To the Editor,
Constricted oxygen and glucose supplies are common conditions that healthy cells following a stress such an ischemic insult as well as cancer cells within a solid tumor need to overcome in order to survive. These conditions are a serious challenge for cells, which accurately measure the impact to make a life or death decision. In cancer cells, aberrant gene expression and/or protein regulation, frequently linked to appearing mutations, may confer selective advantages to survive under these adverse conditions. In the past years, cumulative evidence indicates that protein modification by covalent attachment of the Small Ubiquitin-like MOdifier (SUMO) plays a role in cell survival after oxygen and glucose deprivation (OGD).1 Sumoylation involves maturation of SUMO, E1 (SAE1/UBA2)-mediated activation and transfer to the E2 conjugating enzyme (UBC9), to be attached to target proteins, which is frequently facilitated by a E3 ligase, with PIAS as the most investigated proteins.2 Specific SUMO proteases such as the SENP family members are involved in maturation and recycling SUMO from targets.2 SUMO is essential in vertebrates, participates in most physiological processes and is associated with cancer.2 Three functional SUMO molecules have been identified in humans: SUMO1, 2 and 3. SUMO2 and 3, usually referred to as SUMO2/3, are highly homologous and undistinguishable, and are abundant in the cell as unconjugated to rapidly modify proteins in response to OGD.1 From the initial observation of increased SUMO2/3 conjugation in squirrel brain during hibernation torpor a number of reports have described protection effects of sumoylation in ischemic/reperfusion models, and established better outcomes in cell viability associated with SUMO or UBC9 overexpression and worse outcomes associated with SUMO silencing or SENP1 overexpression.1 However, sumoylation of heterochromatin protein 1 α (HP1α) has been linked to senescence in cancer cells.3 To define molecular relevant aspects of SUMO-mediated response to OGD, we have investigated gene expression regulation of the sumoylation pathway components. Notably we found that Senp7 gene and protein are downregulated in OGD-treated cancer cells. Interestingly, SENP7 overexpression under these conditions increased cell survival, while downregulation potentiated OGD-triggered apoptosis. In contrast, SENP3 promoted cell death. These observations are in agreement with prevalent amplification of SENP7 and deletion of SENP3 genomic regions in many cancer types. More importantly, in colon cancer, high expression of SENP7 correlated with both, poor prognosis and higher transformation degree.
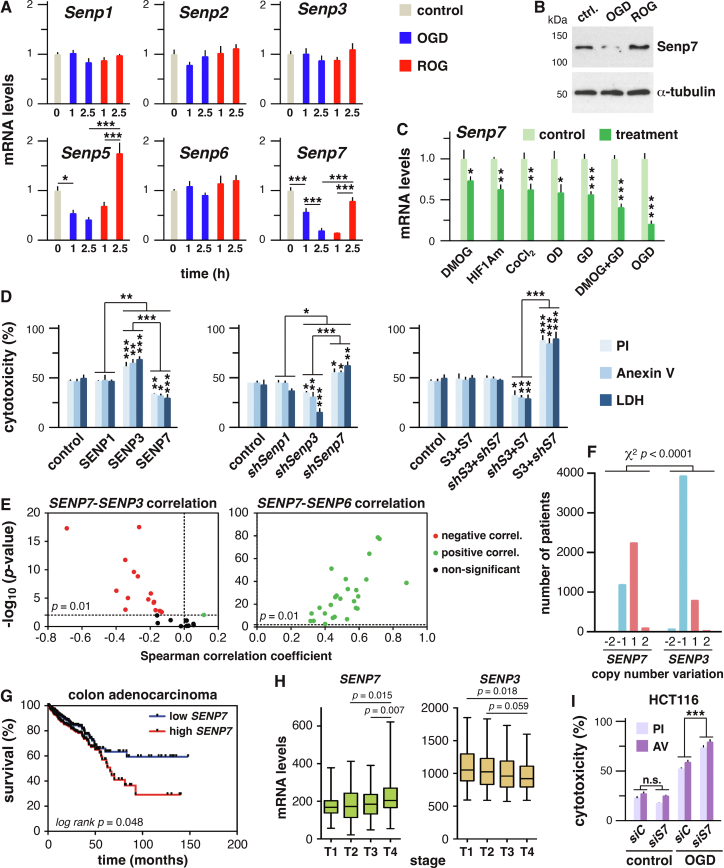
To analyze the impact of OGD on mRNA levels of genes coding for components of the sumoylation pathway, cells were exposed for 1 and 2.5 h (h) to OGD, and for 1 and 2.5 h to restored oxygen and glucose (ROG) after 2.5 h of OGD. These are non-severe OGD conditions, without consequences on cell viability, but putatively affecting gene expression. We chose pluripotent murine embryonic carcinoma cells P19, which are also suitable for sumoylation studies as previously reported. We observed regulation of Sumo2 and genes coding for some ligases and proteases (Fig. 1A, S1A), substantiating the notion that ligases and proteases are main actors of sumoylation regulation. Two SUMO protease genes showed a marked regulation, Senp5 and Senp7 (Fig. 1A). The most prominent effect was observed on Senp7, which displayed a 5-fold decrease at 2.5 h of OGD, while 2.5 h of ROG restored mRNA levels. These changes were accompanied by similar alterations at the protein level (Fig. 1B). Senp7 expression was also downregulated by: 1) DMOG, an inhibitor of prolyl hydroxilases, which blocks degradation of the hypoxia inducible factor 1A (HIF1A), therefore mimicking hypoxia under normoxia conditions, 2) Expression of a degradation resistant mutant variant of HIF1A, 3) The hypoxia mimetic cobalt chloride, 4) Oxygen deprivation (OD), 5) Glucose deprivation (GD) and 6) A combination of GD and DMOG, which displayed the more drastic effects (Fig. 1C, S2A). Arsenite, a potent cytotoxic agent for P19 cells (Fig. S3A), and heat shock (42 °C) also inhibited Senp7 expression (Fig. S3B). Ligase genes Pias1 and Pias4 were slightly blunted by OGD, an effect normalized by ROG (Fig. S1A). In contrast to OGD, treatment with cobalt chloride and arsenite prompted a robust and transient increase in Pias1, 2 and 4 mRNAs (Fig. S1B), indicating that different stress conditions lead to diverse effects on Pias expression.
