Abstract
Annexin A1, a well-known endogenous anti-inflammatory mediator, plays a critical role in a variety of pathological processes. Fibrosis is described by a failure of tissue regeneration and contributes to the development of many diseases. Accumulating evidence supports that Annexin A1 participates in the progression of tissue fibrosis. However, the fundamental mechanisms by which Annexin A1 regulates fibrosis remain elusive, and even the functions of Annexin A1 in fibrotic diseases are still paradoxical. This review focuses on the roles of Annexin A1 in the development of fibrosis of lung, liver, heart, and other tissues, with emphasis on the therapy potential of Annexin A1 in fibrosis, and presents future research interests and directions in fibrotic diseases.
Keywords: Annexin A1, Anti-inflammatory, Fibrosis, Fibrotic diseases, Sequence alignment
Introduction
With a large number of individuals affected, human fibrosis constitutes a major health problem and poses a serious economic burden worldwide.1 Fibrosis is a process of wound reparation which is characterized by the excessive deposition of extracellular matrix (ECM) components in tissues and organs, particularly collagen, fibronectin and alpha smooth muscle actin.2 Fibrotic tissue remodelling often leads to the disruption of normal architecture, ultimately to organ failure,3 which is commonly associated with high morbidity and mortality.4 As a principal cause of mortality, fibrotic diseases can virtually affect every organ system.5 It is usually detected after tissue injury in lung, liver, heart, skin, and eyes.6 Unfortunately, the pathological mechanisms of persistent fibrosis are not fully understood and there are no effective antifibrotic therapies.
Extensive evidence indicates that the progressive fibrotic disease is associated with various etiologies. The examples include, but not limited to, tissue ischemia, infection/inflammation, toxic exposures, inflammation oxidative stress, and endoplasmic reticulum stress.7 Among all the factors, inflammation is known to be a pioneer to fibrosis. A common feature to all fibrotic diseases is the activation of inflammatory wound-healing program that can lead to a temporary excess deposition of ECM components in the affected tissues.5,8 Persistent inflammation results in tissue injury and aberrant repair that promotes prolonged tissue remodeling and subsequent organ dysfunction. Inflammation during acute wound injury releases inflammatory mediators that activate the transition of fibroblasts to myofibroblasts and recruit inflammatory cells.9 Innate immune cells, such as macrophages and neutrophils, release pro-inflammatory factors or pro-fibrotic factors, including transforming growth factor-β (TGF-β), tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1) and matrix metalloproteinases (MMPs), which contribute to ECM deposition and fibrosis.8 In this case, controlling inflammation is a therapeutic strategy to attenuate fibrosis that has attracted much attention.
For the past few years, emerging studies indicates the close links between Annexin A1 and fibrosis. For example, a recent paper reported that Annexin A1 deficiency aggravated kidney injuries and exhibited more severe fibrosis in diabetic mice.10 In another study, the N-terminal-derived peptide of Annexin A1, Ac2-26, acts as an endogenous brake against exaggerated cardiac fibrosis following myocardial infarction.11 Nevertheless, the underlying mechanism involved Annexin A1 in fibrosis remains to be elucidated. Here, we gathered the knowledge of Annexin A1, presented some of the novel experimental findings in different fibrotic organs or tissues, and discussed the potential mechanisms between Annexin A1 and fibrosis. Further discussion focuses on whether Annexin A1 can be used as a therapeutic target to facilitate the development of broadly effective antifibrotic drugs.
Overview of the annexins family
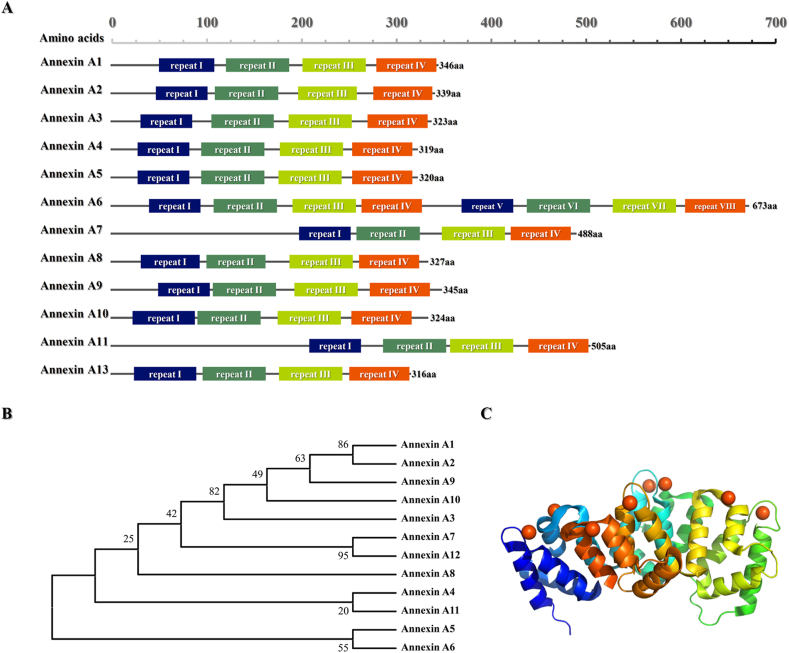
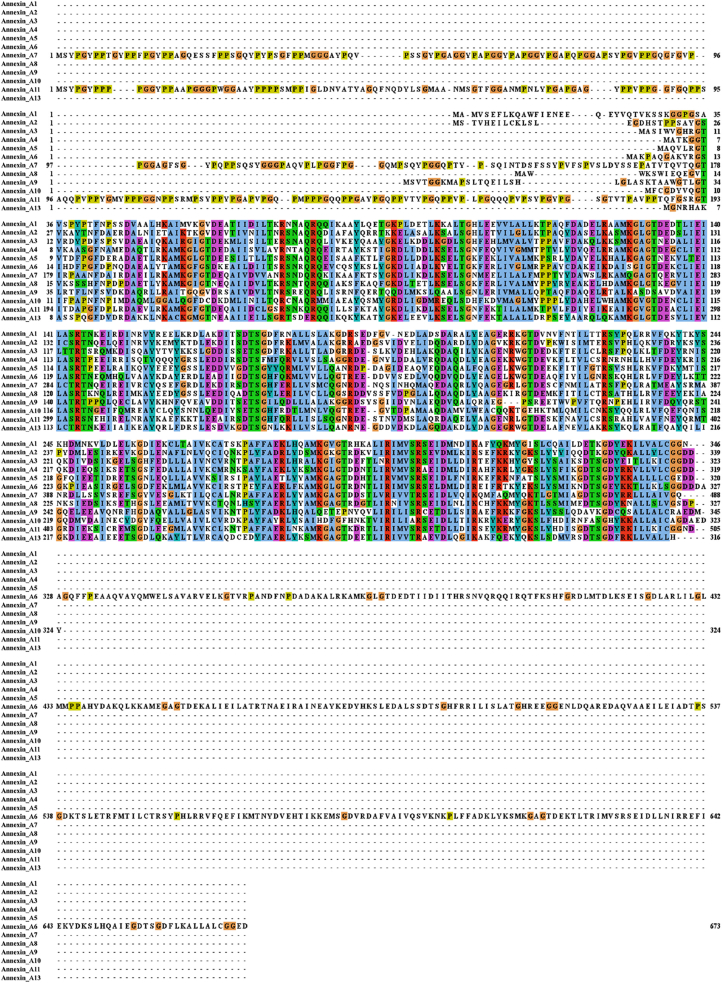
Annexins are an evolutionary conserved superfamily of ubiquitous calcium and phospholipid binding proteins connected to various membrane-related events and cellular functions. Since Creutz's group in 1978 first discovered and purified annexin isolated from bovine adrenal glands,12 more than 1000 different members have been identified so far.13 The protein family was initially called lipocortins, chromobindins, calcimedins, calpactins or synexins. Currently it is mostly known as annexins because of they can annex to the phospholipid membrane of the cell.14 They are widely distributed in various tissues and organs of eucaryotes, and highly expressed in immune cell, such as neutrophils, monocytes, eosinophils and plasma cells.13,15 Although they are usually localized inside the cell, extracellular annexins can be secreted and may bind to the extracellular surface of plasma membrane.16 All annexins share structurally similarities, they consist of two distinct domains, N-terminal and C-terminal domains (Fig. 1A, B). Among the different members of annexins superfamily, the C-terminal domain is relatively conserved (Fig. 2). It is usually made up of four repetitive sequences (except Annexin A6), each of which contains about 70 amino acids, considered to represent a Ca2+ regulated membrane binding module that enables the interaction with negatively charged phospholipids.17,18 The N-terminus in all kinds members of annexins are significantly distinctive in length and sequence, which is unique for a given member and is closely related to their specific biological function. The N-terminus is concealed within the C-terminus in the absence of calcium, whereas the presence of calcium, the N-terminus is exposed because of conformational change.19
Figure 1.
The profile of Annexin A family. (A) Schematic overview of Annexins. The long columns of different colors represent Annexin repeats. (B) The Neighbor-Joining phylogenetic tree of Annexin proteins family was performed by using MEGA software vs 11.0.8. (C) The three-dimensional structure diagrams of the one monomer of full-length human Annexin A1 in the presence of calcium ions (PDB code: 1MCX). Bound calcium ions are illustrated as orange spheres. 3D-structures are visualized with the PyMOL software vs. 2.24.6.
