Abstract
Ubiquitin-like modifier 1 ligating enzyme 1 (UFL1) is a unique E3 ligase of the UFMylation system. Recent studies have shown that this enzyme plays a crucial role in the processes of endoplasmic reticulum stress (ER stress) and apoptosis. Lipopolysaccharide (LPS) can cause injury to ovarian granule cells and hinder follicular development by triggering ER stress and apoptosis. Our study aimed to investigate the mechanism by which UFL1 alleviates ER stress and apoptosis caused by LPS in human granulosa-like cells (KGNs). In this study, we found that the protein levels of UFL1 were increased obviously under LPS stimulation in KGNs and that ER stress and apoptosis were further aggravated when UFL1 was knocked down; in contrast, these events were rescued when UFL1 was overexpressed. Next, we showed that the levels of ferroptosis-related proteins were relatively altered, accompanied by the accumulation of reactive oxygen species (ROS) and Fe2+, following the inhibition of UFL1 expression. In contrast, the overexpression of UFL1 reversed the ferroptosis process by regulating the P53/SLC7A11 (solute carrier family 7, member 11, SLC7A11) system and autophagy in response to LPS stimulation. Furthermore, apoptosis and ER stress in KGNs are rescued by the administration of the ferroptosis inhibitor ferrostatin-1 (Fer-1). Collectively, our research demonstrated a new mechanism for UFL1 that can alleviate ER stress and apoptosis stimulated by LPS; this occurred via the regulation of the ferroptosis pathway in KGNs and may provide a new strategy for research in the field of reproduction.
Keywords: UFL1, ER stress, Apoptosis, Ferroptosis
Introduction
Ubiquitin-like modifier 1 ligating enzyme 1 (UFL1), also known as KIAA0776 or Maxer, is a unique E3 ligase of the UFMylation ubiquitin-like modification system, which conjugates ubiquitin fold modifier 1 (UFM1) to its substrate during the process of UFMylation (Tatsumi et al. 2010; Wei and Xu 2016; Xie et al. 2019). In addition to playing an indispensable role in the UFMylation system, UFL1 also participates in multiple other cellular responses, including DNA damage, inflammation, endoplasmic reticulum (ER) stress, apoptosis, autophagy, and oxidative stress in the hematopoietic, heart, breast, ovary, and other important organs (Li et al. 2019, 2018; Wang et al. 2020; Zhang et al. 2015). Recent research has reported that the depletion of UFL1 could promote the excessive activation of autophagy in bone marrow cells (BMCs) stimulated by lipopolysaccharides (LPS), increase mitochondrial mass and reactive oxygen species (ROS), and ultimately lead to increased oxidative stress and cell death (Li et al. 2019). In addition, knockout of the UFL1 gene in BMCs led to increased ER stress and unfolded protein response (UPR), an enhanced DNA damage response, p53 activation, and cell death in hematopoietic stem cells (HSCs) (Li et al. 2019, 2018). Interestingly, the activation of P53 and autophagy are closely related to the occurrence of ferroptosis (Hu et al. 2020; Jiang et al. 2015; Kang et al. 2019). Thus, existing research indicates that UFL1 is a key regulator of the cellular stress response and may be related to the occurrence of ferroptosis in the process of maintaining cell homeostasis.
Unlike canonical cell programmed death (apoptosis), ferroptosis is a newly discovered form of cell death that is characterized by iron dependence, increased ROS, and lipid peroxidation, and involves the activation of Xc-/GPX4, P62-Keap1-NRF2 (kelch-like ECH-associated protein 1, Keap1; nuclear factor (erythroid-derived 2)-like 2, NRF2), P53/SLC7A11 (solute carrier family 7, member 11, SLC7A11), and other pathways (Cao and Dixon 2016; Guan et al. 2021; Stockwell et al. 2017; Xie et al. 2016). Although ferroptosis and apoptosis are quite different, some studies have reported that there is a mutual relationship between these processes in that inducer of ferroptosis can promote apoptosis via tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) and that this can switch apoptosis to ferroptosis (Lee et al. 2019; Zheng et al. 2017). Recent studies have shown that the ER stress response induced by ferroptosis may mediate other types of cell death, such as apoptosis (Chen et al. 2019; Lee et al. 2018; Su et al. 2019). Nevertheless, the specific mechanisms linking ferroptosis, ER stress, and apoptosis have yet to be elucidated.
Different physiological and pathological stress conditions can disrupt the homeostasis of the ER; these stressors can cause the accumulation of unfolded or misfolded proteins, thus inducing the unfolded protein response (UPR) which ultimately results in ER stress (Di Conza and Ho 2020; Rashid et al. 2015). The maintenance of ER homeostasis is crucial during follicular development and maturation in granulosa cells (GCs); ER stress can cause follicular atresia and premature ovarian follicle (POF) (Huang et al. 2016). In addition, excessive ER stress can elevate the UPR transcription factor C/EBP homologous protein (CHOP) and subsequently activate the apoptosis pathway, thus resulting in a reduction in the number of normal follicles (Hetz 2012; Xiong et al. 2020; Zeng et al. 2017). Lipopolysaccharide (LPS) was recently demonstrated to induce ER stress both in vivo and in vitro (Huang et al. 2020; Pang et al. 2019). Once induced, ER stress can result in the activation of PKR-like ER kinase (PERK), one of the transmembrane sensors in the UPR, thus leading to the overexpression of CHOP and the subsequent initiation of apoptosis (Lebeaupin et al. 2015). Recent studies have shown that UFL1 can regulate LPS-induced ER stress and apoptosis in BMCs (Li et al. 2019). But until now, the mechanism of UFL1 regulating ER stress and apoptosis in GCs has not been determined. Considering that mouse GCs do not passage well during culture, we selected human granulosa-like cells (KGNs) for this study; this is a human granulosa-like tumor cell line that is considered to be a very useful model for understanding the regulation of GC growth and apoptosis and can retain the physiological characteristics of normal GCs, multiply, and undergo passage in culture (Nishi et al. 2001).
In this study, we first investigated the effects of UFL1 on ER stress and apoptosis by using LPS-treated KGNs as an in vitro cell model. Then, we investigated the protective function of UFL1 in KGNs and found that this protein can alleviate the ER stress and apoptosis stimulated by LPS by regulating the ferroptosis pathway.
