Abstract
Decreased estrogen levels in menopause are associated with anthropometric, metabolic, and inflammatory impairments, predisposing women to cardiometabolic risk factors such as diabetes. Menopause and type two diabetes (DM2) are marked by altered heat shock response (HSR), shown by decreased expression of the 70-kDa heat shock protein in the intracellular milieu (iHSP70). While iHSP70 plays an anti-inflammatory role, extracellular HSP70 (eHSP70) may mediate pro-inflammatory pathways and has been associated with insulin resistance in DM2. Considering the roles of these proteins according to localization, the eHSP70-to-iHSP70 ratio (H-index) has been proposed as a biomarker for HSR. We, therefore, evaluated whether this biomarker is associated with glycemic and inflammatory status in postmenopausal women. In this transversal study, 36 postmenopausal women were grouped according to fasting glycemia status as either the control group (normoglycemic, ≤ 99 mg/dL) or DM2 (prediabetic and diabetic, glycemia ≥ 100 mg/dL). DM2 group showed higher triglyceride/glucose (TyG) index and plasma atherogenic index (PAI), both of which are indicators of cardiometabolic risk. In addition, we found that the eHSP70-to-iHSP70 ratio (plasma/peripheral blood mononuclear cells–PBMC ratio) was higher in the DM2 group, compared with the control group. Furthermore, blood leukocyte and glycemia levels were positively correlated with the eHSP70-to-iHSP70 ratio in women that presented H-index values above 1.0 (a.u.). Taken together, our results highlight the eHSP70-to-iHSP70 ratio as a biomarker of altered HSR in DM2 postmenopausal women.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12192-022-01288-8.
Keywords: Menopause, H-index, Glycemia, HSP70, Diabetes
Introduction
Menopause, a physiological event defined by the interruption of menstrual cycles and caused by cessation of ovarian 17-β-estradiol (E2) production, is associated with weight gain and abdominal fatty deposition. In turn, abdominal/visceral adiposity is related to the glycemic and lipidemic impairments found in postmenopausal women, contributing to the development of metabolic diseases, such as type two diabetes (DM2) (Hendryx et al. 2020).
As women’s life expectancy is 73.6 years and menopause occurs on average at 48.7 years old (Schoenaker et al. 2014), women live approximately one-third of their lives in hypoestrogenic conditions. According to estimates, in 2050, approximately 16% of populations in developing countries will be older than 65 years, with a significant increase in the number of postmenopausal women (ONU 2019) and consequent increased risk of metabolic complications.
The effects of hypoestrogenism include modification of the inflammatory profile, resulting in cellular signaling alterations, as heat shock response (HSR) is related to metabolic impairment. Decreased glycolysis and glucose metabolism and increased innate immune responses are observed due to dysregulation of E2 (Wang et al. 2020). At least in part, the cardiometabolic protective effects of E2 are related to its capacity to induce HSR (Miragem and de Bittencourt 2017). For example, maintenance of the cytoprotective ability of many cells is mediated by increased levels of the 70-kDa heat shock protein in the intracellular milieu (iHSP70), which is potentiated or induced by E2 (Knowlton and Sun 2001; Hamilton et al. 2004a, b; Stice et al. 2011). HSP70 is particularly sensitive to stress conditions and is highly expressed by different cell types (mainly the inducible isoform, aka HSP72 or HSPA1A), including blood leukocytes (Hunter-Lavin et al. 2004; Ireland et al. 2007), while its antioxidant, anti-inflammatory, and immune-modulatory roles are mediated by stabilizing the nuclear factor-kappa B (NF-kB) complex and preventing its translocation to the nucleus (Chen et al. 2005; Heck et al. 2011, 2017). Thus, the reduction in E2 affects immune cells in menopausal women.
Decreased iHSP70 levels in patients (Rodrigues-Krause et al. 2012; Cangeri Di Naso et al. 2015) and animal models (Chung et al. 2008) have been demonstrated in the context of obesity/diabetes. Increased iHSP70 levels have also been shown to prevent obesity-induced hyperglycemia, hyperinsulinemia, glucose intolerance, and insulin resistance (Chung et al. 2008; Henstridge et al. 2014; Tsuzuki et al. 2017). Thus, strategies to recover iHSP70 levels (and consequently metabolic health) have been under investigation for cardiometabolic diseases, including atherosclerosis (Bruxel et al. 2019), diabetes (Chung et al. 2008; Hooper et al. 2014), and menopause (Lissarassa et al. 2020), demonstrating the relevance of monitoring iHSP70 levels as a biomarker of health.
However, HSP70 is also found in extracellular fluids (eHSP70), as it is released by immune cells under stress conditions (Hunter-Lavin et al. 2004; Ireland et al. 2007; Heck et al. 2017). High levels of eHSP70 are found in the plasma of patients with diabetes (Rodrigues-Krause et al. 2012; Krause et al. 2014; Heidari et al. 2020) and atherosclerosis (Costa-Beber et al. 2020). Extracellular HSP70 may mediate inflammatory pathways by binding to cell surface receptors (e.g., toll-like receptors, TLR) and promoting NF-kB and AP-1 activation (González-Ramos et al. 2013). Therefore, high levels of eHSP70 represent a warning signal related to metabolic (Alemi et al. 2019) and cardiovascular diseases (Zhang et al. 2010; Krepuska et al. 2011). Together, menopause and diabetes synergistically influence metabolic and oxidative blood markers, while eHSP72 levels are associated with number of years postmenopause (Anklam et al. 2021).
Considering the dual roles of eHSP70 and iHSP70 (Costa-Beber et al. 2020), their relative levels may be relevant for evaluating inflammatory status. Predominance of eHSP70 over iHSP70 indicates unfavorable conditions, low-grade inflammation, and susceptibility to insulin resistance (Krause et al. 2015a). Taking this into consideration, the eHSP70-to-iHSP70 ratio (H-index) should present a baseline value close to 1.0 (1:1 eHSP70-to-HSP70 ratio, when measuring plasma vs. intracellular levels). Indirect calculations have previously shown that higher eHSP70-to-iHSP70 ratios are present in many acute and chronic stress situations or diseases (Heck et al. 2017). Independently, metabolic diseases and challenges to the organism have been shown to be sufficient to increase the H-index to values considered risky (Krause et al. 2015b, a; Bittencourt and Porto 2017; Heck et al. 2017; Mai et al. 2017; Bruxel et al. 2019; Kostrycki et al. 2019). However, it is not yet known whether the eHSP70-to-iHSP70 ratio can assist in the evaluation of inflammatory and metabolic profiles in humans. We, therefore, investigated whether the eHSP70-to-iHSP70 ratio could be used as a biomarker of glycemic and inflammatory status in postmenopausal women.
Methods
Study design
Women participating in care groups in the Family Health Strategies of a town in southern Brazil were recruited to take part in our study. Initially, 93 postmenopausal women were interviewed face to face in order to obtain sociodemographic information, medical history of chronic diseases, time of amenorrhea, use of medications, and dietary information. Postmenopausal status was established by an amenorrhea period of at least 12 months and confirmed by circulating levels of estrogen (17β-estradiol; 25.3 ± 18.6 pg/mL, reference value [RV]: up to 28 pg/mL) and follicle-stimulating hormone (FSH; 55 ± 28.27 mUI/mL, RV: 26.72–133.4 mUI/mL). Furthermore, we applied the following exclusion criteria: current use of hormonal replacement therapy, occurrence of menstrual cycles in the last 12 months, cancer, autoimmune diseases, acute infection, non-treated hypertension, smoking, undergoing chemotherapy treatment, and regular or eventual use of insulin. Finally, 36 women (64.9 ± 6.5 years old) were eligible to participate in this study.
Women were first distributed into two groups according to glycemic profile: control group (normoglycemic, ≤ 99 mg/dL and HbA1c < 5.7%, n = 18) or DM2 (prediabetic and diabetic, glycemia ≥ 100 mg/dL and HbA1c ≥ 5.7% n = 18), for the characterization of metabolic, anthropometric, hematological, and inflammatory parameters, as well as analysis of eHSP70 and iHSP70 levels and individual evaluation of H-index. The participants were further stratified according to H-index: H ≤ 1.0 (n = 19) or H > 1.0 (n = 17) to verify any association with metabolic, anthropometric, hematological, or inflammatory parameters.
This study was designed based on the expected difference for the essential variable in this study, the eHSP72-to-iHSP70 ratio. We expected a mean difference between groups of around 50% (i.e., 1.00 vs. 1.50, based on several indirect data; see Supplementary Table S2 in Heck et al. 2017), and a standard deviation of approximately 50% of the mean, signifying an expected effect size of 1.0; we therefore used a statistical power of 80% with a significance level of 0.05%. The sample size was defined as 18 subjects per group.
