Abstract
Vitamin C has recently been identified as an epigenetic regulator by activating ten eleven translocases (TETs), enzymes involved in generating DNA hydroxymethylcytosine (5hmC). Currently we investigated whether high dose Vitamin C promotes neuroprotection through epigenetic modulation of 5hmC, if there are sex-specific differences in outcome, and the therapeutic potential of ascorbate in stroke-related comorbidities in adult mice. Post-stroke treatment with ascorbate (reduced form), but not dehydroascorbate (oxidized form), increased TET3 activity, 5hmC levels and reduced infarct following focal ischemia. Hydroxymethylation DNA immunoprecipitation-sequencing showed that ascorbate increased 5hmC across the genome and specifically in promoters of several stroke pathophysiology-related genes, particularly anti-inflammatory genes. Ascorbate also decreased markers of oxidative stress, mitochondrial fragmentation and apoptosis in cortical peri-infarct neurons and promoted motor and cognitive functional recovery in both sexes via TET3. Furthermore, post-stroke ascorbate treatment reduced infarct volume and improved motor function recovery in aged, hypertensive and diabetic male and female mice. Delayed ascorbate treatment at 6h of reperfusion was still effective at reducing infarct volume and motor impairments in adult mice. Collectively, this study shows that post-stroke treatment with high dose ascorbate protects the brain through epigenetic reprogramming and may function as a robust therapeutic against stroke injury.
Keywords: Cerebral ischemia, oxidized methylcytosine, neuroprotection, hypertension, diabetes
INTRODUCTION
Epigenetics play a major role in the development, progression and pathological outcome of ischemic stroke (1). Modulation of epigenetic modifications, such as DNA methylation and histone acetylation, have previously been shown to reduce secondary brain damage and improve recovery after focal ischemia (2, 3). The 5-hydroxymethylcytosine (5hmC) is a brain-enriched epigenetic mark associated with increased gene expression and neuroprotection in various neurological diseases (4-9). We have recently shown that 5hmC is globally increased in gene regulatory regions involved in neuroprotective pathways following focal ischemia (10). Furthermore, we and others have shown that 5hmC provides endogenous protection against brain injury after stroke (11-13).
In mammals, Vitamin C is essential for collagen synthesis, antioxidant defense, amino acid metabolism and protein synthesis. Vitamin C is highly concentrated in the brain and has been shown to play protective roles in both acute brain injury and neurodegenerative diseases (14, 15). Vitamin C is transported into the brain in its reduced form (ascorbate) by sodium-dependent vitamin C transporter-2 (SVCT-2), which is found in neuron rich areas of the CNS (15, 16). The oxidized form of Vitamin C, dehydroascorbate (DHA), is transported by the ubiquitous glucose transporters (GLUTs), and is rapidly reduced to ascorbate by glutathione and other reductases (Fig. 1A) (17, 18). Since ascorbate scavenges free radicals, its neuroprotective effects have mainly been attributed to antioxidant defense for several decades (19). More recently, ascorbate was identified as an epigenetic regulator through its ability to modulate ten-eleven translocases (TETs), enzymes that convert 5-methylcytosine (5mC) to 5hmC in DNA. Ascorbate acts a cofactor for TETs, and thus increases TET activity and enhances 5hmC levels (Fig. 1A) (20, 21). However, ascorbate as an epigenetic modulator of neuroprotection has not yet been investigated in many neurological diseases, including stroke.
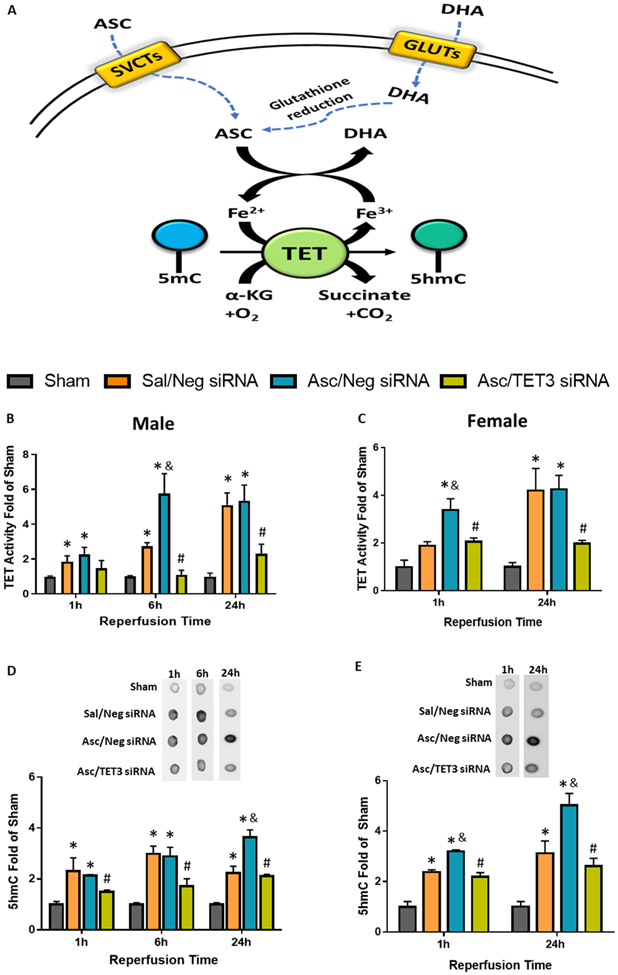
Fig. 1: Ascorbate Increased Post-Stroke 5hmC via TET3.
(A) Vitamin C promotes 5hmC production. Sodium-vitamin C co-transporters (SCVTs) transport ascorbate (ASC; the reduced form of Vitamin C) which acts as a cofactor to stimulate the activity of TETs, dioxygenases that convert methylated DNA (5-methylcytosine; 5mC) to hydroxymethylated DNA (5-hydroxymethylcytosine; 5hmC). Glucose transporters (GLUTs) transport dehydroascorbate (DHA; the oxidized form of Vitamin C) which will be reduced to ASC by glutathione or other reduction agents. (B-E) SiRNA (TET3 or Negative control siRNA) was injected 48h before the induction of MCAO. Saline or ascorbate (500 mg/kg; i.p.) was injected at 30 min reperfusion in adult mice following transient MCAO. (B, C) TET activity at 1h, 6h or 24h reperfusion in male (n=5-7/group) and female (n=3/group) mice following transient MCAO. (D, E) Representative dot blots and quantifications of 5hmC at 1h, 6h or 24h reperfusion in adult male (n=3-6/group) and female mice (n=3/group). p<0.05 compared to sham (*), Sal/Neg siRNA (&), or Asc/Neg siRNA (#) groups by two-way ANOVA (Sidak’s post-test).
Previous studies have indicated that high dose Vitamin C treatment provides protection against stroke-related brain injury (11, 22-25). However, the form of Vitamin C (ascorbate or DHA) and the timing of treatment were important to modulate treatment outcomes. For example, DHA treatment before focal ischemia, or after the induction of permanent ischemia was shown to protect the rodent brain (22-24). However, DHA is dependent on antioxidant defense systems for conversion to ascorbate and consequently DHA accumulates in neurons under conditions of high oxidative stress (Fig. 1A) (26). Thus, DHA injected during the reperfusion phase following focal ischemia where oxidative stress is high, failed to mitigate brain damage in primates (27). Alternatively, ascorbate is active during oxidative stress and has been shown to protect the rodent brain when administered during the reperfusion phase after focal ischemia (11, 25), but the therapeutic effectiveness of ascorbate as a post-stroke therapy has not been assessed.
The ability of ascorbate to act as an epigenetic regulator has never been studied in the post-stroke brain. Thus, we currently investigated whether a major mechanism of ascorbate-mediated protection after stroke is through its ability to induce the epigenetic mark 5hmC in DNA. Furthermore, the Stroke Treatment Academic Industry Roundtable (STAIR) have suggested criteria for rigorous testing of new therapeutic paradigms in preclinical models (28). Hence, we also evaluated the efficacy of ascorbate treatment after stroke by rigorously testing the effect of sex, age and comorbid conditions (type-2 diabetes and hypertension) in adult mice.
METHODS
Focal Ischemia:
All surgical protocols were approved by the Research Animal Resources and Care Committee of the University of Wisconsin-Madison. Mice were cared for in accordance with the Guide for the Care and Use of Laboratory Animals (U.S. Department of Health and Human Services Publication 86-23, revised). Adult (12 to 13 weeks) and aged (64 to 68 weeks) male and female C57Bl/6J mice, BPH/2J mice (hypertensive; 12-13 weeks) and db/db mice (type-2 diabetic; 12-13 weeks) obtained from Jackson Laboratories were used for inducing transient middle cerebral artery occlusion (MCAO) under isoflurane anesthesia by intraluminal suture method using a silicon-coated nylon monofilament (Doccol) as described previously (29). Briefly, the MCA was occluded for 60 min in adult mice, 45 min in aged mice and hypertensive mice, and 40 min in diabetic mice followed by reperfusion. Mortality for each cohort with the indicated occlusion times can be found in Supplementary Table 1. Saline or ascorbate (500mg/kg) or DHA (500mg/kg) was injected i.p. at 30 min or 6h of reperfusion following MCAO. Cohorts of mice were euthanized under isoflurane anesthesia at 1h, 6h, 24h, 48h, 72h, 7 days and 28 days of reperfusion as indicated in the experimental timeline (Supplementary Fig. 1). Sham-operated mice treated with saline served as controls. Mice were randomly assigned to the experimental groups. Ipsilateral peri-infarct cortex (the region directly adjacent to the necrotic ischemic core) from ischemic mice or tissue from similar coordinates in sham mice was dissected as described previously (30).
Motor and Cognitive Function:
Post-ischemic motor function was evaluated by rotarod test (4 min on a cylinder rotating at 8 r/min), beam-walk test (number of foot faults while crossing a 120-cm-long beam) and/or adhesive removal test (time taken to remove a small adhesive sticker placed on each forepaw) at a timepoint between days 1 and 7 of reperfusion as described earlier (31, 32). Neurological score was determined on day one of reperfusion by Modified Neurological Scoring System (MNSS) as described earlier (30) using the following criteria: no neurological deficit observed=0, failure to fully extend right forepaw=1, turning to right=2, circling to right=3, unable to spontaneously walk=4, and death from stroke=5. Post-ischemic cognitive function was analyzed using the Morris water maze test on days 21-25 of reperfusion by examining spatial learning and memory as described previously (32). Escape latency (the duration to reach the platform) was evaluated on 4 consecutive days by placing a mouse into the pool at a different start location and where it was allowed to swim until a hidden platform was located within a maximum period of 60 sec. On the last day of testing, the probe test was conducted with the platform removed and the time spent in the platform quadrant (the quadrant where the platform had previously been located) was recorded. As mice were subjected to focal ischemia, swimming capabilities were confirmed prior to water maze testing. Sham mice did not display observable deficits on any of the behavioral tasks.
Infarct Volume:
Post-ischemic infarct volume was estimated using either triphenyltetrazolium chloride (TTC) staining or cresyl violet staining of the serial brain sections from each mouse as described earlier (33). Sections were scanned and infarct volume was quantified using NIH Image J software and corrected to account for edema using the Swanson formula as described previously (29).
TET3 Knockdown:
Two days before the induction of transient MCAO, a cocktail of three in vivo grade siRNAs (Thermofisher Scientific) targeting non-overlapping regions of TET3 was injected stereotaxically (8 nmol; intracerebral; from bregma −0.2 mm posterior, 1.5 mm dorsoventral, and 3.0 mm lateral) with a Hamilton syringe at 0.5 μl/min as described previously (11, 30). A non-targeting negative control siRNA cocktail was used as a control.
