Abstract
Bacterial wilt (Ralstonia pseudosolanacearum sp. nov.) is a major disease devastating global potato production. Proposed management options are mostly expensive and ineffective. This has necessitated efforts to develop cheaper and eco-friendly management options such as use of botanicals. Antibacterial activity of ethanol and acetone plant extracts from guava (Psidium guajava), drumstick (Moringa oleifera), camphor bush (Tarchonanthus camphoratus) and pelargonium (Pelargonium zonale) against R. pseudosolanacearum sp. nov. was evaluated in-vitro at a concentration of 100 mg/mL of 1 % Dimethlysulfoxide (DMSO) using disk diffusion technique. The R. pseudosolanacearum sp. nov was isolated from infected haulms collected from potato growing field at the University of Nairobi. The most effective extracts were subjected to further screening at different concentrations to determine their minimum inhibitory concentrations (MICs). All the four plant extracts showed varied antibacterial efficacy. P. zonale leaves extract was the most effective with growth inhibition zone of 18.73 mm and 18.60 mm for ethanol and acetone solvents respectively. The average of growth inhibition zones for each plant extract was not significantly different at p ≤ 0.05 among extraction solvents. The minimum inhibitory concentration (MIC) results showed that antibacterial activity of P. zonale and P. guajava leaf started at 6.25 mg/mL with growth inhibition zones of 7.67 and 8.0 mm for ethanol and acetone solvents respectively. P. zonale and P. guajava leaf extracts exhibited significantly higher antibacterial activity at p ≤ 0.05 compared to other extracts. Thus, further research should be conducted to assess their antibacterial potency against R. pseudosolanacearum sp. nov. both in-vivo and under field condition.
Keywords: Antibacterial activity, Bacterial wilt, Bio-control, Plant extracts
1. Background
Ralstonia solanacearum is a heterogeneous species of plant pathogenic bacteria causing vascular wilt to more than 200 plant species from over 50 families globally (Safni et al., 2014). Within its species complex, R. solanacearum (phylotype II), Ralstonia pseudosolanacearum sp. nov. (R. solanacearum (phylotypes I and III), and Ralstonia syzygii subsp. indonesiensis subsp. nov (R. solanecearum (phylotype IV) are known to affect global production of solanaceous crops mainly in the tropical and temperate regions (Rahman et al., 2010, Safni et al., 2014, Boschi et al., 2017). R. pseudosolanacearum sp. nov. is ranked second after late blight caused by Phytophthora infestans among plant pathogenic diseases affecting potato production in tropical, sub-tropical and cool temperate regions (Muthoni et al., 2012, Karim and Hossain, 2018, Mutimawurugo et al., 2020). Globally, R. pseudosolanacearum sp. nov. is estimated to affect more than 1.7 M hectares of land under potato production with annual losses of more than USD 950 million (Muthoni et al., 2012, Mwankemwa, 2015). In Kenya, for example, the pathogen affects more than 70 % of potato farms resulting in high yield losses ranging from 50 to 100 % (Kaguongo et al., 2010).
Because of its soil-borne nature and ability to persist in the soil over a long period of time, bacterial wilt management in potato fields has proved difficult (Gutarra et al., 2017, Sharma et al., 2017). Various management options such as crop rotation and field hygiene (Katafiire et al., 2005, Kassa, 2016, Sharma et al., 2017, Choudhary et al., 2018), use of resistant varieties (Fock et al., 2000), positive and negative selection (Gildemacher et al., 2011, Sharma et al., 2017), biological control (Whipps, 2001, Karim and Hossain, 2018) and chemical control (Sarkar and Chaudhuri, 2016, Biswal and Dhal, 2018) have been suggested for adoption and implementation. However, most of the proposed management options have various limitations such as high phytosanitary standards and negative environmental effects associated with chemical control (Mwankemwa, 2015, Muthoni et al., 2014), increased labor requirements associated with cultural control, limited commercialized biological control agents and lack of complete immunity to R. pseudosolanacearum sp. nov. from resistant varieties due to genetic variability of its species (Patil et al., 2012).
Due to these limitations especially high phytosanitary standards and negative environmental effects associated with few commercialized chemical bactericides as well as inadequate number of commercialized botanicals (plant extracts) for management of bacterial wilt of potatoes, efforts have been focused on development of botanicals as eco-friendly management options against bacterial wilt pathogen (Oboo et al., 2014, Karim and Hossain, 2018). The phytobiocidal effect of various plant extracts against R. pseudosolanacearum sp. nov. has been demonstrated by several researchers both in-vitro and in-vivo (Hassan et al., 2009, Oboo et al., 2014, Din et al., 2016, Mutimawurugo et al., 2020, Wamani, 2020). However, the phytochemical composition of these bioactive compounds varies from one plant to another and their effectiveness is also affected by variation in agro-climatic conditions coupled with varied abiotic factors during plant growth (Liu et al., 2016a, Liu et al., 2016b, Kumar et al., 2017, Gololo, 2018).
Despite the documented positive efficacy results of antibacterial activity of various plant extracts against R. pseudosolanacearum sp. nov. of potatoes, this research area has not been fully explored (Borges et al., 2018, Mutimawurugo et al., 2020). Therefore, the purpose of this work was to test the antibacterial activity of acetone and ethanol extracts of various plant species in vitro against Ralstonia pseudosolanacearum sp. nov. [R. solanacearum (phylotype I)] isolated from infected potato haulms collected from potato growing field at the University of Nairobi, Kenya.
2. Materials and methods
2.1. Sample collection
Infected plants showing typical bacterial wilt symptoms were collected from potato growing field at the University of Nairobi, Upper Kabete Campus. The station is located at a mean altitude of 1980 m, latitude 1° 15 S and longitude 36° 41′ E, in Lower Highland Zone II (LH2) of the Agro-ecological zone (AEZs) of Kenya (Jaetzold et al., 2007). Ten infected plant samples were collected in khaki paper bags, placed in a cool box and taken to the food microbiology laboratory at the University of Nairobi.
