Abstract
The nucleus accumbens shell (NAcSh) and its afferent and efferent neuronal projections control key aspects of motivation for cocaine. A recently described regulator of γ-aminobutyric acid (GABA) projections from the dorsal raphe nucleus (DRN) to the NAcSh (DRN → NAcSh) is the neuropeptide neuromedin U (NMU). Here, we find that systemic administration of NMU decreases breakpoint for cocaine on a progressive ratio schedule of reinforcement in male rats. Employing a retrograde adeno-associated virus (AAV), we found that RNAi-mediated knockdown of the NMU receptor 2 (NMUR2) in afferent DRN projections to the NAcSh increases the breakpoint for cocaine. Our previous studies demonstrated that NMU regulates GABA release in the NAcSh, and our current investigation found that systemic NMU administration suppresses cocaine-evoked GABA release in the NAcSh and increases phosphorylated c-Fos expression in neurons projecting from the NAcSh to the ventral pallidum (VP). To further probe the impact of NMU/NMUR2 on neuroanatomical pathways regulating motivation for cocaine, we employed multi-viral transsynaptic studies. Using a combination of rabies virus and retrograde AAV helper virus, we mapped the impact of NMU across three distinct brain regions simultaneously and found a direct connection of GABAergic DRN neurons to the NAcSh → VP pathway. Together, these data reveal that NMU/NMUR2 modulates a direct connection within the GABAergic DRN → NAcSh → VP circuit that diminishes breakpoints for cocaine. These findings importantly advance our understanding of the neurochemical underpinnings of pathway-specific regulation of neurocircuitry that may regulate cocaine self-administration, providing a unique therapeutic perspective.
Subject terms: Motivation, Brain
Introduction
The nucleus accumbens (NAc) and its associated “direct” and “indirect” pathways are key neuroanatomical pathways engaged in mediating motivational aspects of cocaine self-administration [1]. The NAc neurons of the direct pathway ultimately disinhibit the thalamus and promote motivated behavior via projections to the ventral mesencephalon, including the ventral tegmental area (VTA), whereas the indirect pathway inhibits motivated behavior via the ventral pallidum (VP) [1–3]. The reinforcing efficacy of cocaine can be measured using a progressive ratio (PR) schedule. In the PR schedule, the first response of the session results in cocaine delivery with response requirements increasing exponentially; the “breakpoint” is the last ratio at which the animal responds [4, 5]. Chemogenetic inhibition of neurons in the NAc increases breakpoints for cocaine measured in a PR assay [6], and optogenetic activation of neurons in the NAc decreases cocaine-taking behavior [6]. While the traditional definitions of direct and indirect pathways are under continuous revision with regard to precise dopamine receptor expression [7–9], the neuroanatomical brain region targets of these pathways remain consistent [10]. The afferent projections in the NAc have been mapped to numerous brain regions, including the dorsal raphe nucleus (DRN) [11], but the downstream targets of these neurons remain unclear. Furthermore, outside of the context of dopamine receptor expression patterns, NAc projections to the VP (NAc → VP) have been implicated in regulating cocaine conditioned place preference [12], a measure of drug-associated memory. Here, we combine viral, pharmacological, and transsynaptic tracing strategies to explore three brain regions that form a circuit (DRN → NAc → VP) and regulate breakpoints for cocaine.
A promising target to regulate cocaine self-administration is the neuropeptide neuromedin U (NMU) and its inhibitory G protein-coupled receptor, NMU receptor 2 (NMUR2) [13, 14]. NMUR2 is expressed on presynaptic GABAergic neurons in the NAc shell (NAcSh), including those that project from the DRN → NAcSh [15]. Direct infusion of NMU into the NAcSh decreases local extracellular GABA and blocks expression of cocaine sensitization [15], a behavior regulated by neurocircuitry which overlaps with cocaine-taking behavior [16]. Conversely, cocaine sensitization is increased upon knockdown of NMUR2 in afferent projections to the NAcSh [15]. Furthermore, systemic NMU decreases alcohol self-administration and blunts alcohol-evoked dopamine release in the NAc [17], indicating the NMU-NMUR2 axis is a significant mechanistic component regulating NAc functionality and drug-taking behavior across drug classes. Still, the identity of the efferent projections from the NAcSh that meditate these behaviors are unknown.
We propose that NMUR2 acts as an inhibitory regulator of GABAergic DRN → NAcSh neurons that synapse directly onto and disinhibit NAcSh → VP neurons (DRN → NAcSh → VP), thereby decreasing the effort rats expend to achieve a discrete cocaine reinforcement measured on a progressive ratio schedule (“breakpoint”). We tested this hypothesis in male Sprague Dawley rats to establish pharmacological control of this circuitry that ultimately regulates cocaine self-administration. We then used transsynaptic tracing to characterize the DRN neurons that directly synapse onto NAcSh → VP and NAcSh → VTA neurons. This study identified NMU as a preferential regulator of a GABAergic DRN → NAcSh → VP circuit that regulates breakpoints for cocaine.
Materials and methods
Animals
Male Sprague Dawley outbred rats (n = 130 total; male) (Envigo, Houston, TX, United States), weighing 200–225 g at arrival were single housed as described previously [15, 18]. All experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals (2011) and with the University of Texas Medical Branch Institutional Animal Care and Use Committee.
Drugs
Cocaine HCl (National Institute on Drug Abuse, Research Triangle Park, NC) was dissolved in sterile 0.9% NaCl to prepare a 0.75 mg/kg/inf solution for self-administration or a 10 mg/kg intraperitoneal (IP) injection. NMU (porcine 8-mer sequence; GenScript, Piscataway, NJ) was prepared in sterile 0.9% NaCl with 10% dimethyl sulfoxide (DMSO) to permeabilize the blood brain barrier with total intraperitoneal (IP) injection volume of three mL.
Cocaine self-administration
Rats were anesthetized and implanted with intravenous catheters with back mounts according to previously established methods [19–23]. Cocaine self-administration training consisted of daily 180-min sessions, during which rats were trained to lever press for intravenous cocaine infusions (0.75 mg/kg/0.1 mL infusion) starting on a fixed ratio (FR) of one press per infusion (FR1) and finally stabilizing on an FR5 schedule. Stability criteria were at least seven infusions each hour of session and less than 10% variability each day for three days [19, 20]. Following FR training, rats were advanced onto a PR schedule of reinforcement where exponentially more lever presses are required for each successive cocaine reinforcement [4, 5]. Rats responding for cocaine on a PR schedule maintain stable number of infusions over time [24], and stability was defined as less than 10% variability in the total daily infusions for three days.