Dot Blot:
DNA was isolated from cortical tissue using an AllPrep DNA/RNA mini kit (Qiagen). DNA (50ng/sample) was blotted onto nitrocellulose membranes, subjected to UV irradiation for 10 min, baked at 80°C for 2h, and blocked with 5% BSA in Tris-buffered saline with 0.1% Tween-20 (TBST). The membranes were probed with antibodies (Active Motif) against 5mC (1:1,000), 5hmC (1: 2,000), 5fC (1:1,000) and 5caC (1:1,000) followed by horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse IgG (1:5,000; Cell Signaling). Chemiluminescence (Life Technologies USA) was used to develop blots on the LI-COR Odyssey Fc platform and quantified with Image Studio software (LI-COR Biotechnology).
Immunohistochemistry:
Mice were euthanized at 1 or 3 days of reperfusion by transcardiac paraformaldehyde perfusion fixation, brains were post-fixed, cryoprotected and sectioned (coronal; 30 μm thick) and immunostained with antibodies against 5hmC (1:500; Active Motif) neuronal nuclear antigen (NeuN; 1:300; Millipore), glial fibrillary acidic protein (GFAP; 1:200; Millipore), transmembrane protein 119 (TMEM119; 1:400; Synaptic Systems), phosphorylated dynamin-related protein-1 (p-Drp1; 1:400; Cell Signaling Technology), 3-nitrotyrosine (3-NT; 1:500; Abcam) and cleaved caspase-3 (1:400; Cell Signaling Technology), as described previously (34). Sections were scanned by Keyence BZX fluorescence microscope (Keyence, USA).
5hmC Sequencing:
DNA samples from the peri-infarct cortex at 24h of reperfusion and sham (n =3/group) were subjected to hydroxymethylation DNA immunoprecipitation-sequencing (hMeDIP-seq) as described previously (Arraystar Inc) (30). Briefly, DNA was fragmented to ~200-800 bp sequences, subjected to Illumina HiSeq 4000 sequencing and libraries were quantified using an Agilent 2100 Bioanalyzer. Clean reads were aligned to Mouse genome (UCSC MM10) and used for peak calling of associated hMeDIP-enriched regions (peaks) with statistically significant peaks identified in each sample using a q-value threshold of 10−4 by MACS v2. The hMeDIP-enriched regions (peaks) were annotated by the nearest gene using the UCSC RefSeq database. diffReps (Cut-off: log2FC_1, p-value 10−4) was used to control for false positives and identify statistically significant DhMRs within promoter regions of the associated genes.
TET Activity Assay:
Nuclear lysates were prepared using a nuclear fractionation kit (Biovision) from the ipsilateral peri-infarct cortical tissue and TET activity was quantified using the TET hydroxylase activity quantification assay (Abcam) as described previously (11). In brief, the amount of hydroxymethylated product formed from the methylated substrate in the presence of the nuclear lysates was quantified using a specific antibody. The ratio of hydroxymethylated product was proportional to TET enzyme activity (colorimetrically measured at an absorbance of 450 nm).
Real-time PCR:
An AllPrep DNA/RNA mini kit (Qiagen) was used to isolate peri-infarct cortical RNA. One μg of total RNA was reverse transcribed into cDNA with the High Capacity cDNA Reverse Transcription kit (Applied Biosystems). The mRNA levels were determined with real-time PCR using the SYBR Green method with gene specific primer pairs (Supplementary Table 2).
Statistics:
Statistical significance between groups was estimated either by Mann Whitney U test (2 groups) or by Kruskal-Wallis test (Dunn’s post hoc) (multiple groups). Two-way ANOVA and repeated measures ANOVA (Sidak’s post hoc) was used to compare differences over time between groups. Mice were randomly assigned to experimental groups. Male and female mice were studied in separate experiments. An investigator blinded to the study groups performed the behavioral and histological analyses.
Data Availability:
Data can be made available upon reasonable request.
RESULTS
Ascorbate induced TET activity and increased 5hmC after stroke
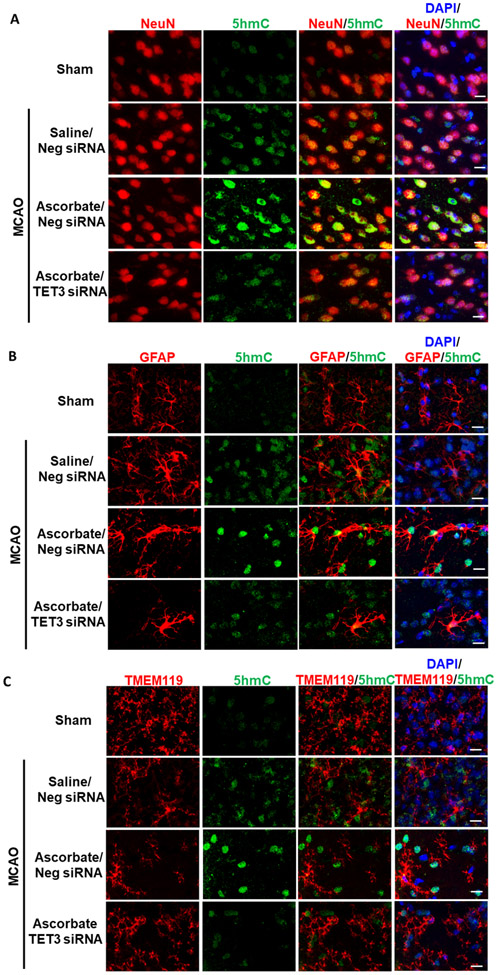
As ascorbate is a TET coactivator (20, 21), we tested the potential role of TET3, the major TET isoform found in the cerebral cortex (35), in ascorbate-mediated TET activation after stroke. To knockdown TET3, we performed intracerebral injection of TET3 siRNA 48h prior to transient MCAO, which results in large cortical spread of the siRNA and ~40% reduction in TET3 expression (30). One hour of transient MCAO increased TET activity and high dose ascorbate treatment (500 mg/kg; i.p. at 30 min of reperfusion) further increased TET activity at 1h of reperfusion in adult female mice and at 6h of reperfusion in adult male mice following transient MCAO (Fig.1B and C) in the peri-infarct cortex (Supplementary Fig. 2). The increases in ascorbate-mediated TET activity were curtailed by TET3 siRNA (Fig.1B and C). Ascorbate treatment had no effect on TET3 expression, which was reduced with TET3 siRNA treatment (Supplementary Fig. 3). Dot blot analysis of isolated DNA showed that transient MCAO increased 5hmC levels, which were further enhanced by ascorbate treatment by 24h reperfusion in both sexes in the peri-infarct cortex (Fig. 1D and E). TET3 siRNA significantly abrogated the ascorbate-induced post-ischemic 5hmC increase in both sexes (Fig. 1D and E). Increased 5hmC was observed to be colocalized with NeuN (mature neuronal nuclear marker), but not GFAP (astrocyte marker) or TMEM119 (microglial marker) in the peri-infarct cortex at 24h of reperfusion following transient MCAO (Fig. 2).
Fig. 2. Ascorbate Increased 5hmC in Neurons of the Post-Ischemic Cortex.
SiRNA (TET3 or Negative control siRNA) was injected 48h before the induction of MCAO. Saline or ascorbate (500 mg/kg; i.p.) was injected at 30 min reperfusion in adult mice following transient MCAO. (A) Increased 5hmC was localized in neurons (NeuN+), (B) but not astrocytes (GFAP+) or (C) microglia (TMEM119+) in the peri-infarct area of the ischemic cortex at 24h reperfusion following transient MCAO (n = 3/group). Scale bar, 15 μm.
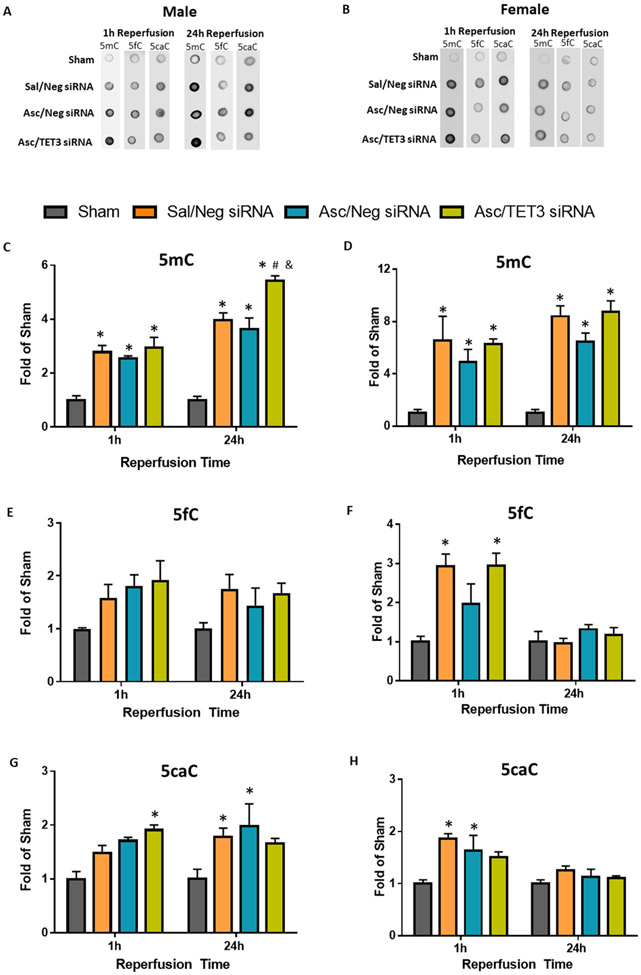
TET enzymes sequentially oxidize 5mC to 5hmC, 5-formylcytosine (5fC) and 5-carboxylcytosine (5caC) (Supplementary Fig.4). The 5fC and 5caC epigenetic marks can then be removed through base excision repair leading to demethylated DNA (Supplementary Fig. 4). DNA dot blot analysis showed global 5mC, 5fC, and 5caC levels were increased in male and female mice at 1h and/or 24h reperfusion following transient MCAO, which were not modulated by ascorbate (Fig. 3). Thus, post-stroke ascorbate treatment specifically modulated global levels of 5hmC, but not 5fC or 5caC.
Fig. 3. Ascorbate Does Not Alter 5mC, 5fC or 5caC.
SiRNA (TET3 or Negative control siRNA) was injected 48h before the induction of MCAO. Saline or ascorbate was injected (i.p.) at 30 min reperfusion in adult mice following transient MCAO. (A, B) Representative dot blots of DNA cytosine methylation and quantifications of (C, D) 5mC, (E, F) 5fC, and (G, H) 5caC at 1h reperfusion or 24h reperfusion in adult male (n=3-6/group) and female mice (n=3/group). p<0.05 compared to sham (*), Sal/Neg siRNA (&), or Asc/Neg siRNA (#) groups by two-way ANOVA (Sidak’s post-test).
