visual acuity of 20/60 right eye with trace RAPD, and 20/20 left eye. Color vision was normal. Ocular motility examination showed full ductions both eyes with orthotropia. External and slit-lamp examination were unremarkable with no cell or flare in either eye. Dilated fundus examination (Fig. 1) showed moderate optic nerve head edema in the right eye with subretinal fluid. The periphery and vessels were otherwise unremarkable. There were no vitreous cells. Fundus examination of the left eye was unremarkable with a normal optic nerve. Optical coherence tomography (OCT) showed thickening of the retinal nerve fiber layer with subretinal fluid in the right eye and was unremarkable in the left eye. Humphrey visual field testing showed an enlarged blind spot in the right eye and was full in the left eye.
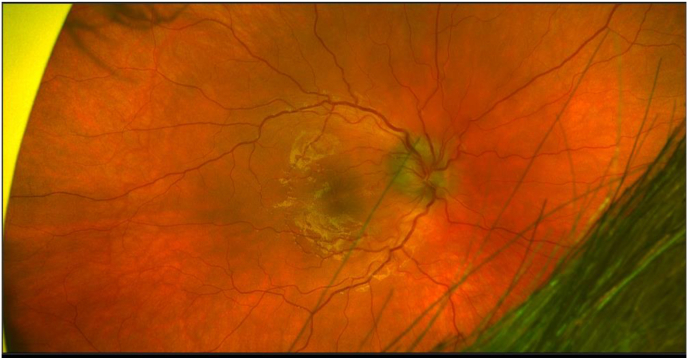
Fig. 1.
Optos widefield photograph of patient's right eye posterior pole on presentation. Note optic disc edema consistent with papillitis with sub-macular fluid.
MRI of the brain and orbits with contrast was unremarkable except for optic disc fullness in the right eye corresponding with the disc edema observed on examination. There was no optic nerve enhancement.
A presumed diagnosis of cat scratch neuroretinitis was made, and the patient received consultation with an infectious disease specialist who recommended treatment with rifampin, azithromycin, and prednisone. Follow-up examination 1 week later showed a macular star exudate in the right eye (Fig. 2), consistent with his diagnosis of neuroretinitis.
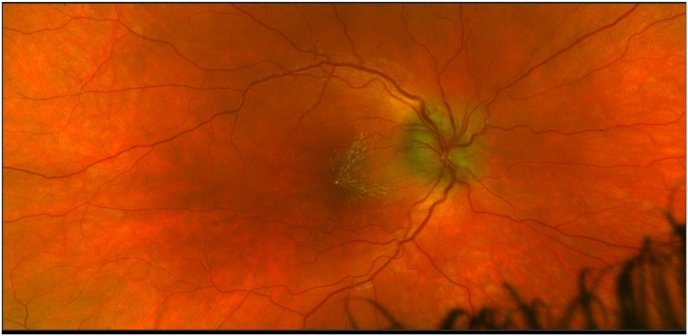
Fig. 2.
Optos widefield photograph of patients's right eye posterior pole one week later. Continued optic disc edema with resolution of sub-macular fluid and subsequent appearance of macular star, suggesting neuroretinitis.
Serology documented elevated Bartonella henselae titers for IgM (1:256) and IgG (1:1024), confirming the diagnosis of cat scratch neuroretinitis.
Upon hearing the diagnosis, the father expressed concern, as his younger 6-year-old daughter was asymptomatic but had also been scratched by the kittens. He asked what to do with the kittens and how to prevent further household infections. The cats were evaluated by a veterinarian, who administered a parasiticide for elimination of fleas. Antibiotic treatment was not recommended.
The boy recovered normal 20/20 vision and had resolution of the exudate in his right eye during the next several months. His sister remained asymptomatic and had a normal ophthalmologic examination with no signs of neuroretinitis. The cats remained well and asymptomatic and were not removed from the home.
