Abstract
Alcohol use disorder (AUD) is a pervasive and devastating mental illness with high comorbidity rates with other mental disorders. Understanding the genetic architecture of this comorbidity could be improved by focusing on intermediate traits that show positive genetic correlation with the disorders. Thus, we aimed to characterize the shared vs. unique polygenicity of AUD, alcohol consumption (AC) and mood instability (MOOD) –beyond genetic correlation, and boost discovery for jointly-associated loci. Summary statistics for MOOD (a binary measure of the tendency to report frequent mood swings), AC (number of standard drinks over a typical consumption week) and AUD GWASs (Ns > 200,000) were analyzed to characterize the cross-phenotype associations between MOOD and AC, MOOD and AUD and AC and AUD. To do so, we used a newly established pipeline that combines (i) the bivariate causal mixture model (MiXeR) to quantify polygenic overlap and (ii) the conjunctional false discovery rate (conjFDR) to discover specific jointly associated genomic loci, which were mapped to genes and biological functions. MOOD was highly polygenic (10.4k single nucleotide polymorphisms, SNPs, SD = 2k) compared to AC (4.9k SNPs, SD = 0.6k) and AUD (4.3k SNPs, SD = 2k). The polygenic overlap of MOOD and AC was twice that of MOOD and AUD (98% vs. 49%), with opposite genetic correlation (−0.2 vs. 0.23), as confirmed in independent samples. MOOD&AUD associated SNPs were significantly enriched for brain genes, conversely to MOOD&AC. Among 38 jointly associated loci, fifteen were novel for MOOD, AC and AUD. MOOD, AC and AUD were also strongly associated at the phenotypic level. Overall, using multilevel polygenic quantification, joint loci discovery and functional annotation methods, we evidenced that the polygenic overlap between MOOD and AC/AUD implicated partly shared biological underpinnings, yet, clearly distinct functional patterns between MOOD&AC and MOOD&AUD, suggesting new mechanisms for the comorbidity of AUD with mood disorders.
Subject terms: Diagnostic markers, Behavioural genetics
Introduction
Alcohol use disorder (AUD) is a mental disorder characterized by a chronic loss of control over the use of alcohol. Alcohol is the most burdensome of addictive substances, contributing to three million deaths each year (Global Burden of Disease, World Health Organization 2018). Alcohol consumption (AC) and AUD do not share the same clinical phenomenology [1], neuropsychology [2], or biological underpinnings - whether on a neuroimaging [3] or a genetic basis [4–6]. AUD is diagnosed in 15% of regular alcohol users [7]. Overall, although AC has been evidenced as a causal factor for AUD [8], it is far from being sufficient [9]–warranting further investigations. With that regard, tobacco smoking could also alter the transition from AC to AUD. A closer look at different AC measures has revealed that considering the frequency vs. quantity of alcohol intake yields different patterns of genetic correlation with other mental phenotypes, despite the significant association between them [10]. Notably, alcohol quantity is positively and strongly correlated with AUD [4, 10] and moderately correlated with depressive symptoms and major depressive disorder [10]. Conversely, alcohol frequency did not show significant genetic correlation with AUD and showed significant, but negative genetic correlation with both depressive symptoms and major depression [10]. Thus, taken together, these data highlight the need for further investigations of the shared and unique molecular underpinnings of AC vs. AUD using genetic analyses.
Accumulating evidence for high genetic correlation [4, 11] and shared environmental factors between AUD and non-substance-related psychiatric disorders [12] have placed greater emphasis on the transdiagnostic vulnerability to mental disorders. Mood instability, both a risk factor and a clinical expression of psychiatric disorders [13, 14], represents one of these transdiagnostic factors. Mood instability has a complex/composite nature that can be measured by several scales, yet, only the single-item measure taken from the Eysenck Neuroticism Scale EPQ-R (“does your mood often goes up and down?”, MOOD henceforward) has been the focus of large genetic studies. The sum-score of EPQ-R has been consistently associated with psychiatric disorders [15], however, item-level phenotypic and genetic analysis evidenced different clusters of interest. Amongst them, depressive affect is strongly driven by MOOD, which shows strong correlation with the EPQ-R sum-score (rg = 0.84) and fairly high SNP-based heritability (h2SNP) = 0.12. The genetic relationships between MOOD and psychiatric disorders [15] was further characterized by our group using newly-established cross-disorder analyses [16], leading us to focus on this phenotype. MOOD has been associated with AUD at the clinical and at the genetic level [17–19], and is a common feature of the psychiatric comorbidities that are commonly associated with AUD, including mood [20], psychotic [21], anxiety [22], and personality [23] disorders. Importantly for the current study, lifetime AUD has further been associated with increased MOOD in a sample of patients with bipolar disorder [24]. Likewise, clinical studies have shown a strong bi-directional relationship between MOOD and AC [25–28]. Therefore, the interaction of MOOD and AC likely increases the risk of developing mood disorders and/or AUD [29]. Taken altogether, these data designate MOOD as a promising endophenotype in psychiatric disorders, especially for comorbid conditions [30]. Studying the genetic liability of MOOD and AC vs. MOOD and AUD may thus yield new insights into the pathophysiology of AUD and open avenues to better model the comorbidity between mood disorders and AUD. These mechanistic hypotheses fit particularly well with the research domain criteria initiative (RDoC [31]) developed by the National Institutes of Mental Health. RDoC are a tentative classification using constructs and domains aimed at better representing the underlying etiology of mental disorders compared to diagnostic categories. Under this framework, MOOD could represent a transdiagnostic factor (the clinical expression of impairments in the arousal construct from the Arousal/Regulatory Systems domains), which might facilitate the transition from regular AC to AUD by increasing the liability toward maladaptive habits (i.e., increasing the impairments in the reward learning construct in the Positive Valence Systems domain), especially in the context of premorbid impairments in the reward responsiveness construct from the Positive Valence Systems. The Hierarchical Taxonomy of Psychopathology (HiTOP) framework also proposes such dimensional framework for psychiatric nosology [32]. Eventually, characterizing the unique vs. shared polygenic liability of these traits and disorder is thus of paramount importance, yet remains unclear [33].
Significant genetic correlation has been shown between mood disorders and AUD [9], between MOOD and mood disorders [34], but not between AC and mood disorders [9]. However, genetic correlation measures only provide a summary measure of genome-wide correlation of effect sizes. They have therefore been unable to quantify the extent of shared versus unique heritability between traits and disorders, the extent of shared genetic factors with concordant and discordant effects on each pair of traits, or identify specific jointly-associated genomic loci. Building on prior findings about genetic correlations between MOOD and AUD, our group applied the bivariate causal mixture model (MiXeR) to quantify the amount of genetic overlap [35], and conjunctional FDR (conjFDR) to identify specific overlapping vulnerability loci [36] to relevant largest-to-date GWASs. For instance, conjFDR has been successfully applied to AC vs. AUD and bipolar disorder or schizophrenia, showing interesting mixed effect directions [37] and calling for extension using transdiagnostic dimensions such as MOOD.
Our aim was to determine the unique vs. shared polygenic liability between AC, an alcohol-related trait, and AUD, an alcohol-related disorder. To do so, we characterized their overlapping and unique polygenic liability with a transdiagnostic trait, MOOD. Further triangulating these traits and disorders based on their genetic architecture may unravel specific relationships across current psychiatric diagnostic categories, and lead to discovery of shared vulnerability and molecular pathways [38]––beyond genetic correlation. We also hypothesized that having MOOD as a primary transdiagnostic phenotype would open avenues toward a better understanding of the comorbidity between mood disorders and AUD.