Ascorbate treatment is neuroprotective after stroke in both sexes
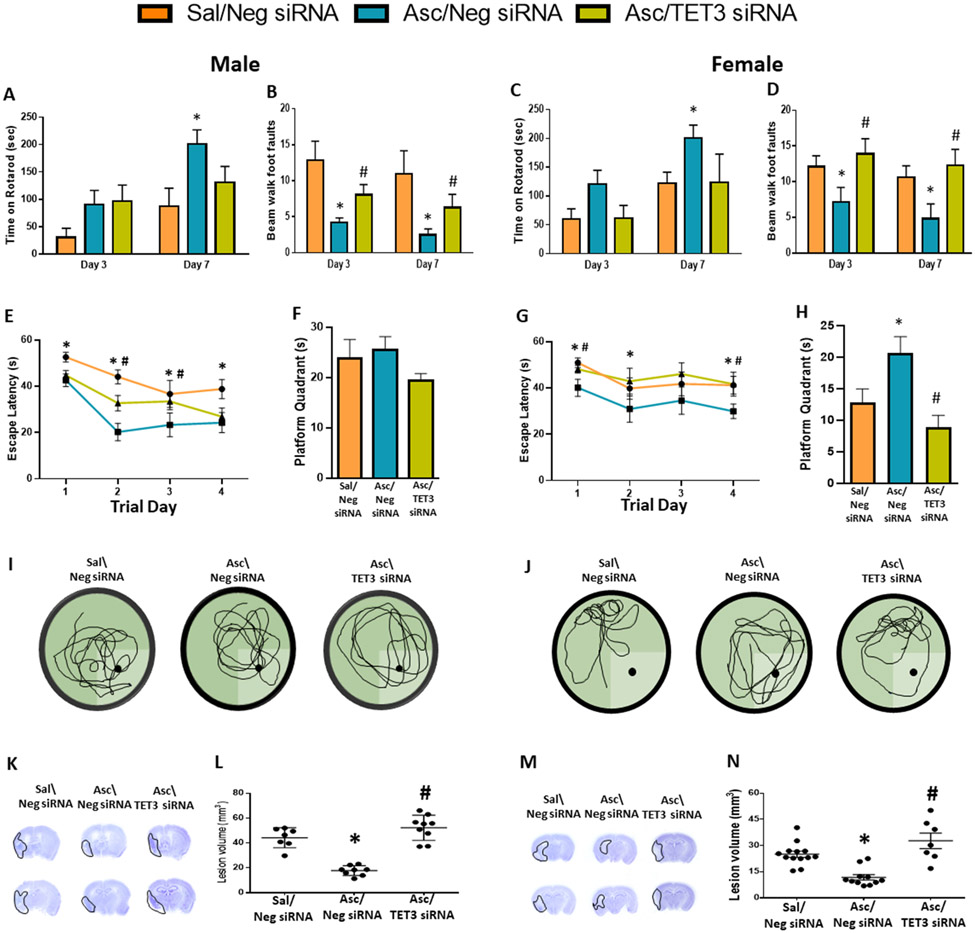
As 5hmC has been shown to be associated with neuroprotection, we next determined whether ascorbate treatment modulates secondary brain damage and functional recovery after stroke through epigenetic modulation of 5hmC. We treated a cohort of adult mice with saline or ascorbate at 30 min reperfusion after transient MCAO. At 3 days of reperfusion, infarct volume was significantly reduced by ascorbate treatment, which was reversed with TET3 inhibition (Supplementary Fig. 5). In another cohort of mice, ascorbate treatment improved motor function on day 3 and day 7 of reperfusion, estimated by rotarod test and beam walk test in both male (Fig. 4A and B) and female mice (Fig. 4C and D) compared with sex-matched saline treated control cohorts. TET3 inhibition reversed the post-stroke motor function improvement on the beam walk test induced by ascorbate (Fig. 4A to D). Cognitive deficits were assessed between days 21 to 25 of reperfusion following transient MCAO using Morris water maze test. Both male and female mice treated with ascorbate at 30 min reperfusion showed improved escape latency (indicates spatial learning) from days 1 to 4 of testing compared to sex-matched saline treated control groups (Fig. 4E and G), but only females stayed in the platform quadrant significantly longer (indicates memory retention) than the saline controls during the probe test on day 4 of testing (Fig. 4F, I, H and J). TET3 knockdown abrogated the ascorbate-mediated improvement in cognitive function (Fig. 4E-J). Both male and female ascorbate-treated mice showed significantly decreased lesion volume compared with the sex-matched saline controls assessed at 28 days of reperfusion after transient MCAO, which was mitigated by TET3 knockdown (Fig. 4K-N).
Fig. 4: Ascorbate Improved Functional Recovery and Ameliorated Cortical Degeneration via TET3.
SiRNA (TET3 or Negative control siRNA) was injected 48h before the induction of MCAO. Saline or ascorbate (500 mg/kg; i.p.) was injected at 30 min reperfusion in adult mice following transient MCAO. (A-D) Recovery of functional motor performance at 3 days and 7 days of reperfusion measured by (A, C) rotarod test and (B, D) beam walk test in male and female adult mice. p<0.05 compared to Sal/Neg siRNA group (*), or Asc/Neg siRNA group (#) by two-way ANOVA (Sidak’s post-test). (E-J) MWM test was performed on days 22-25 of reperfusion. (E, G) Escape latency during training trials. p<0.05 compared to Asc/Neg siRNA vs Sal/Neg siRNA group (*), or Asc/TET3 siRNA vs Asc/Neg siRNA group (#) by repeated-measures ANOVA followed by Sidak’s post-test. (F, H) Time spent in platform quadrant during probe trial. (I, J) Representative trace maps from each treatment group. p<0.05 compared to Sal/Neg siRNA group (*), or Asc/Neg siRNA group (#) by Kruskal-Wallis test (Dunn’s post hoc). (K, M) Representative cresyl violet–stained serial sections (L, N) and lesion volume at 28 days reperfusion. p<0.05 compared to Sal/Neg siRNA group (*), or Asc/Neg siRNA group (#) by Kruskal-Wallis test (Dunn’s post hoc), n= 7-9/group (male) and n=7-13/group (female).
Ascorbate enhanced 5hmC in genes involved in neuroprotection via TET3
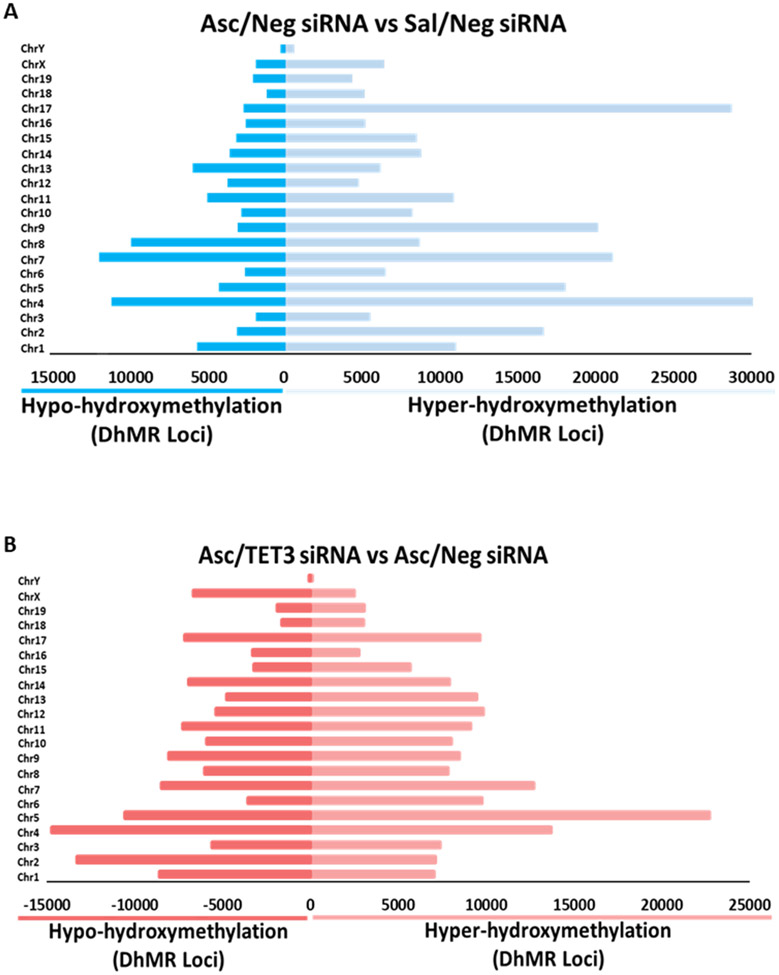
We have previously shown that TET3 modulates 5hmC in both the sham and ischemic peri-infarct cortex (30). To assess the specific genomic regions modulated by the ascorbate-TET3 pathway after stroke, we employed hMeDIP-seq on peri-infarct cortical tissue harvested at 24h reperfusion. Assessments of the differential hydroxymethylated regions (DhMRs) across the genome showed a 29% reduction in hyper-hydroxymethylation in the ascorbate/TET3 siRNA group compared with the ascorbate/Neg siRNA group (Fig. 5A and Supplementary Fig. 6). TET3 inhibition also reduced 5hmC across the genome with a 60% increase in hypo-hydroxymethylation of DhMRs in the ascorbate/TET3 siRNA group compared with the ascorbate/neg siRNA group (Fig. 5B and Supplementary Fig. 6) further confirming our previous experiment that showed 5hmC modulation by the ascorbate-TET3 pathway (Fig. 1).
Fig. 5: Ascorbate Enhanced DhMRs Across the Genome Following Focal Ischemia.
SiRNA (TET3 or Negative control siRNA) was injected 48h before the induction of MCAO. Saline or ascorbate (500 mg/kg; i.p.) was injected at 30 min reperfusion in adult mice following transient MCAO. Significant hyper-hydroxymethylated or hypo-hydroxymethylated DhMR loci group comparisons by chromosome at 24h of reperfusion in peri-infarct cortical tissue in the (A) Ascorbate/Neg siRNA vs Saline/Neg siRNA group and (B) the Asc/TET3 siRNA vs Asc/Neg siRNA group. Statistically significant DhMRs were identified by differential analysis with a cut-off of log2 FC = 1.0, p-value 10−4.
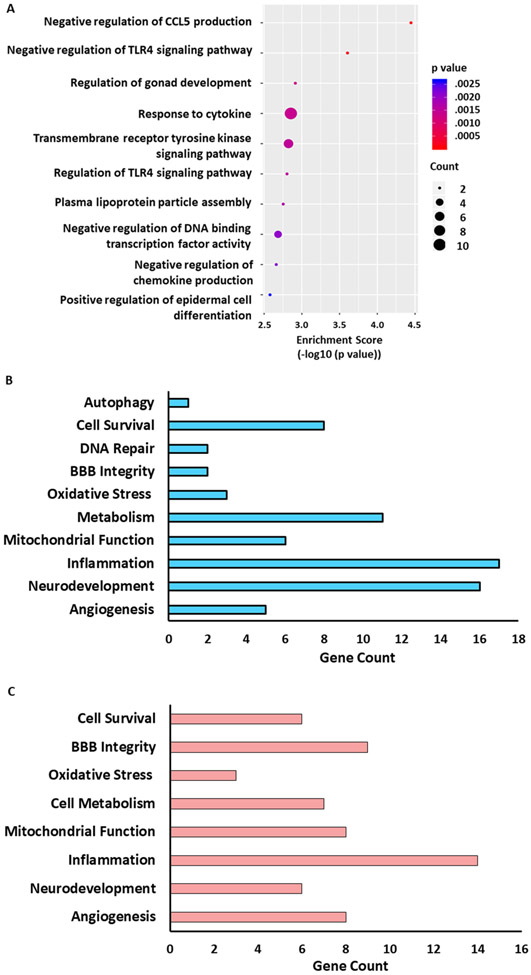
We have previously shown that TET3-modulated 5hmC is associated with genes involved in ischemic neuroprotection (11, 30). Gene ontology (GO) analysis of ascorbate-mediated upregulated DhMRs in protein-coding gene promoters identified hundreds of mRNA genes involved in cellular components (Supplementary Fig. 7), molecular functions (Supplementary Fig. 8) and biological processes (Supplementary Fig. 9). The top ten ascorbate-mediated upregulated DhMRs in biological processes (ranked by enrichment score) included a number of biological processes related to pathological pathways that promote protection in cerebral ischemia (Fig. 6A). For example, the ‘transmembrane tyrosine kinase signaling pathway’, that included fibroblast growth factors genes, which enhance neuronal survival after stroke (36) and the ‘negative regulation of chemokine (C-C motif) ligand 5’ (CCL5; aka RANTES), a chemokine which has been shown to exacerbate ischemic brain damage (37, 38) (Fig. 6A). Interestingly, ascorbate also enhanced DhMRs in the ‘negative regulation of DNA binding transcription factor activity’ biological process. This category included genes such as secreted frizzled-related protein 4 (sFRP4), which is anti-angiogenic and pro-apoptotic (39, 40) and forkhead box h1 (Foxh1) which represses genes involved in vascular formation (41). This indicates ascorbate may be involved in inhibiting the actions of these genes. Further classification of the ascorbate-mediated upregulated DhMRs identified a number of genes related to processes involved in ischemia pathophysiology such as cell survival, oxidative stress, mitochondrial function and angiogenesis (Fig. 6B). TET3 knockdown during ascorbate treatment showed downregulated gene promoter DhMRs in a majority of these pathophysiology-related pathways (Fig. 6C). Many of the ascorbate-TET3 modulated genes involved in these pathways have been previously shown to promote survival and recovery after ischemic injury (Table 1). These include muellerian-inhibiting factor precursor (Amh) that promotes neuroserpin expression, prevents excitotoxicity and reduces infarct volume (42, 43), interferon regulatory factor 8 (Irf8) that attenuates post-ischemic oxidative stress and inflammation (44), the prolactin (Prlr) neuropeptide that lowers edema (45), and platelet-derived growth factor subunit B (Pdfgb) that prevents delayed neuronal death and promotes angiogenesis after cerebral ischemia (46, 47).
