Abstract
Understanding the association between the genetic and clinical phenotypes in children with nephrotic syndrome (NS) of different etiologies is critical for early clinical guidance. We employed whole-exome sequencing (WES) to detect monogenic causes of NS in a multicenter cohort of 637 patients. In this study, a genetic cause was identified in 30.0% of the idiopathic steroid-resistant nephrotic syndrome (SRNS) patients. Other than congenital nephrotic syndrome (CNS), there were no significant differences in the incidence of monogenic diseases based on the age at manifestation. Causative mutations were detected in 39.5% of patients with focal segmental glomerulosclerosis (FSGS) and 9.2% of those with minimal change disease (MCD). In terms of the patterns in patients with different types of steroid resistance, a single gene mutation was identified in 34.8% of patients with primary resistance, 2.9% with secondary resistance, and 71.4% of children with multidrug resistance. Among the various intensified immunosuppressive therapies, tacrolimus (TAC) showed the highest response rate, with 49.7% of idiopathic SRNS patients achieving complete remission. Idiopathic SRNS patients with monogenic disease showed a similar multidrug resistance pattern, and only 31.4% of patients with monogenic disease achieved a partial remission on TAC. During an average 4.1-year follow-up, 21.4% of idiopathic SRNS patients with monogenic disease progressed to end-stage renal disease (ESRD). Collectively, this study provides evidence that genetic testing is necessary for presumed steroid-resistant and idiopathic SRNS patients, especially those with primary and/or multidrug resistance.
Keywords: Clinical phenotypes, Genetic phenotypes, Multicenter cohort, Nephrotic syndrome, Pediatric, Whole-exome sequencing
Introduction
Nephrotic syndrome (NS) is defined by the presence of severe proteinuria, hypoalbuminemia, edema, and hyperlipidemia.1 Many glomerular diseases and a few renal tubule reabsorption disorders present with NS in childhood.2 At present, NS is divided into several categories based on the age at onset and etiology, including congenital nephrotic syndrome (CNS), genetically-associated nephropathy, idiopathic NS (INS) and secondary NS. Genetic nephropathies, CNS and some forms of INS can be monogenic diseases, while secondary NS involves multigene interactions, immune responses, the environment, infection, allergens and so on.3,4
The mechanisms underlying the development of INS are unclear. The disease affects approximately 2–5 per 100,000 children per year. Corticosteroids are the standard therapy for INS, and approximately 80–90% of INS patients initially respond to steroids.1,5 However, nearly half of the children with steroid-sensitive nephrotic syndrome (SSNS) develop frequently-relapsing nephrotic syndrome (FRNS) or steroid-dependent nephrotic syndrome (SDNS). Additionally, 10–15% of affected children already have or later develop steroid-resistant nephrotic syndrome (SRNS).1,3 The management of SRNS is a great challenge due to its heterogeneous etiology. Patients often require several courses of steroid treatment and additional use of one or more immunosuppressants. Despite the fact that nearly 50–70% of SRNS patients will achieve remission with calcineurin inhibitor (CNI) therapy, some patients present with CNI-resistant SRNS, or may exhibit a multidrug-resistant phenotype.6,7 The clinical treatment of these patients is difficult, and the end-stage renal disease (ESRD) free survival rate is low. Approximately 30–40% of SRNS patients progress to ESRD within 10 years, requiring renal replacement therapy.8, 9, 10, 11, 12
At present, SRNS is believed to have a monogenic or immune etiology. With the improvement of molecular diagnostic techniques, more data suggest that the genotype can determine the clinical efficacy of different treatments and can be used to determine the prognosis of these children. It has become apparent that up to 30% of patients with SRNS may have genetic mutations. To date, more than 80 causative genes have been identified, resulting in defects in the podocyte slit diaphragm, actin cytoskeleton, lysosomal proteins, mitochondrial proteins, nuclear transcription factors and/or the glomerular basement membrane.13, 14, 15, 16, 17, 18, 19 Evaluation of genes by panel sequencing is limited to approximately 30 genes. However, whole exome sequencing allows for the evaluation of all genes, including those that may phenocopy steroid-resistant nephrotic syndrome and provide the opportunity for novel gene discovery.13 Individuals with monogenic diseases usually present with refractory nephropathy and more rapidly progress to renal failure, but the condition does not recur after renal transplantation. In contrast, a high risk of post-transplantation recurrence is found in patients without any identified genetic alterations.20,21 It is hypothesized that these patients have circulating immunological factors, including systemic factors (e.g., suPAR) and podocyte-related factors (e.g., ANGPTL4), that can act on the podocyte or basement membrane and disrupt glomerular permeability.22, 23, 24, 25, 26
The clinical manifestations of CNS or genetically-associated nephropathy are often similar to INS. In addition, NS is sometimes the only evident clinical sign during the early stages of disease. These genetic nephropathies are often misclassified and treated with steroids. Therefore, further exploration of the association between the genetic phenotypes and clinical phenotypes in patients with NS of different etiologies is critical for early clinical guidance on the optimal diagnosis and treatment strategies. Of note, the clinical and genetic phenotypes of NS in children vary in different regions and ethnic groups.27 Previous studies lacked large samples and detailed analyses of the associations between genetic and clinical phenotypes. Our present study evaluated the utility of employed whole-exome sequencing (WES) in a bidirectional, multicenter clinical cohort with different clinical types of NS. This study was conducive to exploring the pathogenesis of refractory nephropathy from the perspective of genetics, and for identifying the causative gene mutation sites. We hope that the information presented here will help others achieve a more accurate diagnosis and prognostic evaluation, reduce the risk of post-transplantation recurrence, ensure individualization of treatment strategies, and provide better information for genetic counseling.