3. Discussion
Bartonella species are known to cause several pathologies in both humans and animals8 This fastidious, pleomorphic, gram-negative rod can be found in the blood of the domestic cat9 and, in ophthalmological practice, is often encountered in CSD-induced neuroretinitis10 and Parinaud's oculoglandular disease.11 Bartonella henselae is classically associated with these pathologies; however, there are other causative species of Bartonella that can be spread by a variety of mammals and arthropod vectors.12 B. henselae is known to inhabit the digestive tract of the cat flea, and is therefore present and infectious via flea feces, and is present in flea salivary glands.13 Initial CSD inoculation occurs when the host's skin is pierced and bacteria are introduced into the wound by flea feces – i.e., when a cat scratches a human with flea fecal matter in its claws, or human auto-inoculation when a wound from a flea or tick bite is tampered with or scratched and contaminated with the bacterium from adjacent flea feces (Fig. 3).14 Another possible mode of B. henselae transmission is via the arthropod (tick) salivary glands, although this has yet to be confirmed in vivo.14 Once the bacteria are introduced into the animal host, they can invade erythrocytes and lymphatic tissues, and cause vasculitis and vasoproliferative lesions.15 Although most human infections result in self-limited febrile illness, bacteremia, anemia, endocarditis, vasculitis, lymphadenitis/lymphadenopathy, and even hepatosplenic and neuropsychiatric disease can occur.8,12,16 This spectrum of disease may be related to the immune status of the host, with immunocompromised humans more likely to develop a vascular proliferative response or atypical, disseminated disease or prolonged bacteremia with sometimes fatal outcomes.17, 18, 19, 20 However, these manifestations have been documented in both immunocompetent and immunosuppressed individuals.8,17,19,21,22
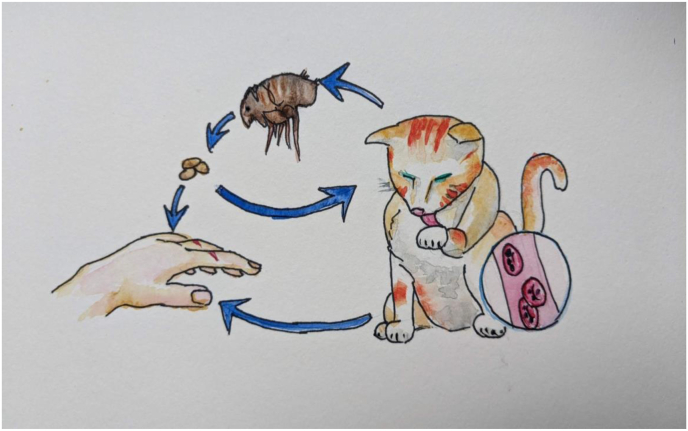
Fig. 3.
Transmission of Bartonella. Mammalian intra-erythrocytic bacteremia leads to bacterial presence in the flea digestive tract following a blood meal. The contaminated flea feces then lead to infection in humans and animals, which can be facilitated by animal scratches or licking.
Unfortunately, current guidelines regarding treatment of the animal are vague and leave many questions unanswered.1, 2, 3 While all authors stress the importance of prevention of transmission by promoting flea eradication, there is a grey area regarding testing and treating the vector animals. According to the Centers for Disease Control (CDC),1 American Association of Feline Practitioners (AAFP),5 and the Advisory Board on Cat Disease (ABCD),3 asymptomatic animals should not be tested except in certain circumstances, such as when the pet owner is immunocompromised. They also concede that in some cases of human infection, testing of the animal may be discussed. However, they do not stipulate fully the circumstances which should prompt testing, and they stress that treatment of a pet is appropriate only if that animal is symptomatic (ie fever, signs of anemia, vasoproliferative lesions, endocarditis and more).11 These vague “guidelines” allow most veterinarians to choose what to do depending on numerous circumstances, including the type of pet, the type of practice (urban or rural), the requests from pet owners, and their own beliefs. Importantly, these recommendations seem to be out of step with what is currently known about transmission and the spectrum of human and animal disease. Literature review demonstrates that bacteremic animals may transmit infection when they have no clinical disease, and this can occur months after their initial inoculation.23 Indeed, evidence of current or prior infection is common in both domestic and stray animals,24 and has been reported in up to 75%25 in certain regions. Even if a pet is not currently infested with fleas, they can potentially still transmit from prior exposures,26 and possibly via saliva.27 Furthermore, infection with B. henselae is beginning to gain recognition as an occupational hazard in veterinary practice.28
