Abstract
Endometrial cancer (EC) is the most common type of gynecologic cancer in women of developed countries. Despite surgery combined with chemo-/radiotherapy regimens, overall survival of patients with high-risk EC tumors is poor, indicating a need for novel therapies. The MEK5-ERK5 pathway is activated in response to growth factors and to different stressors, including oxidative stress and cytokines. Previous evidence supports a role for the MEK5-ERK5 pathway in the pathology of several cancers. We investigated the role of ERK5 in EC. In silico analysis of the PanCancer Atlas dataset showed alterations in components of the MEK5-ERK5 pathway in 48% of EC patients. Here, we show that ERK5 inhibition or silencing decreased EGF-induced EC cell proliferation, and that genetic deletion of MEK5 resulted in EC impaired proliferation and reduced tumor growth capacity in nude mice. Pharmacologic inhibition or ERK5 silencing impaired NF-kB pathway in EC cells and xenografts. Furthermore, we found a positive correlation between ERK5 and p65/RELA protein levels in human EC tumor samples. Mechanistically, genetic or pharmacologic impairment of ERK5 resulted in downregulation of NEMO/IKKγ expression, leading to impaired p65/RELA activity and to apoptosis in EC cells and xenografts, which was rescued by NEMO/IKKγ overexpression. Notably, ERK5 inhibition, MEK5 deletion or NF-kB inhibition sensitized EC cells to standard EC chemotherapy (paclitaxel/carboplatin) toxicity, whereas ERK5 inhibition synergized with paclitaxel to reduce tumor xenograft growth in mice. Together, our results suggest that the ERK5-NEMO-NF-κB pathway mediates EC cell proliferation and survival. We propose the ERK5/NF-κB axis as new target for EC treatment.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-022-04541-6.
Keywords: Map kinase, ERK5, NF-kB, Apoptosis, Endometrial cancer, Anticancer drug
Introduction
Endometrial cancer (EC) is the most common type of gynecologic cancer and the fourth most frequent neoplasia in women of developed countries [1]. EC originates from the uterine endometrial epithelium and is classified into two different histological subtypes [2]. Endometrioid EC accounts for the majority of ECs. These tumors express estrogen and/or progesterone receptors, which are used as targets for hormonal therapy. Non-endometrioid EC (NEEC) represents ~ 20% of cases and comprises serous and clear cell carcinomas, among others. Non-endometrioid EC tumors tend to be estrogen-independent and show enhanced aggressiveness and worse prognosis than endometrioid ECs [2].
Advanced and hormone-independent ECs are currently treated with systemic cytotoxic therapy with carboplatin/paclitaxel [3]. However, its clinical effectiveness is poor in advanced, recurrent or metastatic EC tumors, indicating a need for novel molecular therapies. Since EC tumors frequently concur with activating mutations of PI3KCA or AKT, the PI3K-AKT-mTOR pathway has been proposed for EC targeted therapies. Unfortunately, EC tumors frequently develop resistance to PI3K, Akt, or mTOR inhibitors, mainly due to hyper-activation of the Ras-MEK1 pathway [4]. Hence, there is an urgent need for new targeted therapies to improve treatment of high-risk EC.
The extracellular regulated kinase 5 (ERK5) is the most recent discovered member of the conventional MAP kinase family. ERK5 is activated by its unique MAP2K MEK5, in response to both mitogens and different forms of stress, including oxidative stress and cytokines. These effectors lead to the activation of the MEK5 upstream activators MAP3K2 and MAP3K3 [5]. ERK5 is involved in the proliferation, survival, apoptosis, and differentiation of different cell types [6]. Recently, ERK5 was shown to play an important role in the tumorigenesis, proliferation and survival of several cancer types [7, 8]. Because ERK5 inhibition or silencing compromises the viability of numerous cancer cell lines and tumor xenografts, ERK5 is emerging as a new target for molecular anticancer therapies [9].
In this work, we have investigated for the first time the role of the MEK5-ERK5 pathway in EC. We also report the preclinical development of the small-molecule JWG-071, a selective ERK5 inhibitor with a good pharmacokinetic profile. We present evidence supporting ERK5 inhibition as a targeted therapy with great potential to treat EC.
Materials and methods
Reagents
JWG-071 was in-house synthesized as previously described [10]. AX-15836, BAY-117082, paclitaxel and carboplatin were obtained from MedChem Express (Monmouth Junction, NJ, USA). Cisplatin, staurosporine and EGF were from Sigma-Aldrich (Germany). Q-VD(OMe)-OPh was from APExBio (Gentaur, Spain).
Cells and cell culture
AN3CA and ARK1 EC cells and Hela cervical cancer cells were purchased from ATCC (Manassas, VA, USA). Ishikawa cells were from ECACC and were purchased from Sigma. T-large antigen-transformed BAX−/−/BAK−/− MEFs were generously provided by Dr Victor Yuste (UAB, Spain). HeLa, ARK1 and MEF cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM ThermoFisher). AN3CA were maintained in DMEM-F12 medium (ThermoFisher). Medium was supplemented with 5% (Ishikawa cells) or 10% (rest of cells) fetal bovine serum (FBS, Gibco) and 1% Penicillin/Streptomycin (Gibco). Cells were maintained at 37 °C in a humidified atmosphere containing 5% CO2. Cell lines were transfected using Lipofectamine 2000™ (ThermoFisher Scientific, Waltham, MA, USA) as described before [11].
Cell lysis and immunoblotting
Cells were lysed in ice-cold RIPA buffer supplemented with 1 mM sodium-orthovanadate, 50 mM NaF and 5 mM sodium-pyrophosphate, sonicated and stored at − 20 °C. Subcellular fractionation was performed following standard procedures, as described before [12]. Proteins were resolved in SDS-PAGE gels and electrotransferred onto a nitrocellulose membrane (Sigma-Aldrich). After incubation with the appropriated primary, detection was performed using horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence reagent (Bio-Rad). Primary antibodies used are given in Supplementary Table S1.
DNA constructs
The pEBG2T vector encoding GST-tagged human ERK5 was previously described [12]. The pcDNA3 vector encoding for SR-IkBα was from J.X. Comella (VHIR Barcelona, Spain) [13]. pGL4-AP1 (containing six copies of an AP1-response element fused to luciferase gene) and PGLA4.32- NF-κB (five copies of a NF-κB response element) vectors were from Promega (Madison, WI, USA).
shERK5 lentiviral production
Stable and efficient silencing of endogenous ERK5 protein was achieved using lentivirus. Two pLKO.1 lentiviral vectors encoding for specific shRNA for two human MAPK7 mRNA sequences were used (TRCN0000010262/pLKO.1 and TRCN0000010275/pLKO.1, Sigma). Lentiviral particles were generated in HEK-293 cells by co-transfecting the virion vectors (psPAX2 and pMD2G) and the pLKO.1 ERK5-shRNA vectors. After 4 h, the medium was replaced with fresh medium. Forty-eight h post transfection, the medium containing the viral particles was collected, centrifuged, filtered to remove cell debris, and stored at − 80 °C until use. Lentiviral production is detailed at Supplementary Materials and Methods.
Gene silencing
Validated siRNAs targeting NEMO were from Sigma (ref. EHU032271) and ThermoFisher (ref. AM51331). Validated siRNAs targeting ERK5 were from ThermoFisher (ref. 4390825 and 4392420). Scrambled (control) siRNA was from ThermoFisher. siRNAs were transfected into cells using Lipofectamine-2000, following the manufacturer’s recommended protocol.
Quantitative real-time PCR
Total RNA was isolated from cells using RNeasy kit (Qiagen). cDNA was obtained using iScript™ cDNA Synthesis Kit (Bio-Rad). Real-time quantitative PCR assays were performed using TaqMan Gene Expression Master Mix and the probes human NEMO/IKBKG (Hs00415849_m1) and human GAPDH (Hs03929097_g1) (ThermoFisher Scientific). Amplifications were run in a Bio-Rad CFX96 real-time PCR system, using the following protocol: 50 °C for 2 min, 95 °C for 10 min, 39 cycles of 95 °C for 15 s, 55 °C for 1 min. Each value was normalized to GAPDH levels. Relative expression levels were determined using the 2−ΔΔCt method. Real-time PCR was controlled by the Bio-Rad CFX Manager v 3.1 software.
Generation of MEK5 knockout cells by CRISPR/Cas9.
To generate CRISPR/Cas9 MEK5−/− Hela or Ishikawa cells, we used a kit from Santa Cruz technology, using vectors containing sgRNAs (TACTTGCTGTTCCATTACTG and CAGATTCACTTCCAAGCAAT) and the Cas9 endonuclease gene (sc-401688), in combination with a HDR-puromycin resistance vector (sc-401688). Cells were selected with 1 µg/ml puromycin (Sigma). Single colonies were picked up and monitored for MEK5 expression. Clones showing MEK5 null expression were selected.
Cell viability, clonogenic and proliferation assays
Cell viability was determined by MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium Blue, Sigma) reduction assay. For clonogenic assays, cells were seeded in 6-well plates, treated for 14 days and stained with 0.5% crystal violet solution. Colonies with > 40 cells were counted. Cell proliferation was measured by cell counting assay, using a TC10TM automated cell counter (Bio-Rad, Hercules, CA, USA) using Trypan Blue. Cell viability was also determined using a LIVE/DEAD viability/cytotoxicity kit assay (ThermoFisher), as reported before [11].
Flow cytometry analysis of apoptosis
Cells were seeded and treated for the indicated times. Cell were then trypsinized, washed sequentially with FBS and binding buffer, and incubated in binding buffer with Annexin V and/or propidium iodide (Invitrogen, Waltham, MA, USA) for 15 min. Samples were analyzed in a Beckman Coulter FC500 flow cytometer. For nuclear staining, cells were fixed with 4% PFA, 0.1% NP40 in PBS, and nuclei were stained with 1 μg/mL Hoechst 33258 (Invitrogen).
Human EC tumor samples
Patients with no previous therapy (50–80 years) underwent surgery for endometrial carcinoma at Hospital Vall d’Hebron (Barcelona, Spain). The protocol was approved by the Institutional Review Boards, and informed consent was obtained from the patients involved. After surgery, endometrial normal tissue and tumor samples were collected from each patient and stored frozen (− 80 °C) until analysis. Samples were lysed in RIPA buffer containing protease and phosphatase inhibitor cocktails (Sigma), and stored at -20˚C until use.