The study was conducted according to the guidelines and regulatory norms for research involving human beings according to resolution no. 466/2012 of the National Health Council and approved by UNIJUI’s Ethics Committee no. 1.173.158.
Anthropometric analysis
Body weight (kg) was verified using a calibrated scale; waist (WC), abdominal (AC), and hip circumference (HC) and height were measured with a standard measuring tape (cm). For HC, measurements were taken at the point of greatest circumference of the gluteal region using a standard, non-elastic measuring tape, keeping the tape in a horizontal plane and avoiding pressing the soft tissues. To analyze WC, we utilized the Brazilian guidelines for obesity and risk values for metabolic complications (WC ≥ 80 cm, Diretrizes brasileiras de obesidade 2016). We also evaluated the waist-to-hip ratio by the direct quotient of waist and hip circumference, classified according to the cutoff points recommended by the World Health Organization.
We calculated body mass index (BMI) using the Quetelet equation, and analyzed it according to Brazilian Association for the Study of Obesity guidelines (Diretrizes brasileiras de obesidade 2016). As a complement to this, we evaluated the adiposity index (AI) using the equation: (Bergman et al. 2011) and the conicity index (CI) according to the formula (Valdez 1991).
Blood collection
Biochemical analyses were performed on blood collected from patients after 10 to 12 h of fasting. Blood was immediately separated in vacuum tubes with or without ethylenediaminetetraacetic acid (EDTA; 2 mg/mL of blood) for whole blood aliquots and plasma separation or to obtain serum, respectively. Aliquots for plasma and serum were centrifuged for 15 min at 3000 rpm. Whole blood was used to measure glycated hemoglobin, erythrocyte sedimentation rate (ESR), and blood count, while serum was used to measure E2 levels and lipid, glycemic, and hepatic profiles. Plasma samples were frozen in liquid nitrogen with 1.74 mg/mL (100 mM) PMSF (phenylmethylsulfonyl fluoride, Sigma P7626) for subsequent measurement of IL-6 and eHSP70 levels.
Hormonal dosage
Quantitative analysis of E2 and FSH was performed on serum samples by chemiluminescence, using the automated ADVIA Centaur XP system (Siemens Healthcare Diagnosis). E2 levels were expressed in pg/mL and FSH levels in mUI/mL. The sensitivity and in vitro test limits for measurement of E2 levels were above 20 pg/mL.
Glycemic, lipid, and hepatic profiles
Total cholesterol, high-density lipoprotein (HDL), and triglyceride (TG) levels and fasting glycemia were analyzed in serum samples by enzymatic-colorimetric assays (BioClin Quibasa), using the automated BS200 system (Shenzhen Mindray Bio-medical Electronics). The Friedewald equation for low-density lipoprotein (LDL) indirectly verified , where represents cholesterol bound to VLDL-C (Sposito, 1997). Results were expressed in mg/dL.
Values obtained for TG and HDL were used to calculate the plasma atherogenic index (PAI) according to the formula (Dobiášová 2004). PAI is a sensitive predictor of coronary atherosclerosis and cardiovascular risk (Dobiášová, 2004). PAI levels are classified as follows: low risk, − 0.3 to 0.1; moderate risk, 0.1–0.24; and high risk, > 0.24.
We also calculated the TyG index using TG and fasting glycemia with the formula (Guerrero-Romero et al. 2016). TyG is proposed to indicate insulin resistance by triglyceride levels and fasting glycemia, with cutoff points of 4.55 for women and 4.68 for men (Guerrero-Romero et al. 2016).
Glycated hemoglobin was measured in whole blood aliquots by high-performance liquid chromatography (HPLC). Estimated mean glycemia (EMG) was calculated by the formula , in agreement with the Brazilian Society of Diabetes (Netto et al. 2015). Results were expressed as percentages (%).
Hepatic enzymes oxalacetic glutamic transaminase (OGT), glutamic pyruvic transaminase (GPT), and gamma-glutamyl transferase (GGT) were measured in serum samples using Bioclin Quibasa kits and the automated BS200 system (as above), by kinetic methods. Results were expressed in U/L.
Hematological parameters and inflammatory status
Hematological analysis was performed automatically using spectrophotometry, and impedance was analyzed by hemoscopic reassessment using the ABX Micros 60 hematology analyzer (HORIBA). With this equipment, we obtained parameters for red, white, and platelet series, as follows. Red series: total red blood cell count (cell/mm3), hemoglobin (g/dL), hematocrit (%), mean corpuscular volume (MCV; μm3), mean corpuscular hemoglobin (MCH; pg), mean corpuscular hemoglobin concentration (MCHM; %), and red cell distribution width (RDW; %). White series: total leukocyte count (mm3), differential leukocyte count (mm3), and platelet count (mm3). We calculated the neutrophil-to-lymphocyte ratio based on the absolute count; both are expressed in mm3. Westergreen’s pipette technique was used to verify ESR in whole blood.
Levels of circulating eHSP72 and IL-6 were determined in plasma samples using high-sensitivity enzyme-linked immunosorbent assays (ELISA): HSP70-specific ELISA kit (Eks-715, Enzo Life Sciences) and IL-6 ELISA kit (RayBio® Human IL-6 kit, Raybiotech, Inc., USA). According to the manufacturer’s recommendations, standard curves were constructed from known dilutions of the respective recombinant protein to allow quantitative assessment of the plasma aliquots. Both quantifications were performed using a microplate reader (Mindray MR-96A) at 450 nm. Levels of eHSP70 were expressed in ng/mL/106 cells and IL-6 levels in pg/mL.
Levels of iHSP70 were assessed in peripheral blood mononuclear cells (PBMCs). PBMCs were isolated from whole peripheral blood by isopycnic separation using Histopaque solution 1077. This method is based on the separation of PBMCs from peripheral blood rich in leukocytes through a discontinuous polysaccharose gradient. For this, we centrifuged whole blood containing EDTA (as previously described) and Histopaque (1:1) at 400 g for 10 min. The opaque layer (containing mononuclear cells) at the interface between plasma (superior layer) and Histopaque solution (median layer) was collected. The mononuclear cells were then transferred to a conical tube containing phosphate buffered saline (PBS) (1:10) and centrifuged at 250 g for 20 min at room temperature in order to precipitate the cells.
One aliquot of PBMCs was used for cell counting, while PMSF and assay buffer (1:100) were added to the other aliquot, which was immediately frozen in liquid nitrogen to await further analysis. The level of iHSP70 in the PBMCs was evaluated using the Eks-715 kit (Enzo Life Sciences) and normalized using the total number of leukocytes from each sample. The mean total blood count for Histopaque was 1.6 × 106 cells.
Furthermore, we used plasma eHSP70 (ng/mL) and PBMC iHSP70 levels (both normalized by total leukocyte numbers, ng/106 cells) to calculate the H-index. The H-index is represented by the eHSP70-to-iHSP70 ratio and can be used to estimate immune-inflammatory balance (Heck et al. 2017). H-index lower than or equal to 1.0 is classified as physiological, while between 1.0 and 5.0 is immune inflammatory, and higher than 5.0 is classified as excessively inflammatory (Schöler et al. 2016).
Statistical analysis
We verified normality using the Kolmogorov–Smirnov test and compared the means of normoglycemic and dysglycemic postmenopausal women using the two-tailed Student’s t-test. We also performed Pearson correlation. Data were expressed as mean ± standard deviation (SD) and were processed using GraphPad 9.0 software, considering P < 0.05.
Results
Postmenopausal subject profiles: alterations associated with dysglycemia
The postmenopausal study participants, divided into control and DM2 groups, presented similar profiles in terms of age, time of menopause, and anthropometric characteristics (BMI, WC, WHC, BAI, CI; see data in Table 1, white background) and medications (Table S1). However, in both groups, the BMI was above 30 kg/m2, and the WC was above 88 cm; these values are characteristic of overweight/obese subjects.
Table 1.
