Abstract
Background
Timely diagnosis of cognitive impairment is a key goal of the National Plan to Address Alzheimer’s Disease, but studies of factors associated with a timely diagnosis are limited.
Objective
To identify patient characteristics associated with a timely diagnosis of dementia and mild cognitive impairment (MCI).
Design
Retrospective observational study using survey data from the Health and Retirement Study (HRS) from 1995-2016 (interview waves 3-13).
Participants
4,760 respondents with incident dementia and 1,864 with incident MCI identified using longitudinal measures of cognitive functioning.
Main measures
Timely or delayed diagnosis based on the timing of a self or proxy report of a healthcare provider diagnosis in relation to respondents first dementia or MCI-qualifying cognitive score, sociodemographic characteristics, health status, health care utilization, insurance provider, and year of first qualifying score.
Key results
Only 26.0% of the 4,760 respondents with incident dementia and 11.4% of the 1,864 respondents with incident MCI received a timely diagnosis. Non-Hispanic Black respondents and respondents with less than a college degree were significantly less likely to receive a timely diagnosis of either dementia or MCI than Non-Hispanic White respondents (dementia odds ratio (OR): 0.61, 95% CI: 0.50, 0.75; MCI OR: 0.40, 95% CI: 0.23, 0.70) and those with a college degree (dementia OR for less than high school degree: 0.30, 95% CI: 0.23, 0.38; MCI OR: 0.36, 95% CI: 0.22, 0.60). Respondents that lived alone were also less likely to receive a timely diagnosis of dementia (OR: 0.69, 95% CI: 0.59, 0.81), though not MCI. Timely diagnosis of both conditions increased over time.
Conclusions
Targeting resources for timely diagnosis of cognitive impairment to individuals from racial and ethnic minorities, lower educational attainment, and living alone may improve detection and reduce disparities around timely diagnosis of dementia and MCI.
KEY WORDS: dementia, cognitive impairment, disparities, diagnosis
INTRODUCTION
An estimated 8.8% of adults 65 and older in the United States (US) have dementia, a progressive syndrome with cognitive impairment sufficiently severe to interfere with one’s ability to function independently.1 An additional 18.8% are estimated to have mild cognitive impairment without dementia (MCI).1 Older adults with MCI experience symptoms of cognitive impairment but are still capable of completing typical activities of daily living and are thus at an earlier stage in the continuum of cognitive decline.2 Risk of dementia is not uniform across the older adult population. Prevalence and incidence of dementia are substantially higher among older adults with lower levels of educational attainment and among non-Hispanic Black individuals when compared with non-Hispanic White individuals.3,4
Although cognitive impairment is relatively common among older adults, health care providers frequently fail to recognize it during visits with their patients. Studies estimate that between 19% and 74% of patients with dementia are not diagnosed by their physician, with the wide variability in rates of diagnosis reflective of the variety of methods used to ascertain both the cognitive status of the patient and the physician diagnosis.5–7 Despite their higher risk, non-Hispanic Black individuals with dementia are significantly less likely to have a physician diagnosis than non-Hispanic White individuals with dementia.8 Similarly, older adults with lower levels of educational attainment are more likely to be underdiagnosed.9
Dementia symptom severity is positively associated with the likelihood of a physician diagnosis.5–7,10 Consequently, most older adults with dementia are not diagnosed until they reach a moderate or severe stage of the disease. While pharmaceutical treatments may be limited in their effectiveness for people living with dementia, identifying people earlier in the disease course may provide more time for patients and family members to prepare, facilitate management of medication regimens and comorbid conditions,11,12 aid in the prevention of adverse financial events,13 allow for early mitigation of safety risks,14 and ultimately, improve patient outcomes. Accordingly, the National Plan to Address Alzheimer’s Disease includes timely diagnosis as a key goal, while also acknowledging that some populations that experience a disproportionate burden of disease may experience greater challenges in obtaining a timely diagnosis.
Understanding the factors associated with a timely diagnosis can guide future efforts, but few studies have addressed this question. Most prior studies have examined prevalent cases of dementia to identify patient and provider characteristics associated with receipt of a formal diagnosis,5–9,15 and therefore miss critical information about the timing of diagnosis. The only prior study to use longitudinal data to examine incident cases of dementia had significant limitations, including a small sample size, limited years of data that are now dated, and extremely restrictive inclusion criteria for the study population.16 Additionally, no studies have examined incident cases of MCI, though a goal of earlier diagnosis of dementia would suggest a greater focus on the timely diagnosis of MCI. To address these limitations in the literature, we use longitudinal survey data and linked claims data to identify patient characteristics associated with a timely diagnosis of dementia and a timely diagnosis of MCI. We focus particularly on those characteristics, including race and ethnicity and education, that could contribute to disparities in diagnoses.
METHODS
Study design and data sources
We conducted a retrospective, observational study of older adults with incident dementia or incident MCI using data from the Health and Retirement Study (HRS) from 1995-2016 (interview waves 3-13).17,18 The HRS is a longitudinal biannual study of aging (grant number NIA U01AG009740) of a nationally representative sample of adults, 50 years of age and above. HRS-linked Medicare claims data from 1991-2012 was also used in our sensitivity analyses. Study procedures were approved by the University of Washington’s Institutional Review Board.