Two cohorts of rats were employed in this behavior procedure. Rats (n = 62) were included in the pharmacological tests with NMU. Rats were randomized for between-subjects pharmacological tests before FR testing and then equally distributed into to new groups before PR testing. A second cohort (n = 20) received viral knockdown with a short hairpin against NMUR2 (shNMUR2) or non-silencing control (shCTRL) in the NAcSh immediately following catheter implantation. The non-silencing control expresses a short hairpin that was designed to lack efficacy to alter any target [25]. Rats were removed from the study if the catheter patency was lost or the viral injection as mistargeted (total n = 18 for both cohorts).
Intracranial viral-mediated gene transfer
Rats were anesthetized and placed in a stereotaxic instrument (Kopf, Tujunga, CA) for intracranial delivery of viruses. The NMUR2 knockdown virus (shNMUR2) and non-silencing control (shCTRL) [15, 26] were packaged into AAV6 which has been demonstrated as transported in the retrograded direction [27, 28] The packaged viruses were purified (AAVpro Purification Kit, 6666, Takara Bio, Mountain View, CA), and bilaterally microinfused into the NAcSh (AP + 1.4 mm, ML + 2.0 mm, DV − 6.8 mm with 10° angle) [15]. shNMUR2 has been previously shown to decrease NMUR2 gene expression by over 90% in vitro and NMUR2 protein does not colocalize with neurons infected by AAV-shNMUR2 [26].
Microdialysis and GABA quantification
Rats (n = 16) were anesthetized and placed in a stereotaxic instrument for implantation of CMA 11 microdialysis probe guide cannula (8309582; Harvard/CMA, Holliston, MA) positioned just above NAcSh (AP + 1.4 mm, ML + 0.8 mm, DV − 5.3 mm with 0° angle) [15], and after recovery, a CMA 11 (6 kDa) microdialysis probe with 2-mm active length (8309582; Harvard/CMA, Holliston, MA) was positioned. The experiment started 120 min after implantation to allow for tissue recovery and pressure equilibration before start of experiment. Artificial cerebrospinal fluid (P000151; Harvard/CMA, Holliston, MA) was perfused through the probe at 0.3 uL/min and samples were taken every 30 min. Then, cocaine (10 mg/kg), NMU (0.3 mg/kg) or vehicle were administered IP to the rats. One rat with misplaced probe was removed from study.
Analysis of GABA was performed on an AB Sciex 6500 Q-trap mass spectrometer (Sciex, Framingham, MA) coupled with an 1260 ultra-high pressure liquid chromatography system (Agilent, Santa Clara, CA). The separation was performed on an HS F5 column (3 μ, 150 × 2.1 mm I.D., Discovery, St. Louis, MO). Endogenous GABA and spiked in GABA-d6 internal standard (50 ng/mL) were monitored using multiple reaction monitoring (MRM) mode using the parent precursor Q1 m/z (104.2, 110.2) and Q3 masses set to m/z 87.1, 93 for GABA and GABA-d6, respectively. The MRM ratios, which are defined as the peak area ratios between primary and secondary ion transitions, were analyzed using the AB Sciex Analyst® and MultiQuant® 2.1 software (Sciex, Framingham, MA). The first two samples acquired at time points −30, and 0 mins were quantified and averaged to determine basal GABA concentration.
Immunohistochemistry
Retrograde tracing studies employed an AAV6 with a CMV promoter expressing GFP or mCherry and viruses were injected (two cohorts with total n = 18) into two separate brain regions that have been shown to project to the NAcSh. Specifically, the ventromedial VP (AP − 0.1 mm, ML + 2.4 mm, DV − 8.4 mm with 0° angle) [29, 30] and parabrachial pigmented nucleus of the VTA (AP − 5.3 mm, ML + 1.3 mm, DV − 8.5 mm with 9° angle) [31]. Rats were excluded from the study if any needle track was not contained in the target brain region. Following 16 days for viral expression, rats received NMU (0.3 mg/kg, IP) or vehicle and were euthanized and perfused 120 min later to quantify phosphorylated c-Fos expression in the NAcSh → VTA and NAcSh → VP pathways.
Perfused brains were cryoprotected, washed, blocked, and incubated overnight with rabbit anti-phosphorylated c-Fos (1:200, RRID:AB_10557109, 5348 S, Cell Signaling, Danvers, MA), anti-green fluorescent protein antibody (GFP) (1:500, RRID:AB_2307313, GFP-1010, Aves Labs, Inc., Davis, CA), and mouse anti-RFP (1:500, RRID:AB_1141717, ab65856, abcam, Cambridge, MA) primary antibodies. After a brief washing, sections were treated for two hrs with the secondary antibodies for goat anti-rabbit 650 (1:150, RRID:AB_2884989, GTxRb-113-G650NHSX, ImmunoReagents, Inc., Raleigh, NC), donkey anti-chicken 488 (1:100, RRID:AB_2340375, 703–545–155; Jackson ImmunoResearch, West Grove, PA), and donkey anti-mouse 555 (1:150, RRID:AB_2536180, A-31570, ThermoFisher Scientific, Waltham, MA). Sections were washed, mounted, and coverslipped. Imaging was done using a Leica True Confocal Scanner SPE in confocal mode with Leica Application Suite x software (Leica Microsystems, Wetzlar, Germany). Stereological survey included ten brain slices containing bilateral NAcSh per rat for quantification.
Transsynaptic tracing
To identify and compare the neuron populations of the DRN efferents that synapse onto neurons in the NAcSh → VTA pathway or the NAcSh → VP pathway, transsynaptic tracing was employed [32]. Rats (n = 14) were anesthetized and placed in a stereotaxic instrument for administration of a transsynaptic “helper” virus expressing an avian receptor protein (TVA), mCherry, and optimized Rabies Glycoprotein [33] (#104330; Addgene, Watertown, MA) packaged into AAV6 for retrograde transport [27, 28]. Rats were divided into two groups and the virus was administered into the VP or VTA and a guide cannula was directed to the NAcSh (AP + 1.4 mm, ML + 2.0 mm, DV − 4.8 mm with 10° angle). Once the helper virus was stably expressed at 16 days post-surgery, rabies enhanced GFP (Salk; with envelope protein avian sarcoma leucosis virus glycoprotein [EnvA], G-Deleted Rabies-eGFP [Addgene #32635]) was injected into the NAcSh with an internal cannula that projects 2 mm beyond the guide cannula. Ten days after rabies treatment, rats were euthanized, perfused and investigated for rabies expression in the DRN, using immunohistochemistry for the rabies GFP colocalization with glutamic acid decarboxylase 67(GAD67) or tryptophan hydroxylase (TPH) to identify GABA and serotonin neurons, respectively. Mouse anti-GAD67 (1:150, RRID:AB_448990, ab26116, Abcam, Cambridge, MA) or mouse anti-TPH (1:1000, RRID:AB_261587, T0678, Sigma–Aldrich, St. Louis, MO) with donkey anti-mouse AF-647 (1:200, RRID:AB_162542, A-31571, ThermoFisher Scientific, Waltham, MA). A survey of the DRN was conducted with 30 slices of tissue from each rat with 15 slices co-labeled for GAD67 and 15 slices co-labeled for TPH. Confocal microscope techniques allow imaging >1 µM thick slices in the Z direction, which is smaller than the soma thickness for a medium spiny neuron. Thus, the GAD staining visualized can be inferred to be expressed in presynaptic terminals or in the soma. The colocalization of the pathway neuron cell bodies with GAD67 or TPH are expressed as a percentage of the total observed pathway neurons across all slices. Rats with misplaced injection site on either side were removed (n = 8).