Anthropometric, metabolic, hepatic, and hematological parameters in postmenopausal study participants
| Parameters | Control (n = 18) | DM2 (n = 18) | P-value |
|---|---|---|---|
| Age (years) | 64.7 ± 1.5 | 65.2 ± 1.6 | 0.820 |
| Time of menopause (years) | 16.5 ± 2.2 | 20.6 ± 2.7 | 0.245 |
| Body mass index, BMI (kg/m2) | 30.3 ± 1.5 | 32.2 ± 1.1 | 0.313 |
| Waist circumference, WC (cm) | 101.9 ± 3.4 | 106.5 ± 2.9 | 0.313 |
| Waist to hip ratio, WHC (cm) | 1.0 ± 0.03 | 1.0 ± 0.03 | 0.781 |
| Body adiposity index, BAI | 35.3 ± 1.2 | 36.7 ± 1.3 | 0.430 |
| Conicity index, CI | 1.4 ± 0.02 | 1.4 ± 0.04 | 0.798 |
| HbA1c (%) | 5.6 ± 0.1 | 8.03 ± 0.5* | < 0.0001 |
| EMG (mg/dL) | 114.7 ± 3.1 | 183.7 ± 15.5* | < 0.0001 |
| Triglycerides (mg/dL) | 146.0 ± 21.5 | 172.7 ± 14.7 | 0.313 |
| Total cholesterol (mg/dL) | 200.8 ± 12.3 | 192.4 ± 9.9 | 0.597 |
| High-density lipoprotein, HDL (mg/dL) | 58.1 ± 2.4 | 53.6 ± 2.3 | 0.190 |
| Low-density lipoprotein, LDL (mg/dL) | 108.0 ± 8.5 | 104.4 ± 8.3 | 0.763 |
| Oxalacetic glutamic transaminase, OGT (U/L) | 26.5 ± 4.5 | 27.4 ± 2.6 | 0.873 |
| Glutamic pyruvic transaminase, GPT (U/L) | 22.4 ± 5.4 | 23.5 ± 3.3 | 0.863 |
| Gamma-glutamil transferase, GGT (U/L) | 30.5 ± 6.7 | 33.3 ± 7.5 | 0.787 |
| Erythrocyte sedimentation rate, ESR (mm/1a h) | 22.2 ± 3.7 | 18.8 ± 3.7 | 0.525 |
| Interleukin 6, IL-6 (pg/mL) | 2.3 ± 0.3 | 2.3 ± 0.3 | 0.976 |
| Hemoglobin, Hb (g/dL) | 13.7 ± 0.3 | 13.8 ± 0.3 | 0.842 |
| Hematocrit, Ht (%) | 41.8 ± 0.7 | 42.2 ± 0.9 | 0.317 |
| Mean corpuscular volume, MCV (fl) | 85.2 ± 3.9 | 90.8 ± 1.0 | 0.179 |
| Mean corpuscular Hb, MCH (pg) | 33.7 ± 4.1 | 29.0 ± 0.7 | 0.272 |
| Mean corpuscular Hb conc., MCHC (%) | 33.1 ± 0.2 | 32.9 ± 0.1 | 0.281 |
| Red cell distribution width, RDW (%) | 14.2 ± 1.2 | 13.0 ± 0.2 | 0.334 |
| Leukocytes (mm3) | 5906 ± 312.5 | 7100 ± 441.8* | 0.034 |
| Band neutrophils (mm3) | 73.4 ± 7.0 | 101.4 ± 15.8 | 0.114 |
| Segmented neutrophils (mm3) | 3350 ± 219.0 | 4244 ± 340.9* | 0.034 |
| Basophils (mm3) | 0 | 0 | |
| Eosinophils (mm3) | 140.1 ± 26.16 | 173.3 ± 26.2 | 0.360 |
| Monocytes (mm3) | 258.8 ± 20.4 | 315.3 ± 19.9 | 0.054 |
| Lymphocytes (mm3) | 2080 ± 143.6 | 2266 ± 132.2 | 0.349 |
| Platelets (1000/mm3) | 218.7 ± 11.3 | 224.8 ± 12.1 | 0.717 |
| Neutrophil-to-lymphocyte ratio | 1.7 ± 0.2 | 1.9 ± 0.1 | 0.453 |
Legend. Postmenopausal women divided into control group (fasting glycemia ≤ 99 mg/dL) and DM2 group (fasting glycemia ≥ 100 mg/dL) groups. Results are expressed as mean ± standard deviation. Two-tailed t-test, P < 0.05
As expected, the DM2 group presented higher (1.58-fold) HbA1c levels than the control group; EMG was therefore approximately 69 mg/dL higher in the DM2 group compared with the control group (Table 1, light gray background). However, the groups presented similar profiles for both serum lipids (TG, TC, HDL, LDL) and hepatic enzymes (OGT, GPT, GGT) (Table 1, medium gray background).
In terms of hematological and inflammatory markers (Table 1, dark gray background), IL-6 levels were above 1.0 pg/mL in both groups, while there was no difference for either IL-6 or ESR between groups. Neutrophils and leukocyte counts were higher for the DM2 group than the control group. No other hematological or inflammatory markers showed any differences between groups (band neutrophils, basophils, eosinophils, monocytes, lymphocytes, platelets, and neutrophil-to-lymphocyte ratio).
Both groups presented TyG index means > 4.55, defined as the cutoff for insulin resistance, while the DM2 group had a higher TyG index than the control group (Fig. 1A). In addition, the PAI was higher in the DM2 group (Fig. 1B), corresponding to moderate cardiovascular risk (PAI = 0.13 ± 0.03), while the control group had a lower cardiovascular risk (PAI = − 0.02 ± 0.07).
Fig. 1.
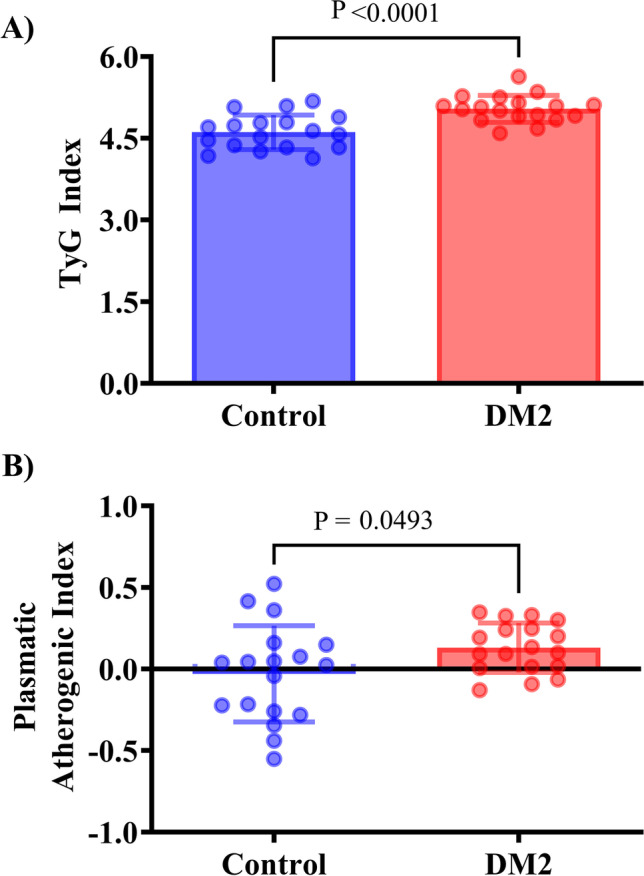
TyG and plasmatic atherogenic index (PAI) in DM2 postmenopausal women. A TyG (mg/dL). B PAI (mmol/L). Data expressed as mean ± SD. Two-tailed t-test, P < 0.05 (n = 18 per group)
DM2 group did not presented either plasma levels of eHSP72 or iHSP70 levels in PBMCs (Fig. 2A and B). When calculating the eHSP70-to-iHSP70 ratio, we found that the DM2 group had an H-index superior to 1.0 (H = 1.369 ± 0.99); this was higher than in the normoglycemic group (H = 0.762 ± 0.55; P = 0.029) (Fig. 2C).
Fig. 2.