Figure 2.
Protein sequence alignment of Annexin A family members. Multiple sequences alignment of Annexin A1-13 proteins was performed by using Jalview software vs 2.11.1.4.
Annexin A1
Considered as the initial member of the Annexin A superfamily (12 in total), Annexin A1 is a 37 kDa Ca2+-regulated phospholipid-binding protein (Fig. 1C). Like the other members, Annexin A1 possesses all the structural characteristics of its siblings, i.e. a conserved C-terminal domain and a relatively unique N-terminal domain. It was originally studied in neutrophils, where it accounts for 2%–4% of total cytoplasmic proteins.20 Then, several investigations had discovered that Annexin A1 is widely expressed in a variety of cells, such as leukocytes, lymphocytes, epithelial cells, and endothelial cells. Numerous groups have reported the existence of three subcellular localizations of Annexin A1, such as cytoplasmic, nuclear and plasma membrane.20 Substantial reports have provided solid evidences that the formyl peptide receptor family (FPRs) is the receptor of Annexin A1, and FPRs/lipoxin A4 receptor (ALX) might serve as a promising therapeutic target. FPRs activated by Annexin A1 initiates a cascade of signaling events, such as mitogen-activated protein kinases (MAPK), phospholipase A2 and phospholipase D.21 The complex role of Annexin A1 could be associated with different phosphorylations.22
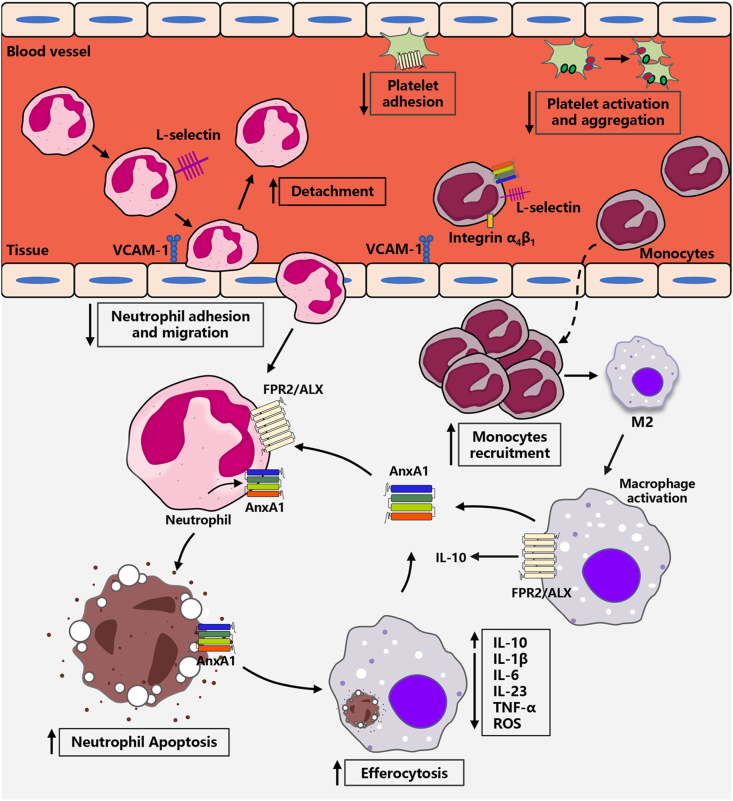
Annexin A1 plays different roles in physiological and pathological states. Firstly, Annexin A1 contributes to the cell membrane formation and rearrangement of the actin cytoskeleton, therefore it may be involved in biological processes that related to cellular membranes.23 Secondly, as a potent endogenous anti-inflammatory protein, Annexin A1 mediates the anti-inflammatory effect (Fig. 3) by i) decreasing adhesion and migration of leukocytes. During the inflammatory responses, transmigration and recruitment of leukocytes (neutrophils and monocytes) pass through blood vessels to the injured site.24 Annexin A1 decreases leukocytes accumulation,25 and adhesion of neutrophils to endothelial cells.26 Neutrophil-derived reactive oxygen species (ROS) generation directly damages the coronary vascular endothelium.27 Annexin A1 or Ac2-26 administration to mice injected with zymosan markedly reduces the degree of neutrophils adhesion and emigration, and promotes detachment of neutrophils adherent to the endothelium.28 The phenotypes mentioned above are closely correlated to Annexin A1's regulation of leukocyte-endothelium interactions, which are molecule-based cell communication, including the molecules on leukocytes (e.g., integrins, and L-selectin) and on endothelial cells (e.g., vascular cell adhesion molecule-1 (VCAM-1)).24 Annexin A1 and Ac2–26, Ac9–25 promote L-selectin shedding on neutrophils, which induces detachment of adherent leukocytes from endothelium,29, 30, 31 likely by competing for binding to the α4β1 integrin with VCAM-1.32 ii) promoting neutrophils apoptosis and removal by macrophages. Deferred neutrophils apoptosis and/or the impaired ability of macrophages phagocytic clearance of apoptotic neutrophils contributes to the development of inflammation. Annexin A1 accelerates neutrophils apoptosis by inducing calcium flux and reducing the expression of the anti-apoptosis factor B-cell lymphoma 2.26,33 At the inflammation site, monocytes differentiate into macrophages to remove abnormal cells and debris (i.e., efferocytosis), which prevents necrosis and release of inflammatory factors,27 including IL-1β, IL-6, TNF-α, and ROS. Annexin A1 induces monocytes differentiation into M2a+M2c− like cells, an anti-inflammatory and pro-resolving macrophage phenotype, produces and secretes IL-10.34,35 Interestingly, endogenous Annexin A1 shuttles back and forth between macrophages and neutrophils that reduces inflammation. An early research from David's group demonstrated that cell surface-exposed Annexin A1 mediates the efficient engulfment of apoptotic cells.36 Similarly, Delaney et al discovered Annexin A1 and its derivatives are released by apoptotic cells induced phagocytosis of apoptotic neutrophils by macrophages.37 iii) regulating the platelet function. Platelet activation as a trigger factor for inflammation.38 Senchenkova et al showed that Annexin A1 promoted resolution of inflammation by decreasing platelet adhesion, diminishing platelet activation and aggregate velocity.39
Figure 3.
Schematic representation of Annexin A1 in inhibiting inflammation.
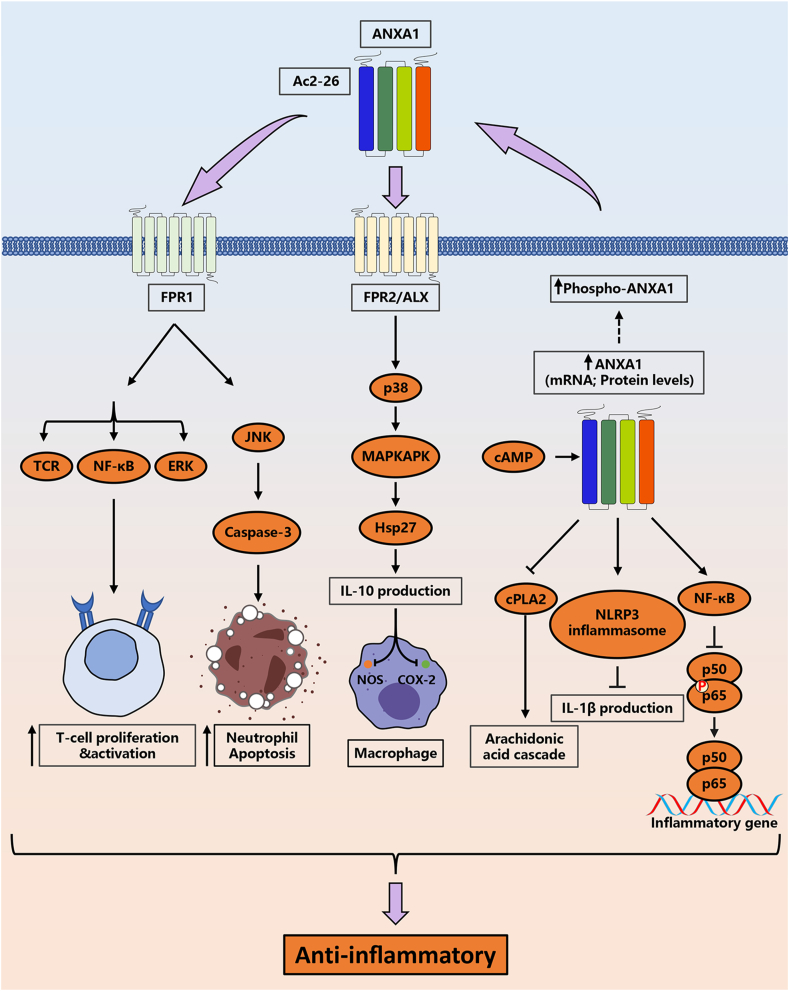
Annexin A1 inhibits inflammatory responses through a variety of signaling pathways (Fig. 4). Lima and colleagues demonstrated that cyclic adenosine monophosphate (cAMP) promoted Annexin A1 expression and its phosphorylation.40 Endogenous Annexin A1 interacts with the transcription factor NF-κB p65 subunit, modulating the inflammatory state by inhibiting its phosphorylation.10 According to Sanches et al, the data suggested that endogenous Annexin A1 attenuated the IL-1β production after nucleotide-binding oligomerization (NOD-), leucine-rich-repeat (LRR-) and pyrin domain-containing protein (NLRP3) inflammasome activation.41 Intracellular Annexin A1 specifically targets cytosolic phospholipase A2 (cPLA2), an initiator enzyme of the arachidonic acid cascade, which resulting in inhibition of cPLA2 activity.42 The phosphorylated Annexin A1 aggregates in the cell membrane and is subsequently externalized. The externalized Annexin A1 drives caspase 3 dependent resolution of neutrophilic inflammation after binding with FPR2/ALXR.40 After binding with FPR1, Annexin A1 exerts their anti-inflammatory effects through regulating the ERK, T cell receptor (TCR) signaling pathways, and NF-κB signaling pathway, which related to the activity, proliferation and differentiation of T cells.43 In addition, peptide Ac2-26 induces neutrophil apoptosis by promoting ALX/FPR1 dimer formation and activating the JNK-caspase-3 pathway.44 Annexin A1 activates the p38/MAPKAPK/Hsp27 signaling to augment production of the anti-inflammatory cytokine IL-10,45 which inhibits the expression of cyclooxygenase-2 (COX-2), prostaglandin E2 (PGE2), and nitric oxide synthase (NOS).46,47
Figure 4.
