Abstract
Acquired hemophilia A (AHA) is a rare autoimmune bleeding disorder. Various autoimmune diseases, including AHA, have been reported to occur after the administration of mRNA COVID-19 vaccines. However, the characteristics of these AHA cases remain unclear. We report a case in which AHA arose in a young patient after the administration of an mRNA COVID-19 vaccine, but improved rapidly. The patient's factor VIII (FVIII) inhibitor titer spontaneously decreased to less than half of that seen at diagnosis. One week after the initial immunosuppressive therapy, the FVIII inhibitor had disappeared. Our case suggests that AHA that arises in young patients after COVID-19 vaccination may resolve spontaneously, and the levels of FVIII inhibitors may decrease more rapidly in such cases than in idiopathic AHA. Unlike for immune thrombocytopenic purpura (ITP), no acute type of AHA has been recognized. This case suggests that just as there is an acute type of ITP that develops in children/after vaccination, there may be an acute type of AHA that arises in young patients that receive mRNA COVID-19 vaccines.
Keywords: COVID-19, Vaccination, Acquired hemophilia A
1. Introduction
Acquired hemophilia A (AHA) is a rare autoimmune bleeding disorder with a high mortality rate [1]. It commonly develops in patients aged >65 years and is caused by the production of autoantibodies against endogenous factor VIII (FVIII) [1]. Although most cases of AHA are idiopathic, AHA is also associated with autoimmune conditions [2]. Various autoimmune diseases may develop after the administration of coronavirus disease 2019 (COVID-19) mRNA vaccines [[3], [4], [5]]. A case of AHA that arose after mRNA COVID-19 vaccine administration has also been reported [6]. However, the characteristics of such AHA cases remain unclear. Here, we report a case of AHA that developed after the administration of an mRNA COVID-19 vaccine in a young patient, but improved rapidly.
2. Case description
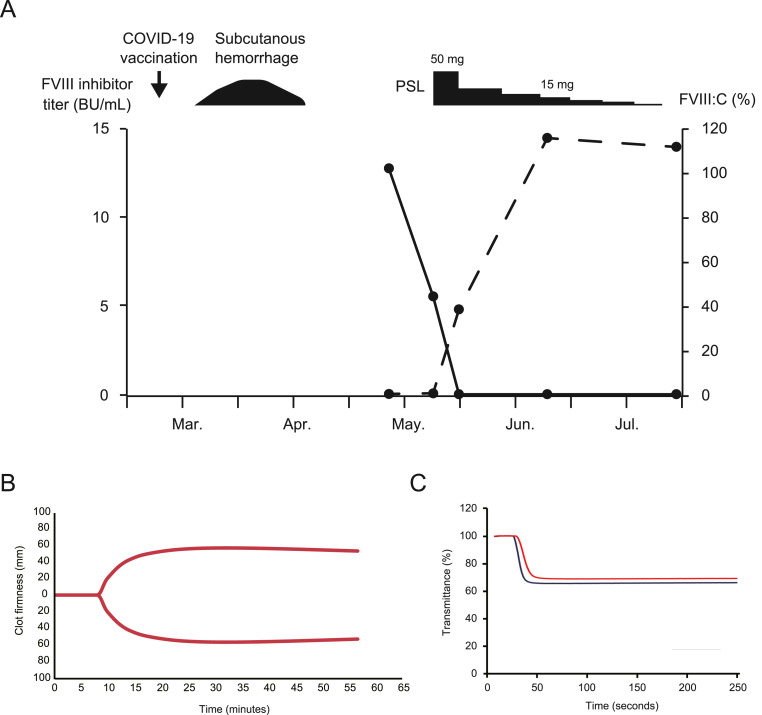
A 45-year-old female developed a subcutaneous hemorrhage caused by minor blunt force trauma. Her last pregnancy had been 5 years ago. Two weeks before onset, she had received her third vaccination for COVID-19. The vaccines administered were Pfizer-BioNTech BNT162b2 for the first and second doses and Moderna mRNA-1273 for the third dose. She visited a clinic one and a half months after symptom onset. Laboratory tests showed a prolonged activated partial thromboplastin time (APTT) (75.1 s). She had a brother and three children, who did not develop AHA after undergoing COVID-19 vaccination. When she visited our hospital, the subcutaneous hemorrhage had resolved. Coagulation tests revealed a prolonged APTT (72.6 s), decreased factor VIII coagulant activity (FVIII:C) (0.9%), and a high FVIII inhibitor titer (12.8 Bethesda units [BU]/mL). Her hemoglobin concentration was 13.4 g/dL, and her platelet count was 256 × 109/L. She was diagnosed with AHA. There was no evidence of underlying diseases, such as malignancies or autoimmune disorders. Twelve days after being diagnosed, she was admitted for treatment.