Gene reporter Luciferase assay
Cells cultured in 12-well plates were transfected with 450 ng of NF-κB-driven (Stratagene) or AP 1-driven luciferase (Promega) reporter plasmids, and 50 ng Renilla luciferase (Promega). Luciferase activity assay was monitored using the dual luciferase kit (Promega), following the manufacturer’s instructions.
EC xenografts
Athymic nude female mice (Hsd: Athymic Nude-Foxn1nu, Envigo, Spain) were injected subcutaneously with 4 × 106 Ishikawa cells into the right flank. When tumors reached 80–100 mm3, mice were randomly distributed into groups and administered with the indicated treatments. JWG-071 was administered once a day intraperitoneally at dose of 50 mg/kg (monotherapy) or 30 mg/kg (combo treatment). Paclitaxel was administered twice a week intraperitoneally at dose of 15 mg/Kg. Tumors volumes were measured as (length × width2)/2, thrice a week. When tumors reached more than 1000 mm3 or body weight decreased 20% of the initial measure, mice were euthanized by cervical dislocation. Tumors were excised from mice, and a fraction of each tumor was homogenized in RIPA buffer containing protease inhibitor cocktail (Sigma), sonicated, centrifuged to remove cell debris, and stored at − 20 °C until use. All procedures involving animals were performed with approval of the UAB Animal Experimentation Committee, according to Spanish official regulations. Individual tumor growth curves are given in Supplementary Fig. 16.
Immunohistochemistry
Samples from tumor xenografts were dissected, formalin-fixed and paraffin-embedded as described previously [14]. Three µm-thickness sections were stained with haematoxylin and analyzed by immunohistochemistry using standard protocols. Briefly, deparaffinized samples were subjected to heat-induced antigen retrieval in citrate buffer. After blocking endogenous peroxidase with 3% H2O2, and reducing unspecific binding with and 5% goat serum and 0,1% Triton X-100, slices were then incubated with anti-cleaved caspase-3 antibody or anti-Ki67 antibody (Cell Signaling) and further processed with secondary antibody (LSAB2 horseradish peroxidase kit; Dako, Copenhagen, Denmark).
Statistical analysis
All in vitro data were assessed using one-way ANOVA followed by Bonferroni multiple comparison test or two-tailed Student’s t test. Tumor volumes of mice were compared using two-way ANOVA followed by Bonferroni, except for combinatorial experiment (two-way ANOVA Tukey´s test). Statistical significance between the groups was assessed with the log-rank test (GraphPad software, San Diego, CA, USA). Data in figures are presented as mean ± SD, unless otherwise indicated. Statistical significance was set at p < 0.05. Synergism analysis was performed using the Compusyn software [15].
Results
ERK5 signaling pathway is altered in EC
To investigate the impact of the MEK5-ERK5 signaling pathway in EC, we performed an in silico data analysis of the components of this pathway. Gene expression data mining from the cBioPortal for Cancer Genomics (Uterine corpus endometrial carcinoma TCGA, PanCancer Atlas) showed alterations (gene copy number, mutations or mRNA alterations) in all components of ERK5 signaling, including the upstream kinase activators MAP3K2 and MAP3K3, the MEK5 and ERK5 genes (MAP2K5 and MAPK7, respectively), and the ERK5 substrates MEF2A, MEF2B, MEF2C, and MEF2D transcription factors [16]. Of note, 48% of human EC patients (250 out of 517) showed alterations of components of ERK5 signaling, mostly associated to gene amplification and high mRNA levels (33%, 171 out of 517) (Fig. 1A). Interestingly, EC is the human cancer that showed the higher percentage of cases wherein ERK5, MEK5, MAP3K2 and MAP3K3 are mutated (Supplementary Fig. 1). Finally, patients with high levels of ERK5 mRNA expression showed lower overall survival (p = 0.000087) and reduced progression-free survival (p = 0.00094) (Fig. 1B). Taken together, these data suggest a role of ERK5 signaling in EC.
Fig. 1.
ERK5 inhibition impairs EC cell proliferation. A Genomic profiles of components of the MEK5-ERK5 pathway in EC patients obtained from Uterine Corpus Endometrial Carcinoma data set (TCGA, PanCancer Atlas). Data set from cBiportal (https://www.cbioportal.org/). MAPK7, ERK5 gene; MAP2K5, MEK5. B Kaplan–Meier plots overall survival (left) and progression-free (right) in uterine corpus endometrial carcinoma patients with high (red), quartile 1) or normal (blue, quartile 2–3) ERK5 mRNA levels (data set from cBioportal). P values were obtained using log-rank test. C Chemical structure of JWG-071. D Immunoblot analysis of the effect of JWG-071 on EGF stimulation. E MTT cytotoxicity (48 h) assay in a panel of human EC cells. F 14-day clonogenic assay. G Effect of ERK5 silencing on the proliferation of EC cell. ERK5 silencing was carried out with two different specific siRNA sequences. Scr., scramble siRNA. Cell proliferation was measured at three days of culture using crystal violet assays. ***, P < 0.001. H LRRK2 or DCLK1 inhibition does not affect human EC cell proliferation. Ishikawa or AN3CA cells were incubated for 48 h with either the specific DCLK1 inhibitor DCLK1-IN-1 or the LRRK2 specific inhibitor MLi-2, and cell proliferation was assessed by crystal violet assay. I Effect of ERK5 inhibitors JWG-071 and AX15836 in EGF-induced cell proliferation. Histograms show number of cells at day 4 referred to cells at day 0. $, P < 0.05; $$$, P < 0.001 EGF vs. vehicle. **, P < 0.01; ***, P < 0.001 ERK5i vs. EGF. J, JWG-071 blocks EGF-induced ERK5 nuclear localization. HeLa cells were pre-incubated with 3 µM JWG-071 prior stimulation with 50 nM EGF (30 min), fractionated into the cytosolic and nuclear fractions, and protein expression analyzed by immunoblot. Hsp90, cytosolic marker; CREB-1, nuclear marker. K ERK5 inhibition impairs EGF-induced c-Jun expression in Ishikawa cells. Immunoblot analysis. L JWG-071 impairs ERK5-mediated AP-1 transcriptional activity. Hela cells transfected with vectors encoding pAP-1–luciferase reporter and pRL-CMV-Renilla were subjected to a dual-luciferase reporter assay. **, P < 0.005
ERK5 inhibition impairs basal and EGF-induced EC cell proliferation
Early work reported that ERK5 mediates serum- and EGF-induced proliferation of cervical cancer HeLa cells [5], by phosphorylating and promoting transcriptional activation of MEF2C and transcription of c-Jun [16]. However, recent development of ERK5 inhibitors has led to question about the role of ERK5 kinase activity in cancer cell proliferation [17–20]. This is due to the discovery that widely used ERK5 ATP-competitive classical inhibitors (such as XMD8-92 and ERK5-IN-1) are unspecific, since they bind the acetyl-lysine domain of Bromodomain-Containing Protein-4 BRD4, a ubiquitous regulator of transcription and mediator of cancer cell proliferation. To address this question, we used the benzo[e]pyrimido-[5,4-b]diazepine-6(11H)-one core to develop a new ATP-competitive ERK5 inhibitor (JWG-071, Fig. 1C), with no BRD4 inhibitory activity [10]. JWG-071 shows selectivity for ERK5 (IC50 90 nM, in vitro kinase assay, Supplementary Fig. 2) over BRD4 (IC50 6 µM, [10]). In this work, we present the preclinical development of JWG-071.
Endometrial carcinoma cells express EGFR, and they represent a good model to investigate the role of EGF in cancer cell proliferation. Therefore, to study the role of ERK5 in basal and EGF-induced proliferation, we tested the JWG-071 compound in two different human endometrioid cancer cell lines that show EGFR expression: Ishikawa and AN3CA cells. We also used HeLa cells as a control, which also express EGFR. JWG-071 potently inhibited EGF-induced ERK5 phosphorylation in the three cancer cell lines tested (active ERK5 auto-phosphorylates, resulting in a slower migrating band), without affecting EGF-induced ERK1/2 phosphorylation (Fig. 1D). Proliferation assays showed that JWG-071 impaired cell proliferation (Fig. 1E) and colony-formation (Fig. 1F) in the cell lines tested. Furthermore, ERK5 silencing with two different specific siRNAs also resulted in a significant impairment of basal proliferation of Ishikawa and AN3CA cells, but not in cells transfected with scramble siRNA (Fig. 1G).
Kinase selectivity of JWG-071 was previously determined by profiling the inhibitor against a panel of 468 human kinases, using an in vitro ATP-site competition binding assay. Apart of ERK5, JWG-071 also inhibited the activity of Doublecortin Like kinases DCLK1 and DCLK2, as well as the activity of the leucine-rich repeat kinase 2 LRRK2 [10]. To investigate whether the antiproliferative effect of JWG-071 was due to DCLK1/2 and/or LRRK2 off-target inhibition, we used the specific inhibitor DLCK1-IN-1 (which targets both DCLK kinases [21]) and the MLi-2 compound (which specifically targets LRRK2 [22]). DLCK1-IN-1 or MLi-2 compounds had no effect on the proliferation of Ishikawa or AN3CA cells (Fig. 1H), indicating that DCLK1/2 or LRRK2 do not mediate the cytotoxicity induced by JWG-071 in EC cells.
Next, we investigated the role of ERK5 activity in EGF-induced cell proliferation. Cell counting experiments showed increased number of Ishikawa or Hela cells in response to EGF, which was significantly impaired by 1 µM JWG-071. Similar results were obtained using 1 µM AX15836 (Fig. 1I), another ERK5 specific inhibitor recently reported [17]. Active ERK5 trans-locates to the nucleus, where it phosphorylates and activates MEF2 transcription factors, which in turn promote c-Jun expression and cell proliferation [16]. In our cell models, EGF treatment resulted in a rapid (30 min) accumulation of active/phosphorylated nuclear ERK5, whereas incubation with JWG-071 abolished nuclear active/phosphorylated ERK5 (Fig. 1J). Accordingly, the ERK5i impaired EGF-induced expression of c-Jun (Fig. 1K) and AP-1 transcriptional activity (Fig. 1L). These results suggest that ERK5 mediates EGF-induced proliferation by activating the MEF2C-c-Jun axis, at least in endometrial and cervical cancer cells that express EGFR.