The signaling pathways associated with the anti-inflammatory effects of Annexin A1 and Ac2-26.
Besides, Annexin A1 is involved in a variety of cellular functions, including cell proliferation, differentiation, apoptosis, autophagy, hormones secretion and regulation of the integrity of the extracellular matrix. Downregulation of Annexin A1 expression by CRISPR/Cas9 inhibited apoptosis via regulation of the IRF3-IFNAR-STAT1-IFIT1 pathway in A549 lung epithelial cells.48 Annexin A1 regulated microglial polarization by modulating IKKα stability via selective autophagy.49 Annexin A1 conferred an added protection against impaired damage stress, which induced DNA damage.50
Although Annexin A1 first appeared as an anti-inflammatory mediator in the late 1970s, it has been demonstrated to be participated in the development of many diseases. For example, Annexin A1 plays a role in tumorigenesis by regulating the process of proliferation,51 differentiation,52 apoptosis,53 chemosensitivity,54 invasion and metastasis.55 Targeting Annexin A1 can abrogate the function of Treg cells and increase the survival rate of patients with triple-negative breast cancer.56 Zhao et al recently showed that Annexin A1 mitigated myocardial ischemia-reperfusion injury by activating signal transducer and activator of transcription 3 (STAT3) signaling pathway to suppress polymorphonuclear neutrophil infiltration and myeloperoxidase activity.57 Purvis et al elegantly suggested that recombinant human Annexin A1 may represent a novel candidate for type-2 diabetes and/or its complications via mediating the RhoA activity, which restores insulin receptor substrate 1 (IRS1) signal transduction.58 As the predominant strategy that treat fibrosis in various organs, anti-inflammatory response gathers multitudinous attention.59 Apart from this immune-system dependent effect, there has been a tremendous infatuation with the idea that Annexin A1 is intimately linked to fibrosis. In recent years, studies had showed that Annexin A1 may take part in many kinds of fibrotic diseases. Details of the relationship between fibrosis and Annexin A1 will be expounded in the next section.
Roles of Annexin A1 in tissue fibrosis
Fibrosis is usually defined as excessive amounts of ECM components deposited in tissues, such as collagen, which is a common pathological outcome of various chronic tissue injury to develop into tissue stiffness and organ failure.60 ECM stiffness can promote tumor cell growth and survival to facilitate invasion and metastasis, and drive malignant transformation.61 The fibrogenic progression is known as a leading cause of morbidity and mortality worldwide.62 However, the mechanisms responsible for leading to tissue fibrosis are still incompletely characterized. Inflammation is a cause of fibrosis, persistent injury and parenchymal cell death provokes tissue inflammation, macrophage activation and immune cell infiltration, the resolution of inflammation is a therapeutic strategy that has gained substantial interest.59
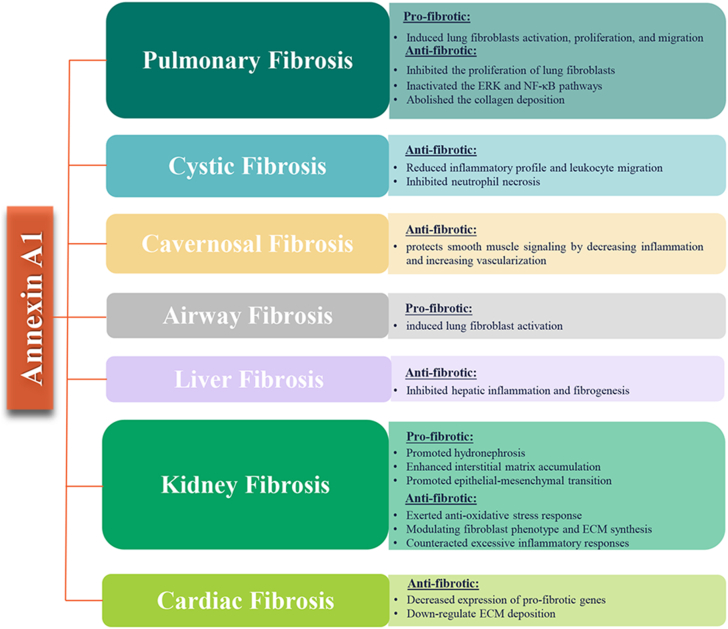
The anti-inflammatory manner of Annexin A1 has been studied for many years in plentiful experimental conditions.25 A growing body of evidences also pointed at anti-fibrosis of Annexin A1, and demonstrated a correlation between Annexin A1 and progression of fibrosis.63, 64, 65, 66 This makes Annexin A1 an accessible target in treating fibrotic conditions. The Annexin A1 full-length protein and its derived molecules might emerge as promising roles in fibrotic diseases, including pulmonary fibrosis, kidney fibrosis, liver fibrosis, cystic fibrosis, cardiac fibrosis.
Annexin A1 and pulmonary fibrosis
Pulmonary fibrosis (PF) is a fatal, devastating and irreversible interstitial disease that the normal lung anatomy is replaced by a process of active remodeling, hyperplasia of fibrous connective tissue in both the fibroblasts and alveolar epithelial cells.67,68 The disease can be idiopathic, called idiopathic pulmonary fibrosis (IPF), which ultimately leading to respiratory failure, lung dysfunction and even death.68 Patients with IPF have a steady increase in both incidence and mortality; the median survival of IPF is approximately 3–5 years after diagnosis.69
In one early study, researchers collected the sera and bronchoalveolar lavage (BAL) fluid of IPF patients to identify the recombinant autoantigens by conducting serological identification of Ags.70 The results showed that Annexin A1 is an autoantigen that elevated both T cell response and antibody production in IPF patients, and the N-terminal of Annexin A1, which have an amino acid sequence QEYVQTVKS and located at 18–26, might play certain roles in the acute exacerbation of IPF. In another study, Jia et al revealed that Annexin A1 silencing using small RNA interfering (siRNA) increased proliferation in lung fibroblasts via activating the ERK and NF-κB pathways, which involved the participation of FPR2.71 Interestingly, studies had focused on the FPR2 on lung fibroblast activation. The ALX/FPR2 antagonist, Tert-butyloxycarbonyl 2 (BOC-2) reverses the beneficial effects of Resolvin D1 in mechanical stretch-induced PF.72 Intratracheal administration of bleomycin-induced animal is the most commonly used models to study mechanisms of pulmonary fibrosis of relevance to IPF.73 The Annexin A1 null mice had been shown to result in exacerbation of lung fibrosis, while the Annexin A1 peptidomimetic Ac2-26 mitigated the most notable fibrotic feature of the animal response to bleomycin.64 The reason of the effect of Annexin A1 in the model may be that it directly alters the phenotype of lung cells, and related to a more aggressive inflammatory reaction. Similar beneficial effects of Ac2-26 on the lung fibrosis were documented in a mouse model of silicosis. The Annexin A1−/− mice that administered silica particles exacerbated fibrotic responses and altered lung function. However, Annexin A1 mimetic peptide, Ac2-26, abolished the fibrosis related variables evoked by silica, including the leukocyte infiltration, collagen deposition, and generation of pro-inflammatory cytokines.74 These results are in contrast to those of Lai et al, who reported that Annexin A1 induced lung fibroblasts activation, proliferation, and migration by upregulating the ERK1/2 and p38 MAPK signaling.75 Further research is warranted in the future to elucidate these controversial outcomes.