On admission, laboratory tests showed that her FVIII inhibitor titer had decreased to 5.6 BU/mL despite no treatment having been administered (Fig. 1 A). An assessment of the clot-formation capacity of her whole blood based on thromboelastography (TEG), which was performed using kaolin and the TEG 6s® system (HAEMONETICS, Boston, MA, U.S.A.), revealed that her reaction rate time was 8.2 min, which was within the reference range (4.6–9.1 min) (Fig. 1B) [7,8]. On the other hand, APTT clot waveform analysis (CWA) showed a slightly prolonged clot time (37.2 s) compared with that of normal plasma (32.0 s). In addition, the minimum absolute value of the first order differential (|min1|; 2.441) and the minimum absolute value of the second order differential (|min2|; 0.324) were lower than those of normal plasma (|min1|: 3.341; |min2|: 0.500) (Fig. 1C). |min1| and |min2| represent the maximum coagulation rate and maximum acceleration of the coagulation rate, respectively [9]. These comprehensive coagulation tests suggested that her coagulation capacity was only slightly impaired. As recommended in the guidelines, immunosuppressive therapy with 1 mg/kg (50 mg/body) of prednisolone (PSL) was initiated according to the general treatment regimen for AHA. One week later, her FVIII inhibitor titer had decreased to 0.1 BU/mL, and her APTT had returned to the normal range. After that, the dose of PSL was tapered rapidly. After 5 weeks of treatment, the PSL dose was <15 mg/day, and the AHA had gone into complete remission (CR) [10]. Two months after the initial treatment, the PSL treatment was discontinued, and no further recurrence was observed.
Fig. 1.
(A) Evaluation of the factor VIII (FVIII) inhibitor titer and FVIII coagulant activity (FVIII:C). The black and dashed lines represent the changes in the FVIII inhibitor titer and FVIII:C, respectively. Two weeks after the third dose of a coronavirus disease 2019 (COVID-19) mRNA vaccine, a subcutaneous hemorrhage developed. After visiting our hospital, the patient's FVIII inhibitor titer and FVIII:C were analyzed over time. Before the initial immunosuppressive therapy (prednisolone; PSL), the patient's FVIII inhibitor titer decreased spontaneously. BU, Bethesda units. (B) Thromboelastography (TEG) performed before the immunosuppressive therapy using kaolin and the TEG 6s® system. The reaction rate time was 8.2 min. (C) Activated partial thromboplastin time (APTT) clot waveform analysis performed before the immunosuppressive therapy. The black and red lines indicate normal plasma and the patient's plasma, respectively. The clot time and |min1| and |min2| values of the normal plasma were 32.0 s, 3.341, and 0.500, respectively. The clot time and |min1| and |min2| values of the patient's plasma were 37.2 s, 2.441, and 0.324, respectively. |min1|, the minimum absolute value of the first order differential; |min2|, the minimum absolute value of the second order differential.