CRISPR/Cas9 MEK5 knockout cells show impaired basal and EGF-induced cell proliferation
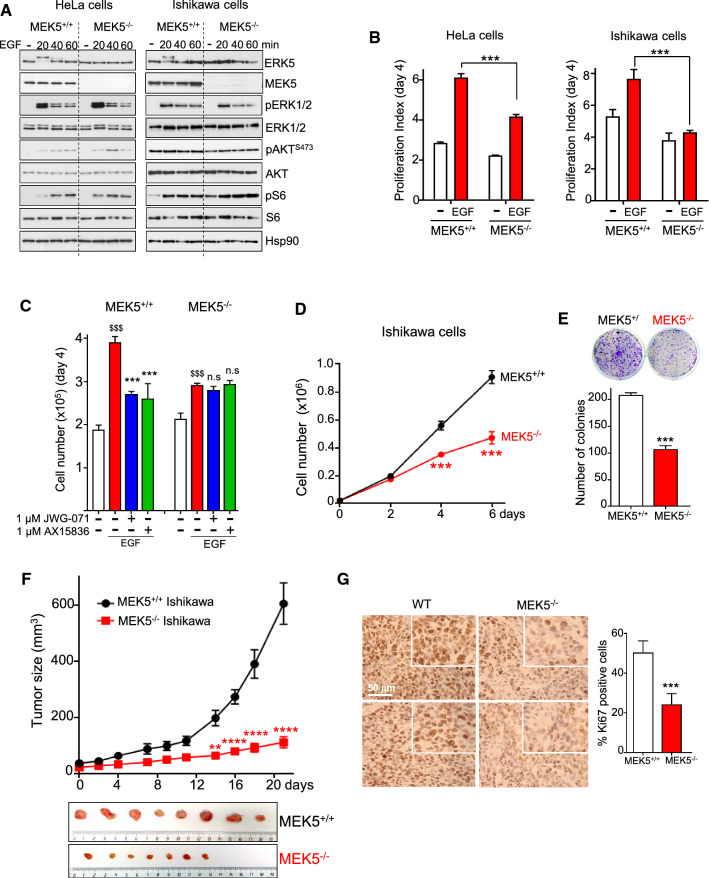
To ensure that the antiproliferative effect of JWG-071 in endometrial and cervical cancer cells expressing EGFR was due to inhibition of ERK5, we analyzed the effect of MEK5 deletion by generating stable CRISPR/Cas9 MEK5 knockout Ishikawa and HeLa cell lines. MEK5-ERK5 constitute a unique signaling module, since MEK5 is the only upstream activating kinase for ERK5, and MEK5 has ERK5 as its only known substrate [23]. Therefore, MEK5−/− cells lack ERK5 kinase activity and represent a useful tool to investigate the impact of ERK5 activity on cell physiology. As expected, and in contrast to wild type cells, MEK5−/− Ishikawa or MEK5−/− HeLa cells did not show ERK5 activation/phosphorylation in response to EGF (Fig. 2A). Importantly, genetic deletion of MEK5 did not affect phosphorylation of ERK1/2, Akt or S6 proteins in response to EGF (Fig. 2A) or insulin (Supplementary Fig. 3A), and neither affected phosphorylation of stress-associated MAP kinases p38 and JNKs in response to osmotic stress (Supplementary Fig. 3B). These results confirm that genetic deletion of MEK5 does not affect the activity of other MAP kinases or the activity of the Akt-mTORC1 axis.
Fig. 2.
MEK5 genetic deletion impairs EC cell proliferation. A CRISPR/Cas9 MEK5−/− cells show impaired ERK5 activation in response to EGF stimulation. Cells were treated with 50 nM EGF and expression of proteins was monitored by immunoblot. B CRISPR/Cas9 MEK5−/− cells show impaired EGF-induced proliferation. Histogram shows the number of cells at day 4 referred to cells at day 0. C ERK5 inhibitors do not impair EGF-induced proliferation in MEK5−/− HeLa cells. $$$, P < 0.001 EGF vs. vehicle. ***, P < 0.001 ERK5i vs. EGF. D CRISPR/Cas9 MEK5−/− Ishikawa cells show impaired proliferation, and impaired number of colonies. E 14-day clonogenic assay. F, In vivo tumor growth of subcutaneous injected MEK5+/+ (n = 8) and MEK5−/− (n = 7) Ishikawa cells. Data are presented as mean ± SEM. **, P < 0.01; ****, P < 0.0001, two-way ANOVA Bonferroni. Pictures show each of the tumors at final day of treatment (21 days). G, Immunohistochemical analysis of Ki67 expression in MEK5+/+ and MEK5−/− Ishikawa cell tumors. Right histogram shows the corresponding quantification. B, D, E, G, ***, P < 0.001
As it happened for ERK5 inhibitor JWG-071, MEK5−/− Ishikawa and MEK5−/− HeLa cells showed significantly impaired proliferation in response to EGF in cell counting experiments (Fig. 2B). More importantly, concentrations of specific ERK5 inhibitors JWG-071 or AX15386 that impaired EGF-induced proliferation in wild type cells, had no effect in the absence of MEK5 (Fig. 2C). These results confirm that activation of the MEK5-ERK5 pathway is needed for EGF-induced proliferation. Regarding basal proliferation, MEK5−/− Ishikawa cells phenocopied the effect of JWG-071, since they showed less proliferation (Fig. 2D) and colony-formation (Fig. 2E) capacity. Finally, to investigate the effect of MEK5 deletion in vivo, wild type MEK5+/+ and MEK5−/− Ishikawa cells were subcutaneously injected in athymic nude mice, and tumor growth was monitored. The deletion of MEK5 caused a drastic reduction on tumor growth in vivo (Fig. 2F), which was also evidenced by a reduction on the levels of the proliferation marker Ki67 (Fig. 2G).
In summary, our results support the use of JWG-071 as a specific ERK5 inhibitor for in vitro and in vivo studies. Furthermore, we provide evidence showing for the first time that ERK5 kinase activity mediates both, basal and EGF-induced proliferation in EC.
ERK5 inhibition induces apoptosis in human EC cell lines
The above results prompted us to investigate the anticancer activity of ERK5 inhibition in EC. To do so, we used the endometrioid cancer cell lines Ishikawa and AN3CA cells (that carry PTEN deficiency and P53 mutation), as well as the more clinically aggressive non-endometrioid serous adenocarcinoma cell line ARK1 (PI3KCA mutation). In cell viability assays, JWG-071 induced cytotoxicity in the three EC cell lines tested, with IC50 values ranging 2–3 µM. In fact, JWG-071 induced cytotoxicity in a wide panel of cancer cells, including prostate, cervical, neuroblastoma, and glioblastoma cancers (Supplementary Fig. 4).
Several authors have reported that the unspecific ERK5 inhibitor XMD8-92 induces apoptosis in different models of solid and hematological cancers (reviewed in [9]). Therefore, we next analyzed whether JWG-071 induces apoptosis in EC cells. Flow cytometry analysis (Annexin V-PI staining) revealed that the ERK5i induced a significant increase of Annexin V staining in Ishikawa, AN3CA, and ARK1 cells (Fig. 3A). Furthermore, chromatin analysis revealed that JWG-071 treatment resulted in a drastic increase in number of cells with condensed and/or fragmented chromatin, a typical hallmark of apoptotic cell death (Fig. 3B). Immunoblot analysis confirmed that JWG-071 induced caspase-3 activation in the three EC cell lines tested, resulting in increased levels of cleaved/active caspase-3 and of cleaved PARP (caspase-3 canonical substrate) (Fig. 3C). To investigate the role of caspase activation in ERK5i-induced cancer cell death, we used the pan caspase inhibitor Q-VD-OPh. Pre-incubation with Q-VD-OPh significantly reverted the cytotoxicity of JWG-071 in Ishikawa, AN3CA, and ARK1 cells (Fig. 3D). We obtained similar results for the human cervical cancer cell line HeLa, where the ERK5i worked as a potent apoptotic inducer (Supplementary Fig. 5A-D). Finally, we undertook a genetic approach to confirm that ERK5i induces apoptosis, using apoptosis-deficient Bax/Bak double knockout MEF cells [24]. JWG-071 did not induce caspase activation (Fig. 3E) or cytotoxicity (Fig. 3F) in MEF Bax−/−/Bak/−, compared to MEF wild type cells. To confirm that ERK5 activity was responsible for the pro-apoptotic effect of JWG-071, we next investigated the effect of MEK5 genetic deletion or ERK5 silencing on EC basal apoptosis. Genetic deletion of MEK5 in either Ishikawa (Fig. 3G) or HeLa (Supplementary Fig. 5E) cells resulted in a significant number of apoptotic cells. Similarly, ERK5 silencing with two different specific shRNAs also resulted in increased number of apoptotic Ishikawa cells (Fig. 3H). Thus, our results demonstrate that ERK5 inhibition induces apoptotic death in EC cells.
Fig.3.