To answer these questions, we surveyed a handful of primary care, tertiary care, and academic veterinarians in our area, and a representative at the CDC. When posing the question of how to address issues related to Bartonella infection in the pet, all were unsure of how to manage these cases, when to seek testing, and the reasons to treat or not. A common response was either that they had not encountered this issue, or that they suggest referral of the pet owner to a veterinary infectious disease specialist. Some academic veterinarians we spoke with recommended removing the animal, others referred us to the CDC, but no one felt comfortable offering definite recommendations. Frequent reference was made by primary and tertiary care veterinarians, and veterinarians at the CDC to Dr. Edward B. Breitschwerdt, as the current foremost veterinary expert on mammalian Bartonella disease in the United States. Dr. Breitschwerdt suggests a One Health approach – a collaborative, transdisciplinary approach to managing a CSD patient and their pets, which would take animals, humans, and their shared environment into account to optimize health outcomes for all.12,29 Accordingly, a suspected animal reservoir in proven cases of human transmission can undergo confirmatory testing and be treated, the goal being to prevent further infection of humans, as well as unnecessary euthanization or abandonment of pets, which would likely worsen Bartonella community transmission. Although this is not currently the formal recommendation of the CDC or AAFP, the purpose of our report is to call attention to the need for renewal and streamlining of recommendations, and for increased communication between the veterinary and medical communities based on the recent findings on this topic. Future directions will assess primary care veterinarians' common practices in response to human pet owners’ CSD infections, update human Bartonella epidemiology considering the recently appreciated heterogeneity of disease, and prospective analysis of the value of therapeutic regimens of pets in human disease transmission.
Table 1 provides illustrative cases and questions derived from clinical practice. They demonstrate scenarios in patient care in which current, available guidelines are unclear, and describe recent findings in the veterinary literature.
Table 1.
Clinical scenarios – questions and answers.
| Scenario | Conundrums | Advice |
|---|---|---|
| A healthy 47-year-old woman develops visually severe neuroretinitis. Serology confirms that the cause is acute CSD due to B. henselae. She is the owner of two healthy cats. | 1. Which cat transmitted the infection? 2. What should she do with the cats? |
In all cases, at all stages of clinical practice, flea elimination should be the foremost recommendation. However, when an animal is exposed to other animals, or spends time outdoors, they may be re-exposed or re-infected with Bartonella, even if they have cleared a prior infection.12,23 For this reason, part of flea control involves keeping the animal indoors and away from other animals.1 Both cats can be tested to determine which is the reservoir and, if infected, our expert recommends treatment. Usually, serology in humans should be enough to confirm exposure and implicate active infection. In animals, PCR from blood is often sufficient to confirm infection.25 A sample treatment regimen for a cat would be doxycycline and fluoroquinolone for 4–6 weeks.1,2,12 |
| A family experiences serial cases of CSD. They own a cat and a dog and both are receiving routine flea prevention products. | 1. Is the dog a suspect in transmission? 2. How should these pets be managed? |
In cases where there are multiple animals involved in potential transmission, or even multiple mammalian species, all animals can be tested for Bartonella bacteremia. There are a number of methods to accomplish this. Diagnostic yield will be greatly improved when two types of tests are utilized (for example, combined PCR and culture).30 Other methods described in the literature for enhancing sensitivity are nested PCR from blood and liquid culture,30 Duplex high-resolution melt (HRM), Taqman,31 and BAPGM followed by PCR12 among others. Confirmatory testing can be followed by dual-agent antibiotic therapy, for dogs as well (doxycycline and fluoroquinolone).4,10 |
| A family living in a rural community has symptoms of CSD in multiple members. They do not know where the exposure came from. | Can people who do not own household pets contract disease from external exposures? | Although less common, a number of alternative modes of transmission have been described. These include mammalian transmission (other than cats), transmission by ticks or other arthropods, and even dog bite and needlestick.14 In settings of heavy flea infestation, it is not necessary for a mammal to be present at all, and disease can be transmitted to humans through broken skin after direct contact with flea feces. |
| A cat owner has recovered from CSD, confirmed to be transmitted by her pet cat. She has subsequently been vigilant about flea control, but does not want to become reinfected. | 1. Why did this occur? 2. How can transmission be prevented in the future? |
