Abstract
As specialists in acute neurology, neurohospitalists are often called upon to diagnose and manage acute viral infections affecting the nervous system. In this broad review covering the neurology of several acute viral infections, our aim is to provide key diagnostic and therapeutic pearls of practical use to the busy neurohospitalist. We will review acute presentations, diagnosis, and treatment of human herpesviruses, arboviruses, enteroviruses, and some vaccine-preventable viruses. The neurological effects of coronaviruses, including COVID-19, are not covered in this review.
Keywords: central nervous system viral diseases, meningoencephalitis, encephalitis, infectious disease medicine, meningitis
Introduction: Approach to the Patient
Accurate diagnosis and treatment of patients with acute neurological disease from viral infections begins with collecting historical data. Most acute infectious neurological syndromes begin and worsen over a short number of days, up to 2–3 weeks. Identification of a patient’s specific symptoms, together with the neurological examination, define a clinical syndrome (eg, encephalitis, meningitis, cerebellitis, acute flaccid myelitis, etc.) which will frame the workup and differential diagnosis. For example, a patient presenting with 3 days of fever and rapidly deteriorating mental status culminating in seizure activity would raise suspicion for an encephalitis. A patient presenting with fever, rash, and paraparesis or focal weakness may raise concern for an infectious myelopathy or polyradiculitis. Before narrowing the etiologic differential diagnosis to a viral infection, the history and examination are used to rule out other causes. Vascular, structural lesions, and acute toxic-metabolic causes can be ruled in or out using a combination of the tempo on presentation and the presence or absence of associated symptoms (fever, constitutional, and other systemic symptoms). In many cases, further acute diagnostic testing (bloodwork, computed tomography, lumbar puncture with cerebrospinal fluid analysis) will be required to exclude competing causes, including non-viral infectious diseases.
Key historical features which help to narrow the infectious differential diagnosis include assessment of host factors such as risk of exposure (eg, travel, sick contacts, zoonotic exposure, injection drug use, sexual history, and occupational history) and immunocompetence (eg, immunosuppressive therapy, hematological malignancy, HIV status, vaccination history). Geographic locale and seasonality are other important pathogen-related factors which need to be considered. These features, along with the results of initial diagnostic testing, help to distinguish viral, bacterial, and fungal causes of infection since the clinical presentations overlap. Sometimes, prodromal symptoms may indicate a specific diagnosis, such as a dermatomal rash in varicella zoster infection. In other cases, symptoms may increase the likelihood of infection but are not specific enough to rely upon (eg, fever, upper respiratory tract symptoms). Localization is an essential, albeit imperfect, tool which can assist in ranking the most likely viral (or non-viral) infectious agent in combination with the patient’s risk factors. For example, a patient with untreated human immunodeficiency virus (HIV) presenting with a polyradiculopathy raises concern for cytomegalovirus (CMV), whereas an immunocompetent patient presenting with an encephalitis brings herpes simplex virus 1 (HSV1) to the top of the list of considerations. Conversely, acute flaccid myelitis (AFM) can occur with numerous viruses. The patient’s age, seasonality, geography, and exposure history allow the clinician to weigh the likelihood of enterovirus vs West Nile virus and confirm the suspicion with targeted diagnostic testing. Table 1 outlines how each step in the clinical evaluation is used to guide the diagnostic search.
Table 1.
Approaching the patient with suspected viral neurological disease.
| Data | Information to collect | How it helps | Examples |
|---|---|---|---|
| History of presenting illness | Temporal course of symptoms, primary area of neurological dysfunction | Raises suspicion for infectious cause, directs clinical examination, triaging | Days of encephalopathy and seizures vs months-to-years of symptoms raises likelihood of infectious encephalitis |
| Associated symptoms | Headache, meningismus, fever, rash, respiratory or gastrointestinal symptoms | Raises or lowers confidence in infectious cause; some symptoms may suggest specific viral infections | Painful, dermatomal rash due to VZV |
| Ability to mount immune response (immunocompetence) | Immunosuppressive medications, HIV status, treatment of HIV, vaccination status, comorbidities | Stratifies patient risk for opportunistic infection and may increase breadth of imaging, blood, and CSF tests used | HIV+ and not treated with cART increases likelihood of CMV and other opportunistic infections |
| Risk of exposure | Season, travel, occupational or recreational activities | Narrows list of etiologic agents, particularly for viral and fungal pathogens | Recent camping and mosquito exposure increases risk of arboviral disease |
| Neurological signs | Altered mental status, dysphasia, pattern of seizure activity, focality of weakness, sensory symptoms, or ataxia | Defines clinical syndrome and allows for precise localization | Diffuse flaccid weakness suggesting polyradiculitis or acute flaccid myelitis |
| Systemic signs | Hemodynamic compromise, skin lesions, ocular or head and neck involvement, respiratory findings | May suggest specific viral or non-viral infectious etiology | Parotitis in an unvaccinated patient suggesting mumps |
| Blood/body fluid analysis | Complete blood count, serology, cultures, body fluid nucleic acid testing (eg, RT-PCR) | Confirms recent or remote exposure, or immunity to infectious agents; can rule sepsis in or out | Stool RT-PCR can confirm enteroviral infection |
| CSF analysis | Pattern and degree of pleocytosis, protein elevation, lactate, nucleic acid testing, cultures, and serology | Identifies an inflammatory response in the CNS; offers confirmation that a virus is replicating in the CSF compartment | HSV PCR confirms diagnosis of HSV encephalitis (particularly >72 h from symptom onset) |
| Diagnostic imaging | Head CT, neuraxial MRI, vessel imaging | Rules in CNS inflammation/infection; rules out alternative causes | MRI brain shows classic limbic encephalitis; enhanced head CT suggests bacterial abscess rather than viral infection |
| Other ancillary tests | Ophthalmologic exam, nerve conduction studies/electromyography | Refine localization, differential | Presence of retinitis suggests CMV in a patient with HIV |
Abbreviations: VZV = varicella zoster virus; HIV = human immunodeficiency virus; cART = combined antiretroviral therapy; CSF = cerebrospinal fluid; CMV = cytomegalovirus; PCR = polymerase chain reaction; RT-PCR = reverse transcription polymerase chain reaction; HSV = herpes simplex virus; CNS = central nervous system; CT = computed tomography; MRI = magnetic resonance imaging.
As our review centers around viral causes, we list the clinical presentations, diagnostic approach, and recommended therapies for each in the sections that follow, organized by virus.
Human Herpesviruses
Viruses in the family Herpesviridae commonly cause neurological disease. They are reviewed in the following sections and in Table 2.
Table 2.
