Abstract
Background
Both catheter left atrial appendage occlusion combined with ablation (COA) and thoracoscopic surgical left atrial appendage clipping combined with ablation (TCA) have shown favorable outcomes in management of patients with atrial fibrillation (AFib). However, studies comparing the endpoints of both techniques are still lacking. Herein, a meta-analysis of safety and efficacy outcomes of COA versus TCA was performed in patients with AFib.
Methods
Pubmed, Embase, Cochrane, and Web of Science databases were searched for retrieving potential publications. The primary outcome was the incidence of stroke during follow-up period of at least 12 months. Secondary outcomes were acute success rate of complete left atrial appendage (LAA) closure by COA or TCA, postprocedural mortality and complications, and all-cause mortality during follow-up period of at least 12 months.
Results
19 studies of COA containing 1,504 patients and 6 studies of TCA with 454 patients were eligible for analysis. No significant difference in stroke and all-cause mortality was found in patients undergoing COA versus TCA after at least a 12-month follow-up (stroke: p = 0.504; all-cause mortality: p = 0.611). COA group had a higher acute success rate compared with TCA group (p = 0.001). COA placed the patients at a higher risk of hemorrhage during the postprocedural period compared with TCA (p = 0.023). A similar risk of other postprocedural complications (stroke/transient ischemic attack and pericardial effusion) and mortality was found in the COA group in comparison with TCA group (p>0.05).
Conclusion
This meta-analysis showed that COA and TCA did not differ in stroke prevention and all-cause mortality in patients with AFib after a follow-up of at least 12 months. Postprocedural complications and mortality were almost comparable between the two groups. In the near future, high-quality randomized controlled trials exploring the optimal surgical strategies for AFib and endpoints of different procedures are warranted.
Systematic review registration
[https://www.crd.york.ac.uk/PROSPERO/], identifier [CRD42022325497].
Keywords: atrial fibrillation, left atrial appendage occlusion, left atrial appendage clipping, stroke, thoracoscopy, meta-analysis, catheter ablation
Introduction
Atrial fibrillation (AFib), the most common sustained cardiac arrhythmia in clinical practice, affects around 2∼4% adults worldwide (1, 2). The prevalence increases markedly with aging (3) and is expected to burgeon in the next few decades (4, 5). Serious complications of AFib include thromboembolism, congestive cardiac failure, and predispose a poorer survival (6). In management of patients with AFib, the major issues are related to the arrhythmia itself and the prevention of thromboembolism.
Antiarrhythmic drugs (AADs) impose a fundamental role in rate- and rhythm-control in patients with AFib. Catheter ablation (CA) or surgical ablation (SA) has been greatly developed for refractory AFib (7–9) and hybrid ablation has shown more favorable outcomes (10). Long-term anticoagulation is recommended as the gold standard of thromboembolism prevention with oral anticoagulation (OAC) therapy.
Left atrial appendage (LAA) is the source of thrombi in > 90% of patients with AFib (11), which provides the rationale for ligation, amputation, or occlusion of LAA. In recent years, various studies have reported that both catheter left atrial appendage occlusion combined with ablation (COA) and thoracoscopic surgical left atrial appendage clipping combined with ablation (TCA) are feasible and safe. Although the outcomes of COA and TCA have been well documented separately, the outcomes of both procedures have not been compared before. Therefore, a meta-analysis was performed to compare the safety and efficacy outcomes of COA versus TCA in patients with AFib after at least 12 months of follow-up.
Methods
This study’s protocol is registered on PROSPERO (CRD42022325497) and reported in accordance with the Meta-analysis of Observational Studies in Epidemiology guidelines (MOOSE) (12) (Supplementary Table 1). This study was conducted under the supervision of the Ethics Committee of Shandong Provincial Hospital.
Eligibility and exclusion criteria
Eligible studies satisfied the following criteria:
(A) studies published as full-text, peer-reviewed articles, with no language restriction;
(B) recruiting adult patients;
(C) patients undergoing catheter left atrial appendage occlusion combined with ablation (COA) or thoracoscopic surgical left atrial appendage clipping combined with ablation (TCA; including hybrid ablation) due to isolated AFib;
(D) reporting the outcomes in preventing stroke in AFib;
(E) interested outcomes including stroke and all-cause mortality during follow-up as well as complications and mortality occurring in the postprocedural period;
(F) study-level data available for statistical analysis.
Exclusion criteria were:
(a) case reports, abstracts, as well as editorial comments and review articles;
(b) studies unpublished or with insufficient data;
(c) follow-up less than 12 months;
(d) surgical procedures concomitant with other intra-cardiac interventions, such as mitral valve or coronary artery bypass surgery.
In case there was overlap in the patient populations in different studies from the same center, we included only the study with the longest follow-up or largest patient cohort.
Search strategy and data extraction
Two authors (Shijie Zhang, Yuqi Cui) searched the Pubmed, Embase, Cochrane, and Web of Science databases from 2012 to 2022 independently, for retrieval of eligible studies reported on the LAA closure in patients with AFib. For the COA group, the search terms used included: “atrial fibrillation,” “occlusion,” “closure,” “left atrial appendage,” “catheter,” “transcatheter,” “percutaneous.” For the TCA group, the search terms used were: “atrial fibrillation,” “exclusion,” “clipping,” “clip,” “occlusion,” “closure,” “left atrial appendage,” “thoracoscopic,” “minimally invasive,” “epicardial.” No search software was used. Authors were not contacted for studies that did not fulfill inclusion criteria or if data were unclear. All identified studies were screened based on their titles and abstracts by these two authors. Next, the full-text articles potentially included in this meta-analysis were re-examined to finally determine the inclusion. The reference lists were also manually checked from retrieved articles for including potential publications. Disagreements were resolved by a further discussion among all authors.
Data extraction was carried out separately by two authors (Xiaochun Ma, Shijie Zhang). Extracted data mainly included the study design and quality, demographics, baseline characteristics, and outcomes of interest. The primary outcome was defined as the incidence of stroke during a follow-up period of at least 12 months. Secondary outcomes were all-cause mortality during follow-up period of at least 12 months, acute success rate, and postprocedural mortality and complications including stroke/transient ischemic attack (TIA), hemorrhage, and pericardial effusion. The acute success of the procedure was defined as successful device implantation with no or minimal residual flow (flow ≤ 5 mm) after ablation was finished. The duration of follow-up was extracted as mean or median length. The patient-year was extracted to calculate the incidence. If reported, the patient-year was retrieved from original articles; if not, was estimated by multiplying the subject number with mean or median follow-up time. Also, disagreements were resolved by a further discussion among all authors.
