Abstract
Glutamate plays an important role in continued use of and relapse to abused substances. However, its involvement in cannabis withdrawal is still unclear. We hypothesize that regional glutamate is associated with the cannabis withdrawal syndrome and recently examined possible association of glutamate with cannabis withdrawal, using magnetic resonance spectroscopy (MRS), in non-treatment-seeking cannabis users. We recruited 26 frequent cannabis users and 11 age-matched non-using controls. Of the 37, 20 users (8f/12m) and 10 controls (5f/5m) completed a verified 21-day abstinence protocol. Dorsal anterior cingulate cortex (dACC) glutamate and γ-amino butyric acid (GABA) were measured with proton MRS at baseline and on abstinent days 7 and 21 in conjunction with measures of cannabis withdrawal and craving (MCQ), sleep difficulties (PSQI) and mood state. We used ANOVA to examine group differences in glutamate and GABA from baseline through day 21 and used linear regression to evaluate correlations between intra-individual glutamate and withdrawal symptoms. We found that self-reported anxiety severity (HAMA) was correlated with urinary THC/Cr ratios at baseline (r = 0.768, p = 0.000076) and abstinent day 7 (r = 0.5636, p = 0.0097), dACC glutamate was significantly lower in the users compared with the controls from baseline through day 21 (F = 5.90, p = 0.022), changes in glutamate between baseline and abstinent day 21 had a significantly negative correlation with corresponding changes in craving (r = −0.72, p = 0.005) after adjusting for age, consumption of alcohol/cigarettes, sleep difficulties, and urinary THC levels. These findings provide preliminary evidence that dACC glutamate is associated with the cannabis withdrawal syndrome.
Subject terms: Human behaviour, Predictive markers
Introduction
Cannabis is the most commonly used recreation drug in the U.S. and recent surveys [1, 2, 3, 4] suggest that the prevalence of cannabis use disorder (CUD) is increasing as many states across the US have legalized recreational cannabis use. Long-term cannabis use, especially with early onset, is associated with poor performance in schoolwork, education, and cognitive functioning [5]. Abrupt cessation of cannabis use often triggers onset of withdrawal symptoms, such as sleep difficulties, irritability, and tension [6–9], which prevent users from remaining abstinent and negatively impact treatment outcome. Increased access and use of cannabis, as well as high risk of relapse of CUD [10], underscore the need to investigate possible intrinsic driving forces, such as neurochemical imbalance, behind cannabis withdrawal.
Animal research has demonstrated that excitatory amino-acid glutamate (Glu) plays an important role in substance dependence, especially in the continuation of and relapse to substance abuse [11–13]. It is known, mostly via animal models, that the glutamatergic system is sensitive to acute and repeated exposure to abused substances such as cocaine and alcohol and is believed to play a critical role in relapse during withdrawal from these substances [14–16]. For example, chronic cocaine use produces a significant reduction in the basal glutamatergic transmission from the prefrontal cortex, including anterior cingulate cortex (ACC), to the nucleus accumbens (Nacc) [17–19] and results in lower extracellular levels of Glu and presynaptic glutamate immunoreactivity [14]. The observed change is believed to be critically involved in relapse-like behavior, since pharmacological restoration of basal extracellular Glu concentrations to those observed in non-withdrawn animals, as with agents such as N-acetylcysteine (NAC), prevented reinstatement of drug seeking induced by a priming injection of cocaine [17]. The altered Glu concentration has been demonstrated in dorsal ACC (dACC) [20] and, recently, Nacc [21] of human cocaine users. NAC-induced dACC Glu change has also been demonstrated in human cocaine users [22].
Measuring Glu in the ACC may also provide important information to better understand possible neurochemical imbalance underlies cannabis withdrawal and frontal lobe alterations, including function, in a CUD population. Previous studies using magnetic resonance spectroscopy (MRS) suggest that baseline Glu was lower in ACC and basal ganglia regions of chronic cannabis users [23, 24], but not in striatal region [25, 27]. Acutely, cannabis actually appeared to increase striatal Glu [26]. We hypothesized that Glu is involved in cannabis withdrawal of chronic users and that MRS can detect the relevant changes in regional Glu and γ-amino butyric acid (GABA) concentrations. We recently reported the association of cannabis withdrawal with striatal glutamate and GABA [27]. Here we report results of Glu and GABA concentrations in dACC collected from the same study. The dACC was targeted because of its known association with integrating information from networks and because abnormal Glu concentrations were previously identified in this region among cocaine users [22]. Given the substantial heterogeneity among individuals in their response to an abrupt cessation of cannabis use [7, 8], we focused on how within-subject changes in withdrawal symptoms are associated with within-subject changes in GABA and Glu over the course of abstinence. In addition to group differences of Glu and GABA concentrations, our primary focus was to evaluate if Glu and GABA changes correlated with craving and cannabis withdrawal symptoms. Secondarily, we also examined if they were associated with other key components of cannabis withdrawal such as sleep difficulties and tension.