Fig. 6: Ascorbate Increased 5hmC in Genes related to Ischemia Pathophysiology via TET3.
SiRNA (TET3 or Negative control siRNA) was injected 48h before the induction of MCAO. Saline or ascorbate (500 mg/kg; i.p.) was injected at 30 min reperfusion in adult mice following transient MCAO. (A) Gene ontological (GO) analysis of top 10 biological processes in the Ascorbate/Neg siRNA vs Saline/Neg siRNA group with hyper-hyroxymethylated DhMRs in pathways ranked by enrichment score (−log10(p value)) at 24h of reperfusion. Statistically significant DhMRs were identified by differential analysis with a cut-off of log2 FC = 1.0, p-value 10−4. The GO biological pathways related to ischemia pathophysiology that are (B) upregulated in the Asc/Neg siRNA versus Sal/Neg siRNA group and (C) downregulated in the Asc/TET3 siRNA group versus Asc/Neg siRNA group at 24h of reperfusion.
Table 1:
Ascorbate/TET3 modulated DhMRs in the promoters of genes putatively involved in cell survival and post-ischemic outcome.
| Gene | Putative function |
|---|---|
| AMH | Hormone, promotes neuroserpin expression and reduces infarct volume after cerebral ischemia |
| IRF8 | Transcription factor, attenuates neuronal apoptosis oxidative stress and inflammation after ischemia |
| INSR | Insulin receptor, mediates signaling that improves neuronal survival |
| LANCL | Glutathione transferase, protects neurons against oxidative stress and prevents apoptosis. |
| PRLR | Neuropeptide, attenuates edema and reduces infarct |
| SFRP4 | Binding protein, decreases pro-apoptotic proteins following ischemia |
| SH3BP5L | Mitochondrial membrane protein, provides endogenous protection in neuronal cells |
| DGAT1 | Metabolic enzyme, reduces inflammation, lipid toxicity and improves recovery after ischemia in cardiomyocytes |
| GFRA4 | Cell receptor, promotes neuronal survival through PI-3 kinase/AKT activation which prevents apoptosis |
| PPPR14A | Phosphatase, expression associated with neuroprotection after stroke |
| TPT1 | Prevents apoptosis, DNA damage and promotes survival following oxidative stress |
| SLA2 | Adapter protein, immune regulator that provides endogenous protection against focal cerebral ischemia |
| Kcnk16 | Potassium channel, may be important in brain repair after cerebral ischemia |
| TRHR | Hormone receptor, signaling prevents oxidative stress, apoptosis and is neuroprotective following ischemia |
| PDGFB | Growth factor, promotes collateralization and reduces oxidative stress following cerebral ischemia |
AMH, muellerian-inhibiting factor precursor; IRF8, interferon regulatory factor 8; INSR, insulin receptor; LANCL, lanC-like protein 1, (lanthionine synthetase-like protein-1); PRLR, prolactin; SFRP4, secreted frizzled-related sequence protein 4 precursor; SH3BP5L, SH3 domain-binding protein 5; DGAT1, diacylglycerol O-acyltransferase 1; GFRA4, GDNF family receptor alpha-4 isoform 4; PPP1R14A, protein phosphatase 1 regulatory subunit 14A; TPT1, translationally-controlled tumor protein; SLA2, src-like-adapter 2; KCNK16, potassium channel, subfamily K, member 16; PDGFB, platelet-derived growth factor subunit B.
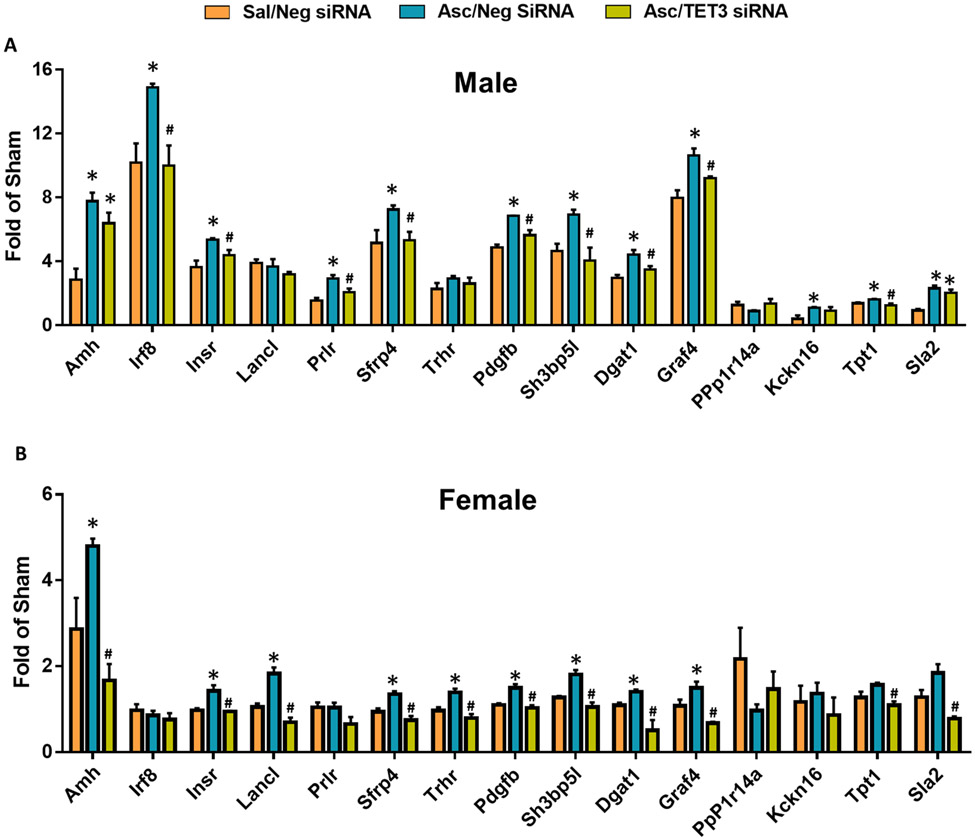
To determine whether the increased gene promoter 5hmC correlates with increased gene expression, we selected 15 genes with known involvement in ischemic neuroprotection (Table 1) and tested the mRNA expression by real-time PCR in the peri-infarct cortex at 2 days of reperfusion following transient MCAO. Ascorbate enhanced 12 of the 15 genes in male mice (Fig. 7A) and 9 of the 15 genes in female mice (Fig. 7B), indicating sex-dependent ascorbate modulation of gene expression. Additionally, 75% of the ascorbate-enhanced genes were dependent on TET3 in male mice (Fig. 7A), while all of the ascorbate-modulated genes were TET3-dependent in female mice (Fig. 7B).
Fig. 7: Ascorbate Increased Expression of Neuroprotective Genes after Stroke in a TET3-dependent Manner.
SiRNA (TET3 or Negative control siRNA) was injected 48h before the induction of MCAO. Saline or ascorbate (500 mg/kg; i.p.) was injected at 30 min reperfusion in adult mice following transient MCAO. (A) Expression of selected genes involved in ischemic outcome that displayed significant DhMRs in male and (B) female mice at 48h reperfusion. *p<0.05 compared to Sal/Neg siRNA group and #p<0.05 compared with Asc/Neg siRNA group by one-way ANOVA followed by Tukey’s post-test. n=3/group.
The majority of genes modulated by ascorbate in the ischemic brain are inflammatory
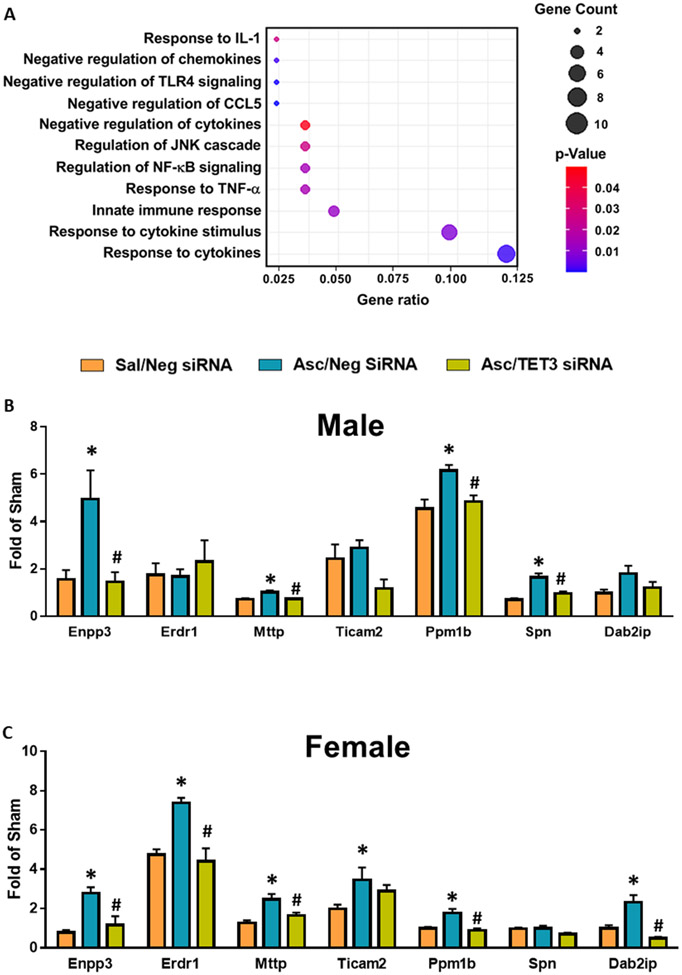
Sixty-percent of the top enriched GO biological processes modulated by ascorbate are involved in inflammatory pathways (Fig. 6A) and the majority of the ischemia pathophysiology-related biological processes modulated by ascorbate are related to inflammation (Fig. 6B). Further analysis identified 11 inflammation-related pathways with ascorbate-mediated increases in DhMRs (Fig. 8A). Many of these genes negatively regulated cytokines and chemokines and other pro-inflammatory transcripts such as nuclear factor kappa B (NF-kB), a transcription factor that induces expression of pro-inflammatory genes, and toll-like receptor 4 (TLR4), a major mediator of inflammatory-mediated pathological progression following ischemia/reperfusion injury (Fig. 8A) (48, 49). We selected seven of these inflammation-related genes and confirmed their modulation by ascorbate at 2 days of reperfusion in the peri-infarct cortex of adult male and female mice subjected to transient MCAO. Ascorbate enhanced expression of 57% of the genes in male and 86% of the genes in female mice (Fig. 8B and C). Almost all of these gene changes were dependent on TET3 (Fig. 8B and C). Some anti-inflammatory genes, such as ectonucleotide pyrophosphatase/phosphodiesterase family member 3 (Enpp3), which limits mast cell post-ischemic inflammatory response (50) and protein phosphatase 1B (Ppm1b) which inhibits NF-kB activation (51) were increased in both male and female mice. Other anti-inflammatory genes, such as leukosialin precursor (Spn, aka CD43) a modulator of immune cell migration (52) and TLR adaptor molecule 2 (Ticam2) that mediates TLR4 signaling (53), were only modulated by ascorbate in male or female mice, respectively (Fig. 8B and C).