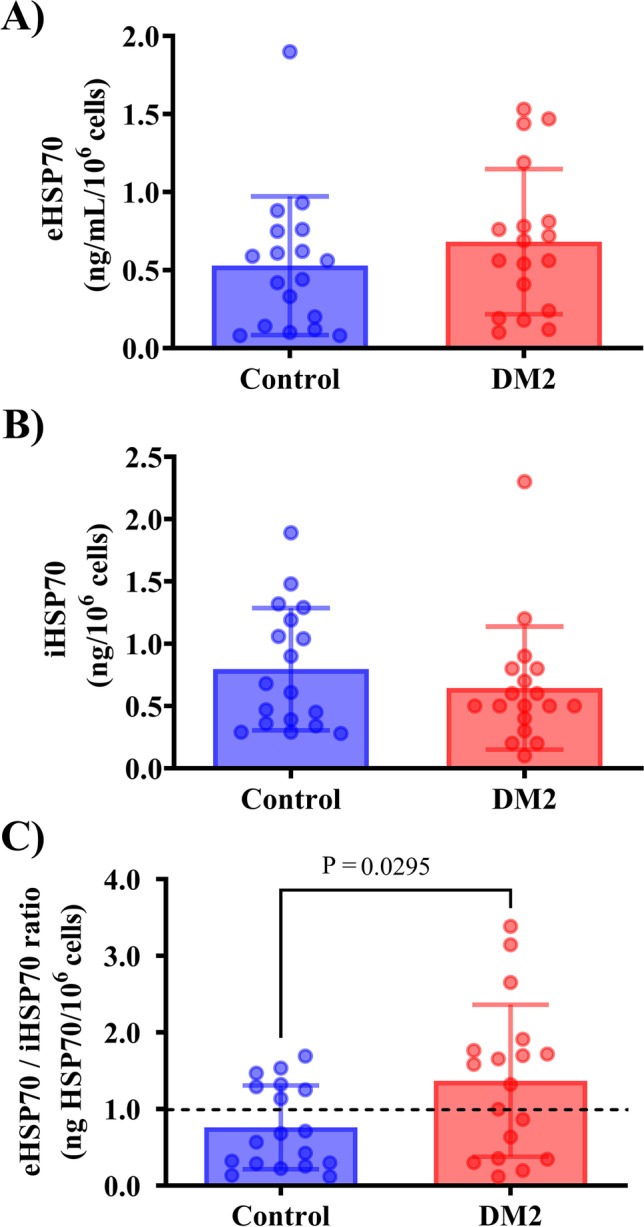
HSP70 levels in DM2 postmenopausal women. A Plasma eHSP70 levels. B PBMC HSP70 levels (iHSP70, ng/10.6 cells). C H-index (eHSP70-to-iHSP70 ratio). Data expressed as mean ± SD. Two-tailed t-test (n = 18 per group)
Postmenopausal subject profiles: changes associated with altered eHSP70-to-iHSP70 ratio
We divided the study participants into two groups, H ≤ 1.0 (n = 19) and H ˃ 1.0 (n = 17), to verify a possible correlation between the eHSP70-to-iHSP70 ratio and metabolic or inflammatory parameters. In the H ˃ 1.0 group, we found a moderately positive correlation between H-index and fasting glycemia levels (r = 0.490), as well as a moderately positive correlation between H-index and total leukocyte count (r = 0.514) (black circles in Fig. 3A and B). The H-index did not correlate with other inflammatory or metabolic variables (Table 2).
Fig. 3.
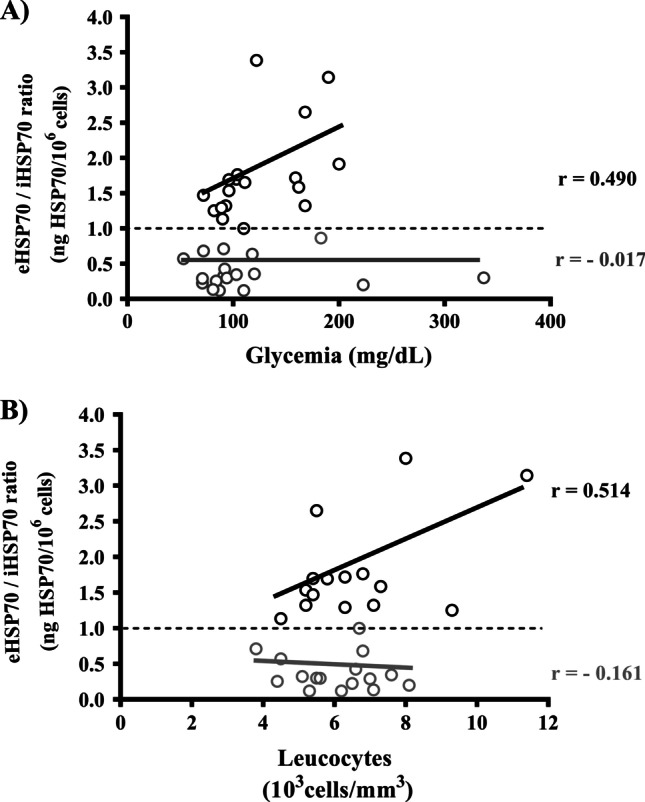
Association between eHSP70-to-iHSP70 ratio and metabolic or inflammatory parameters in normoglycemic and dysglycemic postmenopausal women. A Pearson correlation between H-index and glycemia, H ≤ 1.0 (gray circles): r = − 0.017, P = 0.9449, n = 19; H ˃ 1.0 (black circles), r = 0.490, P = 0.0457, n = 17. B Pearson correlation between H-index and leukocyte count, H ≤ 1.0, r = − 0.161, P = 0.5524, n = 19; H ˃ 1.0, r = 0.514, P = 0.0499, n = 17
Table 2.
Metabolic and inflammatory markers in postmenopausal women stratified by eHSP70-to-iHSP70 ratio
| H-index ≤ 1.0 (n = 19) | H-index > 1.0 (n = 17) | |||
|---|---|---|---|---|
| R value | P value | R value | P value | |
| HbA1c (%) | − 0.131 | 0.603 | 0.412 | 0.099 |
| CT (mg/dL) | 0.088 | 0.719 | 0.022 | 0.930 |
| HDL (mg/dL) | − 0.213 | 0.381 | 0.030 | 0.906 |
| LDL (mg/dL) | 0.223 | 0.357 | − 0.009 | 0.971 |
| TG (mg/dL) | − 0.080 | 0.743 | 0.156 | 0.549 |
| BMI (kg/m2) | − 0.379 | 0.109 | 0.175 | 0.501 |
| WC (cm) | − 0.190 | 0.436 | − 0.064 | 0.806 |
| OGT (U/L) | − 0.070 | 0.773 | − 0.336 | 0.203 |
| GPT (U/L) | − 0.033 | 0.891 | − 0.149 | 0.568 |
| GGT (U/L) | 0.102 | 0.686 | − 0.185 | 0.475 |
| Neutrophil-to-lymphocyte ratio | 0.147 | 0.546 | 0.315 | 0.218 |
| IL-6 (pg/mL) | − 0.284 | 0.304 | − 0.234 | 0.382 |
| ESR (mm/1ª h) | − 0.165 | 0.540 | − 0.045 | 0.872 |
Legend. WC, waist circumference; BMI, body mass index; GGT, gamma-glutamyl transferase; GPT, glutamic pyruvic transaminase; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; OGT, oxalacetic glutamic transaminase; CT, total cholesterol; TG, triglycerides. IL-6, interleukin 6; ESR, erythrocyte sedimentation rate. Pearson’s correlation, P < 0.05
Discussion
Our study found that the eHSP70-to-iHSP70 ratio is increased in DM2 postmenopausal women, supporting the hypothesis that H-index can be a biomarker of altered HSR in DM2. Although there is some evidence from cell culture and animal models, to the best of our knowledge, this is the first evidence from humans that measuring the ratio of HSP70 found in plasma and leukocytes (i.e., the eHSP70-to-iHSP70 ratio) may be helpful to indicate altered HSR and its association with cardiometabolic risk factors.
Previous studies demonstrated that eHSP70 levels are higher in diabetes and inflammatory conditions (Nakhjavani et al. 2012; Morteza et al. 2013; Rodrigues-Krause et al. 2012) and that the iHSP70 levels are decreased in obese/diabetic animals and humans (Chung et al. 2008; Rodrigues-Krause et al. 2012). Also, the relevance of eHSP70 levels as a biomarker in long-standing diabetes, mainly in women, was demonstrated early, but after stratifying women in pre-and post-menopausal profiles, no alteration in eHSP70 levels was found (Nakhjavani et al. 2011). Also, a correlation between blood glucose and inflammatory markers in PBMC was demonstrated, but in the same study was not found a correlation between glycemia and eHSP70 levels (Mahmoud et al. 2018). Our study complemented the investigation in this field by showing that, among postmenopausal women, the glycemic status does not affect eHSP70 levels, but is properly represented by a significant increase in H-index. In our study, after stratifying the sample according to H-index, we found a correlation between such index and glycemia, as well as the leukocyte account. Taken together, this evidence suggests that the H-index, the main focus of our study, must be a better proposal than each measurement (intra or extra HSP70) alone.
Our study innovates in terms of measuring the levels of eHSP70 and iHSP70 in each subject, and calculating the eHSP70/iHSP70 ratio individually, for the first time in humans. This index was made early between cells and culture media (Heck et al. 2017), and it was calculated indirectly using data from sixteen studies (mean of the whole group, shown in Heck et al. 2017, in the Supplemental Table S2) or in animal studies not using blood leukocytes (Goettems-Fiorin et al. 2016; Schöler et al., 2016; Mai et al. 2017; Baldissera et al. 2018; Kostrycki et al. 2019; Goettems-Fiorin et al. 2019; Soares et al. 2020; Stefani et al. 2021; Costa-Beber et al. 2021a, b). Although these previous studies investigated the eHSP70/iHSP70 ratio in different situations, tissues, and procedures, our study showed measured evidence about the real existence of this balance at the individual level.