Methods and materials
Subjects
Twenty-six adult cannabis users (10 females, 16 males, age 21–40) and 11 age-matched, non-using controls (5 females, 6 males) were recruited through local advertisements under a Partners-Healthcare-Institutional-Review-Board approved protocol. Potential candidates were initially provided a description of the study via online advertisements and then interested individuals were invited to complete a brief phone screen, followed by an in-person screening visit and interview using DSM-5 to verify eligibility. Common exclusion criteria for both groups were (1) major medical, neurological, or psychiatric illness including major depression, psychotic disorder, or ADHD, head injuries including loss of consciousness; (2) substance use disorders other than CUD, including stimulants, or history of misuse of other substances other than cannabis within 1 year prior to this study, or cannabis use with medical license; (3) current use of psychoactive medications; (4) insufficient English fluency; (5) complications at birth; (6) current pregnancy; (7) MRI contraindications (e.g., metallic implants, claustrophobia, etc.); (8) prior to or during the study if they smoked more than 10 tobacco cigarettes/day, or if they engaged in binge drinking, characterized by regularly consuming ≥4 drinks for females and ≥5 drinks for males in a 2-h period. In addition, participants of the user group had to meet either DSM-5 criteria for CUD based on the SCID-5 or current usage requirement of cannabis use at least 5 days per week for at least 1 year; and were willing to abstain from using cannabis during the study and smoking nicotine cigarettes within 8 h of MR scan visits. The controls had to meet the additional criteria of no history or current use of abused substances, including cannabis, within 1 year prior to, or during, the study except mild consumption of alcohol and/or cigarettes; and were willing to abstain from smoking nicotine cigarettes within 8 h of MR scan visits.
All participants provided written informed consent and were asked to record their consumption of alcohol and cigarettes in a daily diary during the study to document average weekly consumption. Cannabis users were also required to refrain from cannabis use for at least 12 h prior to their baseline visit to avoid acute intoxication interference. Of the consented 37 participants, six users and one control discontinued after the baseline visit for reasons including relapse of cannabis use (4), dissatisfaction with compensation (1), lost to follow up (1), and unable to keep time commitment (1). The remaining 20 cannabis users (12 males, 8 females) and ten non-using controls (5 males, 5 females) completed a 21-day verified abstinence study protocol. The user completers had an average age of onset for regular cannabis use of (mean ± SD) 18 ± 3, average days of monthly use 28 ± 3, and average amount of 0.9 ± 0.7 gram per use with methods of smoke via bowl, joint, blunt, bong (19); vape only (1); smoke and vape (3) [27]. An abbreviated version of the urinary screen and demographics are included in the Supplementary (Table S1) as they have previously been described elsewhere [27].
Clinical measures
All participants were assessed at baseline and again on abstinent days 7 and 21 using the marijuana craving questionnaire (MCQ) [28] and the Cannabis Withdrawal Scale (CWS) [29]. The target of abstinent day 7 was selected because our prior study demonstrated that withdrawal symptoms peaked at day 7 of abstinence [6]. In addition to CWS, Hamilton rating scale for anxiety (HAMA) [30] and Hamilton rating scale for depression (HAMD) [31] reported previously [27], sleep difficulties were assessed using the Pittsburg Sleep Quality Index (PSQI) [32]. Tension was assessed using the Profile of Mood States (POMS)[33]. More specifically, tension (measured with POMS_tension) was identified as a potential predictor for relapse during cannabis abstinence [29]. In this study, we focused on its possible relation with GABA and Glu during early phase of abstinence. As there is substantial heterogeneity among individuals and their response to abrupt marijuana cessation, we focused on changes of these measures.
MRS measurement
MRS procedure was detailed in the Supplementary as it is, except voxel location, similar to the procedure for the striatum detailed previously [27]. MRS measurement on substance abusers may present a challenge to data collection due to excessive movement. To mitigate these potential problems and avoid missing data points, we adopted a short-TE scheme, to minimize the signal losses due to J-coupling and T2 relaxation and optimize spectral quality, followed by a MEGA-PRESS scan to ensure data quality within a relatively short data collection time. To ensure consistent voxel location over time, vendor-provided auto-align function on the scanner and anatomic landmark were applied in this study.
MRS data were processed offline and quantitatively analyzed with LC model spectral fittings using a simulated basis set, including Glu, glutamine (Gln), and other metabolites [34]. Voxel tissue analysis included segmenting the T1-weighted anatomical images into gray matter, white matter, and cerebral-spinal fluid (CSF) using FSL (FMRIB)™ (www.fmrib.ox.ac.uk). In house software generated a voxel mask in Matlab (Mathworks, Natick, MA, USA) based on the voxel coordinates, orientation, and dimensions to quantitatively derive the relative percentages of each tissue-type in the voxel and CSF volume fraction was corrected prior to deriving Glu and GABA concentrations.
Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences, version 28 (SPSS Inc., Chicago, IL, USA, www.spss.com). A two-way mixed ANOVA was applied to evaluate difference between the users and the controls across the three measurement occasions in Glu and GABA, with a Bonferroni correction applied for multiple comparisons, after examining for normality to ensure appropriateness of parametric statistics. For a group difference reaching statistical significance, Cohen’s d was calculated to evaluate the effect size. Analysis was repeated using analyses of covariance, controlling for age, sex, alcohol/cigarette consumption, and mean THC/Cr ratio (mTHC/Cr), calculated by averaging values of baseline, abstinent days 7 and 21, to assess the potential confounding impact of these variables on levels of Glu and GABA. Significance testing was conducted at α < 0.05 level (two tailed). Linear regression was applied to evaluate the possible relationship between Glu and GABA changes and corresponding changes in CWS, MCQ, PSQI, and POMS_tension. Bonferroni corrections for multiple comparisons were applied to control for possible Type I errors due to multiple regression comparisons. Specifically, an adjusted α = 0.05/4 = 0.0125 was used for both the primary hypothesis (MCQ, CWS) and for the secondary analysis (PSQI, POMS_tension). All relationships that remained significant after Bonferroni correction were then assessed potential confounding impact of age, sex, consumption of alcohol/cigarettes, and mTHC/Cr.