ERK5 inhibition induces apoptotic death in endometrioid and cervical cancer cells. A Flow cytometry analysis of apoptosis induced by JWG-071 in EC cells. Q1, necrosis; Q2, late apoptosis; Q3, live cells; Q4, early apoptosis. B Representative images of nuclear morphology (Hoescht 33258) at 48-h post-treatment with JWG-071 or vehicle. Arrowheads point condensed or fragmented nuclei. Right histograms, quantification of the results (four representative fields of each condition, n = 3). C Immunoblot analysis s of cells treated with vehicle or 5 μM JWG-071 (48 h). Staurosporine (STP, 18-h) was used as a positive control. D The pan-caspase inhibitor Q-VD-OPh impairs JWG-071-induced cytotoxicity. Cell viability (MTT) assay. Values of each experimental condition are referred to untreated cells (100% cell viability). E, F Apoptosis-deficient cells are resistant to JWG-071 cytotoxicity. Bax+/+/Bak+/+ and Bax−/−/Bak−/− MEF cells were treated with vehicle, 5 µM JWG-071 (48-hous) or 1 µM staurosporine (16 h). Active caspase-3 (cCaspase 3) levels were monitored by immunoblot (E). Cell viability was determined by MTT assay (F). G CRISPR/Cas9 MEK5−/− Ishikawa cells show increased apoptosis, compared to MEK5+/+ cells. H ERK5 knock down (lentiviral shRNAs) induce apoptosis in Ishikawa cells. G, H Flow cytometry analysis of cells maintained in low serum (1% FBS) media, Right histograms show quantification of apoptotic cells (Q2 and Q3). B, D, F–H, *, P < 0.05; **, P < 0.005; ***, P < 0.001
ERK5 inhibition impairs NF-κB and activates JNK apoptotic pathways in human endometrioid cells
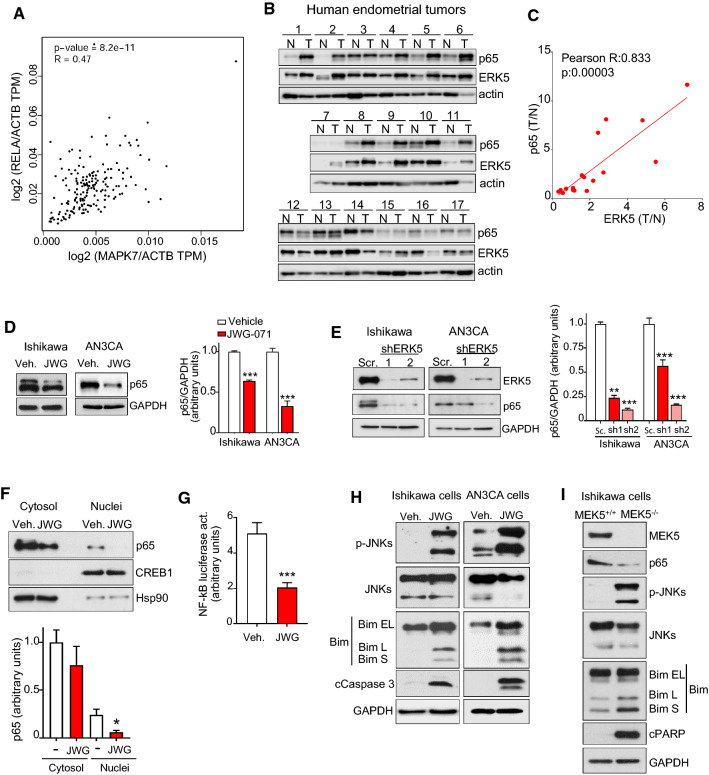
Several authors have shown a role of the MEK5-ERK5 pathway in regulating the NF-κB canonical pathway in cancers, such as leukemia [25] and colon adenocarcinoma [26]. In these cancer models, ERK5 activity induces p65/RELA nuclear translocation and transcriptional activity, whereas ERK5 inhibition or silencing impairs p65/RELA activity. NF-κB pathway regulates, among other cancer hallmarks, proliferation and survival of numerous cancer types. Because it has been suggested that the NF-κB pathway controls proliferation and viability of EC cells [27], we next investigated the role of NF-κB pathway in the anticancer activity of ERK5i.
Gene expression data mining from the cBioPortal for Cancer Genomics (Uterine corpus endometrial carcinoma TCGA, PanCancer Atlas), using the database GEPIA2 (http://gepia2.cancer-pku.cn/#index), showed a strong positive correlation between MAPK7 and RELA mRNA expression levels (R, 0.47; Spearman, 8.2e−11: Fig. 4A). We next analyzed ERK5 and p65/RELA protein levels in tumoral and non-tumoral (peritumoral) samples from 17 endometrioid cancer patients (Supplementary Fig. 6A). Immunoblot analysis revealed that 12 of 17 tumor samples showed increased p65/RELA (p = 0.031) and ERK5 (p = 0.0562) levels, compared with peri-tumoral tissue (Fig. 4B and Supplementary Fig. 6B). No differences in ERK5 protein levels were observed among different cancer grades (Supplementary Fig. 6C). Of note, we found a strong correlation between relative endometrial tumoral/non-tumoral ERK5 and p65 levels (R, 0.835; p, 0.00003, Fig. 4C).
Fig. 4.
ERK5 inhibition impairs NF-kB pathway and JNK-induced apoptosis in endometrioid cancer cells. A Spearman’s correlation analysis of ERK5 and p65/RELA mRNA expression in uterine corpus endometrial cancer patients was analyzed and plotted by GEPIA website (https://gepia.cancer-pku.cn/index.htlm). B Immunoblot analysis of samples from 17 different patients with endometrioid cancer. T, tumor area; N, non-tumor area. C Pearson’s pairwise correlation analysis of relative levels of ERK5 and p65 protein expression in non-tumoral and tumoral endometrial samples. D ERK5 inhibition impairs p65 levels in EC cells. Right histograms show the quantification of the results. E ERK5 silencing impairs endogenous p65 levels. Immunoblot analysis. Cells were infected with lentiviral shRNA particles encoding for either scramble or two different shERK5. Right histogram shows the quantification of p65 expression. F ERK5 inhibition impairs nuclear p65. Ishikawa cells treated with JWG-071 for 24 h were fractionated to obtain cytosolic and nuclear fractions. Expression of indicated proteins was monitored by immunoblot. The histogram below shows the corresponding quantification of cytosolic and nuclear p65, relative to Hsp90 and CREB levels, respectively. Results are the mean ± SD of three independent experiments. G ERK5 inhibition impairs NF-κB transcriptional activity. Cells were transfected with plasmids encoding for a Luciferase-NF-κB reporter and pRL-CMV-Renilla, treated with JWG-071 for 24 h, and subjected to dual-luciferase reporter assay. H ERK5 inhibition activates JNK apoptotic pathway. Cells were treated with vehicle or JWG-071 (48 h) and proteins analyzed by immunoblot. I MEK5 genetic deletion in Ishikawa cells results in impaired p65 levels, and in activation of JNKs and JNK-apoptotic pathway. Protein levels were monitored by immunoblot analysis. D, E–G *, P < 0.05; **, P < 0.005; ***, P < 0.001
Next, we investigated the impact of ERK5 inhibition on the NF-κB canonical pathway in EC cells. In Ishikawa and AN3CA EC cells, the ERK5i induced a significant reduction of p65 levels (Fig. 4D), whereas ERK5 silencing with two different shRNAs also induced a significant decrease in p65 protein levels (Fig. 4E). Moreover, the ERK5i treatment reduced nuclear p65 levels (subcellular fractionation assay, Fig. 4F) and NF-κB transcriptional activity (Fig. 4G). Since impairment of NF-κB canonical pathway results in activation of the JNK-mediated apoptotic pathway in several cancers [28, 29], we investigated if this was also the case in our EC cell models. Treatment of Ishikawa and AN3CA cells with the ERK5i resulted in activation/phosphorylation of JNKs, and in the appearance of Bim-L and Bim-S, the more cytotoxic forms of the pro-apoptotic JNK substrate (Fig. 4H). Interestingly, genetic deletion of MEK5 in Ishikawa (Fig. 4I) or HeLa (Supplementary Fig. 7) cells also resulted in impaired p65 levels, and in activation of JNKs and JNK-apoptotic pathway.
To explore the role of the NF-κB pathway in endometrioid cancer cell viability, we investigated the effect of inhibition of NF-κB. The IκB kinase (IKK) inhibitor BAY11-7082 induced cytotoxicity (MTT assay) and apoptosis (flow cytometry assay) in Ishikawa and AN3CA cells, with IC50 values ranging 2–3 µM (Fig. 5A, B). Furthermore, as it happened for JWG-071, treatment of EC cell lines with BAY11-7082 resulted in impaired p65 levels and in activation of the JNK pro-apoptotic pathway (Fig. 5C). Parallel experiments demonstrated that overexpression of the dominant negative mutant SR- IκBα (a non-degradable form of IκBα, [13]) impaired p65 total expression and activated the JNK-apoptotic pathway (Fig. 5D) and resulted in impaired nuclear p65 levels (Supplementary Fig. 8). Together, these results also suggest an important role of the NF-κB pathway in EC cell viability.
Fig. 5.
The NF-kB inhibition induces apoptotic cell death in endometrioid cancer cells. A Ishikawa and AN3CA cells were treated with BAY11-7082 (NF-kB pathway inhibitor) for 48 h and cell viability was assessed by MTT assay. Similar results were obtained in three separate experiments. B Representative results of flow cytometry analysis of BAY11-7082-induced cell apoptosis. Ishikawa or AN3CA cells treated with BAY11-7082 10 µM for 48 h were stained with Annexin V and propidium iodide (left panel). Histograms represent the percentage of apoptotic cells (early, Q4 quadrant; and late apoptosis, Q2 quadrant), obtained in response to vehicle or BAY11-7082 treatment. Mean values ± SD of assays performed in duplicate are shown. C Expression of proteins from cells treated with NF-kB inhibitor BAY-118072 (10 µM, 48 h) was evaluated by immunoblotting. D Impairment of p65/RELA expression activates JNK apoptotic pathway. Immunoblot analysis of cells transiently transfected with an empty vector or a vector encoding for a non-degradable IKBα protein (SR-IKBα). Right histograms show the corresponding quantification of p65 levels, referred to GAPDH. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Student’s t test)
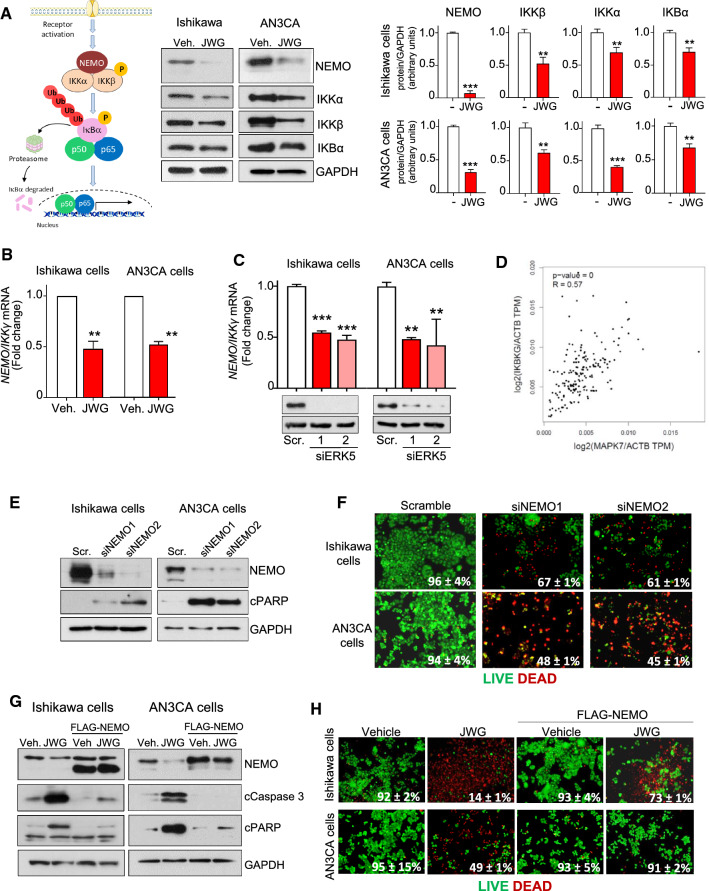
IKKɣ/NEMO mediates ERK5i-induced EC cell death
To investigate the mechanism involved in ERK5i-induced p65/RELA impairment, we next analyzed the expression levels of proteins of the NF-κB canonical pathway. In response to cytokines, such as TNFα or IL-1, the NF-κB essential modulator NEMO/IKKγ recruits and allows the activation of the IkB kinases IKKα and IKKβ. Then, phosphorylated IkB undergoes ubiquitylation and proteasomal degradation, allowing nuclear translocation of the NF-kB complex (p50/p65) and activation of transcription [30]. ERK5i treatment resulted in a drastic reduction of NEMO/IKKγ protein levels, as well as in reduced IKKα /IKKβ/IkBα levels (Fig. 6A). Importantly, ERK5 inhibition (Fig. 6B) or silencing (Fig. 6C) impaired NEMO/IKKγ mRNA levels in EC cells. Furthermore, analysis of the uterine corpus endometrial carcinoma database (TCGA, PanCancer Atlas), using the tool GEPIA2, showed a strong positive correlation between MAPK7 and NEMO/IKKγ mRNA expression levels (Fig. 6D). These results suggest a role for ERK5 in regulating NEMO/IKKγ transcription.