The use of eHSP70/iHSP70 ratio as biomarkers is reasoned by the discussion about the chaperone duality in terms of protein function dependent on the millie in which HSPs are found (Costa-Beber et al. 2020; Krause et al. 2015a, b). While iHSP70 normal expression or induced is associated with better glycemic control and normal insulin sensitivity in animals (Chung et al. 2008; Kavanagh et al. 2011; Marshall et al. 2018) and humans (Hoekstra et al. 2018; Rodrigues-Krause et al. 2012), the eHSP70 may have opposite: the elevated eHSP70 levels are associated with duration of diabetes (Nakhjavani et al. 2010), metabolic comorbidities as obesity (Rodrigues-Krause et al. 2012). While iHSP70 has protective, antiapoptotic, anti-inflammatory proprieties by blocking NF-KB and JNK pathways and regulates cell function as mitochondrial integrity, unfolded protein response and proteostasis, and oxidative capacity (for review, please see Heck et al. 2011; Archer et al. 2018), the eHSP70 may have opposite roles: eHSP70 is related to a pro-inflammatory response, decreased expression of the anti-inflammatory iHSP70, and reduced insulin sensitivity by activation via MyD88 and TIRAP that signal downstream to NF-κB via IRAK4, TRAF6, and IKK and inducing JNK activation via MEKK4/7 (for review, see Krause et al. 2015a, b; Archer et al. 2018). Thus, investigating the ratio of compartmental distributions of HSP70 between extra- and intracellular locations may determine the outcome of the inflammation and its associated insulin resistance (Krause et al. 2015a, b).
The eHSP70/iHSP70 ratio can be also named “the chaperone balance” applied to investigations of low-grade inflammatory conditions (Krause et al. 2015a, b). It is well known that adipose tissue expansion leads to a chronic release of proinflammatory adipokines that can participate in impaired iHSP70 synthesis in insulin-dependent tissues and increases eHSP70 levels by releasing these proteins from immune cells as danger signals. This inflammatory profile implicates in the reduction of HSF-1 activation and eventually reduced iHSP70, reducing the antioxidant and anti-inflammatory capacity of cells and tissues (Krause et al. 2015a, b). Thus, when the eHSP70/iHSP70 ratio chronically is higher (in favor of eHSP70), the insulin sensitivity is impaired while treatments such as exercise (Heck et al. 2017; Schöler et al., 2016) and heat therapy (Miragem et al. 2015; Bruxel et al. 2019; Lissarassa et al. 2020) may induce iHSP70 expression and controlled release levels of eHSP70, change eHSP70/iHSP70 ratio into an anti-inflammatory status (in favor of iHSP70). Thus, this biomarker may be useful for observation of the effects of non-pharmacological intervention in diabetes (Hirsch and Heck et al. 2022).
The main metabolic and cardiovascular complications in postmenopause are related to the weight gain induced by reduced levels of E2. Indeed, postmenopausal women tend to develop abdominal and visceral adiposity, which can be monitored by abdominal and waist circumference (Polotsky and Polotsky 2010). In agreement with this, we did not find any difference in anthropometric parameters for the study participants. However, we found that independent of fasting glycemia, the women had BMI higher than 30 kg/m2 and abdominal circumference higher than 88 cm, both of which are markers of obesity and metabolic impairment (Diretrizes brasileiras de obesidade 2016). Accordingly, we used glycemia as a criterion to stratify the study participants into control and DM2 groups. As expected, the prediabetic and diabetic (DM2 group) subjects presented a higher TyG index, suggesting higher metabolic risk; however, both groups presented TyG superior to 4.55, defined as the cutoff of insulin resistance (Guerrero-Romero et al. 2016). Therefore, these data indicate that both postmenopausal groups had a background of insulin resistance.
It is well known that menopause is a risk factor for cardiovascular and metabolic diseases. Decreased E2 and FSH levels trigger several metabolic changes and predispose to the development of an atherogenic lipid profile, which becomes exacerbated over time (Wang et al. 2020). We did not find any difference in standard lipid parameters between groups. However, DM2 group presented higher PAI, an indicator of cardiovascular risk. According to PAI guidelines, DM2 group presented a moderate risk, while normoglycemic postmenopausal women have a low risk of cardiovascular outcomes (Dobiášová 2004). Together these data highlight the need to carefully monitor women during menopause, particularly if dysglycemia is observed.
Although PAI and TyG indexes are not frequently used in clinical practice or biomedical studies, they have been associated with the early stages of insulin resistance and cardiovascular injury, respectively. The TyG index is easily applicable in the clinical routine because it is based on the measurement of triglycerides and fasting glycemia levels and does not require the follow-up of the insulin levels (Guerrero-Romero et al. 2016). It strongly correlates with HOMA-IR in women and men (Guerrero-Romero et al. 2016) and has been used as an alternative to insulin tolerance test (ITT) in animal models (Costa-Beber et al. 2021a, b). Similarly, the PAI index is inversely correlated with insulin sensitivity (HOMA-S) and reflects the delicate metabolic interactions within the whole lipoprotein complex (Guerrero-Romero et al. 2016; Costa-Beber et al. 2021a, b). Since they present the best cost/performance ratio to verify the insulin resistance in a clinical population, we used TyG and PAI as alternatives to HOMA-IR and HOMA-S to evaluate women’s insulin resistance and sensitivity indirectly, respectively.
In our study, the DM2 group presented higher levels of leukocytes and neutrophils than the control group as a characteristic of low-grade inflammatory process-related DM2 induced by obesity. Increased leukocyte counts were found in women with BMI > 30 (Farhangi et al. 2013). Also, the leukocytes in the bloodstream tended to be higher in subjects that present impaired glucose tolerance test (GTT) in comparison with subjects with normal GTT and in women compared with men, after adjustment for percent body fat, and total leukocytes are positively correlated with adiposity and fasting plasma insulin concentration (Vozarova et al. 2002). In summary, higher leucocyte levels are related to unfavorable metabolic profiles (Pratley et al. 1995). Among the leukocyte types, the neutrophils have the ability to infiltrate tissues using several strategies including the neutrophil extracellular traps (NETs), which are increased in the serum of obese animals and patients (Freitas et al. 2022). Thus, higher adiposity may stimulate a neutrophilic inflammatory environment increasing several adipokines, which is associated with increased peripheric neutrophils, superoxide radical production, and NET formation due to its pro-inflammatory properties. Also, extracellular HSP90 secretion is stimulated by oxidative stress and cytokines, conditions observed in obesity, and promotes inflammation thus activating NET signaling pathways (Freitas et al. 2022). Thus, the correlation found between H-index and glycemia represents a linearity only in a diabetic state, not in normoglycemic subjects. Together, these studies and our data, support the hypothesis that the eHSP70/iHSP70 ratio is a biomarker of an inflammatory state. Also, to control the influence of leukocyte number on the HSP70 levels, we considered the plasma eHSP70 (ng/mL) and PBMC iHSP70 levels normalized by total leukocyte count (HSP/ng/106 cells) to calculate the H-index.
Indeed, there is a requirement for a better index to assess certain metabolic, immune, and inflammatory conditions during menopause, which is a natural yet challenging phase of women’s lives. In this context, monocytes can provide important information, since the level of iHSP70 in monocytes is related to downregulation of kinase proteins and anti-inflammatory activity in obese people (Simar et al. 2012), while release of eHSP70 from PBMCs is correlated with insulin resistance (Chichester et al. 2015). Considering these factors, we assessed iHSP70 levels in PBMCs from postmenopausal women as well as eHSP70 in plasma, but, surprisingly, found no difference between control and DM2 groups.
A previous study showed that eHSP70 is sensitive to menopausal status (Anklam et al. 2021). Thus, we investigated whether its levels predominated over iHSP70 in PBMCs by calculating the ratio of extracellular to intracellular HSP70 and showed that the H-index was indeed higher in DM2 postmenopausal women. Higher levels of eHSP70 compared with iHSP70 are detrimental, leading to an enhanced H-index, and are associated with a pro-inflammatory state (Heck et al. 2017). Accordingly, the H-index has already been suggested to be elevated in metabolically unfavorable scenarios, such as DM2 (Rodrigues-Krause et al. 2012; Krause et al. 2014, 2015b, a). These effects are related to the role of eHSP70 in binding TLR-2 and TLR-4 and activating NF-κβ-dependent immune and inflammatory pathways, while, in contrast, iHSP70 acts in the opposite way by preventing NF-κβ activation. Therefore, our results support the use of the H-index for determining metabolic and inflammatory status in postmenopausal women. Significantly, although the additional effect of high glycemia levels in postmenopausal women was not indicated by standard biomarkers of low-grade inflammation, such as IL-6 and ESR (Berliner et al. 2002), it was shown with high sensitivity by the H-index.
