Abstract
Introduction/objectives
To evaluate the three-year efficacy and safety of ixekizumab with and without concomitant conventional synthetic disease-modifying antirheumatic drug (csDMARD) use in patients with active psoriatic arthritis (PsA).
Method
Patients with PsA who were biologic-naïve (SPIRIT-P1, NCT01695239) or had prior inadequate response to tumor necrosis factor inhibitors (SPIRIT-P2, NCT02349295) were randomized to receive 80-mg ixekizumab every four weeks after receiving 160-mg ixekizumab at baseline. Efficacy, safety, and immunogenicity were evaluated in this post-hoc analysis in three subgroups: (1) ixekizumab monotherapy, (2) ixekizumab and methotrexate (MTX), (3) ixekizumab and any csDMARD (including MTX). Missing data were imputed using multiple imputation for continuous variables and modified non-responder imputation for categorical variables.
Results
Efficacy was similar across the three subgroups with 59.1%, 67.0%, and 66.1% of ixekizumab-treated patients achieving 20% improvement in the American College of Rheumatology scale score at week 156. Radiographic progression of structural joint damage (SPIRIT-P1 only) was similarly inhibited across the three subgroups with several outliers. No new safety signals were reported, and 91.0%, 84.1%, and 83.2% in the three subgroups reported ≥ 1 treatment-emergent adverse event. At week 156, 15.9%, 13.1%, and 11.0% in the three subgroups had antidrug antibodies; most had low titer status.
Conclusions
Ixekizumab showed sustained efficacy in treating patients with PsA for up to three years in monotherapy or in combination with MTX or any csDMARD. The three subgroups had similar safety and immunogenicity profiles, which supports that the use of concomitant MTX or csDMARDs does not seem to impact the benefit/risk profile of ixekizumab.
|
Key Points • Ixekizumab treatment led to improved clinical responses over time when used as monotherapy or in combination with concomitant MTX or any concomitant csDMARD (including MTX) in patients with active PsA. • Ixekizumab monotherapy has similar radiographic efficacy as ixekizumab with MTX or ixekizumab with other csDMARDs (including MTX); similar inhibition of radiographic progression was observed between the subgroups of patients receiving ixekizumab monotherapy or ixekizumab with MTX or other csDMARDs. • The long-term safety profile of ixekizumab used as monotherapy or in combination with MTX or any other csDMARDs is consistent with what has been previously reported. The addition of MTX or any csDMARD to ixekizumab treatment did not negatively impact the favorable long-term safety profile of ixekizumab. |
Supplementary Information
The online version contains supplementary material available at 10.1007/s10067-022-06218-8.
Keywords: Antirheumatic agents, Ixekizumab, Methotrexate, Psoriatic arthritis
Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory, progressive, destructive disease that results in deformities, impaired physical function, and decreased quality of life [1]. Biologic disease-modifying antirheumatic drugs (bDMARDs) have demonstrated efficacy in treating patients with PsA. bDMARDs are often prescribed in combination with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs); however, there is little evidence to support guidance on when to use biologic monotherapy versus concomitant treatment with csDMARDs. Although no differences in efficacy have been observed between patients treated with biologic drugs with or without methotrexate (MTX), specifically, or any concomitant csDMARD in randomized controlled trials [2–5], registry studies have shown that long-term differences in effectiveness via drug survival may be observed with tumor necrosis factor inhibitors [6–9]. The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) 2015 Treatment Recommendations for Psoriatic Arthritis notes limited data available on combining therapies in PsA and that the use of concomitant MTX with bDMARDs does not appear to improve clinical symptoms beyond bDMARD monotherapy; however, results from registry studies have demonstrated greater drug survival when certain bDMARDs, particularly infliximab, are used with concomitant MTX [10]. The 2018 American College of Rheumatology(ACR)/National Psoriasis Foundation Guideline for the Treatment of Psoriatic Arthritis recommends bDMARD monotherapy over the use of concomitant MTX, noting that concomitant MTX may be advisable if the patient has severe psoriasis, a partial response to current MTX, or uveitis [11]. Concomitant MTX may also be suitable for patients receiving TNFi, particularly infliximab and adalimumab, to lessen immunogenicity [11]. The 2019 European League Against Rheumatism (EULAR) guideline’s stance on concomitant MTX aligns with the above recommendations from ACR [11]. Despite limited and conflicting evidence, MTX is the most common first-line treatment for PsA [12].
Ixekizumab, a specific inhibitor of the IL-17A cytokine, is approved in adults for the treatment of active PsA [13, 14]. In SPIRIT-P1 and SPIRIT-P2 24-week studies, ixekizumab demonstrated efficacy both as monotherapy and with background concomitant csDMARDs [9, 15]. Similar results were observed in a 52-week SPIRIT-P1 and SPIRIT-P2 study when ixekizumab was used as monotherapy or when added to concomitant MTX [16]. In a head-to-head study of ixekizumab versus adalimumab, ixekizumab had consistent efficacy regardless of concomitant MTX while ADA efficacy numerically increased with concomitant MTX [17]. The aim of this integrated analysis was to evaluate the long-term clinical efficacy, inhibition of radiographically assessed progression of structural damage, safety, and immunogenicity of ixekizumab through three years (156 weeks) in patients with active PsA enrolled in SPIRIT-P1 and SPIRIT-P2 according to concomitant csDMARD received in the following subgroups: (1) ixekizumab monotherapy (no concomitant MTX or other csDMARDs), (2) ixekizumab and MTX, (3) ixekizumab and any csDMARD (including MTX).
Materials and methods
Study design
This analysis included integrated data from the SPRIT-P1 (NCT01695239) and SPIRIT-P2 (NCT02349295) [18, 19] multicenter, double-blind, randomized, placebo-controlled, phase 3 trials evaluating the efficacy and safety of ixekizumab in patients with active PsA. In SPIRIT-P1, patients were randomized 1:1:1:1 to receive subcutaneous injections of placebo (data not reported here), adalimumab 40 mg once every two weeks (Q2W) (data not reported here), ixekizumab 80 mg Q2W (data not reported here), or ixekizumab 80 mg once every four weeks (Q4W) for 24 weeks. After week 24, patients receiving ixekizumab remained on their originally assigned dose, and those receiving placebo or adalimumab were re-randomized 1:1 to receive ixekizumab Q2W or Q4W through week 156. In SPIRIT-P2, patients were randomized 1:1:1 to receive subcutaneous injections of placebo (data not reported here), ixekizumab 80 mg Q2W (data not reported here), or ixekizumab 80 mg Q4W for 24 weeks. In both studies at week 16, patients who failed to meet predefined criteria for change in tender joint count (TJC) and swollen joint count (SJC) from baseline were classified as inadequate responders (< 20% improvement from baseline in TJC and SJC) and were administered rescue therapy through week 24 [16]. Patients who failed to demonstrate a ≥ 20% improvement from baseline in both tender joint and swollen joint counts at week 32 or thereafter were discontinued from the studies. Changes in concomitant medications were not allowed from weeks 0 through 24 with the exception of inadequate responders who were administered rescue therapy or patients who changed medication due to safety reasons; changes were allowed after week 24 through week 156. Additional details on the study designs have been published previously [18, 19].