Material and methods
We applied a newly established pipeline for characterizing the polygenic architecture of MOOD and AC or AUD, based on cross-trait overlap and improved power for the discovery of overlapping loci. We used the bivariate causal mixture model (MiXeR), and the Conjunctional False Discovery Rate analysis (conjFDR), investigating genome-wide association studies (GWAS) summary statistics data from relevant phenotypes. The manuscript follows the statement of STrengthening the REporting of Genetic Association studies (STREGA) [39]. Effective sample sizes and SNPs are summarized in Table 1. More methodological details are given in the Supplementary Methods File.
Table 1.
Characteristics of original GWASs on mood instability and alcohol-related phenotypes.
| Database/survey | Phenotype | Sample size (n) | Age, gender |
Number of SNPs | h2SNP |
|---|---|---|---|---|---|
| Discovery samples | |||||
| UK Biobank | Mood instability |
Total = 363,705 Cases = 170,180 Controls = 163,525 |
30–69 years 56% women |
9.9 million | 0.077 |
| Million Veterans Program | Alcohol consumption (AUDIT-C) | Total = 200,680 |
30–75 years 8% women |
6.8 million | 0.068 |
| Alcohol use disorder (ICD-9/10) |
Total = 202,004 Cases = 34,658 Controls = 167,346 |
0.094 | |||
| Validation samples | |||||
|
GSCAN With/without UK Biobank data |
Alcohol consumption (drinks/week) |
537,352 (226,223) |
14.1 million | 0.04 | |
|
PGC with UK Biobank |
Alcohol dependence (DSM-IV) |
Total = 52,848 Cases = 14,904 Controls = 37,944 |
39% women Mostly >18 |
10.9 million | 0.098 |
| PGC with UK Biobank + MVP | Alcohol use disorder (ICD-9/10) or alcohol dependence (DSM-IV) | Meta-analysis of the two samples used only for testing the power of MiXeR analyses with these traits due to low h2SNP | |||
SNP numbers are rounded at the 105 level. GWAS summary statistics were obtained from previous publications [4, 44, 45].
SNP single nucleotide polymorphism, h2SNP SNP-based heritability, AUDIT-C Alcohol Use Disorders Identification Test - concise, ICD International Classification of Disease, DSM Diagnostic and Statistical Manual of mental disorders, PGC Psychiatric Genetics Consortium, GSCAN GWAS & Sequencing Consortium of Alcohol and Nicotine use (without 23andMe data).
Samples and phenotypes
The genotyped sample of the UK Biobank consists of 488,377 subjects from the community assessed for a wide range of measures. The Million Veterans Program (MVP) consists of a dataset of more than 200,000 genotyped individuals from the US Veterans Health Care System, whose phenotypic information was obtained during clinical appointments and was extensively recorded in electronic health records. The recruitment and genetic analysis for both samples required written informed consent. They fulfilled ethical standards of the Helsinki declaration 1989. Recruitment was approved by the National Health Service National Research Ethics Service, Ref. 16/NW/0274, and genetic analyses by the Veteran Affairs Central Institutional Review Board.
The primary phenotype was MOOD, defined in the UK Biobank by answering yes/no to the question “does your mood often go up and down?”, as used in a previously-published GWAS [34]. Despite the simplicity of this measure, it was evidenced to be a strong predictive factor for both bipolar and major depressive disorders in a prospective community sample [14]. More generally, single-item measurements of sadness and psychological distress have been found to be useful in the general population [40] and in clinical samples with recurrent depression [41]. Finally, the reliable identification of cross-trait genetic overlap requires very large sample sizes that were not available with more extensive measurements of mood/affective instability at the time of the current study [42].
We focused on two secondary alcohol-related phenotypes: a continuous trait, AC, and a binary disorder, AUD from the MVP survey [43]. AC was measured using the Alcohol Use Disorders Identification Test-Concise (AUDIT-C), which measures typical quantity (item 1) and frequency (item 2) of drinking, and frequency of heavy or binge drinking (item 3) over the past year. AUDIT-C yields a continuous score ranging from 0 to 12. AUD was defined as having at least one inpatient or two outpatient diagnoses of alcohol abuse or dependence or two diagnoses of alcohol intoxication according to the International Classification of Disease (ICD)‐9 or ‐10.
Statistical analysis
We extracted effect sizes and p-values from GWASs performed on each of the selected phenotypes. All participants were of European ancestry in order to maintain linkage disequilibrium (LD) homogeneity across samples, since inconsistency of LD between the reference panel and GWAS sample may bias both MiXeR and conjFDR outcomes. Firstly, we used MiXeR to quantify total polygenic overlap between MOOD and alcohol-related phenotypes (AC, AUD) at the genome-wide level [35]. The method takes GWAS summary statistics on two phenotypes and provides maximum likelihood estimates for the total number of shared and phenotype-specific for non-null variants, and genetic correlation between phenotypes accounting for sample overlap. MiXeR assumes that each phenotype has a fraction of non-null (“causal”) variants uniformly distributed throughout the genome with effect sizes drawn from the same normal distribution, while the complement of this fraction has no effect. Q-Q plots are then produced to estimate cross-phenotype enrichment. This analysis uses variable p-value thresholds for the SNPs association with a given trait as a function of their association with a second trait. If the Q-Q curves for phenotype one increasingly deflect left from the theoretical null line as the significance in phenotype two increases (i.e. become more stringent, reflecting stronger associations), this supports enrichment due to overlapping genomic signal and adds data to the visualization of the proportion of shared vs. unique polygenic signal provided by MiXeR. Conditional Q-Q plots are a model-free approach for visual assessment of shared genetic background between two phenotypes which provides complementary support for MiXeR estimates of genetic overlap [36]. Existence of genetic overlap between pair of phenotypes warrants combining these phenotypes in conjFDR analysis.
Secondly, we performed conjFDR [36] to discover specific genomic loci jointly associated with each alcohol-related phenotype (AC, AUD) and MOOD. ConjFDR estimates posterior probability that a given SNP is null for either phenotype or both phenotypes simultaneously when the p-values for both phenotypes are as small or smaller than the p-values observed in the original GWAS. To control for spurious enrichment in the conjFDR analyses, random pruning was averaged over 100 iterations (noticing that a higher number of iterations yielded identical results), and one SNP in each LD block (r2 > 0.1) was randomly selected from each iteration. SNPs within the major histocompatibility complex (MHC; genome build 19 location 25652429–33368333), the chromosomal region 8p23 (location 7200000–12500000), owing to their complex LD structures, to avoid biased FDR estimation. In the specific context of the current study, conjFDR was deemed particularly relevant in order to identify genomic loci associated with MOOD that were either shared or unique to each of the alcohol-related phenotypes.
Statistical analyses were conducted on Linux®-based servers. Plotting and downstream analyses were performed with R 3.6.3 and R Studio 1.4.1103.
Genomic loci definition
We defined genomic loci according to the Functional Mapping and Annotation of Genome-Wide Association Studies (FUMA GWAS, https://fuma.ctglab.nl/), based on the r2 statistic for measuring LD.
