Abstract
Background
Decades of debate surround the use of intraoperative cholangiography (IOC) during cholecystectomy. To the present day, the role of IOC is controversial as regards decreasing the rate of bile duct injury (BDI). We aimed to review and analyse the available literature on the benefits of IOC during cholecystectomy.
Methods
A systematic literature search was performed until 19 October 2020 in five databases using the following search keys: cholangiogra* and cholecystectomy. The primary outcomes were BDI and retained stone rate. To investigate the differences between the groups (routine IOC vs selective IOC and IOC vs no IOC), we calculated weighted mean differences (WMD) for continuous outcomes and relative risks (RR) for dichotomous outcomes, with 95% confidence intervals (CI).
Results
Of the 19,863 articles, 38 were selected and 32 were included in the quantitative synthesis. Routine IOC showed no superiority compared to selective IOC in decreasing BDI (RR = 0.91, 95% CI 0.66; 1.24). Comparing IOC and no IOC, no statistically significant differences were found in the case of BDI, retained stone rate, readmission rate, and length of hospital stay. We found an increased risk of conversion rate to open surgery in the no IOC group (RR = 0.64, CI 0.51; 0.78). The operation time was significantly longer in the IOC group compared to the no IOC group (WMD = 11.25 min, 95% CI 6.57; 15.93).
Conclusion
Our findings suggest that IOC may not be indicated in every case, however, the evidence is very uncertain. Further good quality research is required to address this question.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00464-022-09267-x.
Keywords: Cholecystectomy, Laparoscopic cholecystectomy, Cholangiography, IOC, Bile duct injury, BDI
Cholecystectomy is one of the most frequently performed surgical interventions. With the advent of laparoscopy, laparoscopic cholecystectomy (LC) became the “gold standard” for the treatment of cholecystolithiasis. It has undeniable advantages over open cholecystectomy: reduced postoperative morbidity, mortality and length of hospital stay, and lower rate of pneumonia and wound infection [1]. Like any surgical intervention, LC carries a risk of complications. Major complications include bile duct injury (BDI), bowel perforation, and vascular injury. Although rare, with an incidence of 0.3% to 0.5%, BDI is a very serious complication of LC [2, 3]. BDI is associated with higher postoperative mortality, morbidity, and decreased quality of life [4]. Several guidelines, meta-analyses, and systematic reviews have been published aiming to provide recommendations for prevention of BDI [4–13]. One of the most investigated methods is intraoperative cholangiography (IOC), but various alternatives (e.g. critical view of safety, laparoscopic ultrasound, and fluorescent cholangiography) have been examined. However, the quality of evidence is low in most of the cases. Most of the literature agrees that IOC has its place in daily practice. Still, due to the low level of evidence and several contradictory articles [14–18], experts have reached no solid consensus.
Advocates of IOC argue that its use during cholecystectomy may reduce the risk of BDI by delineating unclear or aberrant biliary anatomy, aid in intraoperative BDI detection [14, 19], and facilitate perioperative bile duct stone detection, thus decreasing the rate of readmission for retained common bile duct (CBD) stones [13]. Some studies have found that intraoperative detection and treatment of BDI have a beneficial effect on morbidity and mortality [10, 17, 20]. Advocates of IOC therefore suggest a routine combination of LC and IOC. Opponents of IOC argue that when performed routinely, it increases the intraoperative detection of previously asymptomatic bile duct stones. This may result in unnecessary management of CBD stones since only a small percentage of preoperatively asymptomatic and intraoperatively missed bile duct stones became symptomatic after surgery [21]. Further, IOC is a time-consuming procedure, and both staff and patients are exposed to radiation [14]. Hence opponents suggest the omission of IOC.
Some authors support the idea of selective IOC arguing that most CBD stones can be detected preoperatively, and the incidence of BDI is low. [22, 23] IOC may therefore not be necessary routinely, except when CBD stones are suspected or in patients at high risk of BDI.
This systematic review and meta-analysis aims to revise the available literature, thereby providing a comprehensive summary of the topic and giving a better understanding of the necessity of IOC. We identified relevant publications to examine the benefits of routine use, selective use, and omission of IOC during cholecystectomy, and to compare these strategies, especially in terms of BDI and prevention of CBD stone-related complications.
Materials and methods
We report our systematic review and meta-analysis in line with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) Statement [24]. Our systematic review and meta-analysis protocol was previously submitted to PROSPERO under the registration number CRD42021240405. Besides analyses declared in the protocol, we performed a subgroup analysis, including only randomized control trials (RCT) and prospective studies investigating bile duct injury (BDI).
Search strategy
A systematic literature search was conducted up until 19 October 2020 in Embase, MEDLINE (via PubMed), the Cochrane Central Register of Controlled Trials (CENTRAL), Scopus, and Web of Science using the following search keys: cholangiogra* and cholecystectomy. We searched in all fields/all texts in every database, except in Scopus where the “Article title, Abstract, Keywords” fields were used. We did not apply any filters (e.g. language).
Selection strategy and eligibility criteria
Software and manual duplicate removal were performed using Endnote X9 (Clarivate Analytics, Philadelphia, PA, USA). The selection process was carried out by two independent authors (BN and NK). The selection process was conducted in stages of selection by title, abstract, and full text. After each step of the selection process, Cohen’s kappa coefficient was calculated to determine the agreement between the two researchers. We removed all unrelated titles, abstracts, and full texts. No report was excluded based on the follow-up period; we only used studies where the follow-up periods were equal or similar for the quantitative synthesis. Grey literature was excluded from our review. Disagreements were resolved by consensus.
The PICO framework was used to define the eligibility criteria. We included articles where the population (P) consisted of laparoscopic cholecystectomy or a mixed population of open and laparoscopic cholecystectomies. Three intervention (I)/comparison (C) groups were formed based on the available literature: IOC vs no IOC, routine IOC vs selective IOC, and selective IOC vs no IOC. All the articles were selected and analysed in which IOC vs no IOC strategy was compared. In the routine IOC group, all the patients received a cholangiography during the cholecystectomy. We considered selective IOC if patients were selected for IOC based on prespecified criteria (clinical, laboratory, or imaging findings). As regards study type, only RCTs and observational studies were considered eligible.
Outcomes
These groups were examined according to primary outcomes (rate of perioperative bile duct injury and retained stone rate) and secondary outcomes (readmission rate, rate of conversion from laparoscopic procedure to open surgery, the success rate of IOC, operation time (minutes), and length of hospital stay (days)).
BDI was defined as “any tissue damage to the biliary system as a result of surgery”. Retained stones were defined as missed bile duct stones during cholecystectomy that were discovered postoperatively.
Subgroup analysis
We performed the following subgroup analyses [1]: studies comprising laparoscopic cholecystectomy cases exclusively [2], prospective studies reporting on BDI, and [3] studies involving major bile duct injury (MBDI). We defined MBDI as an injury of the CBD, common hepatic duct, left or right main hepatic duct, or BDI requiring surgical repair.
Data extraction
The data extraction was performed by two independent authors (BN and NK). Disagreements were resolved by consensus. We used a standardized data collection sheet to collect all the necessary data: first author, publication year, study design, Digital Object Identifier (DOI), type of surgical intervention, nature of comparison (IOC vs no IOC, routine IOC vs selective IOC, and selective IOC vs no IOC), the definition of selective IOC, age and gender distribution in each group, number of patients in each comparison group, and number of events in each group in terms of primary and secondary outcomes.
