Abstract
Inflammation is a protective response of the body to pathogens and injury. Hence, it is particularly important to explore the pathogenesis and key regulatory factors of inflammation. BMP9 is a unique member of the BMP family, which is widely known for its strong osteogenic potential and insensitivity to the inhibition of BMP3. Recently, several studies have reported an underlying pivotal link between BMP9 and inflammation. What is clear, though not well understood, is that BMP9 plays a role in inflammation in a carefully choreographed manner in different contexts. In this review, we have summarized current studies focusing on BMP9 and inflammation in various tissues and the latest advances in BMP9 expression, signal transduction, and crystal structure to better understand the relationship between BMP9 and inflammation. In addition, we also briefly summarized the inflammatory characteristics of some TGF-β superfamily members to provide better insights and ideas for the study of BMP9 and inflammation.
Keywords: Bone morphogenetic protein 9 (BMP9), Crystal structure, Expression profile, Inflammation, Transforming growth factor-β (TGF-β)
Introduction
Inflammation is a microcirculation-based physiological response that is required to fight invading pathogens and regulate damage repair; it is involved in almost all disease processes, including DNA access and epigenetic regulation.1,2 Necrotic tissue fragments and pathogens can be removed by various immune cells and inflammatory factors; however, inflammatory diseases such as inflammatory bowel disease and cancer may still occur if inflammation regulatory mechanisms are dysregulated or pathogen stimulation is strong.3,4 As inflammation also initiates and guides damage repair in the body, tissue regeneration engineering using inflammatory signals has been explored. Numerous endogenous or exogenous factors, such as TGF-β, NF-κB, and MAPKs, have been reported to promote appropriate levels of inflammatory response.5, 6, 7
BMPs belong to the TGF-β superfamily, which currently has over 20 members.8 In addition to osteogenesis and cartilage functions, BMPs play a vital role in several processes, such as embryogenesis and tissue homeostasis; thus, they are also known as body morphogenetic proteins.9 BMP2, BMP6 and BMP7 are deeply involved in inflammatory disorders, including fibrosis, inflammatory bowel disease, ankylosing spondylitis, and rheumatoid arthritis.10 BMP9 is considered a unique member of the BMP family as it has the strongest osteogenic effect on mesenchymal stem cells (MSCs), is resistant to the BMP signaling inhibitors, noggin and BMP3,11,12 and significantly affects vascular homeostasis,13 angiogenesis,14 metabolism,15 neurogenesis,16,17 and pro- or anti-tumorigenesis.18,19 In recent years, several studies have reported that BMP9 is associated with the occurrence and development of inflammation, particularly in the liver, blood vessels, bone, cartilage, and dental tissues. It has been reported that endothelin, a high-affinity BMP9 co-receptor, is expressed in various immune cells.20 MAPKs, which are also widely involved in regulating inflammation, are currently considered to be involved in Smads-independent BMP9 signaling pathways.21,22 In this paper, we have reviewed the relationships between BMP9 and inflammation, and summarized the expression profile of BMP9 in different species, as well as the latest achievements in crystal structure and signal transduction, for an in-depth understanding of BMP9.
BMP9 overview: expression profile, signaling pathways, and crystal structure
BMP9 expression profile
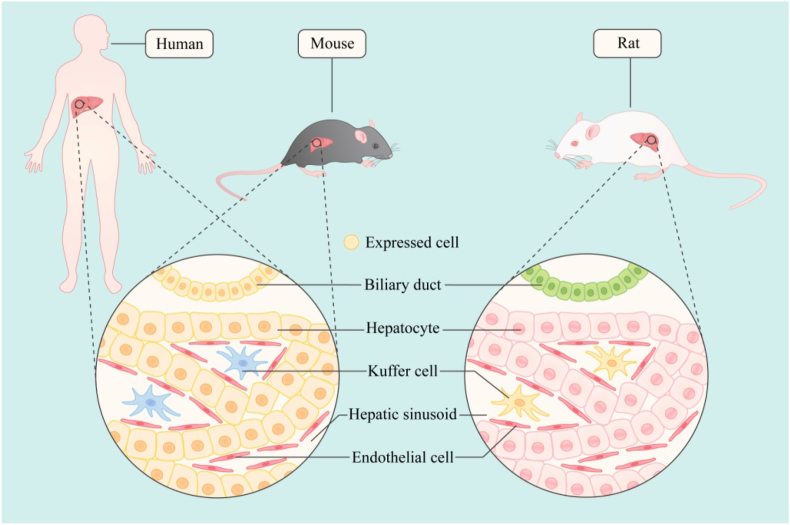
BMPs are widely distributed and increasingly recognized as body morphogenetic proteins.23 BMP2- and BMP7-knockout (KO) mice do not survive in utero and during early postnatal life, respectively. Although BMP5-deficient mice are healthy, they have many skeletal and soft tissue defects. BMP6 overexpression causes skin lesions, while BMP12, BMP13, and BMP14 may be critical for normal tendon repair.24,25 In addition, BMP2, BMP4, BMP5, and BMP6 are expressed in sundry tissues, including that of the brain, skin, heart, liver, and lung. BMP10 is mainly produced in the heart, and BMP7 is abundant in the adult kidney. BMP9, cloned initially from a mouse liver cDNA library, is considered the least characteristic member of the family, which in adult animals is generated by autocrine and paracrine glands, and then released into the circulation; it existed in both active and inactive forms.24,26,27 We do not yet have a clear understanding of the role of BMP9 in embryonic development and its distribution in adulthood. Both BMP9 and BMP10 influence mouse embryogenesis and are functionally redundant in promoting vascular development.28 However, while BMP9 is dispensable in mouse embryos and neonates, BMP10-deficient mice die between embryonic day (E) 9.5 and E10.5, due to cardiac dysplasia.29 Anti-BMP9 antibodies alone weakly affect retinal and tracheal vasculature development in postnatal mice, while a combined BMP9 and BMP10 neutralization treatment can apparently affect both blood and lymphatic vascular system.30,31 In stomatology, BMP9 is also essential for odontogenesis, which is extensively developed in odontoblasts, ameloblasts, dental pulp cells, and osteoblasts in alveolar bones.31 BMP9-KO mice exhibite dentine dysplasia, which is characterized by thin dentin, enlarged pulp canals, and shortened roots, resembling the phenotypes of the common hereditary dental disease, dentinogenesis imperfecta, and alveolar bone with reduced volume and decreased mineral density and trabecular thickness.31 However, the distribution of BMP9 in different species needs to be further studied. A study detected BMP9 in rat hepatic endothelial cells and hepatic stellate cells,32 while another confirmed that it is produced mainly in human and mouse hepatocytes and biliary epithelial cells.27 This may be related to the use of different research methods, which are based on RNA datas obtained after in vitro cell culture and the results of tissue immunohistochemical staining. Interestingly, BMP9 has also been reported to be highly expressed in the liver and lung tissues of young adult mice, which decreases in older mice.33 Moreover, it has also been shown that BMP9 expression in developing bones is weak to moderate, although it is the most potent osteogenic factor in the BMP family. In general, it has been thought that, unlike the multi-tissue expression of other BMP family members, BMP9 is mainly expressed in the liver of adult individuals, but the facts are still worth exploring. A recent study using immunohistochemical techniques in rats showed that BMP9 was strongly expressed in most neurons and axons.34 Our group also found high levels of BMP9 in the odontoblast cell layer in adult pulp tissues by in situ hybridization and immunohistochemistry.35 It seems that the site of BMP9 expression may not be histologically confined, and it may be scattered. Another related fact that cannot be ignored is the presence of BMP9 in the circulation, whether it is related to BMP9 levels in other local tissues, and is there any involvement of BMP9 in the inflammatory site of the system since blood is an easy means of travel to other parts of the body (Fig. 1).
Figure 1.
The main expression sites of BMP9 in different species (current cognition). BMP9 is expressed in hepatocytes and bile duct cells in human and mouse, and is mainly expressed in hepatic stellate cells in rats.
BMP9 signaling pathways
TGF-β superfamily ligands are powerful multipotent cytokines. It includes three subclasses of TGF-β, activins, BMPs, and concrete exceeding 30 members in the human body that exceed the number of corresponding receptors, which consist of seven type I and five type II.6,36,37 Although it has fewer receptors, the high degree of complexity between ligand-receptor interactions favors signal transduction.38 One ligand can bind to multiple receptors and vice versa.39 Signal transduction in the cells can enhance Smads phosphorylation and Smads complex formation, and are then transferred into the nucleus to activate downstream target genes.40,41 Moreover, previous studies have reported that the BMP9 signal transduction pathway has complex ligand-receptor binding, complex intracellular conduction, and insensitivity to existing inhibitors with high tissue and cell specificity.