2.2. Isolation and purification of Ralstonia pseudosolanacearum sp. Nov pathogen
To confirm their infection, the samples were tested for bacterial ooze production and bacterial wilt pathogen isolated from samples which produced bacterial ooze. The bacterial wilt pathogen was isolated on a selective medium; Triphenyl tetrazolium chloride (Kelman’s TZC agar) as described by Kelman, 1954, Karim and Hossain, 2018.
2.3. Bacterial wilt pathogen identification and confirmatory tests
The isolated bacterium was identified based on morphological, physiological, cultural, biochemical and pathogenicity tests according to She et al., (2017). The virulent and non-virulent colonies were differentiated using colony characteristic on Kelman’s TZC medium. A loopful of the test bacterium was smeared on a clean glass slide with a drop of sterile water to determine Gram staining. The smeared bacterium was air-dried and then heat-fixed over a Bunsen flame. The smear was then stained with crystal violet, Lugol’s iodine and safranin with 3 rinses using water according to Rahman et al., (2010). After counterstaining, the slide was blot-dried and examined under a light microscope at x100 magnification with a drop of immersion oil. The Potassium hydroxide (KOH) solubility test was carried out according to Priou et al., (1999) but with slight modification. As opposed to Priou et al., (1999) who carried out KOH directly on bacterial ooze from potato tuber, in this study, KOH test was conducted using isolated bacteria on a sterile glass slide. Biovar identification through carbohydrate fermentation test was carried out according to Rahman et al., (2010) but with slight modifications. In their study, they used microtiter plates and bromothymol blue as an indicator as opposed to this study in which the experiment was carried out using universal bottles and phenol red as an indicator.
2.4. Pathogenicity test
Pathogenicity test was performed using one week old seedlings from certified seed potato tubers (Shangi variety) as described by Priou et al., (1999). Ten certified seed potato tubers were planted in pots (polythene sleeves) containing sterile soil media in a greenhouse and allowed to emerge. From the emerged seedlings, 5 seedlings were inoculated while the remaining 5 non-inoculated seedlings were used as checks. Bacterial cultures of 4.5 × 108 CFU/mL were prepared by culturing the isolated bacteria on casamino peptone glucose (CPG) media without triphenyl tetrazolium chloride (TZC) at 28 ± 1 °C for 48 h. The bacterial cells were harvested by washing the cultures in sterile distilled water. Two days prior to inoculation, the test plants were starved without irrigation and wounds created around the root zone using sterile scalpels. 10 mL of 4.5 × 108 CFU/mL bacterial suspension was inoculated around the root zone of 5 emerged seedlings using a syringe while the other 5 seedlings were inoculated with 10 mL of sterile distilled water. The plants were maintained and monitored for symptom development for a period of 2–5 weeks. The temperatures inside the glass house ranged from 25 to 32 °C while the relive humidity ranged from 80 to 90 %. Bacterial pathogen was re-isolated from the symptomatic seedlings using TZC media.
2.5. Preparation of plant extracts
Four plants namely guava (Psidium guajava), drumstick (Moringa oleifera), camphor bush (Tarchonanthus camphoratus) and pelargonium (Pelargonium zonale) were used in this study Table 1. The identities of the test plants were confirmed by a taxonomist and voucher samples kept at the Department of Crops, Horticulture and Soil Sciences (CHS), Egerton University, Kenya. The crude extracts were extracted from leaves in all the selected plants except for Moringa oleifera in which the extracts were taken both from leaves and seeds as described by (Biswal 2015) but with slight modifications. As opposed to Biswal (2015) who used only water (a polar solvent) as an extraction solvent, in this study-two solvents; ethanol (polar solvent) and acetone (non-polar solvent) were used as extractants. Additionally, the extracted compounds were concentrated to pastes through solvent evaporation to estimate extract yields. Healthy plant parts were collected and washed under a running tap water followed by shade drying at room temperature for three weeks. After complete drying, the plant materials were ground into fine powders. Before extraction, the pH of each plant extract was determined according to Silas et al., (2012) with slight modifications. As opposed to Silas et al., (2012) who soaked 100 g of each plant sample in 5 L of distilled water for a period of 90 days with continuous pH testing on a daily basis, in this study 5 g of each ground plant material was dissolved in 50 mL of distilled water in the ratio of 1:10. The mixtures were shaken on a mechanical shaker (end to end reciprocating shaker) at 600 revolutions per minute for 1 h and the pH of each solution measured using a pH meter. After pH determination, 20 g of fine powder of each plant material was soaked in 200 mL of each extraction solvent (ethanol and acetone) with regular stirring for 48 h. After 48 h, the solutions were filtered through double layers of muslin cloth and the filtrates collected in different sterile bottles. The filtrates were centrifuged at 9000 rpm for 10 min and the supernatants filtered through Whatman filter papers to remove the remaining particles. The filtrates were concentrated to pastes at temperatures slightly below the boiling points of each solvent (50 °C for acetone extracts and 60 °C for ethanol extracts) in two different water baths. The boiling point of acetone is 56 °C while that of ethanol is 78 °C. The beakers were stirred regularly to prevent the pastes from sticking on their walls. The pastes were air-dried overnight, weighed and stored at 4 °C. Percent extract yields were calculated using Eq. (1) as outline by Mostafa et al., (2018).
| (1) |
Table 1.