Determination of incident dementia or MCI
The HRS assesses respondents’ cognitive functioning at every interview wave. Self-respondents are administered an adaptation of the telephone interview for cognitive status (TICS). Proxy respondents are asked for their assessment of the respondent’s memory and functional status. For self-respondents only, missing cognitive measures are imputed by HRS.19 We classified cognitive scores as either normal, MCI without dementia, or dementia according to the Langa-Weir scale, a measure validated through a comprehensive clinical and neuropsychological examination of a subset of HRS respondents.1,20Self-respondent scores were based on a subset of the TICS elements, including immediate and delayed noun recall tests, a serial 7 subtraction test, and a backward counting from 20 test, with scores of 0-6 indicating dementia, 7-11 indicating MCI, and 12-27 indicating normal cognitive functioning.
Scores for respondents represented by proxy were based on the proxy’s assessment of the respondent’s memory, the number of instrumental activities of daily living that the respondent had difficulty carrying out, and the interviewer’s assessment of the respondent’s cognitive limitations, with scores of 6-11 indicating dementia, 3-5 indicating MCI, and 0-2 indicating normal cognitive functioning. The interviewer assessment was added in interview wave 5. For waves 3 and 4, the Langa-Weir scale classified scores according to a 9-point scale based on the proxy’s assessment of the respondent’s memory and difficulties with instrumental activities of daily living.21 Under the 9-point scale, scores of 5-9 indicated dementia, 3-4 indicated MCI, and 0-2 indicated normal cognitive functioning.
A respondent was included in our incident dementia cohort if they had a dementia-qualifying score in one of the interview waves preceded by a normal cognition or MCI score on the interview wave immediately prior. (Figure 1) To be included in our incident MCI cohort, a respondent needed to have a MCI-qualifying score preceded by a normal cognition score on the interview wave immediately prior as well as a confirmatory MCI or dementia-qualifying score in the interview wave immediately following the first qualifying- MCI score. The confirmatory assessment improved the accuracy of our classification by excluding respondents who reverted back to normal cognitive functioning.22
Figure 1.
Study design
Determination of diagnosis of dementia or MCI
At each interview wave, respondents are asked if a doctor has ever told them whether they have any one of nine common chronic conditions. The HRS inquired about a provider diagnosis of a memory-related disease in interview waves 4-9; the wording was updated to reference a provider diagnosis of Alzheimer’s disease or dementia in interview waves 10-13. As memory-related disease broadly captures both MCI and dementia, we used this to define the outcome for both cohorts. A provider diagnosis of Alzheimer’s disease or dementia was used to define the outcome for our dementia cohort only.
As a sensitivity analysis for the incident dementia cohort, we also examined claims data for an ICD-based diagnosis of dementia among HRS respondents who consented to linkage of their Medicare data and had Medicare Parts A and B fee-for-service coverage in the 12 months following the interview date of the first dementia-qualifying score (see Appendix - Table 4 for list of diagnosis codes).
Table.4.
ICD-9-CM diagnostic codes used to identify subjects with dementia
| Diagnosis code | Description |
|---|---|
| 331.0 | Alzheimer’s disease |
| 331.11 | Pick’s disease |
| 331.19 | Other frontotemporal dementia |
| 331.2 | Senile degeneration of brain |
| 331.7 | Cerebral degeneration in diseases classified elsewhere |
| 290.0 | Senile dementia, uncomplicated |
| 290.10 | Presenile dementia, uncomplicated |
| 290.11 | Presenile dementia with delirium |
| 290.12 | Presenile dementia with delusional features |
| 290.13 | Presenile dementia with depressive features |
| 290.20 | Senile dementia with delusional features |
| 290.21 | Senile dementia with depressive features |
| 290.3 | Senile dementia with delirium |
| 290.40 | Vascular dementia, uncomplicated |
| 290.41 | Vascular dementia with delirium |
| 290.42 | Vascular dementia with delusions |
| 290.43 | Vascular dementia with depressed mood |
| 294.0 | Amnestic disorder in conditions classified elsewhere |
| 294.10 | Dementia in conditions classified elsewhere without behavioral disturbance |
| 294.11 | Dementia in conditions classified elsewhere with behavioral disturbance |
| 294.20 | Dementia, unspecified, without behavioral disturbance |
| 294.21 | Dementia, unspecified, with behavioral disturbance |
| 294.8 | Other persistent mental disorders due to conditions classified elsewhere |
| 797 | Senility without mention of psychosis |
Timely diagnosis of dementia or MCI
We defined a timely dementia diagnosis as receiving a self- or proxy-reported health care provider diagnosis in the same interview as the first dementia-qualifying score or in a prior interview. We defined a timely MCI diagnosis as receiving a self- or proxy-reported health care provider diagnosis prior to or in the same interview as the confirmatory MCI score. In sensitivity analysis using the claims, timely dementia diagnosis was defined as a dementia diagnosis code either prior to or within 12 months following the first dementia-qualifying score.