Fig. 6.
IKKɣ/NEMO mediates ERK5i-induced EC cell death. A ERK5 inhibition decreases protein levels of IKKɣ/NEMO and downstream components of the NF-kB canonical pathway. Protein levels of cells treated with either vehicle or JWG-071 (48 h) were analyzed by immunoblotting. Histograms show the quantification of the results. B-C ERK5 inhibition (B) or silencing (C) impairs NEMO/IKKɣ mRNA levels. mRNA levels were analyzed by qRT-PCR and normalized by GAPDH mRNA levels. D Spearman correlation analysis of ERK5 and p65/RELA mRNA expression in uterine corpus endometrial cancer patients was analyzed and plotted using GEPIA website (htpp://gepia.cancer-pku.cn/index.htlm). E NEMO/IKKγ silencing induces apoptosis. EC cells were transfected with control siRNA (Scr) or NEMO/IKKγ-selective siRNAs and levels of proteins were monitored by immunoblotting. F NEMO/IKKγ silencing induces EC cell death. Cells were stained with LIVE/DEAD reagent, and alive (green) and dead (red) were visualized by fluorescence microscopy. Figure shows representative fields, and percentage of alive cells is given. G NEMO/IKKγ overexpression prevents caspase-3 activation in response to JWG-071. Cells transfected with either an empty vector or a vector encoding for human NEMO/IKKγ were treated with vehicle or JWG-071 (48 h), and levels of the indicated proteins were monitored by immunoblotting. H, NEMO/IKKγ overexpression prevents EC cell death induced by JWG-071. A–C **, P < 0.005; ***, P < 0.001
Next, we studied the impact of NEMO in EC cell viability. Silencing of NEMO/IKKγ with two different siRNAs resulted in activation of caspase-3, as judged by the cleavage of the caspase-3 substrate PARP (Fig. 6E) and in a drastic reduction of cell viability (Fig. 6F). Conversely, overexpression of NEMO protein protected EC cells from the cytotoxicity and apoptosis (active caspase-3) induced by the ERK5i (Fig. 6G, H). Of note, DLCK1 or LRRK2 inhibition did not affect NEMO/IKKγ or p65 protein expression levels in Ishikawa or AN3CA cells (Supplementary Fig. 9). All in all, these results suggest that NEMO/IKKγ expression mediates the cytotoxicity induced by ERK5 inhibition in EC cells.
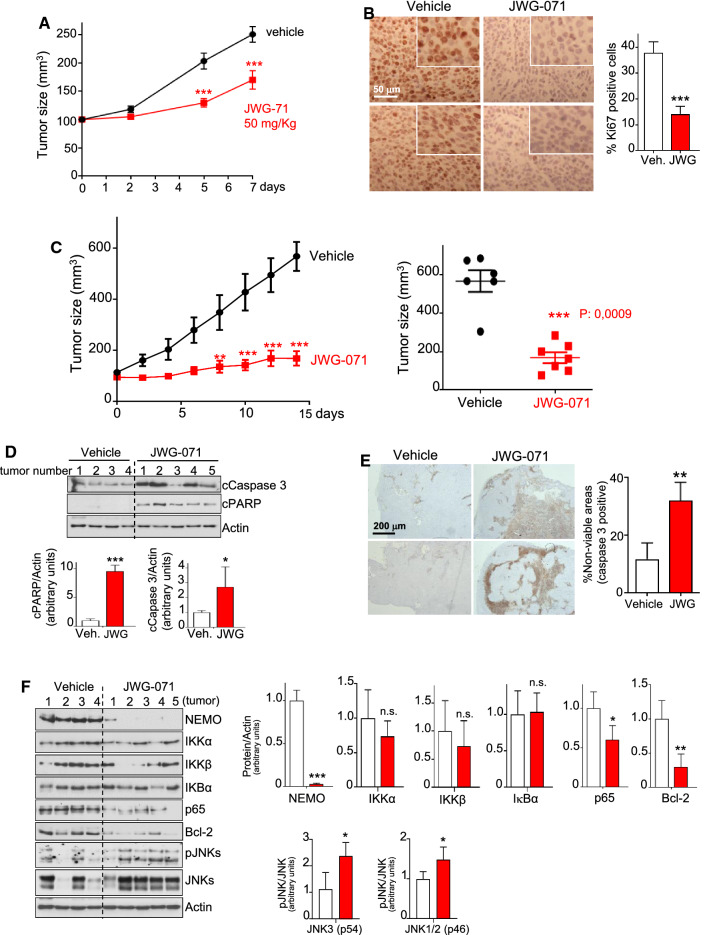
Antitumor activity of ERK5i in EC xenografts
To determine the antitumor activity of JWG-071 in vivo, we selected the Ishikawa cells as a representative EC cell line. The suitability of JWG-071 for in vivo use was assured by pharmacokinetic analysis. JWG-071 demonstrated a favorable pharmacokinetic profile in mice, with 84% oral bioavailability, a Tmax of 1 h, and a half-life of 4,34 h (Supplementary Fig. 10). We first tested the effect of JWG-071 systemic administration on the proliferation of EC tumors in short-term experiments. JWG-071 significantly impaired the growth of human endometrial tumor xenografts (Ishikawa cells) after 5- or 7-day treatment (Fig. 7A). Immunohistochemical analysis showed a significant decrease in levels of the proliferation marker Ki67 in tumors treated with the ERK5i, suggesting that JWG-071 treatment impaired the proliferation of EC tumor cells in vivo (Fig. 7B).
Fig. 7.
ERK5 inhibition impairs growth of EC tumor xenografts in vivo. A Tumor growth curve of subcutaneous xenograft (Ishikawa cells), treated i.p. with either vehicle or 50 mg/Kg JWG-071 daily. N = 6 mice per group. Data are presented as mean ± SEM. ****, P < 0.0001, two-way ANOVA Bonferroni. B Immunohistochemical analysis of Ki67 expression of xenograft tumors. Right histograms show Ki67 quantification. ***, P < 0.001. C, Tumor growth curves. Nude mice bearing Ishikawa cell subcutaneous xenograft tumors were daily treated i.p. with either vehicle (n = 6) or 50 mg/Kg JWG-071 (n = 6). Values represent the mean ± SEM. *, P < 0.05; ***, P < 0.001, two-way ANOVA Bonferroni. Right panel shows the corresponding scatter plot for tumors at day 14 of treatment. D–E Immunoblot (D) and cleaved caspase-3 immunohistochemical (E) analysis of the endometrioid xenograft tumors. Histograms show the corresponding quantification. F Immunoblot analysis of NF-κB pathway proteins from xenograft tumors. Histograms show the corresponding quantifications. B, D–F *, P < 0.05; **, P < 0.01; ***, P < 0.001
In long-term experiments in mice with established subcutaneous Ishikawa tumors, JWG-071 (50 mg/kg once per day) potently impaired tumor growth, with ~ 85% tumor growth inhibition (Fig. 7C), without affecting mouse weight (Supplementary Fig. 11). Immunoblot (Fig. 7D) and immunohistochemical (Fig. 7E) analysis showed that administration of JWG-071 potently activated apoptosis in EC xenograft tumors. Furthermore, ERK5i treatment also resulted in a drastic impairment of NEMO/IKKγ and p65/RELA protein levels in tumors, as well as of the p65/RELA transcriptional substrate Bcl-2 (Fig. 7F). No significant changes in IKKα /IKKβ/IkBα protein levels were observed. Interestingly, ERK5i systemic treatment also resulted in activation of JNKs in tumors. Together, these results demonstrate that effective ERK5 inhibition impairs the NF-κB canonical pathway, as well as cell and tumor viability.
ERK5i sensitizes endometrioid cancer cells and tumor xenografts to chemotherapy
Recent evidence has led to propose ERK5 inhibition as a promising strategy to sensitize lung, breast, or colorectal cancer cells to standard chemotherapy (see [8] for review). To investigate whether this was also the case for EC, we used paclitaxel and carboplatin, two chemotherapies used in combination as standard of care to treat patients with advanced EC (1). ERK5 inhibition synergized with either paclitaxel or carboplatin to promote cytotoxicity in Ishikawa and AN3CA EC cells (Fig. 8A, see combination index values lower than 1). We obtained similar results in colony-formation assays, where combined ERK5i and chemotherapy treatment resulted in a drastic reduction in the number of colonies, compared to single treatments (Fig. 8B). Importantly, CRISPR MEK5−/− cells, which lack ERK5 kinase activity, also showed enhanced sensitivity to paclitaxel and carboplatin treatment, reinforcing the notion that pharmacologic (ERK5i) or genetic (MEK5 knockout) inhibition of ERK5 kinase activity sensitizes EC cells to standard chemotherapy (Fig. 8C). Of note, the NF-κB inhibitor BAY11-7082 also sensitized Ishikawa and AN3CA EC cells to paclitaxel and carboplatin (Supplementary Fig. 12).
Fig. 8.