Quality assessment
The quality of all included studies was assessed by two authors independently (Hongbo Tian, Jinzhang Li). In all observational studies and non-randomized clinical trials, the risk of bias was assessed with the use of Methodological Index for Non-Randomized Studies (MINORS) score (13). In articles reporting randomized controlled trials (RCTs), the risk of bias was assessed using the RoB2 (Revised Cochrane risk of bias tool for randomized trials) (14).
Statistical analysis
All statistical analyses were performed using the software Open Meta-Analyst for Windows 8 (2015). Study characteristics were presented as raw values and percentages for categorical variables, and as mean and 95% confidence interval (CI) for continuous variables. The p-values of continuous variables were computed using the metric “TX Mean” for the weighted mean difference, whereas “Untransformed Proportions” were used for calculating the p-values of categorical data. Due to the low complication rates and high success rate, primary and secondary outcomes were analyzed using the metric “Freeman–Tukey Double Arcsine Proportion” (15). Follow-up outcomes were measured by calculating the event number per 100 patient-years and the incidences of postprocedural complications were defined as event number divided by subject number. To compare the outcomes between two groups, the study type was used as a covariate factor for running all meta regressions (16). All statistical values were computed with 95% confidence intervals (CIs) in a binary random-effects model, and the p-values less than 0.05 was considered statistically significant for all estimates. For the Chi-square test of heterogeneity, a p-value less than 0.10 was considered statistically significant. Besides, I2 metric estimates the percentage of total variation between studies, and I2 more than 50% indicates significant heterogeneity.
Results
Study selection and inclusion
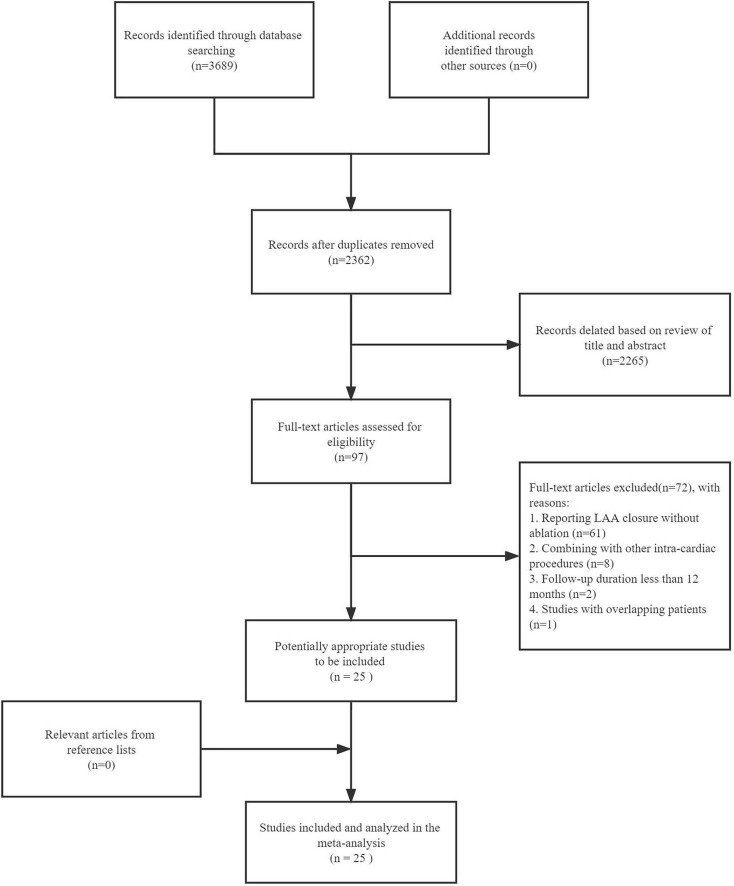
After a digital literature search, a total of 2,362 articles were retrieved from the search after duplicates were discarded. The titles and abstracts of these studies were carefully examined and 2,265 studies were categorized as irrelevant, leaving 97 studies pending re-examination. After retrieval and check of full texts, 72 studies were omitted for not satisfying the eligibility criteria (61 for reporting LAA closure without ablation; 8 for combining with other intra-cardiac procedures; 2 for follow-up duration less than 12 months; 1 study for reporting duplicate data). Two studies (17, 18) were not excluded because their follow-up time was very close to 12 months. A consensus was reached among all authors regarding the final inclusion of studies. Figure 1 presents the flow diagram of study selection and inclusion.
FIGURE 1.
Flow-chart of systematic literature search and study inclusion.
Study characteristics and quality assessment
Ultimately, 19 studies of COA containing 1,504 patients and 6 studies of TCA with 454 patients satisfied our eligibility criteria and were included in this meta-analysis. Characteristics of included studies were detailed in Table 1 (17–41). For all included studies, thrombus exclusion in the left atrium or LAA and measurements of LAA was conducted by transesophageal echocardiography (TEE) or angiography before procedures. Pulmonary vein isolation was performed which represents the cornerstone of AFib ablation and successful LAA closure was confirmed by TEE. The detailed ablation strategies of both approaches were shown in Supplementary Table 2. All included patients were administrated with a short-term OAC therapy. In the COA group, patients were prescribed an oral anticoagulant for 45 days or 3 months, followed by a single or dual antiplatelet strategy.
TABLE 1.