Statistical analysis
We used multivariable logistic regression to identify factors associated with a timely diagnosis of dementia or MCI. Covariates were selected based on prior studies and were determined using the interview wave of the first dementia- or MCI-qualifying score, including sociodemographic characteristics (age, gender, race/ethnicity, level of educational attainment, marital status, and whether the respondent was living alone), health status (self-rated health and number of self-reported conditions from the eight conditions captured by the HRS, including high blood pressure, diabetes, cancer, lung disease, heart problems, stroke, psychiatric problems, and arthritis), health care utilization in the prior two years (any hospitalizations, any nursing home stays, and any visits with a doctor), insurance provider(s) (Medicare, Medicaid, CHAMPVA/TRICARE, and employer-sponsored), and year of first qualifying score. In the MCI cohort analysis, health care utilization variables were expanded to include utilization in the two years prior to the confirmatory MCI score. We chose to exclude marital status from our final models because of collinearity with the living alone variable. We surmised that daily contact with another person who might recognize symptoms of cognitive decline was the more important factor to capture.
We limited analyses to respondents with any self-reported health care utilization in the two years prior to the first dementia-qualifying score for the incident dementia cohort (355 of the 5,133 (6.9%) incident dementia respondents excluded) and to respondents with any self-reported health care utilization in the two years prior to either the first MCI-qualifying score or the confirmatory MCI score for the incident MCI cohort (41 of the 1,906 (2.2%) incident MCI respondents excluded). Respondents with missing covariate data were also excluded (18 and 1 incident dementia and incident MCI respondents, respectively). All analyses were conducted in STATA 16 (StataCorp LP, College Station, TX).
RESULTS
Characteristics of the incident dementia and incident MCI cohorts
Just over one-quarter (26.0%) of the 4,760 respondents with incident dementia received a timely diagnosis. (Table 1) Respondents with a timely diagnosis were older than those with a delayed diagnosis (76.8% 75+ years vs. 61.2%, p <0.001) and in poorer health. Additionally, a larger percentage of respondents with a timely diagnosis were non-Hispanic White and had any college-level education.
Table.1.
Characteristics of respondents with a timely and delayed diagnosis
| Incident Dementia | Incident MCI | |||||
|---|---|---|---|---|---|---|
| Timely diagnosis | Delayed diagnosis | p-value | Timely diagnosis | Delayed diagnosis | p-value | |
| (n=1,237) | (n=3,523) | (n=213) | (n=1,651) | |||
| Age in years, n (%) | ||||||
| < 65 | 94 (7.6) | 625 (17.7) | <0.001 | 32 (15.0) | 431 (26.1) | <0.001 |
| 65 – 74 | 193 (15.6) | 741 (21.0) | 43 (20.2) | 433 (26.2) | ||
| 75 – 84 | 485 (39.2) | 1,203 (34.2) | 95 (44.6) | 552 (33.4) | ||
| 85 + | 465 (37.6) | 954 (27.1) | 43 (20.2) | 235 (14.2) | ||
| Male, n (%) | 479 (38.7) | 1,372 (38.9) | 0.891 | 80 (37.6) | 673 (40.8) | 0.370 |
| Race/Ethnicity, n (%) | ||||||
| White, not Hispanic | 955 (77.2) | 2,084 (59.2) | <0.001 | 171 (80.3) | 1,075 (65.1) | <0.001 |
| Black, not Hispanic | 156 (12.6) | 875 (24.8) | 16 (7.5) | 330 (20.0) | ||
| Hispanic | 103 (8.3) | 481 (13.7) | 23 (10.8) | 207 (12.5) | ||
| Other, not Hispanic | 23 (1.9) | 83 (2.4) | 3 (1.4) | 39 (2.4) | ||
| Education, n (%) | ||||||
| Less than high school | 370 (29.9) | 1,806 (51.3) | <0.001 | 64 (30.1) | 734 (44.5) | <0.001 |
| High school/GED | 427 (34.5) | 1,052 (29.9) | 77 (36.2) | 551 (33.4) | ||
| Some college | 226 (18.3) | 433 (12.3) | 37 (17.4) | 244 (14.8) | ||
| College degree + | 214 (17.3) | 232 (6.6) | 35 (16.4) | 122 (7.4) | ||
| Marital status, n (%) | ||||||
| Married or partnered | 545 (44.1) | 1,542 (43.8) | 0.025 | 120 (56.3) | 915 (55.4) | 0.026 |
| Separated/divorced | 101 (8.2) | 375 (10.6) | 8 (3.8) | 159 (9.6) | ||
| Widowed | 551 (44.5) | 1,462 (41.5) | 81 (38.0) | 537 (32.5) | ||
| Never married | 40 (3.2) | 144 (4.1) | 4 (1.9) | 40 (2.4) | ||
| Living alone, n (%) | 467 (37.8) | 1,287 (36.5) | 0.444 | 68 (31.9) | 499 (30.2) | 0.612 |
| Fair or poor self-rated health, n (%) | 856 (69.2) | 2,013 (57.1) | <0.001 | 103 (48.4) | 731 (44.3) | 0.260 |
| Number of conditions, mean (sd) | 3.2 (1.6) | 2.7 (1.5) | <0.001 | 2.5 (1.5) | 2.1 (1.4) | <0.001 |
| Any hospital stay in 2 years prior to first qualifying score, n (%) | 787 (63.6) | 1,639 (46.5) | <0.001 | 96 (45.1) | 547 (33.1) | 0.001 |
| Any nursing home stay in 2 years prior to first qualifying score, n (%) | 559 (45.2) | 642 (18.2) | <0.001 | 14 (6.6) | 69 (4.2) | 0.111 |
| Insurance*, n (%) | ||||||
| Medicare | 1,144 (92.5) | 2,948 (83.7) | <0.001 | 182 (85.5) | 1,251 (75.8) | 0.002 |
| Medicaid | 256 (20.7) | 794 (22.5) | 0.179 | 23 (10.8) | 196 (11.9) | 0.647 |
| CHAMPVA/TRICARE | 67 (5.4) | 151 (4.3) | 0.102 | 6 (2.8) | 59 (3.6) | 0.571 |
| Employer-sponsored | 312 (25.2) | 809 (23.0) | 0.107 | 70 (32.9) | 526 (31.9) | 0.767 |
*Insurance is not mutually exclusive categories.