Materials and methods
Cell culture and treatment
KGN cells were purchased from the Cell Bank of the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). The KGNs were cultured in DMEM supplemented with 10% fetal bovine serum (Gibco, Staley Rd Grand Island, NY, USA) and 200 U/mL of penicillin and streptomycin (Solarbio) and incubated at 37 °C in a humidified atmosphere containing 5% CO2. KGNs were treated with different doses of LPS for 16 h and then subjected to various assays. After the concentration gradient assay, we selected 5 μg/mL LPS for subsequent experiments. KGNs were treated with 2 μM Fer-1 for 16 h.
Cell viability assay
KGNs were seeded into a 96-well plate at the concentration of 2000 cells/well. The cells were cultured for 24 h, and then treated with LPS (Sigma) and Fer-1 (Sigma) in different concentrations for 16 h followed by the addition of 20μL of CCK-8 solution (TransGen Biotech, Beijing, China) directly into the medium (200 μL per well) and incubation at 37 °C for 1–2 h. Eventually, we use a microplate reader to detect the absorbance at 450 nm.
Cell transfection
We used UFL1 shRNA to knock down the expression of UFL1 and used the pEX-3 vector to clone the UFL1 cDNA plasmid to overexpress UFL1. The sequence and antisense sequence of the shRNA were 5′-GAAACACTTCTGTGTCAGAAA-3′ and 3′-GCTCTGGAACATGGGTTGATA-5′ and were purchased from Sigma. Lentiviruses were made in accordance with the manufacturer’s protocol. KGNs were cultured to a confluency of 50–60% in 6-well dishes and then transfected with prepared lentiviruses. After 48 h of transfection, the successful depletion of UFL1 protein expression was confirmed by western blot analysis.
The UFL1 plasmid was constructed by GenePharma (Shanghai, China) and was sequenced by JinsiruiBio Company (Nanjing, China). The amplified products were purified and cloned into the pEX-3 vector. KGN cells were cultured to a confluency of 50–60% in 6-well dishes and transfected with 2 µg of the pcDNA3.1-UFL1 or the pcDNA3.1 empty vector in OptiMEM (Gibco, Carlsbad, CA, USA) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. After 48 h of transfection, the successful overexpression of UFL1 protein expression was confirmed by western blot analysis.
Western blot
Proteins in KGN samples were extracted by RIPA Lysis Buffer (Applygen, Beijing, China). Protein concentrations were measured with a BCA kit (Applygen, China). The proteins underwent polyacrylamide gel electrophoresis and were then transferred to the PVDF membrane (Millipore, Darmstadt, Germany). The membranes were blocked with 5% bovine serum albumin solution for 6 h and then the primary antibodies were incubated. The primary antibodies used in this study included UFL1 (ab226216, Abcom, Cambridge, UK, 1:2000 for western blot), BIP (66,574–1-Ig, Proteintech, Wuhan, China, 1:5000 for western blot), XBP1s (ab37152, Abcom, Cambridge, UK, 1:2000 for western blot), CHOP (15,204–1-AP, Proteintech, Wuhan, China, 1:1000 for western blot), BAX (WL01637, Wanleibio, Shenyang, China, 1:2000 for western blot), BCL-2 (12,789–1-AP, Proteintech, Wuhan, China, 1:1000 for western blot), P53 (10,442–1-AP, Proteintech, Wuhan, China, 1:5000 for western blot), SLC7A11 (26,864–1-AP, Proteintech, Wuhan, China, 1:2000 for western blot), GPX4 (67,763–1-Ig, Proteintech, Wuhan, China, 1:2000 for western blot), P62 (WL02385, Wanleibio, Shenyang, China, 1:1000 for western blot), NRF2 (WL02135, Wanleibio, Shenyang, China, 1:1000 for western blot), LC3 (WL01506, Wanlei, Shenyang, China, 1:1000 for western blot), FTH1 (DF6278, Affinity, Cincinnati, USA, 1:2000 for western blot), and TUBULIN (10,094–1-AP, Proteintech, Wuhan, China, 1:5000 for western blot). After incubation of the secondary antibody (Affinity Biosciences, Cincinnati, USA), the blots were imaged using the EasySee Western Blot Kit (DW101-01, TransGen Biotech, China). Analyzer Image AI600 and Image J were used to scan and analyze the images.
Detection of intracellular levels of reactive oxygen species
Dihydrorhodamine (DHR, KeyGen BioTECH, China) was used to measure the total levels of intracellular ROS in accordance with the manufacturer’s guidelines. In brief, 10 μM of DHR working solution was added to the wells containing KGNs and incubated for 1 h in the dark. Hoechst 33,342 was then used to stain the nuclei. A NIKON Eclipse 80i fluorescence microscope was used to observe the staining intensity.
Detection of the levels of intracellular Fe2+
FerroOrange (DojinDo, Japan) was used to detect the levels of intracellular Fe2+ in accordance with the manufacturer’s protocol. KGNs were seeded onto confocal dishes and washed with Hank’s balanced salt solution (HBSS) (Gibco, USA) to remove residual reagents. Cells were then treated with 1 μmol/L of FerroOrange with HBSS for 30 min at 37 °C. The cells were finally observed under a confocal laser scanning microscopy (Nikon A1, Japan).
Statistical analysis
The statistical analysis was performed using GraphPad Prism 8 software. One-way ANOVA was used to detect statistical differences between multiple sets of data. A p value < 0.05 was considered statistically significant. All data are presented as the mean ± standard error from at least three independent experiments. It is considered that p < 0.05 was a significant difference.