Once an animal has been confirmed as the reservoir of human infection, the question of treatment arises. Unfortunately, the end point of treatment is not always clear, since determination of eradication after an antibiotic course is not well defined and does not always occur.12 However, it is possible that a resulting reduction in bacterial load would help in preventing further transmission, which would be especially important for at-risk individuals. Additionally, the question of treatment utility has not been comprehensively addressed since 2006,32 and much of our understanding of Bartonella transmission and disease manifestations has changed substantially since then. In spite of these issues, there does seem to be a consensus regarding the necessity of administering two types of antibiotics, for example doxycycline along with a fluoroquinolonea for a course of 4–6 weeks.1,2,12 Once an animal has been treated, it is crucial to maintain optimal flea control to prevent reinfection. |
| Two children in a household with a new kitten are scratched. The kitten's flea history is unknown. | Why don't we routinely see multiple members of one family with symptomatic CSD if it is indeed transmitted by the family cat? | Animals may have had prior flea exposures of which the owner is unaware, and for this reason, when adopting pets, it is best to attempt to adopt an animal which has not had prior flea exposure. Reports on whether age of the animal is important in the likelihood of bacteremia appear to be inconsistent. Strains of Bartonella may vary in virulence,12 which might help explain the spectrum of disease manifestations in humans and animals. Other factors which apparently are important in disease manifestations are age of the host (very young and very old humans have more severe disease), as well as immunocompromised status.1 Finally, the diversity of clinical syndromes associated with bartonelloses is just being uncovered. It is possible that more humans are infected with, and affected by, the Bartonella genus than was previously appreciated.33 |
Pradofloxacin for cats.
4. Conclusions
After review of the recent literature and consultation with a veterinary expert in Bartonella, we propose the following when evaluating a patient with CSD. First, counsel your patient to eradicate fleas from their pets as well as keep pets indoors and away from wild animals that can also be a reservoir for fleas and Bartonella species. This will considerably minimize the possibility of pets becoming infected or re-infected and thereby minimize transmission to other humans. Second, minimize human exposure (particularly the very young, very old, and immune-compromised) to stray or poorly cared-for animals to prevent future infection, and encourage safe play with pets. Third, when a cat scratch or other wound occurs, wash immediately with warm, soapy water, and do not let animals lick wounds. Fourth, when humans are infected, particularly those at risk for severe infection, our veterinary expert (EBB) recommends testing and treating all infected pets. To accomplish this, counsel patients to consult with their animals’ veterinarian regarding options for testing and treatment, which can be achieved with serology and PCR, followed by a course of combination antimicrobials for the pet.
Funding
Dr. Biousse and Dr. Newman are supported in part by the National Institutes of Health's National Eye Institute core grant P30-EY06360 (Department of Ophthalmology, Emory University School of Medicine) and a departmental grant from Research to Prevent Blindness.
Dr. Okrent: Emory Eye Center Departmental Research Grant, 2021–2022.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Patient consent
Written consent to publish this case has not been obtained.
Declaration of competing interest
EBB: Holds U.S. Patent No. 7,115,385 In conjunction with Sushama Sontakke and North Carolina State University; Media and Methods for cultivation of micro-organisms, issued 10/3/2006. Chief scientific officer for Galaxy Diagnostics (provides serological and microbiological diagnostic testing for the detection of Bartonella species infection in animals and human patients).
Dr. Biousse and Dr. Newman are consultants for GenSight and Neurophoenix. Dr. Newman is a consultant for Santhera/Chiesi and Stoke.
The following authors have no financial disclosures: ALOS, PHP.
Acknowledgements
None
References
- 1.For Veterinarians | Bartonella | CDC https://www.cdc.gov/bartonella/veterinarians/index.html Accessed October 18, 2021.
- 2.Lappin M.R., Elston T., Evans L., et al. 2019 AAFP feline zoonoses guidelines. J Feline Med Surg. 2019;21(11) doi: 10.1177/1098612X19880436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feline bartonellosis http://www.abcdcatsvets.org/feline-bartonellosis/ Accessed March 20, 2022.