3. Discussion
We reported the case of a young patient who developed AHA after receiving an mRNA COVID-19 vaccine. Interestingly, her FVIII inhibitor titer decreased spontaneously without immunosuppressive therapy. At that time, slight impairment of her coagulation capacity was detected by CWA. After one week's PSL treatment, her APTT had returned to the normal range. The time required to achieve CR in this case was less than half of the previously reported median time. Furthermore, the patient's mild subcutaneous bleeding resolved spontaneously before immunosuppressive therapy was started, and hemostatic agents were not required.
AHA is a rare disorder, but it is more frequent in the elderly. Although the age distribution of the condition shows a small peak in women aged 20–40 years because of pregnancy-related cases, the median age at diagnosis is approximately 70 years [11]. Cases of AHA involving young patients that are unrelated to pregnancy are extremely rare. According to the PubMed database, 18 cases of AHA that developed after the administration of an mRNA COVID-19 vaccine had been reported by the end of June 2022 (Table 1 ) [6,[12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23]]. As with idiopathic cases, the majority of cases of AHA that arose after the administration of such vaccines involved elderly patients. However, in addition to our patient, another young female was reported to develop AHA after receiving an mRNA COVID-19 vaccine [18]. More than 80% of people aged ≥20 in Japan have received COVID-19 vaccines. Our case suggests that although it is uncommon, AHA may also occur after the administration of mRNA COVID-19 vaccines in young adults.
Table 1.
Reported cases of acquired hemophilia A that arose after the administration of mRNA COVID-19 vaccines.
| Age | Sex | Time to onset from last dose | Vaccine | Diagnosis and |
Symptomsa | Severityb | FVIII inhibitor |
Immunosuppressive |
Outcome | References |
|---|---|---|---|---|---|---|---|---|---|---|
| treatment | (BU/mL) | treatment | ||||||||
| 69 | M | 9 days after 1st dose | N.A. | After 2nd dose | Hematoma in rectus femoris muscle | Mild | 80 | PSL | After 4 weeks, the inhibitor level had decreased to 2 BU/mL. | Radwi M et al. [6] |
| 85 | M | 1 week after 1st dose | Moderna mRNA-1273 | After 2nd dose | Hematoma in iliopsoas muscle | Severe | 2.2 | PSL, Rit | Died | Cittone MG et al. [12] |
| 86 | F | 3 weeks after 2nd dose | Moderna mRNA-1273 | After 2nd dose | Hemothorax | Severe | 1.1 | PSL | After 17 days, the FVIII:C level had increased to 178%. | Cittone MG et al. [12] |
| 72 | F | 2 weeks after 1st dose | Moderna mRNA-1273 | After 1st dose | Cutaneous hematomas, anemia | Severe | 12.4 | PSL, Rit | After 3 weeks, the inhibitor level had decreased to 5.6 BU/mL. | Cittone MG et al. [12] |
| 76 | F | 4 days after 2nd dose | Moderna mRNA-1274 | After 2nd dose | Ecchymoses, melanotic stool, anemia | Severe | 11.2 | mPSL, PSL | After 26 days, the inhibitor level had decreased to <0.5 BU/mL. | Portuguese AJ et al. [13] |
| 67 | M | 19 days after 2nd dose | Pfizer BioNTech | After 2nd dose | Hematoma in the leg | Mild | 110 | PSL, Rit | After 34 days, the inhibitor level had decreased to <0.5 BU/mL. | Farley S et al. [14] |
| 70 | M | 8 days after 1st dose | Moderna mRNA-1273 | After 1st dose | Ecchymoses | Mild | 39.9 | PSL, CPA | After 6 days, the inhibitor level had decreased to 11.4 BU/mL. | Lemoine C et al. [15] |
| 86 | M | 14 days after 2nd dose | Pfizer BioNTech | After 2nd dose | Hematoma, anemia | Severe | 2.1 | mPSL | After 7 months, the inhibitor became undetectable. | Leone MC et al. [16] |