Only 11.4% of the 1,864 respondents with incident MCI received a timely diagnosis. (Table 1) Respondents with a timely diagnosis of MCI were older than those with a delayed diagnosis (64.8% 75+ years vs. 47.7%, p <0.001) and in somewhat poorer health. Similar to the incident dementia cohort, a larger percentage of respondents with a timely MCI diagnosis were non-Hispanic White and had any college-level education.
Timely diagnosis by race/ethnicity and education, unadjusted
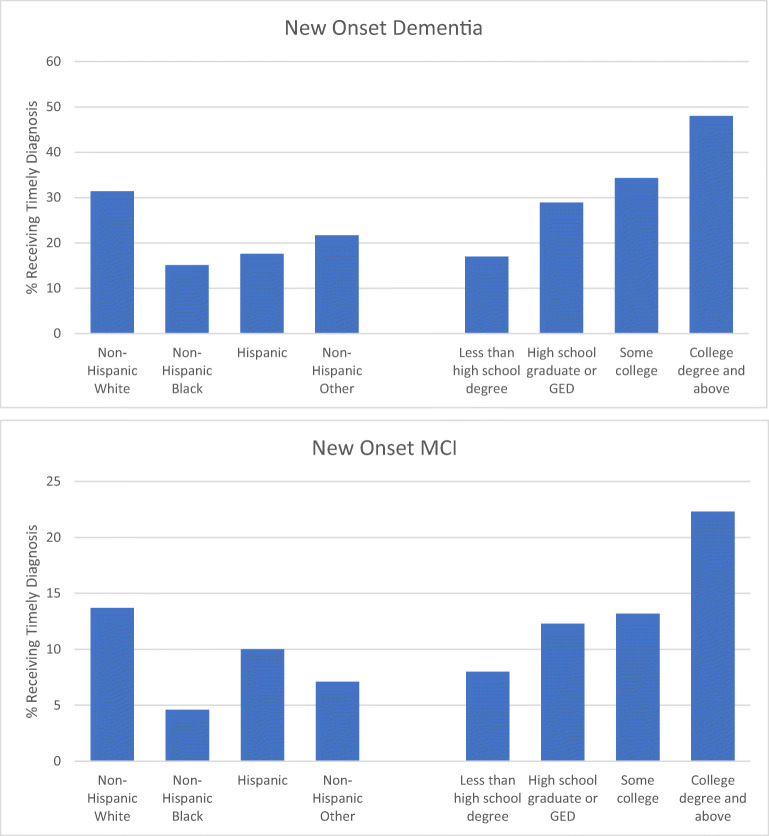
Among all non-Hispanic White respondents with incident dementia, 31.4% received a timely diagnosis as compared to only 15.1% of non-Hispanic Black respondents, 17.6% of Hispanic respondents, and 21.7% of non-Hispanic respondents of other racial groups (Fig. 2). The proportion of respondents with a timely diagnosis of dementia was positively associated with education; only 17.0% of respondents with less than a high school degree had a timely diagnosis, but just under half of respondents with a college degree (48.0%) did.
Figure 2.
Timely diagnosis within sociodemographic groups
Among all non-Hispanic White respondents with incident MCI, 13.7% received a timely diagnosis as compared to only 4.6% of non-Hispanic Black respondents, 10.0% of Hispanic respondents, and 7.1% of non-Hispanic respondents of other racial groups (Fig. 2). The proportion of respondents with a timely diagnosis of MCI increased as educational attainment increased, such that the percentage of respondents with a college degree that received a timely diagnosis (22.3%) was nearly three-fold that of respondents with less than a high school degree (8.0%).