Patients
Patients eligible for SPIRIT-P1 and SPIRIT-P2 were 18 years of age or older with an established diagnosis of PsA for at least 6 months, met Classification for Psoriatic Arthritis criteria, had active psoriatic skin lesions or a documented history of plaque psoriasis, and had active PsA as defined by the presence of at least 3/68 tender and 3/66 swollen joints. bDMARD-naïve patients in SPIRIT-P1 were stratified by csDMARD experience into naïve, past-use, and current-use groups. Patients in SPIRIT-P2 were bDMARD-experienced, were previously treated with ≥ 1 csDMARD, and had an inadequate response (≥ 12 weeks on therapy) or intolerance to 1 or 2 tumor necrosis factor (TNF) alpha inhibitors. Patients must have been on a stable dose of a csDMARD for at least 8 weeks prior to baseline and were not permitted to use more than 1 csDMARD upon study entry. In SPIRIT-P1, radiographs were taken of both hands and feet at screening and were reviewed centrally (by 2 primary readers and an adjudicator when necessary) for evidence of erosive bony changes. As part of the inclusion criteria, patients were required to have at least 1 PsA-related joint erosion on hand or foot radiographs or a C-reactive protein (CRP) level of at least 6 mg/L to be enrolled into SPIRIT-P1.
The maximum allowed doses of concomitant csDMARDs were 25 mg/week for MTX, 400 mg/day for hydroxychloroquine, 20 mg/day for leflunomide, and 3 g/day for sulfasalazine. Simultaneous use of MTX and leflunomide was prohibited for safety reasons. During the double-blind treatment period from weeks 0 to 24, modifying the dose of a concomitant csDMARD and/or the introduction of a new csDMARD was not allowed except for safety reasons or rescue therapy. Lowering or stopping doses of csDMARDs during the double-blind treatment period was allowed if the investigator believed any adverse events or laboratory abnormalities could be attributable to the concomitant csDMARD. During the extension and long-term extension periods from weeks 24 to 52 and weeks 52 to 156, respectively, adjustment of csDMARDs (dose change, introduction, or withdrawal) was allowed, though more than one adjustment of csDMARD at a time within a period of 12 weeks was discouraged. Any changes in concomitant csDMARDs administered were recorded. Additional patient-related details, including blinding, randomization, and other eligibility criteria, have been published previously [18, 19].
Assessments and outcomes
The efficacy and safety of ixekizumab Q4W were evaluated in the following subgroups of patients with active PsA according to the concomitant csDMARD they received: (1) ixekizumab monotherapy (no concomitant MTX or other csDMARDs), (2) ixekizumab and MTX, (3) ixekizumab and any csDMARD (MTX, MTX sodium, sulfasalazine, leflunomide, ciclosporin, hydroxychloroquine, or hydroxychloroquine sulfate), through 156 weeks [20]. Patients in the ixekizumab and MTX subgroup had uninterrupted MTX use (no more than 14 days without using MTX) but were allowed to switch MTX medications and doses. Patients in the ixekizumab and any csDMARD group could have taken concomitant MTX; that is, the ixekizumab and MTX subgroup is a subset of the ixekizumab and any csDMARD subgroup. Categorical outcomes measured include the proportions of patients achieving American College of Rheumatology (ACR) 20/50/70 responses; low disease activity (LDA) as indicated by a score ≤ 14 on the Disease Activity in Psoriatic Arthritis (DAPSA), which is measured by the sum of patient global and pain visual analogue scales (cm), swollen joint count (SJC) of 66 joints, tender joint count (TJC) of 68 joints, and CRP level (mg/dl) [21, 22]; minimal disease activity (MDA), which is achieved if ≥ 5 of the following 7 criteria are met: TJC ≤ 1, SJC ≤ 1, Psoriasis Area and Severity Index (PASI) ≤ 1 or body surface area (BSA) ≤ 3%, patients assessment of pain visual analogue scale (VAS) ≤ 15, patient’s global assessment of disease activity VAS ≤ 20, Health Assessment Questionnaire-Disability Index (HAQ-DI) ≤ 0.5, tender entheseal points ≤ 1; PASI 75/90/100 responses; Nail Psoriasis Severity Index (NAPSI) (0) response; and HAQ-DI improvement from baseline ≥ 0.35 response. Continuous outcomes measured included changes from baseline in NAPSI score and the 36-item Short Form Survey (SF-36) mental and physical functioning domains. For patients in SPIRIT-P1, hand and foot radiographs performed at screening and weeks 52, 108, and 156 were used to evaluate radiographic progression over 3 years. Scoring of radiographs was performed by 2 independent readers, blinded to chronology and clinical data [23]. Structural progression in peripheral joints was measured using the Bone Erosion Score (ES), Joint Space Narrowing (JSN) score, and the van der Heijde modified Total Sharp Score (mTSS), with higher scores indicating greater damage [24]. The initial radiographs obtained at screening served as the baseline radiographs for this analysis.
Safety was evaluated using the incidence of treatment-emergent adverse events (TEAEs) (total, mild, moderate, and severe), serious adverse events (SAEs), adverse events (AEs) leading to discontinuation, and AEs of special interest, which were prespecified. Immunogenicity was evaluated by assessing the number of patients who were positive for treatment-emergent (TE) ADA. Of these patients, neutralizing antibody (Nab) status was also assessed.
Statistical analyses
The post hoc analyses reported here included all patients who were initially randomized to ixekizumab Q4W treatment. Subgroups were comprised of patients treated with ixekizumab who concomitantly received the following treatments from baseline: (1) ixekizumab monotherapy (no concomitant MTX or csDMARDs); (2) ixekizumab and MTX; or (3) ixekizumab and any csDMARD (including MTX). Categorical variables were reported as percentages, and modified non-responder imputation was used to impute missing data. Continuous variables were reported with multiple imputation (MI) used to impute missing data. Radiographic analyses were conducted in patients enrolled in SPIRIT-P1 who received at least 1 dose of the study drug in the long-term extension period starting at week 24. Linear extrapolation was used to impute missing radiographic progression data if patients had baseline and at least one post-baseline value at week 52, 108, or 156. Cumulative probability plots were presented for radiographic progression through 156 weeks. Safety and immunogenicity were summarized using descriptive statistics. The safety analysis population consisted of all randomized patients who received at least 1 dose of study treatment and who were initially randomized to ixekizumab Q4W at week 0. Statistical analyses were performed using SAS® Version 9.2 or higher.