Statistical analysis
All data were analyzed using GraphPad Prism v9 software with established methods [15, 18]. Cocaine breakpoints (measured by infusions) were analyzed by one-way analysis of variance (ANOVA) or unpaired t test, while lever presses were analyzed by Kruskal–Wallis or Mann–Whitney, dependent upon the design of the experiment. The GABA concentrations were analyzed using a mixed model, repeated measures ANOVA with preplanned comparisons employed to compare baseline and cocaine-evoked GABA concentrations. For immunohistochemistry, total neuronal staining was analyzed using unpaired t tests by treatment (vehicle or NMU). Data sets for protein colocalization with c-Fos were analyzed using the Mann–Whitney test by retrograde tracer origin (VP or VTA) and by treatment (vehicle or NMU). Comparison of the GABAergic and serotonergic innervation of circuits was analyzed with chi-square tests. All experimenters were blinded to the treatment allocation (e.g., knockdown vs. control) through the conduct and statistical analyses of the studies.
Results
NMU decreases cocaine breakpoints
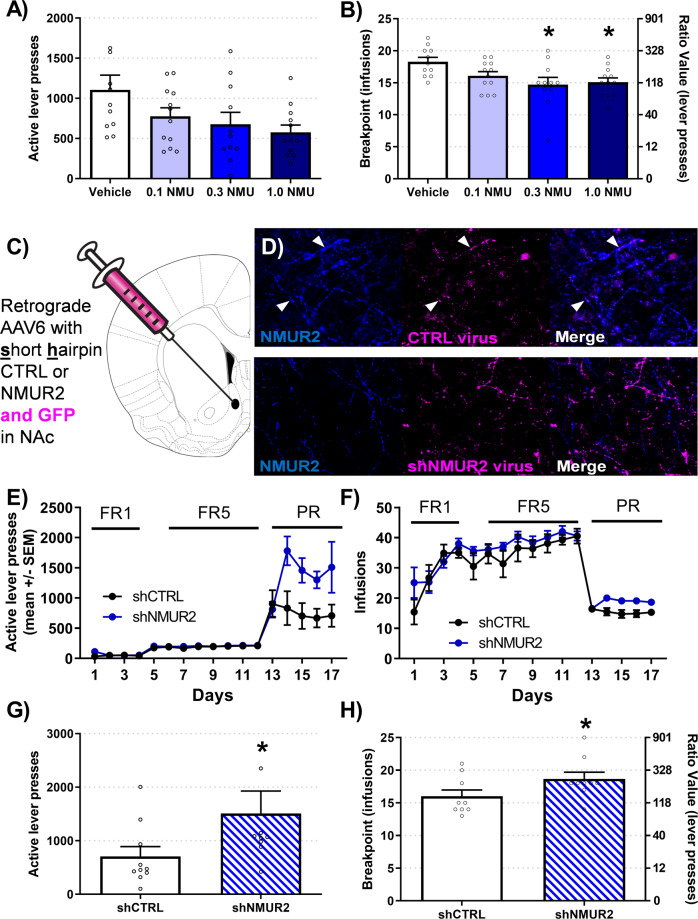
Rats were trained to stability criteria for responding on a FR5 schedule of cocaine self-administration (Fig. S1A). Rats were then trained on a PR schedule and injected with NMU (0.1, 0.3, or 1.0 mg/kg, IP) 10 min before the start of operant session. This time was selected due to the rapid and active transport of NMU across the blood-brain barrier [34]. NMU did not alter total lever presses (H = 7.23; p = 0.065) (Fig. 1A). NMU decreased breakpoints for cocaine (F3,42 = 3.90; p = 0.015) and NMU at 0.3 and 1.0 mg/kg decreased breakpoints compared to vehicle (p = 0.011; p = 0.014) (Fig. 1B). There was no effect of NMU on inactive lever presses (Fig. S1B). These data indicate that NMU decreases breakpoints for cocaine with 0.3 mg/kg NMU as the lowest effective dose.
Fig. 1. NMU and NMUR2 controls breakpoints for cocaine.
A NMU decreased test day lever-presses for cocaine (0.75 mg/kg/inf) on a PR schedule (n = 10–12 per group; one individual Vehicle rat at 2536 lever presses not shown). B NMU decreased test day data breakpoints for cocaine. The breakpoint value is identified on the left axis and the corresponding ratio value of lever presses needed to receive one infusion is located on the right axis. Statistical comparisons were conducted on total infusions. C The schematic diagram for administration of retrograde knockdown of NMUR2 in the NAcSh is clarified. D Representative immunohistochemistry images of control hairpin (top) and NMUR2 hairpin (bottom) are illustrated with NMUR2 (blue) and virus GFP (magenta). E–F Daily acquisition of self-administration is illustrated by active lever presses and infusions. G–H Knockdown of NMUR2 increased test day active lever presses and breakpoints for cocaine (n = 10 per group; one individual shNMUR2 rat at 4585 lever presses not shown). Statistical comparisons were conducted on total infusions. Bar graph shows mean ± SEM. *p < 0.05.
Knockdown of NMUR2 increases cocaine breakpoints
Presynaptic neurons in the NAcSh contain 71% of the NMUR2 protein relative to total NMUR2 localization in this region [15], indicating that NMUR2 is positioned to regulate neurotransmitter release into the NAcSh. We tested the hypothesis that NMUR2 expressed on afferent projections to the NAcSh, including the DRN → NAcSh pathway, regulates cocaine progressive ratio responding. Rats received AAV6 retrograde [27, 28] expressing a short hairpin for NMUR2 (shNMUR2; Fig. 1C, S1C) or non-silencing control (shCTRL; Fig. 1C, S1C) into the NAcSh to knockdown NMUR2 expression on neurons that provide afferent projections to the NAcSh (Fig. 1D). Rats were initially trained to stability on a FR5 cocaine self-administration (Fig. 1E); both exhibited comparable active lever presses and infusions during the operant task prior to training on the PR schedule (Fig. 1E, F). Intra-NAcSh knockdown of NMUR2 increased total lever presses (U = 20; p = 0.022) (Fig. 1G) and breakpoints for cocaine (t = 1.87; p = 0.043) (Fig. 1H), while no change in inactive lever presses was observed (Fig. S1D). These findings demonstrate that the NAcSh is a key node of NMUR2-mediated influence on effort expended for cocaine.