ERK5 inhibition sensitizes endometrioid cancer cells to standard chemotherapeutic agents. A Histograms show cell viability (MTT) assays performed in EC cells treated for 48 h with the indicated concentrations of JWG-071 (µM), cisplatin (CPT, µM), carboplatin (Carb, µM) or paclitaxel (PTX, nM). Lower tables show the combination index (CI) analysis, obtained using the Compusyn software (CI > 1, antagonism; CI = 1, summary effect; CI < 1, synergism). B 14-day clonogenic assay. Left, representative images of single-cell clone proliferation, stained with crystal violet. Right, quantification of number of colonies. C MEK5 deletion sensitizes EC cells to chemotherapy. Cell viability (MTT) assay of MEK5+/+ and MEK5−/− Ishikawa cells. A–C *, P < 0.05; **, P < 0.005; ***, P < 0.001. D ERK5i sensitizes EC tumor xenografts to chemotherapy. Athymic nude mice bearing EC tumor xenografts (Ishikawa cells) were treated with vehicle, 30 mg/Kg JWG-071 (i.p., daily), 15 mg/Kg paclitaxel (i.p., BIW) or combo. Values represent the mean ± SEM of 6 mice for each group. Low panel shows the corresponding scatter plot for tumors at day 15 of treatment. **, P < 0.05; ****, 0.0001, two-way ANOVA Tukey. E Tumors were collected and cleaved caspase-3 was evaluated by immunohistochemical analysis. Right histogram shows the corresponding quantification. $, P < 0.05 combo treatment vs. single treatments. * P < 0.05; ** P < 0.001 ERK5i or paclitaxel vs. vehicle
Finally, to evaluate the effectiveness of ERK5 inhibition and chemotherapy as a combinatorial treatment in vivo, we investigated the effect of JWG-071 and/or paclitaxel in athymic nude mice subcutaneously injected with human endometrioid Ishikawa cells. Single treatments with low doses of paclitaxel (15 mg/Kg) or of JWG-071 (30 mg/Kg) produced 40–50% tumor growth inhibition, compared to the control group. However, combinatorial treatment using both paclitaxel and JWG-071 resulted in a significantly greater inhibition of tumor growth compared with vehicle-treated, tumor-bearing mice (combo vs paclitaxel p < 0.0001; combo vs JWG-071 p = 0.0059) (Fig. 8D). Both mono- and combinatorial treatments were well tolerated by mice, without significant weight loss or other overt effects (Supplementary Fig. 11B). In line with results obtained in EC cultured cells, tumors from mice receiving combinatorial therapy exhibited significantly greater staining for the apoptotic marker active caspase-3, compared with tumors from mice receiving single treatment (Fig. 8E). Thus, ERK5 inhibition sensitizes EC cells and tumors to standard chemotherapy in vitro and in vivo.
Discussion
EC is the most common gynecologic malignancy in the western world, accounting for one third of the deaths related to gynecologic cancers [1]. Early-stage EC carcinomas are treated by surgery and radiation, but the advanced stage has a very poor prognosis [3]. Standard pharmacological treatment of advanced endometrial carcinoma uses a combined regimen of taxanes (paclitaxel) and platinums (carboplatin) [3]. Often, treatments fail due to the emergence of chemoresistance, and other therapeutic options are limited. Therefore, there is an urgent need for new therapeutic therapies to improve the treatment of advanced EC. Understanding the signal transduction pathways dysregulated in EC might be of help in identifying novel therapeutic targets to improve patients’ prognosis and treatment outcomes.
In this study, we propose ERK5 inhibition as a new potential targeted therapy to treat EC, both as monotherapy and in combination with standard chemotherapy. Hence, we report the preclinical development of the small-molecule JWG-071, a potent and selective ERK5 competitive inhibitor with a good pharmacokinetic profile. JWG-071 is the first specific ERK5 kinase inhibitor (no BRD4 activity when used up to 10 µM concentrations in cells, [10]) that shows antitumor activity in animal models.
Most of the ERK5 inhibitors developed so far, including JWG-071, are type I competitive inhibitors that target the ATP-binding site. In in vitro kinase (cell-free) assays, JWG-071 showed an IC50 value of ~ 90 nM or ~ 500 nM when using 50 µM or 200 µM of ATP, respectively (Supplementary Figure S2). Taking in account that the homeostatic levels of intracellular ATP range from 0.5 to 5 mM [31], it is very likely that ERK5 competitive inhibitors (such as JWG-071) should require µM concentrations to displace ATP from the active site in cells. In this regard, Knight and Shokat [32] described that ATP-competitive inhibitors should be active in cells at concentrations 10- to 100-fold above their Ki (determined in cell-free in vitro assays). Furthermore, ERK5 possesses an unusual high Km for ATP (~ 360 μM), so ERK5 kinase activity is sensible to changes in intracellular ATP.
In our study, we found that 1–3 µM concentrations of JWG-071 were enough to block EGF-induced ERK5 activation (Fig. 1D) and proliferation (Fig. 1I) in EC cells. However, it was necessary to use higher concentrations to impair EC basal cell proliferation (Fig. 1E) and colony-formation capacity (Fig. 1F). This might be well due to differences on the experimental conditions used. To investigate the effect of JWG-071 on EGF-mediated ERK5 phosphorylation, cells were serum-starved for 16 h, previous stimulation with EGF. In contrast, colony-formation experiments were performed in cells cultured in complete medium (10% FBS). Since serum starvation results in lower levels of ATP concentration [33], it is plausible that ERK5 competitive inhibitors show higher potency in cells with low ATP levels, such as those subjected to serum starvation.
Here, we demonstrate the requirement of ERK5 kinase activity for EC cell proliferation and survival. In line with this, EC patients with high levels of ERK5 mRNA expression had significant lower overall survival (OS) and progression-free survival (Uterine corpus endometrial carcinoma TCGA, PanCancer Atlas, cBioPortal). This is different to that described by Monti et al., whose in silico analysis reported that high ERK5 expression was protective for OS in all UCEC patients [34]. The reason for this discrepancy could be due of the type of analysis performed. In our study, we used the UCEC TCGA-PanCancer Atlas dataset to compare UCEC cases showing exclusively high (1st quartile) ERK5 mRNA expression (25 out of 477), with those cases showing unaltered ERK5 expression. This analysis rendered a significant lower overall survival for patients with high ERK5 mRNA levels (Fig. 1 A). To support our observations, we also performed similar analysis using the other endometrial carcinoma datasets available at the cBioPortal server. Two of these datasets (MSK 2018 and CPTAC Cell 2020) had no mRNA data for ERK5. The other two datasets, Firehose Legacy and Nature 2013, contain few EC cases showing high mRNA ERK5 expression (7 and 14 cases, respectively). However, and in agreement with the results showed in Fig. 1B, Kaplan–Meier overall survival curves of both datasets showed lower overall survival for patients with high ERK5 mRNA (Supplementary Fig. 13).
Early work reported a role for ERK5 in mediating EGF- and serum-induced proliferation in cervical cancer cells [5]. Interestingly, EGFR is commonly expressed in normal endometrium, and its overexpression in EC (43–67% of EC patients) is associated with advanced stage and poor prognosis [35]. Here, we show for the first time that ERK5 inhibition impaired basal and EGF-induced proliferation of EC cells that express EGFR (Figs. 1, 2). Specific ERK5 inhibition was achieved using either the JWG-071 small compound or EC cells lacking the ERK5 upstream kinase MEK5. MEK5−/− EC cells showed a reduced basal proliferation activity and clonogenicity, as well as a drastic reduced tumor growth in nude mice (Fig. 2), phenocopying the effect of the ERK5i. Importantly, MEK5 deletion in EC cells did not affect activation of ERK1/2, Akt or mTORC1 in response to EGF or insulin, nor did it affect basal activity of these kinases. Our results suggest a genuine role of the MEK5-ERK5 pathway in mediating EC proliferation, at least in epithelial EC cells that express EGFR, as it has been reported for other carcinoma cancers, such as hepatocellular carcinoma [36], prostate adenocarcinoma [37] or non-small cell lung cancer [20]. Of note, it has been reported that ERK5 is dispensable for proliferation of colorectal cancer cells carrying KRAS/BRAF mutations [38], suggesting that genetic mutational background must be considered to establish the exact role of ERK5 in proliferation of different cancer types.
Mechanistically, ERK5 inhibition of EC cells resulted in impairment of EGF-induced c-Jun expression and AP-1 transcriptional activity, as reported for other ERK5i in cancer types, such as breast cancer [39] and hepatocellular carcinoma [36]. Interestingly, analysis of the UCEC dataset using the database GEPIA2 showed a strong positive correlation between MAPK7 and MEF2A (R, 0.62; Spearman, < e−20) MEF2C (R, 0.55, Spearman, 4.4e−15) and MEF2D (R, 0.65, Spearman, < e−20) mRNA expression levels (Supplementary Fig. 14). Since active ERK5 phosphorylates and activates nuclear MEF2 transcription factors, promoting c-Jun expression and cell proliferation [16], these findings support the ERK5-MEF2C-c-Jun axis as a mediator for EGF-driven EC proliferation. Thus, ERK5 inhibition, by preventing EGF-induced ERK5 nuclear translocation, impaired the ERK5-MEF2C-c-Jun axis, leading to slow down proliferation. In this regard, it will be important to investigate whether EGFR overexpression correlates with nuclear ERK5 IHC staining in EC tumors from patients.
ERK5 also participates in the proliferative signaling in response to activation of HER2 receptors in breast cancer cells, [40], and ERK5 inhibition sensitizes these cells to anti-HER2 treatments [41]. 20%-40% of patients with advanced non-endometrioid EC show HER2 overexpression [42], and Trastuzumab added to standard chemotherapy improved survival of patients with HER+ aggressive serous EC, according to a recent phase 2 study [43]. Thus, it will be relevant to investigate whether ERK5 also mediates HER2 signaling in aggressive EC, and whether ERK5 inhibition could be of help to sensitize these cells to anti-HER2 therapies.
The NF-κB pathway is a key regulator of proliferation and survival of many types of cancers, by controlling the expression of a plethora of genes involved in proliferation, angiogenesis, inflammation, and survival, among others [44]. Early work suggested a role for the NF-κB pathway in endometrial carcinogenesis [27]. This was later confirmed by an IHC study that found increased nuclear NF-κB expression in patients with EC, compared with benign and hyperplasic endometrial tissues, and suggested a role for NF-κB pathway in the development of malign endometrial changes [45]. These observations are in agreement with those reported for other gynecologic cancers, such ovary [46] and cervical [47] cancers, where NF-κB is involved in tumor progression.