Statement of human and animal rights
The SPIRIT-P1 and SPIRIT-P2 clinical trials followed Good Clinical Practice, the Declaration of Helsinki, and local regulations. Approval was given by each additional site. All patients provided written informed consent before participating in the trials.
Results
Baseline demographics and disease characteristics
Of 229 patients randomized to ixekizumab Q4W treatment in SPIRIT-P1 and SPIRIT-P2, 202 were categorized into one of three subgroups and included in this integrated post hoc analysis. Of 107 patients initially randomized to ixekizumab Q4W treatment in SPIRIT-P1, 97 completed the double-blind treatment period (weeks 0 to 24), and 63 completed the combined extension and long-term extension period (weeks 24 to 156). Of 122 patients initially randomized to ixekizumab Q4W treatment in SPIRIT-P2, 70 completed the double-blind treatment period, and 70 completed the extension period (weeks 24 to 156). The numbers of patients receiving ixekizumab monotherapy, ixekizumab and MTX, and ixekizumab and any csDMARD (including MTX), comprising the 3 treatment subgroups, were 89, 88, and 113, respectively. In the third subgroup of patients receiving any concomitant csDMARD that includes patients receiving MTX, 24 did not receive MTX continuously. Baseline characteristics were similar across the 3 subgroups (Table 1). Of patients treated with ixekizumab and MTX, the mean MTX dose at baseline was 15.7 mg/week. Concomitant medications in the ixekizumab and any csDMARD subgroup included MTX, sulfasalazine, leflunomide, ciclosporin, hydroxychloroquine, and hydroxychloroquine sulfate.
Table 1.
Demographics and baseline disease characteristics of patients from SPIRIT-P1 and SPIRIT-P2 treated with ixekizumab Q4W
| Concomitant background treatment | Ixekizumab monotherapya Ns = 89 |
Ixekizumab + MTX Ns = 88 |
Ixekizumab + any csDMARDb Ns = 113 |
|---|---|---|---|
| Age (years) | 51.1 (11.9) | 51.0 (12.1) | 50.1 (12.4) |
| Male, n (%) | 43 (48.3) | 41 (46.6) | 54 (47.8) |
| Race, n (%) | |||
| White | 86 (96.6) | 80 (90.9) | 103 (91.2) |
| Asian | 1 (1.1) | 3 (3.4) | 5 (4.4) |
| American Indian or Alaska Native | 0 | 2 (2.3) | 2 (1.8) |
| Native Hawaiian or other Pacific Islander | 0 | 1 (1.1) | 1 (0.9) |
| Black or African American | 1 (1.1) | 0 | 0 |
| Multiple | 1 (1.1) | 2 (2.3) | 2 (1.8) |
| Weight, kg | 87.8 (22.7) | 87.4 (20.1) | 87.3 (20.3) |
| BMI, kg/m2 | 30.0 (7.3) | 30.6 (6.7) | 30.5 (6.8) |
| Previous PsA systemic therapy, n (%) | |||
| No prior treatment | 8 (9.0%) | 25 (28.4%) | 29 (25.7%) |
| Non-biologic only | 28 (31.5%) | 23 (26.1%) | 30 (26.5%) |
| TNFi only | 0 | 21 (23.9%) | 24 (21.2%) |
| TNFi and non-biologic | 53 (59.6%) | 19 (21.6%) | 30 (26.5%) |
| Corticosteroid use, n (%) | 13 (14.6) | 12 (13.6) | 14 (12.4) |
| Time since PsA diagnosis, years | 17.5 (13.8) | 14.6 (12.8) | 14.8 (12.7) |
| Baseline disease characteristics | |||
| n = 88 | n = 88 | n = 113 | |
| Tender joints (68 assessed) | 22.0 (13.7) | 20.7 (14.8) | 21.0 (14.4) |
| n = 88 | n = 88 | n = 113 | |
| Swollen joints (66 assessed) | 12.2 (8.6) | 11.6 (10.6) | 12.3 (11.2) |
| n = 89 | n = 88 | n = 113 | |
| LEI > 0, n (%) | 49 (55.1) | 54 (61.4) | 67 (59.3) |
| n = 87 | n = 87 | n = 110 | |
| LDI-B > 0, n (%) | 24 (27.6) | 26 (29.9) | 35 (31.8) |
| n = 87 | n = 82 | n = 107 | |