NMU decreases cocaine-evoked NAcSh GABA efflux
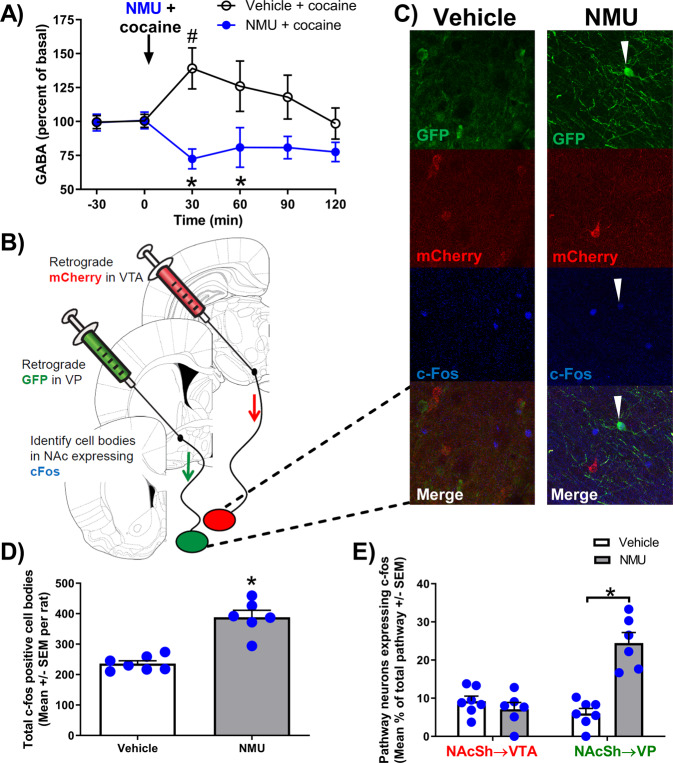
The inhibitory NMUR2 is expressed on presynaptic terminals of GABAergic neurons in the NAcSh [15] and is therefore positioned to regulate NAcSh GABA release. We previously demonstrated that NMU injected directly into the NAcSh decreases local GABA [15], while others have reported increased GABA in NAc following systemic cocaine administration [35]. We hypothesized that systemic NMU blunts cocaine-evoked GABA release in the NAcSh. Following cannula implantation and post-surgical recovery, a microdialysis probe was inserted into the NAcSh (Fig. S2A). After 120 min, basal samples were collected at time points −30 and 0 min to establish baseline levels of GABA (Fig. 2A), followed by systemic administration of NMU (0.3 mg/kg, IP) or vehicle. Immediately following the injection, cocaine (10 mg/kg, IP) was administered (Fig. 2A). A mixed-model repeated measures ANOVA indicates a main effect of treatment (F1,13 = 11.67; p = 0.005), no effect of time (F5,65 = 0.74; p = 0.59), and a treatment X time interaction (F5,65 = 3.49; p = 0.008). In the vehicle-treated group, cocaine increased NAcSh GABA levels at the 30 min time point relative to basal levels within subjects (p = 0.035). NMU-treated rats exhibited significantly lower levels of GABA following cocaine administration than vehicle-treated rats at the 30 (p = 0.0002), and 60 min (p = 0.0258) time points. These data suggest NMU attenuates GABA release in the NAcSh even in the presence of cocaine.
Fig. 2. NMU prevented cocaine-induced increase in GABA and preferentially increased phosphorylated c-Fos on NAcSh→VP projection neurons.
A Cocaine and NMU alter GABA concentrations in the NAcSh measured by microdialysis and mass spectrometry. The arrow indicates time of cocaine (10 mg/kg, IP), NMU (0.3 mg/kg, IP) or vehicle administration. #p < 0.05 compared to basal and *p < 0.05 between groups. B This illustration depicts microinjection sites of retrograde viral vector tracers into target brain regions (VTA or VP) and presents confocal images at 20X objective in the NAcSh. C Representative NAcSh confocal images of vehicle (left) and NMU-treated (right) illustrate NAcSh → VP cell bodies labeled with GFP (green), NAcSh → VTA cell bodies with mCherry (red), and phosphorylated c-Fos (blue). D NMU increased the average quantity of phosphorylated c-Fos positive cell bodies per rat in the NAcSh (n = 6–7 per group). E NMU preferentially increased phosphorylated c-Fos colocalization in the NAcSh → VP (GFP) pathway but not in the NAcSh → VTA (mCherry) pathway quantified from images of tissue from control or NMU-treated rats (n = 3–4 per group). Bar graph shows mean ± SEM. *p < 0.05.
NMU preferentially increases phosphorylated c-Fos on NAcSh → VP projections
The NAc projections to the VP and VTA are key neuroanatomical pathways engaged in cocaine self-administration [1]. To determine which pathways are regulated by NMU, we utilized two different AAV6 retrograde tracers [27, 28] to fluorescently label the NAcSh → VP and NAcSh → VTA pathways. During surgery, we bilaterally administered an AAV6 expressing mCherry into the VTA and an AAV6 expressing green florescent protein (GFP) into VP (Fig. 2B; S2B). This viral procedure resulted in mCherry-labeled neurons in the NAcSh → VTA pathway and GFP-labeled neurons in the NAcSh → VP pathway (Fig. 2B). Rats were then challenged with vehicle or NMU (0.3 mg/kg, IP) and perfused 120 min later to examine NMU-evoked phosphorylated c-Fos in these pathways using immunohistochemistry (Fig. 2C). Overall, NMU increased the total number of phosphorylated c-Fos-positive cell bodies in the NAcSh compared to vehicle (p < 0.0001) (Fig. 2D), consistent with the corresponding decrease in NAcSh GABA (Fig. 2A). A nonparametric Mann–Whitney test indicated that NMU significantly increased the percentage of pathway neurons expressing phosphorylated c-Fos in the NAcSh → VP pathway (p = 0.0023), but not for the NAcSh → VTA pathway (p = 0.3660) (Fig. 2E). The increase in phosphorylated c-Fos presence in the NAcSh → VP pathway following NMU treatment suggests the decrease in GABA (Fig. 2A) preferentially disinhibited the NAcSh → VP pathway (Fig. 2E).
Elucidation of a transsynaptic GABAergic DRN → NAcSh → VP pathway
Transsynaptic tracing tools were employed to label DRN neurons that project directly onto NAcSh → VP neurons (Fig. 3). An AAV6 helper virus expressing three helper proteins was infused into the VP of rats. This helper-protein system has been validated previously in anterograde viruses [36], and we have enhanced this protocols to employ a retrograde virus AAV6 [27, 28] to specifically target the NAcSh efferent pathway. The three helper proteins include: (1) the rabies-docking protein avian tumor virus receptor A (TVA), (2) rabies-infection protein (glycoprotein), and (3) a red fluorescent protein (mCherry) (Fig. 3A). The AAV6 helper virus infected synaptic VP terminals and traveled back to their cell bodies in the retrograde direction [27, 28]. Thus, the helper proteins were expressed only in the NAcSh → VP neurons. Once expressed, the helper proteins transported by the AAV6 helper virus enabled docking and replication of the rabies virus (Fig. 3B).