Results
LPS treatment caused UFL1 elevation, ER stress, and apoptosis
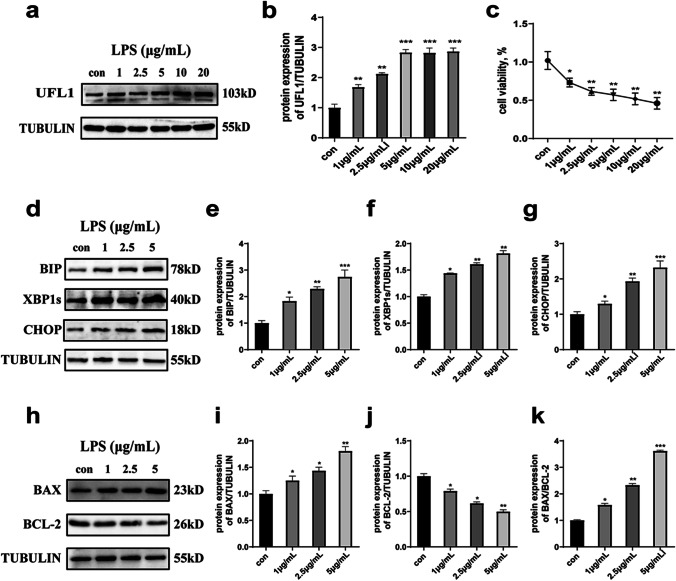
UFL1 is a key regulator of ER stress (Zhang et al. 2015). Firstly, we confirmed the protein expression changes of UFL1 and cell viability in KGNs following LPS stimulation. In order to investigate the changes of UFL1 under different drug concentrations, the dose of LPS was gradually increased from 0 to 20 μg/mL. We found that the expression of UFL1 was significantly increased in an LPS dose-dependent manner, at least to a certain extent (5 μg/mL), but did not change when the concentration of LPS was increased further (Fig. 1a, b). Furthermore, LPS stimulation was shown to inhibit cell viability; this also depended on the dose of LPS (Fig. 1c). Therefore, we chose a low concentration range from 0 to 5 μg/mL (where UFL1 changed significantly) to explore the influence of ER stress. The protein levels of specific markers of ER stress (BIP, XBP1s, and CHOP) were significantly increased after LPS stimulation (Fig. 1d-g). The expression levels of BAX and the ratio of apoptosis BAX/BCL-2 also increased (Fig. 1h-k). In general, the expression of UFL1 increased in the presence of ER stress and following the appearance of apoptosis, thus indicating that UFL1 may be involved in the process of ER stress and apoptosis.
Fig. 1.
LPS stimulation led to UFL1 elevation, ER stress, and the occurrence of apoptosis. a, b Changes in UFL1 protein levels after 16 h of treatment with different LPS concentrations. c Changes in cell activity under different LPS doses. d–g The effect of different concentrations of LPS on the marker proteins BIP, XBP1s, and CHOP. h–k Changes in the levels of key apoptosis proteins (BAX and BCL-2) under different LPS concentrations. *p < 0.05, **p < 0.01, and ***p < 0.001
The knockdown of UFL1 aggravated LPS-induced ER stress and apoptosis
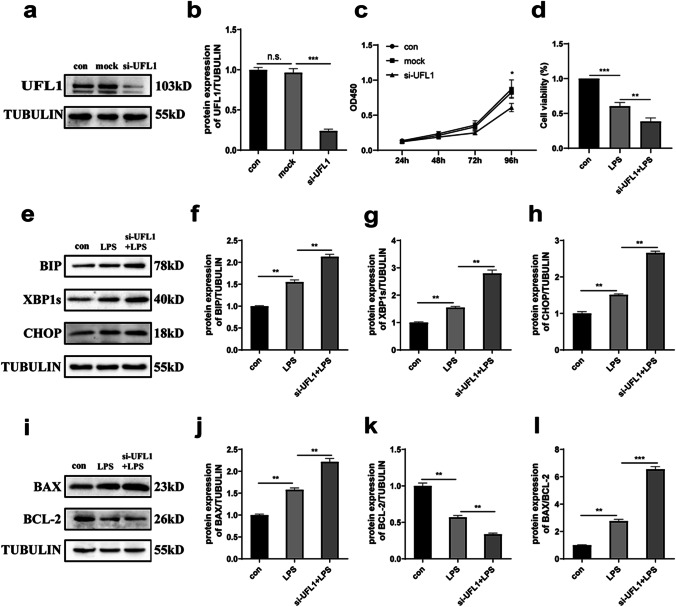
To verify that UFL1 is involved in the process of cellular ER stress and apoptosis in response to LPS treatment, we knocked down the expression of UFL1 in KGNs (Fig. 2a, b). The proliferation of KGN cells was successfully inhibited after UFL1 knockdown (Fig. 2c) (p < 0.05). Compared with the LPS treatment group alone, the combination of UFL1 knockdown and LPS treatment resulted in reduced cell viability (Fig. 2d) (p < 0.01). Compared with the control group, the UFL1 knockdown group was more vulnerable to ER stress and apoptosis when treated with LPS. As shown in Fig. 2e-h, the levels of protein markers of ER stress (BIP, XBP1s, and CHOP) were significantly increased; a similar trend was observed for BAX and the ratio of BAX/BCL-2 (Fig. 2i-l). These results prove that the level of UFL1 may ameliorate ER stress and apoptosis.
Fig. 2.
The inhibition of UFL1 expression aggravated LPS-induced apoptosis and ER stress. a, b UFL1 siRNA changed the expression levels of UFL1 n KGNs. c The cell growth curve in KGNs in which UFL1 had been knocked down. d Cell viability, as detected by CCK-8. e–h Changes in the protein expression of BIP, XBP1s, and CHOP with LPS stimulation in KGNs in which UFL1 had been knocked down. i–k Changes in the protein expression of BAX and BCL-2 with LPS stimulation in KGNs in which UFL1 had been knocked down. l The ratio of BAX/BCL-2 in LPS-stimulated KGNs after UFL1 knockdown. *p < 0.05, **p < 0.01, and ***p < 0.001
The overexpression of UFL1 effectively alleviated LPS-induced ER stress and apoptosis
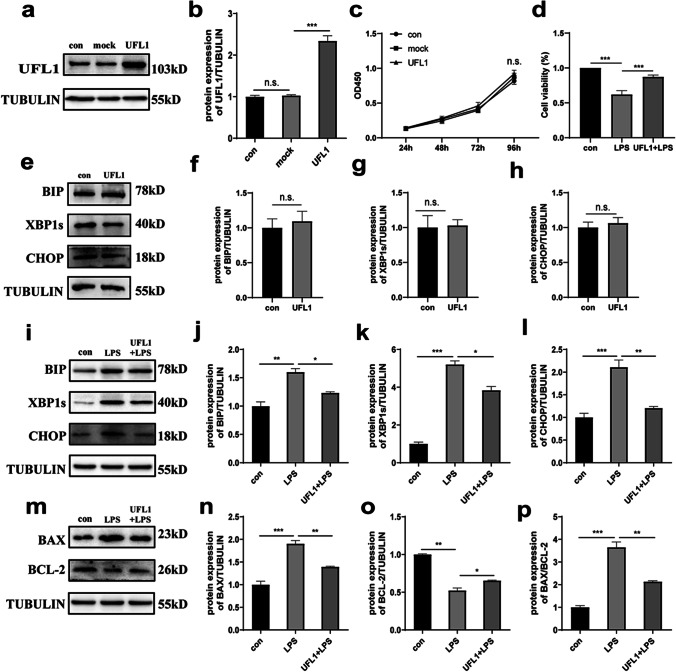
Next, we overexpressed UFL1 to investigate the protective effect of UFL1 against LPS stimulation in KGNs. As shown in Fig. 3a and b, we successfully overexpressed UFL1. The CCK8 assay showed that the overexpression of UFL1 did not affect the growth of KGNs (Fig. 3c). However, compared with the LPS treatment group, the overexpression of UFL1 alleviated the LPS-induced decline in the cell viability of KGNs (Fig. 3d) (p < 0.001). Furthermore, the overexpression of UFL1 alone did not affect cellular endoplasmic reticulum homeostasis when compared to controls; there was no change in the protein levels of ER stress indicators (Fig. 3e-h). These results showed that the protein levels of ER stress markers in the UFL1 overexpression group were obviously reduced when compared with the LPS group, although the levels of ER stress proteins had not been recovered to a normal level, thus suggesting that UFL1 can only rescue LPS-induced ER stress to a certain extent in KGNs (Fig. 3i-l). In addition, the degree of apoptosis had been alleviated by the overexpression of UFL1; this was similar to the trend we observed for the effects of UFL1 on ER stress (Fig. 3m-p). Together, our data indicated that UFL1 can protect KGNs from the ER stress and apoptosis caused by LPS stimulation.