BMP9 signals are transduced through type II (BMPR-II, ActRIIa, and ActRIIb) and type I (ALK1 and ALK2) transmembrane serine/threonine kinase receptors. After the formation of BMP9-type I/II heterogeneous polymers, the type I receptor is transphosphorylated by constitutively active type II receptors. ALK1, an endothelium-specific receptor, has a high affinity for BMP9 in the vascular system.42,43 In addition, recent studies have confirmed that BMP10, another circulating factor, binds to ALK1.44 Endothelin, serving as a type III co-receptor of the TGF-β superfamily, binds to BMP9 and BMP10 with high affinity45,46 and acts as an inhibitor of BMP9-BMPRII coalition, because of overlap of their action sites on BMP9.46 Interestingly, BMP9 loses its strong osteogenic effect during circulation, which may be related to its low circulating concentration. This could illuminate the need for ALK1 and endothelin high-affinity receptors. The susceptibility of BMP9 to redox-dependent cleavage may be one reason for this low concentration.47 Other studies have suggested that increased BMP9 concentration or inflammation activates the ALK2 receptor in non-endothelial cells, such as MSCs and periodontal ligament cells, and ALK1 and ALK2 are necessary for BMP9-mediated cellular events.48,49 In addition, ovarian cancer cell proliferation is facilitated by BMP9–ALK2 signaling. The type II receptor is not particularly unique as BMPRII binds to most BMP ligands, while ActRIIa and ActRIIb have affinity for BMPs and activins. The combination of the DNA-mutant technology and RNAi method has indicated that BMPRII and ActRIIa may be the functional receptors required for BMP9-induced osteogenic differentiation of C3H10T1/2 cells. Although ActRIIb plays a similar role, it is not expressed in C3H10T1/2 stem cells.50 Another study showed that BMP9 has greater affinity for ActRIIb than to BMPRII or ActRIIa, using thermodynamic analysis.51
Similar to other BMPs, BMP9-mediated intracellular signaling consists of Smads-dependent (Smad1/5/8) and Smads-independent (ERK1/2, p38, JNK) signaling pathways. Some studies utilizing selective inhibitors or RNAi technology have demonstrated synergistic or antagonistic interactions between Smads and MAPKs.52, 53, 54 However, whether there are cross-talks among these key intracellular signaling molecules and their specific targets needs to be further studied in different cell contexts. The general thought, traditionally, is that phosphorylated Smads1/5/8 allies with Smad4 (Co-Smad), to form the heterogeneous tetramer and then translocate into the nucleus, activating downstream gene expression, such as inhibitors of DNA-binding proteins (IDs). Interestingly, Smad2/3 has also been confirmed to be involved in the induction of osteogenic differentiation by BMP9 in synovial mesenchymal stem cells, and this study, unfortunately, did not mention whether Smad1/5/8 is phosphorylated. In BMP9-treated human chorionic cancer cells, Smad1/5/8 and Smad2/3 were simultaneously activated and similar phenomena also occurs in human pulmonary artery endothelial and vascular smooth muscle cells.55, 56, 57 How did this event, which is different from the conventional view, occurs, directly or indirectly, deserves further study. Combined with the above statement, we can summarize several potentially indirect characteristics of BMP9 and inflammation. Endothelin is constitutively expressed on endothelial cells of all vascular types and regulates neutrophil and macrophage responses, thereby regulating immune and vascular functions during inflammation,58 and noncanonical MAPKs members, ERK, p38, and JNK are all classic pathways related to inflammation regulation.59,60 Furthermore, we also found that the pathways of BMP9 may be dysregulated in an inflammatory environment.
BMP9 appears to be significantly different from other members of the BMP subfamily. Common antagonists of the BMP family, such as noggin and BMP3, appear to be insensitive to BMP9. CV2 (Crossveinless 2) has been proven to combine and preferentially inhibit BMP9 in vascular endothelial cells, thereby providing strong feedback inhibition for BMP9/ALK1 signaling.61 CV2 has long been considered a potential ligand trap for BMP9; however, a previous study reported the opposite result in pulmonary artery endothelium and C2C12 cells, in which the application of relatively high concentrations of CV2 did not inhibit BMP9-induced Smad1/5 phosphorylation and ALP activity.62 The former differs from the latter as the BMP9-mediated intracellular Smad1/5/8 phosphorylation level was not detected, but the specific reasons still need further study.
BMP9 crystal structure
Owing to the interesting properties of BMP9, researchers have focused on its crystal structure. It possesses the typical structure of proteins belonging to the TGF-β superfamily, comprising two large prodomains and a mature dimer at the C-terminal, which resembles a big butterfly.27,63 The prodomains can be digested by furin proteases,64 forming a non-covalent combination or separation with mature growth factor (GF) domains after secretion and is pivotal in the biosynthesis, stabilization, transport, and signal transduction of GFs. BMP9 remains tightly associated with the prodomain and exerts effects similar to those of BMP9-GF.65
To date, several key sites or residues involved in BMP9 signaling, including two finger regions and an α3-helix in GF and arm domains, and α2 and α5 helix in the prodomain, have been identified.63,65 It has been shown that the BMP9 predomain complex exists in the open or crossed arm conformations. It has also been speculated that the active state of the open arm complex binds directly to the receptor, and the storage condition of crossed arms complex is linked immediately with the extra cellular matrix components, such as heparin, proteoglycan, and fibrillin.63 It has been confirmed that the arm domain and α2 helix occupy the type II receptor binding site, while the α5 helix blocks the type I receptor binding site. Thus, pro-BMP9 (predomain-BMP9) can readily bind to type I receptors, displacing the α5 helix, and further binding to type II receptors, leading to complete prodomain dissociation.62,63 In addition, the chordin family member CV2 binding site has been found on the BMP9 interface and is commonly occupied by the predomain. The von Willebrand factor C (VWC) domain binds to a similar site on the GF finger in the arm domain.63 Thus, the prodomain plays an essential role in BMP9 signaling activation, including binding to receptors and inhibitors, which also seems to be fundamental to some of its unique properties. In addition, a function mapping analysis of the different endoglin domains established that endoglin and type II receptors bind to overlapping sites on BMP946; however, this does not prevent BMP9 signal transduction. The endoglin extracellular domain can be cleaved and circulated as soluble ENG (sENG) during inflammation, which is speculated to be a ligand trap for BMP9. Interestingly, a recent study confirmed that increased levels of circulating sENG might preferentially direct BMP9 signaling via cell surface ENG at the endothelium, instead of having an inhibitory effect.66 This study suggested that cell surface ENG can capture and present circulating BMP9 and BMP10, thereby increasing their local concentrations; however, the key mechanism by which endothelin is replaced by type II receptors remains unknown. Endothelin is not replaced; rather, they bind together as a whole. Further studies on structural research will clarify the function of BMP9 prodomain and its mechanism of binding replacement with different receptors, which will further explain the unique functions of BMP9.
Relationships between BMP9 and inflammation in different tissues
Since the topic of BMP9 and inflammation is still a relatively new field, before elaborating, we first reviewed other important members of the transforming growth factor-β (TGF-β) superfamily, such as TGF-β, to gain more likely consistent family characteristics and beneficial insights.
TGF-β–a key node of inflammatory regulatory signaling
As a powerful representative of the super and sub clans, TGF-β has been studied for decades and is recognized as an effective regulator of inflammation and fibrosis, two processes that occur together. Previous studies have established that TGF-β is involved in the development of inflammation in tissues and organs throughout the body, and extensively investigated the phenotypes and mechanisms.67 Over the past decade, studies aimed at diversified inflammation further confirmed that TGF-β is a key node for different signal regulation or interventions in tissue inflammation, which will provide the basis for clinical targeted drug therapy.
TGF-β appears in the inflammatory areas of all parts of the body, and is essential for many functions. In mice arthritis, TLR4-mediated IL-12 production enhanced IFN-γ and IL-1β expression, suppressed TGF-β production, and promoted antibody-induced inflammation.68 Resolvin D1 (RvD1) level was greatly increased in the peripheral nervous system (PNS) during the recovery stage of experimental autoimmune neuritis (EAN), which eventually upregulates TGF-β levels and improves inflammation resolution and disease recovery.69 In mouse and human lung fibroblasts, integrinαvβ8-mediated activation of TGF-β regulates airway inflammation and fibrosis.70 In various renal diseases, TGF-β is involved in various signaling pathways, such as that involving angiotensin (Ang)-converting enzyme (ACE) 2 and thrombospondin-2 (TSP-2), which both prevent TGF-β and inflammation activation.71,72 TGF-β also mediates inflammatory responses to various external stimuli, including microorganisms. Dysregulation of the microbiome can drive the occurrence of tumors. Bacteria and even viral metabolites can activate TGF-β to regulate the biological characteristics of tumor cells.73 TGF-β also plays a nodal role in other microbial-induced inflammatory diseases. River blindness is a chronic infection with the filarial nematode Onchocer cavolvulus that affects the skin, lymphatic system, and eyes and occurs in sub-Saharan Africa, Yemen, and Latin America.74 TGF-β expression was weak in all infection sites and then showed an association with Th2-mediated hyperreactivity inonchocerciasis.74 In the intestinal system, Lactobacillus acidophilus attenuates Salmonella-induced intestinal inflammation via TGF-β signaling, and impaired TGF-β signaling enhances peritoneal infection induced by E. coli (Escherichia coli) in rats.75,76 In the central nervous system, astrocytic TGF-β signaling can withstand inflammation and reduce neuronal damage caused by Toxoplasma infection. Nowadays, traditional Chinese medicine has attracted attention in the diagnosis and treatment of diseases, affecting the targeting of TGF-β. Tanshinone IIA, the rhizome of Salvia miltiorrhiza (Danshen), is used to treat chronic kidney disease (CKD) and was found to suppress renal fibrosis and inflammation by altering the expression of TGF-β/Smads and NF-κB pathways, and further supports the potential of tanshinone IIA as a new therapeutic agent for slowing the progression of CKD.77 Moreover, naringin, a dihydroflavonoid found in the dry outer pericarp of pomelo and the citrus of Rutaceae, can antagonize renal interstitial fibrosis by regulating the TGF-β/Smads pathway and the expression of inflammatory factors.78 Wen-pi-tang-Hab-Wu-ling-san (WHW) extract, another important herbal medicine was used to treat renal diseases, and has been shown to inhibit mouse fibrosis associated with inflammatory responses, oxidative stress, and the TGF-β/Smad2/3 signaling pathway.79 Theacrine, a compound in tea that is consumed worldwide, also exerted a superior antiarthritic effect through the suppression of IL-6 and the activation of TGF-β. These herbal medicines have a good effect in clinical application, and one of their common targets is TGF-β.80 The anti-inflammatory mechanism of western medicine also seems to be inseparable from TGF-β. Rivaroxaban (RIVA) is a factor Xa inhibitor with cardioprotective action, which inhibits atherosclerosis and numerous inflammatory cascades. To cope with sunitinib-induced cardiotoxicity, RIVA blocks TGF-β and Smads signaling and inhibits oxidative stress-mediated inflammation.81 In addition, TGF-β bound with synthesized short peptide polymers used for material surface modification can continuously release TGF-β and regulate material-induced inflammation, which would provide key functions for tissue regeneration and repair.82
Furthermore, the use of bone morphogenetic protein-2 (BMP-2), the only approved osteoinductive growth factor for clinical application by the Food and Drug Administration (FDA), has also highlighted various adverse events, including ectopic bone formation, osteoclast-mediated bone resorption, inappropriate adipogenesis, and postoperative inflammation.83,84 TGF-β and BMP2 are the best representatives of the TGF-β superfamily members. One is an immune-related pleiotropic factor, and the other has a strong osteogenic potential, but both have anti-inflammatory or pro-inflammatory properties. Data on the link between BMP9 and inflammation are scarce, but there are indications; it need to be studied further.