Annexin A1 and kidney fibrosis
Kidney fibrosis is the hallmark of any ongoing, chronic kidney injury or maladaptive repair.76 It is considered as the underlying pathological process of chronic kidney disease (CKD), which is highly prevalent around the world.77 Deposition of pathological matrix in kidney promotes functional demise of the nephron and its surrounding vasculature, thus leading to organ failure.78
The accumulation of oxidative stress enhances the production of reactive oxygen species (ROS), which is a common pathway underlying various types of kidney disease and promotes the pathogenesis of renal fibrosis.79 Protein DJ-1 exerts anti-oxidative stress function in renal fibroblasts and epithelial cells administration with the profibrogenic agonist angiotensin II (ANG II) or platelet derived growth factor (PDGF). The Annexin A1 playes an important role in anti-oxidative stress response of DJ-1.80 As Neymeyer et al elegantly reported that cortical fibroblasts that CD73-positive are the principal Annexin A1-expressing cell type in the tubulointerstitium.66 Activation of Annexin A1 exerts antifibrotic effects by modulating fibroblast phenotype and ECM synthesis activity in angiotensin receptor antagonist (ARAnp)-treated rats, which induces fibrosis and end-stage renal disease.66 Progressive diabetic nephropathy (DN) may occur after acute kidney injury due to the failed resolution of inflammation.81 As an endogenous mediator, Annexin A1 counteracts excessive inflammatory responses and stimulates pro-resolving mechanisms.82 A very recent report also suggested that the protein levels of Annexin A1 in kidney cortical tissues significantly elevated in DN patients.10 Overexpression of Annexin A1 or treatment of Ac2-26 in diabetic mice and diabetic Annexin A1 knockout mice had therapeutic potential for mitigating kidney injuries as well as its secondary complications, such as fibrosis. Subsequent mechanism studies had found Annexin A1 modulating the inflammatory state by binding to the transcription factor NF-κB p65 subunit and hindering its activation, which is positively correlated with inflammation and tumorigenesis.10,83 The opposite decisive role of Annexin A1 in kidney fibrosis development and progression has been highlighted in a unilateral ureteral obstruction (UUO) model,84 leading to hydronephrosis and interstitial inflammatory infiltration followed by interstitial matrix accumulation.85 The levels of Annexin A1 were upregulated in urine and obstructed kidneys of UUO 3-week group.84 Similar results were observed that the Annexin A1 is increased in the renal parenchyma after 2 and 8 days following UUO rat model.86 In another study, overexpression of miR-206 that targeting Annexin A1 inhibits epithelial-mesenchymal transition and glomerular interstitial fibrosis by JAK/STAT signaling pathway, and downregulation of miR-206 and upregulation of Annexin A1 correlated with aggressiveness of tumor progression in patients with kidney fibrosis.87
Annexin A1 and liver fibrosis
Liver fibrosis is a pathological condition that activated hepatic stellate cells in the liver and characterized by excessive accumulation of extracellular matrix proteins. The condition is the common outcome of most progressive liver diseases leading to cirrhosis and even liver cancer, and there are no available treatments except liver transplantation.88,89
The contribution of Annexin A1 in fibrosis remodeling during nonalcoholic steatohepatitis (NASH) and liver regeneration had been well documented by Locatelli and coworkers. Annexin A1 selectively produced in liver macrophages inhibited hepatic inflammation and fibrogenesis during NASH progression. They found the enhanced degree of liver fibrosis in methionine and choline-deficient diet (MCD)-fed Annexin A1 knockout mice by stimulation of galectin-3 production. Furthermore, in vitro addition of recombinant Annexin A1 down-regulates M1 polarization through secreting interleukin-10.90 Zagoura et al uncovered that mesenchymal stem/stromal cells secreting Annexin A1 exerted beneficial effects in liver regeneration by inhibiting inflammation and fibrosis.91 These findings delineated a previously unrealized role of Annexin A1 in liver fibrosis.
Annexin A1 and cystic fibrosis
Cystic fibrosis (CF) is a classic life-limiting autosomal recessive genetic disease that caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR), which located on the apical surfaces of luminal epithelia and encoded a cyclic adenosine monophosphate (cAMP)-activated chloride-conducting transmembrane channel.92 It affects multiple organ systems–the lungs, pancreas, upper airways, liver, intestine, and reproductive organs–to varying degrees.93
Annexin A1 is a key protein involved in CF pathogenesis especially in relation to the inflammation. An early study from Tsao's group reported that the level of N-terminal cleaved Annexin A1 was obviously increased in the BAL of cystic fibrosis patients.94 It is intriguing to speculate that the N-terminal degradation of intracellular protein Annexin A1, particularly to a 33 kDa breakdown product, could be used as a sensitive biomarker to measure neutrophil necrosis in lung transplant recipient patients with cystic fibrosis.21 Another early study from Edelman's group demonstrated that a down-regulation of Annexin A1 protein in cystic fibrosis transmembrane conductance regulator knockout (CFTR−/−) mice as well as in epithelial cells of CF patients contributes to the worsening of the CF phenotype.63 The CFTR−/− animals or administration of mice with the CFTR inhibitor-172 (CFTR172) exacerbates the acute peritonitis promoted by zymosan, while these animals treated with hrAnnexin A1 or its peptidomimetic presented reduces inflammatory profile and leukocyte migration of CF.95 Further, Liu et al recently showed that Annexin A1 was the most significantly downregulated and identified to be a promising target of CFTR in the injured tendon tissue of DF508 mice with CF through a 2D gel electrophoresis and mass-spectrometry-based comparative proteomics analysis.96
Annexin A1 and cardiac fibrosis
Cardiac fibrosis is a key hallmark of the progression to heart failure following ischemic insults. Upon ischemic injury, the heart undergoes a dynamic remodeling process that evokes sustained fibrotic response, eventually leading to distorted heart architecture, arrhythmias and cardiac dysfunction.97,98
Several lines of evidence indicated that the pro-resolving Annexin A1 protein acted as an endogenous brake against fibrosis. The cardiac ischaemic injury is constructed in adult Annexin A1−/− mice to investigate the correlation between Annexin A1 and collagen deposition.99 The results showed that Annexin A1−/− mice after myocardial infarction exhibited significantly increased expression of pro-fibrotic genes and ECM deposition.99 Furthermore, in another study by Ritchie et al, the Annexin A1 N-terminal peptide, Ac2-26, blunts the ischemia-reperfusion, thus inducing left ventricular collagen deposition and cardiac fibrosis in vivo.11
Annexin A1 and other fibrotic diseases
An early study from Facio's group, by assessing changes in the intracavernous pressure and karyometry, demonstrated that Annexin A1 effectively protected against the irreversible damage of cavernous tissue through significantly repressing the tissue fibrosis.65 According to Kosicka et al,100 the attenuation of plasma Annexin A1 is found in human obesity. Remarkably, fibrosis is recognized as the major pathological change during the development of obesity and a kind of obesity-related metabolic disorders.101,102 It raises the possibility that targeting Annexin A1 may be benefit for the treatment of obesity-related metabolic diseases. Another study also revealed that Annexin A1 may be involved in airway fibrosis of chronic obstructive pulmonary disease (COPD).75 These findings might give rise to new therapeutic strategy for fibrosis related diseases through upregulating the Annexin A1 level.
Therapy potential of Annexin A1 in fibrosis
As mentioned above, there are different roles of Annexin A1 in fibrosis. The conflicting results in the same organ or tissue should be taken into serious consideration. There are a variety of reasons for the paradoxical results. Firstly, the discrepancy may be interpreted by differences in the fibrotic grade. Under the onset of the injury or mild stress conditions, upregulating of endogenous Annexin A1 might exert antifibrotic effects, and elevated expression was detected. Under extremely severe stress or the anaphase of fibrosis, the expression of Annexin A1 is reduced due to the inability of the damaged tissue to produce the protein properly. Secondly, it does not accurately reflect the true state of Annexin A1 in the body by using recombinant proteins or peptides, and the proteins derived from different expression methods and the different concentrations used in the different cell or animal models may also affect the results. Finally, the discrete and obvious differences in the individual characteristics of different fibrosis may lead to different outcomes in the experiment.