Fig. 3.
The overexpression of UFL1 alleviated LPS-induced apoptosis and ER stress. a–b The overexpression of UFL1 in KGNs. c The cell growth curve in KGNs overexpressing UFL1. d Cell viability, as detected by CCK-8 assays. e–h Changes in the protein expression of BIP, XBP1s, and CHOP after the overexpression of UFL1 alone. i–l Changes in the protein expression of BIP, XBP1s, and CHOP under LPS stimulation after UFL1 overexpression. m–o Changes in the protein levels of BAX and BCL-2 under LPS stimulation after UFL1 overexpression. p The ratio of BAX/BCL-2 under LPS stimulation after UFL1 overexpression. *p < 0.05, **p < 0.01, and ***p < 0.001
The knockdown of UFL1 led to ferroptosis and oxidative stress
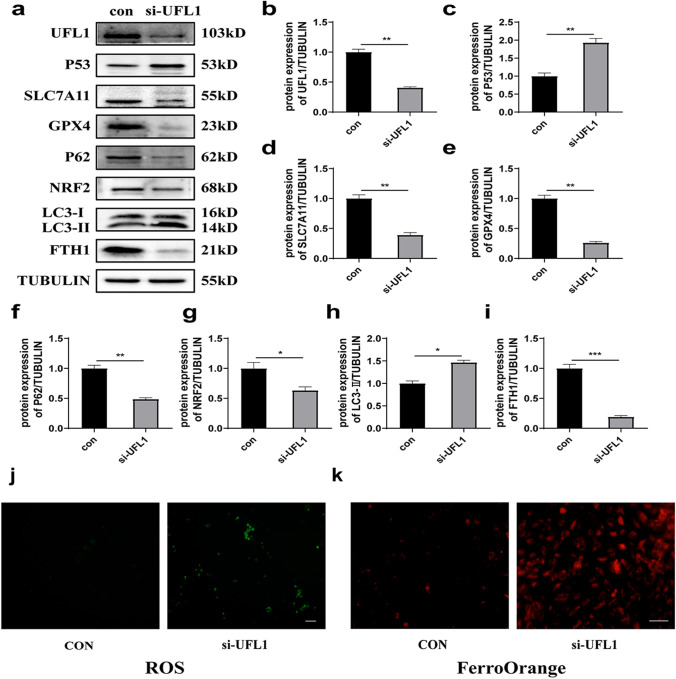
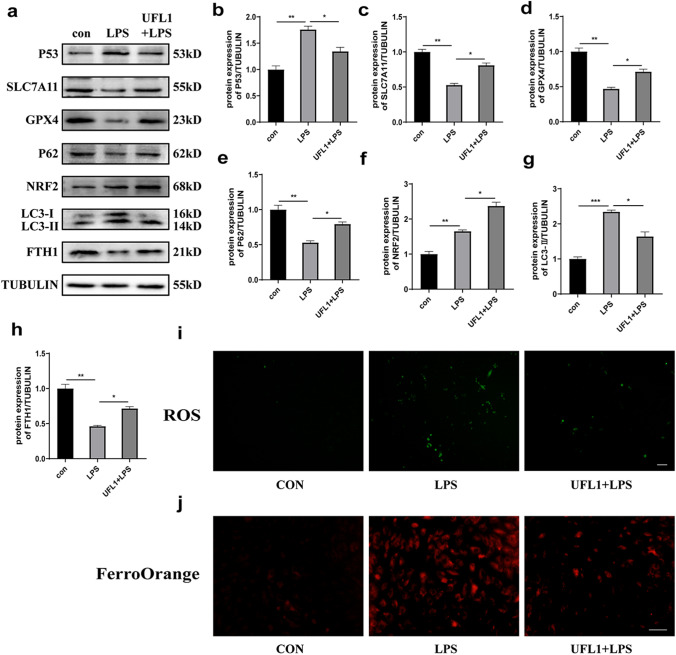
Next, we investigated how UFL1 might relieve ER stress and apoptosis in KGNs. To do this, we detected the levels of ferroptosis-related proteins and the products of lipid peroxidation after knocking down the expression of UFL1. Our results showed that the expression of P53 was significantly increased whereas the protein levels of SLC7A11 and GPX4 were downregulated, thus proving that ferroptosis occurred following the inhibition of UFL1 (Fig. 4a-e). Autophagy is a crucial mechanism of ferroptosis; therefore, we investigated the expression levels of ferroptosis-related autophagy proteins. The knockdown of UFL1 increased the protein levels of LC3-II and downregulated the protein levels of P62, NRF2, and FTH1 (Fig. 4f-i). The analysis also showed that UFL1 knockdown increased the production of ROS and the accumulation of Fe2+ (Fig. 4j, k). Collectively, these data indicated that the silencing of UFL1 triggered ferroptosis and oxidative stress and that UFL1 may be a key factor regulating ferroptosis and oxidative stress.
Fig. 4.