Study characteristics.
| Source | Design | Approach | Patients (n) | Male n (%) | Age, y (Mean ± SD) | Percentage of persAFib and (or) LSPAFib n (%) | Use of OAC before admission n (%) | CHADS2-VASc score | Follow-up (months) | Total patient-years of follow-up |
| Walker et al. (19) | Single-center, prospective | COA | 26 | 20 (76.9) | 63 ± 7 | 12 (46.2) | NA | 2.6 ± 0.8 | 12 | 26 |
| Swaans et al. (20) | Single-center, prospective | COA | 30 | 21 (70.0) | 62.8 ± 8.5 | 17 (56.7) | 28 (93) | 3 (3–5) a | 12 | 30 |
| Alipour et al. (21) | Single-center, prospective | COA | 62 | 40 (64.5) | 64 ± 8 | 23 (37.1) | 50 (80.6) | 3.0 (2.75–4.00) a | 38c | 196.3 |
| Calvo et al. (22) | Single-center, prospective | COA | 35 | 25 (71.4) | 70 ± 7 | 25 (71.4) | 24 (68.6) | 3.1 + 1.1 | 13 | 37.9 |
| Romanov et al. (23) | Single-center, RCT | COA | 45 | 28 (62.2) | 60 ± 5 | 21 (46.7) | NA | 2.2 ± 0.6 | 24 | 78 |
| Panikker et al. (24) | Multicenter, prospective | COA | 20 | 13 (65.0) | 68 ± 7 | 20 (100.0) | NA | 3.1 ± 1.2 | 12 | 20 |
| Phillips et al. (25) | Single-center, prospective | COA | 98 | 67 (68.4) | 65 ± 7 | 42 (42.9) | NA | 2.6 ± 1.0 | 26.7 | 218.3 |
| Pelissero et al. (26) | Single-center, prospective | COA | 21 | 14 (66.7) | 66.9 ± 10.4 | 17 (81.0) | 21 (100.0) | 2.8 ± 1.22 | 14.93 | 26.1 |
| Wintgens et al. (27) | Multicenter, prospective | COA | 349 | 202 (57.9) | 63.1 ± 8.2 | 152 (43.6) | NA | 3.0 (2.0–4.0) a | 34.5c | 1,003.4 |
| Fassini et al. (28) | Single-center, prospective | COA | 49 | 32 (65.3) | 69 ± 8 | 24 (49.0) | 16 (22.9) | 2.8 ± 1.2 | 24 | 98 |
| Liu et al. (29) | Multicenter, prospective | COA | 50 | 33 (66.0) | 64.9 ± 7.7 | 23 (46.0) | 30 (60.0) | 3.7 ± 1.4 | 20.2 | 84.2 |
| Du et al. (17) | Multicenter, retrospective | COA | 122 | 73 (59.8) | 66.4 ± 8.8 | 61 (50.0) | 122 (100.0) | 4.3 ± 1.4 | 11.5 | 116.9 |
| Chen et al. (30) | ingle-center, prospective | COA | 178 | 94 (52.8) | 68.9 ± 8.1 | 90 (50.6) | 78 (43.8) | 3.3 ± 1.5 | 12 | 72 |
| Kita et al. (31) | Single-center, retrospective | COA | 42 | 28 (66.7) | 71.1 ± 8.5 | NA | NA | 3.3 ± 1.1 | 18.6 | 65.1 |
| Liu et al. (32) | Single-center, prospective | COA | 27 | 20 (74.1) | 64.7 ± 6.3 | 10 (37.0) | NA | 4.8 ± 1.4 | 18 | 40.5 |
| Mo et al. (33) | Single-center, retrospective | COA | 76 | 39 (51.3) | 69.9 ± 7.9 | 39 (51.3) | NA | 3.6 ± 1.3 | 24 | 152 |
| Phillips et al. (34) | Multicenter, prospective | COA | 142 | 77 (54.2) | 64.2 ± 7.2 | 43 (30.3) | NA | 3.4 ± 1.4 | 24.2 | 286.4 |
| Ren et al. (35) | Single-center, retrospective | COA | 76 | 47 (61.8) | 67.0 ± 7.5 | 25 (32.9) | 37 (48.7) | 3.4 ± 1.9 | 23.7 | 150.1 |
| Chen et al. (36) | Single-center, prospective | COA | 56 | 32 (57.1) | 69.4 ± 7.5 | 0 (0) | NA | 4.0 (3.0–5.0) a | 12 | 56 |
| Mokracek et al. (18) | Single-center, retrospective | TCA | 30 | 20 (66.7) | NA | 28 (93.3) | NA | 1.7 (0–4) b | 11.09 | 20.9 |
| Ellis et al. (37) | Single-center, retrospective | TCA | 65 | 50 (76.9) | 64.5 ± 8.8 | 53 (81.5) | 57 (86.7) | 2.48 ± 1.54 | 34.3 | 183 |
| van Laar et al. (38) | Multicenter, prospective | TCA | 222 | 152 (68.5) | 66 ± 9 | 179 (80.6) | NA | 2.3 ± 1.0 | 20c | 369 |
| Osmancik et al. (39) | Single-center, prospective | TCA | 40 | 23 (57.5) | 62.6 ± 8.6 | 40 (100.0) | NA | 2.2 ± 1.47 | 12.1 | 40.3 |
| Salzberg et al. (40) | Single-center, prospective | TCA | 42 | 14 (33.3) | 65 ± 7 | 27 (64.3) | 38 (90.5) | 2 (0–4) b | 20 | 70 |
| Haldar et al. (41) | Multicenter, RCT | TCA | 55 | 44 (80.0) | 63.8 ± 8.9 | 55 (100.0) | NA | NA | 12 | 55 |
persAFib, persistent AFib; LSPAFib, longstanding persistent AFib; OAC, oral anticoagulation; NA, not applicable.
aMedian (with interquartile range) was reported. bMedian (with minimum and maximum values) was reported. cMedian was reported.
Differences in baseline characteristics between COA and TCA studies were presented in Table 2. Age (66.2 versus 64.7 years, p = 0.209), male gender distribution (61.7 versus 64.6%, p = 0.486), left ventricular ejection fraction (LVEF; 61.3 versus 58.5%, p = 0.209) and comorbidities including hypertension (75.5 versus 67.6%, p = 0.162) and diabetes mellitus (19.8 versus 20.4%, p = 0.729) were comparable between two groups. The left atrial dimension was significantly larger in TCA group compared to COA group (48.3 versus 43.4 mm, p = 0.009). The percentage of persistent AFib and (or) longstanding persistent AFib was significantly lower in COA studies (48.3 versus 87.9%, p < 0.001). Furthermore, the CHADS2-VASc score (3.3 versus 2.3, p = 0.012) and the percentage of prior stroke/TIA (52.5 versus 57.9%, p = 0.005) was higher in patients undergoing COA. The supplementary baseline characteristics were summarized in Supplementary Table 3.
TABLE 2.