Here, we found a significant and positive correlation between ERK5 and p65/RELA protein levels in human EC tumor samples, and that ERK5 inhibition or silencing, or MEK5 genetic deletion, resulted in impairment of the NF-κB canonical pathway in EC cells and tumor xenografts. These results are in agreement with those reported for colorectal [26] and leukemic [25] cancers, where MEK5/ERK5 overexpression results in enhanced p65/RELA nuclear localization and transcriptional activity. Furthermore, ERK5 is essential for NF-κB-induced survival in leukemic cells [25], and ERK5 protein levels positively correlate with p65/RELA levels in colon carcinoma tumor samples [26]. However, the mechanism by which ERK5 modulates NF-κB pathway in several cancer types remains to be described. In this work, we report that ERK5 inhibition or silencing results in downregulation of NEMO/IKKγ expression, leading to impaired p65/RELA activity and apoptosis, which was rescued when NEMO/IKKγ was transiently overexpressed. Although we found that ERK5 inhibition or silencing significantly reduced NEMO/IKKγ mRNA levels, we cannot discard a role of ERK5 in regulating NEMO/IKKγ stability, as described for other ERK5 substrates such as KLF2 [48]. Further work will be necessary to establish if ERK5 phosphorylates NEMO/IKKγ, and the implications on the stability of this protein.
We also provide evidence showing, for the first time, that the NEMO-IKKβ-p65 axis mediates EC cell survival. Hence, NEMO silencing, IKKβ inhibition, or p65 inhibition (by the SR-IkB repressor) resulted in apoptotic death of EC cells. In all the cases, we observed activation of the JNK-Bim apoptotic pathway in response to canonical NF-kB inhibition, as it has been previously reporter [28, 29]. Interestingly, MEK5 genetic deletion also resulted in activation of the JNK-Bim apoptotic pathway, supporting a role for ERK5 in regulating NF-kB canonical activity. However, the specific JNK inhibitor JNK-IN-8 did not rescue the cytotoxicity induced by the ERK5 inhibitor (Supplementary Fig. 15), indicating that other pathways might contribute to the cytotoxicity induced by the ERK5i. In this regard, we have recently described that ERK5 inhibition induces cytotoxic autophagy in human pancreatic cancer cells [11]. Hence, it will be important to investigate whether impairment of the ERK5 pathway also results in cytotoxic autophagy in EC cells and tumors.
Our results point to NF-κB as an interesting target for EC treatment, as it has been proposed for other cancer types [44]. However, several IKKα or IKKβ inhibitors have failed in clinical trials, mainly due to toxicity concerns [49]. This was probably due to the fact that these inhibitors block the activation of the IKK complex (IKKα /IKKβ, necessary for the activation of the canonical NF-κB pathway) as well as the activity of individual IKKβ and IKKα (necessary for the activation of the non-canonical pathway) [49]. On the contrary, NEMO/IKKγ inhibition affects the activity of the NF-κB pathway mediated by the IKK complex (canonical pathway), but not the activity of individual IKKα or IKKβ or the non-canonical pathway. Concordantly, a small compound that blocks the binding of NEMO/IKKγ to IKKβ has shown anticancer activity in colorectal cancer tumor xenografts [50]. Considering that ERK5 inhibition or silencing impaired NEMO/IKKγ expression in EC cells and xenograft tumors, we speculate that ERK5 inhibition could be an effective and safe strategy to impair the NF-κB pathway in those cancers where NF-κB acts as a driver, such as EC.
Finally, here we report that ERK5 inhibition sensitizes EC cells and xenograft tumors to the standard chemotherapy agents paclitaxel and carboplatin (1), suggesting that ERK5i could be used as an effective targeted therapy to improve chemotherapy of EC patients. Our results are in line with those reported for other cancer types. Thus, among others, ERK5 inhibitor XMD8-92 improves the cytotoxicity of docetaxel and/or doxorubicin in lung [51], triple negative breast [52] and squamous skin [53] cancer cells and tumors, as well as the antitumor action of 5’-fluoroacil in p53-dependent colorectal cancer cells [54]. Furthermore, ERK5 silencing also potentiates doxorubicin and cisplatin therapies in malignant mesothelioma cells [55], or doxorubicin, cyclophosphamide, and paclitaxel in TNBC cells [56]. The mechanism by which ERK5 inhibition sensitizes several types of cancer cells remains to be described, although it has been suggested that it could be due to inhibition of some of the ABC transporters [57]. Since we found that pharmacologic inhibition of the NF-κB pathway (IKKβ inhibitor BAY-117082) also potentiated the cytotoxic effect of paclitaxel and carboplatin in EC cells, it is plausible that NF-κB inhibition may mediate the sensitization of EC cells to chemotherapy exerted by ERK5 inhibitors. Moreover, because activation of the NF-κB pathway is implicated in resistance to taxanes and platinum-based drugs in several preclinical models of cancer [58], it is tempting to speculate that ERK5 inhibition might prevent NF-κB-mediated resistance to chemotherapy in EC cells. Further work will be necessary to investigate this possibility.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
S. Espinosa-Gil is a recipient of a fellowship from FI-AGAUR (2020-FISDU-00575). N. Dieguez-Martinez and I. Bolinaga-Ayala are recipients of PIF fellowships from UAB. Gokhan Gorgisen is a recipient of the 2219 International Postdoctoral Research Fellowship Program for Turkish Citizens from the Scientific and Technological Research Council of Turkey (TUBİTAK). We are grateful to Cristina Gutierrez, and Neus Ontiveros for tissue culture, to Mar Hernández for IHC assistance. We also thank Miguel Segura and Victor Yuste for helpful discussions. We thank the following UAB Services: Servei de Cultius Cel·lulars INc, Laboratori de Luminiscència Espectroscopia and Servei Genòmica Informàtica. We also thank to the UAT Service from VHIR.
Author contributions
Conceived and designed the experiments: ND-M, SE-G, XD and JML. Performed experiments: ND-M, SE-G, GY, EM-R, IB-A, MV-C, GG, ID-O and JML. Analysis of the data: ND-M, SE-G, HP-M, EC, JRB, XD and JML. Writing–original draft: ND-M, SE-G and JML. All the authors read, critically revised, and approved the final manuscript.
Funding
Open Access Funding provided by Universitat Autonoma de Barcelona. The JM Lizcano research group was supported by grants from the Spanish Ministry of Economy and Competitiveness (MINECO, grant SAF2015-64237-R), and the Spanish Ministry of Science and Innovation (grant PID2019-107561RB-I00), and co-funded by the European Regional Development Fund (ERDF).
Data availability
The original contributions presented in the study are included in the article/supplementary material, and further inquiries can be directed to the corresponding author.
Declarations
Conflict of interest
Elisabet Megias-Roda and Héctor Pérez-Montoyo are Ability Pharmaceuticals employees; Jose M Lizcano serves as an uncompensated member of Ability Pharmaceuticals advisory board, and holds shares of the company.
Ethical approval
The animal study was approved by the Ethics Committee on Animal Experiments of the Universitat Autònoma de Barcelona (CEEAH) and the Ethics Commission in Animal Experimentation of the Generalitat de Catalunya, Spain.
Consent to participate
The use of samples from patients was approved by the Institutional Review Boards of Hospital Vall d’Hebron (Barcelona, Spain), and written informed consent was obtained from the patients involved.
Consent to publish
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Colombo N, Creutzberg C, Amant F, et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Ann Oncol. 2016;27:16–41. doi: 10.1093/annonc/mdv484. [DOI] [PubMed] [Google Scholar]
- 3.Morice P, Leary A, Creutzberg C, et al. Endometrial cancer. Lancet. 2016;387:1094–1108. doi: 10.1016/S0140-6736(15)00130-0. [DOI] [PubMed] [Google Scholar]
- 4.Bregar AJ, Growdon WB. Emerging strategies for targeting PI3K in gynecologic cancer. Gynecol Oncol. 2016;140:333–344. doi: 10.1016/j.ygyno.2015.09.083. [DOI] [PubMed] [Google Scholar]
- 5.Kato Y, Tapping RI, Huang S, et al. Bmk1/Erk5 is required for cell proliferation induced by epidermal growth factor. Nature. 1998;395:713–716. doi: 10.1038/27234. [DOI] [PubMed] [Google Scholar]
- 6.Drew BA, Burow ME, Beckman BS. MEK5/ERK5 pathway: the first fifteen years. Biochim Biophys Acta. 2012;1825:37–48. doi: 10.1016/j.bbcan.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lochhead PA, Gilley R, Cook SJ. ERK5 and its role in tumour development. Biochem Soc Trans 40:251–256. Biochem Soc Trans. 2012;40:251–256. doi: 10.1042/BST20110663. [DOI] [PubMed] [Google Scholar]
- 8.Stecca B, Rovida E. Impact of ERK5 on the hallmarks of cancer. Int J Mol Sci. 2019;20:1426. doi: 10.3390/ijms20061426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pereira DM, Rodrigues CMP. Targeted avenues for cancer treatment: the MEK5-ERK5 signaling pathway. Trends Mol Med. 2020;26:394–407. doi: 10.1016/j.molmed.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Erazo T, Ferguson FM, et al. Structural and atropisomeric factors governing the selectivity of pyrimido-benzodiazipinones as inhibitors of kinases and bromodomains. ACS Chem Biol. 2018;13:2438–2448. doi: 10.1021/acschembio.7b00638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gámez-García A, Bolinaga-Ayala I, Yoldi G, et al. ERK5 inhibition induces autophagy-mediated cancer cell death by activating ER stress. Front Cell Dev Biol. 2021;9:1–16. doi: 10.3389/fcell.2021.742049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erazo T, Moreno A, Ruiz-Babot G, et al. Canonical and kinase activity-independent mechanisms for extracellular signal-regulated kinase 5 (ERK5) nuclear translocation require dissociation of Hsp90 from the ERK5-Cdc37 complex. Mol Cell Biol. 2013;33:1671–1686. doi: 10.1128/MCB.01246-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marques-Fernandez F, Planells-Ferrer L, Gozzelino R, et al. TNFα induces survival through the FLIP-L-dependent activation of the MAPK/ERK pathway. Cell Death Dis. 2013;4:e493. doi: 10.1038/cddis.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erazo T, Lorente M, Lopez-Plana A, et al. The new antitumor drug ABTL0812 inhibits the Akt/mTORC1 axis by upregulating tribbles-3 pseudokinase. Clin Cancer Res. 2016;22:2508–2519. doi: 10.1158/1078-0432.CCR-15-1808. [DOI] [PubMed] [Google Scholar]