The results of several reports conflicted with each other, but most of the data collected so far showed that the levels of Annexin A1 were down-regulated in the pathologic process of several fibrotic organs.10,63,66,71,74 In most cases, enhancement/promotion of Annexin A1 signaling may be advantageous. A large body of literature have indicated that recombinant Annexin A1 or its N-terminal derived peptide was used to treat different inflammation-related diseases models in vivo. In contrast to annexin A1 protein, which is difficult to manufacture in large quantities, a small peptide derived from the N-terminal region that retains much of biological activity of annexin A1 particularly attractive for pharmacologist.103 So far, there are three analogues of N-terminal derived peptide reported in literature, termed Ac2-26, Ac2-12, and Ac9-25. Among them, Ac2-26 has sparked great interest and intense investigation in pathological conditions,103,104 such as cerebral ischemia-reperfusion injury,105 diabetic nephropathy,106 and pneumococcal meningitis.107 However, only a few studies have shown that Ac2-26 has a therapeutic effect against fibrotic disease.74,108 As a peptide compound, poor stability has limited its clinical application.109 To improve their stability and delay of proteolytic degradation, many technologies have been developed, including isomerization, lipidation, cyclization, glycosylation, terminal modification and multimerization.109 Another strategy to improve their stability and targetability is to design various targeted polymeric nanoparticles (NPs).110 Because Collagen IV (Col IV) is exposed upon injury, Ac2-26 is encapsulated within nanoparticles that targeted type IV collagen to ensure deposition of the peptide in lesions, Nazila and coworkers created Col IV–targeted nanoparticles containing Ac2-26 (Ac2-26 Col IV NPs).111 Then, a series of impressive study from their group suggested that Ac2-26 Col IV NPs have potential for treatment of chronic inflammatory diseases such as atherosclerosis and intestinal inflammation, and promote epithelial wound repair and anastomotic healing.111, 112, 113 Li et al developed a ROS responsive nanotherapy (AON) packaged Ac2-26, which targeted treatment of inflammatory bowel disease through decreasing the expression of proinflammatory mediators, attenuating trafficking and infiltration of inflammatory cells.114 Further reports demonstrated that Annexin A1 mediates its biological effects by the receptor FPR2/ALXR.105,115 With respect to the research progress of FPR2/ALX agonists, we like to redirect the interested readers to comprehensive reviews from Olivier group.116
Concluding remarks and future perspectives
As an endogenous anti-inflammatory mediator, Annexin A1 gathers multitudinous attention both in academia and pharmaceutical industry because of its functional diversity.116 This review summarizes that Annexin A1 is involved in the pathogenesis of fibrosis in different organs, including pulmonary, kidney, liver, and heart (Fig. 5 and Table 1). Is Annexin A1 a novel therapeutic target for fibrosis? Given the amounts of diverse types of information we've collected so far, it seems like a tough question to answer. In most cases, the fibrotic models have shown that Annexin A1 have a beneficial role in fibrotic disease. Hence, it seems to us that Annexin A1 would be a molecular marker of disease development. We suggest applying Annexin A1 agonists for the treatment and intervention of fibrotic disease.
Figure 5.
Annexin A1 and fibrotic diseases.
Table 1.
Overview of Annexin A1 in different types of fibrosis.
| Organ/Tissue | Model | Annexin A1 | Function | References |
|---|---|---|---|---|
| Pulmonary | IPF patients | Up | – | 70 |
| TNF-induced human lung fibroblasts with siRNA | Down | Annexin A1 inhibits TNF-induced proliferation and inflammatory responses | 71 | |
| BLM-induced Annexin A1 null mice | Down | Augmented inflammation and fibrosis | 64 | |
| Silica-induced Annexin A1 KO mice | Down | Exacerbated pathological changes | 74 | |
| Lung fibroblasts with Ac2-26 | Up | Reduced IL-13, MCP-1 and collagen | 74 | |
| COPD patients | Up | Promoted lung fibroblasts proliferation | 75 | |
| Kidney | ARAnp-treated rats | Up | Antifibrotic effects | 66 |
| Cultured renal cortical fibroblasts of Annexin A1 KO mice | Down | Increased a-smooth muscle actin and collage | 66 | |
| DN patients | Up | – | 10 | |
| HFD/STZ-treated Annexin A1 KO mice | Down | Exacerbated kidney injuries and fibrosis | 10 | |
| UUO 3-week | Up | Pro-fibrotic | 84 | |
| UUO 2–8days | Up | Pro-fibrotic | 86 | |
| CKD model | Up | Involve EMT | 87 | |
| Liver | MCD-fed Annexin A1 KO mice | Down | Enhanced degree of liver fibrosis | 90 |
| AF-MSCs and HPL cell | Up | Inhibiting inflammation and fibrosis | 91 | |
| CF patients | Up | – | 94 | |
| Cystic | CFTR−/− mice and epithelial cells of CF patients | Down | Worsening of the CF phenotype | 63 |
| Injured tendon tissue of DF508 mice with cystic fibrosis | Down | Anti-inflammation mediator | 96 | |
| Cardiac | Annexin A1−/− mice after myocardial Infarction | Down | Increased pro-fibrotic genes and ECM deposition | 99 |
ARAnp, angiotensin receptor antagonist; BLM, bleomycin; CF, cystic fibrosis; CFTR, cystic fibrosis transmembrane conductance regulator; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DN, diabetic nephropathy; ECM, extracellular matrix; EMT, epithelial-mesenchymal transition; HFD, high-fat diet; IL-13, interleukin-13; IPF, idiopathic pulmonary fibrosis; KO, knockout; MCD, methionine and choline deficient; MCP-1, monocyte chemoattractant protein-1; STZ, streptozocin; TNF, tumor necrosis factor; UUO, unilateral ureteral obstruction.
The current studies not only shed light on the biological function of Annexin A1 in fibrosis but also provided a basis of understanding of Annexin A1 as potential therapeutic target for fibrosis. Unfortunately, however, there has been very little research about the underlying mechanism between Annexin A1 and fibrosis. What is more, the translation of the Annexin A1 or its peptides into the clinic have yet to be performed. Accordingly, more work in the future is needed to bring insight into the role of Annexin A1 in the mechanism of fibrotic diseases. A profound knowledge of these processes will be crucial to elucidate whether modulating Annexin A1 could provide an ideal therapeutic approach to fibrosis.
Author contributions
Zhibin Yan: Literature search, Writing-Original Draft. Xurui Cheng: Literature search, Manuscript editing. Tao Wang: Manuscript review. Xiangyu Hong: Manuscript review. Gang Shao: Manuscript editing. Caiyun Fu: Conceptualization, Supervision, Funding acquisition. All authors read and approved the final manuscript.
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81770176), the Special Support Plan for Zhejiang Province High-level Talents (No. 2019R52011), the Zhejiang Provincial Natural Science Foundation of China (No. LD22H310004), and the Scientific Research Foundation of Zhejiang Sci-Tech University (No. 21042100-Y).
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Rosenbloom J., Macarak E., Piera-Velazquez S., Jimenez S.A. Human fibrotic diseases: current challenges in fibrosis research. Methods Mol Biol. 2017;1627:1–23. doi: 10.1007/978-1-4939-7113-8_1. [DOI] [PubMed] [Google Scholar]
- 2.Zhu H., Zhao H., Xu S., et al. Sennoside A alleviates inflammatory responses by inhibiting the hypermethylation of SOCS1 in CCl4-induced liver fibrosis. Pharmacol Res. 2021;174:105926. doi: 10.1016/j.phrs.2021.105926. [DOI] [PubMed] [Google Scholar]
- 3.Peng L., Agogo G.O., Guo J., Yan M. Substance P and fibrotic diseases. Neuropeptides. 2019;76:101941. doi: 10.1016/j.npep.2019.101941. [DOI] [PubMed] [Google Scholar]
- 4.Distler J.H.W., Györfi A.H., Ramanujam M., Whitfield M.L., Königshoff M., Lafyatis R. Shared and distinct mechanisms of fibrosis. Nat Rev Rheumatol. 2019;15(12):705–730. doi: 10.1038/s41584-019-0322-7. [DOI] [PubMed] [Google Scholar]