Publication bias and risk of bias assessment
A funnel plot and Egger’s test were used to assess the presence of publication bias, where the number of articles allowed it. A funnel plot was created when at least six studies were pooled. We used Egger’s test when at least ten were pooled.
The risk of bias assessment was performed independently by two authors using the ROBINS-I (Risk of Bias in Non-randomized Studies—of Interventions) [25] tool for non-randomized studies and the RoB 2 tool for randomized controlled trials recommended by the Cochrane collaboration [26]. Disagreements were resolved by consensus.
Statistical analysis
We used the methods recommended by the Cochrane Collaboration working group for data synthesis [27]. A meta-analysis was performed; the calculated effect sizes were visualized on forest plots.
We calculated weighted mean differences (WMD) for continuous outcomes and relative risks (RR) for dichotomous outcomes, both with 95% confidence intervals (CI), to investigate the differences between the three groups (IOC vs no IOC, routine IOC vs selective IOC, and selective IOC vs no IOC).
Heterogeneity was tested both by performing Cochran’s Q test and calculating Higgins’ I2 indicator. The Q statistics were calculated as the squared deviations from the pooled effect of the weighted sum of individual study effects, with the weights being used as part of the pooling method; p-values were obtained by comparing the test statistics with a chi-square with k-1 degrees of freedom (where k was the number of studies). A p-value of less than 0.10 was considered suggestive of significant heterogeneity. The I2 index corresponds to the percentage of the total variability across studies that is due to heterogeneity. Based on Cochrane’s handbook, a rough classification of its value is the following: not important (0–40%), moderate (30–60%), substantial (50–90%), and considerable (75–100%) [28]. All the statistical analyses were performed using StataIC (version 16).
Certainty of evidence
Certainty of evidence was evaluated according to the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) workgroup recommendations [29]. The outcome assessment was performed for each endpoint by two independent authors (BN and NK), with every disagreement resolved by consensus.
We developed several GRADE evidence profile tables using the GRADEpro GDT software [30] for each comparison group (routine vs selective IOC and IOC vs no IOC) separately. The first table comprises articles that involved a mixed population of open and laparoscopic cholecystectomies. The second table is a subgroup containing only the articles that investigated LC.
Results
Results of search and selection
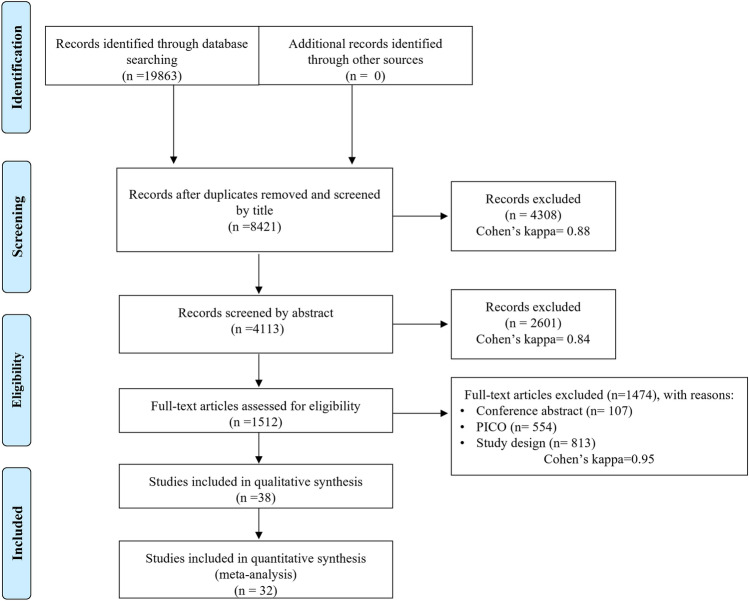
A systematic literature search identified 19,863 articles. The results of the selection process and Cohen’s kappa coefficients are shown in detail in the PRISMA flowchart (Fig. 1). At the end of the selection process, we identified 38 eligible articles [14–19, 21, 31–61], of which 32 were included in the quantitative synthesis [14–19, 21, 31–35, 37, 39–44, 46, 47, 49, 50, 52–59, 61].
Fig. 1.
PRISMA flow diagram
Characteristics of the studies included
The characteristics of the included studies are summarized in 3 tables, divided by the different approaches. Table 1, Supplementary Table 1 and 2 include routine IOC vs selective IOC, IOC vs no IOC, and selective IOC and no IOC, respectively. Eleven of the articles reported on both open and laparoscopic cholecystectomy [15–18, 21, 33–35, 37, 44, 54], whilst 27 of them covered laparoscopic cholecystectomy cases exclusively [14, 19, 31, 32, 36, 38–43, 45–53, 55–61]. We have summarized the different indications for selective IOC in Supplementary Table 3.
Table 1.
Characteristics of included studies (routine IOC vs selective IOC)
| Study | Study design | Centre(s) | Type of procedure | Comparison | Number of patients (female %, mean age ± SD) | Number of SIOC (n) | Outcomes | Follow-up |
|---|---|---|---|---|---|---|---|---|
| Alkhaffaf et al. 2011 | Prospective cohort |
Multicentric (4) in UK |
LC | Routine IOC | 463(80%, 47.8 ± 14.8) | - | BDI, conversion rate to open surgery, LOHS | N/A |
| Selective IOC | 1159(80%, 50.2 ± 15.7) | 263 | ||||||
| Amott et al. 2005 | Quasi-randomized trial | Single centre in Australia | LC | routine IOC | 148 | - | BDI, retained stone rate, success rate of IOC, operation time | N/A |
| Selective IOC | 155 | 45 | ||||||
| Buddingh et al. 2011 | Retrospective cohort | Single centre in the Netherlands | Cholecystectomy | Routine IOC | 435 (63.9%, 53 ± 17) | - | BDI, conversion rate to open surgery, success rate of IOC, operation time | N/A |
| Selective IOC | 421(64.4%, 53 ± 16) | 25 | ||||||
| Carlson et al. 1993 | Prospective cohort |
Multicentric (2) in USA |
LC | Routine IOC | 164 | - | BDI, retained stone rate | A inst: 9–28 months, B inst: 16–31 months |
| Selective IOC | 155 | 21 | ||||||
| Guerra-Filho et al. 2007 | Prospective cohort | Single centre in Brazil | LC | Routine IOC | 127(73.2%, 48.8) | - | Success rate of IOC | N/A |
| Selective IOC | 127(74%, 47.9) | 71 | ||||||
| Nickkholgh et al. 2006 | Retrospective cohort | Single centre in Iran | LC | Routine IOC | 1133 | BDI, retained stone rate, success rate of IOC | N/A | |
| Selective IOC | 800 | 159 | ||||||
| Pham et al. 2016 | Retrospective cohort | Multicentric (2) in China | LC | Routine IOC | 246 (81%, 40, range: 33–57) | Retained stone rate, readmission rate, operation time | 30-day | |
| Selective IOC | 274 (76%, 44, range: 31–53) | 15 | ||||||
| Ragulin-Coyne et al. 2013 | Retrospective cohort | Multicentric (NIS) in USA | Cholecystectomy | Routine IOC | 13,025 (66.9%, 53.5) | - | BDI, LOHS | N/A |
| Selective IOC | 98,790 (66%, 52.5) | N/A | ||||||
| Snow et al. 2001 | Retrospective cohort | Multicentric (4) in USA | LC | Routine IOC | 1522 | - | BDI, retained stone rate, success rate of IOC | 11 year |
| Selective IOC | 487 | 139 |
IOC intraoperative cholangiography,LC laparoscopic cholecystectomy, BDI bile duct injury, LOHS length of hospital stay
Primary outcome(s)
Bile duct injury (BDI)
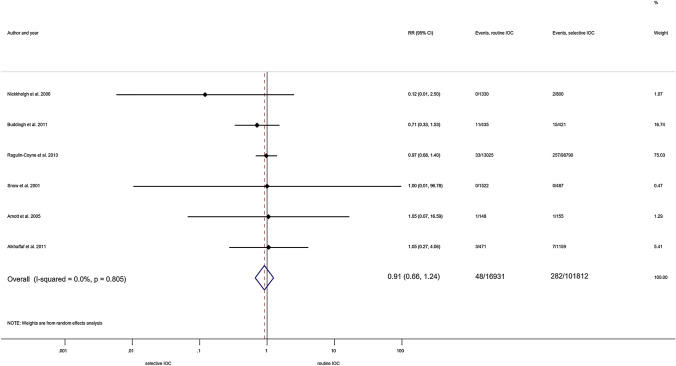
Routine IOC vs selective IOC. We pooled six articles with 118,742 patients for this comparison [21, 31, 32, 35, 49, 55]. We detected no protective effect against BDI in either group (RR = 0.91, 95% CI 0.66; 1.24) along with statistically not significant heterogeneity (I2 = 0.0%, p = 0.805) (Fig. 2).
