Introduction
Stevens Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) is a severe cutaneous adverse reaction. We report a case of TEN and concurrent COVID-19-associated hyperinflammatory syndrome (cHIS) successfully treated with systemic corticosteroids and etanercept.
Case report
A 46-year-old man with a history of ascending aortic dissection status post aortic root and aortic valve replacement on warfarin presented with a 2-day history of painful rash. His only new medication was trimethoprim-sulfamethoxazole 160-800 mg twice daily, started 4 days prior to rash onset and discontinued the following day. Two days preceding the rash, he developed a fever to 38 °C. Review of systems was negative for cough, sore throat, rhinorrhea, or congestion. He was vaccinated and boosted with the BNT162b2 (Pfizer-BioNTech) vaccine. Nasopharyngeal polymerase chain reaction test for SARS-CoV-2 was negative.
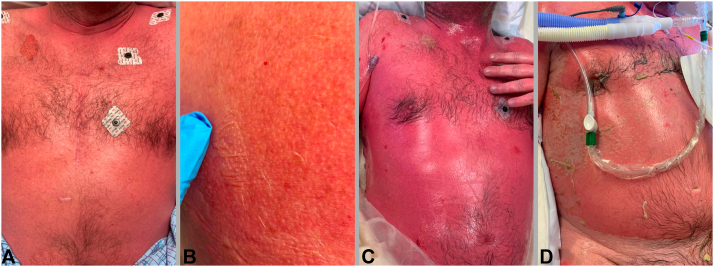
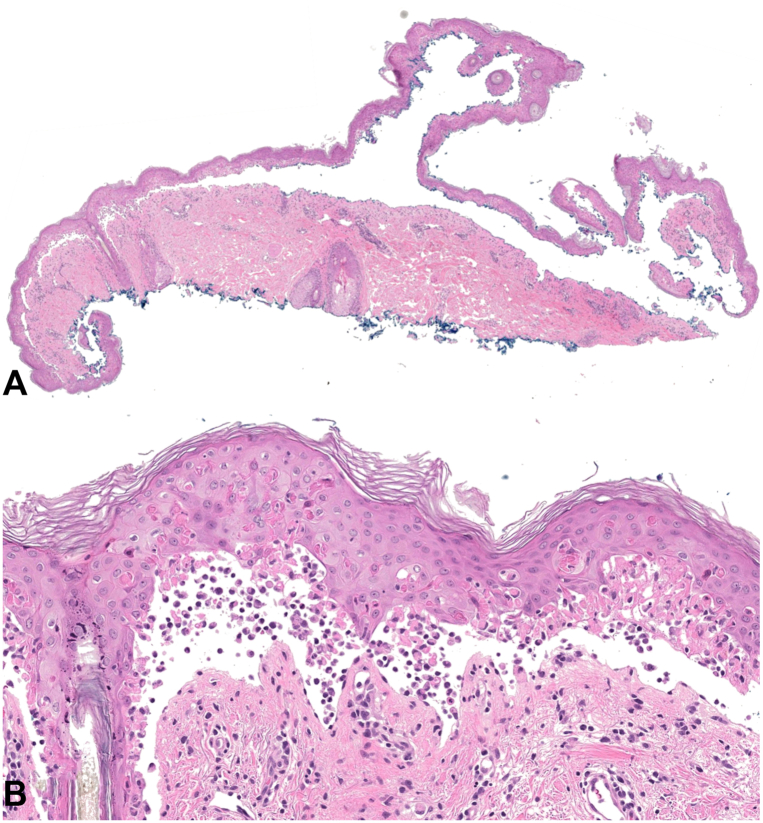
On admission, his vital signs were unremarkable. His dermatologic examination (Fig 1) demonstrated bilaterally injected sclera with yellow-white crusting around the eyelid margins and a 3-cm erosion on the hard palate. Painful red macules coalescing into patches covered >90% of his body surface area, with extensive flaccid bullae creating a “cigarette paper” appearance. Nikolsky sign was positive. Laboratory testing was notable for serum bicarbonate of 19 mmol/L (normal 20-30 mmol/L). Skin biopsy of the right upper back demonstrated an acute basket-woven stratum corneum, widespread, confluent keratinocyte necrosis with focal adnexal involvement, and modest inflammation (Fig 2).