We therefore stratified the postmenopausal study participants according to H-index, using two groups: H ≤ 1.0, representing a normal physiological state, and H > 1.0, indicating unfavorable metabolic and inflammatory conditions and a warning sign (Schöler et al. 2016; Heck et al. 2017). In agreement with our hypothesis, we found a direct association between H-index and leukocyte counts in the H > 1.0 group. Circulating leukocytes play active roles in inflammatory pathways and, when present in high numbers, can predict DM2 development (Nakanishi et al. 2002). Indeed, in our study, dysglycemic postmenopausal women presented higher counts of leukocytes, mainly neutrophils; these data therefore reinforce the idea of additional inflammatory risk represented by glycemic levels above to recommended (> 99 mg/dL).
Accordingly, the anti-/pro-inflammatory balance can be monitored through analysis of the secretory phenotype of PBMCs. HSP70 can be released by such cells under conditions of stress, while lymphocytes account for almost 100% of eHSP70 in serum (Hunter-Lavin et al. 2004; Ireland et al. 2007). Subsequently, eHSP70 acts as a damage-associated molecular pattern (DAMP), propagating a sterile inflammatory response and affecting cell survival (Hulina et al. 2018). We found a positive correlation between total leukocytes and H-index in women with H-index ˃ 1.0. Considering that the release of eHSP70 by PBMCs in elderly monkeys was found to be associated with insulin resistance (Chichester et al. 2015), the correlation between the H-index in PBMCs and total leukocytes identified here highlights the role of altered HSR (measured by eHSP70-to-iHSP70 ratio) in indicating the inflammatory and metabolic impairment suffered by women after menopause.
The H-index shown in our study can be verified in laboratories equipped with a centrifuge and a microplate reader and calculated by a simple procedure. Both iHSP70 and eHSP70 can be measured in one blood sample: iHSP70 in the PBMCs, separated by the Histopaque technique, and eHSP70 in the plasma aliquot (Costa-Beber et al. 2021a, b; Madden et al. 2010). Similarly to other inflammatory markers, H-index has the power to combine the information provided by the iHSP70 and eHSP70 levels in one index, and it was shown to be more sensitive than each one alone, and to match IL-2/IL-10 ratios (Heck et al. 2017; Butkowski and Jelineck 2017; Mahat et al. 2019). Until now, since H-index still does not have established cutoff values, we first needed to establish Rc = [eHSP70]c/[iHSP70]c as the control condition and Rj = [eHSP70]j/[iHSP70]j as the intervention condition, and further calculate H-index by the quotient between Rj and Rc. In the control group, we divided Rc by Rc, so that we use RC = 1 (Goettems-Fiorin et al. 2016; Schöler et al., 2016; Mai et al. 2017; Baldissera et al. 2018; Kostrycki et al. 2019; Goettems-Fiorin et al. 2019; Soares et al. 2020; Stefani et al. 2021; Costa-Beber et al. 2021a, b). This procedure was applied to investigate intervention or disease conditions in comparison with control situations and it was expressed in “how many times the ratio increases/decreases” in comparison with the control situation, in different studies, independent of the method of HSP70 detection and target samples (serum, plasma or culture media for eHSP70; cells and tissues for iHSP70) (Heck et al. 2017). However, in our study, we simplified the calculation of this index and provide the first evidence of a real level of eHSP70/iHSP70 ratio, by calculating for each subject, considering the same method, and the number of cells (normalization by cell count). Thus, our study may help the establishment of a reference level of the H-index with simpler calculations than in previous studies, at least as an indicator of subclinical challenges for DM2 subjects.
Conclusion
Our study indicates that prediabetes and diabetes increase eHSP70-to-iHSP70 ratio postmenopausal women, indicative of altered HSR response, which is correlated with metabolic and inflammatory markers. For further extrapolation, additional shreds of evidence in the field are needed, as new studies with a higher sample size, investigation of the influence of medications and comorbidities, and comparison with pre-menopause women groups are recommended since these are limitations of our study.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
PS, CFVA, AMBS, LMS, MMS, and MNF completed all the interviews, blood collection, and performed biometric profile procedures. FKS, AMBS, and CFVS completed biochemical analysis. MNF, CFVA, and PS performed the hematological procedures. PS, LMS, MSL, and TGH performed the sample processing for eHSP70 and iHSP70 detection in blood and leukocytes. MSL completed statistical analysis. TGH, PBGF, MSL, and MNF designed the study and provided material and laboratory support. LCCB and PBGF helped with manuscript revision. TGH and MSL cowrote the manuscript. All the authors approved the final version.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES), the Regional University of the Northwestern Rio Grande do Sul State (UNIJUI), and by grants awarded to MSL from The Brazilian National Council for Scientific and Technological Development (UNIVERSAL MCTI/CNPq 28/2018 process #427559/20189). MMS, LMS, and LCCB were recipients of scholarships from CAPES. AMBS and PS were recipients of scholarships from PROPG-UNIJUI.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Thiago Gomes Heck, Email: thiago.heck@unijui.edu.br.
Mirna Stela Ludwig, Email: ludwig@unijui.edu.br.
References
- Alemi H, Khaloo P, Rabizadeh S, et al. Association of extracellular heat shock protein 70 and insulin resistance in type 2 diabetes; independent of obesity and C-reactive protein. Cell Stress Chaperones. 2019;24:69–75. doi: 10.1007/s12192-018-0942-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anklam CFV, Lissarassa YPS, dos Santos AB, et al. Oxidative and cellular stress markers in postmenopause women with diabetes: the impact of years of menopause. J Diabetes Res. 2021;2021:3314871. doi: 10.1155/2021/3314871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer AE, Von Schulze AT, Geiger PC (2018) Exercise, heat shock proteins and insulin resistance. Philos Trans R Soc B Biol Sci 373. 10.1098/rstb.2016.0529 [DOI] [PMC free article] [PubMed]
- Baldissera FG, dos Santos AB, Sulzbacher MM, et al. Subacute exposure to residual oil fly ash (ROFA) increases eHSP70 content and extracellular-to-intracellular HSP70 ratio: a relation with oxidative stress markers. Cell Stress Chaperones. 2018;23:1185–1192. doi: 10.1007/s12192-018-0924-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman RN, Stefanovski D, Buchanan TA, et al. A better index of body adiposity. Obesity. 2011;19:1083–1089. doi: 10.1038/oby.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berliner S, Zeltser D, Shapira I, et al. A simple biomarker to exclude the presence of low grade inflammation in apparently healthy individuals. Eur J Cardiovasc Prev Rehabil. 2002;9:281–286. doi: 10.1177/174182670200900509. [DOI] [PubMed] [Google Scholar]
- Bittencourt A, Porto RR. eHSP70/iHSP70 and divergent functions on the challenge: effect of exercise and tissue specificity in response to stress. Clin Physiol Funct Imaging. 2017;37:99–105. doi: 10.1111/cpf.12273. [DOI] [PubMed] [Google Scholar]
- Bruxel MA, Tavares AMV, Zavarize Neto LD, et al. Chronic whole-body heat treatment relieves atherosclerotic lesions, cardiovascular and metabolic abnormalities, and enhances survival time restoring the anti-inflammatory and anti-senescent heat shock response in mice. Biochimie. 2019;156:33–46. doi: 10.1016/j.biochi.2018.09.011. [DOI] [PubMed] [Google Scholar]
- Butkowski EG, Jelinek HF. Hyperglycaemia, oxidative stress and inflammatory markers. Redox Rep. 2017;22:257–264. doi: 10.1080/13510002.2016.1215643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangeri Di Naso F, Rosa Porto R, SarubbiFillmann H, et al. Obesity depresses the anti-inflammatory HSP70 pathway, contributing to NAFLD progression. Obesity. 2015;23:120–129. doi: 10.1002/oby.20919. [DOI] [PubMed] [Google Scholar]
- Chen HW, Kuo HT, Wang SJ, et al. In vivo heat shock protein assembles with septic liver NF-κB/I- κB complex regulating NF-κB activity. Shock. 2005;24:232–238. doi: 10.1097/01.shk.0000174020.87439.f2. [DOI] [PubMed] [Google Scholar]
- Chichester L, Wylie AT, Craft S, Kavanagh K. Muscle heat shock protein 70 predicts insulin resistance with aging. J Gerontol A Biol Sci Med Sci. 2015;70:155–162. doi: 10.1093/gerona/glu015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J, Nguyen A-K, Henstridge DC, et al. HSP72 protects against obesity-induced insulin resistance. PNAS. 2008 doi: 10.1073/pnas.0705799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Beber LC, Hirsch GE, Heck TG, Ludwig MS. Chaperone duality: the role of extracellular and intracellular HSP70 as a biomarker of endothelial dysfunction in the development of atherosclerosis. Arch Physiol Biochem. 2020 doi: 10.1080/13813455.2020.1745850. [DOI] [PubMed] [Google Scholar]
- Costa-Beber LC, Goettems-Fiorin PB, dos Santos JB, et al. Ovariectomy reduces the cardiac cytoprotection in rats exposed to particulate air pollutant. Environ Sci Pollut Res. 2021;28:23395–23404. doi: 10.1007/s11356-021-12350-w. [DOI] [PubMed] [Google Scholar]
- Costa-Beber LC, Goettems-Fiorin PB, dos Santos JB et al (2021b) Ovariectomy enhances female rats’ susceptibility to metabolic, oxidative, and heat shock response effects induced by a high-fat diet and fine particulate matter. Exp Gerontol 145. 10.1016/j.exger.2020.111215 [DOI] [PubMed]
- Diretrizes brasileiras de obesidade 2016 (2016) Diretrizes brasileiras de obesidade 2016. VI Diretrizes Brasileiras de Obesidade.