Results
Participant profile
Demographics of the participants as well as THC concentrations and alcohol consumption have been detailed previously [27]. In brief, 18 of the 20 users were diagnosed as CUD using SCID-5. The remaining two were qualified by their cannabis use amount and frequency. Between the users and the controls, there were no significant differences in sex (p(χ2) = 0.80), age (Mann–Whitney, p = 0.71), and use of cigarettes (Mann–Whitney, p = 0.08). There was a significant difference in alcohol use (Mann–Whitney, p = 0.001), Years of Education (Mann–Whitney, p = 0.007), CWS (Mann–Whitney, p = 0.005), MCQ (Mann–Whitney, p < 0.0005), HAMA (Mann–Whitney, p = 0.033), and HAMD (Mann–Whitney, p = 0.005) at baseline between the cannabis users and controls (Table S1, Supplementary).
Abstinence time course and verification
During acute abstinence, there was an initial fast decrease (days 0 = >7; 100% = >10.9%) and followed by a slower one (days 8 = >21; 10.9% = >4.9%) in the urinary THC/Cr ratios among the cannabis users. No significant changes were found in consumption of alcohol (F = 0.79, p = 0.47) and cigarettes (F = 0.41, p = 0.67) at days 7 and 21, compared to the baseline levels, among the cannabis users. At baseline and abstinent day 7, but not on abstinent day 21, THC/Cr ratio had a positive correlation with cannabis users’ HAMA scores (Baseline r = 0.768, p = 0.000076; day 7 r = 0.5636, p = 0.0097; day 21 r = −0.028, p NS) and HAMD scores (Baseline r = 0.451, p = 0.046; day 7 r = 0.4094, p = 0.073; day 21 r = 0.216, p NS). After Bonferroni correction, only the relationship with HAMA at baseline and day 7 remained significant. Further analysis revealed that the HAMA and THC/Cr relationship at baseline and day 7 was not impacted by age, sex, or consumption of alcohol/cigarettes.
Withdrawal symptoms during abstinence
Sleep difficulties (PSQI) and tension (POMS_tension) of the cannabis users, illustrated in Fig. 1a, started at a higher level and rose by ~36% and 32%, respectively, on day 7 compared to their baselines before returning toward the baselines on day 21 while those of the respective measures of the controls remained relatively flat at a significantly lower level. These findings are consistent with previously reported characteristics of cannabis withdrawals [35]. Neither PSQI (F = 2.0, p = 0.14) nor POMS_tension (F = 2.9, p = 0.07) scores, including the rest of the POMS subscales (e.g., depression, anger, vigor, fatigue, and confusion (data not shown here)), had significant changes from baseline through day 21 among the cannabis users even though significant group differences existed between the users and non-using controls (PSQI, F = 10.6, p = 0.003; POMS_tension, F = 10.1, p = 0.004). PSQI did not correlate with urinary THC/Cr ratios during abstinence except for the baseline (r = 0.535, p = 0.015). POMS_tension did not correlate with THC/Cr ratios from baseline through day 21.
Fig. 1. Change in withdrawal symptoms over time and representative MRS spectra.
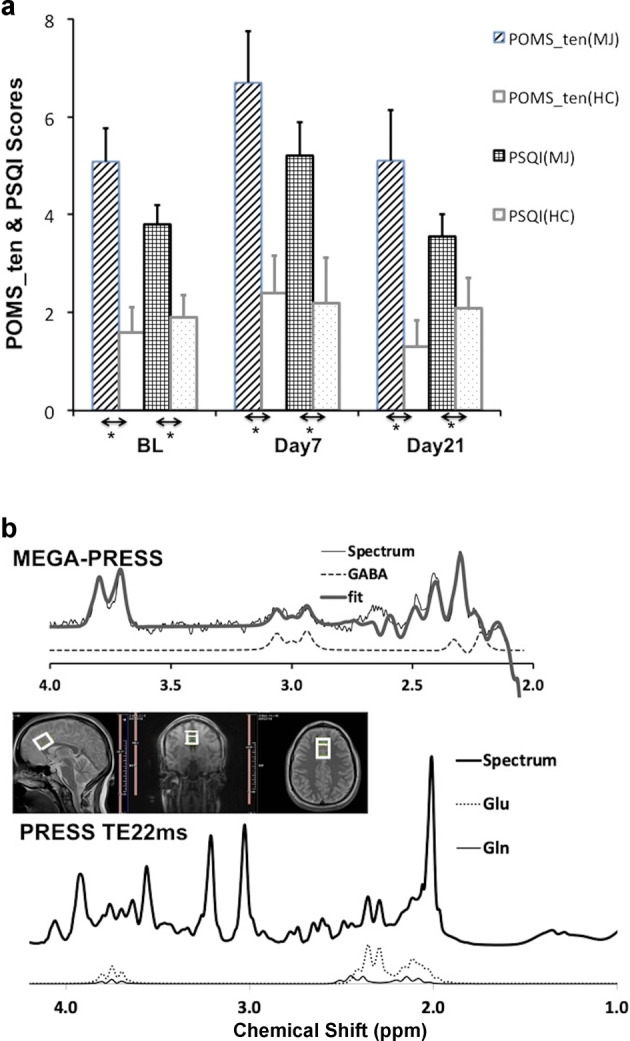
Group averages of POMS_tension and PSQI scores in cannabis users (MJ, dashed and grid respectively) and non-using controls (HC, open & dotted) on baseline (BL), days 7 and 21 (a). *represents p < 0.05. Representative spectra of mega-press (top) and short TE (22 ms) press (bottom) collected from a 18.75-cm3 voxel (middle) placed in dACC region and corresponding fits, including Glu and Gln, with LCM (b).