- 5.Wynn T.A., Ramalingam T.R. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18(7):1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang E., He X., Zeng M. The role of S1P and the related signaling pathway in the development of tissue fibrosis. Front Pharmacol. 2019;9:1504. doi: 10.3389/fphar.2018.01504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu H.H., Chen D.Q., Wang Y.N., et al. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem Biol Interact. 2018;292:76–83. doi: 10.1016/j.cbi.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Shihata W.A., Putra M.R.A., Chin-Dusting J.P.F. Is there a potential therapeutic role for caveolin-1 in fibrosis? Front Pharmacol. 2017;8:567. doi: 10.3389/fphar.2017.00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stone R.C., Chen V., Burgess J., Pannu S., Tomic-Canic M. Genomics of human fibrotic diseases: disordered wound healing response. Int J Mol Sci. 2020;21(22):8590. doi: 10.3390/ijms21228590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu L., Liu C., Chang D.Y., et al. Annexin A1 alleviates kidney injury by promoting the resolution of inflammation in diabetic nephropathy. Kidney Int. 2021;100(1):107–121. doi: 10.1016/j.kint.2021.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin C.X., Rosli S., Deo M., et al. Cardioprotective actions of the annexin-A1 N-terminal peptide, Ac2-26, against myocardial infarction. Front Pharmacol. 2019;10:269. doi: 10.3389/fphar.2019.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creutz C.E., Pazoles C.J., Pollard H.B. Identification and purification of an adrenal medullary protein (synexin) that causes calcium-dependent aggregation of isolated chromaffin granules. J Biol Chem. 1978;253(8):2858–2866. [PubMed] [Google Scholar]
- 13.Xi Y., Ju R., Wang Y. Roles of Annexin A protein family in autophagy regulation and therapy. Biomed Pharmacother. 2020;130:110591. doi: 10.1016/j.biopha.2020.110591. [DOI] [PubMed] [Google Scholar]
- 14.Gerke V., Moss S.E. Annexins: from structure to function. Physiol Rev. 2002;82(2):331–371. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- 15.Laohavisit A., Davies J.M. Annexins. New Phytol. 2011;189(1):40–53. doi: 10.1111/j.1469-8137.2010.03533.x. [DOI] [PubMed] [Google Scholar]
- 16.Mirsaeidi M., Gidfar S., Vu A., Schraufnagel D. Annexins family: insights into their functions and potential role in pathogenesis of sarcoidosis. J Transl Med. 2016;14:89. doi: 10.1186/s12967-016-0843-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grewal T., Rentero C., Enrich C., Wahba M., Raabe C.A., Rescher U. Annexin animal models-from fundamental principles to translational research. Int J Mol Sci. 2021;22(7):3439. doi: 10.3390/ijms22073439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bendix P.M., Simonsen A.C., Florentsen C.D., et al. Interdisciplinary synergy to reveal mechanisms of annexin-mediated plasma membrane shaping and repair. Cells. 2020;9(4):1029. doi: 10.3390/cells9041029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foo S.L., Yap G., Cui J., Lim L.H.K. Annexin-A1 - a blessing or a curse in cancer? Trends Mol Med. 2019;25(4):315–327. doi: 10.1016/j.molmed.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Boudhraa Z., Bouchon B., Viallard C., D'Incan M., Degoul F. Annexin A1 localization and its relevance to cancer. Clin Sci (Lond) 2016;130(4):205–220. doi: 10.1042/CS20150415. [DOI] [PubMed] [Google Scholar]
- 21.D'Acquisto F., Piras G., Rattazzi L. Pro-inflammatory and pathogenic properties of Annexin-A1: the whole is greater than the sum of its parts. Biochem Pharmacol. 2013;85(9):1213–1218. doi: 10.1016/j.bcp.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 22.D'Acunto C.W., Gbelcova H., Festa M., Ruml T. The complex understanding of Annexin A1 phosphorylation. Cell Signal. 2014;26(1):173–178. doi: 10.1016/j.cellsig.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 23.McArthur S., Yazid S., Christian H., et al. Annexin A1 regulates hormone exocytosis through a mechanism involving actin reorganization. FASEB J. 2009;23(11):4000–4010. doi: 10.1096/fj.09-131391. [DOI] [PubMed] [Google Scholar]
- 24.Sugimoto M.A., Vago J.P., Teixeira M.M., Sousa L.P. Annexin A1 and the resolution of inflammation: modulation of neutrophil recruitment, apoptosis, and clearance. J Immunol Res. 2016;2016:8239258. doi: 10.1155/2016/8239258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheikh M.H., Solito E. Annexin A1: uncovering the many talents of an old protein. Int J Mol Sci. 2018;19(4):1045. doi: 10.3390/ijms19041045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruschi M., Petretto A., Vaglio A., Santucci L., Candiano G., Ghiggeri G.M. Annexin A1 and autoimmunity: from basic science to clinical applications. Int J Mol Sci. 2018;19(5):1348. doi: 10.3390/ijms19051348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin C., Yang Y.H., May L., et al. Cardioprotective potential of annexin-A1 mimetics in myocardial infarction. Pharmacol Ther. 2015;148:47–65. doi: 10.1016/j.pharmthera.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Lim L.H., Solito E., Russo-Marie F., Flower R.J., Perretti M. Promoting detachment of neutrophils adherent to murine postcapillary venules to control inflammation: effect of lipocortin 1. Proc Natl Acad Sci USA. 1998;95(24):14535–14539. doi: 10.1073/pnas.95.24.14535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walther A., Riehemann K., Gerke V. A novel ligand of the formyl peptide receptor: annexin I regulates neutrophil extravasation by interacting with the FPR. Mol Cell. 2000;5(5):831–840. doi: 10.1016/s1097-2765(00)80323-8. [DOI] [PubMed] [Google Scholar]
- 30.Hayhoe R.P., Kamal A.M., Solito E., Flower R.J., Cooper D., Perretti M. Annexin 1 and its bioactive peptide inhibit neutrophil-endothelium interactions under flow: indication of distinct receptor involvement. Blood. 2006;107(5):2123–2130. doi: 10.1182/blood-2005-08-3099. [DOI] [PubMed] [Google Scholar]
- 31.Strausbaugh H.J., Rosen S.D. A potential role for annexin 1 as a physiologic mediator of glucocorticoid-induced L-selectin shedding from myeloid cells. J Immunol. 2001;166(10):6294–6300. doi: 10.4049/jimmunol.166.10.6294. [DOI] [PubMed] [Google Scholar]
- 32.Solito E., Romero I.A., Marullo S., Russo-Marie F., Weksler B.B. Annexin 1 binds to U937 monocytic cells and inhibits their adhesion to microvascular endothelium: involvement of the alpha 4 beta 1 integrin. J Immunol. 2000;165(3):1573–1581. doi: 10.4049/jimmunol.165.3.1573. [DOI] [PubMed] [Google Scholar]
- 33.Filep J.G., El Kebir D. Neutrophil apoptosis: a target for enhancing the resolution of inflammation. J Cell Biochem. 2009;108(5):1039–1046. doi: 10.1002/jcb.22351. [DOI] [PubMed] [Google Scholar]
- 34.Li Y., Cai L., Wang H., et al. Pleiotropic regulation of macrophage polarization and tumorigenesis by formyl peptide receptor-2. Oncogene. 2011;30(36):3887–3899. doi: 10.1038/onc.2011.112. [DOI] [PubMed] [Google Scholar]
- 35.Cooray S.N., Gobbetti T., Montero-Melendez T., et al. Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proc Natl Acad Sci USA. 2013;110(45):18232–18237. doi: 10.1073/pnas.1308253110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arur S., Uche U.E., Rezaul K., et al. Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev Cell. 2003;4(4):587–598. doi: 10.1016/s1534-5807(03)00090-x. [DOI] [PubMed] [Google Scholar]
- 37.Scannell M., Flanagan M.B., deStefani A., et al. Annexin-1 and peptide derivatives are released by apoptotic cells and stimulate phagocytosis of apoptotic neutrophils by macrophages. J Immunol. 2007;178(7):4595–4605. doi: 10.4049/jimmunol.178.7.4595. [DOI] [PubMed] [Google Scholar]
- 38.Delaney C., Davizon-Castillo P., Allawzi A., et al. Platelet activation contributes to hypoxia-induced inflammation. Am J Physiol Lung Cell Mol Physiol. 2021;320(3):L413–L421. doi: 10.1152/ajplung.00519.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Senchenkova E.Y., Ansari J., Becker F., et al. Novel role for the AnxA1-Fpr2/ALX signaling axis as a key regulator of platelet function to promote resolution of inflammation. Circulation. 2019;140(4):319–335. doi: 10.1161/CIRCULATIONAHA.118.039345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lima K.M., Vago J.P., Caux T.R., et al. The resolution of acute inflammation induced by cyclic AMP is dependent on annexin A1. J Biol Chem. 2017;292(33):13758–13773. doi: 10.1074/jbc.M117.800391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanches J.M., Branco L.M., Duarte G.H.B., et al. Annexin A1 regulates NLRP3 inflammasome activation and modifies lipid release profile in isolated peritoneal macrophages. Cells. 2020;9(4):926. doi: 10.3390/cells9040926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakaguchi M., Murata H., Sonegawa H., et al. Truncation of annexin A1 is a regulatory lever for linking epidermal growth factor signaling with cytosolic phospholipase A2 in normal and malignant squamous epithelial cells. J Biol Chem. 2007;282(49):35679–35686. doi: 10.1074/jbc.M707538200. [DOI] [PubMed] [Google Scholar]
- 43.Han P.F., Che X.D., Li H.Z., Gao Y.Y., Wei X.C., Li P.C. Annexin A1 involved in the regulation of inflammation and cell signaling pathways. Chin J Traumatol. 2020;23(2):96–101. doi: 10.1016/j.cjtee.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rossi A.G., Sawatzky D.A., Walker A., et al. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat Med. 2006;12(9):1056–1064. doi: 10.1038/nm1468. [DOI] [PubMed] [Google Scholar]