Human Herpesviruses: syndromes, diagnosis, and treatment.
| Herpesvirus | Main Neurological Syndromes | Laboratory Diagnosis | Imaging Diagnosis | Treatment of Choice | Special Considerations |
|---|---|---|---|---|---|
| HSV1 | Encephalitis | CSF PCR | Brain MRI | IV Acyclovir | False negative CSF PCR in first 72 h of illness |
| HSV2 | Recurrent meningitis | CSF PCR | — | Acyclovir/valacyclovir | Value of chronic suppressive treatment uncertain |
| — | Polyradiculitis/myelitis | CSF PCR | Spinal MRI | Acyclovir/valacyclovir | — |
| — | Acute retinal necrosis | Ophthalmology consultation; consider vitreous tap | — | Acyclovir | Clinical diagnosis by ophthalmologic exam |
| VZV | Shingles (Herpes Zoster) | — | Valacyclovir (IV acyclovir for immunocompromised) | Clinical diagnosis | |
| — | Meningoencephalitis/Disseminated zoster | CSF PCR; serum:CSF IgG index | Brain MRI | IV Acyclovir | Immunocompromised patients |
| — | Cerebellitis | CSF PCR; serum:CSF IgG index | Brain MRI | Expectant | Post-infectious syndrome in children |
| — | Vasculopathy | CSF PCR; serum:CSF IgG index | CT or MR angiography +/- vessel wall MRI | IV Acyclovir +/- corticosteroids | Can be delayed days-weeks after reactivation |
| EBV | Meningoencephalitis | CSF PCR; Viral capsid antigen IgM, IgG; EBNA IgG | MRI relevant part of neuraxis | Value of antiviral therapy unknown | Consider whether bystander or culprit |
| — | Post-transplantation lymphoproliferative disorder | CSF PCR; Viral capsid antigen IgM, IgG; EBNA IgG | MRI relevant part of neuraxis | Variable: rituximab, chemotherapies, reduction of immunosuppression | Body CT or PET CT to detect multisystem involvement; may require biopsy |
| CMV | Congenital encephalitis (neonates) | Urine or saliva PCR (first 21 days of life) | Head ultrasound; Brain MRI | Ganciclovir/valganciclovir with confirmed CNS or severe disease | Testing and treatment of infected mothers, those at risk for HIV; hearing and vision testing of infants |
| — | Encephalitis | CSF PCR | Brain MRI | Ganciclovir +/- foscarnet | cART in persons with HIV |
| — | Polyradiculitis/myelitis | CSF PCR | Spinal MRI | Ganciclovir +/- foscarnet | cART in persons with HIV |
| — | Retinitis | Ophthalmology consultation; consider vitreous tap | Valganciclovir/ganciclovir +/- foscarnet; consider intravitreal treatment | Ophthalmologic exam; cART in persons with HIV | |
| HHV6 | Limbic encephalitis | CSF PCR; quantitative CSF:blood PCR replication ratio | Brain MRI | Ganciclovir or foscarnet | Chromosomal integration |
Abbreviations: HSV1 = herpes simplex virus 1; HSV2 = herpes simplex virus 2; VZV = varicella zoster virus; EBV = Epstein-Barr virus; CMV = cytomegalovirus; HHV6 = human herpesvirus 6; CSF = cerebrospinal fluid; PCR = polymerase chain reaction; IgG = immunoglobulin G; IgM = immunoglobulin M; EBNA = Epstein-Barr nuclear antigen; MRI = magnetic resonance imaging; CT = computed tomography; HIV = human immunodeficiency virus; cART = combined antiretroviral therapy.
Herpes Simplex Virus 1 (HSV1)
Background
HSV1 is the most common cause of sporadic acute encephalitis in adults, with most cases representing reactivation of latent disease. HSV encephalitis has an estimated annual incidence of 24 per million people with no seasonal variation and tends to affect those over age 50. 1 Historically HSV encephalitis was fatal in greater than 70% of untreated patients. 2 Timely treatment with antiviral therapy reduces mortality to less than 20%,3,4 although many survivors live with residual disability. 5
Clinical Syndromes And Diagnostic Evaluation
The most common presenting symptoms include fever, headache, altered mental status, focal neurological deficits, and seizures. 4 Manifestations can be nonspecific, and early consideration is key in making the diagnosis. Cerebrospinal fluid (CSF) polymerase chain reaction (PCR) to detect HSV deoxyribonucleic acid (DNA) has become a diagnostic standard due to its excellent sensitivity and specificity (98% sensitive, 94% specific).6,7 PCR is incompletely sensitive less than 72 hours from symptom onset, 8 so CSF PCR can be repeated after 72 hours when clinical suspicion remains high despite an early negative result. CSF usually shows a lymphocytic pleocytosis and elevated protein, but rare patients may have a normal CSF profile. 9 Head computed tomography (CT) may be normal but is insensitive. Brain magnetic resonance imaging (MRI) is abnormal in virtually all cases, with rare exceptions early in the disease course. 4 In a prospective cohort study, brain MRI was found to be 81% sensitive and 100% specific for HSV encephalitis. 10 Typical imaging findings include FLAIR and T2-hyperintensity and gadolinium enhancement in the mesial temporal lobe(s) (Figure 1). Diffusion weighted imaging may be abnormal, and changes can precede those seen on T2-sequences. Insular, inferior frontal lobe, and cingulate hyperintensity can be seen; while brainstem, cerebellar and widespread cortical involvement have been reported in a minority of immunocompromised patients. 11 Electroencephalography (EEG) often shows nonspecific findings, but the presence of lateralized slowing or periodic discharges can suggest HSV encephalitis in the appropriate context (sensitivity ∼86% based on studies from the late 1970s and early 1980s). 12
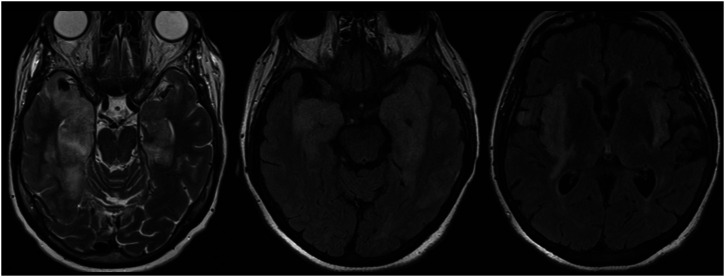
Figure 1.
Herpes simplex virus (HSV) encephalitis. Characteristic bilateral T2/FLAIR hyperintensity and edema of the temporal lobes with extension into the insular cortices and limbic system.
Treatment
Intravenous (IV) acyclovir is the treatment of choice for HSV encephalitis and should be empirically started as soon as the diagnosis of HSV encephalitis is suspected. Treatment should not be delayed until definitive diagnostic studies are obtained, since delayed treatment is associated with poorer outcomes.5,13 IV acyclovir is dosed at 10 mg/kg q8h and continued for 14–21 days, with renal dose adjustment as required. 14 The use of 14 vs 21 days depends on illness severity. Empiric acyclovir can be discontinued if a negative CSF HSV PCR test is obtained more than 72 hours after symptom onset in patients with a low probability of HSV encephalitis after diagnostic evaluation. 3 Therapy should not be discontinued in patients with a high suspicion of HSV encephalitis and a negative CSF HSV PCR unless an alternate diagnosis is made. Adjunctive corticosteroids are of unproven benefit.