| PASI | 7.4 (8.7) | 6.4 (6.3) | 6.2 (6.2) |
| n = 60 | n = 65 | n = 78 | |
| NAPSIc | 24.2 (22.7) | 17.9 (16.3) | 18.7 (16.2) |
| n = 89 | n = 83 | n = 106 | |
| % BSA | 13.3 (18.4) | 14.0 (15.8) | 13.6 (15.7) |
| SF-36 | n = 87 | n = 85 | n = 110 |
| PCS | 32.5 (9.6) | 32.6 (9.5) | 32.7 (9.5) |
| MCS | 47.3 (13.6) | 46.4 (12.5) | 46.6 (12.3) |
| Baseline radiographic scoresd | n1/N1 = 23/36 | n1/N1 = 32/48 | n1/N1 = 41/59 |
| ESd | 9.7 (13.6) | 10.8 (17.4) | 11.0 (17.0) |
| JSNd | 6.7 (13.3) | 8.4 (19.7) | 8.5 (18.4) |
| mTSSd | 16.5 (26.8) | 19.2 (36.4) | 19.5 (34.8) |
Values are mean (SD) unless otherwise indicated
All patients were initially randomized to ixekizumab
aPatients receiving no MTX or other csDMARDs
bPatients receiving any csDMARD, including MTX
cPatients initially randomized to ixekizumab with fingernail involvement at baseline
dPatients from the SPIRIT-P1 trial
BSA, body surface area; csDMARD, conventional synthetic disease-modifying antirheumatic drug; ES, Bone Erosion Score; IXE, ixekizumab; JSN, Joint Space Narrowing score; LDI-B, Leeds Dactylitis Index-Basic; LEI, Leeds Enthesitis Index; MCS; mental component score; mTSS, modified Total Sharp Score; MTX, methotrexate; Ns, number of patients in the treatment subgroup; N1, number of patients in the specified treatment subgroup from SPIRIT-P1; n, number of patients in the specified category; n1, number of patients in the specified category from SPIRIT-P1; NAPSI, Nail Psoriasis Severity Index; PCS, physical component score; PASI, Psoriasis Area and Severity Index; Q4W, every 4 weeks; SD, standard deviation; SF-36, 36-Item Short Form Health Survey; TNFi, tumor necrosis factor inhibitor
Responses and changes from baseline in composite measures
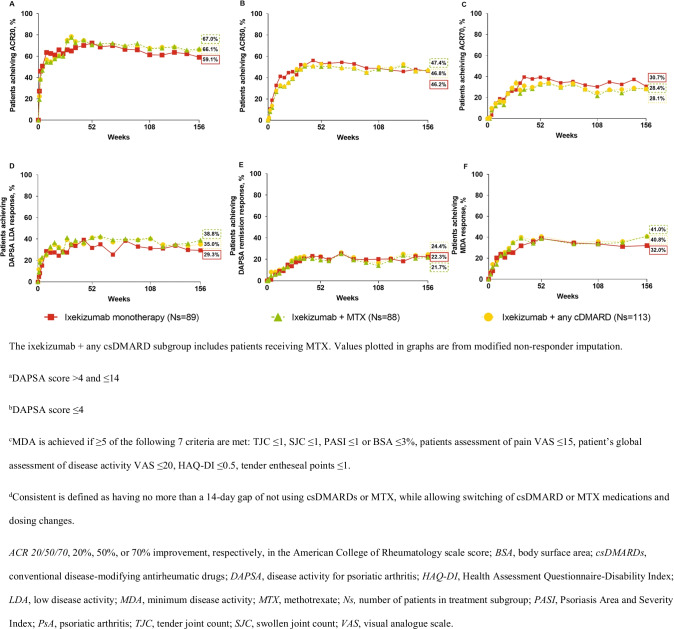
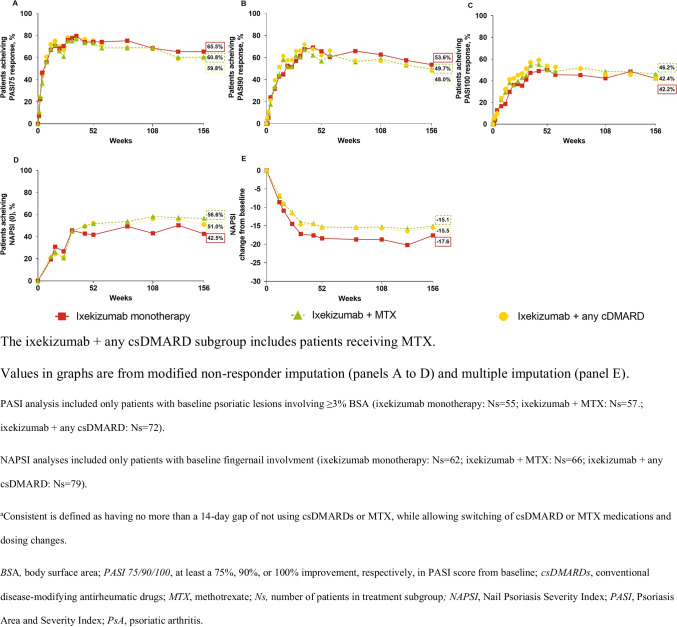
Improvement in signs and symptoms of PsA in patients treated with ixekizumab was observed through week 156 regardless of whether ixekizumab was used as monotherapy or with concomitant MTX or other csDMARDs as assessed by ACR 20/50/70 responses (Fig. 1A–C). Similar results were observed for DAPSA LDA and remission responses and MDA responses (Fig. 1D–F). Psoriasis severity, as measured by PASI 75/90/100 responses, and fingernail involvement, as measured by NAPSI (0) response and NAPSI change from baseline, improved through week 156 whether ixekizumab was used as monotherapy or with concomitant MTX or other csDMARDs (Fig. 2A–D). With respect to the quality of life as measured by changes from baseline (MI analysis) in SF-36 PCS and MCS and in functional disability as measured by improvement from baseline of at least 0.35 in HAQ-DI, benefits were observed whether ixekizumab was used as monotherapy or with MTX or other csDMARDs through week 156 regardless of concomitant csDMARD use (Suppl. Fig. 1A–C).
Fig. 1.
Clinical response and disease control. A ACR20, B ACR50, C ACR70, D DAPSA LDAa, E DAPSA remissionb, and F MDAc % response in patients with PsA and treatment with ixekizumab Q4W and either ixekizumab monotherapy or consistentd concomitant MTX or any csDMARD (including MTX) through three years (156 weeks)
Fig. 2.
Psoriasis skin lesions and nail involvement. A PASI 75, B PASI 90, C PASI 100, D NAPSI (0) % response, and E NAPSI mean change from baseline in patients with PsA and treatment with ixekizumab Q4W and either ixekizumab monotherapy or consistenta concomitant MTX or any csDMARD (including MTX) through three years (156 weeks)