Plant species and plant parts used in the study.
| Plant species | Family | Local name | Common name | Plant part |
|---|---|---|---|---|
| Moringa oleifera | Moringaceae | Moringa | Drumstick tree | leaves and seeds |
| Tarchonanthus camphoratus | Asteraceae | Leleshwa | Camphor bush | leaves |
| Psidium guajava | Myrtaceae | Mapera | Common guava | Leaves |
| Pelargonium zonale | Geraniaceae | Geraniums | Pelargoniums | Leaves |
2.6. Antibacterial bioassays
The antibacterial activity experiment was laid out in a completely randomized design (CRD) with 5 replicates (5 disks per plate) and 9 treatments Table 2. The antibacterial activity of extracts from the four plants was tested using disk diffusion technique according to Mostafa et al., (2018). 100 mg of each plant extract (paste) was reconstituted in 1 mL of 1 % Dimethlysulfoxide (DMSO). Twenty milliliters of molten TZC medium were poured on each sterile petri dish and allowed to cool and solidify. About 100 µL of bacterial suspension at a concentration of 4.2 × 105 CFU/mL was added on the surface of each petri dish and spread gently and uniformly using a sterile l-shaped glass rod. Sterile disks of 6 mm diameter prepared through punching and sterilization of Whatman filter papers were impregnated with 15 µL of each plant extract and allowed to drain for 30 min. For negative control, the disks were impregnated with 15 µL of sterile distilled water and 1 % DMSO respectively while those of positive control were impregnated with 15 µL of KOBE 1.2 SL (Chrysophanol 12 g/l) (registered plant extract in management of bacterial wilt of potatoes) and ENRICH BM (Bronopol 27 %w/w) registered bactericide for management of bacterial wilt of potatoes. The disks were placed on the inoculated TZC media and plates refrigerated at 4 °C for 2 h for optimal diffusion of applied treatments and then incubated at 28 ± 1 °C for 48 h. Presence of growth inhibition zones were observed, the diameters measured using a calibrated ruler and considered as a sign of antibacterial activity.
Table 2.
Treatment description of antibacterial assay experiment.
| Treatments | Treatment type |
|---|---|
| Plant extracts Conc. 100 mg/mL | |
| Treatment 1 | Pelargonium zonale leaves |
| Treatment 2 | Psidium guajava leaves |
| Treatment 3 | Tarchonanthus camphoratus leaves |
| Treatment 4 | Moringa oleifera leaves |
| Treatment 5 | Moringa oleifera seeds |
| Positive controls at commercial rates | |
| Treatment 6 | ENRICH BM (Bronopol 27 %w/w) |
| Treatment 7 | KOBE 1.2 SL (Chrysophanol 12 g/l) |
| Negative controls | |
| Treatment 8 | Distilled water |
| Treatment 9 | 1 % DMSO |
Positive control comprised registered conventional bactericide (ENRICH BM (Bronopol 27 %w/w)) and botanical (KOBE 1.2 SL (Chrysophanol 12 g/l)) form management bacterial wilt of potatoes at commercial rates. BM (Bronopol 27 %w/w) was sourced from Osho Chemical Industries Limited while KOBE 1.2 SL (Chrysophanol 12 g/l) was sourced from Amiran Kenya limited. Negative control comprised distilled water and 1 % Dimethlysulfoxide (DMSO).
2.7. Determination of minimum inhibitory concentration (MIC) of effective plant extracts
The minimum inhibitory concentration (MIC) experiment of most effective plant extracts was laid out in a complete randomized design (CRD) with 5 replicates (5 disks per plate) and 5 treatments (different concentrations) Table 3. The most effective extracts (Pelargonium zonale and Psidium guajava) which exhibited high antimicrobial activity at 100 mg/mL were subjected to further screening to determine the minimum inhibitory concentration (MIC) using disk diffusion method as described by Mostafa et al., (2018). Different concentrations of these two plant extracts were prepared by further dilution of 100 mg/mL concentrations to attain 50 mg/mL, 25 mg/mL, 12.50 mg/mL, 6.25 mg/mL and 3.13 mg/mL. Twenty milliliters of molten TZC medium per petri dish was poured on sterile petri dishes and allowed to cool and solidify. 100 µL of bacterial suspension at a concentration of 4.2 × 105 CFU/mL was added on the surface of each petri dish and spread gently and uniformly using a sterile glass rod. Different sterile disks (6 mm diameter) were impregnated with 15 µL of each concentration and allowed to drain for 30 min. The disks were then placed on the inoculated TZC media and plates were placed in the refrigerator at 4 °C for 2 h for optimal diffusion of applied treatments and then incubated at 28 ± 1 °C for 48 h. Presence of growth inhibition zones was observed and their diameters were measured and recorded.
Table 3.
Treatment description of minimum inhibitory concentration (MIC) experiment.
| Treatments | Treatment type |
|---|---|
| Conc. Mg/mL | |
| Treatment 1 | 50.00 |
| Treatment 2 | 25.00 |
| Treatment 3 | 12.50 |
| Treatment 4 | 6.25 |
| Treatment 5 | 3.13 |
Pelargonium zonale and Psidium guajava extracts were used for MIC experiment.
2.8. Data analysis
The antibacterial activity data were subjected to analysis of variance (ANOVA) using R software, version 4.1.0 (R Studio Team, 2020). The treatment means were separated using Tukey’s Honest significant difference (HSD) at p ≤ 0.05 with the agricolae package.