Post-Infectious Autoimmune Encephalitis
Late worsening or apparent relapse of encephalitis in survivors should prompt consideration of persistent viral infection and autoimmune encephalitis. Along with a repeat HSV PCR on CSF, serum and CSF testing for neuronal cell-surface and synaptic autoantibodies (using commercially available autoimmune encephalitis panels), is recommended. Autoimmune encephalitis has been reported following HSV encephalitis in about to 30% of cases, with anti-N-methyl-D-aspartate (NMDA) receptor antibodies being the most common. 15 In the authors’ experience, test performance by a laboratory which also does immunofluorescence-based screening to detect unclassified autoantibodies is preferred.
Herpes Simplex Virus 2 (HSV2)
Background
HSV2 is an alpha herpesvirus that most commonly causes genital sores (genital herpes). HSV2 is predominantly sexually transmitted, and it causes neurological disease during primary infection or reactivation from latent HSV2 in sensory ganglia. Neurological manifestations of HSV2 can occur without any history of previous mucocutaneous HSV2 lesions and it is estimated that only 5-30% of patients present with a previous history of HSV2 mucocutaneous lesions or concurrent/antecedent lesions at neurological presentation.16-18
Clinical Syndromes
In adults, the most common neurological manifestation of HSV2 is a lymphocytic meningitis. HSV2 is also the most common cause of recurrent lymphocytic meningitis (eg, Mollaret’s meningitis).19,20 Twenty to thirty percent of patients presenting with HSV2 meningitis will have subsequent recurrent episodes of meningitis.16,17 In most patients with recurrent HSV2 meningitis, the severity of meningitis, degree of pleocytosis, and likelihood of a positive HSV2 PCR decrease with each recurrence. Less common neurological complications include meningoencephalitis or encephalitis (particularly in the immunocompromised patient), sacral polyradiculitis (Elsberg syndrome), acute retinal necrosis, or myelitis. 21
Diagnosis
HSV2 PCR testing in the CSF is the diagnostic test of choice for patients with meningitis, with a sensitivity of 87% in primary and 70% in recurrent meningitis. 22 CSF typically demonstrates a lymphocytic pleocytosis with elevated CSF protein levels. MRI brain may demonstrate diffuse leptomeningeal enhancement in cases of meningitis but is most often noncontributory.
Treatment
There is no consensus on the optimal treatment of HSV2 meningitis, as demonstrated by the various combinations of IV acyclovir and oral valacyclovir that have been reported.17,18 The authors often administer IV acyclovir at a dose of 5-10 mg/kg q 8 hours while the patient is symptomatic (eg, for 48-72 hours) and transition to oral valacyclovir (1g PO TID) as symptoms improve to complete a 10–14 day total course of antiviral treatment. Most patients have a benign/favorable outcome, but neurological sequelae have been well documented.16,18
Special Considerations
The prevention of HSV2 meningitis recurrences in individual patients remains a challenging clinical issue. Data from a randomized controlled trial found no benefit in preventing recurrent episodes of meningitis in patients prescribed oral valacyclovir suppression treatment over 2 years. 16 The authors offer suppression treatment to patients with recurrent mucocutaneous HSV2 eruptions. Suppressive therapy directed against HSV2 also reduces shedding of human immunodeficiency virus 1 (HIV1) in genital secretions of HIV-positive individuals, likely due to synergy between HSV2 and HIV1. 23 Whole exome sequencing in patients with recurrent HSV2 meningitis has detected rare genetic variants related to ubiquitin proteasome, autophagy, and cell proliferation pathways, suggesting that affected patients may have an underlying immune defect.24,25
Varicella Zoster Virus (VZV)
Background
Primary infection with VZV is best known for causing chickenpox in children, now a much rarer occurrence in regions where vaccination has been routine since the mid-1990s. Following primary infection, the virus remains latent in dorsal root ganglia and can subsequently reactivate, especially when immunity against VZV wanes with increasing age or immunocompromise. 26
Clinical Syndromes
Neurological manifestations of reactivated VZV, known collectively as herpes zoster, are commonplace in practice, yet can be difficult to identify. VZV can involve any segment of the neuraxis and patients do not always have the classic dermatomal vesicular rash (eg, zoster sine herpete).27,28 VZV vasculopathy and other syndromes may present many weeks to months following reactivation of latent VZV. 27 VZV can present in a disseminated or chronic manner in patients with diminished cell-mediated immunity, such as persons living with AIDS or organ transplant recipients. 29
While VZV can present as a cerebellitis in children and encephalitis in adults, meningitis without parenchymal brain involvement is a common manifestation. VZV vasculopathy can complicate infection, causing ischemic stroke or intracerebral hemorrhage (Figure 2). Additional clinical syndromes include zoster ophthalmicus, outer retinal necrosis, cranial neuritis (as with Ramsay Hunt syndrome), myelitis, radiculitis, and segmental motor neuropathy (zoster paresis). If present, the characteristic herpetiform rash may be a clue to the expected level of neuraxial involvement. 27 Postherpetic neuralgia, characterized by persistent pain lasting for at least 3 months following a shingles eruption, remains a significant cause of post-zoster morbidity. 27
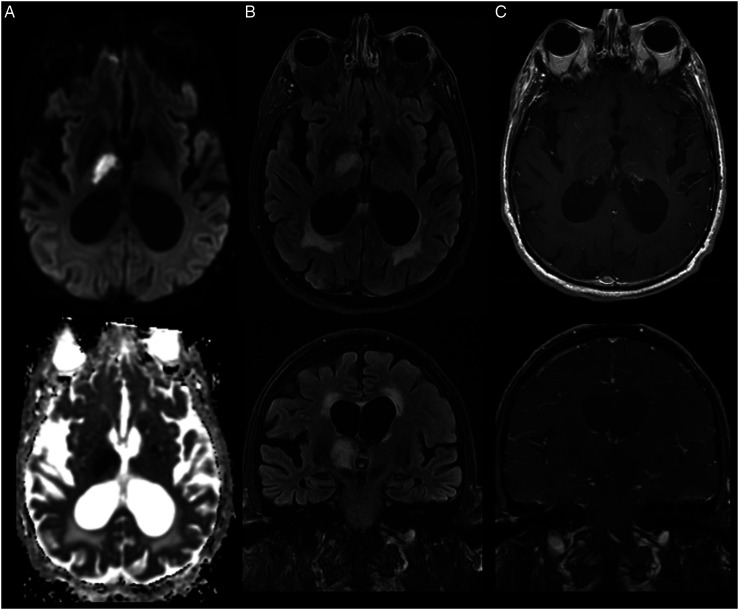
Figure 2.
Varicella zoster virus (VZV) meningitis complicated by ischemic stroke. Brain MRI illustrates T2/FLAIR hyperintensity and diffusion restriction consistent with an ischemic stroke in the right anterior thalamus (A-B). T1 post-gadolinium images demonstrate subtle perivascular and leptomeningeal enhancement (C).