Radiographic progression
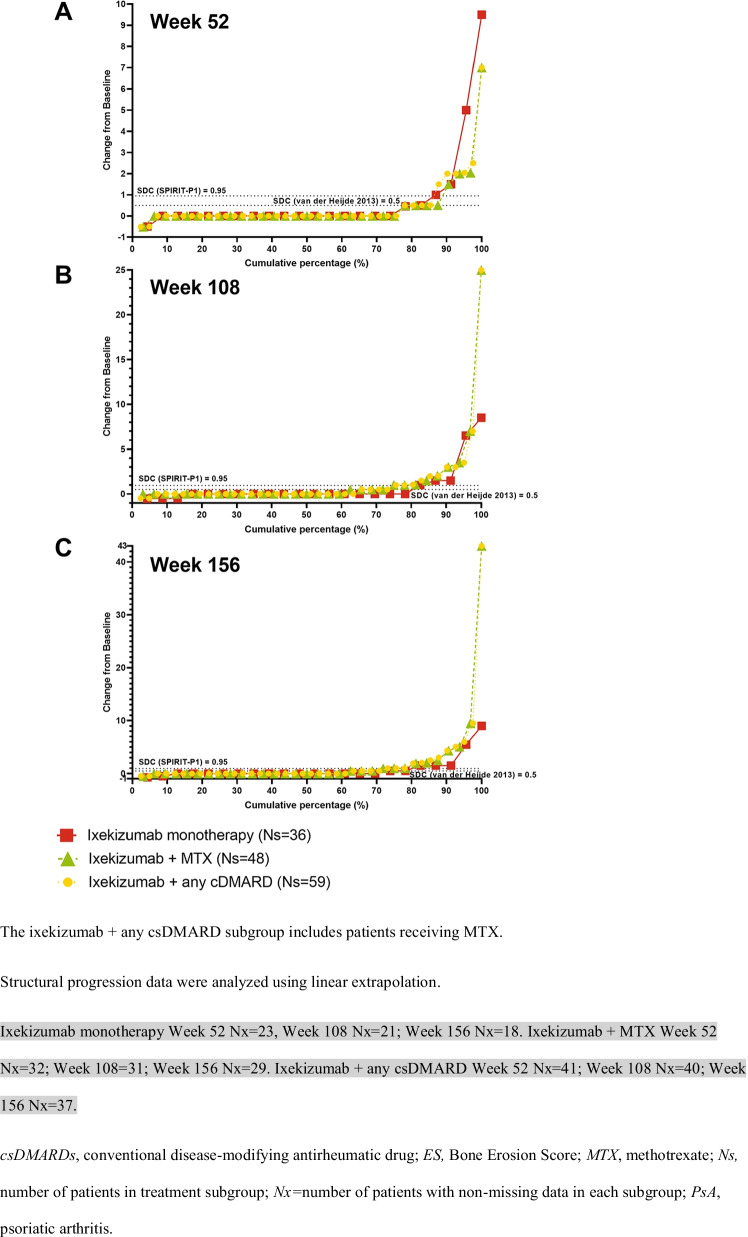
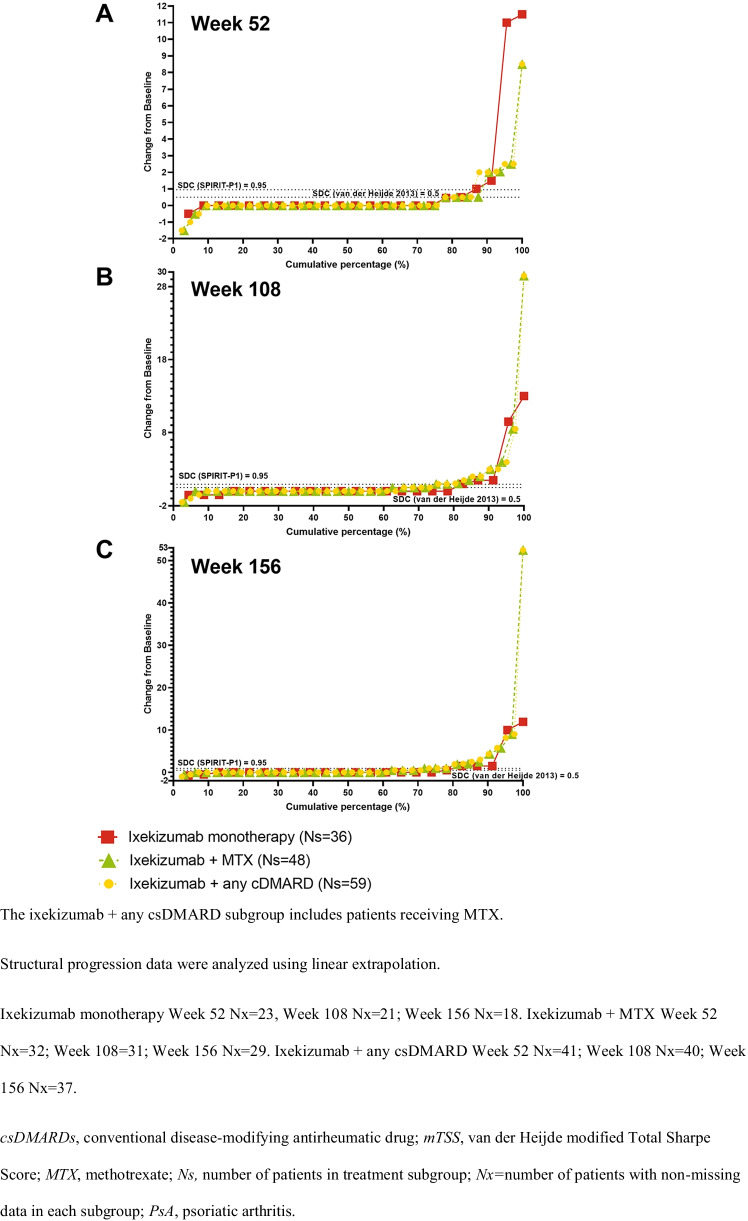
Radiographic progression of structural joint damage was assessed in the SPIRIT-P1 trial only. Changes from baseline in ES, JSN, and mTSS were similar across the three subgroups through 156 weeks with several notable outliers who had significant damage at baseline (Suppl. Table 2).
Figure 3, Supplemental Fig. 3, and Fig. 4 show cumulative probability plots for changes in baseline in Bone ES, JSN, and mTSS, which illustrate the impact of the outlier scores of several patients in the ixekizumab and MTX (only) and ixekizumab and csDMARDs (any) subgrouTwo patients receiving ixekizumab monotherapy and one patient receiving ixekizumab and MTX (all enrolled in SPIRIT-P1) experienced continued worsening of ES, JSN, and mTSS scores at 1 year and through 3 years despite treatment. All three patients shared higher TJC and SJC counts at baseline, and one patient had comorbid osteopenia. Mean baseline TJC and SJC for the 3 treatment subgroups ranged from 20.7 to 22.0 and 11.6 to 12.3, respectively, and mean baseline ES, JSN, and mean mTSS scores ranged from 9.7 to 11.0, 6.7 to 8.5, and 16.5 to 19.5, respectively (Table 1). In comparison, the baseline TJC and SJC scores for the patients who were outliers ranged from 25 to 32 and 12 to 33, respectively. The baseline ES, JSN, and mTSS scores for the patients who were outliers ranged from 27.5 to 53, 22 to 46.4, and 49.5 to 99.9, respectively.
Fig. 3.
Cumulative probability of change from baseline in structural joint damage as measured by ES in patients from SPIRIT-P1 with PsA and treatment with ixekizumab Q4W as monotherapy or with concomitant MTX or any csDMARD (including MTX) at A 52, B 108, and C 156 weeks
Fig. 4.
Cumulative probability of change from baseline in structural joint damage as measured by mTSS in patients from SPIRIT-P1 with PsA and treatment with ixekizumab Q4W as monotherapy or with concomitant MTX or any csDMARD (including MTX) at A 52, B 108, and C 156 weeks
Safety
Most TEAEs were mild or moderate in severity (Table 2). Similar proportions of patients experienced at least one TEAE across all ixekizumab treatment subgroups, though incidence rates (IRs) of moderate TEAEs were numerically higher for patients with ixekizumab monotherapy than those with ixekizumab and concomitant MTX or any csDMARD, Incidence rates (IRs) of SAEs were also similar across the subgroups. Rates of discontinuation due to AEs were numerically higher for patients receiving ixekizumab monotherapy compared to those receiving ixekizumab and MTX or ixekizumab and any csDMARD (Table 2). IRs of infections were numerically higher for patients receiving ixekizumab monotherapy compared to the other two subgroups; however, IRs of serious infections were similar across the three subgroups. Injection site reactions were also numerically higher for patients receiving ixekizumab monotherapy compared to the other subgroups. Through three years, IRs of infections and injection site reactions in patients receiving ixekizumab monotherapy decreased year by year, and most of these adverse events were mild in severity.
Table 2.