3. Results
3.1. Confirmatory and biovar identification through carbohydrate fermentation tests
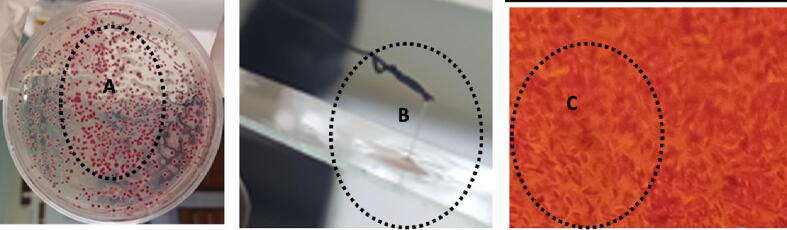
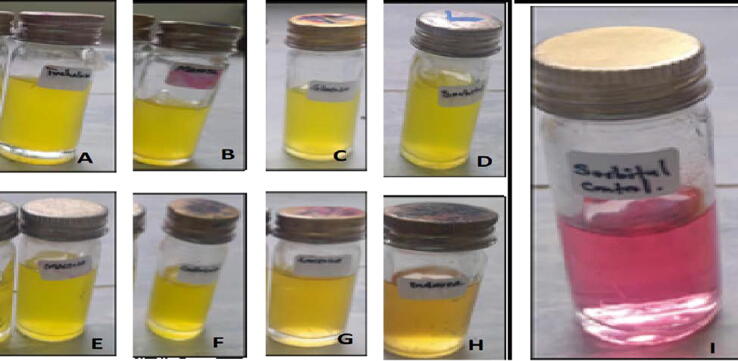
The bacterial isolate colonies had an irregular, spherical, white fluidal appearance with pink cores Fig. 1A. A mucoid (elastic and viscous) thread was observed when a wire loop was raised from the bacterial solution a few centimeters from the glass slide used for potassium hydroxide (KOH) test Fig. 1B. The microscopic result indicated that the bacterial isolate cells did not preserve the crystal violet color in the Gram stain test, but they did retain the pink color of the counter strain, and their form was rod shaped. This indicates that the bacterial isolate was a Gram-negative bacterium Fig. 1C. The isolated bacterial pathogen oxidized all the disaccharide sugars and hexose alcohols. This was indicated by color change from red to yellow in inoculated universal bottles compared to the checks (non-inoculated bottles) Fig. 2.
Fig. 1.
A) Colony characteristics of R. pseudosolanacearum sp. nov. on TZC medium, B) The formation of R. pseudosolancearum sp. nov. mucoid threads as a result of a KOH solubility test and C) Rod-shaped Gram-Negative R. pseudosolancearum sp. nov. bacterium under a light microscope.
Fig. 2.
Biovar identification of the isolated bacterium based on oxidation of disaccharides sugars and hexose alcohols. A) Trehalose, B) Mannitol, C) Dextrose, D) Sorbitol, E) Maltose, F) Celluboise, G) Lactose, H) Dulcitol I) negative check.
3.2. Pathogenicity test
The inoculated seedlings began to exhibit wilt symptoms at 7 days after inoculation. The initial symptoms appeared as wilted apical leaves during the day but the affected seedlings recovered at night. As the symptoms progressed, the wilted leaves failed to recover and the whole infected plants withered two weeks later. No symptoms were observed on non-inoculated plants Fig. 3. The isolated bacteria from the symptomatic plants displayed same morphological characteristics with that of the original pathogen on TZC media. The isolated bacterium was identified as R. pseudosolanacearum sp. nov. based on morphological and biochemical traits as well as pathogenicity tests.
Fig. 3.
Symptom expression in test plants used for pathogenicity test.
3.3. Plant extract yields
The pH and yield percentage of plant extracts extracted by both acetone and ethanol are shown in Table 4. Extracts from 20 g of dried and ground powder of plant material produced varied yields ranging from 0.22 to 3.02 g for those extracted using acetone solvents and 1.39–4.59 g for those extracted using ethanol solvents. Except for Moringa seed powder, Ethanol solvent recorded high percent yields compared to acetone.
Table 4.
The pH of plant extracts and their extract yields per extraction solvent.
| Extract yield (g) |
Percent extract yield (%) |
||||
|---|---|---|---|---|---|
| Plant species | pH | Acetone | Ethanol | Acetone | Ethanol |
| Moringa oleifera leaves | 5.75 | 2.41 | 4.59 | 12.05 | 22.95 |
| Moringa oleifera seeds | 5.33 | 3.02 | 2.93 | 15.10 | 14.65 |
| Tarchonanthus camphoratus | 5.51 | 0.50 | 1.39 | 2.50 | 6.95 |
| Psidium guajava | 5.26 | 1.62 | 3.40 | 8.10 | 17.00 |
| Pelargonium zonale | 4.78 | 0.22 | 3.81 | 1.10 | 19.05 |
3.4. Antibacterial bioassays
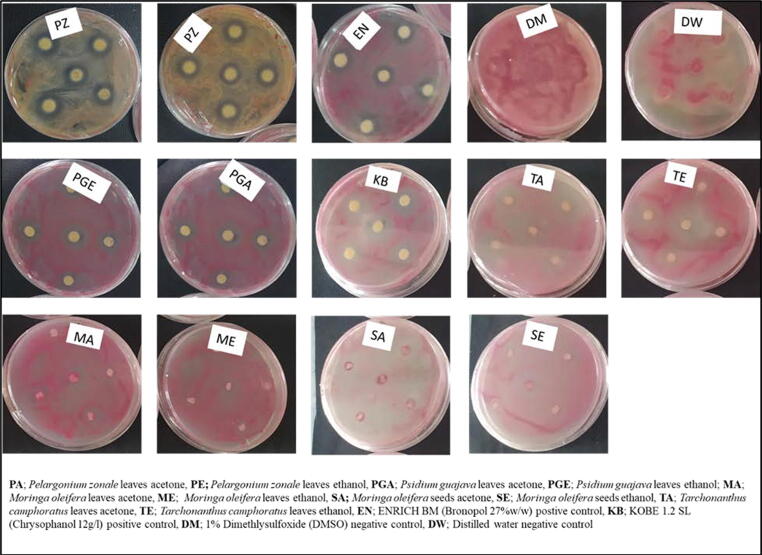
Antibacterial activity of plant extracts from four different plant species and the controls are presented in Table 5. All the tested extracts displayed varied antibacterial potency against R. pseudosolanacearum sp. nov. The antibacterial activity of the four plant extracts differed significantly at p ≤ 0.05 compared to negative and positive controls. From both solvents, extracts from Pelargonium zonale leaves were the most effective at 100 mg/mL of 1 % DMSO based on average of growth inhibition zones followed by Psidium guajava. The antibacterial activity of P. zonale was significantly different at p ≤ 0.05 to those of negative controls and positive controls except ENRICH BM (Bronopol 27 % w/w). The antibacterial activity of P. guajava was significantly different at p ≤ 0.05 to those of negative and positive controls respectively. Antibacterial activity of each plant extract and controls were depicted by clear zones around the impregnated disks Fig. 4.