Diagnosis
Most patients with acute CNS manifestations of herpes zoster have a lymphocytic pleocytosis in CSF, but this can be absent. In patients with meningoencephalitis, testing CSF for VZV DNA by PCR has a sensitivity of 80-95% and specificity >95%. 3 Confirming intrathecal production of VZV IgG using the VZV antibody index may improve diagnostic sensitivity when PCR is negative since clinical symptoms may begin weeks after VZV reactivation (when the yield of PCR may wane). The VZV antibody index requires measurement of CSF and serum VZV IgG along with the total CSF and serum IgG or albumin. Intrathecal VZV IgG synthesis is considered increased when the index quotient is 1.5 or greater.30,31 The VZV antibody index is a diagnostic adjunct and should not be considered a definitive test. VZV IgM is generally not useful for confirming a clinical diagnosis of herpes zoster neurological disease because of poor specificity. Particularly when focal deficits are present, consider CT or MR angiography (plus or minus vessel wall MRI, where expertise exists) to detect VZV vasculopathy.
Treatment
Treatment of VZV neurological infection depends on the area of involvement and whether the patient is immunocompromised. 29 Cutaneous manifestations, zoster ophthalmicus, and zoster oticus without CNS involvement can be treated with oral valacyclovir 1g TID for 7-10 days. Disseminated disease, meningoencephalitis, myelitis, and vasculopathy are treated with IV acyclovir 10 mg/kg q8h for 14–21 days. 3 With vasculopathy, corticosteroids can be considered, but the benefit is uncertain. 32
Prevention
Vaccination prevents VZV neurological disease and morbidity from postherpetic neuralgia. An inactivated recombinant zoster subunit vaccine is preferred for individuals over age 50 or those at increased risk due to immunocompromise. Live virus vaccine is contraindicated in immunocompromised people but continues to be standard in childhood vaccination series.
Epstein-Barr Virus (EBV)
EBV is a ubiquitous virus which causes self-limited infection (infectious mononucleosis) in children and adolescents. Neurological disease from EBV occurs rarely as a primary infection in children 33 or in immunocompromised adults. 34 Manifestations are variable and can involve the CNS or PNS, including meningoencephalitis, cerebellitis, optic neuritis, transverse myelitis, facial palsy, and polyradiculoneuropathy. 35 EBV infection is also associated with post-transplant lymphoproliferative disorder (PTLD) and primary CNS lymphoma. 29 Prior EBV infection is a risk factor for the development of multiple sclerosis (MS), however the mechanism linking EBV to MS remains unclear. 36
The challenge often lies in determining whether EBV is the causative agent in an acute neurological syndrome and deciding whether it requires directed treatment. Serology and viral loads in the blood can determine the timing and presence of systemic infection but are nonspecific regarding nervous system involvement. As reviewed elsewhere, 37 the presence of IgM antibody against the viral capsid antigen (VCA), and IgG antibody against Epstein-Barr early antigen (EA) in acute blood samples are often markers of acute infection. IgM VCA antibodies usually disappear within 4-6 weeks of EBV infection and those against EA within 3–6 months. Conversely, the presence of IgG antibodies against VCA and the detection of antibodies against Epstein-Barr nuclear antigen (EBNA) are generally indicative of prior infection and persist for life. Monospot testing is not reliable in evaluating potential neurological EBV infections as both false positive and false negative results are common. Imaging of relevant neuraxial compartments with MRI is often useful, and certain radiologic patterns, such as reversible T2-hyperintense, diffusion-restricting splenial lesions have been identified in patients with EBV encephalitis. 38 As with imaging, CSF analysis may be inconclusive, since findings can be normal. EBV DNA detected by CSF PCR may be supportive of neurological infection; it is also found incidentally in healthy people and may reactivate during other illnesses. CSF EBV PCR is 100% sensitive and 98.5% specific for HIV-associated primary CNS lymphoma, 39 but the specificity is likely lower for EBV meningoencephalitis. Patients with EBV meningoencephalitis and CNS lymphoma have a high EBV load in CSF, which can help distinguish them from persons with postinfectious neuroinflammation. 40 A caveat is that co-infection with other viral and non-viral pathogens may also increase EBV replication in CSF. 41 Low level evidence suggests that measuring a baseline EBV load in CSF in presumptive infection helps to correlate with response to treatment. 42 The lack of normal reference values may limit interpretation.
No treatment guidelines are available, but antiviral therapy can be considered when neurological symptoms are attributed to EBV. Ganciclovir and derivatives are preferred by some experts since valganciclovir was found to suppress EBV oral shedding. 43 Other antivirals, such as acyclovir have activity against EBV but the clinical benefit is unknown. 44 IVIG has been used anecdotally for suspected post- or para-infectious manifestations; however high-level evidence for its efficacy is lacking. 45 When PTLD is suspected, rituximab, other chemotherapies, or reduction of immunosuppression are sometimes used.
Cytomegalovirus (CMV)
Cytomegalovirus has a high seroprevalence in the general population of 40-100%. 46 Nervous system involvement occurs in two major contexts: congenital infection and in severely immunocompromised adults (eg, organ transplant recipients, advanced HIV). Congenitally acquired CMV causes encephalitis with seizures, sensorineural hearing loss, and developmental delay and can be missed since not all neonates (10%) display classic features. 47 It may be first suspected when periventricular calcifications are seen on cranial ultrasound, or when an infant does poorly on routine hearing screening. In neonates, detection of CMV DNA by PCR of urine or saliva is diagnostic during the first 21 days of life. The introduction of combined antiretroviral therapy (cART) in people living with HIV has decreased the burden of symptomatic CMV in that population. 48 Neurological manifestations in people with advanced HIV most commonly include retinitis, polyradiculomyelitis, mononeuritis multiplex, and less commonly encephalitis with ventriculitis.49-51
Neuraxial MRI and CSF analysis can support the diagnosis. Punctate periventricular lesions with persistent diffusion restriction on MRI may be the imaging correlate of intracellular viral inclusions seen on histopathology. 52 CSF analysis shows a pleocytosis with a high proportion of neutrophils, low glucose, and elevated protein. The pattern of lymphocytic pleocytosis, normal glucose, and elevated protein may also be seen. The diagnosis requires detection of CMV DNA by CSF PCR (sensitivity 62-100%, specificity 89–100%), 53 with no established role for serology.
Ganciclovir +/- foscarnet IV for 14‒21 days is recommended for patients with CMV neurological infection. Foscarnet is preferred by some experts due to its superior CNS penetration and when there is concern for antiviral resistance. 3 The authors avoid combining ganciclovir and foscarnet due to toxicity except in clinically severe cases. Patients who remain severely immunocompromised (eg, CD4 count below 100 cells/mm3) may benefit from continuing with maintenance valganciclovir. 49
Human Herpesvirus 6 (HHV6)
HHV6 is found ubiquitously in the general population. 54 Acquired by most children prior to 3 years of age, it causes the common febrile illness roseola but may also be asymptomatic. HHV6 usually causes neurological disease when it reactivates in immunocompromised patients, particularly those with depressed T-cell mediated immunity (eg, stem cell transplant recipients). 55 In the post-transplant setting, an acute limbic encephalitis associated with HHV6 in CSF has been well described. 56 Clinical features are compatible with other causes of limbic encephalitis, and brain MRI shows T2-hyperintensity involving the mesial temporal lobe(s). 56 Rare cases of limbic encephalitis attributed to HHV6 in non-transplant patients can also occur. CSF typically shows a mild lymphocytic pleocytosis.