Fig. 2.
Forest plot comparing risk of BDI between routine IOC and selective IOC groups (population: both types of cholecystectomy). RR: relative risk; p: P value; CI confidence interval; I-squared: I2
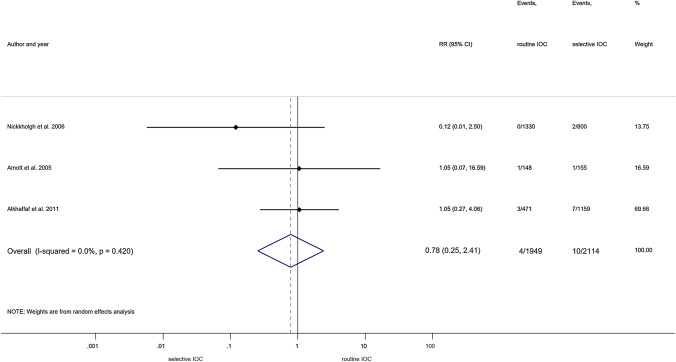
The lack of protective effect against BDI still remained after excluding the articles reporting on open cholecystectomy (RR = 0.78, 95% CI 0.25; 2.41) [31, 32, 49]. This analysis was carried out amongst articles with insignificant statistical heterogeneity (I2 = 0.0%, p = 0.420) (Fig. 3).
Fig. 3.
Forest plot comparing risk of BDI between routine IOC and selective IOC groups (population: laparoscopic cholecystectomy). RR: relative risk; p: P value; CI confidence interval; I-squared: I2
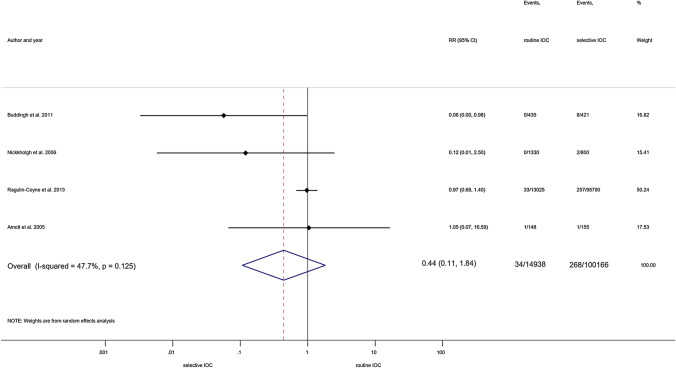
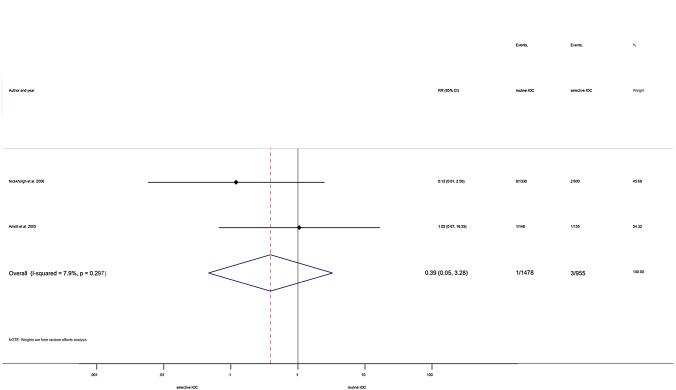
In the same comparison, additional subgroup analyses were performed investigating MBDI. Investigating open and laparoscopic cholecystectomy cases, we found no differences between groups (RR = 0.44, 95% CI 0.11; 1.84; heterogeneity: I2 = 47.7%, p = 0.125) (Fig. 4) [21, 32, 35, 49]. Similarly, no difference was detected when including only laparoscopic cholecystectomy cases (RR = 0.39, 95% CI 0.05; 3.28; heterogeneity: I2 = 7.9%, p = 0.297) (Fig. 5) [32, 49].
Fig. 4.
Forest plot comparing risk of MBDI between routine IOC and selective IOC groups (population: both types of cholecystectomy). RR: relative risk; p: P value; CI confidence interval; I-squared: I2
Fig. 5.
Forest plot comparing risk of MBDI between routine IOC and selective IOC groups (population: laparoscopic cholecystectomy). RR: relative risk; p: P value; CI confidence interval; I-squared: I2
IOC vs no IOC. Based on our analysis of 14 articles with 3,155,940 patients, the use of IOC was not associated with a reduced risk of BDI (RR = 1.03, 95% CI 0.77; 1.37) in substantially heterogeneous publications (I2 = 96.5%, p = 0.000) (Supplementary Fig. 1) [14–19, 34, 37, 40, 46, 47, 53, 56, 61].
The subgroup analysis of ten studies reporting on laparoscopic cholecystectomy exclusively found no difference between the two strategies with 706,336 patients included (RR = 1.19, 95% CI 0.79; 1.79); however, significant heterogeneity was identified (I2 = 82.4%, p = 0.000) (Supplementary Fig. 2) [14, 19, 37, 39, 40, 46, 47, 53, 56, 61].
We performed three additional subgroup analyses with only the prospective studies (RR = 1.09, 95% CI 0.77; 1.54; heterogeneity: I2 = 0.0%, p = 0.965) (Supplementary Fig. 3) [19, 40, 46, 56, 61] and all the studies reported on MBDI (RR = 1.01, 95% CI 0.70; 1.45; heterogeneity: I2 = 96.7%, p = 0.000) (Supplementary Fig. 4) [15, 16, 18, 19, 34, 46, 53, 56, 61], and then we pooled the studies with MBDI in LC only (RR = 1.09, 95% CI 0.35; 3.34; heterogeneity: I2 = 74.8%, p = 0.003) (Supplementary Fig. 5). [19, 39, 46, 53, 56] Neither of them found significant differences between the groups under investigation.
Retained biliary stones after cholecystectomy
Comparing IOC and no IOC, five pooled studies with 2,069 cases found no difference (RR = 0.51, 95% CI 0.12; 2.11) within a one-year follow-up period, nor was statistically significant heterogeneity found (I2 = 13.7%, p = 0.327) (Supplementary Fig. 6) [19, 33, 56–58].