Fig. 3. The schematic diagram of transsynaptic study to label all neurons that project onto the NAcSh → VP pathway is presented.
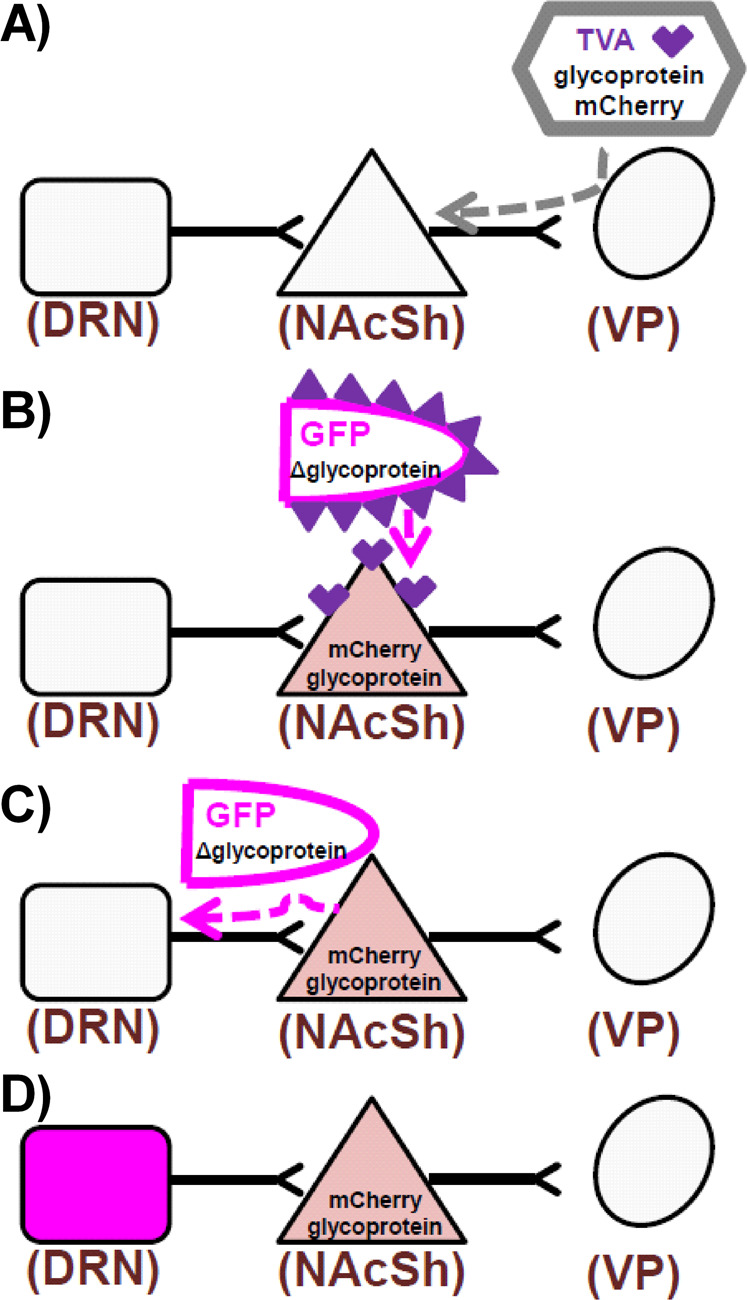
A Retrograde AAV6 was administered to the VP and infected neurons to express TVA, rabies glycoprotein, and mCherry. B Neurons in the NAcSh that express TVA were infected with EnvA pseudotyped rabies virus expressing GFP. Only neurons with TVA can be infected by this pseudotyped rabies. C Rabies replicated in neurons with glycoprotein and transsynaptically “jumps” one synapse in the retrograde direction. D Neurons that express GFP (pseudocolored to magenta) directly synapsed onto NAcSh neurons that project to the VP.
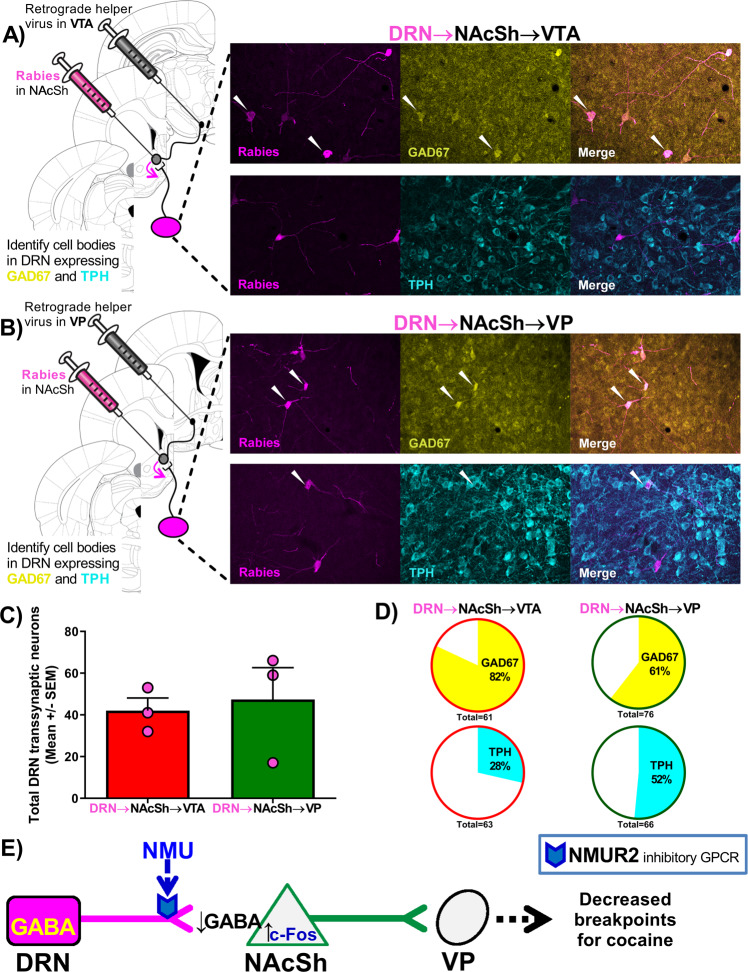
A rabies virus pseudotyped with avian sarcoma leukosis virus envelope glycoprotein (EnvA) and expressing GFP was then injected into the NAcSh (Fig. 3B). The EnvA rabies virus bound to TVA receptors expressed by the helper virus, and thus docked and replicated only in the NAcSh → VP neurons that expressed the helper proteins. This rabies virus lacks the glycoprotein gene (Δ glycoprotein) needed to infect subsequent presynaptic neurons, but the helper virus supplied the needed glycoprotein required for the rabies virus to infect subsequent neurons. Once the rabies virus replicated in the NAcSh, the rabies virus traveled transsynaptically (“jumping” a synapse; Fig. 3C) and retrogradely labeled the soma of neurons that project onto the infected neuron by expressing GFP (Fig. 3D). Thus, the rabies virus labeled all neurons, including those in the DRN that synapse onto the infected NAcSh neurons, confirming the DRN → NAcSh pathway connectivity to VP neurons. This experiment was conducted in parallel with a separate group of rats in which the AAV6 helper virus was injected into the VTA and 16 days later the rabies was injected into the NAcSh (Fig. S3), allowing the comparison of DRN neurons that contribute to the DRN → NAcSh → VP circuit (Fig. 4A) with the DRN → NAcSh → VTA circuit (Fig. 4B). Rabies staining in both circuits was distributed throughout the DRN, regardless of subregion.