Confirming HHV6 as the pathogenic agent can be challenging because viral DNA is detected in the blood and CSF of healthy people due to asymptomatic HHV6 chromosomal integration into telomeres. 57 Furthermore, immunocompromised individuals are prone to HHV6 reactivation which may be unrelated to acute neurological symptoms. A recent study suggested that a CSF/blood replication ratio of >1 using quantitative PCR may support a diagnosis of HHV6 encephalitis in immunocompromised patients by confirming that viral replication is increased in the CSF compartment (and not a bystander). 58
Intravenous ganciclovir or foscarnet is recommended to treat HHV6 encephalitis, with treatment choice depending on the clinical context and patient comorbidities. 59 Combination therapy can be considered in some cases but carries a greater risk of toxicity.
Arboviruses
Arthropod-borne viruses (arboviruses) are transmitted to humans by vectors including mosquitos, ticks, and sandflies. These ribonucleic acid (RNA) viruses may come from different viral families (eg, Flaviviridae, Togaviridae, Peribunyaviridae, etc.). Infection may be asymptomatic, symptomatic with a non-specific febrile illness, or neuroinvasive. Neuroinvasive arboviral infections are difficult to distinguish from one another based on clinicoradiologic features, but seasonality along with the patient’s travel/recreational history can guide the diagnostic search (Table 3).
Table 3.
Arbovirus epidemiology and at-risk populations. Reproduced with permission from Schultz JS, Sparks H & Beckham JD. Arboviral central nervous system infections. Curr Opin Infect Dis. 2021 Jun 1;34(3):264-271. doi: 10.1097/QCO.0000000000000729. Published originally by Wolters Kluwer Health, Inc. 77
| Family/Virus | Vector | Host | Distribution | At-risk populations |
|---|---|---|---|---|
| Flaviviridae / West Nile virus | Mosquito: Culex pipiens | Passerine birds | Continental United States, Southern Europe | Age >65 years, immunocompromised, seasonal exposure |
| Flaviviridae / Usutu virus | Mosquito: Culex species | Passerine birds | Northern Africa, Southern Europe | Age >65 years, immunocompromised, seasonal exposure |
| Flaviviridae / Japanese encephalitis virus | Mosquito: Culex species | Avian and mammalian species | Southeast Asia, Philippines, Oceania | Age <18 years, immunocompromised, rural and seasonal exposure |
| Flaviviridae / Tick-borne encephalitis virus | Tick: Ixodes species | Small rodents | Eastern and Northern Europe, Northern Russia, Eastern China | Outdoor and seasonal exposure to ticks |
| Flaviviridae / Powassan virus | Tick: Ixodes species | Small rodents | Northern United States, Canada, and Northeast Asia | Outdoor and seasonal exposure to ticks |
| Togaviridae / Eastern equine encephalitis virus | Mosquito: Culex and Aedes species | Avian species | North and Eastern United States | Outdoor exposure during seasonal epidemic cycle |
| Togaviridae / Venezuelan encephalitis virus | Mosquito: Culex and Aedes species | Small rodents | Central and South America | Outdoor exposure during seasonal epidemic cycle |
| Togaviridae / Chikungunya virus | Mosquito: Aedes species | Primates | Africa, India, Southeast Asia, Caribbean | Endemic regional exposure |
| Peribunyaviridae / La Crosse virus | Mosquito: Aedes species | Small mammals/rodents | Midwestern United States | Children with outdoor exposure |
West Nile Virus (WNV)
Background
WNV is a flavivirus closely related to Japanese encephalitis and St. Louis encephalitis viruses. 60 Humans are infected with WNV predominantly by mosquito bites, although infrequent cases of transmission through blood products, organ transplantation, and vertical/placental transmission have been reported. 60 WNV infections are reported each year in most states within the United States and in most provinces in Canada. 61
Clinical Syndromes
Approximately 25% of patients infected with WNV develop symptomatic illness characterized by fever, malaise, widespread maculopapular rash, and gastrointestinal symptoms, known as West Nile fever (WNF). 60 Less than 1% of patients with WNV infection will develop neuroinvasive disease (WNND) characterized by meningitis, encephalitis, and/or acute flaccid myelitis (AFM). 62 Movement disorders including tremor, opsoclonus-myoclonus syndrome and parkinsonism have also been reported to complicate WNND, possibly due to tropism for subcortical gray matter structures including the substantia nigra pars compacta, basal ganglia, cerebellum, and thalamus.63,64 Clinical risk factors for severe WNF or WNND include older age, immunosuppression, male sex, hypertension and diabetes. 65
Diagnosis
The appropriate testing for the immunocompetent adult patient with suspected WNND is serum and CSF WNV IgM. 66 Due to the transient and low grade viremia found in infected patients, the sensitivity and utility of WNV reverse transcription-polymerase chain reaction (RT-PCR) testing is limited. 66 One caveat is the absence of a humoral immune response in patients who are immunosuppressed/immunocompromised (eg, with rituximab), in which case serum/CSF WNV RT-PCR is often necessary for diagnosis as seroconversion may be absent or delayed.67,68 Furthermore, while CSF WNV IgM is the most helpful test in diagnosing WNND with a sensitivity approaching 100%, 69 caution is warranted in atypical clinical presentations since both serum and CSF WNV IgM may persist for months after acute infection – a clinical situation in which acute and convalescent serologies can be particularly helpful.70,71 IgG and IgM cross reactivity to related viruses in the same genus (Flavivirus) or non-specific reactivity is another potential pitfall in WNND diagnosis which can be resolved by confirmatory plaque reduction neutralization testing (PRNT) at a reference laboratory. A CSF pleocytosis is commonly detected in WNND (>90%) and a neutrophilic predominance (>50% polymorphonuclear cells) is seen in about 50% of cases. 72 Brain MRI in WNND can be normal. Abnormal findings are common and variable in localization, including T2/FLAIR hyperintensities of deep gray structures (Figure 3), meningeal involvement, non-specific white matter T2/FLAIR hyperintensities, or abnormal signal of the cauda equina and spinal cord with AFM cases. 73
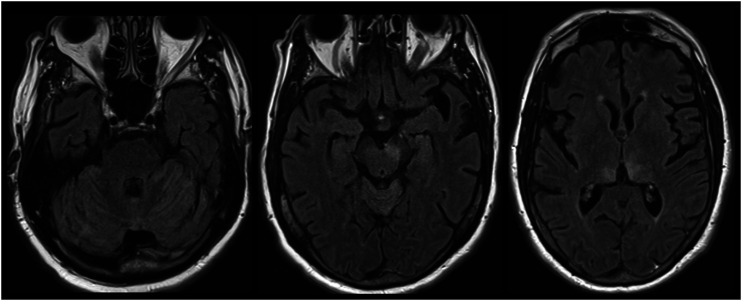
Figure 3.