Methods
Patient cohort
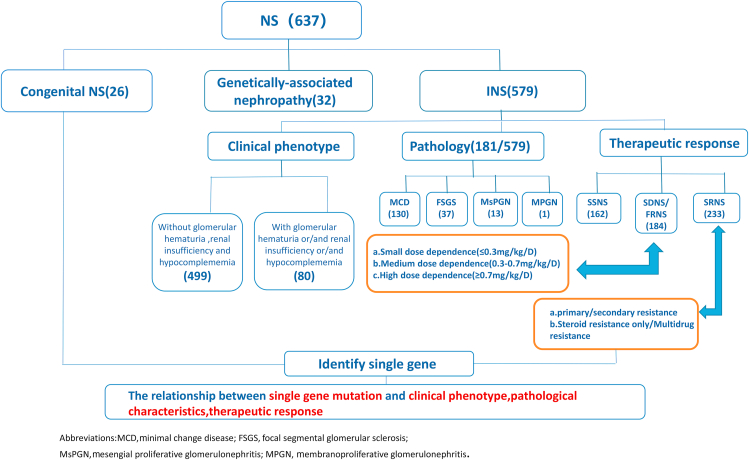
The study recruited patients from the Children's Hospital affiliated with Chongqing Medical University, ShenZhen Children's Hospital, and Chengdu Women's and Children's Central Hospital from January 2010 to June 2020. There were a total of 637 patients with nephrotic syndrome enrolled in the study (Fig. 1).28 The inclusion criteria were: onset of symptoms before 18 years of age and a clinical diagnosis of NS or nephrotic range proteinuria with kidney histology suggesting focal segmental glomerulosclerosis (FSGS), minimal change disease (MCD) or diffuse mesangial sclerosis. The exclusion criteria were: (1) evidence of clinical, laboratory, or kidney biopsy signs of secondary nephrotic syndrome (e.g., systemic lupus erythematosus, Henoch-Schonlein purpura nephritis, anti-neutrophil cytoplasmic antibodies-associated nephritis, or hepatitis B virus-associated nephritis); (2) untraceable parents' DNA.
Figure 1.
Flowchart for the selection of 637 patients included in the study.
Demographic information, the family history, laboratory data, biopsy results, medication use, recurrence and therapeutic responses were retrospectively collected from inpatient records, routine outpatient examinations and the patient's family self-test reports. After obtaining the consent of the children and written informed consent from their parents, WES was performed in all patients after the clinical phenotypes were determined (a 2–3 ml blood sample was required), and blood samples were also collected during the follow-up. The study protocol was approved by the Ethics Committee of the Children's Hospital of Chongqing Medical University (No. 2018–95) and the study was registered in the Chinese Clinical Trial Registry (http://www.chictr.org.cn/, ChiCTR2000029210).
The WES testing procedure is detailed in Supplement 1.
Definitions
Nephrotic syndrome was defined as the presence of edema with protein excretion >40 mg/m2 per h or a urine protein:creatinine ratio ≥2000 mg/g (≥200 mg/mmol) or >3+ proteinuria on dipstick with serum albumin <2.5 g/dL (25 g/L). FRNS was defined as ≥2 relapses within 6 months of an initial response or ≥4 relapses in any 12-month period. SDNS was defined by 2 consecutive relapses occurring while weaning to alternate day steroids or within 2 weeks of steroid discontinuation. SRNS was indicated by persistent proteinuria despite treatment with 60 mg/m2 or 2 mg/kg of a steroid for 4 weeks, after ensuring that there was no infection or non-adherence to the treatment. Secondary resistance was diagnosed in children with initial steroid sensitivity who in subsequent relapses developed SRNS. Presumed steroid resistance was diagnosed in those affected by CNS or genetic nephropathies, regardless of whether they had received treatment with steroids. CNI-resistant SRNS was considered to be present in those without at least a partial remission after 6 months of treatment with a CNI at an adequate dose and/or level. Multidrug resistance was diagnosed in patients without a complete remission after 12 months of treatment with 2 mechanistically distinct steroid-sparing agents (including CNIs) administered at standard doses. CNS was diagnosed in patients with NS with an age of onset younger than 3 months, except for those with intrauterine infections.1
A complete remission was defined by a urine protein-to-creatinine ratio <200 mg/g, a urine albumin-to-creatinine ratio <30 mg/g, or negative/trace dipstick proteinuria during therapy. A partial remission was defined as a urine protein-to-creatinine ratio <200 mg/g or a dipstick ≥1+ but with a plasma albumin >2.5 g/dL1,3.
Podocytopathy genes were identified in the Online Mendelian Inheritance in Man (OMIM; https://www.omim.org) as causing “nephrotic syndrome” or “FSGS”. Phenocopy genes were those identified in the OMIM as causing a syndromic disorder, with nephrotic syndrome being only one among many other clinical signs or even not mentioned at all.13,14
Statistical analyses
Most of the analyses are of a descriptive nature. Continuous variables are presented as median with interquartile range. Categorical variables are presented as number and percentage. Statistical analyses were performed using the SPSS 26.0 software. For group comparison of categorical variables, the Chi-square test or Fisher's exact t test was used.
Kaplan–Meier curves were used to determine the renal survival time. A P-value less than 0.05 was considered significant.
Results
Patient characteristics
A total of 637 patients affected by NS were selected for analysis. The baseline characteristics of the study population are shown in Supplement 2. Among the 637 patients, there were 26 patients with CNS, 32 patients with genetically-associated nephropathy, 162 patients with SSNS, 184 patients with SDNS/FRNS, and 233 patients with idiopathic SRNS.
We detected a causative mutation in 22 of the 26 (84.6%) CNS patients (10 NPHS1, 8 WT1, 3 NPHS2, 1 LAMB2). None of the patients had pathological data. Most families chose to abandon active treatment after the diagnosis. Six CNS patients were lost during the follow-up. CNS patients had the worst outcome; 30.0% (n = 6/20) of the children died and the renal survival rate was only 35.0% (n = 7/20 patients) by the end of the 3.7-year follow-up.
Genetically-associated nephropathy was identified in 32 patients based on the clinical assessment and genetic findings. There were 17 patients with Alport syndrome, 5 patients with Dent disease, 3 patients with oculocerebrorenal syndrome of Lowe, 3 patients with atypical hemolytic uremic syndrome (aHUS), 3 patients with C3 glomerulonephritis, and 1 patient with Fabry disease. The majority (71.9%) of the patients with genetically-associated nephropathy had glomerular hematuria and/or renal insufficiency and/or hypocomplementemia at the onset. Fifteen of these patients showed extrarenal symptoms (e.g., sensorineural hearing loss, ocular abnormalities, developmental retardation, or hemolytic anemia). Moreover, 53.1% (n = 17/32) of the patients had a family history of kidney disease. As expected, this group had the highest detection rate of causative mutations (96.9%).
Minimal change disease was the most common histological pattern in all INS groups. However, 38.5% (n = 37/96) of idiopathic SRNS patients showed FSGS in the histological findings, making renal biopsy valuable for distinguishing patients belonging to different clinical groups. None of the SSNS patients showed pathogenic variants on genetic analysis. Only one of the SDNS/FRNS patients had a detected pathogenic gene (NPHS1 compound heterozygous mutation), which was manifested by high-dose steroid dependence. By contrast, of the 233 idiopathic SRNS patients, 70 (30.0%) patients had monogenic disease. During the 4.2-year follow up, 16 idiopathic SRNS patients progressed to ESRD, and 15 of them were single-gene cases.