BMP9 in liver fibrosis and injury
Fibrosis is a pathological process typically induced by an inflammatory response.85 Liver fibrosis has been implicated in many chronic liver diseases and is a common phenomenon that leads to progressive liver dysfunction. Several serum factors, such as TGF-β and hyaluronic acid (HA), have been evaluated for monitoring liver fibrosis,86 and BMP9 is also expected to be one of them. Both in vivo and in vitro evidence confirmed that BMP9 promotes liver fibrosis.87 A previous study showed that patients with advanced liver fibrosis had elevated BMP9 levels. Furthermore, BMP9 overexpression accelerates liver fibrosis, and adenovirus-mediated BMP9 knockdown attenuates liver fibrogenesis in carbon tetrachloride (CCL4)-induced mouse models.88 Similarly, deletion of the BMP9 gene can reduce 3,5-diethoxicarbonyl-1,4 dihydrocollidine (DDC)-induced cholestatic liver fibrosis.89 However, analysis of multiple publicly available microarray datasets has indicated that BMP9 does not have a major fibrogenic effect.90 Although the function of BMP9 is controversial, several studies have reported that BMP9 regulates inflammation associated with liver fibrosis.
In various mouse models of liver injury, BMP9 expression decreases briefly during the acute inflammatory phase, which could be caused by lipopolysaccharide (LPS) and might be necessary for the repair and regeneration of the damaged area.87 This phenomenon also occurs in the vasculature, which is discussed later in this study. Liver inflammation is also enhanced in DDC-fed BMP9-KO mice.89,91 However, the effect of BMP9 on LPS-stimulated hepatic inflammation is complex and variable, involving fine regulation of numerous liver cells.92 Inflammation and macrophage infiltration are critical elements for the development of non-alcoholic fatty liver disease, which may develop into liver fibrosis in some cases. Nevertheless, BMP9 overexpression exacerbates steatohepatitis in mice on a methionine choline deficiency (MCD) diet, as indicated by hepatic inflammatory gene expression and M1 macrophage recruitment, during which monocyte chemoattractant protein-1 (MCP-1) may play an important role.93 The function of BMP9 in causing inflammation in liver disease is indisputable; therefore, we can speculate that BMP9 can influence liver disease progression (e.g., liver fibrosis), repair, and regeneration by regulating inflammation. Fortunately, this new function has attracted the attention of scholars. Future studies will reveal the mechanisms of this process, and the liver may become the first relatively complete model of BMP9-regulated inflammation.
BMP9 in vascular endothelial inflammation
BMP9 exists in the vasculature in a biologically active form, which maintains vascular system homeostasis and endothelial cell quiescence. A major study focused on the relationships between BMP9 and diseases associated with vascular endothelial inflammation, including pulmonary artery hypertension (PAH), hereditary hemorrhagic telangiectasia (HHT), atherosclerosis, and vascular calcification. In the 21st century, several mutations in genes such as BMPR2, SMAD9, ACVRL1, ENG, and GDF2, have been found to cause PAH.94, 95, 96 Analogously, HHT, an autosomal dominant inheritance, is clearly related to mutations in ENG and ACVRL1, represented as HHT1 and HHT2, respectively. BMP9 treatment reduces alveolarization, septal thickness, inflammation, and type III collagen deposition in rat models of bronchopulmonary dysplasia (BPD). Corresponding in vitro experiments confirmed the anti-inflammatory effects of BMP9.97 Thus, it can be concluded that these vascular endothelial inflammation-associated diseases are specifically associated with BMP9 or its high-affinity receptors. Several studies have focused on this aspect, and the current knowledge can be summarized in the following aspects to illustrate the relationship between BMP9 and vascular inflammation.
BMP9 regulates cytokines associated with inflammation
Direct and overall data of cDNA microarrays obtained after BMP9 treatment of primary human endothelial cells confirmed the variation among chemokine, adhesion, and inflammation pathways, which are closely related to the development of inflammation.98 Quantitative mass spectrometry analysis also revealed changes in the expression levels of related secretory proteins. This almost confirms that there is an association between BMP9 and inflammation in the vasculature, but the exact mechanism remains unclear. Another study on vascular endothelial cells showed that BMP9 can regulate the CCL2/CCR2 inflammatory signaling axis, thereby inhibiting CCL2 and upregulating CCR2; another chemokine, CCL5, was also upregulated transiently.99 A recent study further noted that in healthy conditions, physiological concentrations of BMP9 can inhibit the expression of CCL2, thus reducing the adhesion of monocytes to endothelial cells and maintaining vascular homeostasis; however, in TNF-α-induced inflammatory conditions, BMP9 had no effect on CCL2, but could slightly upregulate IL-8, which does not necessarily promote the inflammatory state.100 Nevertheless, in PAH, BMP9 appears to be dysregulated in the regulation of inflammation, and it can promote endothelial mesenchymal transformation through the increase of IL-6 signaling axis.101 At this point, we can clearly conclude that the role of BMP9 in normal and pathological contexts is different. Vascular calcification is an inflammatory disease that can be regulated by BMP9 through COX2, a proinflammatory factor, and one of the important enzymes in prostaglandin synthesis.102
BMP9 activates inflammation-associated immune cells
BMP9 can promote the recruitment of monocytes to endothelial cells, mainly through the synergistic effect of BMP9 and TNFα to promote the expression of endothelial selectin and adhesion molecules, which involves activation of ALK2 receptor and inhibition of Smad1/5 signaling molecule.103 In addition, 5 ng/ml BMP9 enhanced neutrophil recruitment in LPS-stimulated endothelial cells by synergistically increasing the expression of selectin, vascular cell adhesion molecule-1 (VCAM-1), IL-8, and IL-6.104 It seems that BMP9 does not work on immune cells in a physiological state, and only does so when inflammation is present.
The inflammatory context can also affect BMP9
Conversely, BMP9 has been shown to be regulated by inflammation. A previous study confirmed that the amount of BMP9 produced by the liver decreased 3 h after intraperitoneal injection of LPS in mice and recovered after 18 h. It is important to note that the expression of BMP9 transiently decreased in the vasculature after intraperitoneal LPS injection, similar to the findings in the liver. Interestingly, although LPS is not injected into the liver or the liver is not the inflammation center, the molecules released from the liver are significantly affected. Whether BMP9 is an important inflammation-related regulator or the inflammation of tissues at a distance from the liver can still affect the liver is worth studying.
In summary, we obtained a complex but seemingly clear picture of BMP9 and vascular endothelial inflammation. BMP9 affects the inflammatory process, which in turn affects the production and survival of BMP9. The other two drivers of this event are endothelial cells and innate immune cells, such as neutrophils and monocytes, which are linked by inflammatory factors, chemokines, and adhesion factors, and chemokines, particularly CCL2, may be key nodes. BMP9 interacts with endothelial cells and immune cells to secrete inflammatory cytokines and chemokines, leading to the migration of immune cells to the endothelium for further interaction, which seems to be the core event of this process.
BMP9 in bone injury and arthritis
Several studies have summarized the osteogenic functions of BMP9, and unique osteogenic properties have been identified. It regulates several downstream targets that may play a role in bone induction; however, the ossification pattern of BMP9-induced bone formation is different from that of other BMP-induced bone formation.43 MSCs treated with adenovirus-loaded BMP9 showed varying degrees of ossification and multiple areas of immature braided bone at 3 weeks, but in the BMP2-treated MSCs, the ossification was significantly decreased and the areas of braided bone were dysplastic and small, and the difference was even more significant at 5 weeks.12 Similarly, BMP9-induced bone formation is distinct from that induced by other BMPs and is similar to physiological ossification.105 Interestingly, a study reported that BMP9 induces heterotopic ossification (HO) only in impaired muscles, while BMP2 promotes HO regardless of skeletal muscle status.106 These findings indicate interesting characteristics of BMP9 and confirm a connection between BMP9 and inflammation, which is worth studying.