Fig. 8: Ascorbate Increased 5hmC in Anti-inflammatory Genes After Stroke in a TET3-dependent Manner.
SiRNA (TET3 or Negative control siRNA) was injected 48h before the induction of MCAO. Saline or ascorbate (500 mg/kg; i.p.) was injected at 30 min reperfusion in adult mice following transient MCAO. (A) Gene ratio of inflammation-related biological pathways with increased promoter DhMRs in ascorbate/neg siRNA group compared to saline/neg siRNA group at 24h of reperfusion following transient MCAO. Statistically significant DhMRs were identified by differential analysis with a cut-off of log2 FC = 1.0 and p<0.05. (B) Expression of selected genes involved in inflammation with significant DhMRs in male mice and (C) female mice at 48h reperfusion. p<0.05 compared to Sal/Neg siRNA group (*), or Asc/Neg siRNA group (#) by one-way ANOVA followed by Tukey’s post-test. n=3/group.
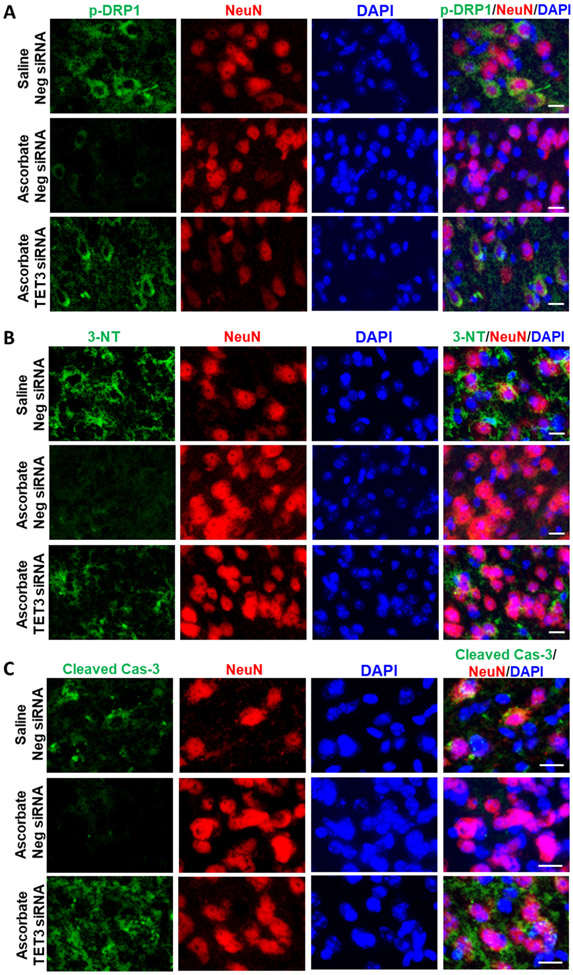
Ascorbate prevented post-stroke pathophysiologic events
In male mice subjected to transient MCAO, ascorbate treatment at 30 min reperfusion reduced abundance of 3-NT (marker of oxidative stress; Fig. 9A), p-DRP1 (marker for mitochondrial fragmentation; Fig. 9B) and cleaved caspase-3 (marker for apoptosis; Fig. 9C) at 3 days of reperfusion compared with saline-treated control groups. Importantly, TET3 knockdown mitigated the ascorbate-mediated reduction of oxidative stress, mitochondrial cleavage and apoptosis indicating a direct role of 5hmC in regulating these beneficial outcomes after stroke. Furthermore, secondary brain damage was significantly reduced through the ascorbate-TET3 pathway at 3 days of reperfusion (Supplementary Fig. 5).
Fig. 9: Ascorbate Decreased Pathophysiological Markers after Stroke via TET3.
SiRNA (TET3 or Negative control siRNA) was injected 48h before the induction of MCAO. Saline or ascorbate (500 mg/kg; i.p.) was injected at 30 min reperfusion in adult mice following transient MCAO. Immunostaining of markers of (A) oxidative stress (3-NT), (B) mitochondrial fragmentation (p-Drp1) and (C) apoptosis (cleaved caspase-3; Cas-3) in the NeuN+ cells (neuronal) in the cortical peri-infarct region of adult mice at 3 days reperfusion. n=3/group; Scale bar is 15 μm.
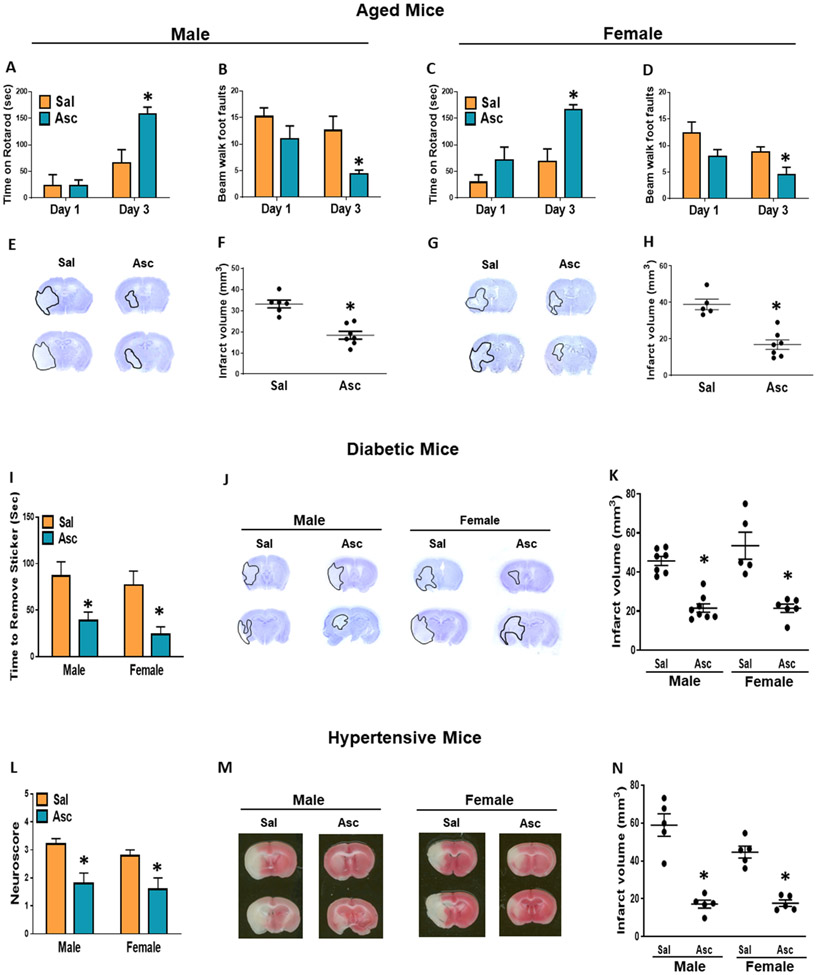
Ascorbate reduced ischemic brain damage and improved recovery in comorbid conditions
STAIR, which stipulates recommendations for new stroke therapies, indicates that preclinical studies should employ aged as well as comorbid animal models to increase translational potential. Hence, we evaluated the efficacy of ascorbate in aged, diabetic and hypertensive male and female mice subjected to transient MCAO. As aged and comorbid mice are highly susceptible to stroke, we decreased the MCAO duration in these cohorts to 45 min in aged mice and hypertensive mice and to 40 min in diabetic mice. In all cohorts, ascorbate (500 mg/kg; i.p.) was injected at 30 min reperfusion following transient MCAO.
Human aging paradigms can be modeled using mice (54). The aged mice employed in this study at 64-68 weeks of age are older than the middle-aged human equivalent age group (~40-56 weeks of age) and slightly younger than the elderly human equivalent age group (72 weeks and above). In aged male and female mice, ascorbate treatment significantly ameliorated post-stroke motor deficits evaluated by rotarod and beam walk tests at day 3 of reperfusion (Fig. 10A-D) and reduced infarct volume (Fig. 10E-H) compared with the sex-matched saline treated controls. The db/db mice that lack the long-form leptin receptor develop severe type 2 diabetes by 12 weeks of age (55). These animals are severely obese and are not able to stay on either the rotarod or walking beam. Hence, we used adhesive removal test in db/db mice to analyze the motor function. Ascorbate treatment in both male and female db/db mice, significantly improved the performance in the adhesive removal test (Fig. 10I) and decreased the infarct volume (Fig. 10J and K) estimated on day 3 of reperfusion compared with the sex-matched saline treated control cohorts. Adult BPH/2J mice display elevated systolic blood pressures due to neurogenic-mediated overactivity of the sympathetic nervous system (56). These mice also fail to perform in motor function tests (57) and therefore we used neuroscores for identifying their functional deficits after stroke. In BPH/2J mice, ascorbate treatment significantly improved neuroscores at 1 day (Fig. 10L) and reduced infarct size at 3 days of reperfusion (Fig. 10M and N) compared to saline-treated sex-matched controls.
Fig. 10: Ascorbate Reduced Secondary Brain Damage in Aged and Comorbid Mice.
(A-H) Male and female aged mice, n=5-7/group. (A, C) Motor function performance at 1 day and 3 days of reperfusion measured by rotarod test and (B, D) beam walk test. (E, G) Representative cresyl violet–stained serial sections (F, H) and infarct volume at 3 days reperfusion. (I-K) Diabetic male and female mice, n=5–8/group. (I) Motor function measured by amount of time taken to remove adhesive from forepaw at 3 days of reperfusion. (J) Representative cresyl violet–stained serial sections (K) and infarct volume at 3 days reperfusion. (L-N) Hypertensive male and female mice, n=5/group. (L) Neuroscore at 24h of reperfusion. (M) Representative cresyl violet–stained serial sections (N) and infarct volume at 3 days reperfusion. p<0.05 compared to saline group (*) by Mann-Whitney test.