Monogenic patients at different ages
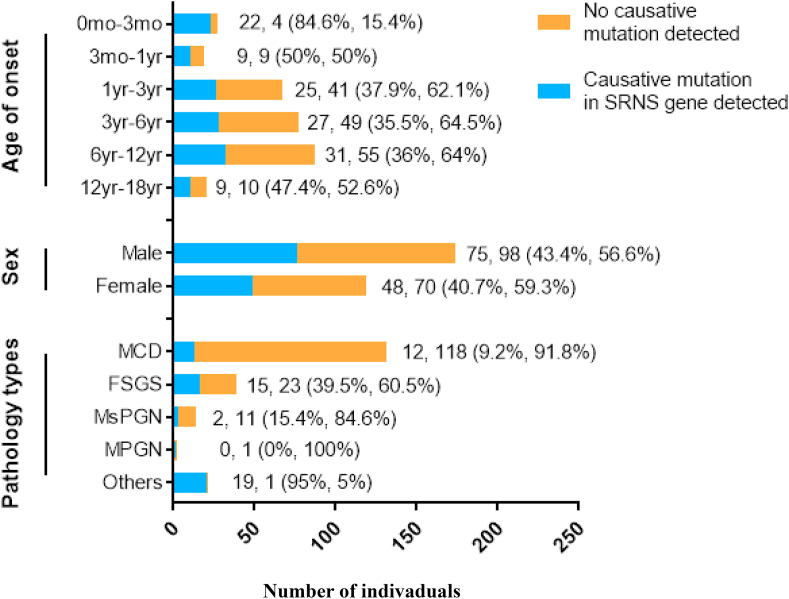
In this study, almost all of the patients with monogenic disease were presumed steroid resistant and had idiopathic SRNS, and the total positive rate of causative mutations was 42.3% (n = 123/291). We analyzed the age and sex characteristics of gene mutations in these two groups (Fig. 2) and found that there was no significant difference in causative mutations detected between the sexes (OR, 0.957; 95%CI, 0.791–1.158; P = 0.65). The detection rate of causative genes was highest in children younger than 3 months old (CNS), at 84.6%. After 3 months of age, the chance of identifying a genetic cause decreased to 50% between the ages of 3 months and 1 year, 37.9% between 1 and 3 years, 35.5% between 3 and 6 years, 36.0% between 6 and 12 years, and 47.4% in those aged 12–18 years. Other than the CNS patients, there was no significant difference in the detection rate among the other age groups.
Figure 2.
Proportion of individuals with causative mutations in presumed steroid resistant and idiopathic SRNS patients, divided by sex, age and pathology.
Monogenic patients with different histopathological findings
A total of 202 children had renal biopsy results available (no biopsy data were available for 435 patients) (Fig. 2). Monogenic disease was seen in 39.5% of the FSGS cases (n = 15/38, 39.5%), 9.2% of those with MCD (n = 12/130), 15.4% of patients with mesangial proliferative glomerulonephritis (MsPGN) (n = 2/13), none of the patients with membranoproliferative glomerulonephritis (MPGN) and in almost all patients with other diseases (Others, n = 19/20). The pathological changes in the Others group included 14 cases of Alport syndrome, 2 of aHUS, 2 of C3 glomerulonephritis and 1 case of Fabry disease. The difference in the mutation detection rate among groups with different pathological changes was statistically significant (Fisher exact test, P < 0.001 across groups).
Causative mutations in patients with idiopathic SRNS
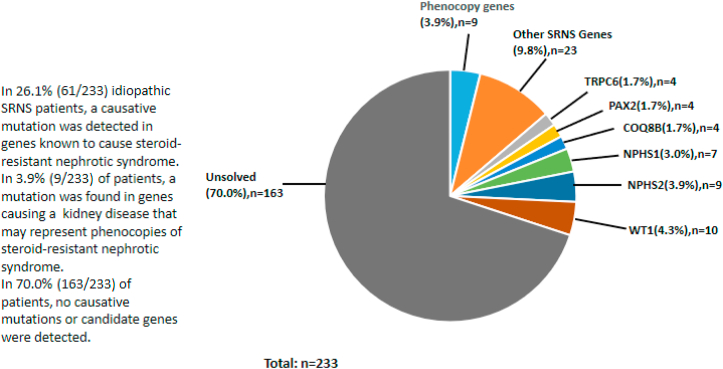
Causative mutations were detected in 30.0% of idiopathic SRNS patients (n = 70/233). Among the identified mutations, 61 were known “nephrotic syndrome” or “FSGS” genes. A list of the pathogenic variants detected is shown in Table 1. Mutations were also found in 9 phenocopy genes, specifically LAGE3, COL4A5, COL4A3, ANKS6, FAT1, and FN1. The proportions of gene distribution in idiopathic SRNS patients can be found in Figure 3. WT1 (n = 10), NPHS2 (n = 9), and NPHS1 (n = 7) were the most commonly mutated genes, containing 37.1% of all mutations identified.
Table 1.
Summary of the whole-exome sequencing in idiopathic SRNS patients.
| WES results (n = 70) | Gene | Inheritance | Disease | OMIM | Case number |
|---|---|---|---|---|---|
| Podocytopathies (n = 61) | WT1 | AD | Nephrotic syndrome, type 4 | 256370 | 10 |
| NPHS2 | AR | Nephrotic syndrome, type 2 | 600995 | 9 | |
| NPHS1 | AR | Nephrotic syndrome, type 1 | 256300 | 7 | |
| TRPC6 | AD | FSGS2 | 603965 | 4 | |
| PAX2 | AD | FSGS7 | 616002 | 4 | |
| COQ8B | AR | Nephrotic syndrome, type 9 | 615567 | 4 | |
| LAMB2 | AR | Nephrotic syndrome, type 5 | 614199 | 3 | |
| PLCE1 | AR | Nephrotic syndrome, type 3 | 610725 | 3 | |
| INF2 | AD | FSGS5 | 613237 | 2 | |
| MAGI2 | AR | Nephrotic syndrome, type 15 | 617609 | 2 | |
| PTPRO | AR | Nephrotic syndrome, type 6 | 614196 | 2 | |
| CRB2 | AR | FSGS9 | 616220 | 2 | |
| ACTN4 | AD | FSGS 1 | 603278 | 2 | |
| AVIL | AR | Nephrotic syndrome, type 21 | 618594 | 1 | |
| NUP107 | AR | Nephrotic syndrome, type 11 | 616730 | 1 | |
| TBC1D8B | XR | Nephrotic syndrome, type 20 | 301028 | 1 | |
| ANLN | AD | FSGS8 | 616032 | 1 | |
| NUP205 | AR | Nephrotic syndrome, type 13 | 616893 | 1 | |
| MYO1E | AR | FSGS6 | 614131 | 1 | |
| CD2AP | AR/AD | FSGS3 | 607832 | 1 | |
| Phenocopies (n = 9) | LAGE3 | AR | Galloway-Mowat syndrome | 614748 | 2 |
| COL4A5 | XR | Alport syndrome/FSGS | 303630 | 2 | |
| COL4A3 | AR | Alport syndrome/FSGS | 120070 | 2 | |
| ANKS6 | AR | Nephronophthisis | 615382 | 1 | |
| FAT1 | AR | FAT1-related glomerulotubular nephropathy | 600976 | 1 | |
| FN1 | AD | Fibronectin glomerulopathy | 135600 | 1 |
All postwhole-exome sequencing diagnoses are defined according to OMIM nomenclature (https://www.omim.org).