Functional annotation
Candidate SNPs for each set of conjFDR analysis (p < 0.1) were annotated with FUMA in three steps:
-
(i)
Mapping loci to SNPs and SNPs to genes based on positional, gene expression and chromatin interaction data (FUMA SNP2GENE)––yielding a list hereafter referred to as credibly mapped genes;
-
(ii)Annotating each candidate SNP for:
- Combined Annotation Dependent Depletion (CADD) scores, which predict SNP deleteriousness on protein structure/function;
- RegulomeDB scores, which predict regulatory functionality (the lower the score, the higher the effect of the SNP);
- Chromatin states, which predict transcription/regulatory effects from chromatin states at the SNP locus;
- Novelty, based on candidate genomic ranges compared to the latest build of GWAScatalog + MEDLINE search, to which we added a manual search in the MRC IEU PheWAS tool as of June 1st, 2022 using the corresponding R package;
-
(iii)
Passing the resulting list of genes into FUMA GENE2FUNCTION to estimate gene-set enrichment. All analyses were corrected for multiple comparisons using the Benjamini-Hochberg method for each pathway/category. For the sake of brevity, we chose to restrict the enrichment testing of credibly mapped genes for positional gene sets, canonical pathways and gene expression as a function of tissue (GTEx data v.8 https://gtexportal.org/home/) and developmental age (https://www.brainspan.org/). Tissue and age-specific gene expression are considered premium datasets in order to describe the functional genomics associated with GWAS data, thus providing valuable insights into psychiatric genomics (see [46] for review);
-
(iv)
Celltype specificity using SNP-epigenomics associations from the Roadmap (http://www.roadmapepigenomics.org/) and the ENCODE (https://www.encodeproject.org/) databases;
-
(v)
DrugBank database, providing hints regarding the druggability of credibly mapped genes and evidence for possible drug repurposing.
An overview of the statistical pipeline is provided in Supplementary Fig. 1, and further details are provided in the following references [36, 47].
Validation in independent alcohol GWASs
We reanalyzed the MOOD x AC and MOOD x AUD relationships using the largest and most recent independent GWASs summary statistics for alcohol-related phenotypes. This included AUD from the Psychiatric Genetics Consortium (AUD-PGC) and AC from the GWAS & Sequencing Consortium of Alcohol and Nicotine use (AC-GSCAN). We also tested the concordance of the effect directions for each lead SNP between the discovery analyses and the re-analyses, using exact binomial tests. The test yields the probability to get observed number of concordant SNPs assuming effect directions are selected randomly.
Exploratory analyses
We leveraged raw genotype data from UK Biobank (accession number 27412) to further dissect the joint genetic architecture of different aspects of AC vs. AUDIT-C total score and MOOD or AUD by a series of exploratory MiXeR and conjFDR analyses. The first series examined either the quantity vs. frequency of drinking as AC phenotypes. The second examined binge drinking as the AC phenotype. Finally, we also ran our AC/AUD and MOOD MiXeR and conjFDR analyses, but separately in ever vs. never lifetime tobacco smokers. All GWASs were performed with regenie v3.0.3 (default settings) in White British samples as identified by UKB FID = 22006 (self-report + genetic principal component analysis). Relatives were kept. Age, sex and first 10 genetic PCs were included as covariates. Of note, since these analyses were deemed exploratory, we did not further correct p-values for these additional tests.
Associations between MOOD, AC and AUD at the phenotypic level
To complete the characterization of the polygenic overlap between our phenotypes of interest, we leveraged the UK Biobank phenotypic data (accession number 27412) to analyze associations between MOOD, AC and AUD, using a series of regression models adjusted for sex, age, and the 10 first genetic principal components. We provide standardized coefficients whenever applicable.
Most of analyses related to secondary objectives are described as Supplementary Methods and Supplementary Data.
Results
A complete overview of the shared polygenicity and genetic correlation for each pair of phenotypes is shown in Supplementary Table 2. Results from the validation analysis are available as supplementary data (text, Supplementary Table 3 and Supplementary Figs. 7–11).
Trait-specific and shared genetic architecture (MiXeR)
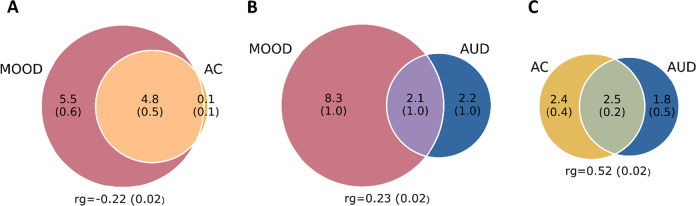
Figure 1 illustrates the high polygenicity of MOOD (10.4k SNPs) as compared to the moderate polygenicity of AC (4.9k SNPs) and AUD (4.3k SNPs). MiXeR showed that almost all AC loci overlapped with MOOD liability whereas only ~50% of AUD genes did so, despite the large overlap in genetic architecture between AC and AUD. This complex picture of mixed effect directions was also in line with the genetic correlation (rg) results, which was significant for all pairs of phenotypes: moderately negative (rg = −0.22) for MOOD and AC (Fig. 1A), moderately positive (rg = 0.23) for MOOD and AUD (Fig. 1B), and strongly positive between AC and AUD (rg = 0.52, Fig. 1C). This indicates different directions of effects between the shared polygenic variants of MOOD and AC versus MOOD and AUD and suggests different molecular mechanisms emerging from partly similar pathways. MiXeR results for MOOD and AC were of high confidence revealing clear optimum in log-likelihood plot (Supplementary Fig. 2A) reflected in tight SD and supported by positive AIC value when compared to the model with minimum overlap. However, the results involving the MVP AUD sample should be interpreted with caution because of erratic behavior of log-likelihood profiles across 20 iterations of the analysis (Supplementary Fig. 2B, C) resulting in high SD of modelled estimates. Importantly, however, in both MOOD and AUD, and AC and AUD analyses, AIC values were positive comparing modelled overlap to complete overlap and negative comparing modelled estimate to minimum possible overlap. Therefore, in both analyses, AIC supports the existence of AUD-specific fraction of “causal” variants.
Fig. 1. Venn diagrams from MiXer analysis.
A Mood instability (MOOD) and alcohol consumption (AC), B MOOD and alcohol use disorder (AUD), and C AC and AUD. Genetic correlation (rg) is also displayed.
Likewise, there was significant enrichment of SNPs associated with MOOD when conditioning on AC and AUD and vice-versa, as evidenced by increasing deviation from the expected null line of SNP strata with increasing significance in the conditional trait on Q-Q plots (Supplementary Fig. 3A, B).
Loci discovery and annotation
Loci discovery (conjFDR)
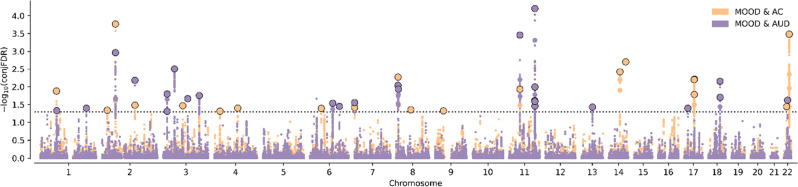
At conjFDR p < 0.05, there were 18 significant lead SNPs in the AC&MOOD analysis and 20 in the AUD&MOOD analysis. Manhattan plots for conjFDR of MOOD&AC and MOOD&AUD analyses are shown in Fig. 2. Among the 38 lead SNPs, only one (rs2312147) was common to both AC and AUD. Functional annotation of these genome-wide significant SNPs is available on Supplementary Table 1.