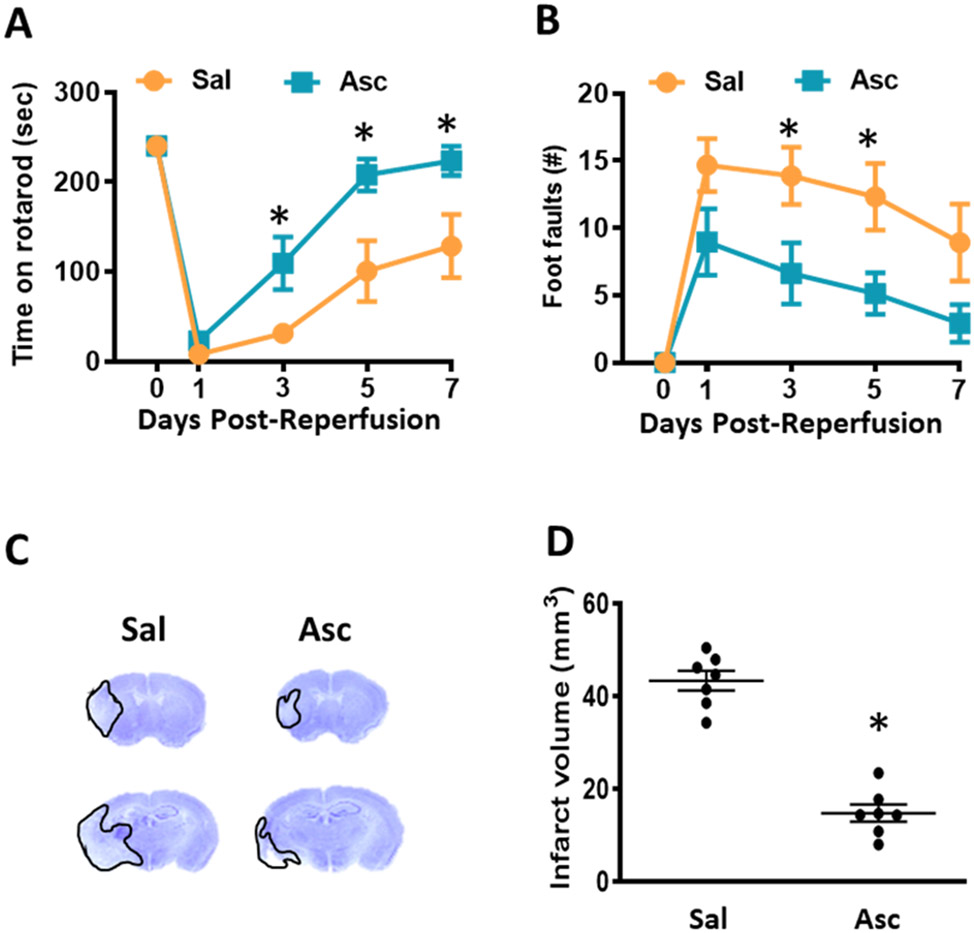
Delayed ascorbate treatment is also efficacious after focal ischemia
For optimal translation, STAIR recommends identifying an effective window of therapeutic opportunity for new stroke therapies. In the above studies, we showed that providing ascorbate at 30 min reperfusion is neuroprotective after focal ischemia. A cohort of adult male mice treated with ascorbate at 6h of reperfusion following 1h transient MCAO also showed significantly improved motor performance in the rotarod test on days 3, 5 and 7 (Fig. 11A) and in the beam walk test on days 3 and 5 of reperfusion (Fig. 11B) and reduced infarct volume on day 7 of reperfusion (Fig. 11C and D) compared with the saline treated control cohort.
Fig. 11: Delayed Ascorbate Treatment at 6h Reperfusion Reduced Infarct and Improves Motor Recovery.
Adult mice treated with ascorbate (500 mg/kg; i.p.) at 6h of reperfusion after transient MCAO. (A, B) Motor function performance at 7 days of reperfusion measured by (A) time on rotarod and (B) number of foot faults on beam walk. p<0.05 compared to saline group (*) by repeated-measures ANOVA followed by Sidak’s post-test. (C) Representative cresyl violet-stained serial sections (D) and infarct volume at 7 days of reperfusion. p<0.05 compared to saline group (*) by Mann-Whitney test. n=7/group.
DHA neither modulates post-ischemic 5hmC nor protects the brain after stroke
DHA is the BBB-permeable oxidized form of Vitamin C that can be reduced and converted to ascorbate (Fig. 1A). Previous studies have reported protection against focal ischemia with 500 mg/kg dose of ascorbate administered prior to transient MCAO (23, 24), but not in the post-ischemic brain. In non-ischemic adult male mice, DHA significantly increased TET activity at 1h post-injection, which was abrogated with TET3 knockdown (Supplementary Fig. 10). However, DHA injection at 30 min reperfusion following 1h transient MCAO neither altered TET activity at 1h reperfusion (Supplementary Fig. 11A) nor 5hmC levels at 1h or 24h reperfusion (Supplementary Fig. 11B) compared to saline control in adult male mice. No difference in infarct size was observed at 24h reperfusion with DHA treatment compared to saline control (Supplementary Fig. 11C and D).
DISCUSSION
Vitamin C provides antioxidant defense in various neurological diseases (58-60). The discovery that Vitamin C acts as a potent regulator of 5hmC suggests that it has potential for therapeutic applications by remodeling the epigenome (61). We show for the first time that ascorbate, the reduced form of Vitamin C, protects the brain after stroke via epigenetic reprogramming of neuroprotective genes. We found that ascorbate treatment modulates several pathways critical for ischemic pathophysiology such as inflammation, mitochondrial function and neuronal survival. Comorbid conditions like hypertension and diabetes significantly worsen stroke outcomes in humans. Consequently, we expanded testing of the translational potential of ascorbate by showing its effectiveness as a post-stroke treatment for abrogating brain damage and promoting recovery in both diabetic and hypertensive male and female mice. We further showed that ascorbate is efficacious to protect the brain after stroke even in aged male and female mice.
Ascorbate induces TET catalytic activity in various mammalian cell types in vitro (20, 21, 62, 63). Our study extended this and importantly showed the in vivo efficacy of ascorbate to enhance TET activity and increase global 5hmC levels in the peri-infarct cortex of mice subjected to transient MCAO. We further showed this effect in both sexes. Interestingly, female mice showed an earlier induction of ascorbate-mediated TET activity, and subsequently more prolonged increases in 5hmC levels. Additionally, while ascorbate prevented brain damage in both sexes, female mice showed better motor and cognitive functional recovery. Female mice have smaller infarcts (nearly half the size) compared to males, and thus protective treatments in general may be more robust. Previous studies indicated sex-specific differences in neuronal 5hmC profiles during development and in response to stress (64, 65). Our data suggests that female mice are more sensitive to post-stroke epigenetic regulation by ascorbate and/or that ascorbate may be modulated to have optimal effectiveness between sexes.
Vitamin C is predominantly present in its reduced form as ascorbate, which is found at a concentration of 40-60 μM (blood) and 200-500 μM (CSF), versus DHA found at 2 μM or less in both fluids in humans and rodents (66-71). Within the brain, SVCT2 is highly expressed in cerebral cortical neurons (16, 72), but SVCT2 is not present on the brain capillary endothelial cells that make up the BBB (73, 74). Thus, ascorbate enters the brain via SVCT2 located on choroid plexus epithelial cells (15, 75). However, during reperfusion, the integrity of the BBB is impaired which may further facilitate the entry of ascorbate into the brain (76). Expression of SVCT2 was previously shown to increase in the peri-infarct region from 2h to 22h of reperfusion following focal ischemia in rats (77). This increase in ascorbate transporter expression might have facilitated the neuroprotection we observed when ascorbate was injected at 6h of reperfusion after transient MCAO.
The hMeDIP-seq analysis indicated that ascorbate modulates 5hmC in genes of a number of pathways associated with post-ischemic neuroprotection. Analysis of mRNA expression confirmed this finding for many of the genes tested. A majority of the ascorbate-mediated upregulated DhMRs occurred in genes associated with neuroprotection after stroke such as Amh, Irf8, Prlr and Pdfgb. Importantly, ascorbate treatment mitigated oxidative stress, mitochondrial fragmentation and apoptosis in the post-ischemic brain. However, increased 5hmC was also observed in some detrimental genes such as mitochondrial ubiquitin ligase activator of NFKB 1 (Mul1) which promotes mitochondrial dysfunction and leads to brain injury following ischemic stroke (78). Another example is voltage-gated hydrogen channel 1 (Hcvn1) which promotes ROS production in inflammatory cells and has been shown to enhance brain damage following focal ischemia (79). Despite increased 5hmC in these potentially detrimental genes, ascorbate overwhelmingly modulated 5hmC in the promoters of putative neuroprotective genes.
In the rodent brain, SVCT2 is only expressed in neuronal cells and thus ascorbate is primarily observed in neurons (26, 80, 81). Our studies also showed that ascorbate-mediated global changes in 5hmC occur predominantly in neurons rather than astrocytes following transient MCAO. Interestingly, the hMeDIP-seq also identified some genes such as junctional adhesion molecule 2 (Jam2) and ubiquitin specific peptidase 34 (USP34) involved in BBB integrity and angiogenesis, which are related to endothelial cell function. Previous studies showed that endothelial cells of the BBB lack SVCT2 under normal conditions, but recent evidence indicates that SVCT2 is expressed in brain endothelial cells between 2-5 days after transient focal ischemia (73, 74, 82). Therefore, post-stroke SVCT2 expression in brain endothelial cells might further promote ascorbate transport across the BBB.
DHA, the oxidized form of Vitamin C, is normally found at low levels within the CNS (81). At high concentrations, DHA can rapidly enter the brain through GLUT transporters in the BBB endothelium where it can be reduced to ascorbate by glutathione (17, 18, 83). Previous studies have shown that DHA treatment either before or during the occlusion decreases secondary brain damage after transient or permanent MCAO in rodents (22-24). Our studies showed that DHA treatment in normal mice (non-ischemic) increased TET activity in a TET3-dependent manner, which may have induced the ischemic tolerance observed with DHA pre-treatment in the past studies. However, we observed that DHA treatment during reperfusion did not enhance TET activity or 5hmC levels. Furthermore, DHA failed to promote neuroprotection which confirms previous findings (27). The difference in DHA effectiveness may be due to the mechanisms of Vitamin C uptake and recycling. In non-pathological conditions, DHA is preferentially taken-up by cells with high reducing capacity, such as astrocytes, which contain high levels of glutathione. Astrocytes then release ascorbate that can enter neurons through the SVCT2 transporter (84-86). However, under conditions that promote oxidative stress, such as during reperfusion following ischemia, overall glutathione levels are decreased and DHA accumulates (26, 85, 87) In vitro studies have shown that DHA induces neuronal death during oxidative stress when astrocytic uptake of DHA is absent (26, 83, 88). Thus, our study shows DHA does not regulate 5hmC in the post-stroke brain and provides further evidence that DHA is not a good candidate for stroke therapy.
In the present study, we employed several criteria recommended by STAIR for developing an effective translational stroke therapy (28). For example, we show that ascorbate provides protection via epigenetic regulation in addition to serving as an antioxidant, which indicates multiple mechanisms of neuroprotection. Additionally, ascorbate-mediated epigenetic reprogramming targets several pathways related to ischemic pathophysiology. We show that peripheral administration (i.p.) of ascorbate induces robust neuroprotection after stroke. While long-term ascorbate pretreatment may provide protection against stroke (89, 90), we specifically determined the effectiveness of ascorbate on post-stroke reperfusion injury, which enhances its translational value as a clinical stroke therapy. Furthermore, we show that ascorbate treatment prevents brain damage and promotes functional recovery in both sexes of aged mice as well as comorbid mice. We further established a time window between 30 min and 6h of reperfusion for the effectiveness of ascorbate. Lastly, ascorbate treatment had no observable effects on mortality in our study. Moreover, human clinical trials for cancer and sepsis have confirmed that administration of high doses of ascorbate does not have clinically relevant toxic or adverse effects (91-93). Thus, our findings suggest that post-stroke ascorbate treatment may represent a promising therapy to abrogate secondary brain damage and to promote functional recovery after stroke.
Supplementary Material
FUNDING
This study was funded in part by National Institutes of Health grants R21 NS095192, R01 NS099531, R01 NS101960, R01 NS109459 and Department of Neurological Surgery, University of Wisconsin. Dr. Vemuganti is the recipient of a Research Career Scientist award (# IK6BX005690) from the US Department of Veterans Affairs.
Footnotes
Conflict of Interests/Competing Interests: Authors declare no conflict of interests and/or competing interests.