Figure 3.
The proportion of gene distribution in idiopathic SRNS.
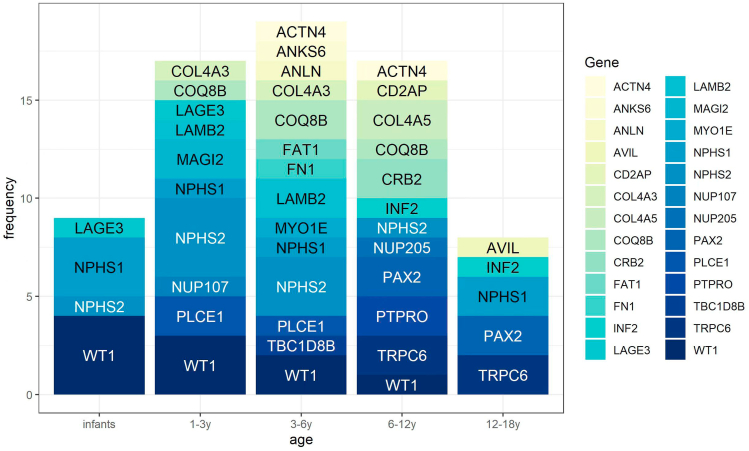
The distribution of causative mutations in each age group of idiopathic SRNS patients is shown in Figure 4. As expected, the majority of patients with WT1 and NPHS1 mutations showed clinical signs in infancy. However, it was previously noted that mutations in these two genes can sometimes cause disease in older children, and this was also true in our study. NPHS2 variants were the main cause of monogenic idiopathic SRNS in children aged 1–6 years old. In our study cohort, the age of onset for patients with MAGI2 and PLCE1 gene mutations was mainly concentrated in early childhood. In contrast, all patients with INF2, TRPC6, PAX2, CRB2 and AVIL mutations were over 6 years old.
Figure 4.
The distribution of causitive mutation genes in different age groups in idiopathic SRNS.
Characteristics of patients with NPHS1, NPHS2 and WT1 mutations
NPHS1, NPHS2 and WT1 were the most commonly mutated genes in our cohort, and these were the causative mutations in almost all cases of monogenic CNS (n = 21) and 37.1% (n = 26/70) of the cases of idiopathic SRNS with monogenic disease. We also compared the characteristics of these three groups (Supplement 3). The median ages of onset for patients with NPHS1, NPHS2 and WT1 mutations were 18, 36, and 14 months respectively. Patients with mutant NPHS2 had a significantly later onset than the other two groups (P < 0.001 across groups). The majority of individuals with mutations of the NPHS1 or NPHS2 genes had complex heterozygous mutations, while only one NPHS2 homozygous mutation was identified. In contrast, single heterozygous mutations were found in all WT1 patients. Notably, 38.9% (n = 7/18) of patients with WT1 mutations showed specific extrarenal involvement (sex reversal/urogenital abnormalities or tumors). Those symptoms were either present at the onset or appeared later on during follow-up. None of the patients with NPHS1 or NPHS2 mutations had extrarenal manifestations. Furthermore, the median renal survival time was 19 months for patients with NPHS1 mutations, 17 months for those with NPHS2 mutations, and 11 months for those with WT1 mutations. Thus, the WT1 patients showed a worse prognosis and progressed to ESRD more quickly (P < 0.001 across groups).
Characteristics of patients with monogenic idiopathic SRNS
The characteristics of idiopathic SRNS patients grouped by genetic results are shown in Table 2. There was no significant difference in the age of onset, sex or clinical classification between the patients with and without a monogenic cause of disease (P > 0.05). Most patients had FSGS (n = 37/96, 38.5%) or MCD (n = 51/96, 53.1%) on biopsy. All of the patients were treated with intensified immunosuppression in the early stage. The median follow-up time was 4.2 years (range, 1–9 years). A total of 98.6% (n = 69/70) of the monogenic patients showed primary resistance, which was significantly higher than in the group without an identified causative gene mutation (n = 129/163, 79.1%) (OR, 0.80, 95%CI, 0.74–0.87, P < 0.001). Only one patient with a NPHS1 compound heterozygous mutation initially presented with high-dose SDNS, then developed secondary resistance during the follow-up. Moreover, with regard to the pattern of steroid resistance, the rate of primary resistance in children with a single gene mutation was 34.8% (n = 69/198), while the gene mutation rate was only 2.9% (n = 1/35) in patients with secondary resistance. Of note, the mutation rate was as high as 71.4% (n = 70/98) in children with multidrug resistance. As expected, complete remission occurred solely in patients without genetic mutations (n = 106/163, 65.0%) (after changing to CNIs, 5 patients who did not respond to the initial treatment with mycophenolate mofetil (MMF) or cyclophosphamide (CTX) achieved a complete response). In contrast, 68.6% (n = 48/70) of the patients with monogenic mutations did not have any response to multiple immunosuppressants, and only 22 patients (31.4%) showed a partial remission. During an average of 4.2 years of follow-up, 99.4% (n = 162/163) of patients without gene mutations had renal survival, while 21.4% (n = 15/70) of the patients with monogenic disease progressed to ESRD (OR, 1.27; 95%CI, 1.12–1.43; P < 0.001).
Table 2.