Fig. 2. Manhattan plot for the conjFDR analysis.
Significant loci at FDR <0.1 are shown for mood instability (MOOD) and alcohol consumption (AC) and for MOOD and alcohol use disorder (AUD).
Fifteen SNPs were novel with regards to MOOD, AC and AUD GWASs that had been published as of June 1st, 2022 (Table 2). Three of these SNPs had potential functional impact due to: deleteriousness (rs34811474, CADD score = 23.7), non-synonymous protein effect (rs8007859) and location in a regulatory region (UTR3, rs11130187). None of the novel SNPs were shared between AC and AUD. Interestingly, one of these SNPs (rs2277840, MOOD&AUD conjFDR) had been associated with the level of response to alcohol, an endophenotype for AUD, in a small-scale GWAS [48].
Table 2.
Significantly associated SNPs for MOOD&AC and for MOOD&AUD, by conjFDR, previously unpublished in GWASs of MOOD, AC or AUD.
| CHR | Start_BP | End_BP | SNP | A1 | A2 | pval_MOOD | pval_Alcohol | zscore_MOOD | zscore_Alcohol | Nearest gene | Function | CADD | RDB | minChrState |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MOOD&AC | ||||||||||||||
| 1 | 72628347 | 72949290 | rs2568957 | A | G | 0.00004 | 0.00013 | −4.1 | 3.8 | RPL31P12 | intergenic | 2.758 | 7 | 14 |
| 4 | 25342606 | 25408838 | rs34811474 | A | G | 0.00052 | 0.00083 | −3.5 | 3.3 | ANAPC4 | exonic | 23.7 | 6 | 4 |
| 4 | 105361545 | 105499837 | rs17034592 | C | T | 0.00004 | 0.00062 | −4.1 | 3.4 | AC004053.1 | ncRNA_intronic | 7.711 | 7 | 5 |
| 6 | 50597378 | 50934086 | rs4642472 | T | C | 0.00079 | 0.00004 | −3.4 | 4.1 | FTH1P5 | intergenic | 0.253 | 5 | 9 |
| 7 | 1221100 | 1228945 | rs12533133 | C | T | 0.00074 | 0.00033 | 3.4 | −3.6 | AC091729.9 | intergenic | 4.537 | 2b | 5 |
| 14 | 69429386 | 69755470 | rs8007859 | G | T | 0.00003 | 0.00003 | −4.2 | 4.2 | EXD2 | exonic | 9.825 | 5 | 4 |
| 22 | 30040197 | 30095706 | rs2857651 | C | T | 0.00069 | 0.00006 | 3.4 | −4 | NF2 | intronic | 11.18 | 7 | 4 |
| MOOD&AUD | ||||||||||||||
| 3 | 48724599 | 49650935 | rs11130187 | T | C | 0.00002 | 0.00002 | 4.2 | 4.2 | C3orf62 | UTR3 | 0.873 | 7 | 4 |
| 6 | 100953047 | 101505612 | rs9390701 | A | G | 0.0002 | 0.00041 | 3.7 | 3.5 | RP3-467N11.2 | intergenic | 7.053 | 6 | 5 |
| 6 | 130544509 | 130768029 | rs7773962 | G | A | 0.00009 | 0.00054 | −3.9 | −3.5 | SAMD3 | intronic | 2.832 | 5 | 1 |
| 7 | 1221100 | 1228945 | rs34527042 | C | T | 0.00057 | 0.00039 | 3.4 | −3.5 | AC091729.9 | intergenic | 10.78 | 6 | 5 |
| 8 | 10876819 | 10955220 | rs7833741 | C | A | 0.00001 | 0.00012 | 4.4 | 3.8 | XKR6 | intronic | 1.985 | 7 | 5 |
| 13 | 68141716 | 68194993 | rs9541161 | T | C | 0.00085 | 0.00053 | −3.3 | 3.5 | LINC00364 | intergenic | 3.989 | 7 | 9 |
| 17 | 15873275 | 16263682 | rs3785620 | C | A | 0.00006 | 0.00064 | 4 | −3.4 | PIGL | intronic | 0.454 | 6 | 4 |
| 22 | 34227271 | 34269594 | rs2277840 | G | A | 0.00005 | 0.00031 | 4.1 | 3.6 | LARGE | intronic | 3.942 | 7 | 5 |
CHR chromosome, BP base pair, SNP single nucleotide polymorphism, A1 alternate allele, A2 reference allele, FDR false discovery rate, SNP single nucleotide polymorphism, MOOD mood instability in the UK Biobank, AC alcohol consumption in the MVP sample, AUD alcohol use disorder in the MVP sample, CADD Combined Annotation Dependent Depletion, RDB RegulomeDB, minChrState chromatin state.
Summary of validation analyses
All MiXeR and genetic correlations analyses fully replicated the patterns of overlap between AC and AUD (Supplementary Fig. 10). Interestingly, the GSCAN-AC & MVP-AUD MiXeR analysis showed a slightly different pattern, with a complete overlap between the traits and the highest correlation of the AC & AUD analyses (rg = 0.73, Supplementary Fig. 11). Importantly, conjFDR associations were replicated for four (AC) and five (AUD) loci, respectively, as reported in Supplementary Table 1 and shown in Supplementary Fig. 12. A complete summary of MiXer results for discovery and validation steps is shown Supplementary Table 2, and the loci that were fully validated for conjFDR are listed in Supplementary Table 3.
Functional analyses of lead SNPs by FUMA
The pattern of genomic localization of SNPs associated with MOOD and alcohol-related phenotypes differed between AC and AUD. In the MOOD&AC analysis, SNPs were significantly enriched for 3’ and 5’ untranslated regions (p = 3 × 10−4 and 9 × 10−8, respectively) and downstream areas (p = 0.002) while SNPs from the MOOD&AUD analysis were significantly enriched for intronic (p = 10−315), intergenic (p = 10−152), exonic coding regions (p = 0.04), non-coding long RNA (p = 0.009), and upstream regions (p = 0.009).