West Nile virus (WNV) encephalitis. Cerebellar, asymmetric midbrain and bilateral medial thalamic involvement are illustrated.
Management and Outcomes
The mortality in patients who develop WNV encephalitis is approximately 10%. 60 Mortality is rare in patients with WNV meningitis. The management for patients with WNF or WNND is supportive – there is no known disease modifying treatment option. A recent trial of high titer WNV IgG intravenous immunoglobulin (IVIG) was stopped due to slow recruitment and unavailability of treatment product, so the benefit of IVIG remains unknown. 74 Following WNV infection and WNND, patients frequently report fatigue and subjective cognitive symptoms for weeks or months. 75 Specific neurological complications of WNND include parkinsonism, cognitive dysfunction, and limb weakness (typically after AFM-related WNND). 75
Non-WNV Arboviruses
Clinical features of WNND are shared by many arboviruses, and the diagnostic process is similar (including the pitfalls regarding IgM/IgG cross reactivity and insensitivity of RT-PCR). CSF in non-WNV neuroinvasive arboviruses often shows a lymphocytic pleocytosis with normal or elevated protein and normal glucose. 76 Disease-specific treatments for neuroinvasive arboviral infections are not available, and management is supportive. Prevention of exposure to mosquito or tick bites in high risk areas is crucial, and there is hope for future vaccine development. 77 Outcomes are variable depending on the involved regions of the nervous system and patient comorbidities.
Enteroviruses
Background
Enteroviruses (EVs) of the Picornaviridae family are a heterogenous group of single-stranded RNA viruses that commonly result in clinical infection in humans worldwide, sometimes with neurological involvement.78,79 Seven species are known to cause clinical disease in humans (enterovirus A, B, C, and D; rhinovirus A, B, and C) and commonly EVs are categorized based on their serotype. 78 While the most notorious enterovirus, poliovirus, has largely been eradicated throughout most of the world, EVs have captured widespread attention in recent years due to the emergence of outbreaks in North America of non-poliovirus EV infections (eg, EV-D68, EV-A71) causing AFM, predominantly in children.80-82
Clinical Syndromes
EVs are a common cause of often mild and self-limited childhood infections resulting in upper respiratory symptoms, hand-foot-mouth disease, rhinitis, or gastrointestinal symptoms. Enteroviral infections occur more frequently in children than adults and are transmitted via respiratory and fecal-oral routes. Occasionally, enteroviral infections can be complicated by CNS involvement presenting with meningitis, encephalitis, AFM, or brainstem encephalitis. 83 In patients with an acquired or inherited humoral immunodeficiency state, EVs can also present with rapidly progressive cognitive impairment or chronic meningitis.84,85
Diagnostic Evaluation
CSF studies usually reveal a lymphocytic pleocytosis. Unique brain MRI findings that may suggest CNS enteroviral infection include dorsal medulla, dorsal pons, and fourth periventricular T2/FLAIR lesions. 86 Spinal MRI may show predominant anterior horn involvement in the setting of AFM. 86 CSF RT-PCR can help confirm CNS enterovirus disease; however, it is increasingly recognized that the yield of RT-PCR in the CSF may be low in specific settings including EV-D68 associated AFM (detected in just 2% of AFM CSF samples). 86 If CNS enterovirus infection is suspected, other bodily fluids (such as nasopharyngeal swab, serum, and stool) in addition to CSF should be tested for EV nucleic acid (increases detection to 20-43% in patients with AFM). 87 If positive, the specific EV might then be typed at an academic or public health laboratory. 86
Treatment
The treatment of CNS EV infections is supportive. Treatments used in clinical practice include IVIG based on case reports or case series (especially in immunosuppressed or immunocompromised patients).86,88 Other agents with anti-enterovirus activity (pleconaril, pirodavir, pocapavir) are experimental or not readily available. The authors trial IVIG treatment in patients with underlying immunodeficiency/immunosuppression and chronic or relapsing CNS enterovirus infection in shared decision making with the patient.
Other Selected Viral Infections With Neurological Effects
An exhaustive review of all viruses causing neuroinvasive or secondary effects on the nervous system is beyond the scope of this article. Included below are several ‘can’t miss’ infections of special interest to the reader because they are common, treatable, or vaccine-preventable.
Acute Manifestations of Human Immunodeficiency Virus (HIV)
Neurological manifestations are commonplace in the setting of primary, acute HIV infection, including during the 24 week window where conventional antibody tests for HIV may be negative or indeterminate. 89 These manifestations can include headaches (reported in 30‒70%), meningitis, meningoencephalitis, cognitive dysfunction, seizures, and peripheral findings mimicking Bell’s palsy, Guillain-Barré syndrome, and myeloradiculopathy. 89 By definition, they will be accompanied by cardinal features of acute HIV seroconversion which resemble infectious mononucleosis. Structured evaluation in a population with primary HIV infection confirmed that while neurological findings were common (occurring in 53%), they are usually mild, suggesting that there may be publication bias favoring severe cases. 90
Findings on MRI range from normal to showing high T2 signal intensity or enhancement in the relevant neuraxial compartment. CSF studies show elevated HIV RNA, consistent with evidence that HIV CNS infiltration occurs within days of infection.91,92 Although HIV does not productively infect neurons, it may gain access to the nervous system through infection of macrophages or microglia, and a CSF macrocytosis is often discovered. 89 Despite the lack of productive infection by HIV, neuronal death can occur via indirect mechanisms through release of neurotoxic molecules from neighboring glial cells or via interaction with HIV proteins. Expert consensus is to treat patients with cART as soon as possible. 89 Some advocate for the selection of agents with superior CNS penetration to address formation of HIV reservoirs and viral escape into the CNS. 93
HIV-associated CD8 encephalitis was first described in 2013 and presents acutely in patients with HIV receiving cART (including well controlled HIV). 94 Brain MRI may show linear gadolinium-enhancing perivascular lesions or diffuse white matter T2/FLAIR hyperintensities. The pathogenesis is not well understood; however, brain biopsies show activation of astrocytes and microglia with CD8+ lymphocytes in perivascular spaces. There appears to be an association with HIV viral escape and IRIS upon re-introduction of cART following an interruption of treatment. 95 Patients with CD8 encephalitis may respond to corticosteroids.
Rabiesvirus
Rabies is caused by infection by viruses from the Lyssavirus genus (family Rhabdoviridae). The virus is transmitted to humans by a bite from an infected dog in more than 99% of cases, with other hosts including bats, foxes, and raccoons. 96 In areas with vigorous canine rabies vaccination protocols, including the United States, exposure is most often linked to bats. In many cases a clear bite is not documented or may be inapparent by history or exam, and exposure alone should be considered a potential risk factor. The 60 000 fatalities reported annually worldwide are likely an underestimation, since cases may not be documented in areas where the disease is endemic in Africa, Asia, and South America – likely due to resource limitations. 96 After an incubation period of 20-90 days, the disease enters the CNS in a retrograde manner from an infected bite, causing death within 5–11 days from onset of the neurological symptoms. 96 The earliest neurological symptoms include encephalopathy alternating with agitation/hyperactivity (furious or encephalitic rabies) and weakness which can occur in the limbs and involve bulbar as well as respiratory muscles (paralytic rabies). Hydrophobia and aerophobia occur in 50% of patients and are considered pathognomonic. Fever, pruritis, paresthesias, and dysautonomia are often seen as part of the clinical course.