Summary of the clinical and pathologic characteristics of the patients with idiopathic SRNS included in the study, divided by genetic group.
| Characteristics (n = 233) | Monogenic disease (n = 70) | Genetic-testing negative (n = 163) |
|---|---|---|
| Age at onset,yr | 5.0 ± 3.4 | 5.4 ± 3.1 |
| Sex (male) | 36/70 (51.4%) | 99/163 (60.7%) |
| Clinical classification | ||
| Without glomerular hematuria, renal insufficiency and hypocomplememia | 43/70 (61.4%) | 116/163 (71.2%) |
| With glomerular hematuria or/and renal insufficiency or/and hypocomplememia | 27/70 (38.6%) | 47/163 (28.8%) |
| Histopathological findings | ||
| MCD | 12/28 (42.9%) | 39/68 (57.3%) |
| FSGS | 14/28 (50.0%) | 23/68 (33.8%) |
| MSPGN | 2/28 (7.1%) | 5/68 (7.4%) |
| MPGN | 0/28 (0%) | 1/68 (1.5%) |
| Pattern of resistance | ||
| Primary resistance | 69/70 (98.6%) | 129/163 (79.1%) |
| Secondary resistance | 1/70 (1.4%) | 34/163 (20.9%) |
| Remission | ||
| Complete | ||
| Steroid + TAC/CsA | 0/45 (0%) | 71/98 (72.5%) |
| Steroid + MMF | 0/7 (0%) | 4/18 (22.2%) |
| Steroid + CTX | 0/18 (0%) | 26/47 (55.3%) |
| Partial | ||
| Steroid + TAC/CsA | 16/45 (35.6%) | 12/98 (12.2%) |
| Steroid + MMF | 0/7 (0%) | 8/18 (44.5%) |
| Steroid + CTX | 0/18 (0%) | 11/47 (23.4%) |
| None | ||
| Steroid + TAC/CsA | 29/45 (64.4%) | 15/98 (15.3%) |
| Steroid + MMF | 7/7 (100%) | 6/18 (33.3%) |
| Steroid + CTX | 18/18 (100%) | 10/47 (21.3%) |
| Multidrug resistance | 70/70 (100%) | 28/163 (17.2%) |
| Length of follow-up, yr | 4.3 ± 2.3 | 4.0 ± 2.1 |
| Renal survival rate | 55/70 (78.6%) | 162/163 (99.4%) |
Abbreviations: Tacrolimus (TAC); Cyclophosphamide (CTX); Mycophenolate-mofetil (MMF); Ciclosporin A (CsA).
Continuous variables are presented as median [interquartile range] and categorical variables are presented as n (%).
All of the patients with idiopathic SRNS received intensified immunosuppression and no children were treated with biological agents at the onset. A majority (60.1%; n = 140/233) of the patients received TAC, 27.9% (n = 65/233) received CTX pulse therapy, 10.7% (n = 25/233) received MMF, and 1.3% (n = 3/233) received cyclosporin A (CsA). Of the 163 patients without detected genetic mutations, 47 received CTX, and a complete response occurred in 55.3% of these patients (n = 26/47). Only 22.2% (n = 4/18) of the mutation-negative patients treated with MMF achieved a complete remission. Treatment with TAC/CsA led to the highest complete remission rate (72.5%, n = 71/98) (P < 0.001 across groups). The overwhelming majority of monogenic patients showed no response to any immunosuppressant. Mutidrug resistance was seen in all (100%) of the monogenic patients compared with 17.2% of the patients without identified mutations (OR, 0.17; CI%, 0.12–0.24; P < 0.001, n = 70/70 monogenic patients and n = 28/163 mutation-negative patients).
Final outcomes
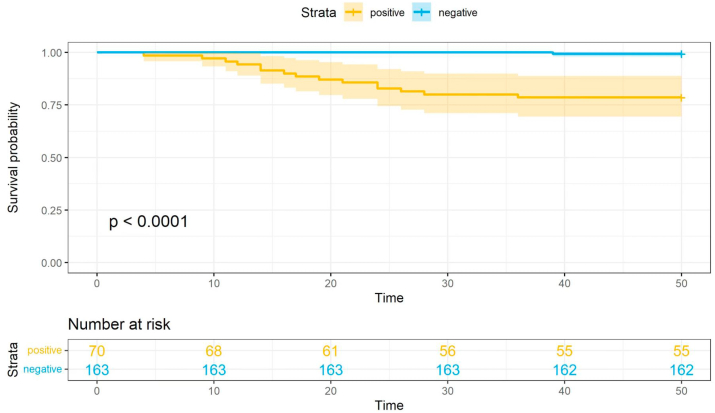
In this study cohort, 9 children died during the follow-up (6 cases of CNS, 2 of idiopathic SRNS, 1 with genetically-associated nephropathy 1) (Supplement 2). The causes of death in patients were severe infection (n = 4), cardiac failure (n = 2), and withdrawal of active treatment (n = 3). At the final follow-up, 30 patients had progressed to ESRD (7 CNS, 7 genetically-associated nephropathy, 16 SRNS) (Supplement 2). Five of the patients with genetically-associated nephropathy had Alport syndrome and 2 had aHUS. The patients with idiopathic SRNS who had monogenic disease also showed a poor outcome, with an average 4.2-year ESRD-free survival rate of 78.6% (Fig. 5).
Figure 5.
Kidney survival in the genetic and genetic mutation negative group of patients.
Among the 16 patients with idiopathic SRNS who progressed to ESRD, the median age at onset was 5.1 years old. Seven of the sixteen (43.8%) were male. Three of these patients already had ESRD at the first visit, and the average time to ESRD in the others was 16 months. Five patients underwent kidney transplantation (2 with a mutation of NPHS2, 2 with a mutation of WT1, and 1 with a mutation of COQ8B), none of them has so far developed a recurrence of their proteinuria. The other 11 children were still on dialysis at the last follow-up (9 on peritoneal dialysis, 2 on hemodialysis). Genetic diagnoses were established in 15 of these patients (93.8%). Mutations in WT1 accounted for 25.0% (n = 4/16) of the identified mutations, while mutations in COQ8B accounted for another 18.9% (n = 3/16) of patients, followed by mutations in PAX2 (n = 2/16, 12.5%), NPHS2 (n = 2/16, 12.5%) and NPHS1 (n = 2/16, 12.5%). The remaining patient had disease attributable to variants of two different genes. Six of the patients had renal biopsy data; all the histopathologic diagnoses were FSGS. Strikingly, patients with FSGS were more likely to progress to ESRD.
Discussion
We herein presented the clinicopathologic and genetic information for pediatric NS, based on 637 pediatric patients enrolled from 3 centers. Our data are from a bidirectional, multicenter cohort and include a large number of patients with NS who underwent full genetic screening based on WES detection in children. Different from previous studies, we first included large numbers of patients with SSNS and SDNS/FRNS, and performed WES detection on all children. This allowed us to have a better understanding of monogenic disease in NS patients with different clinical phenotypes. We also analyzed patients with multidrug-resistant SRNS. Using the data from this cohort, we were able to predict the disease course at an early stage after diagnosis, and provide better options for targeted treatment.