Fig. 4. GABAergic and serotonergic DRN → NAcSh neurons differentially innervated NAcSh → VTA and NAcSh → VP pathways.
A schematic diagram illustrates the viral placement with representative data in the DRN showing rabies (magenta) infected neurons that project onto the (A) NAcSh → VTA pathway or (B) NAcSh → VP pathway, colocalizing with GAD67 (yellow) or TPH (cyan). White arrows indicate colocalization events. C Total number of rabies positive neurons in the DRN from each rat in a survey of the DRN (15 coronal slices per rat and n = 3 per group). D Percentage of the rabies-positive neurons colocalizing with GAD67 or TPH. E The illustration summarizes the proposed pathway.
Quantification of immunohistochemical staining was used to compare the total number of rabies-positive neurons in a survey of the DRN (Fig. 4C); we found no significant difference in the number of DRN rabies-positive neurons that synapse onto the NAcSh → VP pathway or the NAcSh → VTA pathway (p = 0.76). These rabies-labeled neurons in the DRN were examined for colocalization with the neurotransmitter markers for GABA (glutamate decarboxylase; GAD67) and serotonin (tryptophan hydroxylase; TPH). The DRN neurons that project onto the NAcSh → VTA had a greater colocalization with GAD67 (82%) than TPH (28%). In contrast, the DRN neurons that project onto the NAcSh → VP exhibited similar colocalization with GAD67 (61%) and TPH (52%), suggesting these DRN projection neurons contribute both GABAergic and serotonergic drive to the NAcSh → VP pathway. The percent of GABA-positive DRN projections was not different between these circuits (χ2 = 3.47; p = 0.177). The fact that these percentages add up to over 100% suggests that a subset of neurons coexpress both GAD67 and TPH.
Discussion
This study established the DRN → NAcSh → VP circuit and identified NMU as a preferential regulator of this circuit toward subsequent targeted control of cocaine self-administration (Fig. 4F). We demonstrated that systemic NMU dose-dependently decreased breakpoints for cocaine. In addition, presynaptic knockdown of NMUR2 in the NAcSh increased breakpoints for cocaine, indicating that NMUR2 in projections to the NAcSh are regulators of effort expended for cocaine. This complements our previous research demonstrating that the NAcSh is a key site of action for NMU-evoked changes in cocaine locomotor sensitization [15]. Systemic NMU signaling also alters amphetamine-evoked locomotion and alcohol self-administration [17, 37], demonstrating NMU controls self-administration behaviors across psychostimulants.
NMU and cocaine modulate neurotransmission in the NAc
Our research demonstrates that experimenter-administered cocaine increased NAcSh GABA efflux, similar to published studies [35]. In addition, the elevated levels of GABA in the NAc following a history of cocaine self-administration support the connection between NAc GABA and motivation for cocaine [35, 38–40]. Previously, we reported that intra-NAcSh NMU decreases basal GABA, and our work here demonstrated that NMU decreases cocaine-evoked GABA as well. There are numerous sources of GABAergic projections to the NAcSh [41], including the DRN → NAcSh [15, 42, 43], that express presynaptic NMUR2 [15]. Indeed, the DRN → NAcSh pathway was found to colocalize with almost 50% of the NMUR2 in the NAcSh as visualized with confocal microscopy [15]. The NMU-evoked decrease in NAcSh GABA is supported by the corresponding increase in NAcSh phosphorylated c-Fos (Fig. 2D). Tract-tracing identified greater phosphorylated c-Fos presence in the NAcSh → VP pathway compared to the NAcSh → VTA pathway in NMU-treated rats, suggesting the decreased NAcSh GABA preferentially disinhibited the NAcSh → VP pathway. In addition, other neurotransmitters, such as dopamine, may also be involved as NMU blocks amphetamine-evoked dopamine in the NAc and intra-NAc NMU blocks alcohol-evoked dopamine release in the NAc [17, 37]. Thus, the NMU microdialysis studies presented here potentially correspond with a hypothesized blunting of cocaine-evoked dopamine release as well as the reported decrease in GABA, and this research is the subject of future studies.
Seemingly conflicting reports demonstrate that cocaine increases c-Fos expression in the NAc [44] and decreases activity of NAc neurons measured with electrophysiology [45]. Cocaine may evoke pathway-specific alterations in neuronal firing beyond actions at dopamine receptors which explains these conflicting studies. By establishing the underlying neurocircuitry involved in cocaine effects, continued research from our group and others will drive our understanding of neuroadaptations that govern cocaine-taking behaviors. Future studies investigating cocaine-evoked activity of specific neurocircuitry can be investigated and compared using viral techniques including the multi-viral transsynaptic tracing technique we employed in this study.
Transsynaptic tracing to identify connectivity
To determine if the DRN → NAcSh pathway synapses directly onto the NAcSh → VP pathway, we employed a transsynaptic tracing procedure which allowed us to label neurons that project onto specific neuronal populations [32, 46]. While transsynaptic protocols offer the ability to identify structural connectivity among neurons, it is best used in combination with immunohistochemistry protocols that examine proteins in the cell bodies (e.g., GAD67) and not with protein trafficked away from the cell body and to the presynaptic terminal (e.g., NMUR2). GAD67 immunoreactivity can be seen pre- and postsynaptic, which complicate interpretation of these data. Transsynaptic tracing is ideal with in situ hybridization techniques and that is an ongoing direction of our research group. Our findings indicate that the GABA neurons of the DRN → NAcSh pathway do not differ in the explored synaptic profile onto the NAcSh → VP and NAcSh → VTA pathways. This positions accumbal GABA as a key regulatory neurotransmitter for these circuits.