- Dobiášová M. Atherogenic index of plasma [log(triglycerides/HDL-cholesterol)]: Theoretical and practical implications. Clin Chem. 2004;50:1113–1115. doi: 10.1373/clinchem.2004.031757. [DOI] [PubMed] [Google Scholar]
- Farhangi MA, Keshavarz SA, Eshraghian M, et al. White blood cell count in women: Relation to inflammatory biomarkers, haematological profiles, visceral adiposity, and other cardiovascular risk factors. J Heal Popul Nutr. 2013;31:58–64. doi: 10.3329/jhpn.v31i1.14749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas DF, Colón DF, Silva RL, et al. Neutrophil extracellular traps (NETs) modulate inflammatory profile in obese humans and mice: adipose tissue role on NETs levels. Mol Biol Rep. 2022;49:3225–3236. doi: 10.1007/s11033-022-07157-y. [DOI] [PubMed] [Google Scholar]
- Goettems-Fiorin PB, Costa-Beber LC, dos Santos JB, et al. Ovariectomy predisposes female rats to fine particulate matter exposure’s effects by altering metabolic, oxidative, pro-inflammatory, and heat-shock protein levels. Environ Sci Pollut Res. 2019;26:20581–20594. doi: 10.1007/s11356-019-05383-9. [DOI] [PubMed] [Google Scholar]
- Goettems-fiorin PB, Grochanke BS, Baldissera FG, et al. Fine particulate matter potentiates type 2 diabetes development in high-fat diet-treated mice : stress response and extracellular to intracellular HSP70 ratio analysis. J Physiol Biochem. 2016;72:643–656. doi: 10.1007/s13105-016-0503-7. [DOI] [PubMed] [Google Scholar]
- González-Ramos M, Calleros L, López-Ongil S, et al. HSP70 increases extracellular matrix production by human vascular smooth muscle through TGF-β1 up-regulation. Int J Biochem Cell Biol. 2013;45:232–242. doi: 10.1016/j.biocel.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Guerrero-Romero F, Villalobos-Molina R, Jiménez-Flores JR, et al. Fasting triglycerides and glucose index as a diagnostic test for insulin resistance in young adults. Arch Med Res. 2016;47:382–387. doi: 10.1016/j.arcmed.2016.08.012. [DOI] [PubMed] [Google Scholar]
- Hamilton KL, Gupta S, Knowlton AA. Estrogen and regulation of heat shock protein expression in female cardiomyocytes: cross-talk with NFκB signaling. J Mol Cell Cardiol. 2004;36:577–584. doi: 10.1016/j.yjmcc.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Hamilton KL, Mbai FN, Gupta S, Knowlton AA. Estrogen, heat shock proteins, and NFκB in human vascular endothelium. Arterioscler Thromb Vasc Biol. 2004;24:1628–1633. doi: 10.1161/01.ATV.0000137188.76195.fb. [DOI] [PubMed] [Google Scholar]
- Heck TG, Schöler CM, de Bittencourt PIH. HSP70 expression: does it a novel fatigue signalling factor from immune system to the brain? Cell Biochem Funct. 2011;29:215–226. doi: 10.1002/cbf.1739. [DOI] [PubMed] [Google Scholar]
- Heck TG, Scomazzon SP, Nunes PR, et al. Acute exercise boosts cell proliferation and the heat shock response in lymphocytes: correlation with cytokine production and extracellular-to-intracellular HSP70 ratio. Cell Stress Chaperones. 2017;22:27–291. doi: 10.1007/s12192-017-0771-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidari F, Rabizadeh S, Sadat Salehi S et al (2020) Serum HSP70 level in patients with endometrial cancer with and without diabetes. Gynecol Endocrinol 36. 10.1080/09513590.2019.1648415 [DOI] [PubMed]
- Hendryx M, Nicholson W, Manson JAE, et al. Social relationships and risk of type 2 diabetes among postmenopausal women. J Gerontol B Psychol Sci Soc Sci. 2020;75:1597–1608. doi: 10.1093/geronb/gbz047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henstridge DC, Bruce CR, Drew BG, et al. Activating HSP72 in rodent skeletal muscle increases mitochondrial number and oxidative capacity and decreases insulin resistance. Diabetes. 2014;63:1881–1194. doi: 10.2337/db13-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch GE, Heck TG. Inflammation, oxidative stress and altered heat shock response in type 2 diabetes: the basis for new pharmacological and non-pharmacological interventions. Arch Physiol Biochem. 2022;128:411–425. doi: 10.1080/13813455.2019.1687522. [DOI] [PubMed] [Google Scholar]
- Hoekstra SP, Bishop NC, Faulkner SH, et al. Acute and chronic effects of hot water immersion on inflammation and metabolism in sedentary, overweight adults. J Appl Physiol. 2018;125:2008–2018. doi: 10.1152/japplphysiol.00407.2018. [DOI] [PubMed] [Google Scholar]
- Hooper PL, Balogh G, Rivas E, et al. The importance of the cellular stress response in the pathogenesis and treatment of type 2 diabetes. Cell Stress Chaperones. 2014;19:447–464. doi: 10.1007/s12192-014-0493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulina A, GrdićRajković M, Jakšić Despot D, et al. Extracellular Hsp70 induces inflammation and modulates LPS/LTA-stimulated inflammatory response in THP-1 cells. Cell Stress Chaperones. 2018;23:373–384. doi: 10.1007/s12192-017-0847-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter-Lavin C, Davies EL, Bacelar MMFVG, et al. Hsp70 release from peripheral blood mononuclear cells. Biochem Biophys Res Commun. 2004;324:511–517. doi: 10.1016/j.bbrc.2004.09.075. [DOI] [PubMed] [Google Scholar]
- Ireland HE, Leoni F, Altaie O et al (2007) Measuring the secretion of heat shock proteins from cells. Methods 43. 10.1016/j.ymeth.2007.06.011 [DOI] [PubMed]
- Kavanagh K, Flynn DM, Jenkins KA, et al. Restoring HSP70 deficiencies improves glucose tolerance in diabetic monkeys. Am J Physiol - Endocrinol Metab. 2011;300:894–901. doi: 10.1152/ajpendo.00699.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton AA, Sun L (2001) Heat-shock factor-1, steroid hormones, and regulation of heat-shock protein expression in the heart. Am J Physiol Heart Circ 280. 10.1152/ajpheart.2001.280.1.h455 [DOI] [PubMed]
- Kostrycki IM, Wildner G, Donato YH et al (2019) Effects of high-fat diet on eHSP72 and extra-to-intracellular HSP70 levels in mice submitted to exercise under exposure to fine particulate matter. J Diabetes Res 2019. 10.1155/2019/4858740 [DOI] [PMC free article] [PubMed]
- Krause M, Heck TG, Bittencourt A et al (2015a) The chaperone balance hypothesis: the importance of the extracellular to intracellular HSP70 ratio to inflammation-driven type 2 diabetes, the effect of exercise, and the implications for clinical management. Mediators Inflamm 2015. 10.1155/2015/249205 [DOI] [PMC free article] [PubMed]
- Krause M, Keane K, Rodrigues-Krause J, et al. Elevated levels of extracellular heat-shock protein 72 (eHSP72) are positively correlated with insulin resistance in vivo and cause pancreatic β-cell dysfunction and death in vitro. Clin Sci (lond) 2014;126:739–752. doi: 10.1042/CS20130678. [DOI] [PubMed] [Google Scholar]
- Krause M, Ludwig MS, Heck TG, Takahashi HK. Heat shock proteins and heat therapy for type 2 diabetes: pros and cons. Curr Opin Clin Nutr Metab Care. 2015;18:374–380. doi: 10.1097/MCO.0000000000000183. [DOI] [PubMed] [Google Scholar]