Due to the rarity of CNS, only 26 infants were included in our cohort. Similar to previous studies, the causative genes were concentrated in NPHS1, NPHS2 and WT1.29,30 Hereditary CNS is mainly an autosomal recessive (AR) disease, and this pattern of inheritance was observed for 63.6% of our patients. This means that there is a 25% risk of recurrence in subsequent births for these families. At present, the treatment of hereditary CNS is still challenging, and these children are prone to severe complications. The expert consensus recommends genetic testing as a key diagnostic tool during the early evaluation for these patients. For CNS patients with a definite genetic diagnosis, it provides the basis for their families to choose the optimal treatment methods and makes it possible to receive genetic counseling regarding future offspring. Based on the diagnosis, most of the families of children with CNS chose to give up active treatment due to the low rate of success. Therefore, the CNS group showed the worst outcome in our cohort. However, 32 children with NS were diagnosed with genetically-associated nephropathy after the clinical assessment and genetic testing. In these patients, the extrarenal involvement, syndromic features and family history need to be specifically assessed. The data showed that glomerular hematuria, renal insufficiency and hypocomplementemia were more common in patients with presumed SRNS. Therefore, early genetic testing is recommended for children with unknown NS associated with hematuria, renal insufficiency, hypocomplementemia and extrarenal manifestations.
With regard to the INS patients, none of the SSNS patients showed pathogenic variants in the genetic analysis, only one patient who started with SDNS and later developed secondary resistance was found to have a causative gene, and none of the patients in these two groups progressed to kidney failure (after a median follow-up of 4 years). Therefore, for children with SSNS or SSNS/FRNS, we do not recommend genetic testing to detect monogenic disease. However, previous studies have shown that a genetic locus on chromosome 6p and single nucleotide polymorphisms in HLA-DQA1 and HLADQB1 were substantially associated with SSNS. It was reported that this locus can explain 4.6% of the genetic risk for SSNS.1,31 Thus, for SSNS or SSNS/FRNS patients, genetic testing can also be carried out with the consent of their families, and may be conducive to early accurate typing and prediction of steroid sensitivity in clinical practice.
In a cohort of Boston Children's Hospital, the rate of mutation detection was 25%. NPHS1, PLCE1 and NPHS2 were the most commonly mutated genes, being together responsible for 10.7% of 300 cases.13 In 21 European countries participating in PodoNet, almost one quarter of the patients tested were screened positive for one of the monogenic disorders known to cause SRNS. NPHS2, WT1 and NPHS1 were the most commonly mutated genes.12 In contrast, 30.0% of patients with idiopathic SRNS had monogenic disease, which is consistent with the previously published studies.13,14 Additionally, the 4.2-year ESRD-free survival rate was 93.1% in these patients. Of note, monogenic SRNS had a more rapid progression than non-monogenic disease. During the follow-up, approximately 21.4% of idiopathic SRNS patients with monogenic disease progressed to ESRD compared with only 0.6% in the group without any identifiable mutations. In general, patients with idiopathic SRNS progressed to ESRD later than those with “presumed SRNS”. Moreover, 5 patients underwent renal transplantation and have had no recurrence to date. Thus, identifying the mutations may help to predict the prognosis.
We also compiled valuable information about the age characteristics of patients with monogenic disease. Previous studies have suggested that the detection rate of causative genes is higher in young children.19 This was true for hereditary CNS in our study, but there were no significant differences among the other age groups. However, it is interesting that the distribution of causative genes was different in each age group. NPHS1, NPHS2 and WT1 mutations are predominant in children younger than one-year old. However, for schoolchildren and adolescents, INF2, TRPC6, PAX2, CRB2 and AVIL mutations need more attention.13,14,29
Renal histopathology has been used as a key criterion for determining the diagnosis and prognosis in NS patients. Here, we provided genetic information for patients with different pathological changes. Genetic abnormalities were found in 39.5% of patients with FSGS, 9.2% of patients with MCD, and 15.4% of patients with MsPGN. It has been reported that with the progression of the disease, over a half of MCD or MsPGN patients may convert to FSGS on repeat biopsy. On the basis of this, we recommend active genetic testing in FSGS patients.32,33 Meanwhile, in patients with presumed steroid resistance, the pathological findings can include Alport syndrome, C3 glomerulonephritis, Fabry disease, or even some tubular diseases. This finding underlines the value of renal biopsy in identifying monogenic disease.
Our data indicated that there was a clear association between the genetic phenotype and therapeutic response in idiopathic SRNS. Almost all children with monogenic disease showed primary resistance to steroids and had a very poor response to multiple immunosuppressants. Interestingly, one child with a NPHS1 mutation showed high-dose steroid dependence at the onset, then developed secondary resistance during the follow-up. It has been reported that a milder clinical phenotype may occur in some patients with this mutation.13 A complete response occurred in 65.0% of the patients without any identified mutations. In contrast, none of the patients with monogenic disease achieved a complete remission. Remarkably, 17.2% of patients without evidence of genetic mutations still showed multidrug resistance. It is possible that some of these patients may have monogenic disease attributable to currently undiscovered gene mutations or in circulating factors. We also performed the first analysis of the relationship between multidrug-resistant SRNS and genetic phenotypes. In children with multidrug resistance, the single-gene mutation-positive rate was as high as 71.4%, and 16.3% of the patients with monogenic disease developed ESRD during a 4.3-year follow-up.
All of the patients with idiopathic SRNS received intensified immunosuppressive therapy at the beginning of their treatment. A total of 60.1% received TAC, 27.9% received CTX pulse therapy, 10.7% received MMF, and 1.3% received CsA. The TAC/CsA group showed the highest response rate, with 49.7% of all patients achieving a complete remission. For patients with idiopathic SRNS without any detected mutations, the response rate could be as high as 72.5%. However, MMF was less efficacious than TAC/CsA and CTX in patients with idiopathic SRNS. Notably, there were 16 patients with idiopathic SRNS with a single gene mutation who seemed to respond to TAC because they achieved a partial remission. To date, there is not sufficient evidence of a response to support the use of intensified immunosuppression in patients with idiopathic SRNS with monogenic disease. Prior reports indicated that 5%–35% of patients with a monogenic etiology might show a partial response to therapy with CNIs.34, 35, 36, 37, 38 Whether the long-term prognosis is improved by any of these treatments still needs additional study in expanded cohorts. Regardless, genetic screening might make it possible to guide the use of specific therapies in some patients (such as those with defects in the coenzyme Q10 pathway).39 Therefore, early identification of children with monogenetic diseases can reduce the unnecessary use of immunosuppressants and achieve personalized and precise treatment.