- 45.Filep J.G. Biasing the lipoxin A4/formyl peptide receptor 2 pushes inflammatory resolution. Proc Natl Acad Sci USA. 2013;110(45):18033–18034. doi: 10.1073/pnas.1317798110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mertz P.M., DeWitt D.L., Stetler-Stevenson W.G., Wahl L.M. Interleukin 10 suppression of monocyte prostaglandin H synthase-2. Mechanism of inhibition of prostaglandin-dependent matrix metalloproteinase production. J Biol Chem. 1994;269(33):21322–21329. [PubMed] [Google Scholar]
- 47.Ferlazzo V., D'Agostino P., Milano S., et al. Anti-inflammatory effects of annexin-1: stimulation of IL-10 release and inhibition of nitric oxide synthesis. Int Immunopharmacol. 2003;3(10–11):1363–1369. doi: 10.1016/S1567-5769(03)00133-4. [DOI] [PubMed] [Google Scholar]
- 48.Yap G.L.R., Sachaphibulkij K., Foo S.L., Cui J., Fairhurst A.M., Lim L.H.K. Annexin-A1 promotes RIG-I-dependent signaling and apoptosis via regulation of the IRF3-IFNAR-STAT1-IFIT1 pathway in A549 lung epithelial cells. Cell Death Dis. 2020;11(6):463. doi: 10.1038/s41419-020-2625-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li X., Xia Q., Mao M., et al. Annexin-A1 SUMOylation regulates microglial polarization after cerebral ischemia by modulating IKKα stability via selective autophagy. Sci Adv. 2021;7(4) doi: 10.1126/sciadv.abc5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swa H.L., Blackstock W.P., Lim L.H., Gunaratne J. Quantitative proteomics profiling of murine mammary gland cells unravels impact of annexin-1 on DNA damage response, cell adhesion, and migration. Mol Cell Proteomics. 2012;11(8):381–393. doi: 10.1074/mcp.M111.011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xia W., Zhu J., Wang X., et al. ANXA1 directs Schwann cells proliferation and migration to accelerate nerve regeneration through the FPR2/AMPK pathway. FASEB J. 2020;34(10):13993–14005. doi: 10.1096/fj.202000726RRR. [DOI] [PubMed] [Google Scholar]
- 52.Li C.Y., Cai J.H., Tsai J.J.P., Wang C.C.N. Identification of hub genes associated with development of head and neck squamous cell carcinoma by integrated bioinformatics analysis. Front Oncol. 2020;10:681. doi: 10.3389/fonc.2020.00681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xia Q., Li X., Zhou H., Zheng L., Shi J. S100A11 protects against neuronal cell apoptosis induced by cerebral ischemia via inhibiting the nuclear translocation of annexin A1. Cell Death Dis. 2018;9(6):657. doi: 10.1038/s41419-018-0686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen L., Yuan Y., Kar S., et al. PPARγ ligand-induced Annexin A1 expression determines chemotherapy response via deubiquitination of death domain kinase RIP in triple-negative breast cancers. Mol Cancer Ther. 2017;16(11):2528–2542. doi: 10.1158/1535-7163.MCT-16-0739. [DOI] [PubMed] [Google Scholar]
- 55.Zhu J.F., Huang W., Yi H.M., et al. Annexin A1-suppressed autophagy promotes nasopharyngeal carcinoma cell invasion and metastasis by PI3K/AKT signaling activation. Cell Death Dis. 2018;9(12):1154. doi: 10.1038/s41419-018-1204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bai F., Zhang P., Fu Y., et al. Targeting ANXA1 abrogates Treg-mediated immune suppression in triple-negative breast cancer. J Immunother Cancer. 2020;8(1):e000169. doi: 10.1136/jitc-2019-000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou C., Lin Z., Cao H., et al. Anxa1 in smooth muscle cells protects against acute aortic dissection. Cardiovasc Res. 2022;118(6):1564–1582. doi: 10.1093/cvr/cvab109. [DOI] [PubMed] [Google Scholar]
- 58.Purvis G.S.D., Collino M., Loiola R.A., et al. Identification of AnnexinA1 as an endogenous regulator of RhoA, and its role in the pathophysiology and experimental therapy of type-2 diabetes. Front Immunol. 2019;10:571. doi: 10.3389/fimmu.2019.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Henderson N.C., Rieder F., Wynn T.A. Fibrosis: from mechanisms to medicines. Nature. 2020;587(7835):555–566. doi: 10.1038/s41586-020-2938-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zaiss D.M.W. Amphiregulin as a driver of tissue fibrosis. Am J Transplant. 2020;20(3):631–632. doi: 10.1111/ajt.15743. [DOI] [PubMed] [Google Scholar]
- 61.Piersma B., Hayward M.K., Weaver V.M. Fibrosis and cancer: a strained relationship. Biochim Biophys Acta Rev Cancer. 2020;1873(2):188356. doi: 10.1016/j.bbcan.2020.188356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parola M., Pinzani M. Pathophysiology of organ and tissue fibrosis. Mol Aspects Med. 2019;65:1. doi: 10.1016/j.mam.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 63.Bensalem N., Ventura A.P., Vallée B., et al. Down-regulation of the anti-inflammatory protein annexin A1 in cystic fibrosis knock-out mice and patients. Mol Cell Proteomics. 2005;4(10):1591–1601. doi: 10.1074/mcp.M500019-MCP200. [DOI] [PubMed] [Google Scholar]
- 64.Damazo A.S., Sampaio A.L., Nakata C.M., Flower R.J., Perretti M., Oliani S.M. Endogenous annexin A1 counter-regulates bleomycin-induced lung fibrosis. BMC Immunol. 2011;12:59. doi: 10.1186/1471-2172-12-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Facio F.N., Jr., Burnett A.L. Protective effect of annexin-A1 against irreversible damage to cavernous tissue after cavernous nerve injury in the rat. BJU Int. 2012;110(9):1346–1351. doi: 10.1111/j.1464-410X.2012.11097.x. [DOI] [PubMed] [Google Scholar]
- 66.Neymeyer H., Labes R., Reverte V., et al. Activation of annexin A1 signalling in renal fibroblasts exerts antifibrotic effects. Acta Physiol (Oxf) 2015;215(3):144–158. doi: 10.1111/apha.12586. [DOI] [PubMed] [Google Scholar]
- 67.Yu G., Tzouvelekis A., Wang R., et al. Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nat Med. 2018;24(1):39–49. doi: 10.1038/nm.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang D., Yan Z., Bu L., et al. Protective effect of peptide DR8 on bleomycin-induced pulmonary fibrosis by regulating the TGF-β/MAPK signaling pathway and oxidative stress. Toxicol Appl Pharmacol. 2019;382:114703. doi: 10.1016/j.taap.2019.114703. [DOI] [PubMed] [Google Scholar]
- 69.Luppi F., Kalluri M., Faverio P., Kreuter M., Ferrara G. Idiopathic pulmonary fibrosis beyond the lung: understanding disease mechanisms to improve diagnosis and management. Respir Res. 2021;22(1):109. doi: 10.1186/s12931-021-01711-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kurosu K., Takiguchi Y., Okada O., et al. Identification of annexin 1 as a novel autoantigen in acute exacerbation of idiopathic pulmonary fibrosis. J Immunol. 2008;181(1):756–767. doi: 10.4049/jimmunol.181.1.756. [DOI] [PubMed] [Google Scholar]
- 71.Jia Y., Morand E.F., Song W., Cheng Q., Stewart A., Yang Y.H. Regulation of lung fibroblast activation by annexin A1. J Cell Physiol. 2013;228(2):476–484. doi: 10.1002/jcp.24156. [DOI] [PubMed] [Google Scholar]
- 72.Yang Y., Hu L., Xia H., et al. Resolvin D1 attenuates mechanical stretch-induced pulmonary fibrosis via epithelial-mesenchymal transition. Am J Physiol Lung Cell Mol Physiol. 2019;316(6):L1013–L1024. doi: 10.1152/ajplung.00415.2018. [DOI] [PubMed] [Google Scholar]
- 73.Liu T., De Los Santos F.G., Phan S.H. The bleomycin model of pulmonary fibrosis. Methods Mol Biol. 2017;1627:27–42. doi: 10.1007/978-1-4939-7113-8_2. [DOI] [PubMed] [Google Scholar]
- 74.Trentin P.G., Ferreira T.P., Arantes A.C., et al. Annexin A1 mimetic peptide controls the inflammatory and fibrotic effects of silica particles in mice. Br J Pharmacol. 2015;172(12):3058–3071. doi: 10.1111/bph.13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lai T., Li Y., Mai Z., et al. Annexin A1 is elevated in patients with COPD and affects lung fibroblast function. Int J Chron Obstruct Pulmon Dis. 2018;13:473–486. doi: 10.2147/COPD.S149766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuppe C., Ibrahim M.M., Kranz J., et al. Decoding myofibroblast origins in human kidney fibrosis. Nature. 2021;589(7841):281–286. doi: 10.1038/s41586-020-2941-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Djudjaj S., Boor P. Cellular and molecular mechanisms of kidney fibrosis. Mol Aspects Med. 2019;65:16–36. doi: 10.1016/j.mam.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 78.Leaf I.A., Duffield J.S. What can target kidney fibrosis? Nephrol Dial Transplant. 2017;32(suppl_1):i89–i97. doi: 10.1093/ndt/gfw388. [DOI] [PubMed] [Google Scholar]
- 79.Nezu M., Suzuki N. Roles of Nrf2 in protecting the kidney from oxidative damage. Int J Mol Sci. 2020;21(8):2951. doi: 10.3390/ijms21082951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eltoweissy M., Dihazi G.H., Müller G.A., Asif A.R., Dihazi H. Protein DJ-1 and its anti-oxidative stress function play an important role in renal cell mediated response to profibrotic agents. Mol Biosyst. 2016;12(6):1842–1859. doi: 10.1039/c5mb00887e. [DOI] [PubMed] [Google Scholar]