In rheumatoid arthritis, fibroblast-like synoviocytes (FLSs) actively participate in inflammation development by producing inflammatory mediators, such as proinflammatory cytokines, TNF-α and IL-6.107 A recent study further indicated that BMP9 expression decreases in rat arthritis models at 24 days, and BMP9 knockdown and overexpression promote and inhibit FLS proliferation and migration, respectively.108 BMP9 may be involved in atherosclerosis by regulating FLSs; however, BMP9 is downregulated in arthritis synovial tissues, which is consistent with the decrease in BMP9 expression during the acute phase of liver injury, although it is not an instantaneous drop. This may be because the inflammatory environment of BMP9 in the two tissues was different. However, whether it is a transient or long-term reduction, it seems that the inflammatory process can proceed smoothly only if BMP9 is downregulated in the initial or entire stage of inflammation. Osteoarthritis (OS) is an inflammatory disease mediated by adipose-derived mesenchymal stem cells (ADMSCs), and BMP9 overexpression promotes cartilage repair of the mouse knee joint with OS.109 Unfortunately, whether BMP9 can influence the surrounding inflammatory environment has not been studied, and previous descriptions suggest a connection between them. Another study confirmed that BMP2 and BMP9 can partially block chondrogenic differentiation of MSCs inhibited by low IL-1β concentrations; however, BMP9 can maintain high Col2A1 expression irrespective of the IL-1β concentration.
Bone fracture is an inflammatory niche that incorporates intricate interactions between signaling molecules. COX-2, a prostaglandin synthetase, is highly expressed at inflammatory sites and plays an important role in the osteogenic differentiation of BMP9-induced bone marrow MSCs.110,111 Another study showed that LPS inhibits BMP9-induced osteogenic differentiation in vitro and affects BMP9 through the MAPKs pathway.112 However, whether the reverse is also true needs to be studied. Thus, it can be concluded that previous studies have focused on the osteogenic capacity of BMP9 in bones and joints, but not on its role in the presence of inflammatory factors in real situations. However, unlike other BMP family members, BMP9 shows more physiologic osteogenic approach, superior osteogenic capacity, and decline in the osteoarthritic process, supporting the hypothesis that BMP9 regulates both inflammation and repair in bone and joint injury, thereby resulting in a better repair effect, which needs to be further validated.
BMP9 in tooth development, pulp and periodontal diseases
The role of BMP9 in dental organs has been studied relatively late, and its expression during tooth development has been reported recently. BMP9 was expressed in mouse tooth germ cells, ameloblast cells, dental pulp cells, and alveolar bone osteoblasts. BMP9-KO mice possesses worn tooth tip, short root, and thin dentin; similarly, the alveolar bone is small and has poor mineral density, trabecular thickness, and bone volume fraction.113 The functions of BMP9 in tooth development have been reported to be linked with pulp cells, periodontal cells,114 and apical papilla cells.115 Periodontal disease and caries are the two main tooth-related diseases, and caries can further develop into pulpitis and periapical periodontitis. However, the relationships between BMP9 and the three types of inflammatory diseases have been poorly studied. TNF-α is released in large amounts in periapical inflammatory areas, and in vitro studies have shown that it can inhibit the osteogenic repair process, while BMP9 can partially restore this inhibitory effect.116 The study also showed that BMP9 may act as a cytokine to combat chronic inflammation-induced bone lesions, which is a critical and forward-looking point. TNF-α weakens BMP9-induced tooth or osteogenic differentiation in human periodontal ligament fibroblasts117,118 or rat tooth sac cells.119 Interestingly, DKK1 alone inhibits BMP9-induced osteogenic differentiation, while promoting it in combination with TNF-α, the mechanism of which remains to be further explored.119 After implantation, the surrounding area can be flooded with inflammatory responses, and pro- and anti-inflammatory factors can interact. A recent study indicated that the surface modification of implants promotes BMP9 expression in the early post-implantation period.120 Considering its performance in other tissues, it is not difficult to imagine that BMP9 interacts with the inflammatory environment at this stage. Pulpitis, periodontitis, and periapical periodontitis are regulated by many cytokines and are good models for studying inflammation and repair processes. BMP9 is a restorative factor for dentin and alveolar bone formation, and coupled with the above evidence of its association with inflammation, it is believed that BMP9 plays a role in tooth-related inflammation development and progression.
BMP9 in other inflammatory contexts
Skin is the body's natural defense barrier, which can resist all kinds of exoteric stimuli, such as bacteria and viruses.121 Once injury occurs, highly integrated cellular and molecular events are programmed to ensure proper and timely wound healing. Although many elements that regulate wound healing have been identified, the exact mechanisms remain unclear. The dermis of BMP9-KO mice was thinner than that of wild-type (WT) controls. When dealing with skin wounds on the backs of mice, the wound closure rate in BMP9-KO mice was significantly reduced, suggesting that BMP9 plays a role in skin wounds. However, in exploring the potential mechanism, the experiment was not conducted in an inflammatory environment, and was different from that in vivo.122 Whether BMP9 has an effect on skin cells in the inflammatory context and whether it has an effect on the inflammatory state still needs to be determined. Some studies have confirmed that cytokines such as parathyroid hormone-related protein (PTH-rp), IL-6, IL-8, and RANKL (the RANK ligand, RANK: receptor activator of nuclear factor-kappa B) can directly or indirectly promote the migration and invasion of cancer cells by enhancing the activity of MAPKs and Akt signaling pathways.123, 124, 125, 126 Breast cancer cells often metastasize to distant organs, including bone, where they interact with cells in bone tissue to cause local microenvironment changes that lead to osteolytic fractures. Based on this, BMP9 was found to inhibit the expression of IL-6, PTH-rp, and MMP9 matrix metalloproteinase (MMP) molecules by inhibiting the MAPKs and Akt signaling axis, thus blocking cancer cell metastasis.127 The most attractive thing is that these signaling pathways and molecules are associated with inflammation, which seems to directly indicate that BMP9 can regulate inflammatory factors. The same conclusion was obtained in the microenvironment of bone tissue in lung cancer bone metastasis; BMP9 can inhibit the expression of IL-6 and IL-8 through MAPK/ERK and NF-κB signaling pathways.128 It is believed that as the presence of BMP9 is found in more tissues and its function is further studied, the association between BMP9 and inflammation will be confirmed in more lesions and other microenvironments.
Potential mechanisms between BMP9 and inflammation
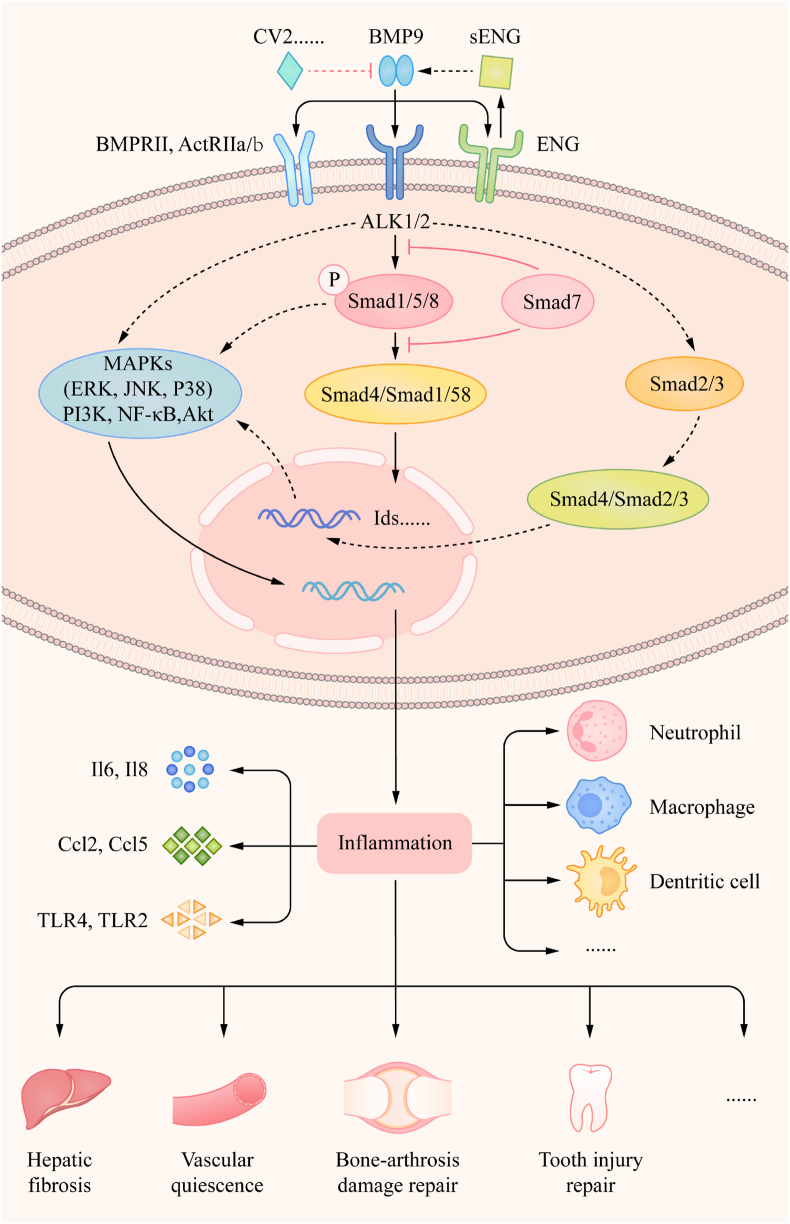
Compared with the relatively thorough studies on TGF-β, the mechanisms by which BMP9 regulates inflammation is not clear, and vice versa. BMP9 signals through classical Smad1/5/8 and non-classical MAPKs regulate many downstream transcription factors, and can interact with multiple signaling molecules, including Wnt, Notch, Hedgehog (Hh), and epidermal growth factor (EGF). All these members work together to regulate skeletal and bone formation, angiogenesis, and the development and homeostasis of multiple organ systems. Some reviews have reported detailed mechanisms of their interaction; however, unfortunately, we do not know whether these also apply to BMP9 and inflammation, which requires further work to understand the mechanisms. Currently, most studies related to BMP9 and inflammation are at the phenotypic stage, but there are still two aspects that can be summarized. Mutations in the BMP9 ligand or its receptors in the vascular system can cause or contribute to diseases with inflammatory phenotypes. Inherited pulmonary hypertension, for example, is mainly caused by mutations in preferred affinity receptor of BMP9, BMPRII, which directly blocks BMP9 signal transduction; although rare, mutations in another high-affinity receptor ALK1 have been identified.96 Recent studies have confirmed that BMP9 gene mutations occur in idiopathic pulmonary arterial hypertension (IPAH).129 In addition, BMP9 can promote monocyte migration by inhibiting Smad1/5 and resistant to the inflammatory environment for tumor cell metastasis, and MAPK/ERK, NF-κB and Akt signaling molecules are regulated in this process.127,128 Therefore, it is not difficult to see that BMP9 regulates the function of immune cells and the expression of inflammatory cytokines through inflammation-related signaling factors. BMP9 also cooperatively promotes TLR4 and TLR2 level to promote the recruitment of LPS- and TNF-α-induced neutrophile granulocyte and monocytes to endothelial cells, respectively, suggesting that BMP9 can directly control the key inflammatory molecules.103,104 In summary, the mechanisms of the interaction between BMP9 and inflammation is not yet complete, but we can learn from other members, which may help provide future research directions (Fig. 2).