Adjusted odds ratios
Table 2 reports adjusted odds ratios for the relationship between timely dementia diagnoses and sociodemographic characteristics. Compared to non-Hispanic Whites, non-Hispanic Blacks had significantly lower odds of receiving a timely diagnosis (odds ratio (OR): 0.61, 95% confidence interval (CI): 0.50, 0.75). Hispanics and non-Hispanic respondents of other racial groups also had lower odds, though the associations were not significant. A dose-response relationship was suggested with level of educational attainment; respondents without a high school degree had less than a third of the odds of a timely diagnosis (OR: 0.30, 95% CI: 0.23, 0.38) compared with respondents with a college degree. Respondents who lived alone were also significantly less likely to have a timely diagnosis (OR: 0.69, 95% CI: 0.59, 0.81). All health status and health care utilization variables were significantly associated with a timely dementia diagnosis, such that those in poorer health and those with higher utilization had higher odds of a timely diagnosis. Finally, year of first qualifying dementia score was a significant predictor (OR: 1.03, 95% CI: 1.02, 1.04), indicating a greater likelihood of a timely dementia diagnosis in more recent years. Sensitivity analyses conducted within the subgroup of incident dementia cases with Medicare fee-for-service coverage in the 12 months following the first dementia qualifying score (n=1,849) produced largely similar results, although health status variables were not found to be significantly associated with a timely dementia diagnosis while gender was significantly associated. (see Appendix – Table 5 for adjusted odds ratios).
Table.2.
Adjusted odds ratio of a timely diagnosis within incident dementia cohort
| Odds ratio (95% CI) | p-value | |
|---|---|---|
| Age in years | ||
| < 65 | reference group | |
| 65 – 74 | 1.43 (1.02, 1.99) | 0.036 |
| 75 – 84 | 1.89 (1.38, 2.59) | <0.001 |
| 85 + | 1.82 (1.32, 2.52) | <0.001 |
| Male | 0.95 (0.82, 1.11) | 0.540 |
| Race/Ethnicity | ||
| Non-Hispanic White | reference group | |
| Non-Hispanic Black | 0.61 (0.50, 0.75) | <0.001 |
| Hispanic | 0.77 (0.60, 1.00) | 0.055 |
| Non-Hispanic Other | 0.83 (0.50, 1.38) | 0.474 |
| Education | ||
| Less than high school | 0.30 (0.23, 0.38) | <0.001 |
| High school graduate or GED | 0.48 (0.38, 0.60) | <0.001 |
| Some college | 0.60 (0.46, 0.78) | <0.001 |
| College degree and above | reference group | |
| Living alone | 0.69 (0.59, 0.81) | <0.001 |
| Fair or poor self-rated health | 1.48 (1.26, 1.74) | <0.001 |
| Number of conditions | 1.09 (1.04, 1.14) | 0.001 |
| Any hospitalization in 2 years prior to first qualifying score | 1.17 (1.00, 1.37) | 0.049 |
| Any nursing home stay in 2 years prior to first qualifying score | 2.63 (2.22, 3.13) | <0.001 |
| Insurance provider | ||
| Medicare | 1.21 (0.89, 1.63) | 0.224 |
| Medicaid | 1.05 (0.87, 1.27) | 0.594 |
| CHAMPVA/TRICARE | 1.02 (0.74, 1.42) | 0.886 |
| Employer-sponsored | 0.98 (0.83, 1.17) | 0.839 |
| Year of first qualifying score | 1.03 (1.02, 1.04) | <0.001 |
Table.5.
Sensitivity analysis - Adjusted odds ratio of a timely diagnosis within incident dementia cohort (n=1,849)
| Odds ratio (95% CI) | p-value | |
|---|---|---|
| Age in years | ||
| < 65 | reference group | |
| 65 – 74 | 3.70 (1.50, 9.14) | 0.005 |
| 75 – 84 | 7.48 (3.07, 18.19) | <0.001 |
| 85 + | 8.11 (3.32, 19.83) | <0.001 |
| Male | 0.68 (0.54, 0.85) | 0.001 |
| Race/Ethnicity | ||
| Non-Hispanic White | reference group | |
| Non-Hispanic Black | 0.63 (0.46, 0.85) | 0.003 |
| Hispanic | 0.74 (0.48, 1.15) | 0.179 |
| Non-Hispanic Other | 0.50 (0.21, 1.19) | 0.118 |
| Education | ||
| Less than high school | 0.28 (0.19, 0.42) | <0.001 |
| High school graduate or GED | 0.47 (0.31, 0.70) | <0.001 |
| Some college | 0.47 (0.30, 0.75) | 0.002 |
| College degree and above | reference group | |
| Living alone | 0.78 (0.62, 0.99) | 0.042 |
| Fair or poor self-rated health | 1.03 (0.82, 1.30) | 0.804 |
| Number of conditions | 1.00 (0.92, 1.08) | 0.939 |
| Any hospitalization in 2 years prior to first qualifying score | 1.17 (0.93, 1.47) | 0.185 |
| Any nursing home stay in 2 years prior to first qualifying score | 3.76 (2.85, 4.95) | <0.001 |
| Insurance provider | ||
| Medicare | 1.58 (0.80, 3.09) | 0.186 |
| Medicaid | 1.08 (0.81, 1.45) | 0.595 |
| CHAMPVA/TRICARE | 1.30 (0.76, 2.24) | 0.340 |
| Employer-sponsored | 1.10 (0.86, 1.41) | 0.451 |
| Year of first qualifying score | 1.05 (1.03, 1.08) | <0.001 |
Among respondents with incident MCI, race and ethnicity and level of educational attainment were the only sociodemographic characteristics significantly associated with a timely MCI diagnosis after adjustment for other covariates. (Table 3) The odds of a timely MCI diagnosis were significantly lower for non-Hispanic Black respondents as compared to non-Hispanic Whites (OR: 0.40, 95% CI: 0.23, 0.70), though not significantly different for Hispanics or non-Hispanic respondents of other racial groups. Respondents without a college degree had lower odds of receiving a timely diagnosis than respondents with a college degree, and odds ratio estimates were suggestive of a dose-response relationship between level of education and timely diagnosis of MCI. Among the health status and health care utilization variables, only number of self-reported conditions and nursing home utilization were significant predictors of a timely MCI diagnosis, such that those with a greater number of self-reported conditions (OR: 1.12, 95% CI: 1.00, 1.26) and those with any nursing home utilization had higher odds of a timely diagnosis (OR: 3.93; 95% CI: 2.70, 5.74). As with the incident dementia cohort, respondents with an initial qualifying MCI score in a more recent year had a higher odds of a timely diagnosis (OR: 1.11, 95% CI: 1.06, 1.18).