Conclusions
Our data demonstrated functional connectivity in the DRN → NAcSh → VP circuit which was regulated by the neuropeptide NMU and that NMU modulates cocaine self-administration. Given the increase in NMU-evoked phosphorylated c-Fos expression in the NAcSh → VP pathway and transsynaptic immunohistochemical colocalization of rabies DRN → NAcSh → VP pathway tracer with NMUR2, NMUR2 localization is appropriately positioned such that pharmacological treatment with NMU activates the NAcSh → VP pathway. Furthermore, while there are DRN serotonin and GABA projections onto the NAcSh → VP pathway, our data demonstrated that the NAcSh → VTA pathway is predominantly innervated by GABAergic neurons from the DRN. These findings emphasize the importance of GABAergic projections to the NAcSh as key regulators efferent pathways and advance our understanding of NMUR2 in neurocircuitry that regulates cocaine.
Supplementary information
Acknowledgements
This work was possible thanks to the facilities and expertize of the University of Texas Medical Branch (UTMB) Center for Addiction Research Rodent In Vivo Assessment Core and the UTMB Mass Spectrometry Facility. We thank Ms. Reyna Collura for her graphic design contributions.
Author contributions
JMK and AES preformed the surgeries, behavior experiments, and microdialysis. JMK and SNM performed the immunohistochemistry. WKR quantified the microdialysis samples. JMK, SNM and JDH performed the data analysis. JMK, A13, KAC and JDH drafted the paper. JMK, KAC, WKR, and JDH conceived the experiments. All have edited the paper.
Funding
This work was supported by the National Institute on Drug Abuse (R03DA033437, P30DA028821, and T32DA07287), Peter F. McManus Charitable Trust, and Clinical and Translational Science Award (UL1TR001439 and KL2TR001441) from the National Center for Advancing Translational Science. The UTMB Mass Spectrometry Facility is supported in part by CPRIT grant RP190682.
Competing interests
Dr. Cunningham has current research funding from VidaLibreBio, Inc., for research unrelated to this study. Additional authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Kathryn A. Cunningham, Jonathan D. Hommel.
Contributor Information
Kathryn A. Cunningham, Email: kcunning@utmb.edu
Jonathan D. Hommel, Email: jdhommel@utmb.edu
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-021-01234-9.
References
- 1.Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat. 2011;5:41. doi: 10.3389/fnana.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lobo MK, Covington HE, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–90. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durieux PF, Bearzatto B, Guiducci S, Buch T, Waisman A, Zoli M, et al. D2R striatopallidal neurons inhibit both locomotor and drug reward processes. Nat Neurosci. 2009;12:393–5. doi: 10.1038/nn.2286. [DOI] [PubMed] [Google Scholar]
- 4.Roberts DC, Morgan D, Liu Y. How to make a rat addicted to cocaine. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1614–24.. doi: 10.1016/j.pnpbp.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 6.Bock R, Shin JH, Kaplan AR, Dobi A, Markey E, Kramer PF, et al. Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nat Neurosci. 2013;16:632–8. doi: 10.1038/nn.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kupchik YM, Brown RM, Heinsbroek JA, Lobo MK, Schwartz DJ, Kalivas PW. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat Neurosci. 2015;18:1230–2. doi: 10.1038/nn.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kupchik YM, Kalivas PW. The direct and indirect pathways of the nucleus accumbens are not what you think. Neuropsychopharmacology. 2017;42:369–70.. doi: 10.1038/npp.2016.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu XY, Ghasemzadeh MB, Kalivas PW. Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens. Neuroscience. 1998;82:767–80.. doi: 10.1016/S0306-4522(97)00327-8. [DOI] [PubMed] [Google Scholar]
- 10.Sesack SR, Grace AA. Cortico-Basal ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z, Chen Z, Fan G, Li A, Yuan J, Xu T. Cell-type-specific afferent innervation of the nucleus accumbens core and shell. Front Neuroanat. 2018;12:84. doi: 10.3389/fnana.2018.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Shen M, Yu Y, Tao Y, Zheng P, Wang F, et al. Optogenetic activation of GABAergic neurons in the nucleus accumbens decreases the activity of the ventral pallidum and the expression of cocaine-context-associated memory. Int J Neuropsychopharmacol. 2014;17:753–63. doi: 10.1017/S1461145713001570. [DOI] [PubMed] [Google Scholar]
- 13.Brighton PJ, Szekeres PG, Wise A, Willars GB. Signaling and ligand binding by recombinant neuromedin U receptors: evidence for dual coupling to Galphaq/11 and Galphai and an irreversible ligand-receptor interaction. Mol Pharm. 2004;66:1544–56. doi: 10.1124/mol.104.002337. [DOI] [PubMed] [Google Scholar]
- 14.Howard AD, Wang R, Pong SS, Mellin TN, Strack A, Guan XM, et al. Identification of receptors for neuromedin U and its role in feeding. Nature. 2000;406:70–4. doi: 10.1038/35017610. [DOI] [PubMed] [Google Scholar]
- 15.Kasper JM, McCue DL, Milton AJ, Szwed A, Sampson CM, Huang M. Gamma-aminobutyric acidergic projections from the dorsal raphe to the nucleus accumbens are regulated by neuromedin U. Biol Psychiatry. 2016;80:878–87. doi: 10.1016/j.biopsych.2016.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steketee JD, Kalivas PW. Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharm Rev. 2011;63:348–65.. doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vallöf D, Ulenius L, Egecioglu E, Engel JA, Jerlhag E. Central administration of the anorexigenic peptide neuromedin U decreases alcohol intake and attenuates alcohol-induced reward in rodents. Addict Biol. 2017;22:640–51.. doi: 10.1111/adb.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasper JM, Smith AE, Hommel JD. In Press (Frontiers in Behavioral Neuroscience, 2018). [DOI] [PMC free article] [PubMed]