According to FUMA the function of the credibly mapped genes from conjFDR mostly differed for MOOD&AC vs MOOD&AUD - in line with MiXeR overall patterns. Firstly, the genes from the MOOD&AC analysis shared enrichment only for the 11p11 region. Secondly, the reactome post-translational protein modification pathway was significantly enriched for both alcohol-related traits, but the MOOD&AC analysis elicited two more pathways related to the TFAP2 family of transcription factors (Supplementary Fig. 4). Thirdly, in the MOOD&AC analysis, the hypothalamus was the only brain tissue with significant enrichment in gene expression, whereas, in the MOOD&AUD analysis, all but four brain tissues were significantly overrepresented (Supplementary Fig. 5A, B) with downregulated genes. Fourthly, for AC (Supplementary Fig. 6A), only 12 genes (18%) showed a progressively decreasing brain expression throughout the lifespan while 19 (28%) showed increasing expression. For AUD, eight (16%) genes show increasing expression throughout the lifespan while 26 (33%) show progressively reduced expression (Chi2 p = 0.031, Supplementary Fig. 6A, B). There was no difference between conjFDR analyses in celltype enrichment using epigenetics databases. Regarding the 15 novel SNPs, one (rs7833741) had previously been associated with neuroticism sum-score at the EPQ-R questionnaire (Supplementary Tables 4 and 5). Six SNPs from the MOOD & AC analysis and five from the MOOD & AUD analysis yielded previous GWAS hits, leaving four fully novel SNPs: rs17034592, rs7773962, rs9541161 and rs2277840. PheWAS associations of the 15 novel SNPs are described in Supplementary Fig. 7 for MOOD & AC and MOOD & AUD, respectively. Briefly, these patterns show that MOOD & AUD SNPs have been previously associated with neuroticism (sum-score and several items), but not with MOOD per se, and with metabolic conditions such as increased BMI and serum lipids levels. Finally, nine genes from the MOOD&AC analysis and 12 from the MOOD&AUD yielded hits in DrugBank. Among the latter, there were multiple DrugBank hits for the genes encoding Inosine monophosphate dehydrogenase 2 (IMPDH2, eight hits), the type 2 dopamine receptor (DRD2, 110 hits) - a classical target in mental health, and the type 2 adenosine receptor (ADORA2B, five hits), one of the targets of the approved AUD medication acamprosate. Other drugs (one hits per gene) were related to the immune and metabolic systems medications as well as to nutrients. None of the novel associations was included in these hits.
The full results from the validation, exploratory and phenotypic analyses are provided as Supplementary Data (Supplementary Figs. 8 to 14) and further discussed below.
Discussion
We leveraged data from large scale GWASs to shed light on the shared and unique polygenic architecture of MOOD, AC and AUD, suggesting shared biological underpinnings between MOOD and alcohol-related phenotypes. We obtained remarkably consistent evidence that the relationship between MOOD and alcohol-related phenotypes strongly differed for AC vs. AUD, thus confirming our main hypothesis. Using MiXeR, we first evidenced a large degree of polygenic overlap between MOOD and alcohol-related phenotypes, which was higher for AC (98%) than for AUD (49%). Next, using conjFDR, 20 SNPs for MOOD&AC and 18 for MOOD&AUD reached genome-wide significance. Importantly, none was common to both analyses, and fifteen were novel at the time of the study for any of the phenotypes––based on a highly conservative checking procedure, which included the latest conjFDR results from our group [37]. At all levels, the functional annotation of conjFDR supported the differences in polygenic overlap between the joint genetic architecture of the vulnerability to MOOD&AC vs. MOOD&AUD. Taken altogether, these findings describe a polygenic scenario with distinctive patterns of effect distributions among the overlapping variants, which may help to define the unique features of each phenotype. Our study was performed using the latest GWASs available for two traits of major relevance for mental health, i.e., MOOD and AC; and characterized how these relate to AUD. The growing number of adequately powered GWASs for alcohol-related phenotypes allowed us to perform a series of validation analyses (Supplementary Figs. 8 to 12), which overall strengthened our discovery results as regards polygenic overlap, shared loci identification, and concordance of effect direction.
Our first main finding was that AC and AUD, beyond confirming their strong, positive genetic correlation [37], showed relevant differences in the proportion of shared vs. unique polygenicity with MOOD. Strikingly, genetic correlation showed opposite directions (negative for AC, positive for AUD). Unfortunately, available AUD samples (MVP, PGC–and their meta-analysis) did not enable enough stability of MiXeR analysis to further discuss the number of overlapping causal variants with MOOD in detail. However, given the high statistical power achieved by meta-analyzing the MVP + PGC samples, this was unlikely due to power issues. Instead, one could hypothesize that the polygenicity of AUD follows a multimodal distribution (including consistent evidence for peak and pleiotropic signals [4, 9, 43, 49]) which, combined with its low SNP-based heritability (<10%), did not allow to generate clear-cut MiXeR outcomes. Future studies using, e.g., multivariate approaches [50] could help strengthening these findings.
The different polygenic overlap between MOOD&AC vs. MOOD&AUD extends genetic correlation results [44] showing that AUDIT-P (a proxy for AUD), but not AC, was associated with most other psychiatric disorders. Additionally, the current findings extend a recent report regarding the polygenicity of AC and AUD and their relationship with schizophrenia and bipolar disorder [37]. Four (out of 38) of the loci that we identified were also reported in this previous study (out of 62). Firstly, this suggests a consistent power for locus discovery between the two studies, that is, 15–20 loci per pair of phenotypes. Secondly, we notice that eight loci identified as novel in the current study were not reported by Wiström et al. [37] and that this previous study did not identify different biological pathways nor gene expression patterns when using either AC or AUD as a secondary phenotype, despite identifying a higher number of jointly-associated loci. Overall, this supports not only the reliability of conjFDR, but also its sensitivity to the phenotypes that are investigated. With that regard, we notice that all of the SNPs we report as novel would reach Bonferroni-corrected genome-wide significance (highest p value = 4.8 × 10−8), suggesting a true boost in discovery power of conjFDR rather than an effect of relaxing the initial significance threshold for candidate loci. The relevance of combining endophenotypes such as MOOD with disorders such as AUD, which has already been suggested by our group [16], should thus be further explored using RDoC constructs.
The current genetic findings add support to the phenomenological differences between alcohol use (AC) and alcohol use disorders (AUD). AC (even when regular and in relatively high amounts) is a normative behavior in most Western world countries, while AUD can develop into a pervasive, devastating mental illness. Half of the polygenic overlap, only two loci and none of the functional analysis results were shared between MOOD&AC and MOOD&AUD conjFDR in our study (although this was based on 38 loci in total as compared to 5–10k representing the overall polygenic signal). Thus, the loci associated with MOOD&AUD were significantly enriched for genes that are typically under-expressed in the putamen, amygdala, substantia nigra and anterior cingulate cortex, which was not the case for any of the MOOD&AC loci. Strikingly, these brain regions are at the core of the neurocircuitry of AUD but not AC [3, 51–53]. Additionally, the genes associated with MOOD&AC showed mostly increasing expression throughout brain development, while a significantly opposite trend was observed for the genes associated with MOOD&AUD. These findings are of paramount importance with regard to the underlying neurobiology of AC compared to that of AUD. There is now consistent evidence that AUD develops from AC through the progressive recruitment of extended brain networks [54] implying different neurotransmitter systems [55].