Table.3.
Adjusted odds ratio of a timely diagnosis within incident MCI cohort
| Odds ratio (95% CI) | p-value | |
|---|---|---|
| Age in years | ||
| < 65 | reference group | |
| 65 – 74 | 1.16 (0.56, 2.40) | 0.685 |
| 75 – 84 | 1.74 (0.84, 3.58) | 0.134 |
| 85 + | 1.42 (0.64, 3.17) | 0.388 |
| Male | 0.99 (0.71, 1.37) | 0.942 |
| Race/Ethnicity | ||
| Non-Hispanic White | reference group | |
| Non-Hispanic Black | 0.40 (0.23, 0.70) | 0.001 |
| Hispanic | 1.23 (0.72, 2.11) | 0.458 |
| Non-Hispanic Other | 0.51 (0.15, 1.76) | 0.285 |
| Education | ||
| Less than high school | 0.36 (0.22, 0.60) | <0.001 |
| High school graduate or GED | 0.50 (0.31, 0.80) | 0.004 |
| Some college | 0.48 (0.28, 0.83) | 0.008 |
| College degree and above | reference group | |
| Living alone | 0.78 (0.55, 1.10) | 0.155 |
| Fair or poor self-rated health | 1.15 (0.83, 1.59) | 0.412 |
| Number of conditions | 1.12 (1.00, 1.26) | 0.050 |
| Any hospitalization in the 2 years prior to first qualifying or confirmatory score | 1.07 (0.76, 1.51) | 0.688 |
| Any nursing home stay in the 2 years prior to first qualifying or confirmatory score | 3.93 (2.70, 5.74) | <0.001 |
| Insurance provider | ||
| Medicare | 0.96 (0.47, 1.93) | 0.900 |
| Medicaid | 1.21 (0.71, 2.05) | 0.490 |
| CHAMPVA/TRICARE | 0.54 (0.22, 1.34) | 0.185 |
| Employer-sponsored | 1.07 (0.77, 1.51) | 0.678 |
| Year of first qualifying score | 1.11 (1.06, 1.18) | <0.001 |
DISCUSSION
Using data from a nationally representative survey our study found that, among older adults with new onset dementia or MCI, timely receipt of a physician diagnosis was infrequent; only 26.0% and 11.4% of our incident dementia and MCI cohorts, respectively, received a timely diagnosis. Encouragingly, the odds of a timely diagnosis for both conditions increased over the study period. In prior studies among prevalent cases of dementia, the most commonly cited reasons for missed diagnoses included: provider lack of knowledge and training in diagnosis of cognitive impairment, particularly in distinguishing normal cognitive aging from impairment; provider beliefs and attitudes toward dementia such as the stigma surrounding a diagnosis, lack of effective treatment options, and utility of a diagnosis; patient beliefs and attitudes toward cognitive evaluation; patient access to care; and insufficient time during health care visits.5
Our study also found consistent and clinically meaningful disparities in timely diagnosis across important sociodemographic characteristics, including race and ethnicity, and educational attainment. Non-Hispanic Black respondents were significantly less likely than non-Hispanic White respondents to receive a timely diagnosis of either condition, as were respondents with less than a college degree when compared with respondents with a college degree. Respondents that lived alone were also significantly less likely to receive a timely diagnosis of dementia, although no association was observed in our incident MCI cohort.
Several prior studies have demonstrated that, among prevalent cases of dementia, non-Hispanic White individuals and individuals with higher levels of education are more likely to have a clinical diagnosis.8,9,15,16,23,24 Our results build on these earlier findings and indicate that for these populations, diagnosis is occurring earlier and closer to the time in which survey cognitive assessment tools are detecting any level of cognitive impairment. The underlying mechanisms for these diagnostic disparities may be multifactorial. For example, studies have found that non-Hispanic Black individuals are more likely to believe that symptoms of dementia are a normal part of aging and less likely to believe that they are at risk of developing dementia than non-Hispanic White individuals, which may delay care seeking.25,26 Historical mistreatment by the medical community and current experiences with discrimination and bias when accessing health care may also discourage racial and ethnic minority populations from seeking care.27,28 Other possible reasons include differences by race and education in access to quality health care, provider recognition and treatment of cognitive decline, and performance of cognitive assessment tools.