- 4.Nelson C.A., Saha S., Mead P.S. Cat-scratch disease in the United States, 2005–2013. Emerg Infect Dis. 2016;22(10) doi: 10.3201/eid2210.160115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Purvin V., Sundaram S., Kawasaki A. Neuroretinitis: review of the literature and new observations. J Neuro Ophthalmol. 2011;31(1) doi: 10.1097/WNO.0b013e31820cf78a. [DOI] [PubMed] [Google Scholar]
- 6.Fraser C.L., Sanchez S., Newman N.J., Biousse V. Cat scratch disease. Ophthalmology. 2012;119(7) doi: 10.1016/j.ophtha.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 7.Nelson C.A., Moore A.R., Perea A.E., Mead P.S. Cat scratch disease: U.S. clinicians' experience and knowledge. Zoonoses and Public Health. 2018;65(1):67–73. doi: 10.1111/ZPH.12368. [DOI] [PubMed] [Google Scholar]
- 8.Cheslock M.A., Embers M.E. Human bartonellosis: an underappreciated public health problem? Tropical Medicine and Infectious Disease. 2019;4(2) doi: 10.3390/tropicalmed4020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacomo V., Kelly P.J., Raoult D. Natural history of Bartonella infections (an exception to Koch's postulate) Clin Diagn Lab Immunol. 2002;9(1) doi: 10.1128/CDLI.9.1.8-18.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Habot-Wilner Z., Trivizki O., Goldstein M., et al. Cat-scratch disease: ocular manifestations and treatment outcome. Acta Ophthalmol. 2018;96(4) doi: 10.1111/aos.13684. [DOI] [PubMed] [Google Scholar]
- 11.Albert D.M., Salman A.R., Winthrop K.L., Bartley G.B. The continuing ophthalmic challenge of bartonella henselae. Ophthalmology Science. 2021;1(3) doi: 10.1016/j.xops.2021.100048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breitschwerdt E.B. Bartonellosis, One Health and all creatures great and small. Vet Dermatol. 2017;28(1) doi: 10.1111/vde.12413. 96-e21. [DOI] [PubMed] [Google Scholar]
- 13.Higgins J.A., Radulovic S., Jaworski D.C., Azad A.F. Acquisition of the cat scratch disease agent bartonella henselae by cat fleas (siphonaptera: pulicidae) J Med Entomol. 1996;33(3):490–495. doi: 10.1093/jmedent/33.3.490. https://academic.oup.com/jme/article/33/3/490/892327 Accessed. [DOI] [PubMed] [Google Scholar]
- 14.Álvarez-Fernández A., Breitschwerdt E.B., Solano-Gallego L. Bartonella infections in cats and dogs including zoonotic aspects. Parasites Vectors. 2018;11(1):1–21. doi: 10.1186/s13071-018-3152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breitschwerdt E.B., Linder K.L., Day M.J., Maggi R.G., Chomel B.B., Kempf V.A.J. Koch's postulates and the pathogenesis of comparative infectious disease causation associated with bartonella species. J Comp Pathol. 2013;148(2-3):115–125. doi: 10.1016/j.jcpa.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma R., Arshad A.M., Sardar S., Zafar A. Hepatosplenic bartonellosis in an immunocompetent teenager: an atypical presentation of cat-scratch disease. Cureus. 2021 doi: 10.7759/cureus.13219. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chomel B.B., Boulouis H.J., Maruyama S., Breitschwerdt E.B. Bartonella spp. in pets and effect on human health. Emerg Infect Dis. 2006;12(3):389. doi: 10.3201/EID1203.050931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chomel B.B., Kasten R.W., Sykes J.E., Boulouis H.J., Breitschwerdt E.B. Annals of the New York Academy of Sciences. vol. 990. 2003. Clinical impact of persistent Bartonella bacteremia in humans and animals. [DOI] [PubMed] [Google Scholar]
- 19.Breitschwerdt E.B., Maggi R.G., Quach C., Bradley J.M. Bartonella spp. bloodstream infection in a Canadian family. Vector Borne Zoonotic Dis. 2019;19(4):234–241. doi: 10.1089/vbz.2018.2353. [DOI] [PubMed] [Google Scholar]