Diagnosis of rabies in areas where the disease is endemic is mostly clinical, but antemortem or postmortem detection of the viral nucleic acid can be confirmatory. 96 The disease is considered lethal once neurological illness is established, except for a handful of documented survivors (for example in Wisconsin in 2004), 97 and supportive care is recommended. Attempts to replicate specific treatment protocols associated with rare cases of survival have had inconsistent results, and it remains unclear what treatment or host factors were critical in these exceptional cases. Immunization of human travelers and infected dogs is the most effective method for preventing illness, since there is no cure. Use of rabies immunoglobulin or post-exposure vaccination is not completely effective at preventing clinical disease but can greatly improve the probability of survival. 96
Measles, Mumps, and Rubella
Measles cases were resurgent in 2019 with 1282 reported in the United States, compared to the year 2000 when measles was declared eliminated by the Centers for Disease Control and Prevention (CDC). 98 This resurgence has been attributed to the unvaccinated population, with some cases brought to the United States from travel-related exposure. Measles encephalitis occurs in 1/1000 measles cases during acute infection after the characteristic febrile illness featuring rash, cough, coryza, and Koplik spots. 98 The encephalitis may be severe with mortality in 15% and residual neurological disability in 25%. 99 Diagnosis can be confirmed by detection of viral DNA by PCR of CSF, nasopharyngeal, throat or urine samples. Serum IgM may be unreliable in the first 4 days after the rash begins. 100 A rare but important late complication is subacute sclerosing panencephalitis (SSPE), felt to be caused by replication of mutated measles virus within the brain 7-10 years after primary infection. 101 SSPE is characterized by progressive neurological deterioration, seizures, generalized periodic discharges on EEG, and diffuse damage to gray and white matter on brain MRI. SSPE eventually progresses to coma, death, or severe neurological disability. 101 Treatment is supportive with both measles encephalitis and SSPE.
Mumps is similarly vaccine-preventable and can present with a self-limited febrile illness featuring characteristic parotitis, orchitis, or oophoritis. Mumps can be associated with meningitis (<10% of cases) or encephalitis (<1% of cases) and fulminant neurological presentations are very rare. 100 Deafness may occur due to involvement of cranial nerve VIII. 102 Diagnosis is often clinical, but can be confirmed based on the presence of viral DNA using PCR of CSF, saliva, or urine within the first week of symptoms. IgM and IgG in serum may be rendered unreliable based on patient vaccination status, however their presence in CSF is a more specific indicator of CNS infection. 100
Rubella causes a characteristic febrile exanthem and can cause severe complications when acquired congenitally by the fetus of a non-immune mother, including cataracts, cardiac anomalies, intellectual, and hearing impairment. 103 Infants may be diagnosed with suspected or probable congenital rubella syndrome based on the presence of the aforementioned clinical features. Congenital rubella syndrome can be confirmed when laboratory evidence of infection is demonstrated by isolation of virus from bodily fluid specimens (or RNA by RT-PCR), rubella-specific IgM, or persistently elevated convalescent antibody titers in the infant. As with measles and mumps, neurological sequelae of rubella infection are best prevented by widespread vaccination.
Conclusion
As we reviewed, neurological manifestations of acute viral infections are protean. Equipped with a diagnostic approach to recall salient features of the discussed neurological infections, neurohospitalists can contribute to early recognition and prompt treatment, resulting in a positive influence on patient outcomes.
Supplemental Material
Supplemental Material - Neurology of Acute Viral Infections by Jonathan D Krett, J David Beckham, Kenneth L Tyler, Amanda L Piquet, Lakshmi Chauhan, Carla J Wallace, Daniel M Pastula, and Ronak K Kapadia in The Neurohospitalist
Supplemental Material - Neurology of Acute Viral Infections by Jonathan D Krett, J David Beckham, Kenneth L Tyler, Amanda L Piquet, Lakshmi Chauhan, Carla J Wallace, Daniel M Pastula, and Ronak K Kapadia in The Neurohospitalist
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Jonathan D Krett https://orcid.org/0000-0003-4964-3341
Daniel M Pastula https://orcid.org/0000-0001-9342-4459
Ronak K Kapadia https://orcid.org/0000-0002-9820-0486
References
- 1.Hjalmarsson A, Blomqvist P, Skoldenberg B. Herpes simplex encephalitis in Sweden, 1990-2001: Incidence, morbidity, and mortality. Clin Infect Dis. 2007;45(7):875-880. doi: 10.1086/521262 [DOI] [PubMed] [Google Scholar]
- 2.Tyler KL. Herpes simplex virus infections of the central nervous system: encephalitis and meningitis, including Mollaret’s. Herpes. 2004;11 Suppl 2(suppl 2):57A-64A. [PubMed] [Google Scholar]
- 3.Tunkel AR, Glaser CA, Bloch KC, et al. The management of encephalitis: clinical practice guidelines by the infectious diseases society of America. Clin Infect Dis. 2008;47(3):303-327. doi: 10.1086/589747 [DOI] [PubMed] [Google Scholar]
- 4.Bradshaw MJ, Venkatesan A. Herpes simplex virus-1 encephalitis in adults: Pathophysiology, diagnosis, and management. Neurotherapeutics. 2016;13(3):493-508. doi: 10.1007/s13311-016-0433-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raschilas F, Wolff M, Delatour F, et al. Outcome of and prognostic factors for herpes simplex encephalitis in adult patients: results of a multicenter study. Clin Infect Dis. 2002;35(3):254-260. doi: 10.1086/341405 [DOI] [PubMed] [Google Scholar]
- 6.Lakeman FD, Whitley RJ. Diagnosis of herpes simplex encephalitis: application of polymerase chain reaction to cerebrospinal fluid from brain-biopsied patients and correlation with disease. J Infect Dis. 1995;171(4):857-863. doi: 10.1093/infdis/171.4.857 [DOI] [PubMed] [Google Scholar]
- 7.DeBiasi RL, Kleinschmidt-DeMasters BK, Weinberg A, Tyler KL. Use of PCR for the diagnosis of herpesvirus infections of the central nervous system. J Clin Virol. 2002;25(suppl 1):S5-11. doi: 10.1016/s1386-6532(02)00028-8 [DOI] [PubMed] [Google Scholar]
- 8.Weil AA, Glaser CA, Amad Z, Forghani B. Patients with suspected herpes simplex encephalitis: rethinking an initial negative polymerase chain reaction result. Clin Infect Dis. 2002;34(8):1154-1157. doi: 10.1086/339550 [DOI] [PubMed] [Google Scholar]