Safety overview after 156 weeks of treatment with ixekizumab Q4W according to concomitant csDMARD or MTX use (incidence rates per 100 PY)
| Parameter | Ixekizumab monotherapya Ns = 89 |
Ixekizumab + MTX Ns = 88 |
Ixekizumab + any csDMARDb Ns = 113 |
|||
|---|---|---|---|---|---|---|
| Total PY | 188.3 | 201.1 | 256.0 | |||
| n (%) | IR | n (%) | IR | n (%) | IR | |
| TEAEs (≥ 1) | 81 (91.0) | 43.0 | 74 (84.1) | 36.8 | 94 (83.2) | 36.7 |
| Mild | 25 (28.1) | 13.3 | 27 (30.7) | 13.4 | 33 (29.2) | 12.9 |
| Moderate | 49 (55.1) | 26.0 | 38 (43.2) | 18.9 | 51 (45.1) | 19.9 |
| Severe | 7 (7.9) | 3.7 | 9 (10.2) | 4.5 | 10 (8.8) | 3.9 |
| SAEs | 13 (14.6) | 6.9 | 12 (13.6) | 6.0 | 13 (11.5) | 5.1 |
| Discontinuations due to AE | 11 (12.4) | 5.8 | 6 (6.8) | 3.0 | 11 (9.7) | 4.3 |
| AEs of special interest | 72 (80.9) | 38.2 | 64 (72.7) | 31.8 | 81 (71.7) | 31.6 |
| Infections | 67 (75.3) | 35.6 | 47 (53.4) | 23.4 | 61 (54.0) | 23.8 |
| Nasopharyngitis | 22 (24.7) | 11.7 | 8 (9.1) | 4.0 | 11 (9.7) | 4.3 |
| Upper respiratory tract infection | 18 (20.2) | 9.6 | 12 (13.6) | 6.0 | 17 (15.0) | 6.6 |
| Sinusitis | 8 (9.0) | 4.2 | 7 (8.0) | 3.5 | 8 (7.1) | 3.1 |
| Bronchitis | 7 (7.9) | 3.7 | 5 (5.7) | 2.5 | 9 (8.0) | 3.5 |
| Serious infections | 3 (3.4) | 1.6 | 3 (3.4) | 1.5 | 3 (2.7) | 1.2 |
| Serious Candida infection | 1 (1.1) | 0.5 | – | – | – | – |
| Serious latent tuberculosis | 1 (1.1) | 0.5 | – | – | – | – |
| Serious pneumonia | 1 (1.1) | 0.5 | 2 (2.3) | 1.0 | 2 (1.8) | 0.8 |
| Serious gastroenteritis | – | – | 1 (1.1) | 0.5 | 1 (0.9) | 0.4 |
| Injection-site reactions | 19 (21.3) | 10.1 | 11 (12.5) | 5.5 | 16 (14.2) | 6.3 |
| Hepatic events | 8 (9.0) | 4.2 | 6 (6.8) | 3.0 | 8 (7.1) | 3.1 |
| Allergic reactions/hypersensitivities | 7 (7.9) | 3.7 | 4 (4.5) | 2.0 | 8 (7.1) | 3.1 |
| Non-anaphylaxis | 7 (7.9) | 3.7 | 4 (4.5) | 2.0 | 8 (7.1) | 3.1 |
| Depression | 3 (3.4) | 1.6 | 5 (5.7) | 2.5 | 6 (5.3) | 2.3 |
| Malignancies | 3 (3.4) | 1.6 | 1 (1.1) | 0.5 | 2 (1.8) | 0.8 |
| Cerebrocardiovascular events | 1 (1.1) | 0.5 | 1 (1.1) | 0.5 | 1 (0.9) | 0.4 |
| Inflammatory bowel disease | – | – | – | – | – | – |
aPatients receiving no MTX or other csDMARDs
bPatients receiving any csDMARD, including MTX
AE, adverse event; csDMARDs, conventional disease-modifying anti-rheumatic drugs; CI, confidence interval; IXE, ixekizumab; IR, incidence rate; PY, patient years; Q4W, every 4 weeks; SAE, serious adverse event; MTX, methotrexate; Ns, number of patients in treatment subgroup; n, number of patients in specified category; TEAE, treatment-emergent adverse event
Immunogenicity
A numerically greater proportion of patients receiving ixekizumab monotherapy (15.9%) were TE-ADA positive compared to those receiving ixekizumab and MTX (13.1%) or ixekizumab and any csDMARD (11.0%) (Supplemental Table 1). Of patients who were TE-ADA positive, most had low titer status (92.9%, 90.9%, and 91.7% in the ixekizumab monotherapy, ixekizumab and MTX, and ixekizumab and any csDMARD subgroups, respectively). Of patients who were TE-ADA positive, 35.7%, 27.3%, and 25.0%, in the three treatment subgroups, respectively, had positive Nab status.
Discussion
The analyses reported here show that ixekizumab improves signs and symptoms of PsA, including manifestations of psoriasis, and quality of life in patients with active PsA up to 156 weeks, whether used as monotherapy or with concomitant MTX or other csDMARDs. These results confirm and extend previous 24- and 52-week analyses [9, 15, 16], showing consistent long-term efficacy of ixekizumab with or without concomitant therapy with MTX or other csDMARDs.
We assessed the radiographic progression of structural joint damage by the mean change from baseline to weeks 52, 108, and 156 in ES, JSN, and mTSS. For the majority of patients (~ 85%), the changes from baseline were similar across the three treatment groups through 156 weeks (Fig. 3, Suppl. Fig. 2, and Fig. 4). It is important to keep in mind that there were outliers who could have influenced the scores. Three patients receiving ixekizumab monotherapy had outlying ES, JSN, and mTSS scores at 156 weeks compared to the mean; these patients also had higher-than-average ES, JSN, and mTSS scores (all 3 with baseline mTSS scores > 40) as well as TJC and SJC at baseline. Previously described low rates of radiographic progression persisted with up to three years of ixekizumab treatment regardless of the addition of background MTX or csDMARD [23].
The overall safety profile presented here is consistent with previously published ixekizumab safety analyses in patients with PsA [18, 19]. All three ixekizumab treatment subgroups had similar safety findings. Similar frequencies of patients in the three subgroups had at least one treatment-emergent adverse event. Even though this post hoc analysis was not powered to evaluate differences in safety between the groups, numerical differences in frequencies of infections and injection site reactions were present, with patients receiving ixekizumab monotherapy reporting higher rates of these adverse events than those receiving ixekizumab and MTX (only) or ixekizumab and csDMARD (any). Because the concomitant treatment subgroups were not randomized, these differences in frequencies of infections and injection site reactions could be due to bias as those at high risk of infections may stop csDMARD use. Additionally, larger studies may be needed to evaluate the frequencies of these TEAEs in these subgroups of patients.