Despite our initial question, we could not examine the routine IOC vs selective IOC groups because follow-up periods were too variable. The results of these articles can be found in the supplementary material (Supplementary Table 4).
Secondary outcome(s)
Routine vs selective IOC
When analysing the success rate of IOC during laparoscopic cholecystectomy in the four studies involved comparing routine IOC and selective IOC, we were not able to identify any statistically significant difference (RR = 0.96, 95% CI 0.86; 1.06; I2 = 88.2%, p < 0.001) (Supplementary Fig. 7) [32, 41, 49, 55].
Comparing the routine and selective approach by operation time, the results did not show us a statistically significant difference (WMD = 14.02, 95% CI –6.96; 35.00, I2 = 98.2%, p < 0.001), including three studies with 2445 patients. These studies only investigated patients who had undergone laparoscopic cholecystectomy (Supplementary Fig. 8) [31, 32, 50].
IOC vs no IOC
The meta-analysis of three studies with 10,735 patients identified a significant difference (RR = 0.64, 95% CI 0.51; 0.78) favouring IOC with a lower risk of conversion to open surgery compared to no IOC group without significant heterogeneity (I2 = 0.4%, p = 0.336) (Supplementary Fig. 9) [19, 46, 61]. The operation time was significantly longer during cholecystectomy in the IOC group compared to the no IOC group (WMD = 11.25 min, 95% CI 6.57; 15.93; heterogeneity I2 = 95.9%, p = 0.000) (Supplementary Fig. 10) [19, 33, 42, 46, 49, 56, 59] .
The meta-analysis comparing the readmission rate after laparoscopic cholecystectomy between IOC and no IOC groups within a follow-up period of 30 days found no statistically significant difference (RR = 0.92, 95% CI 0.79; 1.06, I2 = 86.9%, p < 0.001) (Supplementary Fig. 11) [14, 42, 52, 56]. Comparing IOC and no IOC by length of hospital stay, no statistically significant differences were found (WMD = -0.03, 95% CI –0.26; 0.20; heterogeneity: I2 = 98.3%, p < 0.001) (Supplementary Fig. 12) [14, 19, 33, 43, 44, 54, 56, 59]. The results were the same when we analysed studies reporting exclusively on LC cases (WMD = 0.04, 95% CI –0.12; 0.19; heterogeneity: I2 = 90.0%, p < 0.001) (Supplementary Fig. 13) [19, 43, 56, 59].
Qualitative synthesis
We included the following endpoints in our qualitative synthesis: BDI, MBDI (routine IOC vs selective IOC: one publication [36]; IOC vs no IOC: one publication) [45], retained stone rate (routine IOC vs selective IOC: five studies [32, 36, 49, 50, 55]; IOC vs no IOC: one publication [38]; selective IOC vs no IOC: three studies) [48, 51, 60], readmission rate (IOC vs no IOC: four studies) [33, 46, 57, 58], conversion rate to open surgery (routine IOC vs selective IOC: two studies) [31, 35], success rate of IOC (routine IOC vs selective IOC: one study) [35], operation time (routine IOC vs selective IOC: one study [35]; IOC vs no IOC: one study) [38] and length of hospital stay (IOC vs no IOC: one study [57]; routine IOC vs selective IOC: three studies) [21, 31, 50]. A summary of studies only included in the qualitative synthesis can be found in the supplementary material (Supplementary Tables 4 and 5).
Publication bias and risk of bias assessment
Based on a visual assessment of funnel plots, there is a high risk of publication bias in the case of MBDI when the population consisted of both types of cholecystectomy and when only LCs were performed, retained biliary stones after cholecystectomy, operation time (population consisted of LC; comparison: IOC vs no IOC), operation time (population consisted of LC, comparison: IOC vs no IOC), and length of hospital stay (population consisted of both types of cholecystectomy; comparison: IOC vs no IOC). The results of publication bias, funnel plots, and Egger’s tests can be found in the supplementary data.
Most of the publications investigated were deemed to have a serious risk of bias because of the presence of uncontrolled confounding factors. We excluded three articles from the quantitative synthesis due to critical risk of bias [36, 38, 45]. A summary of the risk of bias assessment can be found in the supplementary data.
Certainty of evidence
All examined outcomes were assessed as having a very low level of evidence. The design of the included studies, the potential presence of uncontrolled confounding factors, the significant level of heterogeneity greatly influenced the quality of evidence. The GRADE evidence profile tables are shown in Tables 2 and 3 summarizing the comparison of routine IOC vs selective IOC and in Supplementary Table 6 and 7 concerning about IOC vs no IOC.
Table 2.
GRADE evidence profile – Comparison: Routine vs selective IOC. Population: both type of cholecystectomy
| Certainty assessment | № of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Routine IOC | selective IOC | Relative (95% CI) |
Absolute (95% CI) |
||
| Bile duct injury (both type of cholecystectomy) (assessed with: RR) | ||||||||||||
| 6 | observational studies | very seriousa | not serious | seriousb | not serious | none | 48/16930 (0.3%) | 282/101812 (0.3%) |
RR 0.91 (0.66 to 1.24) |
0 fewer per 1 000 (from 1 fewer to 1 more) |
⨁◯◯◯ Very low |
CRITICAL |
| Major bile duct injury (both type of cholecystectomy) (assessed with: RR) | ||||||||||||
| 4 | observational studies | very seriousa | not serious | seriousb | not serious | publication bias strongly suspectedc | 34/14938 (0.2%) | 268/100166 (0.3%) |
RR 0.44 (0.11 to 1.84) |
1 fewer per 1 000 (from 2 fewer to 2 more) |
⨁◯◯◯ Very low |
CRITICAL |
CI confidence interval, RR risk ratio
aBias is likely due to the presence of confounding factors
bMost patients had acute biliary disease
cDue to the low number of articles publication bias was not assessed
Table 3.
GRADE evidence profile – Comparison: Routine vs selective IOC. Population: laparoscopic cholecystectomy
| Certainty assessment | № of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Routine IOC | selective IOC | Relative (95% CI) |
Absolute (95% CI) |
||
| Bile duct injury (laparoscopic cholecystectomy) (assessed with: RR) | ||||||||||||
| 3 | observational studies | very seriousa | not serious | not serious | not serious | publication bias strongly suspectedb | 4/1949 (0.2%) | 10/2114 (0.5%) |
RR 0.78 (0.25 to 2.41) |
1 fewer per 1 000 (from 4 fewer to 7 more) |
⨁◯◯◯ Very low |
CRITICAL |
| Major bile duct injury (laparoscopic cholecystectomy) (assessed with: RR) | ||||||||||||
| 2 | observational studies | very seriousa | not serious | not serious | not serious | publication bias strongly suspectedb | 1/1478 (0.1%) | 3/955 (0.3%) |
RR 0.39 (0.05 to 3.28) |
2 fewer per 1 000 (from 3 fewer to 7 more) |
⨁◯◯◯ Very low |
CRITICAL |
| Success rate of IOC (laparoscopic cholecystectomy) (assessed with: RR) | ||||||||||||
| 4 | observational studies | very seriousa | very seriousc | not serious | very seriousd | publication bias strongly suspectede | 2846/3127 (91.0%) | 372/414 (89.9%) |
RR 0.96 (0.86 to 1.06) |
36 fewer per 1 000 (from 126 fewer to 54 more) |
⨁◯◯◯ Very low |
IMPORTANT |
| Operation time (laparoscopic cholecystectomy) (assessed with: WMD) | ||||||||||||
| 3 | observational studies | very seriousa | very seriousc | seriousf | seriousg | publication bias strongly suspectedh | 857 | 1588 | - |
WMD 14.02 min. more (6.96 fewer to 35 more) |
⨁◯◯◯ Very low |
IMPORTANT |
CI confidence interval, RR risk ratio
aBias is likely due to the presence of confounding factors
bDue to the low number of articles publication bias was not assessed
cInconsistency is likely due to the presence of statistically significant heterogeneity
dConfidence intervals cross the benefit/harm line and 0−effect line
ePublication bias is likely due to funnel plot asymmetry
fIndirect population is likely due to the variable inclusion and exclusion criteria
gImprecision is likely due to the overall effect estimate lies between benefit and harm
hThe risk of publication bias was not assessed due to the low number of studies included
Discussion
Our results suggest that selective IOC may not be inferior to routine IOC in the prevention of BDI. The success rate of IOC and the operation time were also similar between these two groups.