Self-reported craving (MCQ) was significantly elevated at baseline (Table S1, Supplementary) and dropped by ~15% by abstinent day 7. It stayed at this level through day 21 while that of the controls remained flat at a significantly lower level from baseline through day 21 (group F = 21.7, p < 0.0005). MCQ did not correlate with THC/Cr ratios from baseline through day 21.
dACC Glu and GABA
High quality spectra of short TE PRESS were collected from most of the participants (S/N (mean ± sem): 46.4 ± 0.9 (users, n = 20), 44.8 ± 0.5 (non-using controls, n = 10)) (Figs. 1b and S1 in the Supplementary). Full-width-half-maximum of NAA resonance was 4.2 ± 0.2 Hz among the users and 3.9 ± 0.3 Hz among the non-using controls. Cramer–Rao lower bounds (CRLBs) of Glu was 2.85 ± 0.08% among the users (n = 20) and 3.0 ± 0.0% among the non-using controls (n = 10). Glx (=Glu + Gln(glutamine)) and Glu were highly correlated (r = 0.941 at baseline, 0.967 on day 7, and 0.914 on day 21) and shared similar correlation significance with withdrawal detailed below. Quality of the MEGA-PRESS spectra was high as well: S/N 44.3 ± 1.2 (users, n = 20), 43.9 ± 1.4 (controls, n = 10), CRLB of GABA was 3.8 ± 0.1 for the users and 4.2 ± 0.1 for the controls. Consistency of voxel location from baseline through day 21 is illustrated in Table S2 in the Supplementary.
Group average of dACC Glu among the users was lower, compared to the controls at the baseline, and remained low at days 7 and 21; that of GABA among the users was mildly lower relative to the controls’ from baseline through day 21, with a slight reduction near day 7 (Table 1). Cohen’s d for the dACC Glu group difference was 0.90 (baseline), 0.96 (day 7), and 0.98 (day 21), respectively. ANOVA analysis indicated that the users’ Glu was significantly lower compared to that of the controls’ (F = 6.86, p = 0.014). Even using age, sex, consumption of alcohol/cigarettes, and mTHC/Cr as covariates, the lower Glu in the cannabis users remained significant (F = 5.90, p = 0.022). However, there was no significant difference in dACC GABA between the users and the controls (F = 2.93, p = 0.098) from baseline through day 21.
Table 1.
ANOVA analysis of dACC glutamate and GABA measures (mean ± sem) from baseline through day 21 and after controlling for consumptions of alcohol and cigarettes (ANCOVA), where BL = baseline, D7 = abstinent day 7th, D21 = abstinent day 21st, MJ = cannabis users, HC = non-use controls.
| Time | BL | D7 | D21 | ANCOVA |
|---|---|---|---|---|
| Glu(MJ) | 7.90 ± 0.13 | 7.87 ± 0.18 | 7.98 ± 0.13 | |
| Glu(HC) | 8.47 ± 0.16 | 8.36 ± 0.11 | 8.27 ± 0.12 | |
| Time | F = 0.22, p = 0.80 | F = 0.23, p = 0.80 | ||
| Time*group | F = 0.76, p = 0.47 | F = 0.78, p = 0.47 | ||
| Groups | F = 6.86, p = 0.014 | F = 5.90, p = 0.022 | ||
| GABA(MJ) | 1.63 ± 0.05 | 1.55 ± 0.06 | 1.64 ± 0.06 | |
| GABA(HC) | 1.67 ± 0.06 | 1.72 ± 0.09 | 1.70 ± 0.06 | |
| Time | F = 0.13, p = 0.88 | F = 0.13, p = 0.88 | ||
| Time*group | F = 0.87, p = 0.42 | F = 0.85, p = 0.43 | ||
| Groups | F = 2.93, p = 0.098 | F = 2.97, p = 0.096 |
Statistically significant p<0.05 values are in bold.