Figure 1.
Oxygen and glucose deprivation regulates expression of SENP7, which promotes cell survival and associates with poor colon cancer prognosis. (A) Relative mRNA levels of the indicated genes, as determined by quantitative PCR (qPCR), in control P19 cells (0) or cells subjected to OGD and ROG (after 2.5 h of OGD) for the indicated time points. Levels were normalized to the levels at 0 h. Values are means ± S.D. of three independent experiments analyzed in triplicate. Statistical significance of changes in gene expression was analyzed by one-way ANOVA (P < 0.05) followed by the Tukey's post-test: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. (B) Protein levels of SENP7 were analyzed by Western blot under control, 2.5 h OGD and 2.5 h ROG (after 2.5 h OGD) conditions. 40 μg of total protein were loaded per lane. α-tubulin levels were determined as a loading control. (C)Senp7 mRNA levels were determined by qPCR in cells subjected to: addition of DMOG, expression of mutant HIF1A (HIF1Am), cobalt chloride, oxygen deprivation (OD), glucose deprivation (GD), combined DMOG and GD, and OGD. Values and statistic were as in (A). (D) The percentage of cytotoxicity was determined in P19 cells subjected to harmful OGD by propidium iodide (PI) or Anexin V labeling, or by measurement of LDH activity, after transfection of (left panel) empty vector (control) and expression constructs for SENP1, 3 and 7, (middle panel) a control shRNA and expression constructs for shRNA molecules (sh) against Senp1, 3 and 7 mRNAs, and (right panel) empty vector (control) and different combinations of expression constructs for SENP7 (S7) and SENP3 (S3), and expression constructs for sh against their corresponding mRNAs. Values are means ± S.D. of three independent determinations. Statistic was as in (A). (E) Volcano plots showing Spearman correlation coefficient versus –log10 (P-value) for the correlation between mRNA levels of SENP7 and SENP3 (left panel) or the SENP7 and SENP6 (right panel) in different types of tumors. Each dot represents a different type of tumor. Negative correlations are depicted in red and positive correlations are depicted in green. For P > 0.01 dots are depicted in black. (F) Copy number variation data for SENP7 and SENP3 in TCGA PanCancer cohort (n = 10528 samples). −2 and −1 indicates deep and shallow deletions respectively. 2 and 1 indicates high level and low level amplification, respectively. (G) Kaplan–Meier survival plots for colon adenocarcinoma patients from TCGA cohort. Patients were divided into two subgroups according to the SENP7 mRNA levels. P-value was calculated by the log-rank test. (H) Boxplot of transcript levels of SENP7 (left panel) or SENP3 (right panel) in colon adenocarcinoma tumors with different histological grades (T1 to T4). Unpaired Student t-test P-values among the indicated distributions are provided. (I) The percentage of cytotoxicity was determined in HCT116 cells subjected to harmful OGD or under normal growth conditions (control) by propidium iodide (PI) and Anexin V (AV) labeling, after transfection of control (siC) or SENP7-directed (siS7) siRNAs. Values are means ± S.D. of three independent determinations. Statistic was as in (A).
