To the Editor:
Aspects of volume management, including larger interdialytic weight gains, higher ultrafiltration rates, and chronic hypervolemia, are associated with adverse outcomes among individuals with kidney failure who are dependent on hemodialysis.1 Prescribing diuretics for volume control is a mainstay of advanced chronic kidney disease treatment and peritoneal dialysis care; however, diuretic use in hemodialysis practice is inconsistent.2,3 Observational studies have shown that diuretic use (vs. nonuse) is associated with lower risks of intradialytic hypotension and hospitalization among people receiving hemodialysis; however, firm conclusions are limited by potential confounding from the benefits of residual kidney function among diuretic users.4,5 A small prospective study suggests that diuretics can increase urine volume in patients producing as little as 100 mL of urine per day.6
Diuretic use in hemodialysis practice is common in many regions, and 45% of European and 48% of Japanese patients continue diuretics after hemodialysis initiation.5 In contrast, diuretic use among US patients declines after dialysis initiation.3 Uncertainty regarding the efficacy and optimal dosing of diuretics likely contributes to this variation in practice. We undertook this study to describe the use of oral diuretics among US patients receiving hemodialysis.
Using 2017 data from the US Renal Data System and a cross-sectional design, we identified adults receiving center-based maintenance hemodialysis on July 1, 2017, with continuous Medicare coverage during the preceding 180 days and excluded those with prior kidney transplants (Fig S1). We used Medicare Part D prescription drug claims to determine the diuretic use status and calculated the proportion of patients taking a diuretic, overall and by diuretic type, on July 1, 2017. Among patients taking a loop diuretic, we determined their daily furosemide-equivalent dose. In a secondary analysis, we linked the US Renal Data System cohort to the database of a large US dialysis organization and evaluated the frequency of 24-hour urine volume monitoring among diuretic users and nonusers. Item S1 reports the detailed methods.
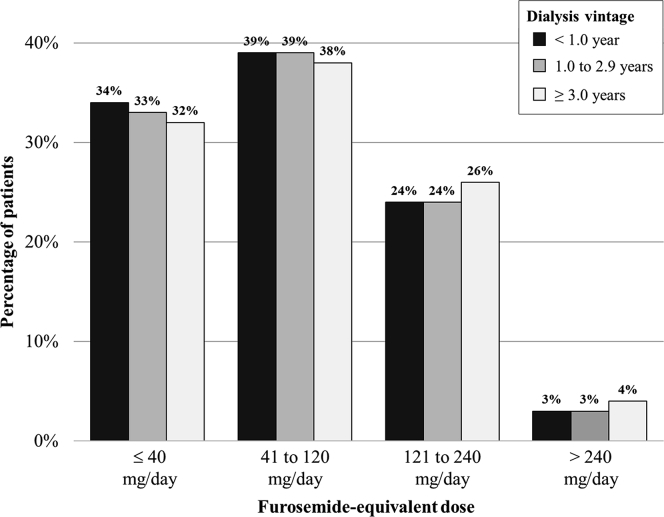
Of 176,448 patients receiving hemodialysis who met the study criteria, 22,296 (13%) were taking a diuretic (Table 1). Overall, the study cohort was representative of the prevalent adult US population receiving hemodialysis.2 Diuretic users (vs nonusers) were older, were newer to hemodialysis, were more likely to be White, and had a higher prevalence of cardiovascular conditions, including heart failure and hypertension. Among diuretic users, 90% were taking a loop diuretic, 8% were taking a thiazide or thiazide-like diuretic, 6% were taking a potassium-sparing diuretic, and <1% were taking a carbonic anhydrase inhibitor. Of the 20,097 loop diuretic users, 83% used furosemide, 9% used bumetanide, 7% used torsemide, and <1% used ethacrynic acid. Furosemide-equivalent dosing among loop diuretic users ranged from ≤20 mg/d (8%) to >320 mg/d (1%), and dosing did not differ by dialysis vintage (Fig 1). Only 28% of the loop diuretic users were taking doses of >80 mg of furosemide-equivalents per day. Moreover, the use of thiazide or thiazide-like diuretics and aldosterone antagonists without concomitant loop diuretic therapy was common (Table S1).