The evidence on how one’s living situation impacts timing of dementia diagnosis is mixed. Primary care providers indicate that concerns of relatives, caregivers, or friends are an important trigger of a comprehensive cognitive examination of their patients.29 However, studies also suggest that relative and caregiver assessments of cognitive functioning frequently differ from health care provider assessments.30 The one prior study that examined patient living situation and receipt of a formal diagnosis among individuals with dementia found that living alone was a significant risk factor for being undiagnosed.31 Our findings are consistent, further indicating that living alone is significantly associated with a later dementia diagnosis.
Our study has limitations. Self- or proxy-report of a health care provider diagnosis of a memory issue or dementia may not accurately reflect the receipt or timing of a diagnosis, especially for respondents experiencing cognitive impairment. However, sensitivity analyses using claims data to identify the presence and timing of a diagnosis code indicate that our results are robust to the definition of timely diagnosis. We do not include an indicator for a proxy respondent in the primary models. However, our results are robust to this indicator with the exception that health controls lose statistical significance, likely due to the correlation between health limitations and use of proxy respondents. Due to data limitations, we were unable to examine provider factors associated with a timely diagnosis of dementia and MCI, which prior studies suggest may play an important role in diagnosis timing.5 Sample size limitations precluded our ability to disaggregate the “Non-Hispanic Other” group, though access to and receipt of health care for cognitive impairment may have differed significantly for subpopulations in this group. Finally, our analysis is correlational in nature and our findings do not necessarily have a causal interpretation.
In the absence of routine screening of older adults for cognitive impairment, targeted approaches aimed at racial and ethnic minorities, individuals with lower educational attainment, and older individuals living alone may improve detection and reduce disparities around a timely diagnosis of dementia and MCI.
Acknowledgements
This study was supported by the National Institute on Aging (R01AG049815). Dr. Coe was also supported by the National Institute on Aging (P30AG012836).
Appendix
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Langa KM, Larson EB, Crimmins EM, et al. A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern Med. 2017;177(1):51–58. doi: 10.1001/jamainternmed.2016.6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: A clinical review. JAMA. 2014;312(23):2551–2561. doi: 10.1001/jama.2014.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meng X, D’Arcy C. Education and dementia in the context of the cognitive reserve hypothesis: A systematic review with meta-analyses and qualitative analyses. PLoS One. 2012;7(6):e38268. doi: 10.1371/journal.pone.0038268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Power MC, Bennett EE, Turner RW, et al. Trends in relative incidence and prevalence of dementia across non-Hispanic Black and White individuals in the United States, 2000-2016. JAMA Neurol. 2021;78(3):275–284. doi: 10.1001/jamaneurol.2020.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradford A, Kunik ME, Schulz P, Williams SP, Singh H. Missed and delayed diagnosis of dementia in primary care: Prevalence and contributing factors. Alzheimer Disease and Associated Disorders. 2009;23(4):306–314. doi: 10.1097/WAD.0b013e3181a6bebc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chodosh J, Petitti DB, Elliott M, et al. Physician recognition of cognitive impairment: Evaluating the need for improvement. J Am Geriatr Soc. 2004;52(7):1051–1059. doi: 10.1111/j.1532-5415.2004.52301.x. [DOI] [PubMed] [Google Scholar]
- 7.Valcour VG, Masaki KH, Curb JD, Blanchette PL. The detection of dementia in the primary care setting. Archives of Internal Medicine. 2000;160(19):2964–2968. doi: 10.1001/archinte.160.19.2964. [DOI] [PubMed] [Google Scholar]
- 8.Gianattasio KZ, Prather C, Glymour MM, Ciarleglio A, Power MC. Racial disparities and temporal trends in dementia misdiagnosis risk in the United States. Alzheimers Dement (N Y) 2019;5:891–898. doi: 10.1016/j.trci.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amjad H, Roth DL, Sheehan OC, Lyketsos CG, Wolff JL, Samus QM. Underdiagnosis of dementia: An observational study of patterns in diagnosis and awareness in US older adults. J Gen Intern Med. 2018;33(7):1131–1138. doi: 10.1007/s11606-018-4377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin JS, O'Connor E, Rossom RC, et al. U.S. Preventive Services Task Force evidence syntheses, formerly systematic evidence reviews. Screening for cognitive impairment in older adults: An evidence update for the U.S. Preventive Services Task Force. Rockville: Agency for Healthcare Research and Quality (US); 2013. [PubMed] [Google Scholar]