Table 5.
Antibacterial activity of plant extracts against Ralstonia pseudosolanacearum sp. nov.
| Plant species | Extraction solvent |
|
|---|---|---|
| Ethanol | Acetone | |
| Inhibition zone (mm) | Inhibition zone (mm) | |
| Plant extracts Conc. 100 mg/mL | ||
| Pelargonium zonaleleaves | 18.73 ± 0.31 a | 18.60 ± 0.20 a |
| Psidium guajava leaves | 14.27 ± 0.12b | 14.13 ± 0.12b |
| Tarchonanthus camphoratus leaves | 8.40 ± 0.00c | 8.73 ± 0.30c |
| Moringa oleifera leaves | 7.37 ± 0.15 d | 7.47 ± 0.12 d |
| Moringa oleifera seeds | 7.33 ± 0.12 d | 7.33 ± 0.06 d |
| Positive controls at commercial rates | ||
| ENRICH BM (Bronopol 27 %w/w) | 18.13 ± 0.46 a | 18.13 ± 0.46 a |
| KOBE 1.2 SL (Chrysophanol 12 g/l) | 8.67 ± 0.12c | 8.67 ± 0.12c |
| Negative controls | ||
| Distilled water | 0.00 ± 0.00 e | 0.00 ± 0.00 e |
| 1 % DMSO | 0.00 ± 0.00 e | 0.00 ± 0.00 e |
| Mean | 10.20 | 9.24 |
| MSD | 0.62 | 0.60 |
| CV | 2.11 | 2.25 |
The values are average growth inhibition zones (mm) ± standard deviation from triplicates of ethanol and acetone extracts of each of the four plant materials and controls. Means within the same column having same letter(s) do not differ significantly at p ≤ 0.05, DMSO = Dimethlysulfoxide, MSD = mean square displacement, CV = coefficient of variation.
Fig. 4.
Growth inhibition zones of various plant extracts and controls against Ralstonia pseudosolanacearum sp. nov.
3.5. Minimum inhibitory concentration (MIC) of effective plant extracts
The minimum inhibitory concentration (MIC) of Pelargonium zonale and Psidium guajava leave extracts are illustrated in Table 6. The inhibitory effect of P. zonale against Ralstonia pseudosolanacearum sp. nov. started at 6.25 mg/mL of 1 % DMSO with inhibition zones of 7.67 and 8.0 mm for ethanol and acetone extracts while P. guajava exhibited inhibitory effect against the same pathogen at 6.25 mg/mL of 1 % DMSO with inhibition zones of 7.67 and 8.0 mm for ethanol and acetone extracts respectively.
Table 6.
Minimum inhibitory concentration (MIC) of effective plant extracts against Ralstonia pseudosolanacearum sp. nov.
| Plant extract | Conc. Mg/mL | Inhibition zones (mm) |
|
|---|---|---|---|
| Ethanol | Acetone | ||
| Pelargonium zonale leaves | 50.00 | 16.17 ± 0.76 | 16 0.00 ± 0.00 |
| 25.00 | 12.83 ± 0.29 | 12.17 ± 0.29 | |
| 12.50 | 10.00 ± 0.87 | 10.33 ± 0.29 | |
| 6.25 | 7.67 ± 0.29 | 8.00 ± 0.50 | |
| 3.13 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Psidium indicum leaves | 50.00 | 11.17 ± 0.29 | 11.83 ± 0.29 |
| 25.00 | 8.67 ± 0.29 | 8.67 ± 0.58 | |
| 12.50 | 8.00 ± 0.00 | 8.17 ± 0.29 | |
| 6.25 | 7.67 ± 0.58 | 8.00 ± 0.00 | |
| 3.13 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
The values are average growth inhibition zones (mm) ± standard deviation from triplicates of ethanol and acetone extracts of each concentration of the two effective plant extracts.
4. Discussion
The isolated bacteria exhibited irregular, round, and white fluidal colonies with pink centers on triphenyl tetrazolium chloride (TZC) media which was consistent with R. pseudosolanacearum sp. nov. characteristics on this medium as described by Kelman (1954). Both the Gram stain and KOH solubility tests confirmed that the isolated bacterium was gram negative and this was in agreement with the research findings by Rahman et al., 2010, Khasabulli et al., 2017, She et al., 2017. The isolated bacterium oxidized all the disaccharide sugars and hexose alcohols. Similar results were reported by various scientists who classified the isolated R. pseudosolanacearum sp. nov. with similar characteristics as biovar III race 1 (Ralstonia solanacearum (phylotype I)) (Rahman et al., 2010, Popoola et al., 2015, Boschi et al., 2017, Khasabulli et al., 2017). Pathogenicity test of the isolated R. pseudosolanacearum sp. nov. to susceptible potato seedlings in the greenhouse produced similar wilt symptoms to those diagnosed in the field. Similarly, morphological characteristics of the re-isolated bacterium from these test plants were identical to those of the original pathogen on TZC media. Similar results were reported in other studies (Rahman et al., 2010, Popoola et al., 2015, Khasabulli et al., 2017, She et al., 2017).
Ethanol as an extraction solvent recorded significantly higher percent extract yields from all the leaves compared to acetone but this was different with the M. oleifera seed extract in which acetone recorded slightly higher yield. Ethanol is a polar solvent thus extracts more diverse secondary metabolites from various plant parts as opposed to acetone which is non-polar (Yusnawan, 2013, Snehlata et al., 2018). Even though polar solvents are documented for high extract yields, the yielded extracts are always low in phenolic and flavonoid content in comparison to extracts from non-polar solvents (Nawaz et al., 2020). High yields of M. oleifera seed extracts from acetone solvent can be attributed to high oil content observed after extract concentration. It is argued that oils are easily extracted by non-polar solvents such as acetone as opposed to polar ones (Nwabueze and Okocha 2008). Similarly, M. oleifera seed extracts might have contained high proportions of phenolic and flavonoid compounds in addition to oils which are highly soluble in non-polar solvents as opposed to polar solvents (Nawaz et al., 2020).