Table 1.
USRDS cohort characteristics overall and by diuretic use status
| Characteristic | Overall N = 176,448 | Diuretic User n = 22,296 | Diuretic Nonuser n = 154,152 | Std Diffa |
|---|---|---|---|---|
| Age, y | 64 ± 14 | 66 ± 13 | 63 ± 14 | 0.17 |
| Female | 80,412 (46%) | 10,527 (47%) | 69,885 (45%) | 0.04 |
| Race | ||||
| White | 94,129 (53%) | 14,464 (65%) | 79,665 (52%) | 0.27 |
| Black | 70,535 (40%) | 6,316 (28%) | 64,219 (42%) | 0.28 |
| Other | 11,784 (7%) | 1,516 (7%) | 10,268 (7%) | 0.01 |
| Hispanic | 29,132 (17%) | 3,611 (16%) | 25,521 (17%) | 0.01 |
| Cause of ESKD | ||||
| Diabetes | 85,305 (48%) | 12,879 (58%) | 72,426 (47%) | 0.22 |
| Hypertension | 54,492 (31%) | 6,011 (27%) | 48,481 (31%) | 0.10 |
| Glomerular disease | 14,819 (8%) | 1,318 (6%) | 13,501 (9%) | 0.11 |
| Cystic disease | 3,884 (2%) | 378 (2%) | 3,506 (2%) | 0.04 |
| Other | 17,948 (10%) | 1,710 (8%) | 16,238 (11%) | 0.10 |
| Dialysis vintage | ||||
| <1.0 y | 6,683 (4%) | 1,530 (7%) | 5,153 (3%) | 0.16 |
| 1.0-2.9 y | 52,797 (30%) | 9,880 (44%) | 42,917 (28%) | 0.35 |
| ≥3.0 y | 116,968 (66%) | 10,886 (49%) | 106,082 (69%) | 0.42 |
| Arrhythmia | 47,894 (27%) | 6,438 (29%) | 41,456 (27%) | 0.04 |
| Conduction disorder | 19,477 (11%) | 2,629 (12%) | 16,848 (11%) | 0.03 |
| Dyslipidemia | 110,427 (63%) | 15,739 (71%) | 94,688 (61%) | 0.19 |
| Heart failure | 70,904 (40%) | 10,917 (49%) | 59,987 (39%) | 0.20 |
| Hypertension | 154,865 (88%) | 20,392 (91%) | 134,473 (87%) | 0.14 |
| Ischemic heart disease | 75,581 (43%) | 10,864 (49%) | 64,717 (42%) | 0.14 |
| Peripheral arterial disease | 57,666 (33%) | 7,634 (34%) | 50,032 (32%) | 0.04 |
| Stroke | 34,309 (19%) | 4,467 (20%) | 29,842 (19%) | 0.02 |
| Valvular disease | 34,703 (20%) | 4,675 (21%) | 30,028 (19%) | 0.04 |
| Asthma or COPD | 46,932 (27%) | 6,982 (31%) | 39,950 (26%) | 0.12 |
| Liver disease | 21,637 (12%) | 2,638 (12%) | 18,999 (12%) | 0.02 |
Note: Values are displayed as count (%) for categorical variables and as mean ± standard deviation for continuous variables. Figure S2 displays percentages of patients receiving hemodialysis who were diuretic users across US regions. Table S2 displays characteristics of the USRDS cross-sectional cohort and the subset of patients treated at the large dialysis organization.
Abbreviations: COPD, chronic obstructive pulmonary disease; ESKD, end-stage kidney disease; Std Diff, standardized difference; USRDS, US Renal Data System.