- Krepuska M, Szeberin Z, Sótonyi P, et al. Serum level of soluble Hsp70 is associated with vascular calcification. Cell Stress Chaperones. 2011;16:257–265. doi: 10.1007/s12192-010-0237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissarassa YPS, Vincensi CF, Costa-Beber LC, et al. Chronic heat treatment positively impacts metabolic profile of ovariectomized rats: association with heat shock response pathways. Cell Stress Chaperones. 2020;25:467–479. doi: 10.1007/s12192-020-01087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai AS, dos Santos AB, Beber LCC et al (2017) Exercise training under exposure to low levels of fine particulate matter: effects on heart oxidative stress and extra-to-intracellular HSP70 ratio. Oxid Med Cell Longev 2017. 10.1155/2017/9067875 [DOI] [PMC free article] [PubMed]
- Madden J, Coward JC, Shearman CP, et al. Hsp70 expression in monocytes from patients with peripheral arterial disease and healthy controls :monocyte Hsp70 in PAD. Cell Biol Toxicol. 2010;26:215–223. doi: 10.1007/s10565-009-9134-x. [DOI] [PubMed] [Google Scholar]
- Mahat RK, Singh N, Rathore V, et al. Cross-sectional correlates of oxidative stress and inflammation with glucose intolerance in prediabetes. Diabetes Metab Syndr Clin Res Rev. 2019;13:616–621. doi: 10.1016/j.dsx.2018.11.045. [DOI] [PubMed] [Google Scholar]
- Mahmoud FF, Haines D, Dashti AA, et al. Correlation between heat shock proteins, adiponectin, and T lymphocyte cytokine expression in type 2 diabetics. Cell Stress Chaperones. 2018;23:955–965. doi: 10.1007/s12192-018-0903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JPS, Estevez E, Kammoun HL, et al. Skeletal muscle-specific overexpression of heat shock protein 72 improves skeletal muscle insulin-stimulated glucose uptake but does not alter whole body metabolism. Diabetes, Obes Metab. 2018;20:1928–1936. doi: 10.1111/dom.13319. [DOI] [PubMed] [Google Scholar]
- Miragem AA, de Bittencourt PIH. Nitric oxide-heat shock protein axis in menopausal hot flushes: Neglected metabolic issues of chronic inflammatory diseases associated with deranged heat shock response. Hum Reprod Update. 2017;23:600–628. doi: 10.1093/humupd/dmx020. [DOI] [PubMed] [Google Scholar]
- Miragem AA, Ludwig MS, Heck TG, et al. Estrogen deprivation does not affect vascular heat shock response in female rats: a comparison with oxidative stress markers. Mol Cell Biochem. 2015;407:239–249. doi: 10.1007/s11010-015-2472-5. [DOI] [PubMed] [Google Scholar]
- Morteza A, Nakhjavani M, Larry M, et al. Heat shock protein 70 and albuminuria in patients with type 2 diabetes: a matched case control study. Cell Stress Chaperones. 2013;18:815–819. doi: 10.1007/s12192-013-0435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhjavani M, Morteza A, Asgarani F, et al. The dual behavior of heat shock protein 70 and asymmetric dimethylarginine in relation to serum CRP levels in type 2 diabetes. Gene. 2012;498:107–111. doi: 10.1016/j.gene.2012.01.085. [DOI] [PubMed] [Google Scholar]
- Nakhjavani M, Morteza A, Khajeali L, et al. Increased serum HSP70 levels are associated with the duration of diabetes. Cell Stress Chaperones. 2010;15:959–964. doi: 10.1007/s12192-010-0204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhjavani M, Morteza A, Meysamie A, et al. Serum heat shock protein 70 and oxidized LDL in patients with type 2 diabetes: does sex matter? Cell Stress Chaperones. 2011;16:195–201. doi: 10.1007/s12192-010-0232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi N, Yoshida H, Matsuo Y, et al. White blood-cell count and the risk of impaired fasting glucose or type II diabetes in middle-aged Japanese men. Diabetologia. 2002;45:42–48. doi: 10.1007/s125-002-8243-1. [DOI] [PubMed] [Google Scholar]
- Netto PA, Posicionamento oficial SBD (2017) São Paulo: Sociedade Brasileira de Diabetes. Avaliable in https://profissional.diabetes.org.br/wpcontent/uploads/2021/09/posicionamento-oficial-sbd-01-2017.pdf
- ONU (2019) World population prospects 2019. Available in https://www.un.org/development/desa/en/news/population/world-population-prospects-2019.html
- Polotsky HN, Polotsky AJ. Metabolic implications of menopause. Semin Reprod Med. 2010;28:426–434. doi: 10.1055/s-0030-1262902. [DOI] [PubMed] [Google Scholar]
- Pratley RE, Wilson C, Bogardus C. Relation of the white blood cell count to obesity and insulin resistance: effect of race and gender. Obes Res. 1995;3:563–571. doi: 10.1002/j.1550-8528.1995.tb00191.x. [DOI] [PubMed] [Google Scholar]
- Rodrigues-Krause J, Krause M, O’Hagan C, et al. Divergence of intracellular and extracellular HSP72 in type 2 diabetes: does fat matter? Cell Stress Chaperones. 2012;17:293–302. doi: 10.1007/s12192-011-0319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenaker DAJM, Jackson CA, Rowlands JV, Mishra GD. Socioeconomic position, lifestyle factors and age at natural menopause: a systematic review and meta-analyses of studies across six continents. Int J Epidemiol. 2014;43:1542–1562. doi: 10.1093/ije/dyu094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöler CM, Marques CV, da Silva GS, et al. Modulation of rat monocyte/macrophage innate functions by increasing intensities of swimming exercise is associated with heat shock protein status. Mol Cell Biochem. 2016;421:111–125. doi: 10.1007/s11010-016-2791-1. [DOI] [PubMed] [Google Scholar]
- Simar D, Jacques A, Caillaud C. Heat shock proteins induction reduces stress kinases activation, potentially improving insulin signalling in monocytes from obese subjects. Cell Stress Chaperones. 2012;17:615–621. doi: 10.1007/s12192-012-0336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares M, do Santos AB, Weich TM, et al. Heat shock response in noise-induced hearing loss: effects of alanyl-glutamine dipeptide supplementation on heat shock proteins status. Braz J Otorhinolaryngol. 2020;86:703–710. doi: 10.1016/j.bjorl.2019.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani GP, Capalonga L, da Silva LR et al (2021) Effects of aerobic and resistance exercise training associated with carnosine precursor supplementation on maximal strength and V̇O2max in rats with heart failure. Life Sci 282. 10.1016/j.lfs.2021.119816 [DOI] [PubMed]
- Stice JP, Chen L, Kim SC, et al. 17β-estradiol, aging, inflammation, and the stress response in the female heart. Endocrinology. 2011;152:1589–1598. doi: 10.1210/en.2010-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzuki T, Kobayashi H, Yoshihara T, et al. Attenuation of exercise-induced heat shock protein 72 expression blunts improvements in whole-body insulin resistance in rats with type 2 diabetes. Cell Stress Chaperones. 2017;22:263–269. doi: 10.1007/s12192-017-0767-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez R. A simple model-based index of abdominal adiposity. J Clin Epidemiol. 1991;44:955–956. doi: 10.1016/0895-4356(91)90059-I. [DOI] [PubMed] [Google Scholar]
- Vozarova B, Stefan N, Lindsay RS, et al. High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51:1889–1895. doi: 10.2337/diabetes.51.6.1889. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mishra A, Brinton R (2020) Transitions in metabolic and immune systems from pre-menopause to post-menopause: implications for age-associated neurodegenerative diseases. F1000Research 2020. 10.12688/F1000RESEARCH.21599.1 [DOI] [PMC free article] [PubMed]
- Zhang X, Xu Z, Zhou L, et al. Plasma levels of Hsp70 and anti-Hsp70 antibody predict risk of acute coronary syndrome. Cell Stress Chaperones. 2010;15:675–686. doi: 10.1007/s12192-010-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.