- 15.Chou TC. Drug combination studies and their synergy quantification using the chou-talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 16.Kato Y, Kravchenko VV, Tapping RI, et al. BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C. EMBO J. 1997;16:7054–7066. doi: 10.1093/emboj/16.23.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin ECK, Amantea CM, Nomanbhoy TK, et al. ERK5 kinase activity is dispensable for cellular immune response and proliferation. Proc Natl Acad Sci USA. 2016;113:11865–11870. doi: 10.1073/pnas.1609019113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rovida E, Di Maira G, Tusa I, et al. The mitogen-activated protein kinase ERK5 regulates the development and growth of hepatocellular carcinoma. Gut. 2015;64:1454–1465. doi: 10.1136/gutjnl-2014-306761. [DOI] [PubMed] [Google Scholar]
- 19.Tusa I, Gagliardi S, Tubita A, et al. The hedgehog-GLI pathway regulates MEK5-ERK5 expression and activation in melanoma cells. Int J Mol Sci. 2021;22:11259. doi: 10.3390/ijms222011259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sánchez-Fdez A, Re-Louhau MF, Rodríguez-Núñez P, et al. Clinical, genetic and pharmacological data support targeting the MEK5/ERK5 module in lung cancer. npj Precis Oncol. 2021;5:78. doi: 10.1038/s41698-021-00218-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferguson FM, Nabet B, Raghavan S, et al. Discovery of a selective inhibitor of doublecortin like kinase 1. Nat Chem Biol. 2020;6:635–643. doi: 10.1038/s41589-020-0506-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fell MJ, Mirescu C, Basu K, et al. MLi-2, a potent, selective, and centrally active compound for exploring the therapeutic potential and safety of lrrk2 kinase inhibition. J Pharmacol Exp Ther. 2015;355:397. doi: 10.1124/jpet.115.227587. [DOI] [PubMed] [Google Scholar]
- 23.Gomez N, Erazo T, Lizcano JM. ERK5 and cell proliferation: nuclear localization is what matters. Front Cell Dev Biol. 2016 doi: 10.3389/fcell.2016.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zong W-X, Lindsten T, Ross AJ, et al. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 2001 doi: 10.1101/gad.897601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garaude J, Cherni S, Kaminski S, et al. ERK5 activates NF-kappaB in leukemic T cells and is essential for their growth in vivo. J Immunol. 2006;177:7607–7617. doi: 10.4049/jimmunol.177.11.7607. [DOI] [PubMed] [Google Scholar]
- 26.Simões A, Pereira DM, Gomes SE, et al. Aberrant MEK5/ERK5 signalling contributes to human colon cancer progression via NF-κB activation. Cell Death Dis. 2015;6:e1718. doi: 10.1038/cddis.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pallares J, Martínez-Guitarte JL, Dolcet X, et al. Abnormalities in the NF-κB family and related proteins in endometrial carcinoma. J Pathol. 2004;204:569–577. doi: 10.1002/path.1666. [DOI] [PubMed] [Google Scholar]
- 28.Tang G, Minemoto Y, Dibling B, et al. Inhibition of JNK activation through NF-κB target genes. Nature. 2001;414:313–317. doi: 10.1038/35104568. [DOI] [PubMed] [Google Scholar]
- 29.Papa S, Zazzeroni F, Pham CG, et al. Linking JNK signaling to NF-κB: a key to survival. J Cell Sci. 2004;117:5197–5208. doi: 10.1242/jcs.01483. [DOI] [PubMed] [Google Scholar]
- 30.Gilmore TD. Introduction to NF-κB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 31.Gribble FM, Loussouarn G, Tucker SJ, et al. A novel method for measurement of submembrane ATP concentration. J Biol Chem. 2000;275:30046–30049. doi: 10.1074/jbc.M001010200. [DOI] [PubMed] [Google Scholar]
- 32.Knight ZA, Shokat KM. Features of selective kinase inhibitors. Chem Biol. 2005;12:621–637. doi: 10.1016/j.chembiol.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 33.Ching JK, Rajguru P, Marupudi N, et al. A role for AMPK in increased insulin action after serum starvation. Am J Physiol Physiol. 2010;299:C1171–C1179. doi: 10.1152/ajpcell.00514.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monti M, Celli J, Missale F, et al. Clinical significance and regulation of ERK5 expression and function in cancer. Cancers. 2022;14:348. doi: 10.3390/cancers14020348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konecny GE, Santos L, Winterhoff B, et al. HER2 gene amplification and EGFR expression in a large cohort of surgically staged patients with nonendometrioid (type II) endometrial cancer. Br J Cancer. 2009;100:89–95. doi: 10.1038/sj.bjc.6604814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rovida E, Di MG, Tusa I, et al. The mitogen-activated protein kinase ERK5 regulates the development and growth of hepatocellular carcinoma. Gut. 2015;64:1454–1465. doi: 10.1136/gutjnl-2014-306761. [DOI] [PubMed] [Google Scholar]
- 37.Mehta PB, Jenkins BL, McCarthy L, et al. MEK5 overexpression is associated with metastatic prostate cancer, and stimulates proliferation, MMP-9 expression and invasion. Oncogene. 2003;22:1381–1389. doi: 10.1038/sj.onc.1206154. [DOI] [PubMed] [Google Scholar]
- 38.Lochhead PA, Clark J, Wang LZ, et al. Tumor cells with KRAS or BRAF mutations or ERK5/MAPK7 amplification are not addicted to ERK5 activity for cell proliferation. Cell Cycle. 2016;15:506–518. doi: 10.1080/15384101.2015.1120915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mulloy R, Salinas S, Philips A, Hipskind RA. Activation of cyclin D1 expression by the ERK5 cascade. Oncogene. 2003;22:5387–5398. doi: 10.1038/sj.onc.1206839. [DOI] [PubMed] [Google Scholar]
- 40.Esparis-Ogando A, Az-Rodriguez E, Montero JC, et al. Erk5 participates in neuregulin signal transduction and is constitutively active in breast cancer cells overexpressing ErbB2. Mol Cell Biol. 2002;22:270–285. doi: 10.1128/MCB.22.1.270-285.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montero JC, Ocana A, Abad M, et al. Expression of Erk5 in early stage breast cancer and association with disease free survival identifies this kinase as a potential therapeutic target. PLoS ONE. 2009;4:e5565. doi: 10.1371/journal.pone.0005565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saito A, Yoshida H, Nishikawa T, Yonemori K. Human epidermal growth factor receptor 2 targeted therapy in endometrial cancer: clinical and pathological perspectives. World J Clin Oncol. 2021;12:868–881. doi: 10.5306/wjco.v12.i10.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fader AN, Roque DM, Siegel E, et al. Randomized phase II Trial of carboplatin-paclitaxel compared with carboplatin–paclitaxel–trastuzumab in advanced (stage III–IV) or recurrent uterine serous carcinomas that overexpress Her2/Neu ( NCT01367002): updated overall survival analysis. Clin Cancer Res. 2020;26:3928–3935. doi: 10.1158/1078-0432.CCR-20-0953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xia Y, Shen S, Verma IM. NF-κB, an active player in human cancers. Cancer Immunol Res. 2014;2:823–830. doi: 10.1158/2326-6066.CIR-14-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yilmaz E, Coskun EI, Gul M, et al. Nuclear factor-kappa β pathway and endometrial cancer: a pilot study. Eur J Gynaecol Oncol. 2017;38:536–540. doi: 10.12892/ejgo3471.2017. [DOI] [Google Scholar]
- 46.Harrington BS, Annunziata CM. NF-κB signaling in ovarian cancer. Cancers (Basel) 2019;11:1182. doi: 10.3390/cancers11081182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tilborghs S, Corthouts J, Verhoeven Y, et al. The role of nuclear factor-kappa b signaling in human cervical cancer. Crit Rev Oncol Hematol. 2017;120:141–150. doi: 10.1016/j.critrevonc.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 48.Brown HA, Williams CAC, Zhou H, et al. An ERK5-KLF2 signalling module regulates early embryonic gene expression and telomere rejuvenation in stem cells. Biochem J. 2021;478:4119–4136. doi: 10.1042/BCJ20210646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramadass V, Vaiyapuri T, Tergaonkar V. Small molecule NF-κB pathway inhibitors in clinic. Int J Mol Sci. 2020;2:5164. doi: 10.3390/ijms21145164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu Z, Gao J, Zhang X, et al. Characterization of a small-molecule inhibitor targeting NEMO/IKKβ to suppress colorectal cancer growth. Signal Transduct Target Ther. 2022;7:71. doi: 10.1038/s41392-022-00888-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang Q, Liao L, Deng X, et al. BMK1 is involved in the regulation of p53 through disrupting the PML–MDM2 interaction. Oncogene. 2013;32:3156–3164. doi: 10.1038/onc.2012.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Al-Ejeh F, Miranda M, Shi W, et al. Kinome profiling reveals breast cancer heterogeneity and identifies targeted therapeutic opportunities for triple negative breast cancer. Oncotarget. 2014;5:3145–3158. doi: 10.18632/oncotarget.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Finegan KG, Perez-Madrigal D, Hitchin JR, et al. ERK5 is a critical mediator of inflammation-driven cancer. Cancer Res. 2015;75:742–753. doi: 10.1158/0008-5472.CAN-13-3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pereira DM, Simoes AE, Gomes SE, et al. MEK5/ERK5 signaling inhibition increases colon cancer cell sensitivity to 5-fluorouracil through a p53-dependent mechanism. Oncotarget. 2016;7:34322–34340. doi: 10.18632/oncotarget.9107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shukla A, Miller JM, Cason C, et al. Extracellular signal-regulated kinase 5: a potential therapeutic target for malignant mesotheliomas. Clin Cancer Res. 2013;19:2071–2083. doi: 10.1158/1078-0432.CCR-12-3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pavan S, Meyer-Schaller N, Diepenbruck M, et al. A kinome-wide high-content siRNA screen identifies MEK5-ERK5 signaling as critical for breast cancer cell EMT and metastasis. Oncogene. 2018;37:4197–4213. doi: 10.1038/s41388-018-0270-8. [DOI] [PubMed] [Google Scholar]
- 57.Wang F, Li D, Zheng Z, et al. Reversal of ABCB1-related multidrug resistance by ERK5-IN-1. J Exp Clin Cancer Res. 2020;39:50. doi: 10.1186/s13046-020-1537-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Godwin P, Baird A-M, Heavey S, et al. Targeting nuclear factor-kappa b to overcome resistance to chemotherapy. Front Oncol. 2013 doi: 10.3389/fonc.2013.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, and further inquiries can be directed to the corresponding author.