Key Teaching Points.
-
•
We present a case of redo pulmonary vein isolation complicated by perforation of the left atrium with a multipolar mapping catheter during electroanatomical mapping at the inferior base of the left atrial appendage (LAA). The cause of the acute cardiac tamponade as demonstrated during surgical repair was a tear in the LAA that might have been facilitated by a local lesion from previous ablation.
-
•
The advent of multipolar mapping catheters has greatly increased the speed of obtaining 3-dimensional maps. Their flexible splines are generally not prone to exert extensive pressure on the myocardium. However, advancing the catheter out of a long sheath in close proximity to the atrial wall may cause the catheter to act “like a spear”; therefore, it is safer to advance the catheter to the tip of the sheath and then withdraw the sheath to expose the catheter.
-
•
Electroanatomical mapping and image integration allow precise catheter visualization during electrophysiology studies. However, single projections may be misleading. Therefore, catheter position should always be ascertained using different view angles.
Introduction
Atrial fibrillation (AF) recurrence is not uncommon after pulmonary vein isolation (PVI) and redo radiofrequency ablation is recommended to maintain sinus rhythm.1 During catheter ablation of AF, cardiac perforation is the most commonly recognized complication. Literature about cardiac perforation during high-resolution mapping with a multipolar catheter (PentaRay) before PVI is limited. We present a case of a redo PVI procedure complicated by PentaRay catheter perforation at the base of the left atrial appendage (LAA).
Case report
A 62-year-old male patient with a history of hypertension and type 2 diabetes was admitted to our electrophysiology lab for his third PVI and second cavotricuspid isthmus (CTI) ablation owing to recurrent paroxysmal AF and typical atrial flutter. Eighteen months later, he developed atypical flutter and returned for another ablation procedure. The previous PVIs had been performed 12 years and 8 years earlier, respectively. CTI ablation had been performed 11 years before. Eight years before, the patient suffered from cardiogenic shock owing to tachycardia-induced cardiomyopathy as a result of recurrent AF and atrial flutter.
During the procedure, a decapolar catheter was placed in the coronary sinus followed by an uneventful transseptal puncture (transseptal needle, Brockenbrough BRX XS; long steerable sheath with guidewire, Agilis medium curve, Abbott). A multipolar catheter (PentaRay; Biosense Webster, Diamond Bar, CA) and a steerable sheath (Agilis medium curl, Abbott, IL, USA) were used to create an electroanatomical map using the CARTO (Biosense Webster) electroanatomical mapping system. Shortly after the mapping procedure was started, the patient’s blood pressure decreased to 70/40 mm Hg, and heart rate increased to 110 beats per minute. The oxygen saturation was 100% on 3 L oxygen per nasal cannula. Bedside echocardiography and fluoroscopy revealed pericardial effusion (PE) with echocardiographic signs of cardiac tamponade. After withdrawal of the multipolar catheter from the left to the right atrium, an emergent pericardiocentesis was performed. Although autotransfusion of the hemorrhagic effusion of more than 1000 mL was immediately performed, the patient’s hemodynamics kept deteriorating. Immediate review of the catheter movement on the CARTO system revealed that the spines of the multipolar catheter had perforated the lateral left atrial (LA) wall at the basal edge of the LAA (Figure 1 and Supplemental Video). Withdrawal of the multipolar catheter from the pericardial space resulted in the deterioration of cardiac tamponade. Thus, a heart team including electrophysiologists, interventional cardiologists, cardiac surgeons, and anesthesiologists decided to proceed with open heart surgery. Inspection of the heart revealed very thin myocardium at the LA wall with a tear at the base of the LAA that could be treated successfully by direct suture. On the second day after the operation, echocardiography showed no signs of fluid reaccumulation. Owing to the paroxysmal AF, anticoagulant treatment was reinitiated under continuous hemodynamic monitoring. The patient was discharged after a 2-week hospitalization. At 3-month follow-up, echocardiography showed no signs of recurred PE.
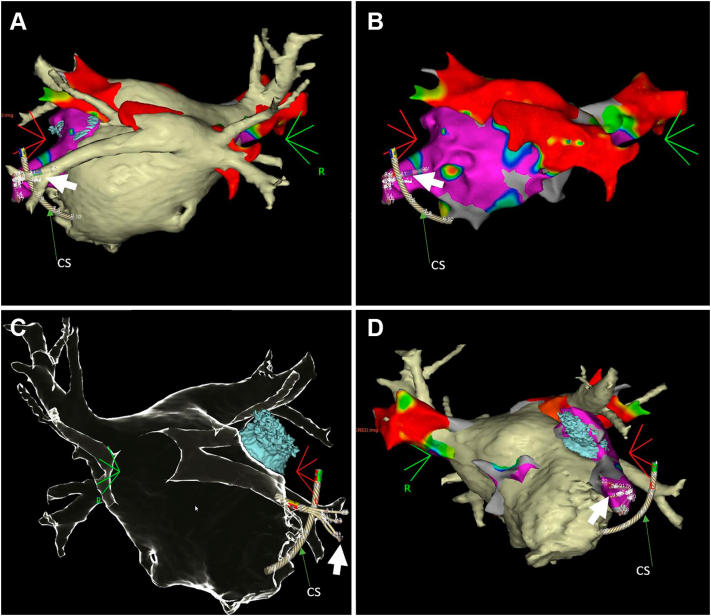
Figure 1.