Results from in to vitro screening of antibacterial activity of the four plant extracts against R. pseudosolanacearum sp. nov. revealed that P. zonale leaves were the most effective extract followed by P. guajava leaves while Moringa oleifera seeds was the least. The varied antibacterial activity between the plant extracts can be attributed to diversity and or difference in concentrations of secondary metabolites in each plat extract (Yihune and Yemata 2019). These in-vitro results were in accordance with those of Oboo et al., 2014, Biswal, 2015 who reported antibacterial activity of these plant extracts against R. pseudosolanacearum sp. nov. in-vitro. However, P. zonale and P. guajava leaf extracts exhibited high growth inhibition zones which contrasted the findings of Biswal (2015) and this can be attributed to difference in plant species, plant parts used in the study, adopted extraction method and varied agro-climatic conditions coupled with diverse abiotic factors during the plant growth (Liu et al., 2016a, Liu et al., 2016b, Kumar et al., 2017, Gololo, 2018, Mutimawurugo et al., 2020). Oboo et al., (2014) reported high antibacterial activity of T. camphoratus against R. solanacearum both in-vitro and in-vivo. In this study, T. camphoratus extract was the third best extract and this contrasted their findings. Its dismal performance can be attributed to failure of the extracted paste to dissolve in the reconstituting solvent.
KOBE 1.2 SL (Chrysophanol 12 g/l) and ENRICH BM (Bronopol 27 %w/w) are documented to control bacterial wilt pathogen through induction of host plant resistance (https://agroduka.com/, xxxx, Liu et al., 2016a, Liu et al., 2016b). Stretton and Manson (1973) reported in-vitro efficacy of bronopol against different strains of bacteria and this was confirmed by the findings of this study which demonstrated its in-vitro antibacterial activity against R. pseudosolanacearum sp. nov. The study results also revealed in-vitro antibacterial potency of Chrysophanol against R. pseudosolanacearum sp. nov.
The minimum inhibitory concentration (MIC) results revealed that the antibacterial activity of the P. zonale and P. guajava leave extracts decreased with decreasing extract concentration. For both extraction solvents, the antibacterial activity of P. zonale and P. guajava leaf extracts against R. pseudosolanacearum sp. nov. started at 6.25 mg/mL of 1 % DMSO. These results were in accordance with the findings of Mutimawurugo et al. (2020), who reported varied MICs ranging from 6.25 to 12.5 mg/mL for different plant extracts against R. pseudosolanacearum sp. nov. Similarly, Mutimawurugo et al. (2020) also reported decreased antibacterial activity of different plant extracts against Ralstonia solanacearum with decreased extract concentrations and this can be attributed to reduced toxicity levels of bioactive compounds due to dilution effect.
Numerous researchers have investigated the antibacterial efficacy of different plant extracts and their respective bioactive compounds against R. pseudosolanacearum sp. nov. both in-vitro and in-vivo (Hassan et al., 2009, Oboo et al., 2014, Biswal, 2015, Din et al., 2016, Mutimawurugo et al., 2020, Wamani, 2020). Some of the documented bioactive compounds against R. pseudosolanacearum sp. nov. include flavonoids and alkaloids (Mutimawurugo et al., 2020), 5-(3-buten-1-ynyl)-2, 2′-bithienyl and 5-(4-acetoxy-1-butynyl)-2, 2′-bithienyl from Tagetes patula (Terblanche and de Villiers, 1998). These bioactive compounds are reported to demonstrate bactericidal effect through interaction with enzymes and proteins of the target bacterial cell membrane causing disruption. Additionally, the bioactive compounds with hydrophobic characteristics can react with proteins of the target cell membrane and mitochondria of the target bacteria thereby changing its membrane permeability (Sánchez et al., 2010, Gonelimali et al., 2018, Mostafa et al., 2018).
5. Conclusion
These results revealed varied antibacterial potency of the tested plant extracts against R. pseudosolanacearum sp. nov. in-vitro. Pelargonium zonale and Psidium guajava leaf extracts displayed significantly high antibacterial activity compared to other extracts. Further research studies should be conducted to assess the antibacterial potency of these two plant extracts against R. pseudosolanacearum sp. nov. both in-vivo and under field condition. Similarly, phytochemical analysis studies should be carried out on these two extracts to identify the bioactive compounds against R. pseudosolanacearum sp. nov.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The Authors appreciates The Centre of Excellence in Sustainable Agriculture and Agribusiness Management (CESAAM), Egerton University for funding the work through World Bank Funded project IDA CREDIT NO-5798.
Footnotes
Peer review under responsibility of King Saud University.