| 73 | F | 14 days after 1st dose | Pfizer BioNTech | After 2nd dose | Hematomas in knee and jaw | Mild | 0.8 | mPSL | After 5 months, the inhibitor became undetectable. | Leone MC et al. [16] |
| 67 | M | 49 days after 2nd dose | Pfizer BioNTech | After 2nd dose | Hematoma of the tongue | Mild | 2.5 | PSL, CPA | Six days after discharge, the inhibitor became undetectable. | Leone MC et al. [16] |
| 77 | M | 52 days after 2nd dose | Pfizer BioNTech | After 2nd dose | Hematuria, anemia | Severe | 6.9 | mPSL, Rit | Died | Leone MC et al. [16] |
| 95 | F | 1 week after 1st dose | Pfizer BioNTech | After 2nd dose | Hematoma in hand | Mild | 5.4 | PSL, Rit | After 22 days, the inhibitor level had decreased to 0.7 BU/mL. | Murali A et al. [17] |
| 39 | F | 10 days after 1st dose | Pfizer BioNTech | After 1st dose | Hematuria | Mild | 17.2 | None | After 2 months, the FVIII:C level had increased to 78.8%. | Soliman DS et al. [18] |
| 80 | M | 2 weeks after 1st dose | Pfizer BioNTech | After 1st dose | Hematoma of the thigh, anemia | Severe | 7.5 | mPSL, PSL, AZ | After 6 weeks, the inhibitor became undetectable. | Ai Vuen L et al. [19] |
| 75 | M | 3 months after 2nd dose | Pfizer BioNTech | After 2nd dose | Ecchymoses, anemia | Severe | 318 | PSL, Rit, CPA, CyA | Improved | Al Hennawi H et al. [20] |
| 77 | M | 3 weeks after 2nd dose | Moderna mRNA-1273 | After 2nd dose | Ecchymoses | Mild | 71.6 | PSL, CPA | After 5 weeks, the inhibitor level had decreased to 49 BU/mL. | Fu PA et al. [21] |
| 72 | M | 9 days after 3rd dose | Moderna mRNA-1273 | After 3rd dose | Ecchymoses, anemia | Severe | 158.6 | PSL, CPA, Rit | After 3 weeks, the inhibitor level had decreased to 20.6 BU/mL. | Plüß M et al. [22] |
| 75 | M | 6 days after 3rd dose | Pfizer BioNTech | After 3rd dose | Ecchymoses, anemia | Severe | N.A. | PSL, CPA | After 12 days, the FVIII:C level had increased to a normal level. | Rashid A et al. [23] |
| 45 | F | 2 weeks after 3rd dose | Moderna mRNA-1273 | After 3rd dose | Subcutaneous hemorrhage | Mild | 12.8 | PSL | After 3 weeks, the inhibitor level had decreased to 0.1 BU/mL. | This case |
COVID-19, coronavirus disease 2019; M, male; F, female; N.A., not applicable; FVIII, factor VIII; BU, Bethesda units; FVIII:C, FVIII coagulant activity.
PSL, prednisolone; Rit, rituximab; mPSL, methylprednisolone; CPA, cyclophosphamide; AZ, azathioprine; CyA, cyclosporine A.
Anemia was defined as a hemoglobin (Hb) level of <8 g/dL or a sudden drop in the Hb level of >2 g/dL that required a blood transfusion.
Including our patient, 19 patients have been reported to develop AHA after receiving COVID-19 vaccines (Table 1). The median age at onset in these cases was 75 years (range: 39–95), and the patients included 12 males and 7 females. Whereas vaccine-induced immune thrombotic thrombocytopenia, a coagulation abnormality that may arise after COVID-19 vaccine administration, is caused by the use of an adenovirus-based vaccine, all of the reported cases of AHA that arose after COVID-19 vaccine administration involved mRNA vaccines [24]. The mechanism responsible for the development of AHA after the administration of mRNA COVID-19 vaccines has not been fully elucidated. A previous study demonstrated that broad toll-like-receptor stimulation may cause polyclonal B-cell activation and trigger autoantibody production instead of cross-reactivity to the vaccine-induced anti-spike immunoglobulin G, which occurs as a molecular mimicry phenomenon [25]. There are no reported AHA cases in which genetic predisposition has been suggested. Although our patient was young, her siblings did not develop AHA after receiving COVID-19 vaccines.