Three-dimensional (3D) reconstruction of a computed tomography (CT) scan for left atrium-pulmonary vein (LA-PV). A: The 3D reconstruction of CT scan for LA-PV displayed in posteroanterior (PA) projection. The inferior veins are located in a posterior position at the LA wall and show a common antrum and narrow lumen diameter. Note the multipolar catheter perforated through the inferior basal left atrial appendage (LAA) (white arrow). B: Voltage amplitude map merged with CT of the left atrium. Note the perforated multipolar catheter was outside of the anatomy (white arrow). C: CT image of the LA-PV and LAA in anteroposterior (AP) projection demonstrating the multipolar catheter in line with the left inferior pulmonary vein, leading to a visual illusion by single projection, which was illustrated by video review, see Supplemental Video: 3D electroanatomical mapping of LA-PV merged with CT in left anterior oblique (LAO) projection. Note that the multipolar catheter is visible anterior to the left inferior pulmonary vein. CS = coronary sinus catheter.
Discussion
We present a case of redo PVI complicated by perforation of the left atrium with a multipolar mapping catheter during electroanatomical mapping at the inferior base of the LAA, which has not been reported in previous literature. The advent of multipolar mapping catheters has greatly increased the speed of obtaining 3-dimensional maps during AF catheter ablation and has been associated with a small risk of complications. Only a rare complication is reported regarding the entrapment of the mapping catheter in a mechanical prosthesis in mitral position.2,3 The present case is the first report of a multipolar PentaRay catheter penetrating the LAA, resulting in pericardial tamponade and the need for surgical suture.
Potential mechanisms for catheter-related cardiac tamponade as a complication of PVI include ablation-induced local ulcer rupture or small pericardial hemorrhage associated with intense postoperative anticoagulation. Local rupture occurs predominantly in thin myocardium, and the most frequent cause of direct cardiac tamponade encountered after AF ablation is a thin posterior LA wall.4
However, LAA perforation is not uncommon in LAA occlusion procedures. In the PROTECTAF (Percutaneous Closure of the Left Atrial Appendage with Warfarin Therapy to Prevent Stroke in Patients with Atrial Fibrillation) trial and the PREVAIL (Prospective Randomised Evaluation of the Watchman LAA Occlusion Device in Patients with Atrial Fibrillation) trial, the incidence of PE with the Watchman device for LAA occlusion was 4.8% and 1.9%, respectively, with 1.3%–1.6% of patients requiring surgical repair.5,6 While there is sufficient literature to report complications during device placement, there are no guidelines or protocols to recommend how to manage them. However, it is highly desirable to avoid the risk of urgent surgical repair with concomitant sternotomy. A double wire to the subxiphoid pericardial channel, with double site drainage and continuous aspiration, may allow a “seal” on the source of bleeding, which could be achieved in a series of patients.7 However, in our case, despite effective pericardial drainage, the bleeding progressed. Consideration was taken by the surgeon to manage the perforation with either a WATCHMAN (Boston Scientific, Marlborough, MA) device or an AtriClip (AtriCure, West Chester, OH). Unfortunately, this was deemed not possible because the location at the LAA was too basal. Thus, an open-chest suture was unavoidable and owing to the thin and fragile myocardium, the suture had to be reinforced with a patch.
The LAA is a component of the left atrium that originates from the anterolateral primordial atrium and is formed by thin pectinate muscles, which makes the LAA more susceptible to perforation by catheter maneuvers, compared to other LA locations. In addition, many patients with persistent AF have underlying early fibrotic substrate changes in the atria, which is also a potential risk factor for perforation. Further, cardiac perforation secondary to warfarin or new oral anticoagulants has been reported.8,9 Based on case reports, spontaneous pericardial hemorrhage is more likely to occur in patients with risk factors for bleeding, including advanced age, chronic kidney disease, and malignancy. In our case, the sudden symptom development and hemorrhagic PE were consistent with an acute course.