Figure 2.
Model diagram of the potential mechanisms by which BMP9 regulates inflammation in different tissues.
Conclusion
Several features of BMP9 have been discovered, which make it a potential target for the treatment of hepatic fibrosis, vascular diseases, such as PAH and HHT, as well as a candidate for promoting bone injury healing and bone regeneration. The most important findings seem to be the transient decrease in BMP9 expression in the liver during different types of inflammation and a long-term decrease in arthritis. However, the bidirectional regulation relationships between BMP9 and inflammation seem to be almost certain considering the evidence available, and further work might be helpful in finding the answer. Thus, BMP9 appears to be a growth and differentiation factor that regulates inflammation and promotes repair, which also provides new insights into tissue damage and repair or regenerative medicine. With further research, BMP9 is likely to become a molecule that can both regulate inflammation and have a strong repair ability and may be used in clinical injury and repair research.
Author contributions
Tianzhu Song was responsible for drafting the manuscript including literature search, reading and writing. Dingming Huang revised the article for important intellectual content. Dongzhe Song guided this project, critically revised the article for important intellectual content and performed the final approval of the version to be submitted.
Conflict of interests
All authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [No. 81900996 (D.Z.S), 81771063 (D.M.H)], and the Postdoc Science Funding of China [No. 2019M653441 (D.Z.S)].
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Dingming Huang, Email: dingminghuang@163.com.
Dongzhe Song, Email: dongzhesong@scu.edu.cn.
References
- 1.Zarrin A.A., Bao K., Lupardus P., Vucic D. Kinase inhibition in autoimmunity and inflammation. Nat Rev Drug Discov. 2021;20(1):39–63. doi: 10.1038/s41573-020-0082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooke J.P. Inflammation and its role in regeneration and repair. Circ Res. 2019;124(8):1166–1168. doi: 10.1161/CIRCRESAHA.118.314669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crusz S.M., Balkwill F.R. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol. 2015;12(10):584–596. doi: 10.1038/nrclinonc.2015.105. [DOI] [PubMed] [Google Scholar]
- 4.Fullerton J.N., Gilroy D.W. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov. 2016;15(8):551–567. doi: 10.1038/nrd.2016.39. [DOI] [PubMed] [Google Scholar]
- 5.Mountziaris P.M., Mikos A.G. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng Part B Rev. 2008;14(2):179–186. doi: 10.1089/ten.teb.2008.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanjabi S., Oh S.A., Li M.O. Regulation of the immune response by TGF-beta: from conception to autoimmunity and infection. Cold Spring Harb Perspect Biol. 2017;9(6) doi: 10.1101/cshperspect.a022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasconcelos D.P., Águas A.P., Barbosa M.A., Pelegrín P., Barbosa J.N. The inflammasome in host response to biomaterials: bridging inflammation and tissue regeneration. Acta Biomater. 2019;83:1–12. doi: 10.1016/j.actbio.2018.09.056. [DOI] [PubMed] [Google Scholar]
- 8.Gomez-Puerto M.C., Iyengar P.V., García de Vinuesa A., Ten Dijke P., Sanchez-Duffhues G. Bone morphogenetic protein receptor signal transduction in human disease. J Pathol. 2019;247(1):9–20. doi: 10.1002/path.5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L., Trebicka E., Fu Y., et al. The bone morphogenetic protein-hepcidin axis as a therapeutic target in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18(1):112–119. doi: 10.1002/ibd.21675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grgurevic L., Christensen G.L., Schulz T.J., Vukicevic S. Bone morphogenetic proteins in inflammation, glucose homeostasis and adipose tissue energy metabolism. Cytokine Growth Factor Rev. 2016;27:105–118. doi: 10.1016/j.cytogfr.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Cheng H., Jiang W., Phillips F.M., et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Joint Surg Am. 2003;85(8):1544–1552. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Kang Q., Sun M.H., Cheng H., et al. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther. 2004;11(17):1312–1320. doi: 10.1038/sj.gt.3302298. [DOI] [PubMed] [Google Scholar]
- 13.David L., Mallet C., Keramidas M., et al. Bone morphogenetic protein-9 is a circulating vascular quiescence factor. Circ Res. 2008;102(8):914–922. doi: 10.1161/CIRCRESAHA.107.165530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Y., Yu H., Yang C., et al. Krüppel-like factor 6 mediates pulmonary angiogenesis in rat experimental hepatopulmonary syndrome and is aggravated by bone morphogenetic protein 9. Biol Open. 2019;8(6) doi: 10.1242/bio.040121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang M., Liang Z., Yang M., et al. Role of bone morphogenetic protein-9 in the regulation of glucose and lipid metabolism. FASEB J. 2019;33(9):10077–10088. doi: 10.1096/fj.201802544RR. [DOI] [PubMed] [Google Scholar]
- 16.Burke R.M., Norman T.A., Haydar T.F., et al. BMP9 ameliorates amyloidosis and the cholinergic defect in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2013;110(48):19567–19572. doi: 10.1073/pnas.1319297110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.López-Coviella I., Berse B., Krauss R., Thies R.S., Blusztajn J.K. Induction and maintenance of the neuronal cholinergic phenotype in the central nervous system by BMP-9. Science. 2000;289(5477):313–316. doi: 10.1126/science.289.5477.313. [DOI] [PubMed] [Google Scholar]
- 18.García-Álvaro M., Addante A., Roncero C., et al. BMP9-induced survival effect in liver tumor cells requires p38MAPK activation. Int J Mol Sci. 2015;16(9):20431–20448. doi: 10.3390/ijms160920431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li F.S., Huang J., Cui M.Z., et al. BMP9 mediates the anticancer activity of evodiamine through HIF-1α/p53 in human colon cancer cells. Oncol Rep. 2020;43(2):415–426. doi: 10.3892/or.2019.7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoonderwoerd M.J.A., Goumans M.T.H., Hawinkels L.J.A.C. Endoglin: beyond the endothelium. Biomolecules. 2020;10(2):289. doi: 10.3390/biom10020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y., Hu T., Ye G., Xiang X.R., Hu N. p38 MAPK pathway mediated BMP9-induced osteogenetic differentiation of hPDLSCs. Shanghai Kou Qiang Yi Xue. 2018;27(6):596–601. [PubMed] [Google Scholar]
- 22.Zheng W., Gu X., Sun X., Wu Q., Dan H. FAK mediates BMP9-induced osteogenic differentiation via Wnt and MAPK signaling pathway in synovial mesenchymal stem cells. Artif Cells Nanomed Biotechnol. 2019;47(1):2641–2649. doi: 10.1080/21691401.2019.1631838. [DOI] [PubMed] [Google Scholar]
- 23.Reddi A.H. BMPs: from bone morphogenetic proteins to body morphogenetic proteins. Cytokine Growth Factor Rev. 2005;16(3):249–250. doi: 10.1016/j.cytogfr.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Wang R.N., Green J., Wang Z., et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014;1(1):87–105. doi: 10.1016/j.gendis.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bolt P., Clerk A.N., Luu H.H., et al. BMP-14 gene therapy increases tendon tensile strength in a rat model of Achilles tendon injury. J Bone Joint Surg Am. 2007;89(6):1315–1320. doi: 10.2106/JBJS.F.00257. [DOI] [PubMed] [Google Scholar]
- 26.Song J.J., Celeste A.J., Kong F.M., Jirtle R.L., Rosen V., Thies R.S. Bone morphogenetic protein-9 binds to liver cells and stimulates proliferation. Endocrinology. 1995;136(10):4293–4297. doi: 10.1210/endo.136.10.7664647. [DOI] [PubMed] [Google Scholar]
- 27.Bidart M., Ricard N., Levet S., et al. BMP9 is produced by hepatocytes and circulates mainly in an active mature form complexed to its prodomain. Cell Mol Life Sci. 2012;69(2):313–324. doi: 10.1007/s00018-011-0751-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levet S., Ouarné M., Ciais D., et al. BMP9 and BMP10 are necessary for proper closure of the ductus arteriosus. Proc Natl Acad Sci U S A. 2015;112(25):E3207–E3215. doi: 10.1073/pnas.1508386112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen H., Shi S., Acosta L., et al. BMP10 is essential for maintaining cardiac growth during murine cardiogenesis. Development. 2004;131(9):2219–2231. doi: 10.1242/dev.01094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niessen K., Zhang G., Ridgway J.B., Chen H., Yan M. ALK1 signaling regulates early postnatal lymphatic vessel development. Blood. 2010;115(8):1654–1661. doi: 10.1182/blood-2009-07-235655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen H., Ridgway J.B., Sai T., et al. Context-dependent signaling defines roles of BMP9 and BMP10 in embryonic and postnatal development. Proc Natl Acad Sci U S A. 2013;110(29):11887–11892. doi: 10.1073/pnas.1306074110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller A.F., Harvey S.A., Thies R.S., Olson M.S. Bone morphogenetic protein-9. An autocrine/paracrine cytokine in the liver. J Biol Chem. 2000;275(24):17937–17945. doi: 10.1074/jbc.275.24.17937. [DOI] [PubMed] [Google Scholar]