Carrying out IOC did not result in significant difference in any of the endpoints under examination compared to the omission of IOC, apart from a lower conversion rate and longer operation time. A significantly higher conversion rate to open surgery appeared in the no IOC group, and a significantly longer operation time was characteristic in the IOC group.
There is a consensus that IOC has its place in surgical practice due to its role in detecting CBD stones and diagnosing BDI. However, the recommendations are not unanimous because there are still doubts about the extent to which IOC can prevent BDI [4–13]. According to the latest meta-analysis published in 2021, IOC should be performed routinely, it has a protective effect against BDI over the selective approach, and it is a cost-effective intervention as well [12]. In contrast, others suggest liberal [9] or selective [5] use of IOC to mitigate the risk of BDI.
Primary outcome(s)
Bile duct injury (BDI)
Our findings do not support the higher protective value of routine IOC compared to selective cholangiography. In addition, our results indicate that IOC has no clear benefit over omission of IOC; a selective policy might be more reasonable rather than the omission of IOC.
Based on our results, the role of intraoperative cholangiography in preventing BDI can be questioned. However, it still plays an important diagnostic role for BDI and CBD stones [40, 54]. From our standpoint, the main question is not whether an IOC should be performed but in whom: whether it should be done in all cases or only when the situation requires it.
Articles concentrating on routine IOC vs selective IOC draw different conclusions. The latest one was published in 2013 by Ragulin-Coyne et al.; it involved 111,815 patients and found that routine IOC does not reduce the rate of BDI but incurs a high cost [21]. In contrast, Buddingh et al., concluded that implementation of routine IOC had a protective effect against major BDI [35]. As mentioned earlier, in a meta-analysis published in 2021 the authors claim that routine IOC decreases the risk and prevents BDI, and is also more cost-effective [12]. However, these results should be handled with caution because the population defined as selective IOC in the meta-analysis appears as patients “without IOC” in the majority of the pooled studies [15, 37, 39, 40, 54, 62].
Sheffield et al. have shown that the link between IOC and common BDI may be due to unmeasured confounding factors [54] and differences in baseline characteristics between the comparator groups. They claim that the relation between IOC and common BDI is sensitive to the statistical method applied [54]. They used the standard risk adjustment method to determine that omission of IOC is linked to BDI, even after controlling for potential confounding factors. At the same time, when instrumental variable methods were applied, the association was no longer significant.
In comparing IOC and no IOC, several publications used data obtained from large databases [14–16, 18, 39, 47, 53]. They frequently used a proxy definition of BDI because these databases have no precise definition of BDI. Lilley et al. claim that the use of a proxy definition for common BDI is not an appropriate method to identify it because this may introduce confounding by indication [16].
Retained biliary stones after cholecystectomy
Our results suggest that IOC does not significantly reduce the rate of postoperatively detected residual CBD stones.
Hope et al. state that IOC should be used freely to detect CBD stones as decided by the surgeon due to its high sensitivity, specificity, positive and negative predictive value, and accuracy [9]. Another meta-analysis suggests that IOC should be used more widely in the diagnosis and management of CBD stones [7]. A previous meta-analysis published in 2012 states that routine IOC can reduce the readmission for retained CBD stones; however, they do not suggest routine IOC in patients without suspicion of CBD stones (based on clinical, biochemical, or radiological findings) [13]. A recent retrospective study found that asymptomatic untreated bile duct stones have a cumulative incidence of biliary complications (6.1% at one year, 11% at three years and 17% at five years). Still, they concluded that doing follow-ups for patients with in situ stones (a “wait-and-see strategy”) is more beneficial than early endoscopic management of asymptomatic stones [63]. A study published in 2013 points out that routine IOC may detect more CBD stones intraoperatively than ideal [21]. The number of endoscopic retrograde cholangiopancreatographies (ERCP) and CBD explorations is thus higher in the case of routine IOC users. They also found a link between the use of routine IOC and an increased overall complication rate. They support the idea of selective IOC because it can reduce the number of unnecessary interventions. Sheffield et al. confirm this; they also found relationship between routine IOC and higher use of ERCP and common duct exploration [54]. According to the ASGE (American Society of Gastrointestinal Endoscopy) guideline, the number of diagnostic ERCPs should be reduced because of their high risk and lack of benefit [64].
The ASGE and ESGE (European Society of Gastrointestinal Endoscopy) recommend two similar pre-operative risk stratification methods to assess the probability of CBD stones [64, 65]. These guidelines might be helpful in terms of indexing patients who need further investigation and possible management of CBD stones. These algorithms allow the surgeon to consider whether an IOC is required or not.
Secondary outcome(s)
When we compared routine to selective IOC, we did not find a significant difference between them as regards the success rate of IOC and operation time.
A significant difference was found between IOC and no IOC in conversion rate to open surgery and operation time. The results indicate a higher conversion rate to open surgery amongst patients who did not receive IOC. Conversion to open surgery frequently happens during a difficult laparoscopic dissection or when BDI is suspected [47]. A previous publication reports that a higher risk of BDI appears with the conversion from a laparoscopic to an open procedure [66]. This could reflect the surgeon’s lack of experience with open cholecystectomy [67] or the overall difficulty of the case [47].
The data suggest that patients who underwent LC and IOC had a significantly longer operation time by almost 13 min. This result contradicts the theory put forth by opponents of IOC: IOC adds a significant amount of time to LC.
Investigating readmission rate and length of hospital stay between IOC and no IOC, we found no significant differences.
Strengths and limitations
Our meta-analysis is characterized by comprehensiveness and a high number of included patients. Compared to the recently published meta-analyses [7, 12] we placed great emphasis on the study of routine IOC vs selective IOC approaches and identified several additional articles [34, 36, 37, 45, 47, 53, 55, 56, 61]. We performed several subgroup analyses (only LC cases exclusively, MBDI and prospective studies) to provide a better quality of evidence and a more thorough review.
Our study has some limitations. The majority of the pooled articles are retrospective cohort studies. They provide data from large-scale databases with potential sources of bias and are not controlled or partially adjusted for confounding variables. Our results should therefore be handled with caution.
Our results continue to lose strength due to the presence of statistical heterogeneity for some endpoints. This phenomenon might be the result of study design, not only laparoscopic cholecystectomy included, variable use of IOC, different definitions of BDI, and varying degrees of confounding factor control. In addition, IOC is used as a diagnostic tool for detecting BDI in some cases, which has a potential distortive effect.