UFL1 knockdown led to ferroptosis and oxidative stress. a) Changes in the protein expression of UFL1, P53, SLC7A11, GPX4, P62, NRF2, LC3, and FHT1 after the silencing of UFL1. b–i Quantitative analysis of UFL1, P53, SLC7A11, GPX4, P62, NRF2, LC3, and FHT1 expression in KGNs in which UFL1 had been knocked down. j The production of ROS in KGNs in which UFL1 had been knocked down. Bar = 200 μm. k FerroOrange was used to detect changes in iron ions (Fe.2+) in KGNs in which UFL1 had been knocked down. Bar = 100 μm. *p < 0.05, **p < 0.01, and ***p < 0.001
UFL1 reduced ER stress and apoptosis via the P53/Xc system and autophagy-dependent ferroptosis
To investigate whether ferroptosis and autophagy are the protective mechanisms induced by UFL1 to rescue ER stress and apoptosis, we treated KGNs with LPS and overexpressed UFL1 simultaneously. The analysis found that UFL1 overexpression inhibited P53 activation and reversed the downregulation of SLC7A11 and GPX4 induced by LPS stimulation (Fig. 5a-d). Apart from affecting the P53/Xc system, the LPS + UFL1 group exhibited a restriction in the upregulation of LC3-II and the downregulation of P62, NRF2, and FTH1 when compared with the LPS group (Fig. 5e-h). Furthermore, FerroOrange experiments showed that fluorescence intensity in the LPS + UFL1 group was obviously increased as compared with the LPS group, thus indicating that UFL1 reduced the accumulation of Fe2+ (Fig. 5i). Furthermore as shown in Fig. 5j, the generation of ROS increased significantly when we overexpressed UFL1. Collectively, these results indicated that the overexpression of UFL1 exerts rescue capability via the P53/Xc system and by autophagy-dependent ferroptosis in LPS-stimulated KGNs.
Fig. 5.
UFL1 regulated the P53/Xc system and autophagy-dependent ferroptosis. a Changes in the protein expression of P53, SLC7A11, GPX4, P62, NRF2, LC3, and FHT1 after UFL1 overexpression. b–h Quantitative analysis of P53, SLC7A11, GPX4, P62, NRF2, LC3, and FHT1 expression after UFL1 overexpression. i The products of ROS after UFL1 overexpression. Bar = 200 μm. j FerroOrange was used to detect changes in iron ions (Fe.2+) in KGNs in which UFL1 had been knocked down. Bar = 100 μm. *p < 0.05, **p < 0.01, and ***p < 0.001
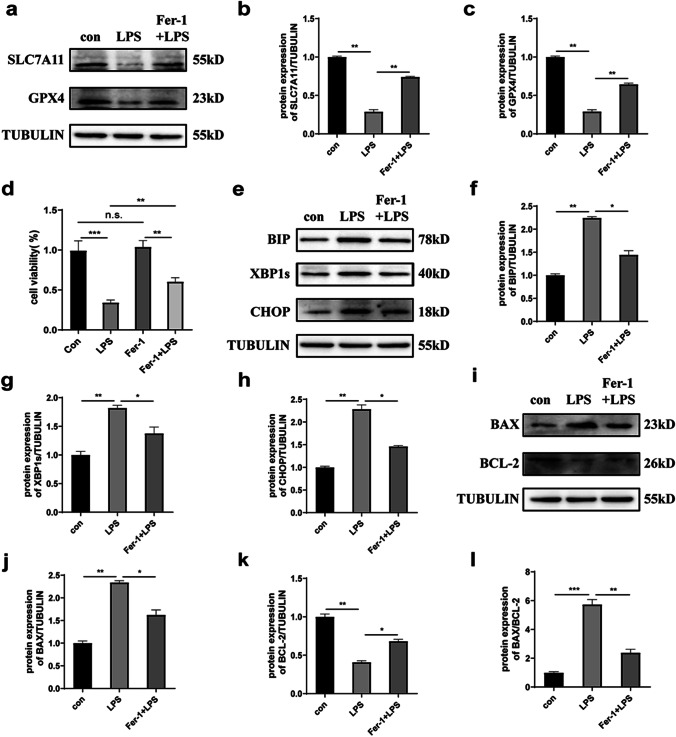
The inhibition of ferroptosis reduced ER stress and apoptosis
To investigate the influence of blocking ferroptosis on ER stress and apoptosis, we treated KGNs with the ferroptosis-specific inhibitor Fer-1 (2 μM, 16 h) under LPS stimulation. Analysis showed that the expression levels of SLC7A11 and GPX4 were upregulated in the LPS + Fer-1 group, thus indicating that Fer-1 effectively inhibited ferroptosis in LPS-treated KGNs (Fig. 6a-c). Compared with the LPS group, the protein levels of the ER stress markers BIP, XBP1s, and CHOP decreased significantly as well as the apoptosis factors BAX and the ratio of BAX/BCL-2 when treated with Fer-1 and LPS simultaneously, thus suggesting that Fer-1 alleviated the damage in KGNs caused by LPS (Fig. 6e-l). In addition, we found that the cell viability of the Fer-1 + LPS group was better than that in the LPS group, but did not completely recover to normal levels, thus indicating that Fer-1 only rescued cell death caused by LPS treatment to a certain extent (Fig. 6d). Taken together, these data suggested that blocking ferroptosis alleviated LPS-induced ER stress and apoptosis and that ferroptosis might play a key role in LPS-induced cell damage.
Fig. 6.
The administration of a ferroptosis inhibitor reduced cell damage caused by LPS stimulation. a–c Changes in the protein expression of SLC7A11 and GPX4 in LPS-stimulated KGNs treated with Fer-1. d Changes in cell activity in LPS-stimulated KGNs treated with Fer-1. e–h Changes in the protein expression levels of BIP, XBP1s, and CHOP in LPS-stimulated KGNs treated with Fer-1. i–l Changes in the protein expression levels of BAX and BCL-2 in LPS-stimulated KGNs treated with Fer-1. *p < 0.05, **p < 0.01, and ***p < 0.001
Discussion
In this study, we first evaluated the injury caused by LPS stimulation to KGNs and found that the mechanism of cell damage was related to ER stress and apoptosis. Interestingly, LPS stimulation resulted in the increased expression of UFL1; therefore, we hypothesized that UFL1 might be involved in cell homeostasis. Next, we investigated the effect of changing UFL1 expression on ER stress and apoptosis and found that the knockdown of UFL1 enhanced the degree of ER stress and apoptosis whereas the overexpression of UFL1 obviously reversed this tendency. These studies indicated that the overexpression of UFL1 protected cells against LPS-induced ER stress and apoptosis to maintain cell homeostasis; these findings are consistent with the outcomes of previous studies relating to UFL1 function in the heart, breast, and other tissues (Li et al. 2019, 2018; Wei and Xu 2016). In addition, many previous studies have demonstrated that ferroptosis is closely associated with ER stress and apoptosis (Lee et al. 2018; Zheng et al. 2017). In the present study, we found that the levels of ferroptosis-related proteins were elevated when the expression of UFL1 was silenced, thus indicating that UFL1 influences the occurrence of ER stress and apoptosis by regulating ferroptosis.