- 33.Liu W., Deng Z., Zeng Z., et al. Highly expressed BMP9/GDF2 in postnatal mouse liver and lungs may account for its pleiotropic effects on stem cell differentiation, angiogenesis, tumor growth and metabolism. Genes Dis. 2019;7(2):235–244. doi: 10.1016/j.gendis.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogawa C., Mikawa S., Hayashi Y., Masumoto K., Katou F., Sato K. BMP9 expression in the adult rat brain. J Chem Neuroanat. 2021;113 doi: 10.1016/j.jchemneu.2021.101933. [DOI] [PubMed] [Google Scholar]
- 35.Li X., Wang L., Su Q., et al. Potential roles of bone morphogenetic protein 9 in the odontogenic differentiation of dental pulp cells. J Endod. 2021;47(3):436–443. doi: 10.1016/j.joen.2020.10.018. [DOI] [PubMed] [Google Scholar]
- 36.Heldin C.H., Moustakas A. Signaling receptors for TGF-beta family members. Cold Spring Harb Perspect Biol. 2016;8(8):a022053. doi: 10.1101/cshperspect.a022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang T., David L., Mendoza V., et al. TGF-β signalling is mediated by two autonomously functioning TbetaRI:TbetaRII pairs. EMBO J. 2011;30(7):1263–1276. doi: 10.1038/emboj.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wood J.H., Guo J., Morrell N.W., Li W. Advances in the molecular regulation of endothelial BMP9 signalling complexes and implications for cardiovascular disease. Biochem Soc Trans. 2019;47(3):779–791. doi: 10.1042/BST20180137. [DOI] [PubMed] [Google Scholar]
- 39.Yadin D., Knaus P., Mueller T.D. Structural insights into BMP receptors: specificity, activation and inhibition. Cytokine Growth Factor Rev. 2016;27:13–34. doi: 10.1016/j.cytogfr.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 40.Itoh S., ten Dijke P. Negative regulation of TGF-beta receptor/Smad signal transduction. Curr Opin Cell Biol. 2007;19(2):176–184. doi: 10.1016/j.ceb.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 41.Ross S., Hill C.S. How the Smads regulate transcription. Int J Biochem Cell Biol. 2008;40(3):383–408. doi: 10.1016/j.biocel.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 42.Miyazono K., Kamiya Y., Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147(1):35–51. doi: 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- 43.Lamplot J.D., Qin J., Nan G., et al. BMP9 signaling in stem cell differentiation and osteogenesis. Am J Stem Cells. 2013;2(1):1–21. [PMC free article] [PubMed] [Google Scholar]
- 44.Tillet E., Ouarné M., Desroches-Castan A., et al. A heterodimer formed by bone morphogenetic protein 9 (BMP9) and BMP10 provides most BMP biological activity in plasma. J Biol Chem. 2018;293(28):10963–10974. doi: 10.1074/jbc.RA118.002968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alt A., Miguel-Romero L., Donderis J., et al. Structural and functional insights into endoglin ligand recognition and binding. PLoS One. 2012;7(2) doi: 10.1371/journal.pone.0029948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castonguay R., Werner E.D., Matthews R.G., et al. Soluble endoglin specifically binds bone morphogenetic proteins 9 and 10 via its orphan domain, inhibits blood vessel formation, and suppresses tumor growth. J Biol Chem. 2011;286(34):30034–30046. doi: 10.1074/jbc.M111.260133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei Z., Salmon R.M., Upton P.D., Morrell N.W., Li W. Regulation of bone morphogenetic protein 9 (BMP9) by redox-dependent proteolysis. J Biol Chem. 2014;289(45):31150–31159. doi: 10.1074/jbc.M114.579771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo J., Tang M., Huang J., et al. TGFbeta/BMP type I receptors ALK1 and ALK2 are essential for BMP9-induced osteogenic signaling in mesenchymal stem cells. J Biol Chem. 2010;285(38):29588–29598. doi: 10.1074/jbc.M110.130518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tantilertanant Y., Niyompanich J., Everts V., Supaphol P., Pavasant P., Sanchavanakit N. Cyclic tensile force stimulates BMP9 synthesis and in vitro mineralization by human periodontal ligament cells. J Cell Physiol. 2019;234(4):4528–4539. doi: 10.1002/jcp.27257. [DOI] [PubMed] [Google Scholar]
- 50.Wu N., Zhao Y., Yin Y., Zhang Y., Luo J. Identification and analysis of type II TGF-β receptors in BMP-9-induced osteogenic differentiation of C3H10T1/2 mesenchymal stem cells. Acta Biochim Biophys Sin (Shanghai) 2010;42(10):699–708. doi: 10.1093/abbs/gmq075. [DOI] [PubMed] [Google Scholar]
- 51.Townson S.A., Martinez-Hackert E., Greppi C., et al. Specificity and structure of a high affinity activin receptor-like kinase 1 (ALK1) signaling complex. J Biol Chem. 2012;287(33):27313–27325. doi: 10.1074/jbc.M112.377960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu D.J., Zhao Y.Z., Wang J., He J.W., Weng Y.G., Luo JY. Smads. p38 and ERK1/2 are involved in BMP9-induced osteogenic differentiation of C3H10T1/2 mesenchymal stem cells. BMB Rep. 2012;45(4):247–252. doi: 10.5483/bmbrep.2012.45.4.247. [DOI] [PubMed] [Google Scholar]
- 53.Li C., Yang X., He Y., et al. Bone morphogenetic protein-9 induces osteogenic differentiation of rat dental follicle stem cells in P38 and ERK1/2 MAPK dependent manner. Int J Med Sci. 2012;9(10):862–871. doi: 10.7150/ijms.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng W., Chen Q., Zhang Y., et al. BMP9 promotes osteogenic differentiation of SMSCs by activating the JNK/Smad2/3 signaling pathway. J Cell Biochem. 2020;121(4):2851–2863. doi: 10.1002/jcb.29519. [DOI] [PubMed] [Google Scholar]
- 55.Wang X., Zong L., Wang W., Yang J., Xiang Y. CD105 overexpression mediates drug-resistance in choriocarcinoma cells through BMP9/Smad pathway. J Cancer. 2020;11(2):272–283. doi: 10.7150/jca.34965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cunha S.I., Pardali E., Thorikay M., et al. Genetic and pharmacological targeting of activin receptor-like kinase 1 impairs tumor growth and angiogenesis. J Exp Med. 2010;207(1):85–100. doi: 10.1084/jem.20091309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu D., Mackenzie N.C., Shanahan C.M., Shroff R.C., Farquharson C., MacRae V.E. BMP-9 regulates the osteoblastic differentiation and calcification of vascular smooth muscle cells through an ALK1 mediated pathway. J Cell Mol Med. 2015;19(1):165–174. doi: 10.1111/jcmm.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meurer S.K., Weiskirchen R. Endoglin: an 'accessory' receptor regulating blood cell development and inflammation. Int J Mol Sci. 2020;21(23) doi: 10.3390/ijms21239247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jing H., Zhang Q., Li S., Gao X.J. Pb exposure triggers MAPK-dependent inflammation by activating oxidative stress and miRNA-155 expression in carp head kidney. Fish Shellfish Immunol. 2020;106:219–227. doi: 10.1016/j.fsi.2020.08.015. [DOI] [PubMed] [Google Scholar]
- 60.Luo W., Jin Y., Wu G., et al. Blockage of ROS and MAPKs-mediated inflammation via restoring SIRT1 by a new compound LF10 prevents type 1 diabetic cardiomyopathy. Toxicol Appl Pharmacol. 2019;370:24–35. doi: 10.1016/j.taap.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 61.Yao Y., Jumabay M., Ly A., et al. Crossveinless 2 regulates bone morphogenetic protein 9 in human and mouse vascular endothelium. Blood. 2012;119(21):5037–5047. doi: 10.1182/blood-2011-10-385906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salmon R.M., Guo J., Wood J.H., et al. Molecular basis of ALK1-mediated signalling by BMP9/BMP10 and their prodomain-bound forms. Nat Commun. 2020;11(1) doi: 10.1038/s41467-020-15425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mi L.Z., Brown C.T., Gao Y., et al. Structure of bone morphogenetic protein 9 procomplex. Proc Natl Acad Sci U S A. 2015;112(12):3710–3715. doi: 10.1073/pnas.1501303112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anderson S.B., Goldberg A.L., Whitman M. Identification of a novel pool of extracellular pro-myostatin in skeletal muscle. J Biol Chem. 2008;283(11):7027–7035. doi: 10.1074/jbc.M706678200. [DOI] [PubMed] [Google Scholar]