Limitations
There were two main limitations associated with the present study. First, the average follow-up time was approximately 4 years, which was relatively short. Second, the number of children who underwent renal transplantation in this study was small, so the evaluation of the long-term prognosis and postoperative recurrence was limited.
Conclusions
In summary, our present data describe the relationship between clinical phenotypes and genotypes in children with NS due to various etiologies. These data could be valuable in identifying monogenic disease in the early stage, judging the course of disease, developing personalized treatment plans, evaluating the risk of recurrence after kidney transplantation and providing genetic counseling for these patients and their families.
Importantly, we found that patients who were presumed to be steroid-resistant showed more extrarenal involvement, syndromic features and family history, and the positive rate of single gene mutations was high. The age of onset due to different causative genes varied, and FSGS was the most common pathological type in patients with idiopathic SRNS with monogenic disease.
We recommend genetic testing for all children with idiopathic SRNS, especially those with primary resistance. In contrast, we do not recommend WES for patients with SSNS and SDNS/FRNS because the findings will not influence the selection of treatment.
Compared with CTX and MMF, CNIs led to the highest remission rate in patients with idiopathic SRNS. We herein provide the first evidence that the incidence of multidrug resistance in children with monogenic disease is higher than in those without identified mutations, and that these monogenic patients have a more rapid progression to ESRD than SRNS patients without identifiable gene mutations. For these children, conservative treatment is recommended due to the poor overall response to the current treatments.
Remarkably, some of the patients with idiopathic SRNS without identifiable gene mutations still showed multidrug resistance and even progressed to renal failure, which suggests that there might be more unknown genes or circulating factors worthy of further exploration. At the same time, multicenter and prospective intervention cohort studies with a larger sample size are needed in the future to address these possibilities and to more clearly delineate the relationships reported in this study. It will be necessary to integrate the whole genome, epigenetics, the spatial transcriptome, proteome and other -omics to more fully understand pediatric NS with different etiologies. Only then will it be possible to achieve accurate DNA–protein–drug metabolism typing, to prescribe tailored treatments (including immunosuppressants and renal replacement), and to provide the optimized selection of new intervention strategies.
Conflict of interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This cohort study is funded by the China National Natural Science Foundation (No. 81970618), China National Clinical Research Centre Foundation (No. NCRC-2019-GP-02), Chongqing Science and Technology Commission project (No. cstc2016jcyjA0440), Chongqing Science and Technology plan project of Yuzhong District (No. 2017045), Science and Technology Research Project of Chongqing Education Commission (No. KJZD-M201900401), and the central government directs special funds for local science and technology development.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2022.03.023.
Contributor Information
Haiping Yang, Email: oyhp0708@163.com.
Qiu Li, Email: Liqiu809@126.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Noone D.G., Iijima K., Parekh R. Idiopathic nephrotic syndrome in children. Lancet. 2018;392(10141):61–74. doi: 10.1016/S0140-6736(18)30536-1. [DOI] [PubMed] [Google Scholar]
- 2.Cattran D.C., Feehally J., Cook H.T., et al. Kidney disease: improving global outcomes (KDIGO) glomerulonephritis work group. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int. 2012;2(2):139–274. [Google Scholar]
- 3.Trautmann A., Vivarelli M., Samuel S., et al. IPNA clinical practice recommendations for the diagnosis and management of children with steroid-resistant nephrotic syndrome. Pediatr Nephrol. 2020;35(8):1529–1561. doi: 10.1007/s00467-020-04519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dufek S., Ylinen E., Trautmann A., et al. Infants with congenital nephrotic syndrome have comparable outcomes to infants with other renal diseases. Pediatr Nephrol. 2019;34(4):649–655. doi: 10.1007/s00467-018-4122-0. [DOI] [PubMed] [Google Scholar]
- 5.Lombel R.M., Gipson D.S., Hodson E.M. Kidney Disease: improving Global Outcomes. Treatment of steroid-sensitive nephrotic syndrome: new guidelines from KDIGO. Pediatr Nephrol. 2013;28(3):415–426. doi: 10.1007/s00467-012-2310-x. [DOI] [PubMed] [Google Scholar]
- 6.Tullus K., Webb H., Bagga A. Management of steroid-resistant nephrotic syndrome in children and adolescents. Lancet Child Adolesc Health. 2018;2(12):880–890. doi: 10.1016/S2352-4642(18)30283-9. [DOI] [PubMed] [Google Scholar]
- 7.Bagga A., Sinha A. Individualizing treatment of steroid-resistant nephrotic syndrome: registries to the fore. Clin J Am Soc Nephrol. 2020;15(7):920–922. doi: 10.2215/CJN.08080520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trautmann A., Schnaidt S., Lipska-Ziętkiewicz B.S., et al. Long-term outcome of steroid-resistant nephrotic syndrome in children. J Am Soc Nephrol. 2017;28(10):3055–3065. doi: 10.1681/ASN.2016101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y., He Q., Wang Y., et al. A systematic analysis of major susceptible genes in childhood-onset steroid-resistant nephrotic syndrome. Ann Clin Lab Sci. 2019;49(3):330–337. [PubMed] [Google Scholar]