- 11.Barthold D, Marcum ZA, Chen S, et al. Difficulty with taking medications is associated with future diagnosis of Alzheimer's disease and related dementias. J Gen Intern Med. 2021;36(4):863–868. doi: 10.1007/s11606-020-06279-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCormick WC, Kukull WA, van Belle G, Bowen JD, Teri L, Larson EB. Symptom patterns and comorbidity in the early stages of Alzheimer's disease. J Am Geriatr Soc. 1994;42(5):517–521. doi: 10.1111/j.1532-5415.1994.tb04974.x. [DOI] [PubMed] [Google Scholar]
- 13.Nicholas LH, Langa KM, Bynum JPW, Hsu JW. Financial presentation of Alzheimer disease and related dementias. JAMA Intern Med. 2020 181:220-227 [DOI] [PMC free article] [PubMed]
- 14.Amjad H, Roth DL, Samus QM, Yasar S, Wolff JL. Potentially unsafe activities and living conditions of older adults with dementia. J Am Geriatr Soc. 2016;64(6):1223–1232. doi: 10.1111/jgs.14164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin PJ, Emerson J, Faul JD, et al. Racial and ethnic differences in knowledge about one's dementia status. J Am Geriatr Soc. 2020;68(8):1763–1770. doi: 10.1111/jgs.16442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Tysinger B, Crimmins E, Zissimopoulos JM. Analysis of dementia in the US population using Medicare claims: Insights from linked survey and administrative claims data. Alzheimers Dement (N Y) 2019;5:197–207. doi: 10.1016/j.trci.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Health and Retirement Study . RAND HRS Longitudinal File 2016 (V1) public use dataset. Ann Arbor: Produced and distributed by the University of Michigan with funding from the National Institute on Aging (grant number NIA U01AG009740); 2019. [Google Scholar]
- 18.RAND HRS Longitudinal File 2016 (V1) Produced by the RAND Center for the Study of Aging. Santa Monica: with funding from the National Institute on Aging and the Social Security Administration; 2019. [Google Scholar]
- 19.McCammon RJ, Fisher GG, Hassan H, Faul JD, Rodgers WL, Weir DR. Health and Retirement Study Imputation of Cognitive Functioning Measures: 1992-2016 (Version 1.0) Ann Arbor: Produced by the University of Michigan Survey Research Center; 2019. [Google Scholar]
- 20.Crimmins EM, Kim JK, Langa KM, Weir DR. Assessment of cognition using surveys and neuropsychological assessment: The Health and Retirement Study and the Aging, Demographics, and Memory Study. J Gerontol B Psychol Sci Soc Sci. 2011;66(Suppl 1):i162–171. doi: 10.1093/geronb/gbr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langa KM, Weir DR, Kabeto M, Sonnega A. Langa-Weir classification of cognitive function (1995 onward) Ann Arbor: Produced by the University of Michigan Survey Research Center; 2018. [Google Scholar]
- 22.Robertson K, Larson EB, Crane PK, et al. Using varying diagnostic criteria to examine mild cognitive impairment prevalence and predict dementia incidence in a community-based sample. J Alzheimers Dis. 2019;68(4):1439–1451. doi: 10.3233/JAD-180746. [DOI] [PubMed] [Google Scholar]
- 23.Borson S, Scanlan JM, Watanabe J, Tu SP, Lessig M. Improving identification of cognitive impairment in primary care. Int J Geriatr Psychiatry. 2006;21(4):349–355. doi: 10.1002/gps.1470. [DOI] [PubMed] [Google Scholar]
- 24.Savva GM, Arthur A. Who has undiagnosed dementia? A cross-sectional analysis of participants of the Aging, Demographics and Memory Study. Age and Ageing. 2015;44(4):642–647. doi: 10.1093/ageing/afv020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts JS, Connell CM, Cisewski D, Hipps YG, Demissie S, Green RC. Differences between African Americans and whites in their perceptions of Alzheimer disease. Alzheimer Dis Assoc Disord. 2003;17(1):19–26. doi: 10.1097/00002093-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Connell CM, Roberts JS, McLaughlin SJ, Akinleye D. Racial differences in knowledge and beliefs about Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23(2):110–116. doi: 10.1097/WAD.0b013e318192e94d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chin AL, Negash S, Hamilton R. Diversity and disparity in dementia: The impact of ethnoracial differences in Alzheimer’s disease. Alzheimer Dis Assoc Disord. 2011;25(3):187–195. doi: 10.1097/WAD.0b013e318211c6c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alzheimer’s Association 2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021;17(3):327–406. doi: 10.1002/alz.12328. [DOI] [PubMed] [Google Scholar]
- 29.Brayne C, Fox C, Boustani M. Dementia screening in primary care: Is it time? JAMA. 2007;298(20):2409–2411. doi: 10.1001/jama.298.20.2409. [DOI] [PubMed] [Google Scholar]
- 30.Stella F, Forlenza OV, Laks J, et al. Caregiver report versus clinician impression: Disagreements in rating neuropsychiatric symptoms in Alzheimer's disease patients. Int J Geriatr Psychiatry. 2015;30(12):1230–1237. doi: 10.1002/gps.4278. [DOI] [PubMed] [Google Scholar]
- 31.Wilkins CH, Wilkins KL, Meisel M, Depke M, Williams J, Edwards DF. Dementia undiagnosed in poor older adults with functional impairment. J Am Geriatr Soc. 2007;55(11):1771–1776. doi: 10.1111/j.1532-5415.2007.01417.x. [DOI] [PubMed] [Google Scholar]