- 65.Brown M.A., Zhao Q., Baker K.A., et al. Crystal structure of BMP-9 and functional interactions with pro-region and receptors. J Biol Chem. 2005;280(26):25111–25118. doi: 10.1074/jbc.M503328200. [DOI] [PubMed] [Google Scholar]
- 66.Lawera A., Tong Z., Thorikay M., et al. Role of soluble endoglin in BMP9 signaling. Proc Natl Acad Sci U S A. 2019;116(36):17800–17808. doi: 10.1073/pnas.1816661116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoshimura A., Wakabayashi Y., Mori T. Cellular and molecular basis for the regulation of inflammation by TGF-beta. J Biochem. 2010;147(6):781–792. doi: 10.1093/jb/mvq043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim H.S., Chung D.H. TLR4-mediated IL-12 production enhances IFN-γ and IL-1β production, which inhibits TGF-β production and promotes antibody-induced joint inflammation. Arthritis Res Ther. 2012;14(5) doi: 10.1186/ar4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luo B., Han F., Xu K., et al. Resolvin D1 programs inflammation resolution by increasing TGF-β expression induced by dying cell clearance in experimental autoimmune neuritis. J Neurosci. 2016;36(37):9590–9603. doi: 10.1523/JNEUROSCI.0020-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kitamura H., Cambier S., Somanath S., et al. Mouse and human lung fibroblasts regulate dendritic cell trafficking, airway inflammation, and fibrosis through integrin αvβ8-mediated activation of TGF-β. J Clin Invest. 2011;121(7):2863–2875. doi: 10.1172/JCI45589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu Z., Huang X.R., Chen H.Y., Penninger J.M., Lan H.Y. Loss of angiotensin-converting enzyme 2 enhances TGF-β/Smad-mediated renal fibrosis and NF-κB-driven renal inflammation in a mouse model of obstructive nephropathy. Lab Invest. 2012;92(5):650–661. doi: 10.1038/labinvest.2012.2. [DOI] [PubMed] [Google Scholar]
- 72.Daniel C., Vogelbacher R., Stief A., Grigo C., Hugo C. Long-term gene therapy with thrombospondin 2 inhibits TGF-β activation, inflammation and angiogenesis in chronic allograft nephropathy. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0083846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pang X., Tang Y.J., Ren X.H., Chen Q.M., Tang Y.L., Liang X.H. Microbiota, epithelium, inflammation, and TGF-β signaling: an intricate interaction in oncogenesis. Front Microbiol. 2018;9 doi: 10.3389/fmicb.2018.01353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Korten S., Hoerauf A., Kaifi J.T., Büttner D.W. Low levels of transforming growth factor-beta (TGF-beta) and reduced suppression of Th2-mediated inflammation in hyperreactive human onchocerciasis. Parasitology. 2011;138(1):35–45. doi: 10.1017/S0031182010000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang I.F., Lin I.C., Liu P.F., et al. Lactobacillus acidophilus attenuates Salmonella-induced intestinal inflammation via TGF-β signaling. BMC Microbiol. 2015;15 doi: 10.1186/s12866-015-0546-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang X., Nie J., Jia Z., et al. Impaired TGF-beta signalling enhances peritoneal inflammation induced by E. coli in rats. Nephrol Dial Transplant. 2010;25(2):399–412. doi: 10.1093/ndt/gfp480. [DOI] [PubMed] [Google Scholar]
- 77.Wang D.T., Huang R.H., Cheng X., Zhang Z.H., Yang Y.J., Lin X. Tanshinone IIA attenuates renal fibrosis and inflammation via altering expression of TGF-β/Smad and NF-κB signaling pathway in 5/6 nephrectomized rats. Int Immunopharmacol. 2015;26(1):4–12. doi: 10.1016/j.intimp.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 78.Wang R., Wu G., Dai T., et al. Naringin attenuates renal interstitial fibrosis by regulating the TGF-β/Smad signaling pathway and inflammation. Exp Ther Med. 2021;21(1) doi: 10.3892/etm.2020.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jung K.J., Kim J., Park Y.K., Yoon Y.R., Park K.M. Wen-pi-tang-Hab-Wu-ling-san reduces ureteral obstructive renal fibrosis by the reduction of oxidative stress, inflammation, and TGF-beta/Smad2/3 signaling. Food Chem Toxicol. 2010;48(2):522–529. doi: 10.1016/j.fct.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 80.Gao M., Zheng J., Zheng C., Huang Z., Huang Q. Theacrine alleviates chronic inflammation by enhancing TGF-β-mediated shifts via TGF-β/SMAD pathway in Freund's incomplete adjuvant-induced rats. Biochem Biophys Res Commun. 2020;522(3):743–748. doi: 10.1016/j.bbrc.2019.11.126. [DOI] [PubMed] [Google Scholar]
- 81.Imam F., Al-Harbi N.O., Khan M.R., et al. Protective effect of RIVA against sunitinib-induced cardiotoxicity by inhibiting oxidative stress-mediated inflammation: probable role of TGF-β and Smad signaling. Cardiovasc Toxicol. 2020;20(3):281–290. doi: 10.1007/s12012-019-09551-8. [DOI] [PubMed] [Google Scholar]
- 82.Shi D., Xiao J., Gu R., Wu G., Liao H. Polyurethane conjugating TGF-β on surface impacts local inflammation and endoplasmic reticulum stress in skeletal muscle. J Biomed Mater Res A. 2017;105(4):1156–1165. doi: 10.1002/jbm.a.35999. [DOI] [PubMed] [Google Scholar]
- 83.James A.W., LaChaud G., Shen J., et al. A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng Part B Rev. 2016;22(4):284–297. doi: 10.1089/ten.teb.2015.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nguyen V., Meyers C.A., Yan N., Agarwal S., Levi B., James A.W. BMP-2-induced bone formation and neural inflammation. J Orthop. 2017;14(2):252–256. doi: 10.1016/j.jor.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rockey D.C., Bell P.D., Hill J.A. Fibrosis—a common pathway to organ injury and failure. N Engl J Med. 2015;372(12):1138–1149. doi: 10.1056/NEJMra1300575. [DOI] [PubMed] [Google Scholar]
- 86.Lurie Y., Webb M., Cytter-Kuint R., Shteingart S., Lederkremer G.Z. Non-invasive diagnosis of liver fibrosis and cirrhosis. World J Gastroenterol. 2015;21(41):11567–11583. doi: 10.3748/wjg.v21.i41.11567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Breitkopf-Heinlein K., Meyer C., König C., et al. BMP-9 interferes with liver regeneration and promotes liver fibrosis. Gut. 2017;66(5):939–954. doi: 10.1136/gutjnl-2016-313314. [DOI] [PubMed] [Google Scholar]
- 88.Li P., Li Y., Zhu L., et al. Targeting secreted cytokine BMP9 gates the attenuation of hepatic fibrosis. Biochim Biophys Acta Mol Basis Dis. 2018;1864(3):709–720. doi: 10.1016/j.bbadis.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 89.Addante A., Roncero C., Almalé L., et al. Bone morphogenetic protein 9 as a key regulator of liver progenitor cells in DDC-induced cholestatic liver injury. Liver Int. 2018;38(9):1664–1675. doi: 10.1111/liv.13879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.John M., Kim K.J., Bae S.D.W., Qiao L., George J. Role of BMP-9 in human liver disease. Gut. 2019;68(11):2097–2100. doi: 10.1136/gutjnl-2018-317543. [DOI] [PubMed] [Google Scholar]
- 91.Pascale R.M., Feo F., Calvisi D.F. The complex role of bone morphogenetic protein 9 in liver damage and regeneration: new evidence from in vivo and in vitro studies. Liver Int. 2018;38(9):1547–1549. doi: 10.1111/liv.13925. [DOI] [PubMed] [Google Scholar]
- 92.Gaitantzi H., Karch J., Germann L., et al. BMP-9 modulates the hepatic responses to LPS. Cells. 2020;9(3) doi: 10.3390/cells9030617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li Q., Liu B., Breitkopf-Heinlein K., et al. Adenovirus-mediated overexpression of bone morphogenetic protein-9 promotes methionine choline deficiency-induced non-alcoholic steatohepatitis in non-obese mice. Mol Med Rep. 2019;20(3):2743–2753. doi: 10.3892/mmr.2019.10508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Trembath R.C., Thomson J.R., Machado R.D., et al. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N Engl J Med. 2001;345(5):325–334. doi: 10.1056/NEJM200108023450503. [DOI] [PubMed] [Google Scholar]
- 95.Shintani M., Yagi H., Nakayama T., Saji T., Matsuoka R. A new nonsense mutation of SMAD8 associated with pulmonary arterial hypertension. J Med Genet. 2009;46(5):331–337. doi: 10.1136/jmg.2008.062703. [DOI] [PubMed] [Google Scholar]