Differences in characteristics between COA and TCA studies at baseline.
| Characteristics | COA | TCA | p-value |
| Age (years) | 66.2 (64.8–67.6) | 64.7 (63.5–65.9) | 0.209 |
| Male (%) | 61.7 (58.5–64.8) | 64.6 (52.9–76.2) | 0.486 |
| PersAFib and (or) LSPAFib (%) | 48.3 (32.2–64.3) | 87.9 (70.0–95.8) | <0.001 |
| Hypertension (%) | 75.5 (71.0–80.1) | 67.6 (55.6–79.6) | 0.162 |
| Diabetes mellitus (%) | 19.8 (17.0–22.5) | 20.4 (10.5–30.3) | 0.729 |
| CHADS2-VASc score | 3.3 (2.9–3.6) | 2.3 (2.2–2.4) | 0.012 |
| LVEF (%) | 61.3 (59.7–62.9) | 58.5 (52.7–64.3) | 0.209 |
| Left atrial dimension (mm) | 43.4 (41.7–45.1) | 48.3 (43.9–52.8) | 0.009 |
| Prior stroke/TIA (%) | 39.8 (29.4–50.1) | 10.1 (7.2–12.9) | 0.005 |
Data are presented as means or proportions followed by the 95% confidence interval.
P-value of the meta-regression was computed using the metric “Untransformed Proportion” in a binary random-effects model using study type as covariate factor.
PersAFib, persistent atrial fibrillation; LSPAFib, longstanding persistent atrial fibrillation; LVEF, left ventricular ejection fraction; TIA, transient ischemic attack.
The methodological quality of 23 non-randomized controlled studies was assessed by the MINORS tool. Twenty studies with non-comparative design were evaluated using 8 items and 3 comparative studies were assessed with an additional 4 criteria. Based on grading standards, quality was assessed as moderate for 21 studies and 2 studies had a high risk of bias (Supplementary Table 4). Notably, no study had a prospective calculation of study size and an unbiased assessment of endpoints, since most studies were single-arm studies. The remaining 2 study was RCTs and the overall risk of bias evaluated using the RoB 2 tool was “some concern” and “low risk,” respectively (Supplementary Table 5).
Primary outcome
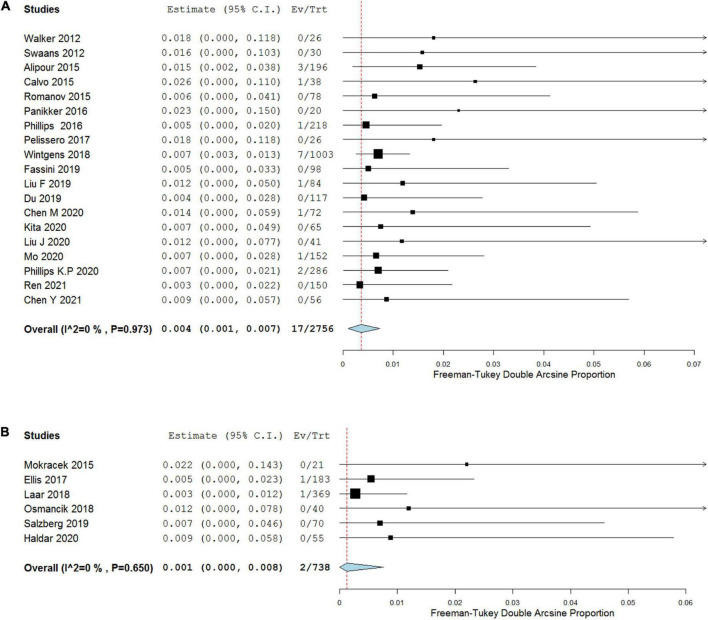
Stroke during the follow-up period of at least 12 months was reported in all included articles. The incidences reported among most included studies were 0, and the highest was 2.6 per 100 patient-years. The pooled incidences of stroke in COA and TCA groups were 0.4 (95% CI, 0.1–0.7) and 0.1 (95% CI, 0–0.8) per 100 patient-years, respectively, and no considerable heterogeneity was found (I2 = 0%). Meta-regression analysis showed that stroke was comparable between COA and TCA groups (p = 0.504; Figure 2 and Table 3).
FIGURE 2.
Forest plots depicting primary outcome of COA (A) versus TCA (B).
TABLE 3.
Differences in outcomes and complications between COA and TCA studies.
| COA | TCA | p-value | |||
|
|
|
||||
| Number of events (n) | 95% CI | Number of events(n) | 95% CI | ||
| Primary outcome | |||||
| Stroke during follow-up | 17 | 0.4(0.1–0.7) per 100 patient-years | 2 | 0.1 (0–0.8) per 100 patient-years | 0.504 |
| Secondary outcome | |||||
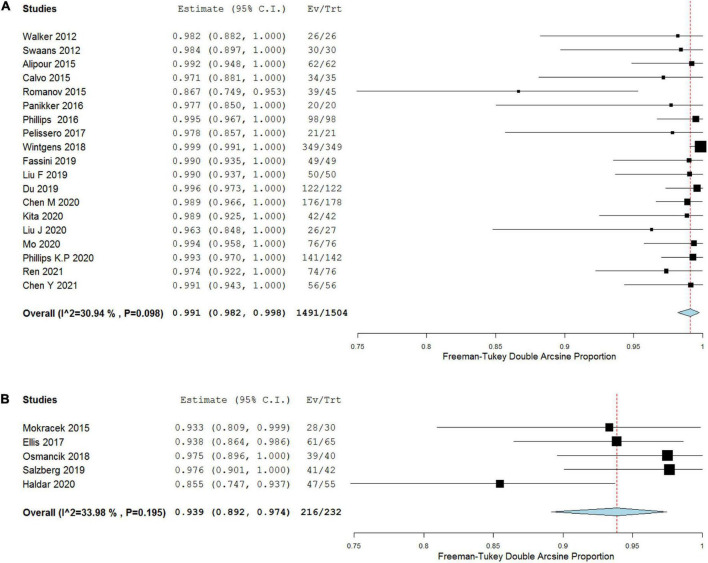
| Acute success rate | 1,491 | 99.1% (98.2–99.8) | 216 | 93.9% (89.2–97.4) | 0.001 |
| Postoperative stroke/TIA | 2 | 0.3% (0–0.8) | 0 | 0.3% (0–1.3) | 0.948 |
| Postoperative hemorrhage | 51 | 3.0% (2.1–4.0) | 7 | 1.6% (0.2–4.0) | 0.023 |
| Postoperative pericardial effusion | 26 | 1.4% (0.7–2.2) | 0 | 0.3% (0.0–1.3) | 0.063 |
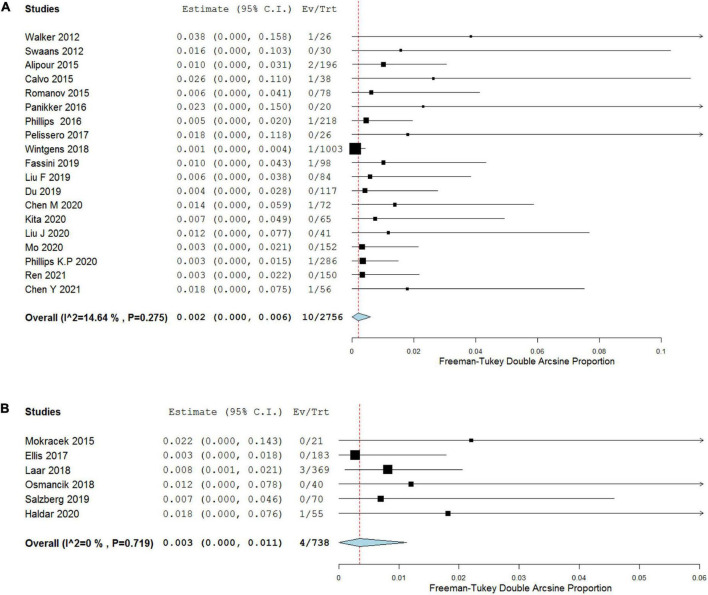
| All-cause mortality during follow-up | 10 | 0.2 (0–0.6) per 100 patient-years | 4 | 0.3 (0–1.1) per 100 patient-years | 0.611 |
Data were presented as total number of patients of COA or TCA group per event, followed by means and 95% CIs in a binary random-effects model. Statistical analysis for primary outcome and secondary outcomes: one-arm meta-regression “Freeman-Tukey Double Arcsine Proportion” using the study type as a covariate to compare the outcomes between two groups. TIA, transient ischemic attack.
Secondary outcome
Acute success rate