Primary outcome
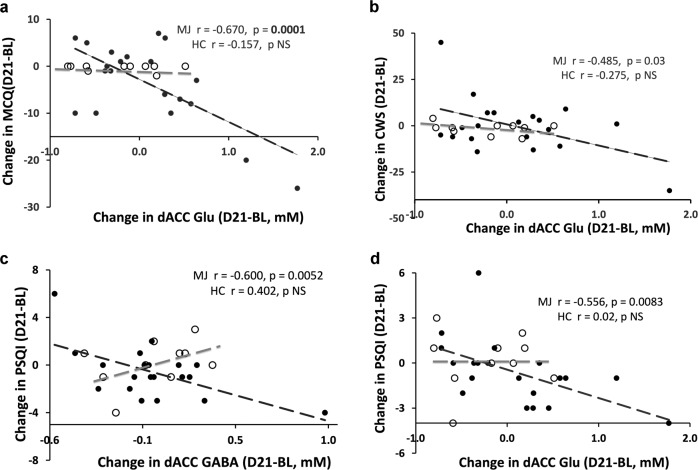
Changes in Glu, baseline to day 21, but not to day 7, negatively correlated with those of MCQ (r = −0.670, p = 0.0001) and CWS (r = −0.485, p = 0.03) (Fig. 2a, b); there was no correlation between changes in Glu and either MCQ or CWS in the controls, and between change in user GABA and MCQ or CWS, from baseline through day 21. After Bonferroni correction, the relationship between Glu and CWS became insignificant (p = 0.03 > 0.013). The MCQ and Glu relationship was confounded by age, consumption of alcohol/cigarettes, sleep difficulties, and mTHC/Cr. Adjusting for these confounders resulted in 13.9%, 14.8%, 11.1%, and 10.7% change, respectively, in the slope. That is increase in age and/or consumption of alcohol/cigarettes, decrease in sleep difficulties, or increase in mTHC/Cr leading to less craving. The adjustment for partial correlation was r = −0.72 (p = 0.005).
Fig. 2. Change in dACC Glu versus changes in withdrawal symptoms.
Change in dACC Glu (D21-BL) versus changes of MCQ (D21-BL) in cannabis users (MJ, solid circles, n=20) and non-using controls (HC, open circles, n=10). Span of the glutamate changes was approximately 2.5 mM among the users and about 1.3 mM for the non-using controls. After adjusting for confounding, the partial correlation was r = −0.72 (p = 0.005). (a) Change in dACC Glu (D21-BL) versus changes of CWS (D21-BL) in cannabis users (MJ, solid circles, n = 20) and non-using controls (HC, open circles, n = 10). Spreads of the glutamate changes was about 2:1 between the MJ and the HC. No bold p value for the MJ here indicates an insignificance after Bonferroni correction. (b) Change in dACC GABA (D21-BL) versus changes of PSQI (D21-BL) in cannabis users (MJ, solid circles, n = 20) and non-using controls (HC, open circles, n = 10). Note the spread of GABA changes was about 1.5 mM for the cannabis users and about 0.7 mM in the controls. After adjusting for confounding, the partial correlation was r = −0.393 (p = 0.101). (c) Change in dACC Glu (D21-BL) versus changes of PSQI (D21-BL) in cannabis users (MJ, solid circles, n = 20) and non-using controls (HC, open circles, n = 10). After adjusting for confounding, the partial correlation was r = −0.332 (p = 0.148) (d).
Secondary analysis
Changes in Glu of the users were also negatively correlated with POMS_tension (p = 0.0195) and PSQI (p = 0.0083) scores, but not among the controls (Fig. 2c, d). Change in GABA of the users was also negatively correlated with PSQI (p = 0.0052) scores. Only the relationships with PSQI remain significant (p < 0.0125) after Bonferroni correction. The PSQI and Glu relationship was confounded by age, consumption of alcohol/cigarettes, and sex. Adjusting for these confounders resulted in 63%, 41%, and 45% changes, respectively, in the slope. That is increase in age and/or consumption of alcohol/cigarettes or male users leading to more sleep difficulties. The adjustment for partial correlation was r = −0.332 (p = 0.148). The PSQI and GABA relationship was also confounded by age, consumption of alcohol/cigarettes, and sex. Adjusting for these confounders resulted in 15%, 13%, and 16% changes, respectively, in the slope. That is increase in age and/or consumption of alcohol/cigarettes or male users leading to more sleep difficulties. The adjustment for partial correlation was r = −0.393 (p = 0.101).
Discussion
The present study examined dACC Glu and GABA in conjunction with self-reported craving and withdrawal symptoms during a verified 21-day abstinence period in a group of non-treatment seeking cannabis users compared to age-matched non-using controls. Our data demonstrated cannabis withdrawal impacted dACC Glu and GABA in the users with the following features: (1) dACC Glu concentration was significantly lower at baseline and abstinent days 7 and 21 even after adjusting for covariates while dACC GABA concentration only trended lower relative to the controls from baseline through day 21 (Table 1); (2) a significantly negative correlation between the changes in dACC Glu and self-reported craving (MCQ) after adjusting for confounds while negative trends between changes in sleep difficulties (PSQI) and dACC Glu as well as GABA after adjusting for confounds; (3) anxiety severity (measured with HAMA) was significantly correlated with urinary THC/Cr ratio at baseline and abstinent day 7.
It is unknown how dACC Glu is affected during cannabis abstinence. Here we present evidence that dACC Glu remained significantly lower, compared to non-using controls, during 21-consecutive days of abstinence. Our data show dACC neurochemical state of the users was still abnormal on day 21 of abstinence. Three weeks may not be a long period for an abstinent process. But for cannabis withdrawal, the first week of abstinence is the most stressful one for many users [6, 7, 35, 36] and the neurochemical abnormality during the first 3 weeks may have significance for developing novel treatment or abstinent strategies. The lower dACC Glu during cannabis abstinence is consistent with previous observation on cannabis users at baseline [23] and cocaine users during protracted withdrawal [20, 22], but is inconsistent with that observed during alcohol withdrawal [37] due to a difference in the glutamate modulating mechanism (i.e., alcohol directly potentiates GABA signaling versus THC modulates CB1 and CB2 receptors). The lower Glu is also consistent with a report of NAC-induced reduction of craving in human cannabis users [38] even though it is currently unclear what mechanism is responsible for the lower Glu.