- 10.Gbadegesin R.A., Winn M.P., Smoyer W.E. Genetic testing in nephrotic syndrome--challenges and opportunities. Nat Rev Nephrol. 2013;9(3):179–184. doi: 10.1038/nrneph.2012.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheong H.I. Genetic tests in children with steroid-resistant nephrotic syndrome. Kidney Res Clin Pract. 2020;39(1):7–16. doi: 10.23876/j.krcp.20.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trautmann A., Bodria M., Ozaltin F., et al. Spectrum of steroid-resistant and congenital nephrotic syndrome in children: the PodoNet registry cohort. Clin J Am Soc Nephrol. 2015;10(4):592–600. doi: 10.2215/CJN.06260614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warejko J.K., Tan W., Daga A., et al. Whole exome sequencing of patients with steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol. 2018;13(1):53–62. doi: 10.2215/CJN.04120417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landini S., Mazzinghi B., Becherucci F., et al. Reverse phenotyping after whole-exome sequencing in steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol. 2020;15(1):89–100. doi: 10.2215/CJN.06060519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giglio S., Provenzano A., Mazzinghi B., et al. Heterogeneous genetic alterations in sporadic nephrotic syndrome associate with resistance to immunosuppression. J Am Soc Nephrol. 2015;26(1):230–236. doi: 10.1681/ASN.2013111155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadowski C.E., Lovric S., Ashraf S., et al. A single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndrome. J Am Soc Nephrol. 2015;26(6):1279–1289. doi: 10.1681/ASN.2014050489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lovric S., Ashraf S., Tan W., Hildebrandt F. Genetic testing in steroid-resistant nephrotic syndrome: when and how? Nephrol Dial Transplant. 2016;31(11):1802–1813. doi: 10.1093/ndt/gfv355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akchurin O., Reidy K.J. Genetic causes of proteinuria and nephrotic syndrome: impact on podocyte pathobiology. Pediatr Nephrol. 2015;30(2):221–233. doi: 10.1007/s00467-014-2753-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bierzynska A., McCarthy H.J., Soderquest K., et al. Genomic and clinical profiling of a national nephrotic syndrome cohort advocates a precision medicine approach to disease management. Kidney Int. 2017;91(4):937–947. doi: 10.1016/j.kint.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Gallon L., Leventhal J., Skaro A., Kanwar Y., Alvarado A. Resolution of recurrent focal segmental glomerulosclerosis after retransplantation. N Engl J Med. 2012;366(17):1648–1649. doi: 10.1056/NEJMc1202500. [DOI] [PubMed] [Google Scholar]
- 21.Francis A., Trnka P., McTaggart S.J. Long-term outcome of kidney transplantation in recipients with focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2016;11(11):2041–2046. doi: 10.2215/CJN.03060316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bullich G., Domingo-Gallego A., Vargas I., et al. A kidney-disease gene panel allows a comprehensive genetic diagnosis of cystic and glomerular inherited kidney diseases. Kidney Int. 2018;94(2):363–371. doi: 10.1016/j.kint.2018.02.027. [DOI] [PubMed] [Google Scholar]
- 23.Trautmann A., Lipska-Ziętkiewicz B.S., Schaefer F. Exploring the clinical and genetic spectrum of steroid resistant nephrotic syndrome: the PodoNet registry. Front Pediatr. 2018;6:200. doi: 10.3389/fped.2018.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mason A.E., Sen E.S., Bierzynska A., et al. Response to first course of intensified immunosuppression in genetically-stratified steroid resistant nephrotic syndrome. Clin J Am Soc Nephrol. 2020;15(7):983–994. doi: 10.2215/CJN.13371019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joshi S., Andersen R., Jespersen B., Rittig S. Genetics of steroid-resistant nephrotic syndrome: a review of mutation spectrum and suggested approach for genetic testing. Acta Paediatr. 2013;102(9):844–856. doi: 10.1111/apa.12317. [DOI] [PubMed] [Google Scholar]
- 26.McCarthy E.T., Sharma M., Savin V.J. Circulating permeability factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2010;5(11):2115–2121. doi: 10.2215/CJN.03800609. [DOI] [PubMed] [Google Scholar]
- 27.Chanchlani R., Parekh R.S. Ethnic differences in childhood nephrotic syndrome. Front Pediatr. 2016;4:39. doi: 10.3389/fped.2016.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravani P., Bonanni A., Rossi R., Caridi G., Ghiggeri G.M. Anti-CD20 antibodies for idiopathic nephrotic syndrome in children. Clin J Am Soc Nephrol. 2016;11(4):710–720. doi: 10.2215/CJN.08500815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lipska B.S., Iatropoulos P., Maranta R., et al. Genetic screening in adolescents with steroid-resistant nephrotic syndrome. Kidney Int. 2013;84(1):206–213. doi: 10.1038/ki.2013.93. [DOI] [PubMed] [Google Scholar]
- 30.Hinkes B.G., Mucha B., Vlangos C.N., et al. Nephrotic syndrome in the first year of life: two thirds of cases are caused by mutations in 4 genes (NPHS1, NPHS2, WT1, and LAMB2) Pediatrics. 2007;119(4):e907–e919. doi: 10.1542/peds.2006-2164. [DOI] [PubMed] [Google Scholar]
- 31.D'Agati V.D., Kaskel F.J., Falk R.J. Focal segmental glomerulosclerosis. N Engl J Med. 2011;365(25):2398–2411. doi: 10.1056/NEJMra1106556. [DOI] [PubMed] [Google Scholar]
- 32.Yu H., Artomov M., Brähler S., et al. A role for genetic susceptibility in sporadic focal segmental glomerulosclerosis. J Clin Invest. 2016;126(3):1067–1078. doi: 10.1172/JCI82592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Büscher A.K., Beck B.B., Melk A., et al. Rapid response to cyclosporin A and favorable renal outcome in nongenetic versus genetic steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol. 2016;11(2):245–253. doi: 10.2215/CJN.07370715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valverde S., Hernandez A.M., Velasquez L., et al. Efficacy of prednison-tacrolimus vs prednisone-cyclosporine in steroid-resistant nephrotic syndrome. Pediatr Nephrol. 2010;25(9):1804. [Google Scholar]
- 35.Gulati A., Sinha A., Gupta A., et al. Treatment with tacrolimus and prednisolone is preferable to intravenous cyclophosphamide as the initial therapy for children with steroid-resistant nephrotic syndrome. Kidney Int. 2012;82(10):1130–1135. doi: 10.1038/ki.2012.238. [DOI] [PubMed] [Google Scholar]
- 36.Sinha A., Gupta A., Kalaivani M., Hari P., Dinda A.K., Bagga A. Mycophenolate mofetil is inferior to tacrolimus in sustaining remission in children with idiopathic steroid-resistant nephrotic syndrome. Kidney Int. 2017;92(1):248–257. doi: 10.1016/j.kint.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 37.Gbadegesin R.A., Adeyemo A., Webb N.J.A., et al. HLA-DQA1 and PLCG2 are candidate risk loci for childhood-onset steroid-sensitive nephrotic syndrome. J Am Soc Nephrol. 2015;26(7):1701–1710. doi: 10.1681/ASN.2014030247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zagury A., Oliveira A.L., Montalvão J.A., et al. Steroid-resistant idiopathic nephrotic syndrome in children: long-term follow-up and risk factors for end-stage renal disease. J Bras Nefrol. 2013;35(3):191–199. doi: 10.5935/0101-2800.20130031. [DOI] [PubMed] [Google Scholar]
- 39.Starr M.C., Chang I.J., Finn L.S., et al. COQ2 nephropathy: a treatable cause of nephrotic syndrome in children. Pediatr Nephrol. 2018;33(7):1257–1261. doi: 10.1007/s00467-018-3937-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.