Immunogenicity against biologics is generally understood to be mitigated by treatment with concomitant immunosuppressants, such as MTX and other csDMARDs [25]; however, concomitant MTX for the sole purpose of preventing or lessening immunogenicity is not currently recommended in psoriasis [26]. While patients receiving ixekizumab monotherapy did have numerically higher ADA compared to those receiving ixekizumab and MTX and ixekizumab and any csDMARD, these differences were very small and not thought to be of clinical consequence. Previous 52-week results from SPIRIT-H2H, a study of ixekizumab versus adalimumab, are consistent with the findings from the present 156-week analysis of bDMARD-naïve and TNFi-experienced patients with PsA enrolled in SPIRIT-P1 and SPIRIT-P2, showing that treatment with ixekizumab demonstrated consistent efficacy with and without concomitant csDMARDs, including MTX [17].
This post hoc analysis was limited as it used RCT data and did not address data from patients in the real world. Because this analysis evaluated efficacy and safety through 3 years, it is potentially biased towards data from patients who remained in the study long term. Subgroup sample sizes were also small. In addition, there were no placebo arms during the long-term extension periods of SPIRIT-P1 and SPIRIT-P2. Radiographic progression of structural joint damage was only assessed in SPIRIT-P1 (enrolling bDMARD-naïve patients) and not in SPIRIT-P2 (enrolling TNFi-experienced patients), restricting the applicability of these results to bDMARD-naïve patients only. In addition, radiographic progression of structural joint damage was only assessed to week 24, limiting our ability to confirm the week 52 results from this study. Safety results were limited by small numbers of patients in the treatment groups, which could have impacted the mean scores for the safety assessments. Safety results were also limited by the possibility of bias by indication, as those at high risk of infections may stop csDMARD use.
This analysis’s strengths include a long treatment duration through 156 weeks as well as the inclusion of data from two clinical trials evaluating both bDMARD-naïve and TNFi-experienced patient populations (with the exception of the radiographic progression analyses, which were only performed on data from bDMARD-naïve patients). In addition, this analysis evaluated immunogenicity, including detecting the presence or absence of Nab, to ixekizumab treatment with or without concomitant MTX or csDMARDs in the post-baseline period through 156 weeks.
In conclusion, this post hoc analysis supports that ixekizumab can offer similar efficacy and safety profiles whether it is used as monotherapy or as combination therapy with MTX or other csDMARDs. More dedicated studies are needed to understand the radiographic progression findings. The safety profile for ixekizumab is similar to what has been previously reported with no new safety signals. The proportions of patients who had ADAs and Nab in the post-baseline period through 156 weeks were small and similar in patients receiving ixekizumab with or without concomitant MTX or csDMARDs. These data suggest that there are no additional clinical or radiographic benefits to concomitant MTX or other csDMARD use in this post hoc analysis, supporting the use of ixekizumab monotherapy.
Supplementary Information
(DOCX 531 kb)
Acknowledgements
The authors thank Nicole Lipitz and Lydia Morris, PhD, employees of Syneos Health, for writing and editorial support. LCC is funded by a National Institute for Health Research Clinician Scientist award. The work was supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Author contribution
Laura C. Coates, Philip Mease, Andris Kronbergs, and Bernard Combe contributed to the interpretation of data for the work and critical revision of the manuscript for important intellectual content. Cameron Helt contributed to the conception of the work, analysis of data for the work, interpretation of data for the work, and critical revision of the manuscript for important intellectual content. David Sandoval contributed to the conception of the work, interpretation of data for the work, and critical revision of the manuscript for important intellectual content. So Young Park contributed to the analysis of data for the work, drafting of the manuscript, and critical revision of the manuscript for important intellectual content. Peter Nash and Atul Deodhar contributed to the acquisition of data for the work, interpretation of data for the work, and critical revision of the manuscript for important intellectual content.
Funding
This study was funded by Eli Lilly and Company. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
LCC has received research grants from AbbVie, Boehringer-Ingelheim, BMS, Eli Lilly, Janssen, and Novartis; consultancies with AbbVie, Amgen, Boehringer-Ingelheim, Eli Lilly, Janssen, Novartis, and Pfizer; and has been a speaker for AbbVie, Amgen, Boehringer-Ingelheim, Celltrion, Eli Lilly and Company, Janssen, Novartis, Pfizer, and Sandoz. PM has received research grants from AbbVie, Amgen, BMS, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, Sun, and UCB; consultancies with AbbVie, Amgen, Boehringer Ingelheim, BMS, Eli Lilly, Galapagos, Gilead, GSK, Janssen, Novartis, Pfizer, Sun, and UCP; and has been a speaker for AbbVie, Amgen, Eli Lilly, Janssen, Novartis, Pfizer, and UCB. AK, KS CH, DS, and SYP report employment and stock ownership with Eli Lilly. BC has received research grants from AbbVie, Eli Lilly, Novartis, and Pfizer; consultancies with AbbVie, BMS, Galapagos, and Eli Lilly; has been an advisor for AbbVie, Galapagos, Celltrion, Chugai, Eli Lilly, Janssen, Novartis, and Chugai; and has been a speaker for AbbVie, BMS, Galapagos, Celltrion, Chugai, Eli Lilly, Merck, Novartis, and Pfizer. PN has received funding for clinical trials, research grants, and honoraria for lectures and advice from AbbVie, Amgen, BMS, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, Roche, Sanofi-Aventis, and UCB. AD reports receiving research grants and/or honoraria for consulting or speaking from AbbVie, Amgen, Aurinia, Celgene, Eli Lilly, GSK, Janssen, MoonLake, Novartis, Pfizer, and UCB.
Data availability
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank, or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
Declarations
Ethical approval
Protocols and consent forms were approved by each site’s institutional review board or ethics committee, which included the Western Institutional Review Board (SPIRIT-P1) and the Bellberry Human Research Ethics Committee (SPIRIT-P2).
Informed consent
SPIRIT-P1 and SPIRIT-P2 were conducted in accordance with the ethical principles of the Declaration of Helsinki as well as local laws and regulations. Patients’ written informed consent was received for participation in SPIRIT-P1 and SPIRIT-P2. Patients’ written informed consent was also received for publication of results from these trials.