There were 19 COA studies and 5 TCA studies that examined the acute success rate and were therefore suitable for meta-analysis. The success rates of both groups were 99.1% (95% CI, 98.2–99.8%, I2 = 30.94%, p = 0.098) in COA group and 93.9% (95% CI, 89.2–97.4%, I2 = 33.98%, p = 0.195) in TCA group. The pooled results showed COA group had a higher acute success rate compared with TCA group (p = 0.001; Figure 3 and Table 3). Two studies (23, 41) showed relatively poor results (86.7 and 85.5%, respectively). The efficiency of complete LAA closure during long-term follow-up could not be calculated because of incomplete reported data in most included studies.
FIGURE 3.
Forest plots depicting acute success rate of COA (A) versus TCA (B).
All-cause mortality during follow-up period
All-cause mortality during the follow-up period of at least 12 months was 0.2 per 100 patient-years (95% CI, 0.0–0.6) in COA group as well as 0.3 per 100 patient-years (95% CI, 0.0–1.1) in TCA group and two groups were not statistically different regarding all-cause mortality (p = 0.611; Figure 4 and Table 3).
FIGURE 4.
Forest plots showing pooled analysis of all-cause mortality of COA (A) versus TCA (B) during follow-up period.
Postprocedural complications
A total of 25 studies reported the occurrence of postprocedural stroke/TIA, hemorrhage, and pericardial effusion events. The meta-analysis demonstrated a similar risk of postprocedural stroke/TIA between COA and TCA groups (0.3 versus 0.3%, p = 0.948; Supplementary Figure 1 and Table 3). Postprocedural hemorrhage events including all major or minor hemorrhage complications after the procedure were reported with incidences ranging from 0.0 to 10.0%. The pooled results of COA and TCA groups were 3.0% (95% CI, 2.1–4.0%) and 1.6% (95% CI, 0.2–4.0%) respectively, and meta-regression analysis showed a higher risk of hemorrhage of COA group during postprocedural period compared with TCA group (p = 0.023; Supplementary Figure 2 and Table 3). By pooling the results of postprocedural pericardial effusion, the incidences of COA and TCA groups were 1.4% (95% CI, 0.7–2.2%) and 0.3% (95% CI, 0–1.3%) respectively, and did not differ statistically between two groups (p = 0.063; Supplementary Figure 3 and Table 3). No patients died during the postprocedural period.
Discussion
Our meta-analysis was the first to compare the safety and efficiency outcomes of COA versus TCA in patients with AFib. No significant difference in stroke prevention was found in patients undergoing COA versus TCA after at least 12-month follow-up. COA and TCA were similar in terms of all-cause mortality during the follow-up period. The COA group had a higher acute success rate than TCA group. COA placed the patients at a higher risk of hemorrhage during the perioperative period. However, postprocedural complications (stroke/TIA and pericardial effusion) and mortality were comparable between the two groups.
Catheter ablation has been shown more efficacious in rhythm controlling of AFib than AADs, with added benefit of reducing medical burden and improving quality of life (42, 43). Recent evidence demonstrated that surgical ablation during intra-cardiac surgery is more effective in rhythm control than catheter ablation. However, surgical ablation is more invasive with higher postoperative complication rates and a longer duration of hospitalization (44, 45). Hybrid ablation, combining thoracoscopic epicardial and transvenous endocardial ablation procedure, has illustrated more favorable outcomes. In a recent meta-analysis of single-arm studies including 3,716 patients (538 undergoing hybrid ablation and 3,178 undergoing catheter ablation), hybrid ablation was more effective than catheter ablation in maintaining sinus rhythm (SR) in patients with persistent or longstanding persistent AFib (10).