Ten of the reported patients (53%) developed severe AHA after receiving mRNA COVID-19 vaccines. We classified the severity of the reported AHA patients’ symptoms according to methods described in previous studies [11,26]. The proportion of patients who developed severe symptoms was slightly lower than has been reported for patients who developed AHA unrelated to COVID-19 vaccines [11]. Our patient only had minor bleeding symptoms. The other reported young patient also had mild bleeding symptoms. There are few reports of AHA occurring after COVID-19 vaccine administration in young patients. Further studies are warranted to assess whether AHA symptoms that arise after COVID-19 vaccination are milder in young patients than in older patients.
Immunosuppressive therapy is recommended for all adults as soon as AHA is diagnosed to reduce the risk of bleeding [1,27]. However, previous studies have shown that FVIII inhibitors disappear spontaneously after several months in some AHA cases [28,29]. A survey of 215 AHA patients showed that FVIII inhibitors spontaneously disappeared in 11 patients [28]. In a natural history study of 16 AHA patients, the conditions of 3 patients improved without treatment [29]. In the majority of these patients that experienced spontaneous improvement, the eradication of inhibitors took more than 10 months from the diagnosis of AHA. In our case, within 2 weeks the patient's FVIII inhibitor levels had decreased to less than half of those seen at diagnosis without treatment. The FVIII inhibitors disappeared within 3 weeks after diagnosis, although she had received one week of PSL treatment. A multicenter prospective observational study demonstrated that the median time to CR was 79 days [10]. Our patient achieved CR 37 days after the initial treatment. A previously reported 39-year-old patient who developed AHA after receiving an mRNA COVID-19 vaccine recovered 2 months after the initial presentation without immunosuppressive therapy [18]. In a young patient, the time to CR in pregnancy-related AHA was reported to be similar to that for AHA with other etiologies [30]. Our study suggests that AHA that arises after the administration of mRNA COVID-19 vaccines may improve spontaneously in young patients, and CR may be achieved more rapidly in such cases than in idiopathic AHA.
Unlike for immune thrombocytopenic purpura (ITP), no acute type of AHA has been recognized. In ITP, a hemorrhagic autoimmune hematological disorder, an acute type of ITP is known to develop after vaccine administration, such as after the administration of a measles-mumps-rubella (MMR) vaccine. ITP is characterized by a decreased platelet count due to the production of antibodies against platelet membrane antigens. ITP is classified as acute or chronic [31]. While ITP in adults is generally chronic, >70% of children with ITP develop acute-type ITP, which resolves irrespective of whether therapy is administered [31,32]. In children, MMR vaccination is associated with an increased risk of ITP. As spontaneous remission is less common in elderly AHA patients, immunosuppressive therapies are administered for AHA, as is case for chronic-type ITP. However, the conditions of the previously reported young patient who developed AHA after receiving an mRNA COVID-19 vaccine and our patient improved more rapidly than idiopathic AHA. As well as ITP, acute-type AHA may develop after the administration of mRNA COVID-19 vaccines in young patients.
In conclusion, our case suggests that AHA that arises after the administration of mRNA COVID-19 vaccines may resolve spontaneously in young patients, like acute-type ITP, and the levels of FVIII inhibitors may decrease more rapidly in such cases than in idiopathic AHA. Further evaluations of large cohorts are warranted to obtain more detailed findings about acute-type AHA that arises after the administration of mRNA COVID-19 vaccines.
Disclosure
This study was supported by the Japan Society for the Promotion of Science (JSPS) (KAKENHI Grant Number 21K16248 to HH). Written consent for publication was obtained from the patient.