- 19.Sholler DJ, Stutz SJ, Fox RJ, Boone EL, Wang Q, Rice KC. The 5-HT2A receptor (5-HT2AR) regulates impulsive action and cocaine cue reactivity in male sprague-dawley rats. J Pharmacol Exp Ther. 2018;368:41–9. doi: 10.1124/jpet.118.251199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swinford-Jackson SE, Anastasio NC, Fox RG, Stutz SJ, Cunningham KA. Incubation of cocaine cue reactivity associates with neuroadaptations in the cortical serotonin (5-HT) 5-HT2C receptor (5-HT2CR) system. Neuroscience. 2016;324:50–61. doi: 10.1016/j.neuroscience.2016.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anastasio NC, Stutz SJ, Fox RG, Sears RM, Emeson RB, DiLeone RJ, et al. Functional status of the serotonin 5-HT2C receptor (5-HT2CR) drives interlocked phenotypes that precipitate relapse-like behaviors in cocaine dependence. Neuropsychopharmacology. 2014;39:370–82. doi: 10.1038/npp.2013.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anastasio NC, Liu S, Maili L, Swinford SE, Lane SD, Fox RG, et al. Variation within the serotonin (5-HT) 5-HT2C receptor system aligns with vulnerability to cocaine cue reactivity. Transl Psychiatry. 2014;4:e369. doi: 10.1038/tp.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anastasio NC, Sholler DJ, Fox RG, Stutz SJ, Merritt CR, Bjork JM, et al. Suppression of cocaine relapse-like behaviors upon pimavanserin and lorcaserin co-administration. Neuropharmacology. 2020;168:108009. doi: 10.1016/j.neuropharm.2020.108009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Depoortere RY, Li DH, Lane JD, Emmett-Oglesby MW. Parameters of self-administration of cocaine in rats under a progressive-ratio schedule. Pharm Biochem Behav. 1993;45:539–48.. doi: 10.1016/0091-3057(93)90503-L. [DOI] [PubMed] [Google Scholar]
- 25.Hommel JD, Sears RM, Georgescu D, Simmons DL, DiLeone RJ. Local gene knockdown in the brain using viral-mediated RNA interference. Nat Med. 2003;9:1539–44. doi: 10.1038/nm964. [DOI] [PubMed] [Google Scholar]
- 26.Benzon CR, Johnson SB, McCue DL, Li D, Green TA, Hommel JD. Neuromedin U receptor 2 knockdown in the paraventricular nucleus modifies behavioral responses to obesogenic high-fat food and leads to increased body weight. Neuroscience. 2014;258:270–9. doi: 10.1016/j.neuroscience.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Low K, Aebischer P, Schneider BL. Direct and retrograde transduction of nigral neurons with AAV6, 8, and 9 and intraneuronal persistence of viral particles. Hum Gene Ther. 2013;24:613–29. doi: 10.1089/hum.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salegio EA, Samaranch L, Kells AP, Mittermeyer G, San Sebastian W, Zhou S, et al. Axonal transport of adeno-associated viral vectors is serotype-dependent. Gene Ther. 2013;20:348–52. doi: 10.1038/gt.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Root DH, Melendez RI, Zaborszky L, Napier TC. The ventral pallidum: Subregion-specific functional anatomy and roles in motivated behaviors. Prog Neurobiol. 2015;130:29–70. doi: 10.1016/j.pneurobio.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sixth Edition. Academic Press; 2007.
- 31.Herin DV, Bubar MJ, Seitz PK, Thomas ML, Hillman GR, Tarasenko YI, et al. Elevated expression of serotonin 5-HT(2A) receptors in the rat ventral tegmental area enhances vulnerability to the behavioral effects of cocaine. Front Psychiatry. 2013;4:2. doi: 10.3389/fpsyt.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Callaway EM, Luo L. Monosynaptic circuit tracing with glycoprotein-deleted rabies viruses. J Neurosci. 2015;35:8979–85. doi: 10.1523/JNEUROSCI.0409-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ciabatti E, González-Rueda A, Mariotti L, Morgese F, Tripodi M. Life-long genetic and functional access to neural circuits using self-inactivating rabies virus. Cell. 2017;170:382–92.e14. doi: 10.1016/j.cell.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gevaert B, Wynendaele E, Stalmans S, Bracke N, D’Hondt M, Smolders I, et al. Blood-brain barrier transport kinetics of the neuromedin peptides NMU, NMN, NMB and NT. Neuropharmacology. 2016;107:460–70.. doi: 10.1016/j.neuropharm.2016.03.051. [DOI] [PubMed] [Google Scholar]
- 35.Xi ZX, Ramamoorthy S, Shen H, Lake R, Samuvel DJ, Kalivas PW. GABA transmission in the nucleus accumbens is altered after withdrawal from repeated cocaine. J Neurosci. 2003;23:3498–505.. doi: 10.1523/JNEUROSCI.23-08-03498.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kohara K, Pignatelli M, Rivest AJ, Jung HY, Kitamura T, Suh J, et al. Cell type-specific genetic and optogenetic tools reveal hippocampal CA2 circuits. Nat Neurosci. 2014;17:269–79.. doi: 10.1038/nn.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vallöf D, Vestlund J, Engel JA, Jerlhag E. The anorexigenic peptide neuromedin U (NMU) attenuates amphetamine-induced locomotor stimulation, accumbal dopamine release and expression of conditioned place preference in mice. PLoS One. 2016;11:e0154477. doi: 10.1371/journal.pone.0154477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu S, Bubar MJ, Lanfranco MF, Hillman GR, Cunningham KA. Serotonin2C receptor localization in GABA neurons of the rat medial prefrontal cortex: implications for understanding the neurobiology of addiction. Neuroscience. 2007;146:1677–88. doi: 10.1016/j.neuroscience.2007.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henry DJ, White FJ. The persistence of behavioral sensitization to cocaine parallels enhanced inhibition of nucleus accumbens neurons. J Neurosci. 1995;15:6287–99. doi: 10.1523/JNEUROSCI.15-09-06287.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Addolorato G, Leggio L, Hopf FW, Diana M, Bonci A. Novel therapeutic strategies for alcohol and drug addiction: focus on GABA, ion channels and transcranial magnetic stimulation. Neuropsychopharmacology. 2012;37:163–77.. doi: 10.1038/npp.2011.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noori HR, Spanagel R, Hansson AC. Neurocircuitry for modeling drug effects. Addict Biol. 2012;17:827–64.. doi: 10.1111/j.1369-1600.2012.00485.x. [DOI] [PubMed] [Google Scholar]
- 42.Bang SJ, Commons KG. Forebrain GABAergic projections from the dorsal raphe nucleus identified by using GAD67-GFP knock-in mice. J Comp Neurol. 2012;520:4157–67.. doi: 10.1002/cne.23146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDevitt RA, Tiran-Cappello A, Shen H, Balderas I, Britt JP, Marino RA, et al. Serotonergic versus nonserotonergic dorsal raphe projection neurons: differential participation in reward circuitry. Cell Rep. 2014;8:1857–69.. doi: 10.1016/j.celrep.2014.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szucs RP, Frankel PS, McMahon LR, Cunningham KA. Relationship of cocaine-induced c-Fos expression to behaviors and the role of serotonin 5-HT2A receptors in cocaine-induced c-Fos expression. Behav Neurosci. 2005;119:1173–83. doi: 10.1037/0735-7044.119.5.1173. [DOI] [PubMed] [Google Scholar]
- 45.White FJ, Hu XT, Henry DJ. Electrophysiological effects of cocaine in the rat nucleus accumbens: microiontophoretic studies. J Pharm Exp Ther. 1993;266:1075–84. [PubMed] [Google Scholar]
- 46.Wickersham IR, Lyon DC, Barnard RJ, Mori T, Finke S, Conzelmann KK, et al. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron. 2007;53:639–47.. doi: 10.1016/j.neuron.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.