Electrophysiologic procedures can be technically demanding and require a detailed understanding of the cardiac anatomy and its 3D mapping correlates. Generally, the flexible spines of the multipolar mapping catheter move freely and therefore are very unlikely to cause perforation. When using a long sheath, advancing the catheter out of the sheath in close contact to the LA wall may increase the risk of perforation if the splines are not fully expanded owing to incomplete exit from the tip of the sheath before getting in contact with the LA wall. However, in our case, postprocedural CARTO review of the catheter movement within the LA demonstrated full expansion of the multipolar catheter before getting into contact with the LA wall. Thus, incomplete expansion of the catheter splines can be excluded as a reason for perforation in this case. The immediate cause of the acute cardiac tamponade as demonstrated during surgical repair was a tear in the LAA that might have been facilitated by a local lesion owing to a previous ablation procedure.
For delayed cardiac tamponade, there are no specific early symptoms during the procedure such as hypotension, which may occur within minutes or days.10 Cardiac electrophysiologists should be aware of the possibility of delayed cardiac tamponade, especially in patients with previous cardiac catheter ablation combined with or without other risk factors. Treatment options for cardiac tamponade include pericardiocentesis and surgical repair, although small hemodynamically stable effusions can be treated conservatively. Cardiac tamponade owing to perforation with a multipolar catheter was associated with an acute tear in an area of thin myocardium at the LA free wall, which had to be managed by surgical repair in this case. There are no reports of delayed PE after mapping procedures with a multipolar catheter.
Conclusion
This case report for the first time describes the rare event of a perforation of the left atrium with a multipolar catheter in a patient undergoing LA mapping for redo PVI after previous PVI and CTI ablation. Since the flexible splines of the multipolar catheter are generally not prone to exert extensive pressure on the myocardium, perforation is most likely to occur in a fragile portion of the LA wall, which might be a previous ablation site or the LAA. Surgical intervention was required, as hemodynamic stability could not be achieved by pericardiocentesis in this case.
Footnotes
Funding Support: None.
Disclosures: None.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrcr.2022.05.026.
Appendix. Supplementary data
Three-dimensional (3D) reconstruction of a computed tomographic (CT) scan for LA-PV displayed in posteroanterior (PA) projection. The inferior veins are located in a posterior position at the LA wall and show a common antrum and narrow lumen diameter. The multipolar catheter perforated was outside of the anatomy through the inferior basal LAA (blue).
References
- 1.Das M., Wynn G.J., Saeed Y., et al. Pulmonary vein re-isolation as a routine strategy regardless of symptoms: the PRESSURE randomized controlled trial. JACC Clin Electrophysiol. 2017;3:602–611. doi: 10.1016/j.jacep.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Sheldon S.H., Good E. PentaRay entrapment in a mechanical mitral valve during catheter ablation of atrial fibrillation. HeartRhythm Case Rep. 2015;2:200–201. doi: 10.1016/j.hrcr.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawaji T., Kato M., Yokomatsu T. How to release PentaRay catheter entrapped in the hinge point of mechanical mitral valve? Europace. 2020;22:204. doi: 10.1093/europace/euz219. [DOI] [PubMed] [Google Scholar]
- 4.Roy B., Suhny A., Andrew B., et al. Left atrial wall thickness variability measured by CT scans in patients undergoing pulmonary vein isolation. J Cardiovasc Electrophysiol. 2011;22:1232–1236. doi: 10.1111/j.1540-8167.2011.02100.x. [DOI] [PubMed] [Google Scholar]
- 5.Holmes D.R., Reddy V.Y., Turi Z.G., et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374:534–542. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]
- 6.Holmes D.R., Jr., Kar S., Price M.J., et al. Prospective randomized evaluation of the Watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1–12. doi: 10.1016/j.jacc.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 7.Macias C., Shivkumar K., Tung R. Novel approach to intraprocedural cardiac tamponade: dual-site drainage with continuous suction. Heart Rhythm. 2016;13:2091–2094. doi: 10.1016/j.hrthm.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Cinelli M., Uddin A., Duka I., et al. Spontaneous hemorrhagic pericardial and pleural effusion in a patient receiving Apixaban. Cardiol Res. 2019;10:249–252. doi: 10.14740/cr902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kham N.M., Song M. Spontaneous, life-threatening hemmorrhagic cardiac tamponade secondary to rivaroxaban. Am J Ther. 2016;23:e1128–e1131. doi: 10.1097/MJT.0000000000000263. [DOI] [PubMed] [Google Scholar]
- 10.Riccardo C., Hugh C., Shih-Ann C., et al. Delayed cardiac tamponade after radiofrequency catheter ablation of atrial fibrillation: a worldwide report. J Am Coll Cardiol. 2011;58:2696–2697. doi: 10.1016/j.jacc.2011.09.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Three-dimensional (3D) reconstruction of a computed tomographic (CT) scan for LA-PV displayed in posteroanterior (PA) projection. The inferior veins are located in a posterior position at the LA wall and show a common antrum and narrow lumen diameter. The multipolar catheter perforated was outside of the anatomy through the inferior basal LAA (blue).