In a previous study, Zhang et al. reported that the loss of UFL1 induced the DNA damage response and p53 activation in HSCs (Zhang et al. 2015). However, Liu et al. demonstrated that P53 is one of the substrates of UFL1 in mouse embryonic fibroblasts (MEF) and that p53 protein levels were significantly reduced when UFL1 was knocked down (Liu et al. 2020a). In addition, Li et al. reported that UFL1 knockdown aggravated LPS-induced autophagy and that LC3-II protein expression was particularly increased while P62 protein expression was significantly reduced (Wang et al. 2020). To investigate the role of UFL1 in LPS-induced damage in KGNs, we detected the alteration of ferroptosis-specific markers related to two classic ferroptosis pathways: the P53/SLC7A11 system and autophagy-dependent ferroptosis (Hou et al. 2016; Hu et al. 2020; Xie et al. 2016). We found that the expression of P53 was activated after UFL1 knockdown; these findings were consistent with those of Zhang’s study but differed from the results of Liu et al. Then, we speculated that UFL1 might perform various functions in different cell lines. Furthermore, our data showed that the expression levels of LC3-II and P62 had increased and decreased, respectively; these findings were similar to those of Li et al. Furthermore, we found that the protein levels of SLC7A11 and GPX4, considered to be key proteins related to ferroptosis (Hadian and Stockwell 2020; Lei et al. 2021), were significantly reduced following the depletion of UFL1. We also observed the significant accumulation of ROS and Fe2+, thus implying the occurrence of ferroptosis (Hou et al. 2016; Kong et al. 2019; Tian et al. 2020). Ferritin heavy chain 1 (FTH1) is one of the components of the main iron storage protein ferritin and can degrade ferritin during autophagy to yield free Fe2+ and trigger the occurrence of ferroptosis. Our data showed that the expression of FTH1 was significantly reduced after UFL1 knockdown, thus indicating that UFL1 may be crucial for the regulation of ferroptosis.
Ferroptosis, a newly discovered type of cell death, has been widely associated with tumorigenesis. However, few studies have explored the specific connection between ferroptosis and tumorigenesis, such as synergy or antagonism between ferroptosis and ER stress/apoptosis (Cao and Dixon 2016; Hassannia et al. 2019; Lee et al. 2019). GPX4 belongs to the glutathione peroxidase family and catalyzes the reduction of lipid peroxides, thereby protecting cells against the ferroptosis caused by oxidative damage (Bersuker et al. 2019; Yang and Stockwell 2016). In our study, the activation of P53 caused a reduction in the downstream molecule SLC7A11, eventually inhibiting the activity of GPX4 after the knockdown of UFL1, thus leading to the accumulation of Fe2+ and ROS, thereby triggering ferroptosis (Lee et al. 2018; Zheng et al. 2017). NRF2, a key regulator of the cellular antioxidant response, and also considered to be an important regulatory factor for ferroptosis, can be activated by the autophagy adaptor protein P62 in selective autophagy (Dodson et al. 2019; Hybertson et al. 2011; Levine and Kroemer 2019; Sun et al. 2016). The depletion of UFL1 led to excessive autophagy, accompanied by a reduction in NRF2 and FTH1, thereby enhancing the degradation of ferritin and promoting the occurrence of ferroptosis (Zhang et al. 2020). In contrast, by inhibiting the p53/SLC7A11 axis and autophagy, the overexpression of UFL1 reversed the ferroptosis process and reduced the accumulation of ROS and Fe2+, thus suggesting that UFL1 exerts antioxidant effects. In contrast to other molecules that were reduced by the overexpression of UFL1 such as P53 and LC3-II, the protein levels of NRF2 increased in the LPS group and remained continuously elevated after the overexpression of UFL1. Previous studies showed that NRF2 appears to play a cytoprotective role against oxidative stress in a variety of diseases and that the activation of NRF2 has emerged as a potential therapeutic target for many diseases (Dong et al. 2016). Lin et al. found that baicalin could alleviate hydrogen peroxide-induced HK-2 cytotoxicity by inhibiting ER stress (decreased BIP and CHOP expression) and by activating NRF2 signaling (increased NRF2 expression) (Lin et al. 2014). These data indicate that the overexpression of UFL1 may further enhance the antioxidant capacity of cells by promoting the release of NRF2, thus enhancing the resistance of cells to ferroptosis. On the other hand, we found that UFL1 overexpression alleviated LPS-induced ER stress (the decreased expression of BIP). BIP, an ER molecular chaperone, can recognize misfolded proteins in the ER and trigger the unfolded protein responses (UPR) to alleviate ER stress (Pobre et al. 2019). When misfolded proteins were reduced or when oxidative stress was alleviated, BIP expression returned to normal levels. In addition, the ferroptosis inhibitor Fer-1 alleviated the ER stress and apoptosis caused by LPS; these findings were consistent with previous studies of acute kidney injury (AKI) and acute lung injury (ALI) (Hu et al. 2020; Liu et al. 2020b; Miotto et al. 2020). Our experiments indicate that the function of UFL1 reduces ER stress and apoptosis via the ferroptosis pathway.
Conclusions
Our present research showed that UFL1 can rescue the ER stress and apoptosis caused by LPS stimulation via the P53/SLC7A11 system and by autophagy. This study highlights the novel role of UFL1 in the regulation of ER stress and apoptosis in KGNs and indicates that UFL1 may be an effective target to reduce LPS-reduced cellular damage by adjusting the ferroptosis pathway.
Acknowledgements
The authors thank all of the members in their lab. We are grateful to the Nanchang University Medical Department for providing technical support and abundant resources for our research group. Thanks to the National Natural Science Foundation for providing financial support for us. The authors would like to express their gratitude to EditSprings (https://www.editsprings.cn) for the expert linguistic services provided.
Author contribution
Conceptualization: Jingyi Li, Xiangting Tang; methodology: Jingyi Li, Xuer Tu; investigation: Jingyi Li, Zhe Jin; statistical analyses: Jingyi Li, Hao Dong; writing—original draft preparation: Jingyi Li, Qi Yang, Ting Yao; writing—review and editing: Jingyi Li, Xiangting Tang; funding acquisition: Zezheng Pan; resources: Zezheng Pan; project administration: Jingyi Li, Zezheng Pan. All authors read and approved the final manuscript.