At the phenotypic level, both AC and AUD have been strongly associated with MOOD, including in the current original analysis. In clinical samples, the association of MOOD and AC was bi-directional and involved both negative and positive reinforcement [56, 57]. Here, we tentatively suggest that individuals with a high genetic load for MOOD will exhibit high levels of AC due to the resulting MOOD phenotype, regardless of their genetic liability toward AC. Accordingly, individuals with a high genetic load for AC may drink more alcohol, whatever their level of genetic vulnerability to MOOD. Exploratory analyses showed variable patterns of polygenic overlap and genetic correlation between MOOD and four measures of AC, namely: total AUDIT-C, frequency of drinks/week, amounts of alcohol per week and binge drinking (Supplementary data and Supplementary Fig. 13A, B). This includes an unexpected negative correlation for alcohol amounts and MOOD, conversely to the literature usually showing positive correlation between alcohol quantity measures and psychiatric traits, as opposed to frequency measures. This suggests a positive correlation between AC quantity and frequency, as supported by their strong positive genetic correlation with AUD, despite different patterns of polygenic overlap (Supplementary Fig. 13C, D). Moreover, the opposite genetic correlation previously reported for these two AC phenotypes was related to psychiatric disorders, i.e., major depression or bipolar disorder. Although the complete item-level analysis of the shared polygenicity of the neuroticism scale with alcohol phenotypes could be of interest, we deemed it was beyond the scope of the current study. We notice that the previous associations of MOOD & AUD SNPs with neuroticism questionnaire items (Supplementary Fig. 7) were related to the ‘tension’ cluster rather than the ‘depressive affects’ cluster [15], making the current findings with MOOD original. Having a single item such as MOOD to identify plausible endophenotypes opens opportunity for applied research. More specifically, we hypothesize that MOOD represents a relevant endophenotype towards mood disorders since it may be relevant for both the frequency and the quantity of alcohol intake. Conversely, although MiXeR was not interpretable for this phenotype, we evidenced that binge alcohol intake shared only one jointly associated locus with the corresponding analyses using total AUDIT-C as an AC measure, suggesting that this phenotype––which has major clinical relevance––may have different genetic underpinnings from other AC measures. Finally, as regards tobacco smoking, results from MiXeR and conjFDR were mostly similar between ever and never-smokers regarding MOOD and AC, noticing that we could not conclude regarding MOOD and AUD in this particular analysis due to power issues (Supplementary Fig. 14).
Our study has limitations. Firstly, both the MOOD and AC traits remain relatively non-specific and may be heterogeneous, although they represent highly relevant constructs for research. Especially, the MOOD phenotype (assessed using a single, binary item) could have been influenced by the respondents’ recent alcohol use and/or alcohol withdrawal symptoms at the time of MOOD assessment. We hope that large GWASs will be conducted based on finer-grained assessment of MOOD in the future. Secondly, the MVP sample is composed of 92% men aged >30, all with military duty, and we could not obtain the phenotypic breakdown for this sample. This limits the generalizability of our findings, since the prevalence, the severity and the relationships of MOOD, AC and AUD are susceptible to change over the lifespan [18, 19, 58]. In line with this, we only considered European ancestry subsamples for analysis. We plan to develop trans-ancestral overlap analyses in the near future to overcome this important limitation for better precision psychiatry. Thirdly, the results regarding AUD in the primary analysis should be interpreted with caution given statistical issues (reflected in high SD value for the number of SNPs evidenced by MiXeR), while noticing that additional analyses suggested that power was not involved. Fourthly, MiXeR does not allow for mapping of SNPs involved in the observed polygenic overlap and only takes two traits as input. To overcome this limitation and better describe complex overlap such as reported in the current paper, a trivariate version of MiXeR is currently under development. Finally, one should note that phenotypic analyses could be confounded by suboptimal alcohol use assessments. New analyses on subgroups with better defined phenotypes (e.g., AC among non-AUD participants) would help to further characterize the links between MOOD, AC and AUD.
Conclusion
By leveraging large GWAS data beyond genetic correlations using MiXeR and conjFDR, the characterization of the polygenic overlap between mood instability and alcohol-related phenotypes improved our understanding of their shared vs. unique polygenicity. Our findings open avenues toward a better understanding of the comorbidity between mental disorders, especially between AUD and mood disorders through MOOD, and extend the growing body of evidence regarding how AC and AUD differ in terms of biological mechanisms. We believe our findings fit within the efforts to conceptualize mental disorders as combinations of relevant psychopathological dimensions [31, 32] to better inform mechanisms. Thus, while confirming that alcohol use disorders, alcohol intake frequency, alcohol intake quantity and binge do not entirely overlap in terms of polygenicity, their genetic relationships with MOOD as the transdiagnostic factor of interest yielded patterns that differed from what is observed in the literature using full psychopathological scales (i.e., neuroticism sum-score) or case-control status for mood disorders. The genetic susceptibility to mood instability could represent a missing link between AC and AUD, providing strong biological support to clinical observations.
Supplementary information
Acknowledgements
The authors would like to thank all the support personnel from the Norwegian center of excellence for mental disorders (NORMENT) and all the researchers that provided valuable input for the current study. The authors would also like the INTPART program for supporting a post-doc exchange for RI.
Author contributions
(CRediT roles) RI, GH, AS, OF and OAA, Conceptualization; AS, Formal analysis; KOC, SD, AMD, TVL, OS and OAA, Funding acquisition; Methodology, AL, OF, SB, WC, CF, AMD, OS, OAA; NK, Project administration; MCH, SD, TVL, Resources; RI, Writing - original draft; AS, BH, GH, OF, OS, OAA, Writing - review & editing. All authors have critically reviewed and approved the manuscript.
Funding
We were funded by the Research Council of Norway (276082, 213837, 223273, 204966/F20, 229129, 249795/F20, 225989, 248778, 249795), the South-Eastern Norway Regional Health Authority (2013-123, 2014-097, 2015-073, 2016-064, 2017-004), Stiftelsen Kristian Gerhard Jebsen (SKGJ-Med-008), The European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (ERC Starting Grant, Grant Agreement No. 802998) and National Institutes of Health (R01MH100351, R01GM104400, NIDA/NCI: U24DA041123). This work was partly performed on the TSD (Tjeneste for Sensitive Data) facilities, owned by the University of Oslo, operated and developed by the TSD service group at the University of Oslo, IT-Department (USIT) (tsd-drift@usit.uio.no). Computations were also performed on resources provided by UNINETT Sigma2—the National Infrastructure for High Performance Computing and Data Storage in Norway. This work used the Extreme Science and Engineering Discovery Environment (XSEDE) including COMET and OASYS resources at the UCSD through allocation TG-IBN200001. The funding source had no role in the conception, the recruitment or the statistical analyses included in the current study. OAA has received speaker’s honorarium from Lundbeck and is a consultant for Healthlytix.
Competing interests
AMD is a founder of and holds equity interest in CorTechs Labs and serves on its scientific advisory board. He is also a member of the Scientific Advisory Board of Healthlytix and receives research funding from General Electric Healthcare (GEHC). The terms of these arrangements have been reviewed and approved by the University of California, San Diego in accordance with its conflict-of-interest policies. The remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-022-01401-6.