- 20.Chomel B.B., Boulouis H.J., Breitschwerdt E.B. Cat scratch disease and other zoonotic Bartonella infections. J Am Vet Med Assoc. 2004;224(8):1270–1279. doi: 10.2460/JAVMA.2004.224.1270. [DOI] [PubMed] [Google Scholar]
- 21.Prutsky G., Domecq J.P., Mori L., et al. Treatment outcomes of human bartonellosis: a systematic review and meta-analysis. Int J Infect Dis. 2013;17(10) doi: 10.1016/J.IJID.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Jackson L.A., Perkins B.A., Wenger J.D. Cat scratch disease in the United States: an analysis of three national databases. Am J Publ Health. 1993;83(12) doi: 10.2105/AJPH.83.12.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huwyler C., Heiniger N., Chomel B.B., Kim M., Kasten R.W., Koehler J.E. Dynamics of Co-infection with bartonella henselae genotypes I and II in naturally infected cats: implications for feline vaccine development. Microb Ecol. 2017;74(2) doi: 10.1007/s00248-017-0936-8. [DOI] [PubMed] [Google Scholar]
- 24.Jameson P., Greene C., Regnery R., et al. Prevalence of bartonella henselae antibodies in pet cats throughout regions of north America. JID (J Infect Dis) 1995;172(4) doi: 10.1093/infdis/172.4.1145. [DOI] [PubMed] [Google Scholar]
- 25.Souza A.M., Almosny N.R.P., Favacho A.R.M., et al. Bartonella spp. and hematological changes in privately owned domestic cats from Rio de Janeiro, Brazil. J Infect Dev Ctries. 2017;11(8) doi: 10.3855/jidc.8152. [DOI] [PubMed] [Google Scholar]
- 26.Kordick D.L., Wilson K.H., Sexton D.J., Hadfield T.L., Berkhoff H.A., Breitschwerdt E.B. Prolonged Bartonella bacteremia in cats associated with cat-scratch disease patients. J Clin Microbiol. 1995;33(12) doi: 10.1128/jcm.33.12.3245-3251.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oskouizadeh K., Zahraei-Salehi T., Aledavood S.J. Detection of Bartonella henselae in domestic cats' saliva. Iran J Microbiol. 2010;2(2):80. Accessed. /pmc/articles/PMC3279777/ [PMC free article] [PubMed] [Google Scholar]
- 28.Mosbacher M.E., Klotz S., Klotz J., Pinnas J.L. Bartonella henselae and the potential for arthropod vector-borne transmission. Vector Borne Zoonotic Dis. 2011;11(5):471–477. doi: 10.1089/VBZ.2010.0106. [DOI] [PubMed] [Google Scholar]
- 29.One Health | CDC https://www.cdc.gov/onehealth/index.html?msclkid=631727a5a92811ec9a76eb96dcc70863 Accessed March 20, 2022.
- 30.Drummond M.R., Lania B.G., de Paiva Diniz P.P.V., et al. Improvement of bartonella henselae DNA detection in cat blood samples by combining molecular and culture methods. J Clin Microbiol. 2018;56(5) doi: 10.1128/JCM.01732-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y.Y., Zhao L.S., Song X.P., et al. Development of fluorogenic probe-based and high-resolution melting-based polymerase chain reaction assays for the detection and differentiation of Bartonella quintana and Bartonella henselae. J Microbiol Methods. 2017;138:30–36. doi: 10.1016/J.MIMET.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 32.Brunt J., Guptill L., Kordick D.L., Kudrak S., Lappin M.R. American Association of Feline Practitioners 2006 Panel report on diagnosis, treatment, and prevention of Bartonella spp. infections. J Feline Med Surg. 2006;8(4) doi: 10.1016/j.jfms.2006.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breitschwerdt E.B., Bradley J.M., Maggi R.G., Lashnits E., Reicherter P. Bartonella associated cutaneous lesions (BACL) in people with neuropsychiatric symptoms. Pathogens. 2020;9(12):1–19. doi: 10.3390/pathogens9121023. [DOI] [PMC free article] [PubMed] [Google Scholar]