Funding
This research was funded by the National Natural Science Foundation of China grant number (81860263) and the Natural Science Foundation of Jiangxi Province grant number (20192BAB205119).
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jingyi Li and Xiangting Tang contributed equally to the article.
References
- Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, Bassik MC, Nomura DK, Dixon SJ, Olzmann JA. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao JY, Dixon SJ. Mechanisms of ferroptosis. Cell Mol Life Sci. 2016;73:2195–2209. doi: 10.1007/s00018-016-2194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Mi Y, Zhang X, Ma Q, Song Y, Zhang L, Wang D, Xing J, Hou B, Li H, Jin H, Du W, Zou Z. Dihydroartemisinin-induced unfolded protein response feedback attenuates ferroptosis via PERK/ATF4/HSPA5 pathway in glioma cells. J Exp Clin Cancer Res. 2019;38:402. doi: 10.1186/s13046-019-1413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Conza G, Ho PC (2020) ER stress responses: an emerging modulator for innate immunity. Cells 9(3):695. 10.3390/cells9030695 [DOI] [PMC free article] [PubMed]
- Dodson M, Castro-Portuguez R, Zhang DD. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019;23:101107. doi: 10.1016/j.redox.2019.101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong D, Reece EA, Yang P. The Nrf2 activator vinylsulfone reduces high glucose-induced neural tube defects by suppressing cellular stress and apoptosis. Reprod Sci (Thousand Oaks, Calif) 2016;23:993–1000. doi: 10.1177/1933719115625846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X, Li Z, Zhu S, Cheng M, Ju Y, Ren L, Yang G, Min D. Galangin attenuated cerebral ischemia-reperfusion injury by inhibition of ferroptosis through activating the SLC7A11/GPX4 axis in gerbils. Life Sci. 2021;264:118660. doi: 10.1016/j.lfs.2020.118660. [DOI] [PubMed] [Google Scholar]
- Hadian K, Stockwell BR. SnapShot: ferroptosis. Cell. 2020;181(1188–1188):e1181. doi: 10.1016/j.cell.2020.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassannia B, Vandenabeele P, Vanden Berghe T. Targeting ferroptosis to iron out cancer. Cancer Cell. 2019;35:830–849. doi: 10.1016/j.ccell.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ, 3rd, Kang R, Tang D. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12:1425–1428. doi: 10.1080/15548627.2016.1187366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Zhang H, Yi B, Yang S, Liu J, Hu J, Wang J, Cao K, Zhang W. VDR activation attenuate cisplatin induced AKI by inhibiting ferroptosis. Cell Death Dis. 2020;11:73. doi: 10.1038/s41419-020-2256-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N, Yu Y, Qiao J. Dual role for the unfolded protein response in the ovary: adaption and apoptosis. Protein Cell. 2016;8:14–24. doi: 10.1007/s13238-016-0312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CY, Deng JS, Huang WC, Jiang WP, Huang GJ (2020) Attenuation of lipopolysaccharide-induced acute lung injury by hispolon in mice, through regulating the TLR4/PI3K/Akt/mTOR and Keap1/Nrf2/HO-1 pathways, and suppressing oxidative stress-mediated ER stress-induced apoptosis and autophagy. Nutrients 12(6):1742. 10.3390/nu12061742 [DOI] [PMC free article] [PubMed]
- Hybertson BM, Gao B, Bose SK, McCord JM. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol Aspects Med. 2011;32:234–246. doi: 10.1016/j.mam.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, Baer R, Gu W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang R, Kroemer G, Tang D. The tumor suppressor protein p53 and the ferroptosis network. Free Radic Biol Med. 2019;133:162–168. doi: 10.1016/j.freeradbiomed.2018.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Z, Liu R, Cheng Y. Artesunate alleviates liver fibrosis by regulating ferroptosis signaling pathway. Biomed Pharmacother. 2019;109:2043–2053. doi: 10.1016/j.biopha.2018.11.030. [DOI] [PubMed] [Google Scholar]
- Lebeaupin C, Proics E, de Bieville CH, Rousseau D, Bonnafous S, Patouraux S, Adam G, Lavallard VJ, Rovere C, Le Thuc O, Saint-Paul MC, Anty R, Schneck AS, Iannelli A, Gugenheim J, Tran A, Gual P, Bailly-Maitre B. ER stress induces NLRP3 inflammasome activation and hepatocyte death. Cell Death Dis. 2015;6:e1879. doi: 10.1038/cddis.2015.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Lee DH, Choudry HA, Bartlett DL, Lee YJ. Ferroptosis-induced endoplasmic reticulum stress: cross-talk between ferroptosis and apoptosis. Mol Cancer Res. 2018;16:1073–1076. doi: 10.1158/1541-7786.MCR-18-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Lee DH, Jeong SY, Park SH, Oh SC, Park YS, Yu J, Choudry HA, Bartlett DL, Lee YJ. Ferroptosis-inducing agents enhance TRAIL-induced apoptosis through upregulation of death receptor 5. J Cell Biochem. 2019;120:928–939. doi: 10.1002/jcb.27456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei G, Zhuang L, Gan B (2021) mTORC1 and ferroptosis: regulatory mechanisms and therapeutic potential. BioEssays News Rev Mol Cell Dev Biol 43(8):e2100093. 10.1002/bies.202100093 [DOI] [PubMed]
- Levine B, Kroemer G. Biological functions of autophagy genes: a disease perspective. Cell. 2019;176:11–42. doi: 10.1016/j.cell.2018.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Yue G, Ma W, Zhang A, Zou J, Cai Y, Tang X, Wang J, Liu J, Li H, Su H. Ufm1-specific ligase Ufl1 regulates endoplasmic reticulum homeostasis and protects against heart failure. Circ Heart Fail. 2018;11:e004917. doi: 10.1161/CIRCHEARTFAILURE.118.004917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Li L, Chen K, Wang Y, Yang F, Wang G. UFL1 alleviates lipopolysaccharide-induced cell damage and inflammation via regulation of the TLR4/NF-kappaB pathway in bovine mammary epithelial cells. Oxid Med Cell Longev. 2019;2019:6505373. doi: 10.1155/2019/6505373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Li L, Zhang Y, Zheng L, Xu M, Rong R, Zhu T. Baicalin ameliorates H2O2 induced cytotoxicity in HK-2 cells through the inhibition of ER stress and the activation of Nrf2 signaling. Int J Mol Sci. 2014;15:12507–12522. doi: 10.3390/ijms150712507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Guan D, Dong M, Yang J, Wei H, Liang Q, Song L, Xu L, Bai J, Liu C, Mao J, Zhang Q, Zhou J, Wu X, Wang M, Cong YS. UFMylation maintains tumour suppressor p53 stability by antagonizing its ubiquitination. Nat Cell Biol. 2020;22:1056–1063. doi: 10.1038/s41556-020-0559-z. [DOI] [PubMed] [Google Scholar]