Fig 1.
Clinical findings. Coalescing red papules on the anterior chest (A) with a positive Nikolsky sign on the right upper back (B), both on initial presentation (day 1). Coalescing red macules and patches with >95% BSA with multiple large flaccid bullae and erosions, demonstrated on the anterior chest on day 2 (C). Significant re-epithelialization after 2 doses of etanercept as demonstrated on the anterior chest on day 11 (D). BSA, Body surface area.
Fig 2.
Histopathological findings. Acute basket-woven stratum corneum, widespread, confluent keratinocyte necrosis with focal adnexal involvement, and modest inflammation, seen at 2× (A) and 200× (B).
The diagnosis based on clinicopathological correlation was TEN, with trimethoprim-sulfamethoxazole as the suspected drug culprit. He was initially treated with a single dose of methylprednisolone 125 mg IV (2 mg/kg prednisone equivalent) and etanercept 50 mg subcutaneously. On day 2, he became febrile with a temperature of 39.3 °C. On day 4, he tested positive for SARS-CoV-2 by nasopharyngeal polymerase chain reaction. Laboratory testing was notable for C-reactive protein of 276.5 mg/L (normal <10 mg/L) and ferritin of 1465 ng/mL (normal 30-400 ng/mL). On day 5, he began a 5-day course of dexamethasone 6 mg and received a second etanercept dose. On day 6, he received tocilizumab 8 mg/kg IV and began a 5-day course of remdesivir 100 mg. On day 7, he required intubation and mechanical ventilation due to agitated delirium requiring sedation. While intubated, he required low positive end-expiratory pressures and a low fraction of inspired oxygen. Initial reepithelization was observed on day 7. His hospital course was complicated by Pseudomonas bacteremia and Clostridioides difficile colitis, for which he received IV piperacillin-tazobactam and PO vancomycin, respectively. Complete reepithelization was observed by day 15. He was extubated on day 17 and discharged home on day 23.
Discussion
This case report describes the first successful treatment of TEN and concurrent cHIS with systemic corticosteroids and etanercept in the literature. His SCORTEN (Score of Toxic Epidermal Necrolysis) was 3 and the ABCD-10 severity-of-illness score (age, bicarbonate, cancer, dialysis, 10% body surface area) was 2, with predicted mortality rates of 35.8% and 12.3%, respectively.1,2 The cHIS score was 2 (based on D-dimer and C-reactive protein). cHIS score >1 is sensitive for mechanical ventilation and mortality.3 Some viruses may contribute to the pathogenesis of SJS/TEN, for example, by increasing expression of Fas ligand or sensitivity to Fas ligand-mediated apoptosis.4 Such a pathogenic role for SARS-CoV-2 remains uncertain.
Consensus regarding SJS/TEN treatment is lacking. A 2022 Cochrane systematic review determined with low-certainty evidence that etanercept, when compared to corticosteroids, may reduce disease-specific mortality in SJS/TEN.5 This determination was based on a randomized controlled trial, which further reported that etanercept, compared with corticosteroids, significantly reduced the skin-healing time in participants with ≥10% body surface area detachment and the incidence of gastrointestinal hemorrhage in all participants.6 The Cochrane systematic review determined an uncertain difference in disease-specific mortality between treatment with and without corticosteroids.5 Since the Cochrane review’s publication, additional studies have evaluated the efficacy of etanercept in the treatment of SJS/TEN. A retrospective study of 242 patients with SJS/TEN found that corticosteroids combined with etanercept, compared with corticosteroid monotherapy, reduced mortality, skin healing time, and corticosteroid-related adverse events.7 A prospective study of 25 patients with SJS/TEN found that corticosteroids combined with etanercept, compared with corticosteroid monotherapy, significantly shortened the course of the initial steroid treatment and the duration of the acute stage, hospitalization stay, and skin reepithelialization.8
Systemic corticosteroids have been shown to reduce mortality in hospitalized patients with COVID-19 infection receiving respiratory support.9 Data on etanercept use during COVID-19 infection are limited; however, a meta-analysis of 35 studies found no difference in hospitalization between patients with COVID-19 infection taking tumor necrosis factor–alpha inhibitors and controls.10 Further research is required to determine the efficacy and safety of systemic corticosteroids and etanercept in patients with SJS/TEN complicated by COVID-19 infection.