The negative correlation between the changes in dACC Glu and craving (Fig. 2a) suggests that lowering Glu leads to increasing craving in cannabis user during early phase abstinence. While other mechanisms could also contribute to the negative relationship, we speculate that brain ACC’s need for more GABA to counter craving and function normally during abstinence is the driving force for this negative relationship. Given Glu’s role as a precursor of GABA in vivo and the driving force, the lower dACC Glu concentration led to a positive correlation between dACC Glu and GABA (Baseline p = 0.0095, day 7 p = 0.0011, day 21 p = 0.0116) and, hence, resulted in the negative relationship with craving (i.e., lowering Glu, lowering GABA, and increasing craving). This negative correlation is also consistent with the finding of preclinical investigation mentioned in the introduction: i.e., dACC Glu increase leads to decrease in craving and cannabis-seeking behavior. This also suggests that the glutamatergic system is a potential target for pharmaceutical intervention to cannabis dependence [38].
We recently reported a mild increase in striatal glutamate of chronic cannabis users during the early phase of abstinence [27]. We now report lower dACC glutamate in the same users during the same verified abstinence period. The diverse, but parallel responses of dACC and striatal glutamate during cannabis abstinence may reflect underlying neurochemical changes associated with impaired function of response inhibition and salience attribution. In addition, elevated striatal glutamate and decreased dACC glutamate are both consistent with an up-regulation of ventral striatal presynatic dopamine levels [39], a self-compensatory gesture of the (CNS) system in the abstinent users toward their significantly higher, self-reported withdrawal symptoms. However, persistently high withdrawal symptoms on day 21, compared to the control group, appeared consistent with a previous finding of low striatal dopamine release in currently chronic cannabis users who are recently abstinent [40]. Future studies are needed to confirm this relationship. Another feature worthy of mentioning is that in the striatum, GABA changes were correlated with severity of withdrawal symptoms (CWS) [27], but in dACC, change in glutamate was correlated with that of craving (MCQ) from the same group of cannabis users going through the same abstinent protocol. dACC is a brain region that subserves cognition and motor control while the striatum contains neuronal activity related to movements, rewards and the conjunction of both movement and reward. We speculate that the unique association of glutamate and GABA with MCQ and CWS is most likely related to the role of the neurotransmitters in networks/brain regions associated with different functions. The combination of the dACC association with those of striatum suggests a more complex role of glutamate and GABA in brain during cannabis withdrawal.
Anxiety and depressive symptoms are extremely common during abstinence. Anxiety severity, measured by HAMA scores, of the cannabis users exhibited a positive correlation with urinary THC/Cr ratios at baseline and day 7, which was consistent with a previous observation using serum metabolites [41]. This relationship was reduced by day 21 when urinary THC metabolites were eliminated from the majority of the abstinent users. A lack of significant relationship between depressive symptoms (HAMD) and urinary THC/Cr ratio during the same time-period suggests that anxiety plays a larger role in the cannabis withdrawal syndrome. Similarly, craving, sleep difficulties, and tension are also prominent elements of cannabis withdrawal syndrome [29]. These three metrics were not correlated with urinary THC/Cr ratio but persist long into abstinence. Their relationships with GABA and Glu were confounded with age, sex, and/or consumption of alcohol/cigarettes, which demonstrated, once again, that these confounders were also important factors to consider in an abstinent study and treatment strategy. These symptoms, including anxiety and depression, were positively correlated with one another from baseline through day 21, i.e., increasing sleep difficulties led to more craving, increasing anxiety and depressive symptoms led to more sleep difficulties and craving. They were associated with distress and impairment of daily activities as well as relapse [29]. Treatments that focus on craving by medication intervention in conjunction with behavior therapy could be more successful to achieve abstinence.
The present study has several caveats that must be acknowledged. First, the sample size of completers is relatively small (N = 20 cannabis users, N = 10 controls). In this initial study, we elected to focus on cannabis users due to limited resource. Second, our study did not include follow up to verify whether the substantial changes in dACC glutamate persisted beyond the abstinence period. Future studies could reveal if recovery of dACC glutamate coincides or precedes diminishing withdrawal symptoms such as craving and sleep difficulties. Third, measuring glutamate at 3T is challenging due to its signal overlap with Gln [42–45]. Short TE PRESS in conjunction with good shimming in the present study provided excellent spectral quality and the separated quantifications of Glu and Gln within reasonable fitting errors (CRLBs ≤3% for Glu and ≤13.1% for Gln). Future studies using more sophisticated techniques such as TE-averaging or 2D-J resolved schemes [46–48] for improving separation of Glu and Gln will be required to further characterize the role of glutamate system during cannabis withdrawal.
In summary, dACC glutamate levels were lower in the cannabis users compared to healthy controls during the early phase of abstinence. The changes in dACC glutamate and craving (MCQ) were significantly and negatively correlated, even after adjusting for the confounding impact of age, sex, and consumption of alcohol/cigarettes during early phase of abstinence. In addition, anxiety severity of the users was positively correlated with urinary THC/Cr ratio at baseline and abstinent day 7. These findings provide preliminary evidence that dACC glutamate is associated with some elements of the cannabis withdrawal syndrome.
Supplementary information
Acknowledgements
The MRS sequences and the fastmap package were developed by Edward J. Auerbach and Małgorzata Marjańska and provided by the University of Minnesota under a C2P agreement. We thank assistance of Dr. B. Frederick on implementing the MRS components of the study, Dr. G. Fitzmaurice for statistical advice, Ms M. Shevenell, J. Tiber, and Ms W. Tartarini provided during data collection.