Clinical maintenance of SR does not reduce the frequency of thrombotic events. The AFFIRM (46) and RACE (47) trials have demonstrated that the incidence of thrombotic events was not altered in patients undergoing rate versus rhythm control by AADs. Even if SR is maintained with AADs, long-term anticoagulation is required. For isolated LAA closure, a meta-analysis (48) including 3 randomized open-label controlled trials (PROTECT AF, PREVAIL, and PRAGUE-17) showed that LAA closure was non-inferior or superior to vitamin K antagonist for all stroke or systemic embolism. Thus, LAA closure with AF ablation aiming at concomitant stroke prevention as well as rhythm control might be a more comprehensive treatment. In recent years, various studies have reported that both COA and TCA are feasible and safe. A recent cohort study (33) using propensity score matching was performed to compare the procedural and long-term outcomes of COA with isolated CA or isolated left atrial appendage closure (LAAO). This study included patients who underwent COA (combined group), CA alone (catheter ablation-only group), or LAAO alone (LAAO-only group). The AFib-free rate of COA group was comparable with that of CA-only group. Compared with LAAO-only group, COA group achieved similar complete occlusion rates immediately after the procedure and 45 days after the procedure. And incidences of stroke and bleeding events of COA group were lower compared with those of CA-only group and LAAO-only group. Stroke events reported in most studies included in our work were clinically rare, further indicating the combined procedure is safe and efficacious. In addition, surgical LAA clipping might provide an “electrical isolation” effect, thereby potentially reducing the stroke risk (11). However, relevant evidence is currently scarce regarding the comparison between catheter LAA closure and surgical LAA clipping. And future investigation comparing the effectiveness and safety of ablation combined with LAA closure with isolated LAA closure or isolated ablation is necessary.
There was no evidence for device thrombus in any patients who had stroke events in COA group. Most stroke events were thought related to incomplete LAA closure. In addition, almost all stroke events occurred after OAC was discontinued. Except for LAA, apical thrombus after myocardial infarction was found in two patients. Alipour et al. (21). reported that 3 patients had plaque formation in carotid arteries, and 1 patient had > 50% stenosis in bilateral carotid arteries. In TCA group, one stroke event occurred when one patient was receiving OAC and in sinus rhythm (38). Another one occurred with confirmed complete LAA closure, without OAC use and cardiac thrombus (37). All-cause mortality during the follow-up period of two groups was also extremely low and only a small fraction of mortality events were related to the cardiac cause or OAC use.
COA had a higher acute success rate than TCA. In COA group, the main rationale for procedural failure was that the occlusion device did not match the size of the LAA. Whereas in TCA group, the procedural failure of LAA closure was mainly attributed to lung or cardiac adhesions or unique anatomical features. However, as a consequence of incomplete reporting of data, the long-term efficacy of the LAA closure could not be measured.
Our meta-analysis reported most postoperative complications available for comparative analysis. In COA group, patients had a higher risk of hemorrhage during the perioperative period in comparison to TCA group. Postoperative hemorrhage events were mainly minor in COA group, such as groin hematoma. The majority of hemorrhage events occurring in TCA group were considered due to bleeding from the thoracic wall incision, which required transfusion or surgical intervention. Although no mortality happened during the postprocedural period, 25 pericardial effusion events including cardiac tamponade developed in COA group. Whereas in TCA group, no pericardial effusion event was reported. One patient had a tear in the right upper pulmonary vein during encircling with the dissector in TCA group. Finally, as previously mentioned, postoperative stroke/TIA rarely occurred in both groups.
Several limitations in this meta-analysis should be paid attention to interpret the results. Firstly, only 2 RCTs have been found by our digital search and included in this meta-analysis. Secondly, this meta-analysis contained a relatively small sample size. Thirdly, observational studies inevitably introduced a source of bias due to their non-randomized, unblinded design. Fourthly, differences between the included studies in the percentages of long-standing persistent AFib, antithrombotic agents, and other variables have been several possible confounders. Fifthly, the arrhythmia-free survival could not be meta-analyzed due to the incompetent data at the end of follow-up or at a specific time point. Finally, evidence is missing regarding the mid-term and long-term efficacy of COA versus TCA.
In summary, this meta-analysis demonstrated that the safety and efficacy endpoints of COA and TCA in patients with AFib are similar. However, data directly comparing both techniques are lacking. Large randomized clinical trials investigating the optimal strategy for AFib and efficacy and safety outcomes of different procedures are warranted in the near future.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
CZ and XM: concept and design. SZ, YC, JL, YY, HT, HF, and XM: acquisition, analysis, or interpretation of data. SZ, YC, HZ, and XM: drafting of the manuscript. All authors: critical revision of the manuscript for important intellectual content.
Acknowledgments
The authors thank Mansen Wang (mansenwang@yahoo.com) for providing the assistance for statistical analysis.
Abbreviations
- AFib
atrial fibrillation
- AADs
antiarrhythmic drugs
- CA
catheter ablation
- SA
surgical ablation
- OAC
oral anticoagulation
- LAA
left atrial appendage
- COA
catheter left atrial appendage occlusion combined with ablation
- TCA
thoracoscopic surgical left atrial appendage clipping combined with ablation
- MOOSE
Meta-analysis of Observational Studies in Epidemiology guidelines
- TIA
transient ischemic attack
- MINORS
Methodological Index for Non-Randomized Studies
- RCTs
randomized controlled trials
- RoB 2
Revised Cochrane risk of bias tool for randomized trials
- TEE
transesophageal echocardiography
- SR
sinus rhythm
- LAAO
left atrial appendage closure.