References
- 1.Goodman A. Addiction: definition and implications. Br J Addict. 1990;85:1403–8. doi: 10.1111/j.1360-0443.1990.tb01620.x. [DOI] [PubMed] [Google Scholar]
- 2.Yücel M, Oldenhof E, Ahmed SH, Belin D, Billieux J, Bowden-Jones H, et al. A transdiagnostic dimensional approach towards a neuropsychological assessment for addiction: an international Delphi consensus study. Addiction. 2019;114:1095–109. doi: 10.1111/add.14424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vollstädt-Klein S, Wichert S, Rabinstein J, Bühler M, Klein O, Ende G, et al. Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction. 2010;105:1741–9. doi: 10.1111/j.1360-0443.2010.03022.x. [DOI] [PubMed] [Google Scholar]
- 4.Walters RK, Polimanti R, Johnson EC, McClintick JN, Adams MJ, 23andMe Research Team. et al. Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nat Neurosci. 2018;21:1656–69. doi: 10.1038/s41593-018-0275-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polimanti R, Walters RK, Johnson EC, McClintick JN, Adkins AE, Adkins DE, et al. Leveraging genome-wide data to investigate differences between opioid use vs. opioid dependence in 41,176 individuals from the Psychiatric Genomics Consortium. Mol Psychiatry. 2020;25:1673–87. doi: 10.1038/s41380-020-0677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson EC, Chang Y, Agrawal A. An update on the role of common genetic variation underlying substance use disorders. Curr Genet Med Rep. 2020;8:35–46. doi: 10.1007/s40142-020-00184-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the National Comorbidity Survey. Exp Clin Psychopharmacol. 1994;2:244. doi: 10.1037/1064-1297.2.3.244. [DOI] [Google Scholar]
- 8.Lohoff FW, Clarke T-K, Kaminsky ZA, Walker RM, Bermingham ML, Jung J, et al. Epigenome-wide association study of alcohol consumption in N = 8161 individuals and relevance to alcohol use disorder pathophysiology: identification of the cystine/glutamate transporter SLC7A11 as a top target. Mol Psychiatry. 2022;27:1754–64. doi: 10.1038/s41380-021-01378-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kranzler HR, Zhou H, Kember RL, Smith RV, Justice AC, Damrauer S, et al. Genome-wide association study of alcohol consumption and use disorder in 274,424 individuals from multiple populations. Nat Commun. 2019;10:1–11.. doi: 10.1038/s41467-019-11916-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marees AT, Smit DJA, Ong J-S, MacGregor S, An J, Denys D, et al. Potential influence of socioeconomic status on genetic correlations between alcohol consumption measures and mental health. Psychol Med. 2020;50:484–98. doi: 10.1017/S0033291719000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou H, Rentsch CT, Cheng Z, Kember RL, Nunez YZ, Sherva RM, et al. Association of OPRM1 functional coding variant with opioid use disorder: a genome-wide association study. JAMA Psychiatry. 2020;77:1072. doi: 10.1001/jamapsychiatry.2020.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirsch DE, Tretyak V, Radpour S, Weber WA, Nemeroff CB, Fromme K, et al. Childhood maltreatment, prefrontal-paralimbic gray matter volume, and substance use in young adults and interactions with risk for bipolar disorder. Sci Rep. 2021;11:123. doi: 10.1038/s41598-020-80407-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aas M, Henry C, Bellivier F, Lajnef M, Gard S, Kahn J-P, et al. Affective lability mediates the association between childhood trauma and suicide attempts, mixed episodes and co-morbid anxiety disorders in bipolar disorders. Psychol Med. 2017;47:902–12. doi: 10.1017/S0033291716003081. [DOI] [PubMed] [Google Scholar]
- 14.Angst J, Gamma A, Endrass J. Risk factors for the bipolar and depression spectra. Acta Psychiatr Scandinavica. 2003;108:15–19. doi: 10.1034/j.1600-0447.108.s418.4.x. [DOI] [PubMed] [Google Scholar]
- 15.Nagel M, Watanabe K, Stringer S, Posthuma D, van der Sluis S. Item-level analyses reveal genetic heterogeneity in neuroticism. Nat Commun. 2018;9:905. doi: 10.1038/s41467-018-03242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hindley G, O’Connell KS, Rahman Z, Frei O, Bahrami S, Shadrin A, et al. The shared genetic basis of mood instability and psychiatric disorders: A cross-trait genome-wide association analysis. Am J Med Genet B Neuropsychiatr Genet 2022:189:207–18. [DOI] [PMC free article] [PubMed]
- 17.Wills TA, Simons JS, Sussman S, Knight R. Emotional self-control and dysregulation: a dual-process analysis of pathways to externalizing/internalizing symptomatology and positive well-being in younger adolescents. Drug Alcohol Depend. 2016;163:S37–45. doi: 10.1016/j.drugalcdep.2015.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chou SP, Lee HK, Cho MJ, Park J-I, Dawson DA, Grant BF. Alcohol use disorders, nicotine dependence, and co-occurring mood and anxiety disorders in the United States and South Korea—a cross-national comparison. Alcohol: Clin Exp Res. 2012;36:654–62. doi: 10.1111/j.1530-0277.2011.01639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of dsm-iv alcohol abuse and dependence in the United States: results from the National epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2007;64:830–42. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- 20.Hunt GE, Malhi GS, Cleary M, Lai HMX, Sitharthan T. Prevalence of comorbid bipolar and substance use disorders in clinical settings, 1990-2015: Systematic review and meta-analysis. J Affect Disord. 2016;206:331–49. doi: 10.1016/j.jad.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–8. doi: 10.1001/jama.1990.03450190043026. [DOI] [PubMed] [Google Scholar]
- 22.Vorspan F, Mehtelli W, Dupuy G, Bloch V, Lépine J-P. Anxiety and substance use disorders: co-occurrence and clinical issues. Curr Psychiatry Rep. 2015;17:4. doi: 10.1007/s11920-014-0544-y. [DOI] [PubMed] [Google Scholar]
- 23.Skodol AE, Oldham JM, Gallaher PE. Axis II comorbidity of substance use disorders among patients referred for treatment of personality disorders. AJP. 1999;156:733–8. doi: 10.1176/ajp.156.5.733. [DOI] [PubMed] [Google Scholar]
- 24.Lagerberg TV, Aminoff SR, Aas M, Bjella T, Henry C, Leboyer M, et al. Alcohol use disorders are associated with increased affective lability in bipolar disorder. J Affect Disord. 2017;208:316–24. doi: 10.1016/j.jad.2016.09.062. [DOI] [PubMed] [Google Scholar]
- 25.Bolton JM, Robinson J, Sareen J. Self-medication of mood disorders with alcohol and drugs in the National Epidemiologic Survey on Alcohol and Related Conditions. J Affect Disord. 2009;115:367–75. doi: 10.1016/j.jad.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Turner S, Mota N, Bolton J, Sareen J. Self‐medication with alcohol or drugs for mood and anxiety disorders: a narrative review of the epidemiological literature. Depress Anxiety. 2018;35:851–60. doi: 10.1002/da.22771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duif M, Thewissen V, Wouters S, Lechner L, Jacobs N. Affective instability and alcohol consumption: ecological momentary assessment in an adult sample. J Stud Alcohol Drugs. 2019;80:441–7. doi: 10.15288/jsad.2019.80.441. [DOI] [PubMed] [Google Scholar]