Authors’ contributions
Hiroki Hosoi: Conceptualization, Data curation, Investigation, Writing – Original Draft; Misato Tane: Data curation, Investigation; Hideki Kosako: Data curation, Investigation; Masaki Ibe: Data curation, Investigation; Masahiro Takeyama: Data curation, Investigation, Writing – Review & Editing; Shogo Murata: Writing – Review & Editing; Toshiki Mushino: Writing – Review & Editing; Takashi Sonoki: Writing – Review & Editing, Supervision.
Declaration of competing interest
The authors declare that they have no conflicts of interest.
Acknowledgments
We thank the patient and clinical staff at Wakayama Medical University Hospital for their participation in this study.
References
- 1.Kruse-Jarres R., Kempton C.L., Baudo F., Collins P.W., Knoebl P., Leissinger C.A., et al. Acquired hemophilia A: updated review of evidence and treatment guidance. Am. J. Hematol. 2017;92:695–705. doi: 10.1002/ajh.24777. https://doi:10.1002/ajh.24777 [DOI] [PubMed] [Google Scholar]
- 2.Collins P.W., Hirsch S., Baglin T.P., Dolan G., Hanley J., Makris M., et al. Acquired hemophilia A in the United Kingdom: a 2-year national surveillance study by the United Kingdom haemophilia centre doctors' organisation. Blood. 2007;109:1870–1877. doi: 10.1182/blood-2006-06-029850. https://doi:10.1182/blood-2006-06-029850 [DOI] [PubMed] [Google Scholar]
- 3.Tabata S., Hosoi H., Murata S., Takeda S., Mushino T., Sonoki T. Severe aplastic anemia after COVID-19 mRNA vaccination: causality or coincidence? J. Autoimmun. 2022;126 doi: 10.1016/j.jaut.2021.102782. https://doi:10.1016/j.jaut.2021.102782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bostan H., Ucan B., Kizilgul M., Calapkulu M., Hepsen S., Gul U., et al. Relapsed and newly diagnosed Graves' disease due to immunization against COVID-19: a case series and review of the literature. J. Autoimmun. 2022;128 doi: 10.1016/j.jaut.2022.102809. https://doi:10.1016/j.jaut.2022.102809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X., Gao L., Tong X., Chan V.K.Y., Chui C.S.L., Lai F.T.T., et al. Autoimmune conditions following mRNA (BNT162b2) and inactivated (CoronaVac) COVID-19 vaccination: a descriptive cohort study among 1.1 million vaccinated people in Hong Kong. J. Autoimmun. 2022;130 doi: 10.1016/j.jaut.2022.102830. https://doi:10.1016/j.jaut.2022.102830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radwi M., Farsi S. A case report of acquired hemophilia following COVID-19 vaccine. J. Thromb. Haemostasis. 2021;19:1515–1518. doi: 10.1111/jth.15291. https://doi:10.1111/jth.15291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nogami K. The utility of thromboelastography in inherited and acquired bleeding disorders. Br. J. Haematol. 2016;174:503–514. doi: 10.1111/bjh.14148. https://doi:10.1111/bjh.14148 [DOI] [PubMed] [Google Scholar]
- 8.Hosoi H., Akagi Y., Mushino T., Takeyama M., Minoura N., Hiroi T., et al. Use of thromboelastography before the administration of hemostatic agents to safely taper recombinant activated factor VII in acquired hemophilia A: a report of three cases. Thromb. J. 2022;20:28. doi: 10.1186/s12959-022-00387-x. https://doi:10.1186/s12959-022-00387-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeyama M., Sasai K., Matsumoto T., Furukawa S., Ogiwara K., Yada K., et al. Comprehensive blood coagulation potential in patients with acquired hemophilia A: retrospective analyses of plasma samples obtained from nationwide centers across Japan. Int. J. Hematol. 2022;15:163–172. doi: 10.1007/s12185-021-03249-w. [DOI] [PubMed] [Google Scholar]