Implications for practice
A selective approach to IOC may be appropriate. Selective IOC combined with measures that aid prevention of BDI (e.g. critical view of safety, fundus-first approach, multi-port laparoscopic technique, and low threshold for conversion to open cholecystectomy) [5, 68] and together with investigations that detect bile duct stone perioperatively (e.g. abdominal ultrasound, laparoscopic ultrasound, endoscopic ultrasound, and magnetic resonance cholangiopancreatography) [5, 64] should be considered.
Implications for research
No uniform indication system for selective IOC has been developed. Such an indication system should consider the risk factors for BDI (e.g. sex, age, experience of surgeons, prolonged laparoscopic cholecystectomy, and indication for cholecystectomy) and the possible presence (based on clinical, laboratory, and imaging findings) and available treatment of biliary stones. Future studies are needed to establish a standard indication system based on which surgeons perform IOC.
Several authors state that BDI cannot be examined with randomized trials due to its low incidence [8, 15]; high-quality prospective studies which also consider the presence of potential biases and confounding factors are therefore needed.
In conclusion, selective IOC may not be inferior to routine IOC in preventing BDI. IOC might not be indicated in every case, and its selective use may stand as an alternative to routine policy, however, the evidence is very uncertain. Further good quality research is required to address this question and to determine the exact selection criteria for IOC.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- ASGE
American Society of Gastrointestinal Endoscopy
- BDI
Bile duct injury
- CBD
Common bile duct
- CI
95% Confidence interval
- ERCP
Endoscopic retrograde cholangiopancreatography
- ESGE
European Society of Gastrointestinal Endoscopy
- IOC
Intraoperative cholangiography
- LC
Laparoscopic cholecystectomy
- MBDI
Major bile duct injury
- RCT
Randomized clinical trial
- RR
Relative risk
- WMD
Weighted mean difference
Author contribution
NK and SA: conceptualization. NK and BN: literature search, screening the records, data extraction, assessing the quality of the included studies. DN: statistical analysis. NK: visualization. NK, SA, and MF: writing the first draft of the current manuscript. SA, MF, PH, BE, SB, JB, AV, and KEM: critical revision of the manuscript and approving the submitted draft. All the authors provided critical conceptual input and approved the final version of the manuscript.
Funding
Open access funding provided by University of Szeged. Funding was provided by the Economic Development and Innovation Operational Programme Grant (GINOP-2.3.2-15-2016-00048 – STAY ALIVE and GINOP-2.3.4-15-2020-00010 Competence Centre for Health Data Analysis, Data Utilization and Smart Device and Technology Development at the University of Pécs) and by a Human Resources Development Operational Programme Grant (EFOP-3.6.1.-16-2016-00004 – Comprehensive Development for Implementing Smart Specialization Strategies at the University of Pécs), both supported by the European Union. The project was co-financed by the European Social Fund.
Declarations
Disclosures
Drs. Norbert Kovács, Dávid Németh, Mária Földi, Bernadette Nagy, Stefania Bunduc, Péter Hegyi, Judit Bajor, Katalin Eszter Müller, Áron Vincze, Bálint Erőss, and Szabolcs Ábrahám have no conflicts of interest or financial ties to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Coccolini F, Catena F, Pisano M, Gheza F, Fagiuoli S, DiSaverio S, et al. Open versus laparoscopic cholecystectomy in acute cholecystitis. Systematic review and meta-analysis. Int J Surg. 2015;18:196–204. doi: 10.1016/j.ijsu.2015.04.083. [DOI] [PubMed] [Google Scholar]
- 2.Rystedt J, Lindell G, Montgomery A. Bile duct injuries associated with 55,134 cholecystectomies: treatment and outcome from a national perspective. World J Surg. 2016;40(1):73–80. doi: 10.1007/s00268-015-3281-4. [DOI] [PubMed] [Google Scholar]
- 3.Flum DR, Cheadle A, Prela C, Dellinger EP, Chan L. Bile duct injury during cholecystectomy and survival in medicare beneficiaries. JAMA. 2003;290(16):2168–2173. doi: 10.1001/jama.290.16.2168. [DOI] [PubMed] [Google Scholar]
- 4.Eikermann M, Siegel R, Broeders IAMJ, Dziri C, Fingerhut A, Gutt C, et al. Prevention and treatment of bile duct injuries during laparoscopic cholecystectomy: the clinical practice guidelines of the European Association for Endoscopic Surgery (EAES) Surg Endosc. 2012;26:3003–3039. doi: 10.1007/s00464-012-2511-1. [DOI] [PubMed] [Google Scholar]
- 5.Brunt LM, Deziel D, Telem D, Strasberg S, Aggarwal R, Asbun H, et al. Safe cholecystectomy multi-society practice guideline and state of the art consensus conference on prevention of bile duct injury during cholecystectomy. Ann Surg. 2020;272:1. doi: 10.1097/SLA.0000000000003791. [DOI] [PubMed] [Google Scholar]
- 6.Buddingh K, Nieuwenhuijs V, Buuren L, Hulscher J, Jong J, Dam G. Intraoperative assessment of biliary anatomy for prevention of bile duct injury: a review of current and future patient safety interventions. Surg Endosc. 2011;25:2449–2461. doi: 10.1007/s00464-011-1639-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donnellan E, Sugrue M, Johnston A, Bucholc M. A meta-analysis of the use of intraoperative cholangiography; time to revisit our approach to cholecystectomy? Surgery. 2020;3:8–15. doi: 10.1016/j.sopen.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ford J, Soop M, Du J, Loveday B, Rodgers M. Systematic review of intraoperative cholangiography in cholecystectomy. Br J Surg. 2012;99:160–167. doi: 10.1002/bjs.7809. [DOI] [PubMed] [Google Scholar]
- 9.Hope W, Fanelli R, Walsh D, Narula V, Price R, Stefanidis D, et al. SAGES clinical spotlight review: intraoperative cholangiography. Surg Endosc. 2017;31:1. doi: 10.1007/s00464-016-5320-0. [DOI] [PubMed] [Google Scholar]
- 10.Ludwig K, Bernhardt J, Steffen H, Lorenz D. Contribution of intraoperative cholangiography to incidence and outcome of common bile duct injuries during laparoscopic cholecystectomy. Surg Endosc. 2002;16:1098–1104. doi: 10.1007/s00464-001-9183-6. [DOI] [PubMed] [Google Scholar]
- 11.Overby D, Apelgren K, Richardson W, Fanelli R. SAGES guidelines for the clinical application of laparoscopic biliary tract surgery. Surg Endosc. 2010;24:2368–2386. doi: 10.1007/s00464-010-1268-7. [DOI] [PubMed] [Google Scholar]