- 28.Dvorak RD, Kuvaas NJ, Lamis DA, Pearson MR, Stevenson BL. Emotionally up and down, behaviorally to and fro: drinking motives mediate the synergistic effects of urgency and emotional instability on alcohol outcomes. J Drug Educ. 2015;45:156–84. doi: 10.1177/0047237916639030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang P, Tao R, He C, Liu S, Wang Y, Zhang X. The risk factors of the alcohol use disorders—through review of its comorbidities. Front Neurosci. 2018;12:303. doi: 10.3389/fnins.2018.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guglielmo R, Miskowiak KW, Hasler G. Evaluating endophenotypes for bipolar disorder. Int J Bipolar Disord. 2021;9:17. doi: 10.1186/s40345-021-00220-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–51. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 32.Watson D, Levin-Aspenson HF, Waszczuk MA, Conway CC, Dalgleish T, Dretsch MN, et al. Validity and utility of Hierarchical Taxonomy of Psychopathology (HiTOP): III. Emotional dysfunction superspectrum. World Psychiatry. 2022;21:26–54. doi: 10.1002/wps.20943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Broome MR, Saunders KEA, Harrison PJ, Marwaha S. Mood instability: significance, definition and measurement. Br J Psychiatry. 2015;207:283–5. doi: 10.1192/bjp.bp.114.158543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ward J, Tunbridge EM, Sandor C, Lyall LM, Ferguson A, Strawbridge RJ, et al. The genomic basis of mood instability: identification of 46 loci in 363,705 UK Biobank participants, genetic correlation with psychiatric disorders, and association with gene expression and function. Molecular Psychiatry. 2019. 2019. 10.1038/s41380-019-0439-8. [DOI] [PMC free article] [PubMed]
- 35.Frei O, Holland D, Smeland OB, Shadrin AA, Fan CC, Maeland S, et al. Bivariate causal mixture model quantifies polygenic overlap between complex traits beyond genetic correlation. Nat Commun. 2019;10:1–11.. doi: 10.1038/s41467-018-07882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smeland OB, Frei O, Shadrin A, O’Connell K, Fan C-C, Bahrami S, et al. Discovery of shared genomic loci using the conditional false discovery rate approach. Hum Genet. 2020;139:85–94. [DOI] [PubMed]
- 37.Wiström ED, O’Connell KS, Karadag N, Bahrami S, Hindley GFL, Lin A, et al. Genome-wide analysis reveals genetic overlap between alcohol use behaviours, schizophrenia and bipolar disorder and identifies novel shared risk loci. Addiction. 2021. 2021. 10.1111/add.15680. [DOI] [PubMed]
- 38.Brainstorm Consortium, Anttila V, Bulik-Sullivan B, Finucane HK, Walters RK, Bras J, et al. Analysis of shared heritability in common disorders of the brain. Science. 2018;360:eaap8757. [DOI] [PMC free article] [PubMed]
- 39.Little J, Higgins JPT, Ioannidis JPA, Moher D, Gagnon F, Elm Evon, et al. STrengthening the REporting of Genetic Association Studies (STREGA)— An Extension of the STROBE Statement. PLOS Med. 2009;6:151–63.. doi: 10.1371/journal.pmed.1000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmad F, Jhajj AK, Stewart DE, Burghardt M, Bierman AS. Single item measures of self-rated mental health: a scoping review. BMC Health Serv Res. 2014;14:398. doi: 10.1186/1472-6963-14-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Rijsbergen GD, Bockting CLH, Berking M, Koeter MWJ, Schene AH. Can a one-item mood scale do the trick? Predicting relapse over 5.5-years in recurrent depression. PLoS One. 2012;7:e46796. [DOI] [PMC free article] [PubMed]
- 42.Mathieu F, Etain B, Dizier MH, Lajnef M, Lathrop M, Cabon C, et al. Genetics of emotional reactivity in bipolar disorders. J Affect Disord. 2015;188:101–6. doi: 10.1016/j.jad.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 43.Justice AC, Smith RV, Tate JP, McGinnis K, Xu K, Becker WC, et al. AUDIT-C and ICD codes as phenotypes for harmful alcohol use: association with ADH1B polymorphisms in two US populations. Addiction. 2018;113:2214–24. doi: 10.1111/add.14374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanchez-Roige S, Palmer AA, Fontanillas P, Elson SL, Adams MJ, Howard DM, et al. Genome-wide association study meta-analysis of the Alcohol Use Disorder Identification Test (AUDIT) in two population-based cohorts. Am J Psychiatry. 2019;176:107–18. doi: 10.1176/appi.ajp.2018.18040369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. 2019;51:237–44. [DOI] [PMC free article] [PubMed]
- 46.Uffelmann E, Posthuma D. Emerging methods and resources for biological interrogation of neuropsychiatric polygenic signal. Biol Psychiatry. 2021;89:41–53. doi: 10.1016/j.biopsych.2020.05.022. [DOI] [PubMed] [Google Scholar]
- 47.Frei O, Holland D, Smeland OB, Shadrin AA, Fan CC, Maeland S, et al. Bivariate causal mixture model quantifies polygenic overlap between complex traits beyond genetic correlation. Nat Commun. 2019;10:2417. doi: 10.1038/s41467-019-10310-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joslyn G, Ravindranathan A, Brush G, Schuckit M, White RL. Human variation in alcohol response is influenced by variation in neuronal signaling genes. Alcohol: Clin Exp Res. 2010;34:800–12. doi: 10.1111/j.1530-0277.2010.01152.x. [DOI] [PubMed] [Google Scholar]
- 49.Ripke S, Neale BM, Corvin A, Walters JTR, Farh K-H, Schizophrenia Working Group of the Psychiatric Genomics Consortium. et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–7. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van der Meer D, Frei O, Kaufmann T, Shadrin AA, Devor A, Smeland OB, et al. Understanding the genetic determinants of the brain with MOSTest. Nat Commun. 2020;11:3512. doi: 10.1038/s41467-020-17368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fouyssac M, Belin D. Beyond drug‐induced alteration of glutamate homeostasis, astrocytes may contribute to dopamine‐dependent intrastriatal functional shifts that underlie the development of drug addiction: a working hypothesis. Eur J Neurosci. 2019;50:3014–27. doi: 10.1111/ejn.14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meyer PJ, King CP, Ferrario CR. Motivational processes underlying substance abuse disorder. Curr Top Behav Neurosci. 2016;27:473–506. doi: 10.1007/7854_2015_391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leong SL, Glue P, Manning P, Vanneste S, Lim LJ, Mohan A, et al. Anterior cingulate cortex implants for alcohol addiction: a feasibility study. Neurotherapeutics. 2020;17:1287–99. doi: 10.1007/s13311-020-00851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ersche KD, Meng C, Ziauddeen H, Stochl J, Williams GB, Bullmore ET, et al. Brain networks underlying vulnerability and resilience to drug addiction. PNAS. 2020;117:15253–61. doi: 10.1073/pnas.2002509117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Volkow ND, Wiers CE, Shokri-Kojori E, Tomasi D, Wang G-J, Baler R. Neurochemical and metabolic effects of acute and chronic alcohol in the human brain: Studies with positron emission tomography. Neuropharmacology. 2017;122:175–88. doi: 10.1016/j.neuropharm.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 56.Dvorak RD, Stevenson BL, Kilwein TM, Sargent EM, Dunn ME, Leary AV, et al. Tension reduction and affect regulation: an examination of mood indices on drinking and non-drinking days among university student drinkers. Exp Clin Psychopharmacol. 2018;26:377–90. doi: 10.1037/pha0000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Leon AN, Dvorak RD, Kramer MP, Peterson R, Pinto DA, Leary AV, et al. Daily patterns of emotional functioning on drinking and nondrinking days. Alcohol Clin Exp Res. 2020;44:2598–610. doi: 10.1111/acer.14480. [DOI] [PubMed] [Google Scholar]
- 58.Marwaha S, Parsons N, Flanagan S, Broome M. The prevalence and clinical associations of mood instability in adults living in England: results from the Adult Psychiatric Morbidity Survey 2007. Psychiatry Res. 2013;205:262–8. doi: 10.1016/j.psychres.2012.09.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.