We next focused on the impact of SENP7 overexpression and knockdown after harmful OGD, and comparing with SENP3 and SENP1 (Fig. 1D, S2, S4A, S5). Consistent with previous studies,4 SENP3 overexpression was deleterious to cell viability (Fig. 1D). However, cells overexpressing SENP7 displayed improved viability (Fig. 1D). Conversely, shRNA-mediated silencing of Senp3 and Senp7 improved and impaired cell viability, respectively (Fig. 1D). SENP1 overexpression or depletion had no effect on cell viability (Fig. 1D). As SENP3 and SENP7 exhibit opposing effects on cell viability, we sought to gauge the combined impact of both factors (overexpression/knockdown) on survival. The simultaneous overexpression or knockdown of both proteases negated individual effects (Fig. 1D). Moreover, combined knockdown of Senp7 with overexpression of SENP3 had the most severe impact on cell viability (Fig. 1D, S5B). Consistent with previous reports,1 SUMO2 and UBC9 overexpression reduced cytotoxicity (Fig. S4). Interestingly, PIAS4 overexpression also led to cell protection (Fig. S4). Since ligases do not promote global sumoylation, this suggests that target-specific sumoylation suffices to improve cell survival. That both a SUMO ligase (PIAS4) and a SUMO protease (SENP7) promote cell protection may be counterintuitive, and could be reconciled by differences in target selection or by sequential action.
Our data indicate that hampering SENP7 downregulation during OGD promotes survival while constitutive low levels decreased it, suggesting that SENP7 downregulation during OGD may be a system to suppress viability of cells subjected to these adverse conditions. Since increased viability under these conditions results on selective advantage for many cancer cells, our observations prompted us to analyze SENP3 and SENP7 in cancer. A negative correlation between SENP7 and SENP3 mRNA levels was observed for most tumors, while a positive correlation was found between SENP7 and all other SENPs (Fig. 1E, S6). In addition, the SENP7 locus often displayed an increase in copy number (gene amplifications) in different types of tumors, while a decrease (gene deletions) was observed for SENP3 (Fig. 1F, S7). As such we pondered whether SENP7 expression was associated to a worse prognosis in cancer patients. Among the different types of cancers, this was especially evident for colorectal cancers from different cohorts (Fig. 1G and Table S1). Even more, mRNA levels of SENP7 positively correlated with tumor stage, while a negative correlation was observed for SENP3 (Fig. 1H). Thus, SENP7 emerges as a new prognosis marker for colon cancer. It has been reported that SENP7 upregulation in breast cancer leads to HP1α desumoylation, which results in decreased senescence.3 Furthermore, SPOP-mediated degradation of SENP7 has been proposed as a tumor suppressor mechanism promoting senescence, and SENP7 is highly expressed in prostate tumor cells with mutations in SPOP.5 Now, our data indicate that cancer cells may escape from OGD-triggered loss of viability by upregulating SENP7.
Finally, to check for colon cancer cells’ dependence on SENP7, we turned to the HCT116 cell line. Analysis of the depmap database (https://depmap.org/portal/) indicates that RNAi-mediated knockdown of SENP7 has a significant negative effect on HCT116 proliferation (DEMETER2 dependency score for gene effect = −0.326). Thus, we sought to investigate in these cells whether SENP7 knockdown has also an effect on cell cytotoxicity under OGD conditions. Interestingly, SENP7 knockdown (Fig. S2B) led to significant increased cytotoxicity in comparison with control conditions (Fig. 1I). Therefore, taken together, our results suggest that acting against SENP7 could be of therapeutic interest to fight colon cancer.
Conflict of interests
Authors declare no conflict of interests.
Funding
This work was supported by MICIN/AEI/10.13039/501100011033, Spain/ERDF “A way to make Europe”, European Union (No. PGC2018-094232-B-I00); CEICE, Junta de Andalucía, Spain (No. CTS 2064). JF C–V was supported by MICIN/AEI/10.13039/501100011033, Spain/ESF “Investing in your future”, European Union (contract No. BES-2016-076500).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2022.02.019.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bernstock J.D., Yang W., Ye D.G., et al. SUMOylation in brain ischemia: patterns, targets, and translational implications. J Cereb Blood Flow Metab. 2018;38(1):5–16. doi: 10.1177/0271678X17742260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie M., Yu J., Ge S., Huang J., Fan X. SUMOylation homeostasis in tumorigenesis. Cancer Lett. 2020;469:301–309. doi: 10.1016/j.canlet.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Bawa-Khalfe T., Lu L.S., Zuo Y., et al. Differential expression of SUMO-specific protease 7 variants regulates epithelial-mesenchymal transition. Proc Natl Acad Sci U S A. 2012;109(43):17466–17471. doi: 10.1073/pnas.1209378109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo C., Hildick K.L., Luo J., Dearden L., Wilkinson K.A., Henley J.M. SENP3-mediated deSUMOylation of dynamin-related protein 1 promotes cell death following ischaemia. EMBO J. 2013;32(11):1514–1528. doi: 10.1038/emboj.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu H., Ren S., Bitler B.G., et al. SPOP E3 ubiquitin ligase adaptor promotes cellular senescence by degrading the SENP7 deSUMOylase. Cell Rep. 2015;13(6):1183–1193. doi: 10.1016/j.celrep.2015.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.