Funding
This study was supported by the grants from the National Natural Science Foundation of China (81800255 to XM), the Nature Science Foundation of Shandong Province (ZR2020MH044 to CZ), Natural Science Foundation of Shandong Province (ZR2018BH002 to XM), Shandong Provincial Medicine and Health Science and Technology Development Plan Project (202003011417 to HT), 2021 Shandong Medical Association Clinical Research Fund – Qilu Special Project (YXH2022ZX02139 to HT), National Nature Science Foundation of China (81600222 to YC), Young experts of Taishan Scholar Program of Shandong Province (tsqn201812142 to YC), Academic Promotion Programme of Shandong First Medical University (2019RC017 to YC), and Jinan Science and Technology Plan Project (202019165 to HZ and XM).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.970847/full#supplementary-material
References
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: a report from the American heart association. Circulation. (2019) 139:e56–528. [DOI] [PubMed] [Google Scholar]
- 2.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J. (2021) 42:373–498. 10.1093/eurheartj/ehab648 [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the anticoagulation and risk factors in atrial fibrillation (ATRIA) Study. JAMA. (2001) 285:2370–5. 10.1001/jama.285.18.2370 [DOI] [PubMed] [Google Scholar]
- 4.Lane DA, Skjøth F, Lip GYH, Larsen TB, Kotecha D. Temporal trends in incidence, prevalence, and mortality of atrial fibrillation in primary care. J Am Heart Assoc. (2017) 6:e005155. 10.1161/JAHA.116.005155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the framingham heart study: a cohort study. Lancet. (2015) 386:154–62. 10.1016/S0140-6736(14)61774-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McManus DD, Rienstra M, Benjamin EJ. An update on the prognosis of patients with atrial fibrillation. Circulation. (2012) 126:e143–6. 10.1161/CIRCULATIONAHA.112.129759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haegeli LM, Calkins H. Catheter ablation of atrial fibrillation: an update. Eur Heart J. (2014) 35:2454–9. 10.1093/eurheartj/ehu291 [DOI] [PubMed] [Google Scholar]
- 8.Barnett SD, Ad N. Surgical ablation as treatment for the elimination of atrial fibrillation: a meta-analysis. J Thorac Cardiovasc Surg. (2006) 131:1029–35. 10.1016/j.jtcvs.2005.10.020 [DOI] [PubMed] [Google Scholar]
- 9.Osmancik P, Budera P, Talavera D, Hlavicka J, Herman D, Holy J, et al. Five-year outcomes in cardiac surgery patients with atrial fibrillation undergoing concomitant surgical ablation versus no ablation. The long-term follow-up of the PRAGUE-12 Study. Heart Rhythm. (2019) 16:1334–40. 10.1016/j.hrthm.2019.05.001 [DOI] [PubMed] [Google Scholar]
- 10.van der Heijden CAJ, Vroomen M, Luermans JG, Vos R, Crijns H, Gelsomino S, et al. Hybrid versus catheter ablation in patients with persistent and longstanding persistent atrial fibrillation: a systematic review and meta-analysis†. Eur J Cardiothorac Surg. (2019) 56:433–43. 10.1093/ejcts/ezy475 [DOI] [PubMed] [Google Scholar]
- 11.Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. (1996) 61:755–9. 10.1016/0003-4975(95)00887-X [DOI] [PubMed] [Google Scholar]
- 12.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational Studies in Epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 13.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. (2016) 355:i4919. 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 15.Schwarzer G, Chemaitelly H, Abu-Raddad LJ, Rücker G. Seriously misleading results using inverse of freeman-tukey double arcsine transformation in meta-analysis of single proportions. Res Synth Methods. (2019) 10:476–83. 10.1002/jrsm.1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallace BC, Schmid CH, Lau J, Trikalinos TA. Meta-Analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med Res Methodol. (2009) 9:80. 10.1186/1471-2288-9-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du X, Chu H, Ye P, He B, Xu H, Jiang S, et al. Combination of left atrial appendage closure and catheter ablation in a single procedure for patients with atrial fibrillation: Multicenter experience. J Formos Med Assoc. (2019) 118:891–7. 10.1016/j.jfma.2018.10.006 [DOI] [PubMed] [Google Scholar]
- 18.Mokracek A, Kurfirst V, Bulava A, Hanis J, Tesarik R, Pesl L. Thoracoscopic occlusion of the left atrial appendage. Innovations. (2015) 10:179–82. 10.1177/155698451501000306 [DOI] [PubMed] [Google Scholar]
- 19.Walker DT, Humphries JA, Phillips KP. Combined catheter ablation for atrial fibrillation and watchman(®) left atrial appendage occlusion procedures: a single centre experience. J Atr Fibrillation. (2012) 5:687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swaans MJ, Post MC, Rensing BJ, Boersma LV. Ablation for atrial fibrillation in combination with left atrial appendage closure: first results of a feasibility study. J Am Heart Assoc. (2012) 1:e002212. 10.1161/JAHA.112.002212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alipour A, Swaans MJ, van Dijk VF, Balt JC, Post MC, Bosschaert MAR, et al. Ablation for atrial fibrillation combined with left atrial appendage closure. JACC Clin Electrophysiol. (2015) 1:486–95. 10.1016/j.jacep.2015.07.009 [DOI] [PubMed] [Google Scholar]
- 22.Calvo N, Salterain N, Arguedas H, Macias A, Esteban A, García de Yébenes M, et al. Combined catheter ablation and left atrial appendage closure as a hybrid procedure for the treatment of atrial fibrillation. Europace. (2015) 17:1533–40. 10.1093/europace/euv070 [DOI] [PubMed] [Google Scholar]
- 23.Romanov A, Pokushalov E, Artemenko S, Yakubov A, Stenin I, Kretov E, et al. Does left atrial appendage closure improve the success of pulmonary vein isolation? Results of a randomized clinical trial. J Interv Card Electrophysiol. (2015) 44:9–16. 10.1007/s10840-015-0030-4 [DOI] [PubMed] [Google Scholar]
- 24.Panikker S, Jarman JW, Virmani R, Kutys R, Haldar S, Lim E, et al. Left atrial appendage electrical isolation and concomitant device occlusion to treat persistent atrial fibrillation: a first-in-human safety, feasibility, and efficacy study. Circ Arrhythm Electrophysiol. (2016) 9:e003710. 10.1161/CIRCEP.115.003710 [DOI] [PubMed] [Google Scholar]
- 25.Phillips KP, Walker DT, Humphries JA. Combined catheter ablation for atrial fibrillation and watchman® left atrial appendage occlusion procedures: five-year experience. J Arrhythm. (2016) 32:119–26. 10.1016/j.joa.2015.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pelissero E, Giuggia M, Todaro MC, Trapani G, Giordano B, Senatore G. [Combined left atrial appendage percutaneous closure and atrial fibrillation ablation: a single center experience]. G Ital Cardiol. (2017) 18(12 Suppl 1.):11s–7s. [DOI] [PubMed] [Google Scholar]