Absolute standardized differences comparing diuretic users to diuretic nonusers. A standardized difference of >0.10 represents an imbalance between groups.
Figure 1.
Furosemide-equivalent dosing among loop diuretic users stratified by dialysis vintage. Because percentages were rounded to the nearest whole number, the sum of the percentages within dialysis vintage categories may not add up to 100%.
A total of 58,079 patients were in both the US Renal Data System and dialysis organization databases, including 6,659 (11%) diuretic users and 51,420 (89%) diuretic nonusers (Table S2). Overall, 3% of diuretic users and 2% of diuretic nonusers had a 24-hour urine volume measurement in the prior 180 days. The median urine volumes were 700 mL (interquartile range, 0-1,300 mL) and 200 mL (interquartile range, 0-1,000 mL) per 24 hours among diuretic users and nonusers, respectively. Among the 176 loop diuretic users with 24-hour urine volume measurements, urine volumes were similar regardless of dose; the median urine volumes were 700 mL (interquartile range, 100-1,200 mL) per 24 hours for patients taking ≤80 mg of furosemide-equivalents per day and 700 mL (interquartile range, 0-1,300 mL) per 24 hours for patients taking >80 mg of furosemide-equivalents per day.
Our analysis reveals substantial variation in diuretic use, dosing, and monitoring in the US hemodialysis practice. We found that diuretic dosing was particularly variable, with the majority of patients prescribed loop diuretics at furosemide-equivalent doses lower than what is recommended for patients with chronic kidney disease who are not dependent on dialysis.7,8 In addition, 24-hour urine collections were strikingly infrequent, and measured urine volumes did not appear to correspond to dosing. Higher loop diuretic dosing is required to overcome physiologic changes related to kidney function decline, such as tubular resistance, impaired gastrointestinal absorption, and secondary hyperaldosteronism.7 However, decreased kidney function impairs both renal and hepatic furosemide elimination pathways, prolonging the elimination half-life of furosemide.9 Using too high of a loop diuretic dose can lead to tinnitus, ototoxicity, and other side effects. In addition, loop diuretics may compete for protein binding sites, increasing the risk of drug-drug interactions.10 Such uncertainty regarding the risk-benefit balance of diuretic therapy in patients receiving hemodialysis likely, in part, underlies the observed variations in diuretic prescribing patterns.
This cross-sectional snapshot of diuretic use among US patients receiving hemodialysis reveals inconsistent and, in some cases, nonsensical prescribing patterns. Although clinicians may monitor urine output by means other than 24-hour urine collections, our findings also suggest the absence of a systematic approach to laboratory-based urine volume monitoring in hemodialysis care. Investigation of the efficacy, safety, and optimal dosing of diuretics in individuals with kidney failure who are dependent on hemodialysis is needed.
Article Information
Authors’ Contributions
Research idea and study design: JEF and MMA; data acquisition: JEF; data analysis/interpretation: JEF and MMA; statistical analysis: MMA; supervision or mentorship: JEF. Each author contributed important intellectual content during manuscript drafting or revision and agrees to be personally accountable for the individual’s own contributions and to ensure that questions pertaining to the accuracy or integrity of any portion of the work, even one in which the author was not directly involved, are appropriately investigated and resolved, including with documentation in the literature if appropriate.
Support
This work and Dr Flythe and Dr Assimon were supported by a grant (R03 DK124651) from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr Flythe and Dr Assimon are supported by a grant (R01 HL152034) from the National Heart, Lung, and Blood Institute of the National Institutes of Health.
Financial Disclosure
In the last 3 years, Dr Flythe has received speaking honoraria from the American Society of Nephrology and multiple universities and investigator-initiated research funding unrelated to this project from the Renal Research Institute, a subsidiary of Fresenius Kidney Care, North America. Dr Flythe serves on a medical advisory board for Fresenius Kidney Care, North America; and a scientific advisory board and a data and safety monitoring board for the National Institute of Diabetes and Digestive and Kidney Diseases. Dr Flythe has received consulting fees from Fresenius Kidney Care, North America; and AstraZeneca. In the last 3 years, Dr Assimon has received investigator-initiated research funding from the Renal Research Institute, a subsidiary of Fresenius Medical Care, North America; and honoraria from the American Society of Nephrology and the International Society of Nephrology.