References
- Biswal G. Studies on antibacterial activity of some aqueous plant extracts against Ralstonia solanacearum causing bacterial wilt and brown rot of potato. International Journal of Plant Sciences (Muzaffarnagar) 2015;10(1):1–6. [Google Scholar]
- Biswal G., Dhal N.K. Management of bacterial wilt disease of potato in coastal plains of Odisha. African Journal of Microbiology Research. 2018;12(12):284–289. [Google Scholar]
- Borges D.F., Lopes E.A., Moraes A.R.F., Soares M.S., Visôtto L.E., Oliveira C.R., Valente V.M.M. Formulation of botanicals for the control of plant-pathogens: A review. Crop Prot. 2018;110:135–140. [Google Scholar]
- Boschi F., Schvartzman C., Murchio S., Ferreira V., Siri M.I., Galván G.A., Smoker M., Stransfeld L., Zipfel C., Vilaró F.L., Dalla-Rizza M. Enhanced bacterial wilt resistance in potato through expression of Arabidopsis EFR and introgression of quantitative resistance from Solanum commersonii. Front. Plant Sci. 2017;8 doi: 10.3389/fpls.2017.01642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary D.K., Nabi S.U., Dar M.S., Khan K.A. Ralstonia solanacearum: A widespread and global bacterial plant wilt pathogen. Journal of Pharmacognosy and Phytochemistry. 2018;7(2):85–90. [Google Scholar]
- Din N., Ahmad M., Siddique M., Ali A., Naz I., Ullah N., Ahmad F. Phytobiocidal management of bacterial wilt of tomato caused by Ralstonia solanacearum (Smith) Yabuuchi. Spanish Journal of Agricultural Research. 2016;14(3):1–13. [Google Scholar]
- Fock I., Collonnier C., Purwito A., Luisetti J., Souvannavong V., Vedel F., Servaes A., Ambroise A., Kodja H., Ducreux G., Sihachakr D. Resistance to bacterial wilt in somatic hybrids between Solanum tuberosum and Solanum phureja. Plant Sci. 2000;160(1):165–176. doi: 10.1016/s0168-9452(00)00375-7. [DOI] [PubMed] [Google Scholar]
- Gildemacher P.R., Schulte-Geldermann E., Borus D., Demo P., Kinyae P., Mundia P., Struik P.C. Seed potato quality improvement through positive selection by smallholder farmers in Kenya. Potato Res. 2011;54(3):253–266. [Google Scholar]
- Gololo S.S. Phytochemistry. Apple Academic Press; 2018. Effects of Environmental Factors on the Accumulation of Phytochemicals in Plants; pp. 267–278. [Google Scholar]
- Gonelimali F.D., Lin J., Miao W., Xuan J., Charles F., Chen M., Hatab S.R. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front. Microbiol. 2018;9:1639. doi: 10.3389/fmicb.2018.01639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutarra L., Herrera J., Fernandez E., Kreuze J., Lindqvist-Kreuze H. Diversity, pathogenicity, and current occurrence of bacterial wilt bacterium Ralstonia solanacearum in Peru. Front. Plant Sci. 2017;8:1221. doi: 10.3389/fpls.2017.01221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan M.A.E., Bereika M.F.F., Abo-Elnaga H.I.G., Sallam M.A.A. Direct antimicrobial activity and induction of systemic resistance in potato plants against bacterial wilt disease by plant extracts. The Plant Pathology Journal. 2009;25(4):352–360. [Google Scholar]
- https://agroduka.com/enrich-bm. Date accessed: 03/10/2021.
- Jaetzold R., Ira F., Schmidt H., Hornet B., Shisanya C. (2nd edition, Ministry of Agriculture/GTZ; Nairobi Kenya: 2007. Farm Management Handbook of Kenya-Natural Conditions and Farm Management Information. Part B: Central Kenya. [Google Scholar]
- Kaguongo, W. P., Ng’ang’a, N. M., Muthoka, N., Muthami, F., & Maingi, G. (2010). Seed potato subsector master plan for Kenya (2009-2014). Seed potato study sponsored by GTZ-PSDA, USAID, CIP and Government of Kenya. Ministry of Agriculture, Kenya.
- Karim Z., Hossain M.S. Management of bacterial wilt (Ralstonia solanacearum) of potato: focus on natural bioactive compounds. Journal of Biodiversity Conservation and Bioresource Management. 2018;4(1):73–92. [Google Scholar]
- Kassa B. Potato bacterial wilt management in the Central Highlands of Ethiopia. Ethiopian Journal of Agricultural Sciences. 2016;26(2):83–97. [Google Scholar]
- Katafiire M., Adipala E., Lemaga B., Olanya M., El-Bedewy R., Ewell P. Management of Bacterial Wilt of Potato Using One-season Rotation Crops in Southwestern Uganda. American Phytopathological Society Press; 2005. pp. 197–204. [Google Scholar]
- Kelman A. The relationship of pathogenicity of Pseudomonas solanacearum to colony appearance in a tetrazolium medium. Phytopathology. 1954;44(12) [Google Scholar]
- Khasabulli B.D., Musyimi D.M., Miruka D.M., Opande G.T., Jeruto P. Isolation and characterisation of Ralstonia solanacearum strains of tomato wilt disease from Maseno. Kenya. Journal of Asian Scientific Research. 2017;7(9):404–420. [Google Scholar]
- Kumar S., Yadav A., Yadav M., Yadav J.P. Effect of climate change on phytochemical diversity, total phenolic content and in vitro antioxidant activity of Aloe vera (L.) Burm. f. BMC research notes. 2017;10(1):1–12. doi: 10.1186/s13104-017-2385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.Y., Liao C.K., Lo C.T., Yang H.H., Lin K.C., Peng K.C. Chrysophanol is involved in the biofertilization and biocontrol activities of Trichoderma. Physiol. Mol. Plant Pathol. 2016;96:1–7. [Google Scholar]
- Liu W., Yin D., Li N., Hou X., Wang D., Li D., Liu J. Influence of environmental factors on the active substance production and antioxidant activity in Potentilla fruticosa L. and its quality assessment. Sci. Rep. 2016;6(1):1–18. doi: 10.1038/srep28591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafa A.A., Al-Askar A.A., Almaary K.S., Dawoud T.M., Sholkamy E.N., Bakri M.M. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi journal of biological sciences. 2018;25(2):361–366. doi: 10.1016/j.sjbs.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthoni J., Shimelis H., Melis R. Management of bacterial wilt [Ralstonia solanacearum Yabuuchi et al., 1995] of Potatoes: Opportunity for host resistance in Kenya. J. Agric. Sci. 2012;4(9):64. [Google Scholar]