- Liu P, Feng Y, Li H, Chen X, Wang G, Xu S, Li Y, Zhao L. Ferrostatin-1 alleviates lipopolysaccharide-induced acute lung injury via inhibiting ferroptosis. Cell Mol Biol Lett. 2020;25:10. doi: 10.1186/s11658-020-00205-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotto G, Rossetto M, Di Paolo ML, Orian L, Venerando R, Roveri A, Vuckovic AM, Bosello Travain V, Zaccarin M, Zennaro L, Maiorino M, Toppo S, Ursini F, Cozza G. Insight into the mechanism of ferroptosis inhibition by ferrostatin-1. Redox Biol. 2020;28:101328. doi: 10.1016/j.redox.2019.101328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi Y, Yanase T, Mu Y, Oba K, Ichino I, Saito M, Nomura M, Mukasa C, Okabe T, Goto K, Takayanagi R, Kashimura Y, Haji M, Nawata HJE (2001) Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor. Endocrinology 142(1):437–445. 10.1210/endo.142.1.7862 [DOI] [PubMed]
- Pang J, Peng H, Wang S, Xu X, Xu F, Wang Q, Chen Y, Barton LA, Chen Y, Zhang Y, Ren J. Mitochondrial ALDH2 protects against lipopolysaccharide-induced myocardial contractile dysfunction by suppression of ER stress and autophagy. Biochim Biophys Acta. 2019;1865:1627–1641. doi: 10.1016/j.bbadis.2019.03.015. [DOI] [PubMed] [Google Scholar]
- Pobre KFR, Poet GJ, Hendershot LM. The endoplasmic reticulum (ER) chaperone BiP is a master regulator of ER functions: Getting by with a little help from ERdj friends. J Biol Chem. 2019;294:2098–2108. doi: 10.1074/jbc.REV118.002804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid HO, Yadav RK, Kim HR, Chae HJ. ER stress: autophagy induction, inhibition and selection. Autophagy. 2015;11:1956–1977. doi: 10.1080/15548627.2015.1091141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascon S, Hatzios SK, Kagan VE, Noel K, Jiang X, Linkermann A, Murphy ME, Overholtzer M, Oyagi A, Pagnussat GC, Park J, Ran Q, Rosenfeld CS, Salnikow K, Tang D, Torti FM, Torti SV, Toyokuni S, Woerpel KA, Zhang DD. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su LJ, Zhang JH, Gomez H, Murugan R, Hong X, Xu D, Jiang F, Peng ZY. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid Med Cell Longev. 2019;2019:5080843. doi: 10.1155/2019/5080843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R, Tang D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63:173–184. doi: 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi K, Sou YS, Tada N, Nakamura E, Iemura S, Natsume T, Kang SH, Chung CH, Kasahara M, Kominami E, Yamamoto M, Tanaka K, Komatsu M. A novel type of E3 ligase for the Ufm1 conjugation system. J Biol Chem. 2010;285:5417–5427. doi: 10.1074/jbc.M109.036814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Lu J, Hao X, Li H, Zhang G, Liu X, Li X, Zhao C, Kuang W, Chen D, Zhu M. FTH1 inhibits ferroptosis through ferritinophagy in the 6-OHDA model of Parkinson's disease. Neurotherapeutics. 2020;17:1796–1812. doi: 10.1007/s13311-020-00929-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li C, Wang Y, Li L, Han Z, Wang G (2020) UFL1 alleviates LPS-induced apoptosis by regulating the NF-κB signaling pathway in Bovine Ovarian Granulosa cells. Biomolecules 10(2):260. 10.3390/biom10020260 [DOI] [PMC free article] [PubMed]
- Wei Y, Xu X. UFMylation: a unique & fashionable modification for life. Genomics Proteomics Bioinformatics. 2016;14:140–146. doi: 10.1016/j.gpb.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, Kang R, Tang D. Ferroptosis: process and function. Cell Death Differ. 2016;23:369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Fang Z, Pan Z. Ufl1/RCAD, a Ufm1 E3 ligase, has an intricate connection with ER stress. Int J Biol Macromol. 2019;135:760–767. doi: 10.1016/j.ijbiomac.2019.05.170. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Yin Q, Jin E, Chen H, He S (2020) Selenium attenuates chronic heat stress-induced apoptosis via the inhibition of endoplasmic reticulum stress in mouse granulosa cells. Molecules (Basel, Switzerland) 25(3):557. 10.3390/molecules25030557 [DOI] [PMC free article] [PubMed]
- Yang WS, Stockwell BR. Ferroptosis: death by lipid peroxidation. Trends Cell Biol. 2016;26:165–176. doi: 10.1016/j.tcb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Sun H, Li Y, Shao M, Han P, Yu X, He L, Xu Y, Li S. Exposure to triptolide affects follicle development in NIH mice: Role of endoplasmic reticulum stress in granulosa cell apoptosis. Hum Exp Toxicol. 2017;36:82–92. doi: 10.1177/0960327116638725. [DOI] [PubMed] [Google Scholar]
- Zhang M, Zhu X, Zhang Y, Cai Y, Chen J, Sivaprakasam S, Gurav A, Pi W, Makala L, Wu J, Pace B, Tuan-Lo D, Ganapathy V, Singh N, Li H. RCAD/Ufl1, a Ufm1 E3 ligase, is essential for hematopoietic stem cell function and murine hematopoiesis. Cell Death Differ. 2015;22:1922–1934. doi: 10.1038/cdd.2015.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Guo M, Li Y, Shen M, Kong D, Shao J, Ding H, Tan S, Chen A, Zhang F, Zheng S. RNA-binding protein ZFP36/TTP protects against ferroptosis by regulating autophagy signaling pathway in hepatic stellate cells. Autophagy. 2020;16:1482–1505. doi: 10.1080/15548627.2019.1687985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng DW, Lei Q, Zhu JY, Fan JX, Li CX, Li C, Xu Z, Cheng SX, Zhang XZ. Switching apoptosis to ferroptosis: metal-organic network for high-efficiency anticancer therapy. Nano Lett. 2017;17:284–291. doi: 10.1021/acs.nanolett.6b04060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.