- 10.Tiede A., Klamroth R., Scharf R.E., Trappe R.U., Holstein K., Huth-Kuhne A., et al. Prognostic factors for remission of and survival in acquired hemophilia A (AHA): results from the GTH-AH 01/2010 study. Blood. 2015;125:1091–1097. doi: 10.1182/blood-2014-07-587089. https://doi:10.1182/blood-2014-07-587089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knoebl P., Marco P., Baudo F., Collins P., Huth-Kuhne A., Nemes L., et al. Demographic and clinical data in acquired hemophilia A: results from the European Acquired Haemophilia Registry (EACH2) J. Thromb. Haemostasis. 2012;10:622–631. doi: 10.1111/j.1538-7836.2012.04654.x. https://doi:10.1111/j.1538-7836.2012.04654.x [DOI] [PubMed] [Google Scholar]
- 12.Cittone M.G., Battegay R., Condoluci A., Terzi di Bergamo L., Fernandes E., Galfetti E., et al. The statistical risk of diagnosing coincidental acquired hemophilia A following anti-SARS-CoV-2 vaccination. J. Thromb. Haemostasis. 2021;19:2360–2362. doi: 10.1111/jth.15421. https://doi:10.1111/jth.15421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Portuguese A.J., Sunga C., Kruse-Jarres R., Gernsheimer T., Abkowitz J. Autoimmune- and complement-mediated hematologic condition recrudescence following SARS-CoV-2 vaccination. Blood Adv. 2021;5:2794–2798. doi: 10.1182/bloodadvances.2021004957. https://doi:10.1182/bloodadvances.2021004957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farley S., Ousley R., Van Wagoner N., Bril F. Autoimmunity after coronavirus disease 2019 (COVID-19) vaccine: a case of acquired hemophilia A. Thromb. Haemostasis. 2021;121:1674–1676. doi: 10.1055/a-1579-5396. https://doi:10.1055/a-1579-5396 [DOI] [PubMed] [Google Scholar]
- 15.Lemoine C., Giacobbe A.G., Bonifacino E., Karapetyan L., Seaman C. A case of acquired haemophilia A in a 70-year-old post COVID-19 vaccine. Haemophilia. 2022;28:e15–e17. doi: 10.1111/hae.14442. https://doi:10.1111/hae.14442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leone M.C., Canovi S., Pilia A., Casali A., Depietri L., Fasano T., et al. Four cases of acquired hemophilia A following immunization with mRNA BNT162b2 SARS-CoV-2 vaccine. Thromb. Res. 2022;211:60–62. doi: 10.1016/j.thromres.2022.01.017. https://doi:10.1016/j.thromres.2022.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murali A., Wong P., Gilbar P.J., Mangos H.M. Acquired Hemophilia A following Pfizer-BioNTech SARS CoV-2 mRNA vaccine, successfully treated with prednisolone and rituximab. J. Oncol. Pharm. Pract. 2022 doi: 10.1177/10781552221075545. https://doi:10.1177/10781552221075545 [DOI] [PubMed] [Google Scholar]
- 18.Soliman D.S., Al Battah A., Al Faridi D., Ibrahim F. Acquired hemophilia A developed post COVID-19 vaccine: an extremely rare complication. J. Med. Cases. 2022;13:1–4. doi: 10.14740/jmc3827. https://doi:10.14740/jmc3827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ai Vuen L., Aun Su-Yin E., Naila Kori A., Shah T.M. Case of acquired haemophilia a in Southeast Asia following COVID-19 vaccine. BMJ Case Rep. 2022;15 doi: 10.1136/bcr-2021-246922. https://doi:10.1136/bcr-2021-246922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al Hennawi H., Al Masri M.K., Bakir M., Albarazi M., Jazaeri F., Almasri T.N., et al. Acquired hemophilia A post-COVID-19 vaccination: a case report and review. Cureus. 