Disclaimer
Some of the data reported here have been provided by the US Renal Data System under Data Use Agreement 2018-23d to Dr Flythe. This letter underwent privacy review by a National Institute of Diabetes and Digestive and Kidney Diseases project officer and received clearance. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the United States government. Additionally, some of the data reported here have been supplied by DaVita Clinical Research. DaVita Clinical Research had no role in the design or implementation of this study or in the decision to publish.
Peer Review
Received March 20, 2022. Evaluated by 3 external peer reviewers, with direct editorial input from the Statistical Editor and the Editor-in-Chief. Accepted in revised form May 28, 2022.
Footnotes
Figure S1: Flow diagram of study cohort creation.
Figure S2: Percentage of patients in the USRDS cross-sectional cohort using a diuretic by region of the U.S.
Item S1: Detailed methods.
Table S1: Combinations of diuretics used by patients in the USRDS cross-sectional cohort.
Table S2: Characteristics of the USRDS cross-sectional cohort and the subset of patients treated at the large dialysis organization.
Supplementary Material
Figures S1 and S2; Item S1; Tables S1 and S2.
References
- 1.Flythe J.E., Chang T.I., Gallagher M.P., et al. Blood pressure and volume management in dialysis: conclusions from a Kidney Disease: improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2020;97(5):861–876. doi: 10.1016/j.kint.2020.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United States Renal Data System . National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2021. 2021 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. [Google Scholar]
- 3.St Peter W.L., Sozio S.M., Shafi T., et al. Patterns in blood pressure medication use in US incident dialysis patients over the first 6 months. BMC Nephrol. 2013;14:249. doi: 10.1186/1471-2369-14-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sibbel S., Walker A.G., Colson C., Tentori F., Brunelli S.M., Flythe J. Association of continuation of loop diuretics at hemodialysis initiation with clinical outcomes. Clin J Am Soc Nephrol. 2019;14(1):95–102. doi: 10.2215/CJN.05080418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bragg-Gresham J.L., Fissell R.B., Mason N.A., et al. Diuretic use, residual renal function, and mortality among hemodialysis patients in the Dialysis Outcomes and Practice Pattern Study (DOPPS) Am J Kidney Dis. 2007;49(3):426–431. doi: 10.1053/j.ajkd.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Medcalf J.F., Harris K.P., Walls J. Role of diuretics in the preservation of residual renal function in patients on continuous ambulatory peritoneal dialysis. Kidney Int. 2001;59(3):1128–1133. doi: 10.1046/j.1523-1755.2001.0590031128.x. [DOI] [PubMed] [Google Scholar]
- 7.Ellison D.H. Clinical pharmacology in diuretic use. Clin J Am Soc Nephrol. 2019;14(8):1248–1257. doi: 10.2215/CJN.09630818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voelker J.R., Cartwright-Brown D., Anderson S., et al. Comparison of loop diuretics in patients with chronic renal insufficiency. Kidney Int. 1987;32(4):572–578. doi: 10.1038/ki.1987.246. [DOI] [PubMed] [Google Scholar]
- 9.Brater D.C. Clinical pharmacology of loop diuretics in health and disease. Eur Heart J. 1992;13(suppl G):10–14. doi: 10.1093/eurheartj/13.suppl_g.10. [DOI] [PubMed] [Google Scholar]
- 10.Mihaila S.M., Faria J., Stefens M.F.J., et al. Drugs commonly applied to kidney patients may compromise renal tubular uremic toxins excretion. Toxins (Basel) 2020;12(6):391. doi: 10.3390/toxins12060391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1 and S2; Item S1; Tables S1 and S2.