- 81.Moreno J.A., Gomez-Guerrero C., Mas S., et al. Targeting inflammation in diabetic nephropathy: a tale of hope. Expert Opin Investig Drugs. 2018;27(11):917–930. doi: 10.1080/13543784.2018.1538352. [DOI] [PubMed] [Google Scholar]
- 82.Perucci L.O., Sugimoto M.A., Gomes K.B., Dusse L.M., Teixeira M.M., Sousa L.P. Annexin A1 and specialized proresolving lipid mediators: promoting resolution as a therapeutic strategy in human inflammatory diseases. Expert Opin Ther Targets. 2017;21(9):879–896. doi: 10.1080/14728222.2017.1364363. [DOI] [PubMed] [Google Scholar]
- 83.Zhang T., Ma C., Zhang Z., Zhang H., Hu H. NF-κB signaling in inflammation and cancer. MedComm. 2021;2(4):618–653. doi: 10.1002/mco2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yuan Y., Zhang F., Wu J., Shao C., Gao Y. Urinary candidate biomarker discovery in a rat unilateral ureteral obstruction model. Sci Rep. 2015;5:9314. doi: 10.1038/srep09314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li S., Ghoshal S., Sojoodi M., et al. The farnesoid X receptor agonist EDP-305 reduces interstitial renal fibrosis in a mouse model of unilateral ureteral obstruction. FASEB J. 2019;33(6):7103–7112. doi: 10.1096/fj.201801699R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kypreou K.P., Kavvadas P., Karamessinis P., et al. Altered expression of calreticulin during the development of fibrosis. Proteomics. 2008;8(12):2407–2419. doi: 10.1002/pmic.200700831. [DOI] [PubMed] [Google Scholar]
- 87.Zhao S.Q., Shen Z.C., Gao B.F., Han P. microRNA-206 overexpression inhibits epithelial-mesenchymal transition and glomerulosclerosis in rats with chronic kidney disease by inhibiting JAK/STAT signaling pathway. J Cell Biochem. 2019;120(9):14604–14617. doi: 10.1002/jcb.28722. [DOI] [PubMed] [Google Scholar]
- 88.Kisseleva T., Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2021;18(3):151–166. doi: 10.1038/s41575-020-00372-7. [DOI] [PubMed] [Google Scholar]
- 89.Yan Z., Wang D., An C., et al. The antimicrobial peptide YD attenuates inflammation via miR-155 targeting CASP12 during liver fibrosis. Acta Pharm Sin B. 2021;11(1):100–111. doi: 10.1016/j.apsb.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Locatelli I., Sutti S., Jindal A., et al. Endogenous annexin A1 is a novel protective determinant in nonalcoholic steatohepatitis in mice. Hepatology. 2014;60(2):531–544. doi: 10.1002/hep.27141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zagoura D., Trohatou O., Makridakis M., et al. Functional secretome analysis reveals Annexin-A1 as important paracrine factor derived from fetal mesenchymal stem cells in hepatic regeneration. EBioMedicine. 2019;45:542–552. doi: 10.1016/j.ebiom.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Elborn J.S. Cystic fibrosis. Lancet. 2016;388(10059):2519–2531. doi: 10.1016/S0140-6736(16)00576-6. [DOI] [PubMed] [Google Scholar]
- 93.Naehrig S., Chao C.M., Naehrlich L. Cystic fibrosis. Dtsch Arztebl Int. 2017;114(33–34):564–574. doi: 10.3238/arztebl.2017.0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tsao F.H., Meyer K.C., Chen X., Rosenthal N.S., Hu J. Degradation of annexin I in bronchoalveolar lavage fluid from patients with cystic fibrosis. Am J Respir Cell Mol Biol. 1998;18(1):120–128. doi: 10.1165/ajrcmb.18.1.2808. [DOI] [PubMed] [Google Scholar]
- 95.Dalli J., Rosignoli G., Hayhoe R.P., Edelman A., Perretti M. CFTR inhibition provokes an inflammatory response associated with an imbalance of the annexin A1 pathway. Am J Pathol. 2010;177(1):176–186. doi: 10.2353/ajpath.2010.091149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu Y., Feng L., Wang H., et al. Identification of an anti-inflammation protein, annexin A1, in tendon derived stem cells (TDSCs) of cystic fibrosis mice: a comparative proteomic analysis. Proteomics Clin Appl. 2018;12(6):e1700162. doi: 10.1002/prca.201700162. [DOI] [PubMed] [Google Scholar]
- 97.Ma Z.G., Yuan Y.P., Wu H.M., Zhang X., Tang Q.Z. Cardiac fibrosis: new insights into the pathogenesis. Int J Biol Sci. 2018;14(12):1645–1657. doi: 10.7150/ijbs.28103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Park S., Nguyen N.B., Pezhouman A., Ardehali R. Cardiac fibrosis: potential therapeutic targets. Transl Res. 2019;209:121–137. doi: 10.1016/j.trsl.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Qin C.X., Finlayson S.B., Al-Sharea A., et al. Endogenous annexin-A1 regulates haematopoietic stem cell mobilisation and inflammatory response post myocardial infarction in mice in vivo. Sci Rep. 2017;7(1):16615. doi: 10.1038/s41598-017-16317-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kosicka A., Cunliffe A.D., Mackenzie R., et al. Attenuation of plasma annexin A1 in human obesity. FASEB J. 2013;27(1):368–378. doi: 10.1096/fj.12-213728. [DOI] [PubMed] [Google Scholar]
- 101.Li X., Zhao Y., Chen C., et al. Critical role of matrix metalloproteinase 14 in adipose tissue remodeling during obesity. Mol Cell Biol. 2020;40(8):e00564-19. doi: 10.1128/MCB.00564-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sakashita H., Yamada S., Kinoshita M., Kajikawa T., Iwayama T., Murakami S. Mice lacking PLAP-1/asporin counteracts high fat diet-induced metabolic disorder and alveolar bone loss by controlling adipose tissue expansion. Sci Rep. 2021;11(1):4970. doi: 10.1038/s41598-021-84512-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mui L., Martin C.M., Tschirhart B.J., Feng Q. Therapeutic potential of annexins in sepsis and COVID-19. Front Pharmacol. 2021;12:735472. doi: 10.3389/fphar.2021.735472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gavins F.N., Hickey M.J. Annexin A1 and the regulation of innate and adaptive immunity. Front Immunol. 2012;3:354. doi: 10.3389/fimmu.2012.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu X., Gao W., Li L., et al. Annexin A1 protects against cerebral ischemia-reperfusion injury by modulating microglia/macrophage polarization via FPR2/ALX-dependent AMPK-mTOR pathway. J Neuroinflammation. 2021;18(1):119. doi: 10.1186/s12974-021-02174-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu L., Liu C., Chang D.Y., et al. The attenuation of diabetic nephropathy by annexin A1 via regulation of lipid metabolism through the AMPK/PPARα/CPT1b pathway. Diabetes. 2021;70(10):2192–2203. doi: 10.2337/db21-0050. [DOI] [PubMed] [Google Scholar]
- 107.Rüger M., Kipp E., Schubert N., et al. The formyl peptide receptor agonist Ac2-26 alleviates neuroinflammation in a mouse model of pneumococcal meningitis. J Neuroinflammation. 2020;17(1):325. doi: 10.1186/s12974-020-02006-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ferreira T.P.T., Guimarães F.V., Sá Y.A.P.J., et al. Annexin-A1-derived peptide Ac2-26 suppresses allergic airway inflammation and remodelling in mice. Cells. 2022;11(5):759. doi: 10.3390/cells11050759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang D., Cheng L., Li J., et al. Peptide DR8 analogs alleviate pulmonary fibrosis via suppressing TGF-β1 mediated epithelial-mesenchymal transition and ERK1/2 pathway in vivo and in vitro. Eur J Pharm Sci. 2021;167:106009. doi: 10.1016/j.ejps.2021.106009. [DOI] [PubMed] [Google Scholar]
- 110.Li Y., Zhang W., Zhao R., Zhang X. Advances in oral peptide drug nanoparticles for diabetes mellitus treatment. Bioact Mater. 2022;15:392–408. doi: 10.1016/j.bioactmat.2022.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kamaly N., Fredman G., Subramanian M., et al. Development and in vivo efficacy of targeted polymeric inflammation-resolving nanoparticles. Proc Natl Acad Sci U S A. 2013;110(16):6506–6511. doi: 10.1073/pnas.1303377110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Leoni G., Neumann P.A., Kamaly N., et al. Annexin A1-containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J Clin Invest. 2015;125(3):1215–1227. doi: 10.1172/JCI76693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Reischl S., Lee J.H., Miltschitzky J.R.E., et al. Ac2-26-nanoparticles induce resolution of intestinal inflammation and anastomotic healing via inhibition of NF-κB signaling in a model of perioperative colitis. Inflamm Bowel Dis. 2021;27(9):1379–1393. doi: 10.1093/ibd/izab008. [DOI] [PubMed] [Google Scholar]
- 114.Li C., Zhao Y., Cheng J., et al. A proresolving peptide nanotherapy for site-specific treatment of inflammatory bowel disease by regulating proinflammatory microenvironment and gut microbiota. Adv Sci. 2019;6(18):1900610. doi: 10.1002/advs.201900610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ding Y., Flores J., Klebe D., et al. Annexin A1 attenuates neuroinflammation through FPR2/p38/COX-2 pathway after intracerebral hemorrhage in male mice. J Neurosci Res. 2020;98(1):168–178. doi: 10.1002/jnr.24478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Corminboeuf O., Leroy X. FPR2/ALXR agonists and the resolution of inflammation. J Med Chem. 2015;58(2):537–559. doi: 10.1021/jm501051x. [DOI] [PubMed] [Google Scholar]