Conflicts of interest
None disclosed.
Footnotes
Drs Choi and Garritano equal contribution, first authorship.
Funding sources: JG was supported in part by NIHF30HG011193 and by US NIH MSTP Training Grant T32GM007205. AJL is supported by a Women’s Health Career Development Award from the Dermatology Foundation and by CTSA grant number KL2 TR001862 from the National Center for Advancing Translational Sciences (NCATS), components of the National Institutes of Health (NIH), and NIH roadmap for Medical Research, through the Yale Center for Clinical Investigation. The publication's contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
IRB approval status: Not applicable.
Prior presentation: This work has not been presented previously.
Consent for the publication of all patient photographs and medical information was provided by the authors at the time of article submission to the journal stating that all patients gave consent for their photographs and medical information to be published in print and online and with the understanding that this information may be publicly available.
References
- 1.Fouchard N., Bertocchi M., Roujeau J.-C., Revuz J., Wolkenstein P., Bastuji-Garin S. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149–153. doi: 10.1046/j.1523-1747.2000.00061.x. [DOI] [PubMed] [Google Scholar]
- 2.Noe M.H., Rosenbach M., Hubbard R.A., et al. Development and validation of a risk prediction model for in-hospital mortality among patients with Stevens-Johnson syndrome/toxic epidermal necrolysis—ABCD-10. JAMA Dermatol. 2019;155:448–454. doi: 10.1001/jamadermatol.2018.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Webb B.J., Peltan I.D., Jensen P., et al. Clinical criteria for COVID-19-associated hyperinflammatory syndrome: a cohort study. Lancet Rheumatol. 2020;2:e754–e763. doi: 10.1016/S2665-9913(20)30343-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimmerman D., Dang N.H. In: Oncologic Critical Care. Nates J., Price K., editors. Springer; Cham: 2019. Stevens–Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) [DOI] [Google Scholar]
- 5.Jacobsen A., Olabi B., Langley A., et al. Systemic interventions for treatment of Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome. Cochrane Database Syst Rev. 2022;3(3):CD013130. doi: 10.1002/14651858.CD013130.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang C.-W., Yang L.-Y., Chen C.-B., et al. Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128:985–996. doi: 10.1172/JCI93349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J., Lu C.-W., Chen C.-B., et al. Evaluation of combination therapy with etanercept and systemic corticosteroids for Stevens-Johnson syndrome and toxic epidermal necrolysis: a multicenter observational study. J Allergy Clin Immunol In Pract. 2022;10:1295–1304.e6. doi: 10.1016/j.jaip.2022.01.038. [DOI] [PubMed] [Google Scholar]
- 8.Ao S., Gao X., Zhan J., et al. Inhibition of tumor necrosis factor improves conventional steroid therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis in a cohort of patients. J Am Acad Dermatol. 2022;86:1236–1245. doi: 10.1016/j.jaad.2022.01.039. [DOI] [PubMed] [Google Scholar]
- 9.Group R.C. Dexamethasone in hospitalized patients with Covid-19. New Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kokkotis G., Kitsou K., Xynogalas I., et al. Systematic review with meta-analysis: COVID-19 outcomes in patients receiving anti-TNF treatments. Aliment Pharmacol Ther. 2022;55:154–167. doi: 10.1111/apt.16717. [DOI] [PubMed] [Google Scholar]