2022;14 doi: 10.7759/cureus.21909. https://doi:10.7759/cureus.21909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu P.A., Chen C.W., Hsu Y.T., Wei K.C., Lin P.C., Chen T.Y. A case of acquired hemophilia A and bullous pemphigoid following SARS-CoV-2 mRNA vaccination. J. Formos. Med. Assoc. 2022 doi: 10.1016/j.jfma.2022.02.017. https://doi:10.1016/j.jfma.2022.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pluss M., Mitteldorf C., Szuszies C.J., Tampe B. Case report: acquired haemophilia A following mRNA-1273 booster vaccination against SARS-CoV-2 with concurrent diagnosis of pleomorphic dermal sarcoma. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.868133. https://doi:10.3389/fimmu.2022.868133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rashid A., Khan Z., Alam J. Acquired hemophilia A with SARS-CoV-2 mRNA vaccine: first case from Pakistan. Scand. J. Clin. Lab. Invest. 2022:1–3. doi: 10.1080/00365513.2022.2092902. https://doi:10.1080/00365513.2022.2092902 [DOI] [PubMed] [Google Scholar]
- 24.Pavord S., Scully M., Hunt B.J., Lester W., Bagot C., Craven B., et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N. Engl. J. Med. 2021;385:1680–1689. doi: 10.1056/NEJMoa2109908. https://doi:10.1056/NEJMoa2109908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirsiger J.R., Martinez M., Tsakiris D.A., Cittone M.G., Graf L., Oldenburg J., et al. Investigating potential mechanisms underlying FVIII inhibition in acquired hemophilia A associated with mRNA COVID-19 vaccines. J. Thromb. Haemostasis. 2022;20:1015–1018. doi: 10.1111/jth.15665. https://doi:10.1111/jth.15665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baudo F., Collins P., Huth-Kuhne A., Levesque H., Marco P., Nemes L., et al. Management of bleeding in acquired hemophilia A: results from the European Acquired Haemophilia (EACH2) Registry. Blood. 2012;120:39–46. doi: 10.1182/blood-2012-02-408930. https://doi:10.1182/blood-2012-02-408930 [DOI] [PubMed] [Google Scholar]
- 27.C P W., Chalmers E., Hart D., Jennings I., Liesner R., Rangarajan S., et al. Diagnosis and management of acquired coagulation inhibitors: a guideline from UKHCDO. Br. J. Haematol. 2013;162:758–773. doi: 10.1111/bjh.12463. https://doi:10.1111/bjh.12463 [DOI] [PubMed] [Google Scholar]
- 28.Green D., Lechner K. A survey of 215 non-hemophilic patients with inhibitors to Factor VIII. Thromb. Haemost. 1981;45:200–203. https://www.ncbi.nlm.nih.gov/pubmed/6792737 [PubMed] [Google Scholar]
- 29.Lottenberg R., Kentro T.B., Kitchens C.S. Acquired hemophilia. A natural history study of 16 patients with factor VIII inhibitors receiving little or no therapy. Arch. Intern. Med. 1987;147:1077–1081. doi: 10.1001/archinte.147.6.1077. https://doi:10.1001/archinte.147.6.1077 [DOI] [PubMed] [Google Scholar]
- 30.Tengborn L., Baudo F., Huth-Kuhne A., Knoebl P., Levesque H., Marco P., et al. Pregnancy-associated acquired haemophilia A: results from the European Acquired Haemophilia (EACH2) registry. BJOG. 2012;119:1529–1537. doi: 10.1111/j.1471-0528.2012.03469.x. https://doi:10.1111/j.1471-0528.2012.03469.x [DOI] [PubMed] [Google Scholar]
- 31.Cines D.B., Blanchette V.S. Immune thrombocytopenic purpura. N. Engl. J. Med. 2002;346:995–1008. doi: 10.1056/NEJMra010501. https://doi:10.1056/NEJMra010501 [DOI] [PubMed] [Google Scholar]
- 32.Provan D., Arnold D.M., Bussel J.B., Chong B.H., Cooper N., Gernsheimer T., et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3:3780–3817. doi: 10.1182/bloodadvances.2019000812. https://doi:10.1182/bloodadvances.2019000812 [DOI] [PMC free article] [PubMed] [Google Scholar]