Author contributions
Study concept and design: SEL and CSZ. CSZ oversaw the study execution, collection and analysis of the MR data, drafted and revised the manuscript. KAD screened and recruited all study participants, collected and documented all self-administered withdrawal ratings, urinary THC data, and daily dairies of alcohol and cigarette use as well as followed up with most of the study participants during the study. She participated in proof of the manuscripts. SEL provided guidance to the study, closely participated in drafting, revising, and proofing the manuscript.
Funding
This study was supported, in part, by the National Institute on Drug Abuse (grant No. DA041574 [to CSZ]). The funding agency had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-022-01321-5.
References
- 1.Compton WM, Han B, Jones CM, Blanco C, Hughes A. Marijuana use and use disorders in adults in the USA, 2002–14: analysis of annual cross-sectional surveys. Lancet Psychiatry. 2016;3:954–64. doi: 10.1016/S2215-0366(16)30208-5. [DOI] [PubMed] [Google Scholar]
- 2.Hasin DS, Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang H, et al. Prevalence of marijuana use disorders in the United States between 2001-2 and 2012-3. JAMA Psychiatry. 2015;72:1235–42. doi: 10.1001/jamapsychiatry.2015.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.SAMHSA, Mental Health Services Administration [SAMHSA], 2019. Results from the 2018 National Survey on Drug Use and Health. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2019.
- 4.Schulenberg J. Monitoring the future national survey results on drug use, 1975-2018: Volume II, college students and adults ages 19-60. 2019.
- 5.Pope HG., Jr Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 2003;69:303–10. doi: 10.1016/S0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- 6.Kouri EM, Pope HG, Jr, Lukas SE. Changes in aggressive behavior during withdrawal from long-term marijuana use. Psychopharmacology. 1999;143:302–8. doi: 10.1007/s002130050951. [DOI] [PubMed] [Google Scholar]
- 7.Allsop DJ, Copeland J, Norberg MM, Fu S, Molnar A, Lewis J, et al. Quantifying the clinical significance of cannabis withdrawal. PLoS ONE. 2012;7:e44864. doi: 10.1371/journal.pone.0044864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Budney AJ. Review of the validity and significance of cannabis withdrawal syndrome. Am J Psychiatry. 2004;161:1967–77. doi: 10.1176/appi.ajp.161.11.1967. [DOI] [PubMed] [Google Scholar]
- 9.Haney M. The marijuana withdrawal syndrome: diagnosis and treatment. Curr Psychiatry Rep. 2005;7:360–6. doi: 10.1007/s11920-005-0036-1. [DOI] [PubMed] [Google Scholar]
- 10.Kadden RM, Litt MD, Kabela-Cormier E, Petry NM. Abstinence rates following behavioral treatments for marijuana dependence. Addictive Behav. 2007;32:1220–36. doi: 10.1016/j.addbeh.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalivas P, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–50. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–72. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 13.Lüscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–63. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochemical Pharmacol. 2008;75:218–65. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leviel V, Gobert A, Guibert B. The glutamate-mediated release of dopamine in the rat striatum: further characterization of the dual excitatory-inhibitory function. Neuroscience. 1990;39:305–12. doi: 10.1016/0306-4522(90)90269-A. [DOI] [PubMed] [Google Scholar]
- 16.O'Neill J, Tobias MC, Hudkins M, London ED. Glutamatergic neurometabolites during early abstinence from chronic methamphetamine abuse. Int J Neuropsychopharmacol. 2015;18:pyu059. doi: 10.1093/ijnp/pyu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–9. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 18.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 19.Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–23. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- 20.Yang S, Salmeron BJ, Ross TJ, Xi ZX, Stein EA, Yang Y. Lower glutamate levels in rostral anterior cingulate of chronic cocaine users—a 1H-MRS study using TE-averaged PRESS at 3 T with an optimized quantification strategy. Psychiatry Res Neuroimaging. 2009;174:171–6. doi: 10.1016/j.pscychresns.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engeli EJ. Impaired glutamate homeostasis in the nucleus accumbens in human cocaine addiction. Mol Psychiatry. 2021;26:5277–85. doi: 10.1038/s41380-020-0828-z. [DOI] [PubMed] [Google Scholar]
- 22.Schmaal L, Veltman DJ, Nederveen A, van den Brink W, Goudriaan AE. N-acetylcysteine normalizes glutamate levels in cocaine-dependent patients: a randomized crossover magnetic resonance spectroscopy study. Neuropsychopharmacology. 2012;37:2143–52. doi: 10.1038/npp.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prescot AP, Locatelli AE, Renshaw PF, Yurgelun-Todd DA. Neurochemical alterations in adolescent chronic marijuana smokers: a proton MRS study. Neuroimage. 2011;57:69–75. doi: 10.1016/j.neuroimage.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang L, Cloak C, Yakupov R, Ernst T. Combined and independent effects of chronic marijuana use and HIV on brain metabolites. J NeuroImmune Pharmacol. 