- 27.Wintgens L, Romanov A, Phillips K, Ballesteros G, Swaans M, Folkeringa R, et al. Combined atrial fibrillation ablation and left atrial appendage closure: long-term follow-up from a large multicentre registry. Europace. (2018) 20:1783–9. 10.1093/europace/euy025 [DOI] [PubMed] [Google Scholar]
- 28.Fassini G, Gasperetti A, Italiano G, Riva S, Moltrasio M, Dello Russo A, et al. Cryoballoon pulmonary vein ablation and left atrial appendage closure combined procedure: a long-term follow-up analysis. Heart Rhythm. (2019) 16:1320–6. 10.1016/j.hrthm.2019.03.022 [DOI] [PubMed] [Google Scholar]
- 29.Liu FZ, Lin WD, Liao HT, Peng J, Xue YM, Zhan XZ, et al. Mid-term outcomes of concomitant left atrial appendage closure and catheter ablation for non-valvular atrial fibrillation: a multicenter registry. Heart Vessels. (2019) 34:860–7. 10.1007/s00380-018-1312-4 [DOI] [PubMed] [Google Scholar]
- 30.Chen M, Wang ZQ, Wang QS, Sun J, Zhang PP, Feng XF, et al. One-stop strategy for treatment of atrial fibrillation: feasibility and safety of combining catheter ablation and left atrial appendage closure in a single procedure. Chin Med J. (2020) 133:1422–8. 10.1097/CM9.0000000000000855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kita K, Carlson S, Huntsinger M, Tun H, Sohn J, Doshi RN. Safety and feasibility of combined atrial fibrillation ablation and left atrial appendage occlusion after left atrial appendage electrical isolation. J Interv Card Electrophysiol. (2020) 57:43–55. 10.1007/s10840-019-00603-1 [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Xia Y, Zhang H, Li X, Zhang S, Fang P. Left atrial appendage closure after cryoballoon ablation in patients with atrial fibrillation. Herz. (2021) 46(Suppl 1.):82–8. [DOI] [PubMed] [Google Scholar]
- 33.Mo BF, Sun J, Zhang PP, Li W, Chen M, Yuan JL, et al. Combined therapy of catheter ablation and left atrial appendage closure for patients with atrial fibrillation: a case-control study. J Interv Cardiol. (2020) 2020:8615410. 10.1155/2020/8615410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips KP, Romanov A, Artemenko S, Folkeringa RJ, Szili-Torok T, Senatore G, et al. Combining left atrial appendage closure and catheter ablation for atrial fibrillation: 2-year outcomes from a multinational registry. Europace. (2020) 22:225–31. 10.1093/europace/euz286 [DOI] [PubMed] [Google Scholar]
- 35.Ren Z, Zhang J, Wang S, Jia P, Li X, Zhang J, et al. Two-year outcome from combining cryoballoon ablation and left atrial appendage closure: CLACBAC study. Front Cardiovasc Med. (2020) 7:610537. 10.3389/fcvm.2020.610537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen YH, Wang LG, Zhou XD, Fang Y, Su L, Wu SJ, et al. Outcome and safety of intracardiac echocardiography guided left atrial appendage closure within zero-fluoroscopy atrial fibrillation ablation procedures. J Cardiovasc Electrophysiol. (2022) 33:667–76. 10.1111/jce.15370 [DOI] [PubMed] [Google Scholar]
- 37.Ellis CR, Aznaurov SG, Patel NJ, Williams JR, Sandler KL, Hoff SJ, et al. Angiographic efficacy of the atriclip left atrial appendage exclusion device placed by minimally invasive thoracoscopic approach. JACC Clin Electrophysiol. (2017) 3:1356–65. 10.1016/j.jacep.2017.03.008 [DOI] [PubMed] [Google Scholar]
- 38.van Laar C, Verberkmoes NJ, van Es HW, Lewalter T, Dunnington G, Stark S, et al. Thoracoscopic left atrial appendage clipping: a multicenter cohort analysis. JACC Clin Electrophysiol. (2018) 4:893–901. 10.1016/j.jacep.2018.03.009 [DOI] [PubMed] [Google Scholar]
- 39.Osmancik P, Budera P, Zdarska J, Herman D, Petr R, Fojt R, et al. Residual echocardiographic and computed tomography findings after thoracoscopic occlusion of the left atrial appendage using the Atriclip Pro device. Interact Cardiovasc Thorac Surg. (2018) 26:919–25. 10.1093/icvts/ivx427 [DOI] [PubMed] [Google Scholar]
- 40.Salzberg SP, van Boven WJ, Wyss C, Hürlimann D, Reho I, Zerm T, et al. “AF HeartTeam” guided indication for stand-alone thoracoscopic left atrial ablation and left atrial appendage closure. J Atr Fibrillation. (2019) 11:2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haldar S, Khan HR, Boyalla V, Kralj-Hans I, Jones S, Lord J, et al. Catheter ablation vs. thoracoscopic surgical ablation in long-standing persistent atrial fibrillation: CASA-AF randomized controlled trial. Eur Heart J. (2020) 41:4471–80. 10.1093/eurheartj/ehaa658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blomström-Lundqvist C, Gizurarson S, Schwieler J, Jensen SM, Bergfeldt L, Kennebäck G, et al. Effect of catheter ablation vs antiarrhythmic medication on quality of life in patients with atrial fibrillation: the captaf randomized clinical trial. JAMA. (2019) 321:1059–68. 10.1001/jama.2019.0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mark DB, Anstrom KJ, Sheng S, Piccini JP, Baloch KN, Monahan KH, et al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the cabana randomized clinical trial. JAMA. (2019) 321:1275–85. 10.1001/jama.2019.0692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gillinov AM, Gelijns AC, Parides MK, DeRose JJ, Jr, Moskowitz AJ, Voisine P, et al. Surgical ablation of atrial fibrillation during mitral-valve surgery. N Engl J Med. (2015) 372:1399–409. 10.1056/NEJMoa1500528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gammie JS, Haddad M, Milford-Beland S, Welke KF, Ferguson TB, Jr, O’Brien SM, et al. Atrial fibrillation correction surgery: lessons from the society of thoracic surgeons national cardiac database. Ann Thorac Surg. (2008) 85:909–14. 10.1016/j.athoracsur.2007.10.097 [DOI] [PubMed] [Google Scholar]
- 46.Van Gelder IC, Hagens VE, Bosker HA, Kingma JH, Kamp O, Kingma T, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. (2002) 347:1834–40. 10.1056/NEJMoa021375 [DOI] [PubMed] [Google Scholar]
- 47.Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. (2002) 347:1825–33. 10.1056/NEJMoa021328 [DOI] [PubMed] [Google Scholar]
- 48.Turagam MK, Osmancik P, Neuzil P, Dukkipati SR, Reddy VY. Left atrial appendage closure versus oral anticoagulants in atrial fibrillation: a meta-analysis of randomized trials. J Am Coll Cardiol. (2020) 76:2795–7. 10.1016/j.jacc.2020.08.089 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.