- 96.Pousada G., Baloira A., Fontán D., Núñez M., Valverde D. Mutational and clinical analysis of the ENG gene in patients with pulmonary arterial hypertension. BMC Genet. 2016;17(1) doi: 10.1186/s12863-016-0384-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen X., Orriols M., Walther F.J., et al. Bone morphogenetic protein 9 protects against neonatal hyperoxia-induced impairment of alveolarization and pulmonary inflammation. Front Physiol. 2017;8 doi: 10.3389/fphys.2017.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Young K., Conley B., Romero D., et al. BMP9 regulates endoglin-dependent chemokine responses in endothelial cells. Blood. 2012;120(20):4263–4273. doi: 10.1182/blood-2012-07-440784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Young K., Tweedie E., Conley B., et al. BMP9 crosstalk with the Hippo pathway regulates endothelial cell matricellular and chemokine responses. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0122892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Upton P.D., Park J., De Souza P., et al. BMP9 suppresses MCP1 production by human pulmonary artery endothelial cells. Ann Am Thorac Soc. 2010;181 A5242. [Google Scholar]
- 101.Upton P.D., Park J.E.S., De Souza P.M., et al. Endothelial protective factors BMP9 and BMP10 inhibit CCL2 release by human vascular endothelial cells. J Cell Sci. 2020;133(14):jcs239715. doi: 10.1242/jcs.239715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.He F., Wang H., Ren W.Y., et al. BMP9/COX-2 axial mediates high phosphate-induced calcification in vascular smooth muscle cells via Wnt/β-catenin pathway. J Cell Biochem. 2018;119(3):2851–2863. doi: 10.1002/jcb.26460. [DOI] [PubMed] [Google Scholar]
- 103.Mitrofan C.G., Appleby S.L., Nash G.B., et al. Bone morphogenetic protein 9 (BMP9) and BMP10 enhance tumor necrosis factor-α-induced monocyte recruitment to the vascular endothelium mainly via activin receptor-like kinase 2. J Biol Chem. 2017;292(33):13714–13726. doi: 10.1074/jbc.M117.778506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Appleby S.L., Mitrofan C.G., Crosby A., et al. Bone morphogenetic protein 9 enhances lipopolysaccharide-induced leukocyte recruitment to the vascular endothelium. J Immunol. 2016;197(8):3302–3314. doi: 10.4049/jimmunol.1601219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Varady P., Li J.Z., Cunningham M., et al. Morphologic analysis of BMP-9 gene therapy-induced osteogenesis. Hum Gene Ther. 2001;12(6):697–710. doi: 10.1089/104303401300057423. [DOI] [PubMed] [Google Scholar]
- 106.Luther G., Wagner E.R., Zhu G., et al. BMP-9 induced osteogenic differentiation of mesenchymal stem cells: molecular mechanism and therapeutic potential. Curr Gene Ther. 2011;11(3):229–240. doi: 10.2174/156652311795684777. [DOI] [PubMed] [Google Scholar]
- 107.Yeremenko N., Zwerina K., Rigter G., et al. Tumor necrosis factor and interleukin-6 differentially regulate Dkk-1 in the inflamed arthritic joint. Arthritis Rheumatol. 2015;67(8):2071–2075. doi: 10.1002/art.39183. [DOI] [PubMed] [Google Scholar]
- 108.Song B., Li X.F., Yao Y., et al. BMP9 inhibits the proliferation and migration of fibroblast-like synoviocytes in rheumatoid arthritis via the PI3K/AKT signaling pathway. Int Immunopharmacol. 2019;74 doi: 10.1016/j.intimp.2019.105685. [DOI] [PubMed] [Google Scholar]
- 109.Liu X., Du M., Wang Y., Liu S., Liu X. BMP9 overexpressing adipose-derived mesenchymal stem cells promote cartilage repair in osteoarthritis-affected knee joint via the Notch1/Jagged1 signaling pathway. Exp Ther Med. 2018;16(6):4623–4631. doi: 10.3892/etm.2018.6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang J.H., Liu Y.Z., Yin L.J., et al. BMP9 and COX-2 form an important regulatory loop in BMP9-induced osteogenic differentiation of mesenchymal stem cells. Bone. 2013;57(1):311–321. doi: 10.1016/j.bone.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 111.Wang H., Hu Y., He F., et al. All-trans retinoic acid and COX-2 cross-talk to regulate BMP9-induced osteogenic differentiation via Wnt/β-catenin in mesenchymal stem cells. Biomed Pharmacother. 2019;118 doi: 10.1016/j.biopha.2019.109279. [DOI] [PubMed] [Google Scholar]
- 112.Daigang L., Jining Q., Jinlai L., et al. LPS-stimulated inflammation inhibits BMP-9-induced osteoblastic differentiation through crosstalk between BMP/MAPK and Smad signaling. Exp Cell Res. 2016;341(1):54–60. doi: 10.1016/j.yexcr.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 113.Huang X., Wang F., Zhao C., et al. Dentinogenesis and tooth-alveolar bone complex defects in BMP9/GDF2 knockout mice. Stem Cells Dev. 2019;28(10):683–694. doi: 10.1089/scd.2018.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang P., Wang Y., Tang W., et al. Bone morphogenetic protein-9 enhances osteogenic differentiation of human periodontal ligament stem cells via the JNK pathway. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0169123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang H., Wang J., Deng F., et al. Canonical Wnt signaling acts synergistically on BMP9-induced osteo/odontoblastic differentiation of stem cells of dental apical papilla (SCAPs) Biomaterials. 2015;39:145–154. doi: 10.1016/j.biomaterials.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang F., Jiang Y., Huang X., et al. Pro-inflammatory cytokine TNF-α attenuates BMP9-induced osteo/odontoblastic differentiation of the stem cells of dental apical papilla (SCAPs) Cell Physiol Biochem. 2017;41(5):1725–1735. doi: 10.1159/000471865. [DOI] [PubMed] [Google Scholar]
- 117.Kusuyama J., Nakamura T., Ohnishi T., Eiraku N., Noguchi K., Matsuguchi T. Low-intensity pulsed ultrasound (LIPUS) promotes BMP9-induced osteogenesis and suppresses inflammatory responses in human periodontal ligament-derived stem cells. J Orthop Trauma. 2017;31(7) doi: 10.1097/01.bot.0000520897.92470.70. [DOI] [PubMed] [Google Scholar]
- 118.Kusuyama J., Nakamura T., Ohnishi T., et al. Low-intensity pulsed ultrasound promotes bone morphogenic protein 9-induced osteogenesis and suppresses inhibitory effects of inflammatory cytokines on cellular responses via Rho-associated kinase 1 in human periodontal ligament fibroblasts. J Cell Biochem. 2019;120(9):14657–14669. doi: 10.1002/jcb.28727. [DOI] [PubMed] [Google Scholar]
- 119.Li X., Chen D., Jing X., Li C. DKK1 and TNF-alpha influence osteogenic differentiation of adBMP9-infected-rDFCs. Oral Dis. 2020;26(2):360–369. doi: 10.1111/odi.13235. [DOI] [PubMed] [Google Scholar]
- 120.Óbice A.L.S., Correa M.G., Feng H.S., et al. The impact of implant abutment surface treatment with TiO(2) on peri-implant levels of angiogenesis and bone-related markers: a randomized clinical trial. Int J Oral Maxillofac Surg. 2019;48(7):962–970. doi: 10.1016/j.ijom.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 121.Gantwerker E.A., Hom D.B. Skin: histology and physiology of wound healing. Facial Plast Surg Clin North Am. 2011;19(3):441–453. doi: 10.1016/j.fsc.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 122.Chai P., Yu J., Wang X., Ge S., Jia R. BMP9 promotes cutaneous wound healing by activating Smad1/5 signaling pathways and cytoskeleton remodeling. Clin Transl Med. 2021;11(1) doi: 10.1002/ctm2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang L., Teng Y., Zhang Y., et al. Proteasome inhibitor bortezomib (PS-341) enhances RANKL-induced MDA-MB-231 breast cancer cell migration. Mol Med Rep. 2012;5(2):580–584. doi: 10.3892/mmr.2011.678. [DOI] [PubMed] [Google Scholar]
- 124.Wang Y.H., Dong Y.Y., Wang W.M., et al. Vascular endothelial cells facilitated HCC invasion and metastasis through the Akt and NF-κB pathways induced by paracrine cytokines. J Exp Clin Cancer Res. 2013;32(1) doi: 10.1186/1756-9966-32-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Seike T., Fujita K., Yamakawa Y., et al. Interaction between lung cancer cells and astrocytes via specific inflammatory cytokines in the microenvironment of brain metastasis. Clin Exp Metastasis. 2011;28(1):13–25. doi: 10.1007/s10585-010-9354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sierra A., Price J.E., García-Ramirez M., Méndez O., López L., Fabra A. Astrocyte-derived cytokines contribute to the metastatic brain specificity of breast cancer cells. Lab Invest. 1997;77(4):357–368. [PubMed] [Google Scholar]
- 127.Wan S., Liu Y., Weng Y., et al. BMP9 regulates cross-talk between breast cancer cells and bone marrow-derived mesenchymal stem cells. Cell Oncol. 2014;37(5):363–375. doi: 10.1007/s13402-014-0197-1. [DOI] [PubMed] [Google Scholar]
- 128.Wang J., Weng Y., Zhang M., et al. BMP9 inhibits the growth and migration of lung adenocarcinoma A549 cells in a bone marrow stromal cell-derived microenvironment through the MAPK/ERK and NF-κB pathways. Oncol Rep. 2016;36(1):410–418. doi: 10.3892/or.2016.4796. [DOI] [PubMed] [Google Scholar]
- 129.Guo K., Xu L., Jin L., et al. Bone morphogenetic protein 9, and its genetic variants contribute to susceptibility of idiopathic pulmonary arterial hypertension. Aging (Albany NY) 2020;12(3):2123–2131. doi: 10.18632/aging.102726. [DOI] [PMC free article] [PubMed] [Google Scholar]