2006;1:65–76. doi: 10.1007/s11481-005-9005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muetzel RL, Marjanska M, Collins PF, Becker MP, Valabregue R, Auerbach EJ, et al. In vivo 1 H magnetic resonance spectroscopy in young-adult daily marijuana users. NeuroImage Clin. 2013;2:581–9. doi: 10.1016/j.nicl.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mason NL, Theunissen EL, Hutten NRPW, Tse DHY, Toennes SW, Stiers P, et al. Cannabis induced increase in striatal glutamate associated with loss of functional corticostriatal connectivity. Eur Neuropsychopharmacol. 2019;29:247–56. doi: 10.1016/j.euroneuro.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Zuo CS, Davis KA, Kuppe MK, Dahlgren MK, Gruber S, Fitzmaurice GM, et al. Elevated striatal glutamate + Glutamine in recreational cannabis users during abstinence. J Psychiatr Res. 2022;146:192–200. doi: 10.1016/j.jpsychires.2021.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heishman SJ, Evans RJ, Singleton EG, Levin KH, Copersino ML, Gorelick DA. Reliability and validity of a short form of the Marijuana Craving Questionnaire. Drug Alcohol Depend. 2009;102:35–40. doi: 10.1016/j.drugalcdep.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allsop DJ, Norberg MM, Copeland J, Fu S, Budney AJ. The Cannabis Withdrawal Scale development: patterns and predictors of cannabis withdrawal and distress. Drug Alcohol Depend. 2011;119:123–9. doi: 10.1016/j.drugalcdep.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 31.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bysse D, Reynolds C, III, Monk T. The Pittsburgh Sleep Quality Index (PSQI): a new instrument for psychiatric research and practice. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 33.Albrecht RR, Ewing SJ. Standardizing the administration of the Profile of Mood States (POMS): development of alternative word lists. J Personal Assess. 1989;53:31–39. doi: 10.1207/s15327752jpa5301_4. [DOI] [PubMed] [Google Scholar]
- 34.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–9. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 35.Kouri EM, Pope HG., Jr Abstinence symptoms during withdrawal from chronic marijuana use. Exp Clin Psychopharmacol. 2000;8:483–92. doi: 10.1037/1064-1297.8.4.483. [DOI] [PubMed] [Google Scholar]
- 36.Budney AJ, Moore BA, Vandrey RG, Hughes JR. The time course and significance of cannabis withdrawal. J Abnorm Psychol. 2003;112:393–402. doi: 10.1037/0021-843X.112.3.393. [DOI] [PubMed] [Google Scholar]
- 37.Mon A, Durazzo TC, Meyerhoff DJ. Glutamate, GABA, and other cortical metabolite concentrations during early abstinence from alcohol and their associations with neurocognitive changes. Drug Alcohol Depend. 2012;125:27–36. doi: 10.1016/j.drugalcdep.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roten AT, Baker NL, Gray KM. Marijuana craving trajectories in an adolescent marijuana cessation pharmacotherapy trial. Addictive Behav. 2013;38:1788–91. doi: 10.1016/j.addbeh.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gleich T, Deserno L, Lorenz RC, Boehme R, Pankow A, Buchert R, et al. Prefrontal and striatal glutamate differently relate to striatal dopamine: potential regulatory mechanisms of striatal presynaptic dopamine function? J Neurosci. 2015;35:9615–21. doi: 10.1523/JNEUROSCI.0329-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Albrecht DS, Skosnik PD, Vollmer JM, Brumbaugh MS, Perry KM, Mock BH, et al. Striatal D2/D3 receptor availability is inversely correlated with cannabis consumption in chronic marijuana users. Drug Alcohol Depend. 2013;128:52–7. doi: 10.1016/j.drugalcdep.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonnet U, Borda T, Scherbaum N, Specka M. Abstinence phenomena of chronic cannabis-addicts prospectively monitored during controlled inpatient detoxification (part II): psychiatric complaints and their relation to delta-9-tetrahydrocannabinol and its metabolites in serum. Drug Alcohol Depend. 2015;155:302–6. doi: 10.1016/j.drugalcdep.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 42.Choi C, Coupland NJ, Bhardwaj PP, Malykhin N, Gheorghiu D, Allen PS. Measurement of brain glutamate and glutamine by spectrally‐selective refocusing at 3 Tesla. Magn Reson Med. 2006;55:997–1005. doi: 10.1002/mrm.20875. [DOI] [PubMed] [Google Scholar]
- 43.Hancu I. Optimized glutamate detection at 3T. J Magn Reson Imaging. 2009;30:1155–62. doi: 10.1002/jmri.21936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mullins PG. Comparative reliability of proton spectroscopy techniques designed to improve detection of J‐coupled metabolites. Magn Reson Med. 2008;60:964–9. doi: 10.1002/mrm.21696. [DOI] [PubMed] [Google Scholar]
- 45.Schubert F. Glutamate concentrations in human brain using single voxel proton magnetic resonance spectroscopy at 3 Tesla. Neuroimage. 2004;21:1762–71. doi: 10.1016/j.neuroimage.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 46.Adalsteinsson E. In vivo 2D J-resolved magnetic resonance spectroscopy of rat brain with a 3-T clinical human scanner. Neuroimage. 2004;22:381–6. doi: 10.1016/j.neuroimage.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 47.Hurd R. Measurement of brain glutamate using TE‐averaged PRESS at 3T. Magn Reson Med. 2004;51:435–40. doi: 10.1002/mrm.20007. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y, Shen J. Simultaneous quantification of glutamate and glutamine by J‐modulated spectroscopy at 3 Tesla. Magn Reson Med. 2016;76:725–32. doi: 10.1002/mrm.25922. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.