- 12.Rystedt J, Wiss J, Adolfsson J, Enochsson L, Hallerbäck B, Johansson P, et al. Routine versus selective intraoperative cholangiography during cholecystectomy: systematic review, meta-analysis and health economic model analysis of iatrogenic bile duct injury. BJS Open. 2021;5:1. doi: 10.1093/bjsopen/zraa032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sajid M, Leaver C, Haider Z, Worthington T, Karanjia N, Singh K. Routine on-table cholangiography during cholecystectomy: a systematic review. Ann R Coll Surg Engl. 2012;94:375–380. doi: 10.1308/003588412X13373405385331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altieri M, Yang J, Obeid N, Zhu C, Talamini M, Pryor A. Increasing bile duct injury and decreasing utilization of intraoperative cholangiogram and common bile duct exploration over 14 years: an analysis of outcomes in New York State. Surg Endosc. 2018;32:667–674. doi: 10.1007/s00464-017-5719-2. [DOI] [PubMed] [Google Scholar]
- 15.Flum D, Dellinger E, Cheadle A, Chan L, Koepsell T. Intraoperative cholangiography and risk of common bile duct injury during cholecystectomy. JAMA. 2003;289:1639–1644. doi: 10.1001/jama.289.13.1639. [DOI] [PubMed] [Google Scholar]
- 16.Lilley E, Scott J, Jiang W, Krasnova A, Raol N, Changoor N, et al. Intraoperative cholangiography during cholecystectomy among hospitalized medicare beneficiaries with non-neoplastic biliary disease. Am J Surg. 2017;214:682–686. doi: 10.1016/j.amjsurg.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 17.Törnqvist B, Strömberg C, Akre O, Enochsson L, Nilsson M. Selective intraoperative cholangiography and risk of bile duct injury during cholecystectomy: Intraoperative cholangiography and bile duct injury during cholecystectomy. Br J Surgy. 2015;102:952–958. doi: 10.1002/bjs.9832. [DOI] [PubMed] [Google Scholar]
- 18.Törnqvist B, Zheng Z, Ye W, Waage A, Nilsson M. Long-term effects of iatrogenic bile duct injury during cholecystectomy. Clin Gastroenterol Hepatol. 2009;7:1013–1018. doi: 10.1016/j.cgh.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Ding G-Q, Cai W, Qin M-F. Is intraoperative cholangiography necessary during laparoscopic cholecystectomy for cholelithiasis? World J Gastroenterol. 2015;21:2147–2151. doi: 10.3748/wjg.v21.i7.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carroll B, Friedman R, Liberman MA, Phillips E. Routine cholangiography reduces sequelae of common bile duct injuries. Surg Endosc. 1997;10:1194–1197. doi: 10.1007/s004649900277. [DOI] [PubMed] [Google Scholar]
- 21.Ragulin-Coyne E, Witkowski ER, Chau Z, Ng SC, Santry HP, Callery MP, et al. Is routine intraoperative cholangiogram necessary in the twenty-first century? A national view. J Gastrointest Surg. 2013;17(3):434–442. doi: 10.1007/s11605-012-2119-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livingston E, Miller J, Coan B, Rege R. Indications for selective intraoperative cholangiography. J Gastrointestinal Surg. 2006;9:1371–1377. doi: 10.1016/j.gassur.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Metcalfe M, Ong T, Bruening M, Iswariah H, Wemyss-Holden S, Maddern G. Is laparoscopic intraoperative cholangiogram a matter of routine? Am J Surg. 2004;187:475–481. doi: 10.1016/j.amjsurg.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. www.training.cochrane.org/handbook. 2021.
- 28.Higgins J G, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. John Wiley & Sons, Ltd: The Cochrane Collaboration. www.handbook.cochrane.org.
- 29.Schünemann H BJ, Guyatt G, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. guidelinedevelopment.org/handbook.
- 30.GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University, 2020 (developed by Evidence Prime, Inc.). gradepro.org.
- 31.Alkhaffaf B, Parkin E, Flook D. Endoscopic retrograde cholangiopancreatography prior to laparoscopic cholecystectomy: a common and potentially hazardous technique that can be avoided. Arch Surg. 2011;146(3):329–333. doi: 10.1001/archsurg.2011.30. [DOI] [PubMed] [Google Scholar]
- 32.Amott D, Webb A, Tulloh B. Prospective comparison of routine and selective operative cholangiography. ANZ J Surg. 2005;75(6):378–382. doi: 10.1111/j.1445-2197.2005.03393.x. [DOI] [PubMed] [Google Scholar]
- 33.Bennion RS, Wyatt LE, Thompson JE., Jr Effect of intraoperative cholangiography during cholecystectomy on outcome after gallstone pancreatitis. J Gastrointest Surg. 2002;6(4):575–581. doi: 10.1016/S1091-255X(01)00017-8. [DOI] [PubMed] [Google Scholar]
- 34.Borie F, Mathonnet M, Deleuze A, Gravié JF, Gugenheim J. The cost and the effectiveness of cholangiography for the diagnosis and treatment of a bile duct injury after difficult identification of the cystic duct. J Gastrointest Surg. 2021;25(6):1430–1436. doi: 10.1007/s11605-020-04640-4. [DOI] [PubMed] [Google Scholar]
- 35.Buddingh K, Weersma R, Savenije R, Dam G, Nieuwenhuijs V. Lower rate of major bile duct injury and increased intraoperative management of common bile duct stones after implementation of routine intraoperative cholangiography. J Am Coll Surg. 2011;213:267–274. doi: 10.1016/j.jamcollsurg.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Carlson MA, Ludwig KA, Frantzides CT, Cattey RP, Henry LG, Walker AP, et al. Routine or selective intraoperative cholangiography in laparoscopic cholecystectomy. J Laparoendosc Surg. 1993;3(1):27–33. doi: 10.1089/lps.1993.3.27. [DOI] [PubMed] [Google Scholar]
- 37.Fletcher DR, Hobbs MS, Tan P, Valinsky LJ, Hockey RL, Pikora TJ, et al. Complications of cholecystectomy: risks of the laparoscopic approach and protective effects of operative cholangiography: a population-based study. Ann Surg. 1999;229(4):449–457. doi: 10.1097/00000658-199904000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flowers JL, Zucker KA, Graham SM, Scovill WA, Imbembo AL, Bailey RW. Laparoscopic cholangiography. Results and indications. Ann Surg. 1992;215(3):209–216. doi: 10.1097/00000658-199203000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flum D, Koepsell T, Heagerty P, Sinanan M, Dellinger E. Common bile duct injury during laparoscopic cholecystectomy and the use of intraoperative cholangiography: adverse outcome or preventable error? Arch Surg. 1960;2001(136):1287–1292. doi: 10.1001/archsurg.136.11.1287. [DOI] [PubMed] [Google Scholar]
- 40.Giger-Pabst U, Ouaissi M, Hsu Schmitz S-F, Krahenbuhl S, Krähenbühl L. Bile duct injury and use of cholangiography during laparoscopic cholecystectomy. Br J Surg. 2011;98:391–396. doi: 10.1002/bjs.7335. [DOI] [PubMed] [Google Scholar]
- 41.Guerra-Filho V, Nunes TA, Araújo ID. Perioperative fluorocholangiography with routine indication versus selective indication in laparoscopic cholecystectomy. Arq Gastroenterol. 2007;44(3):271–275. doi: 10.1590/S0004-28032007000300017. [DOI] [PubMed] [Google Scholar]
- 42.Halawani HM, Tamim H, Khalifeh F, Mailhac A, Jamali FR. Impact of intraoperative cholangiography on postoperative morbidity and readmission: analysis of the NSQIP database. Surg Endosc. 2016;30(12):5395–5403. doi: 10.1007/s00464-016-4896-8. [DOI] [PubMed] [Google Scholar]