REFERENCES
- 1.Bertogliat MJ, Morris-Blanco KC, and Vemuganti R. Epigenetic mechanisms of neurodegenerative diseases and acute brain injury. Neurochem Int. 2020;133:104642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qureshi IA, and Mehler MF. Emerging role of epigenetics in stroke: part 1: DNA methylation and chromatin modifications. Arch Neurol. 2010;67(11):1316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris-Blanco KC, Kim T, Bertogliat MJ, Mehta SL, Chokkalla AK, and Vemuganti R. Inhibition of the Epigenetic Regulator REST Ameliorates Ischemic Brain Injury. Mol Neurobiol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu H, Su Y, Shin J, Zhong C, Guo JU, Weng YL, et al. Tet3 regulates synaptic transmission and homeostatic plasticity via DNA oxidation and repair. Nat Neurosci. 2015;18(6):836–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Zhang Z, Li L, Xu K, Ma Z, Chow HM, et al. Selective loss of 5hmC promotes neurodegeneration in the mouse model of Alzheimer's disease. Faseb j. 2020;34(12):16364–82. [DOI] [PubMed] [Google Scholar]
- 6.Li L, Qiu Y, Miao M, Liu Z, Li W, Zhu Y, et al. Reduction of Tet2 exacerbates early stage Alzheimer's pathology and cognitive impairments in 2×Tg-AD mice. Hum Mol Genet. 2020;29(11):1833–52. [DOI] [PubMed] [Google Scholar]
- 7.Carella A, Tejedor JR, García MG, Urdinguio RG, Bayón GF, Sierra M, et al. Epigenetic downregulation of TET3 reduces genome-wide 5hmC levels and promotes glioblastoma tumorigenesis. Int J Cancer. 2020;146(2):373–87. [DOI] [PubMed] [Google Scholar]
- 8.Mellen M, Ayata P, Dewell S, Kriaucionis S, and Heintz N. MeCP2 binds to 5hmC enriched within active genes and accessible chromatin in the nervous system. Cell. 2012;151(7):1417–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kriaucionis S, and Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science. 2009;324(5929):929–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris-Blanco KC, Chokkalla AK, Bertogliat MJ, and Vemuganti R. TET3 regulates DNA hydroxymethylation of neuroprotective genes following focal ischemia. J Cereb Blood Flow Metab. 2021;41(3):590–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris-Blanco KC, Kim T, Lopez MS, Bertogliat MJ, Chelluboina B, and Vemuganti R. Induction of DNA Hydroxymethylation Protects the Brain After Stroke. Stroke. 2019; 50(9):2513–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miao Z, He Y, Xin N, Sun M, Chen L, Lin L, et al. Altering 5-hydroxymethylcytosine modification impacts ischemic brain injury. Hum Mol Genet. 2015;24(20):5855–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei X, Yu L, Zhang Y, Li X, Wu H, Jiang J, et al. The Role of Tet2-mediated Hydroxymethylation in Poststroke Depression. Neuroscience. 2021;461:118–29. [DOI] [PubMed] [Google Scholar]
- 14.Covarrubias-Pinto A, Acuña AI, Beltrán FA, Torres-Díaz L, and Castro MA. Old Things New View: Ascorbic Acid Protects the Brain in Neurodegenerative Disorders. Int J Mol Sci. 2015;16(12):28194–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrison FE, and May JM. Vitamin C function in the brain: vital role of the ascorbate transporter SVCT2. Free Radic Biol Med. 2009;46(6):719–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caprile T, Salazar K, Astuya A, Cisternas P, Silva-Alvarez C, Montecinos H, et al. The Na+-dependent L-ascorbic acid transporter SVCT2 expressed in brainstem cells, neurons, and neuroblastoma cells is inhibited by flavonoids. J Neurochem. 2009;108(3):563–77. [DOI] [PubMed] [Google Scholar]
- 17.Agus DB, Gambhir SS, Pardridge WM, Spielholz C, Baselga J, Vera JC, et al. Vitamin C crosses the blood-brain barrier in the oxidized form through the glucose transporters. J Clin Invest. 1997;100(11):2842–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin H, Carroll VN, Sriram R, Villanueva-Meyer JE, von Morze C, Wang ZJ, et al. Imaging glutathione depletion in the rat brain using ascorbate-derived hyperpolarized MR and PET probes. Sci Rep. 2018;8(1):7928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Getoff N. Vitamin C: electron emission, free radicals and biological versatility. In Vivo. 2013;27(5):565–70. [PubMed] [Google Scholar]
- 20.Yin R, Mao SQ, Zhao B, Chong Z, Yang Y, Zhao C, et al. Ascorbic acid enhances Tet-mediated 5-methylcytosine oxidation and promotes DNA demethylation in mammals. J Am Chem Soc. 2013;135(28):10396–403. [DOI] [PubMed] [Google Scholar]
- 21.Minor EA, Court BL, Young JI, and Wang G. Ascorbate induces ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine. J Biol Chem. 2013;288(19):13669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song J, Park J, Kim JH, Choi JY, Kim JY, Lee KM, et al. Dehydroascorbic Acid Attenuates Ischemic Brain Edema and Neurotoxicity in Cerebral Ischemia: An in vivo Study. Exp Neurobiol. 2015;24(1):41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang J, Agus DB, Winfree CJ, Kiss S, Mack WJ, McTaggart RA, et al. Dehydroascorbic acid, a blood-brain barrier transportable form of vitamin C, mediates potent cerebroprotection in experimental stroke. Proc Natl Acad Sci U S A. 2001;98(20):11720–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mack WJ, Mocco J, Ducruet AF, Laufer I, King RG, Zhang Y, et al. A cerebroprotective dose of intravenous citrate/sorbitol-stabilized dehydroascorbic acid is correlated with increased cerebral ascorbic acid and inhibited lipid peroxidation after murine reperfused stroke. Neurosurgery. 2006;59(2):383–8; discussion −8. [DOI] [PubMed] [Google Scholar]
- 25.Chang CY, Chen JY, Wu MH, and Hu ML. Therapeutic treatment with vitamin C reduces focal cerebral ischemia-induced brain infarction in rats by attenuating disruptions of blood brain barrier and cerebral neuronal apoptosis. Free Radic Biol Med. 2020;155:29–36. [DOI] [PubMed] [Google Scholar]
- 26.García-Krauss A, Ferrada L, Astuya A, Salazar K, Cisternas P, Martínez F, et al. Dehydroascorbic Acid Promotes Cell Death in Neurons Under Oxidative Stress: a Protective Role for Astrocytes. Mol Neurobiol. 2016;53(9):5847–63. [DOI] [PubMed] [Google Scholar]
- 27.Ducruet AF, Mack WJ, Mocco J, Hoh DJ, Coon AL, D'Ambrosio AL, et al. Preclinical evaluation of postischemic dehydroascorbic Acid administration in a large-animal stroke model. Transl Stroke Res. 2011;2(3):399–403. [DOI] [PubMed] [Google Scholar]
- 28.Albers GW, Goldstein LB, Hess DC, Wechsler LR, Furie KL, Gorelick PB, et al. Stroke Treatment Academic Industry Roundtable (STAIR) recommendations for maximizing the use of intravenous thrombolytics and expanding treatment options with intra-arterial and neuroprotective therapies. Stroke. 2011;42(9):2645–50. [DOI] [PubMed] [Google Scholar]
- 29.Nakka VP, Lang BT, Lenschow DJ, Zhang DE, Dempsey RJ, and Vemuganti R. Increased cerebral protein ISGylation after focal ischemia is neuroprotective. J Cereb Blood Flow Metab. 2011;31(12):2375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris-Blanco KC, Chokkalla AK, Bertogliat MJ, and Vemuganti R. TET3 regulates DNA hydroxymethylation of neuroprotective genes following focal ischemia. J Cereb Blood Flow Metab. 2020:271678x20912965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehta SL, Chokkalla AK, Kim T, Bathula S, Chelluboina B, Morris-Blanco KC, et al. Long Noncoding RNA Fos Downstream Transcript Is Developmentally Dispensable but Vital for Shaping the Poststroke Functional Outcome. Stroke. 2021;52(7):2381–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim T, Mehta SL, Morris-Blanco KC, Chokkalla AK, Chelluboina B, Lopez M, et al. The microRNA miR-7a-5p ameliorates ischemic brain damage by repressing α-synuclein. Sci Signal. 2018;11(560). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopez MS, Morris-Blanco KC, Ly N, Maves C, Dempsey RJ, and Vemuganti R. MicroRNA miR-21 Decreases Post-stroke Brain Damage in Rodents. Transl Stroke Res. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan YP, Sailor KA, Lang BT, Park SW, Vemuganti R, and Dempsey RJ. Monocyte chemoattractant protein-1 plays a critical role in neuroblast migration after focal cerebral ischemia. J Cereb Blood Flow Metab. 2007;27(6):1213–24. [DOI] [PubMed] [Google Scholar]
- 35.Hahn MA, Qiu R, Wu X, Li AX, Zhang H, Wang J, et al. Dynamics of 5-hydroxymethylcytosine and chromatin marks in Mammalian neurogenesis. Cell Rep. 2013;3(2):291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dordoe C, Chen K, Huang W, Chen J, Hu J, Wang X, et al. Roles of Fibroblast Growth Factors and Their Therapeutic Potential in Treatment of Ischemic Stroke. Front Pharmacol. 2021;12:671131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terao S, Yilmaz G, Stokes KY, Russell J, Ishikawa M, Kawase T, et al. Blood cell-derived RANTES mediates cerebral microvascular dysfunction, inflammation, and tissue injury after focal ischemia-reperfusion. Stroke. 2008;39(9):2560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dénes A, Humphreys N, Lane TE, Grencis R, and Rothwell N. Chronic systemic infection exacerbates ischemic brain damage via a CCL5 (regulated on activation, normal T-cell expressed and secreted)-mediated proinflammatory response in mice. J Neurosci. 2010;30(30):10086–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saran U, Mani KP, Balaguru UM, Swaminathan A, Nagarajan S, Dharmarajan AM, et al. sFRP4 signalling of apoptosis and angiostasis uses nitric oxide-cGMP-permeability axis of endothelium. Nitric Oxide. 2017;66:30–42. [DOI] [PubMed] [Google Scholar]
- 40.Zeng W, Cao Y, Jiang W, Kang G, Huang J, and Xie S. Knockdown of Sfrp4 attenuates apoptosis to protect against myocardial ischemia/reperfusion injury. J Pharmacol Sci. 2019;140(1):14–9. [DOI] [PubMed] [Google Scholar]
- 41.Choi J, Dong L, Ahn J, Dao D, Hammerschmidt M, and Chen JN. FoxH1 negatively modulates flk1 gene expression and vascular formation in zebrafish. Dev Biol. 2007;304(2):735–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lebeurrier N, Launay S, Macrez R, Maubert E, Legros H, Leclerc A, et al. Anti-Mullerian-hormone-dependent regulation of the brain serine-protease inhibitor neuroserpin. J Cell Sci. 2008;121(Pt 20):3357–65. [DOI] [PubMed] [Google Scholar]
- 43.Wu J, Echeverry R, Guzman J, and Yepes M. Neuroserpin protects neurons from ischemia-induced plasmin-mediated cell death independently of tissue-type plasminogen activator inhibition. Am J Pathol. 2010;177(5):2576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiang M, Wang L, Guo S, Lu YY, Lei H, Jiang DS, et al. Interferon regulatory factor 8 protects against cerebral ischaemic-reperfusion injury. J Neurochem. 2014;129(6):988–1001. [DOI] [PubMed] [Google Scholar]