Footnotes
The original online version of this article was revised: Retrospective open access
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
7/11/2022
A Correction to this paper has been published: 10.1007/s10067-022-06271-3
References
- 1.FitzGerald O, et al. Psoriatic arthritis. Nat Rev Dis Primers. 2021;7(1):59. doi: 10.1038/s41572-021-00293-y. [DOI] [PubMed] [Google Scholar]
- 2.Antoni C, et al. Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann Rheum Dis. 2005;64(8):1150–1157. doi: 10.1136/ard.2004.032268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mease PJ, et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a Phase 3 double-blind randomised placebo-controlled study (RAPID-PsA) Ann Rheum Dis. 2014;73(1):48–55. doi: 10.1136/annrheumdis-2013-203696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mease PJ, et al. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2005;52(10):3279–3289. doi: 10.1002/art.21306. [DOI] [PubMed] [Google Scholar]
- 5.Mease PJ, et al. Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum. 2004;50(7):2264–2272. doi: 10.1002/art.20335. [DOI] [PubMed] [Google Scholar]
- 6.Glintborg B, et al. Impact of different infliximab dose regimens on treatment response and drug survival in 462 patients with psoriatic arthritis: results from the nationwide registries DANBIO and ICEBIO. Rheumatology (Oxford) 2014;53(11):2100–2109. doi: 10.1093/rheumatology/keu252. [DOI] [PubMed] [Google Scholar]
- 7.Glintborg B, et al. Treatment response, drug survival, and predictors thereof in 764 patients with psoriatic arthritis treated with anti-tumor necrosis factor α therapy: results from the nationwide Danish DANBIO registry. Arthritis Rheum. 2011;63(2):382–390. doi: 10.1002/art.30117. [DOI] [PubMed] [Google Scholar]
- 8.Fagerli KM, et al. The role of methotrexate co-medication in TNF-inhibitor treatment in patients with psoriatic arthritis: results from 440 patients included in the NOR-DMARD study. Ann Rheum Dis. 2014;73(1):132–137. doi: 10.1136/annrheumdis-2012-202347. [DOI] [PubMed] [Google Scholar]
- 9.Nash P, et al. Ixekizumab is efficacious when used alone or when added to conventional synthetic disease-modifying antirheumatic drugs (cDMARDs) in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor inhibitors. RMD Open. 2018;4(2):e000692. doi: 10.1136/rmdopen-2018-000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coates LC, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 Treatment Recommendations for Psoriatic Arthritis. Arthritis Rheumatol. 2016;68(5):1060–1071. doi: 10.1002/art.39573. [DOI] [PubMed] [Google Scholar]
- 11.Singh JA, et al. Special Article: 2018 American College of Rheumatology/National Psoriasis Foundation Guideline for the Treatment of Psoriatic Arthritis. Arthritis Care Res (Hoboken) 2019;71(1):2–29. doi: 10.1002/acr.23789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coates LC, et al. Methotrexate in psoriasis and psoriatic arthritis. J Rheumatol Suppl. 2020;96:31–35. doi: 10.3899/jrheum.200124. [DOI] [PubMed] [Google Scholar]
- 13.Liu L, et al. Generation and characterization of ixekizumab, a humanized monoclonal antibody that neutralizes interleukin-17A. J Inflamm Res. 2016;9:39–50. doi: 10.2147/jir.s100940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taltz (ixekizumab) [package insert]. (2021) Indianapolis, IN: Eli Lilly and Company
- 15.Coates LC, et al. Ixekizumab efficacy and safety with and without concomitant conventional disease-modifying antirheumatic drugs (cDMARDs) in biologic DMARD (bDMARD)-naïve patients with active psoriatic arthritis (PsA): results from SPIRIT-P1. RMD Open. 2017;3(2):e000567. doi: 10.1136/rmdopen-2017-000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Combe B, et al. Ixekizumab, with or without concomitant methotrexate, improves signs and symptoms of PsA: week 52 results from Spirit-P1 and Spirit-P2 studies. Arthritis Res Ther. 2021;23(1):41. doi: 10.1186/s13075-020-02388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smolen JS, et al. Efficacy and safety of ixekizumab with or without methotrexate in biologic-naïve patients with psoriatic arthritis: 52-week results from SPIRIT-H2H study. Rheumatol Ther. 2020;7(4):1021–1035. doi: 10.1007/s40744-020-00250-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mease PJ, et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis. 2017;76:79–87. doi: 10.1136/annrheumdis-2016-209709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nash P, et al. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet. 2017;389(10086):2317–2327. doi: 10.1016/s0140-6736(17)31429-0. [DOI] [PubMed] [Google Scholar]
- 20.Coates LC, et al. SAT0410 Efficacy and safety of ixekizumab in patients with active psoriatic arthritis based on concomitant conventional disease-modifying antirheumatic drugs (cDMARD) use: results from Spirit-P1 and Spirit-P2. Ann Rheum Dis. 2020;79(Suppl 1):1157–1158. doi: 10.1136/annrheumdis-2020-eular.3994. [DOI] [Google Scholar]
- 21.Mease PJ. Measures of psoriatic arthritis: Tender and Swollen Joint Assessment, Psoriasis Area and Severity Index (PASI), Nail Psoriasis Severity Index (NAPSI), Modified Nail Psoriasis Severity Index (mNAPSI), Mander/Newcastle Enthesitis Index (MEI), Leeds Enthesitis Index (LEI), Spondyloarthritis Research Consortium of Canada (SPARCC), Maastricht Ankylosing Spondylitis Enthesis Score (MASES), Leeds Dactylitis Index (LDI), Patient Global for Psoriatic Arthritis, Dermatology Life Quality Index (DLQI), Psoriatic Arthritis Quality of Life (PsAQOL), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), Psoriatic Arthritis Response Criteria (PsARC), Psoriatic Arthritis Joint Activity Index (PsAJAI), Disease Activity in Psoriatic Arthritis (DAPSA), and Composite Psoriatic Disease Activity Index (CPDAI) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S64–85. doi: 10.1002/acr.20577. [DOI] [PubMed] [Google Scholar]
- 22.Schoels MM, et al. Disease activity in psoriatic arthritis (PsA): defining remission and treatment success using the DAPSA score. Ann Rheum Dis. 2016;75(5):811–818. doi: 10.1136/annrheumdis-2015-207507. [DOI] [PubMed] [Google Scholar]
- 23.Chandran V, et al. Ixekizumab treatment of biologic-naïve patients with active psoriatic arthritis: 3-year results from a phase III clinical trial (SPIRIT-P1) Rheumatology (Oxford) 2020;59(10):2774–2784. doi: 10.1093/rheumatology/kez684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol. 2000;27(1):261–263. [PubMed] [Google Scholar]
- 25.Thomas SS, et al. Comparative immunogenicity of TNF inhibitors: impact on clinical efficacy and tolerability in the management of autoimmune diseases. Syst Rev Meta-Analysis BioDrugs. 2015;29(4):241–258. doi: 10.1007/s40259-015-0134-5. [DOI] [PubMed] [Google Scholar]
- 26.Jullien D, Prinz JC, Nestle FO. Immunogenicity of biotherapy used in psoriasis: the science behind the scenes. J Invest Dermatol. 2015;135(1):31–38. doi: 10.1038/jid.2014.295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 531 kb)
Data Availability Statement
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank, or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.