- Muthoni J., Kabira J., Shimelis H., Melis R. Spread of bacterial wilt disease of potatoes in Kenya: Who is to blame? International Journal of Horticulture. 2014;4(3):10–15. [Google Scholar]
- Mutimawurugo M.C., Ogweno J.O., Muhinyuza J.B., Wagara I.N. In vitro antibacterial activity of selected plant extracts against potato bacterial wilt (Ralstonia solanacearum Smith) in Rwanda. Journal of Applied Horticulture. 2020;22(3) [Google Scholar]
- Mwankemwa Z. Sokoine University of Agriculture); 2015. Occurrence and distribution of potato bacterial wilt disease and variability of its causal agent in southern highlands of Tanzania. Doctoral dissertation. [Google Scholar]
- Nawaz H., Shad M.A., Rehman N., Andaleeb H., Ullah N. Effect of solvent polarity on extraction yield and antioxidant properties of phytochemicals from bean (Phaseolus vulgaris) seeds. Brazilian. J. Pharm. Sci. 2020;56 [Google Scholar]
- Nwabueze T.U., Okocha K.S. Extraction performances of polar and non-polar solvents on the physical and chemical indices of African breadfruit (Treculia africana) seed oil. Afr. J. Food Sci. 2008;2(10):119–125. [Google Scholar]
- Oboo H., Muia A.W., Kinyua Z.M. Effect of selected essential oil plants on bacterial wilt disease development in potatoes. Journal of Applied Biosciences. 2014;78:6666–6674. [Google Scholar]
- Patil V.U., Gopal J., Singh B.P. Improvement for bacterial wilt resistance in potato by conventional and biotechnological approaches. Agric. Res. 2012;1(4):299–316. [Google Scholar]
- Popoola A.R., Ganiyu S.A., Enikuomehin O.A., Bodunde J.G., Adedibu O.B., Durosomo H.A., Karunwi O.A. Isolation and characterization of Ralstonia solanacearum causing bacterial wilt of tomato in Nigeria. Nigerian Journal of Biotechnology. 2015;29:1–10. [Google Scholar]
- Priou S., Aley P., Chujoy E., Lemaga B.A., French E.R., French E. International Potato; 1999. Integrated Control of Bacterial Wilt of Potato. [Google Scholar]
- Rahman M.F., Islam M.R., Rahman T., Meah M.B. Biochemical characterization of Ralstonia solanacearum causing bacterial wilt of brinjal in Bangladesh. Progressive Agriculture. 2010;21(1–2):9–19. [Google Scholar]
- RStudio Team . RStudio; PBC, Boston, MA: 2020. RStudio: Integrated Development for R. http://www.rstudio.com/ [Google Scholar]
- Safni, I., Cleenwerck, I., De Vos, P., Fegan, M., Sly, L., & Kappler, U. (2014). Polyphasic taxonomic revision of the Ralstonia solanacearum species complex: proposal to emend the descriptions of Ralstonia solanacearum and Ralstonia syzygii and reclassify current R. syzygii strains as Ralstonia syzygii subsp. syzygii subsp. nov., R. solanacearum phylotype IV strains as Ralstonia syzygii subsp. indonesiensis subsp. nov., banana blood disease bacterium strains as Ralstonia syzygii subsp. celebesensis subsp. nov. and R. solanacearum phylotype I and III strains as International Journal of Systematic and Evolutionary Microbiology, 64(Pt_9), 3087-3103. [DOI] [PubMed]
- Sánchez E., García S., Heredia N. Extracts of edible and medicinal plants damage membranes of Vibrio cholerae. Appl. Environ. Microbiol. 2010;76(20):6888–6894. doi: 10.1128/AEM.03052-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Chaudhuri S. Bacterial wilt and its management. Curr. Sci. 2016;110(8):1439–1445. [Google Scholar]
- Sharma K., Shawkat B., Miethbauer T., Schulte-Geldermann E. Management in Potato Field. Farmers’ guide; 2017. Strategies for bacterial wilt (Ralstonia solanacearum) [Google Scholar]
- She X., Yu L., Lan G., Tang Y., He Z. Identification and genetic characterization of Ralstonia solanacearum species complex isolates from Cucurbita maxima in China. Front. Plant Sci. 2017;8:1794. doi: 10.3389/fpls.2017.01794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silas M.N.E., Murungi J.I., Wanjau R.N. The pH of leaf water extracts and amount of acid required lowering the pH of leaf water extracts to 5.0. American International Journal of contemporary research. 2012;2(11):72–78. [Google Scholar]
- Snehlata K., Sheel R., Kumar B. Evaluation of phytochemicals in polar and nonpolar solvent extracts of leaves of Aegle marmelos (L.) Journal of Biotechnology and Biochemistry. 2018;4(5):31–38. [Google Scholar]
- Stretton R.J., Manson T.W. Some Aspects of the Mode of Action of the Antibacterial Compound Bronopol (2-bromo-2-nitropropan-1, 3-diol) J. Appl. Bacteriol. 1973;36(1):61–76. doi: 10.1111/j.1365-2672.1973.tb04073.x. [DOI] [PubMed] [Google Scholar]
- Terblanche J., de Villiers D.A. In: Bacterial Wilt Disease. Prior P., Allen C., Elphinstone J., editors. Springer Berlin Heidelberg; Berlin, Heidelberg: 1998. The suppression of Ralstonia solanacearum by marigolds; pp. 325–331. [Google Scholar]
- Wamani A.O. University of Nairobi); 2020. Screening of Microbial Antagonists and Plant Extracts Against Selected Tomato Pathogens and Their Potential in the Management of Bacterial Wilt (Doctoral dissertation. [Google Scholar]
- Whipps J.M. Microbial interactions and biocontrol in the rhizosphere. J. Exp. Bot. 2001;52(suppl_1):487–511. doi: 10.1093/jexbot/52.suppl_1.487. [DOI] [PubMed] [Google Scholar]
- Yihune E., Yemata G. Antibacterial activity of medicinal plant extracts against Ralstonia solanacearum (Smith) that causes bacterial wilt in hot pepper (Capsicum annuum L.) Acta Scientiarum. Biological Sciences. 2019;41:e45402–e. [Google Scholar]
- Yusnawan E. The effectiveness of polar and non-polar fractions of Ageratum conyzoides l. to control peanut rust disease and phytochemical screenings of secondary metabolites. Jurnal Hama dan Penyakit Tumbuhan Tropika. 2013;13(2):159–166. [Google Scholar]