- 45.Vermani B, Mukherjee S, Kumar G, and Patnaik R. Prolactin attenuates global cerebral ischemic injury in rat model by conferring neuroprotection. Brain Inj. 2020;34(5):685–93. [DOI] [PubMed] [Google Scholar]
- 46.Krupinski J, Issa R, Bujny T, Slevin M, Kumar P, Kumar S, et al. A putative role for platelet-derived growth factor in angiogenesis and neuroprotection after ischemic stroke in humans. Stroke. 1997;28(3):564–73. [DOI] [PubMed] [Google Scholar]
- 47.Iihara K, Hashimoto N, Tsukahara T, Sakata M, Yanamoto H, and Taniguchi T. Platelet-derived growth factor-BB, but not -AA, prevents delayed neuronal death after forebrain ischemia in rats. J Cereb Blood Flow Metab. 1997;17(10):1097–106. [DOI] [PubMed] [Google Scholar]
- 48.Howell JA, and Bidwell GL 3rd. Targeting the NF-κB pathway for therapy of ischemic stroke. Ther Deliv. 2020;11(2):113–23. [DOI] [PubMed] [Google Scholar]
- 49.Famakin BM, and Vemuganti R. Toll-Like Receptor 4 Signaling in Focal Cerebral Ischemia: a Focus on the Neurovascular Unit. Mol Neurobiol. 2020;57(6):2690–701. [DOI] [PubMed] [Google Scholar]
- 50.Parrella E, Porrini V, Benarese M, and Pizzi M. The Role of Mast Cells in Stroke. Cells. 2019;8(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun W, Yu Y, Dotti G, Shen T, Tan X, Savoldo B, et al. PPM1A and PPM1B act as IKKbeta phosphatases to terminate TNFalpha-induced IKKbeta-NF-kappaB activation. Cell Signal. 2009;21(1):95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Velázquez F, Grodecki-Pena A, Knapp A, Salvador AM, Nevers T, Croce K, et al. CD43 Functions as an E-Selectin Ligand for Th17 Cells In Vitro and Is Required for Rolling on the Vascular Endothelium and Th17 Cell Recruitment during Inflammation In Vivo. J Immunol. 2016;196(3):1305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oshiumi H, Sasai M, Shida K, Fujita T, Matsumoto M, and Seya T. TIR-containing adapter molecule (TICAM)-2, a bridging adapter recruiting to toll-like receptor 4 TICAM-1 that induces interferon-beta. J Biol Chem. 2003;278(50):49751–62. [DOI] [PubMed] [Google Scholar]
- 54.Flurkey K, J M. Currer, and Harrison DE. In: Fox JG, Davisson MT, Quimby FW, Barthold SW, Newcomer CE, and Smith AL eds. The Mouse in Biomedical Research (Second Edition). Burlington: Academic Press; 2007:637–72. [Google Scholar]
- 55.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84(3):491–5. [DOI] [PubMed] [Google Scholar]
- 56.Davern PJ, Nguyen-Huu TP, La Greca L, Abdelkader A, and Head GA. Role of the sympathetic nervous system in Schlager genetically hypertensive mice. Hypertension. 2009;54(4):852–9. [DOI] [PubMed] [Google Scholar]
- 57.Hartman RE, Kamper JE, Goyal R, Stewart JM, and Longo LD. Motor and cognitive deficits in mice bred to have low or high blood pressure. Physiol Behav. 2012;105(4):1092–7. [DOI] [PubMed] [Google Scholar]
- 58.Qiu S, Li L, Weeber EJ, and May JM. Ascorbate transport by primary cultured neurons and its role in neuronal function and protection against excitotoxicity. J Neurosci Res. 2007;85(5):1046–56. [DOI] [PubMed] [Google Scholar]
- 59.Rebec GV, Barton SJ, Marseilles AM, and Collins K. Ascorbate treatment attenuates the Huntington behavioral phenotype in mice. Neuroreport. 2003;14(9):1263–5. [DOI] [PubMed] [Google Scholar]
- 60.Wagner GC, Carelli RM, and Jarvis MF. Ascorbic acid reduces the dopamine depletion induced by methamphetamine and the 1-methyl-4-phenyl pyridinium ion. Neuropharmacology. 1986;25(5):559–61. [DOI] [PubMed] [Google Scholar]
- 61.Brabson JP, Leesang T, Mohammad S, and Cimmino L. Epigenetic Regulation of Genomic Stability by Vitamin C. Front Genet. 2021;12:675780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blaschke K, Ebata KT, Karimi MM, Zepeda-Martínez JA, Goyal P, Mahapatra S, et al. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature. 2013;500(7461):222–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shenoy N, Bhagat T, Nieves E, Stenson M, Lawson J, Choudhary GS, et al. Upregulation of TET activity with ascorbic acid induces epigenetic modulation of lymphoma cells. Blood Cancer J. 2017;7(7):e587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Papale LA, Li S, Madrid A, Zhang Q, Chen L, Chopra P, et al. Sex-specific hippocampal 5-hydroxymethylcytosine is disrupted in response to acute stress. Neurobiol Dis. 2016;96:54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spiers H, Hannon E, Schalkwyk LC, Bray NJ, and Mill J. 5-hydroxymethylcytosine is highly dynamic across human fetal brain development. BMC Genomics. 2017;18(1):738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Okamura M Uptake of L-ascorbic acid and L-dehydroascorbic acid by human erythrocytes and HeLa cells. J Nutr Sci Vitaminol (Tokyo). 1979;25(4):269–79. [DOI] [PubMed] [Google Scholar]
- 67.Dhariwal KR, Hartzell WO, and Levine M. Ascorbic acid and dehydroascorbic acid measurements in human plasma and serum. Am J Clin Nutr. 1991;54(4):712–6. [DOI] [PubMed] [Google Scholar]
- 68.Schenk JO, Miller E, Gaddis R, and Adams RN. Homeostatic control of ascorbate concentration in CNS extracellular fluid. Brain Res. 1982;253(1–2):353–6. [DOI] [PubMed] [Google Scholar]
- 69.Miele M, and Fillenz M. In vivo determination of extracellular brain ascorbate. J Neurosci Methods. 1996;70(1):15–9. [DOI] [PubMed] [Google Scholar]
- 70.Stamford JA, Kruk ZL, and Millar J. Regional differences in extracellular ascorbic acid levels in the rat brain determined by high speed cyclic voltammetry. Brain Res. 1984;299(2):289–95. [DOI] [PubMed] [Google Scholar]
- 71.Iwama M, Amano A, Shimokado K, Maruyama N, and Ishigami A. Ascorbic acid levels in various tissues, plasma and urine of mice during aging. J Nutr Sci Vitaminol (Tokyo). 2012;58(3):169–74. [DOI] [PubMed] [Google Scholar]
- 72.Mun GH, Kim MJ, Lee JH, Kim HJ, Chung YH, Chung YB, et al. Immunohistochemical study of the distribution of sodium-dependent vitamin C transporters in adult rat brain. J Neurosci Res. 2006;83(5):919–28. [DOI] [PubMed] [Google Scholar]
- 73.García Mde L, Salazar K, Millán C, Rodríguez F, Montecinos H, Caprile T, et al. Sodium vitamin C cotransporter SVCT2 is expressed in hypothalamic glial cells. Glia. 2005;50(1):32–47. [DOI] [PubMed] [Google Scholar]
- 74.Qiao H, and May JM. Development of ascorbate transporters in brain cortical capillary endothelial cells in culture. Brain Res. 2008;1208:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Angelow S, Haselbach M, and Galla HJ. Functional characterisation of the active ascorbic acid transport into cerebrospinal fluid using primary cultured choroid plexus cells. Brain Res. 2003;988(1–2):105–13. [DOI] [PubMed] [Google Scholar]
- 76.Yang GY, and Betz AL. Reperfusion-induced injury to the blood-brain barrier after middle cerebral artery occlusion in rats. Stroke. 1994;25(8):1658–64; discussion 64-5. [DOI] [PubMed] [Google Scholar]
- 77.Berger UV, Lu XC, Liu W, Tang Z, Slusher BS, and Hediger MA. Effect of middle cerebral artery occlusion on mRNA expression for the sodium-coupled vitamin C transporter SVCT2 in rat brain. J Neurochem. 2003;86(4):896–906. [DOI] [PubMed] [Google Scholar]
- 78.Ren KD, Liu WN, Tian J, Zhang YY, Peng JJ, Zhang D, et al. Mitochondrial E3 ubiquitin ligase 1 promotes brain injury by disturbing mitochondrial dynamics in a rat model of ischemic stroke. Eur J Pharmacol. 2019;861:172617. [DOI] [PubMed] [Google Scholar]
- 79.Wu LJ, Wu G, Akhavan Sharif MR, Baker A, Jia Y, Fahey FH, et al. The voltage-gated proton channel Hv1 enhances brain damage from ischemic stroke. Nat Neurosci. 2012;15(4):565–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Castro M, Caprile T, Astuya A, Millán C, Reinicke K, Vera JC, et al. High-affinity sodium-vitamin C co-transporters (SVCT) expression in embryonic mouse neurons. J Neurochem. 2001;78(4):815–23. [DOI] [PubMed] [Google Scholar]
- 81.Rice ME, and Russo-Menna I. Differential compartmentalization of brain ascorbate and glutathione between neurons and glia. Neuroscience. 1998;82(4):1213–23. [DOI] [PubMed] [Google Scholar]
- 82.Gess B, Sevimli S, Strecker JK, Young P, and Schäbitz WR. Sodium-dependent vitamin C transporter 2 (SVCT2) expression and activity in brain capillary endothelial cells after transient ischemia in mice. PLoS One. 2011;6(2):e17139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Song JH, Shin SH, and Ross GM. Oxidative stress induced by ascorbate causes neuronal damage in an in vitro system. Brain Res. 2001;895(1–2):66–72. [DOI] [PubMed] [Google Scholar]
- 84.Kim EJ, Park YG, Baik EJ, Jung SJ, Won R, Nahm TS, et al. Dehydroascorbic acid prevents oxidative cell death through a glutathione pathway in primary astrocytes. J Neurosci Res. 2005;79(5):670–9. [DOI] [PubMed] [Google Scholar]
- 85.Nualart FJ, Rivas CI, Montecinos VP, Godoy AS, Guaiquil VH, Golde DW, et al. Recycling of vitamin C by a bystander effect. J Biol Chem. 2003;278(12):10128–33. [DOI] [PubMed] [Google Scholar]
- 86.Astuya A, Caprile T, Castro M, Salazar K, García Mde L, Reinicke K, et al. Vitamin C uptake and recycling among normal and tumor cells from the central nervous system. J Neurosci Res. 2005;79(1–2):146–56. [DOI] [PubMed] [Google Scholar]
- 87.Rice ME. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000;23(5):209–16. [DOI] [PubMed] [Google Scholar]
- 88.Song JH, Shin SH, and Chung IM. Effects of glutamate on dehydroascorbate uptake and its enhanced vulnerability to the peroxidation in cerebral cortical slices. Exp Mol Med. 2002;34(6):419–25. [DOI] [PubMed] [Google Scholar]
- 89.Iwata N, Okazaki M, Xuan M, Kamiuchi S, Matsuzaki H, and Hibino Y. Orally administrated ascorbic acid suppresses neuronal damage and modifies expression of SVCT2 and GLUT1 in the brain of diabetic rats with cerebral ischemia-reperfusion. Nutrients. 2014;6(4):1554–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ranjan A, Theodore D, Haran RP, and Chandy MJ. Ascorbic acid and focal cerebral ischaemia in a primate model. Acta Neurochir (Wien). 1993;123(1–2):87–91. [DOI] [PubMed] [Google Scholar]
- 91.Fowler AA 3rd, Syed AA, Knowlson S, Sculthorpe R, Farthing D, DeWilde C, et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med. 2014;12:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hoffer LJ, Levine M, Assouline S, Melnychuk D, Padayatty SJ, Rosadiuk K, et al. Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann Oncol. 2008;19(11):1969–74. [DOI] [PubMed] [Google Scholar]
- 93.Monti DA, Mitchell E, Bazzan AJ, Littman S, Zabrecky G, Yeo CJ, et al. Phase I evaluation of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. PLoS One. 2012;7(1):e29794. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data can be made available upon reasonable request.