- 43.Ingraham AM, Cohen ME, Ko CY, Hall BL. A current profile and assessment of north American cholecystectomy: results from the American college of surgeons national surgical quality improvement program. J Am Coll Surg. 2010;211(2):176–186. doi: 10.1016/j.jamcollsurg.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Johnson PM, Walsh MJ. The impact of intraoperative cholangiography on recurrent pancreatitis and biliary complications in patients with gallstone pancreatitis. J Gastrointest Surg. 2012;16(12):2220–2224. doi: 10.1007/s11605-012-2041-0. [DOI] [PubMed] [Google Scholar]
- 45.Khalili TM, Phillips EH, Berci G, Carroll BJ, Gabbay J, Hiatt JR. Final score in laparoscopic cholecystectomy. Cholangiogram 1207, no cholangiogram 116. Surg Endosc. 1997;11(11):1095–1098. doi: 10.1007/s004649900538. [DOI] [PubMed] [Google Scholar]
- 46.Khan OA, Balaji S, Branagan G, Bennett DH, Davies N. Randomized clinical trial of routine on-table cholangiography during laparoscopic cholecystectomy. Br J Surg. 2011;98(3):362–367. doi: 10.1002/bjs.7356. [DOI] [PubMed] [Google Scholar]
- 47.Mangieri C, Hendren B, Strode M, Faler B. Bile duct injuries (BDI) in the advanced laparoscopic cholecystectomy era. Surg Endosc. 2019;33:724. doi: 10.1007/s00464-018-6333-7. [DOI] [PubMed] [Google Scholar]
- 48.Misra M, Schiff J, Rendon G, Rothschild J, Schwaitzberg S. Laparoscopic cholecystectomy after the learning curve: what should we expect? Surg Endosc. 2005;19(9):1266–1271. doi: 10.1007/s00464-004-8919-5. [DOI] [PubMed] [Google Scholar]
- 49.Nickkholgh A, Soltaniyekta S, Kalbasi H. Routine versus selective intraoperative cholangiography during laparoscopic cholecystectomy: a survey of 2,130 patients undergoing laparoscopic cholecystectomy. Surg Endosc. 2006;20(6):868–874. doi: 10.1007/s00464-005-0425-x. [DOI] [PubMed] [Google Scholar]
- 50.Pham XD, de Virgilio C, Al-Khouja L, Bermudez MC, Schwed AC, Kaji AH, et al. Routine intraoperative cholangiography is unnecessary in patients with mild gallstone pancreatitis and normalizing bilirubin levels. Am J Surg. 2016;212(6):1047–1053. doi: 10.1016/j.amjsurg.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 51.Robinson BL, Donohue JH, Gunes S, Thompson GB, Grant CS, Sarr MG, et al. Selective operative cholangiography. Appropriate management for laparoscopic cholecystectomy. Arch Surg. 1995;130(6):625–630. doi: 10.1001/archsurg.1995.01430060063012. [DOI] [PubMed] [Google Scholar]
- 52.Rosero EB, Joshi GP. Hospital readmission after ambulatory laparoscopic cholecystectomy: incidence and predictors. J Surg Res. 2017;219:108–115. doi: 10.1016/j.jss.2017.05.071. [DOI] [PubMed] [Google Scholar]
- 53.Russell J, Walsh S, Mattie A, Lynch J. Bile duct injuries, 1989–1993: a statewide experience. Arch Surgry. 1996;131:382. doi: 10.1001/archsurg.1996.01430160040007. [DOI] [PubMed] [Google Scholar]
- 54.Sheffield K, Riall T, Han Y, Kuo Y-F, Townsend C, Goodwin J. Association between cholecystectomy with vs without intraoperative cholangiography and risk of common duct injury. JAMA. 2013;310:812–820. doi: 10.1001/jama.2013.276205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Snow LL, Weinstein LS, Hannon JK, Lane DR. Evaluation of operative cholangiography in 2043 patients undergoing laparoscopic cholecystectomy: a case for the selective operative cholangiogram. Surg Endosc. 2001;15(1):14–20. doi: 10.1007/s004640000311. [DOI] [PubMed] [Google Scholar]
- 56.Soper NJ, Dunnegan DL. Routine versus selective intra-operative cholangiography during laparoscopic cholecystectomy. World J Surg. 1992;16(6):1133–1140. doi: 10.1007/BF02067079. [DOI] [PubMed] [Google Scholar]
- 57.Tabone LE, Sarker S, Fisichella PM, Conlon M, Fernando E, Yi S, et al. To 'gram or not'? Indications for intraoperative cholangiogram. Surgery. 2011;150(4):810–819. doi: 10.1016/j.surg.2011.07.062. [DOI] [PubMed] [Google Scholar]
- 58.Verma S, Wichmann MW, Gunning T, Beukes E, Maddern G. Intraoperative cholangiogram during laparoscopic cholecystectomy: a clinical trial in rural setting. Aust J Rural Health. 2016;24(6):415–421. doi: 10.1111/ajr.12279. [DOI] [PubMed] [Google Scholar]
- 59.Wewelwala C, Cashin P, Blamey S, Gribbin J, Low L, Croagh D. Effect of contrast injection into the biliary tract during intraoperative cholangiogram on postoperative liver function tests: LFT derangement due to cholangiogram. Asian J Endosc Surg. 2015;8:158–163. doi: 10.1111/ases.12174. [DOI] [PubMed] [Google Scholar]
- 60.Zang J-f, Yuan Y, Zhang C, Gao J. Elective laparoscopic cholecystectomy without intraoperative cholangiography: role of preoperative magnetic resonance cholangiopancreatography - a retrospective cohort study. BMC Surg. 2016;16:45. doi: 10.1186/s12893-016-0159-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Z'Graggen K, Wehrli H, Metzger A, Buehler M, Frei E, Klaiber C. Complications of laparoscopic cholecystectomy in Switzerland A prospective 3-year study of 10,174 patients. Swiss Association of Laparoscopic and Thoracoscopic Surgery. Surg Endosc. 1998;12(11):1303–1310. doi: 10.1007/s004649900846. [DOI] [PubMed] [Google Scholar]
- 62.Waage A, Nilsson M. Iatrogenic bile duct injury: a population-based study of 152 776 cholecystectomies in the Swedish Inpatient Registry. Arch Surg. 2006;141(12):1207–1213. doi: 10.1001/archsurg.141.12.1207. [DOI] [PubMed] [Google Scholar]
- 63.Hakuta R, Hamada T, Nakai Y, Oyama H, Kanai S, Suzuki T, et al. Natural history of asymptomatic bile duct stones and association of endoscopic treatment with clinical outcomes. J Gastroenterol. 2020;55(1):78–85. doi: 10.1007/s00535-019-01612-7. [DOI] [PubMed] [Google Scholar]
- 64.Buxbaum J, Fehmi S, Sultan S, Fishman D, Qumseya B, Cortessis V, et al. ASGE guideline on the role of endoscopy in the evaluation and management of choledocholithiasis. Gastrointes Endosc. 2019;89:1075. doi: 10.1016/j.gie.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Manes G, Paspatis G, Aabakken L, Anderloni A, Arvanitakis M, Ah-Soune P, et al. Endoscopic management of common bile duct stones: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2019;51(5):472–491. doi: 10.1055/a-0862-0346. [DOI] [PubMed] [Google Scholar]
- 66.Wolf AS, Nijsse BA, Sokal SM, Chang Y, Berger DL. Surgical outcomes of open cholecystectomy in the laparoscopic era. Am J Surg. 2009;197(6):781–784. doi: 10.1016/j.amjsurg.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 67.Schulman CI, Levi J, Sleeman D, Dunkin B, Irvin G, Levi D, et al. Are we training our residents to perform open gall bladder and common bile duct operations? J Surg Res. 2007;142(2):246–249. doi: 10.1016/j.jss.2007.03.073. [DOI] [PubMed] [Google Scholar]
- 68.Roviaro GCMM, Rebuffat C, Varoli F, Vergani V, Rabughino G, Scarduelli A. Complications following cholecystectomy. J R Coll Surg Edinb. 1997;27:360. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.