- 9.Bewersdorf JP, Koedel U, Patzig M, et al. Challenges in HSV encephalitis: Normocellular CSF, unremarkable CCT, and atypical MRI findings. Infect. 2019;47(2):267-273. doi: 10.1007/s15010-018-1257-7 [DOI] [PubMed] [Google Scholar]
- 10.Granerod J, Davies NWS, Mukonoweshuro W, et al. Neuroimaging in encephalitis: analysis of imaging findings and interobserver agreement. Clin Radiology. 2016;71(10):1050-1058. doi: 10.1016/j.crad.2016.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan IL, McArthur JC, Venkatesan A, Nath A. Atypical manifestations and poor outcome of herpes simplex encephalitis in the immunocompromised. Neurol. 2012;79(21):2125-2132. doi: 10.1212/WNL.0b013e3182752ceb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai C-W, Gragasin ME. Electroencephalography in herpes simplex encephalitis. J Clin Neurophysiol. 1988;5(1):87-104. doi: 10.1097/00004691-198801000-00003 [DOI] [PubMed] [Google Scholar]
- 13.Hughes PS, Jackson AC. Delays in initiation of acyclovir therapy in herpes simplex encephalitis. Can J Neurol Sci. 2012;39(5):644-648. doi: 10.1017/s0317167100015390 [DOI] [PubMed] [Google Scholar]
- Mondal D. Acyclovir. Reference Module in Biomedical Sciences; 2016. [Google Scholar]
- 15.Armangue T, Spatola M, Vlagea A, et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: A prospective observational study and retrospective analysis. Lancet Neurol. 2018;17(9):760-772. doi: 10.1016/s1474-4422(18)30244-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aurelius E, Franzen-Rohl E, Glimaker M, et al. Long-term valacyclovir suppressive treatment after herpes simplex virus type 2 meningitis: A double-blind, randomized controlled trial. Clin Infect Dis. 2012;54(9):1304-1313. doi: 10.1093/cid/cis031 [DOI] [PubMed] [Google Scholar]
- 17.Landry ML, Greenwold J, Vikram HR. Herpes simplex type-2 meningitis: Presentation and lack of standardized therapy. Am J Med. 2009;122(7):688-691. doi: 10.1016/j.amjmed.2009.02.017 [DOI] [PubMed] [Google Scholar]
- 18.Miller S, Mateen FJ, Aksamit AJ., Jr. Herpes simplex virus 2 meningitis: A retrospective cohort study. J NeuroVirol. 2013;19(2):166-171. doi: 10.1007/s13365-013-0158-x [DOI] [PubMed] [Google Scholar]
- 19.Tedder DG, Ashley R, Tyler KL, Levin MJ. Herpes simplex virus infection as a cause of benign recurrent lymphocytic meningitis. Annals of Internal Medicine. 1994;121(5):334-338. doi: 10.7326/0003-4819-121-5-199409010-00004 [DOI] [PubMed] [Google Scholar]
- 20.Shalabi M, Whitley RJ. Recurrent benign lymphocytic meningitis. Clin Infect Dis. 2006;43(9):1194-1197. doi: 10.1086/508281 [DOI] [PubMed] [Google Scholar]
- 21.Berger JR, Houff S. Neurological complications of herpes simplex virus type 2 infection. Archives Neurol. 2008;65(5):596-600. doi: 10.1001/archneur.65.5.596 [DOI] [PubMed] [Google Scholar]
- 22.Franzen-Rohl E, Tiveljung-Lindell A, Grillner L, Aurelius E. Increased detection rate in diagnosis of herpes simplex virus type 2 meningitis by real-time PCR using cerebrospinal fluid samples. J Clin Microbiol. 2007;45(8):2516-2520. doi: 10.1128/JCM.00141-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagot N, Ouédraogo A, Foulongne V, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med. 2007;356(8):790-799. doi: 10.1056/NEJMoa062607 [DOI] [PubMed] [Google Scholar]
- 24.Hait AS, Thomsen MM, Larsen SM, et al. Whole-exome sequencing of patients with recurrent HSV-2 lymphocytic mollaret meningitis. J Infect Dis. 2020;223:1776-1786. doi: 10.1093/infdis/jiaa589 [DOI] [PubMed] [Google Scholar]
- 25.Goyal T, Ali I. Recurrent herpes simplex virus 2 lymphocytic meningitis in patient with IgG subclass 2 deficiency. Emerg Infect Dis. 2020;26(4):748-750. doi: 10.3201/eid2604.190406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilden D, Nagel MA, Cohrs RJ. Varicella-zoster. Neurovirology. 2014;123:265-283. doi: 10.1016/b978-0-444-53488-0.00012-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilden D, Nagel MA, Cohrs RJ, Mahalingam R. The variegate neurological manifestations of varicella zoster virus infection. Curr Neurol Neurosci Rep. 2013;13(9):374. doi: 10.1007/s11910-013-0374-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis GW. Zoster sine herpete. Bmj. 1958;2(5093):418-421. doi: 10.1136/bmj.2.5093.418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baldwin KJ, Cummings CL. Herpesvirus infections of the nervous system. Continuum (Minneap Minn). 2018;24(5):1349-1369. doi: 10.1212/CON.0000000000000661 [DOI] [PubMed] [Google Scholar]
- 30.Nagel MA., Forghani B, Mahalingam R, et al. The value of detecting anti-VZV IgG antibody in CSF to diagnose VZV vasculopathy. Neurology. 2007;68(13):1069-1073. doi: 10.1212/01.wnl.0000258549.13334.16 [DOI] [PubMed] [Google Scholar]
- 31.Reiber H, Lange P. Quantification of virus-specific antibodies in cerebrospinal fluid and serum: Sensitive and specific detection of antibody synthesis in brain. Clinical Chemistry. 1991;37(7):1153-1160. [PubMed] [Google Scholar]
- 32.Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. Varicella zoster virus vasculopathies: Diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol. 2009;8(8):731-740. doi: 10.1016/S1474-4422(09)70134-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng H, Chen D, Peng X, Wu P, Jiang L, Hu Y. Clinical characteristics of Epstein-Barr virus infection in the pediatric nervous system. BMC Infect Dis. 2020;2520(1):886. doi: 10.1186/s12879-020-05623-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kennedy PGE. An overview of viral infections of the nervous system in the immunosuppressed. Journal of Neurology. 2020;268:3026-3030. doi: 10.1007/s00415-020-10265-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.CDC . Epstein-Barr Virus and Infectious Mononucleosis. Centers for Disease Control and Prevention; 2020. September https://www.cdc.gov/epstein-barr/hcp.html. Updated. [Google Scholar]
- 36.Bjornevik K, Cortese M, Healy BC, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 2022;375(6578):296-301. doi: 10.1126/science.abj8222 [DOI] [PubMed] [Google Scholar]
- 37.De Paschale M, Clerici P. Serological diagnosis of Epstein-Barr virus infection: Problems and solutions. World Journal of Virology. 2012;1(1):31-43. doi: 10.5501/wjv.v1.i1.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo Y, Wang S, Jiang B, et al. Encephalitis with reversible splenial and deep cerebral white matter lesions associated with Epstein–Barr virus infection in adults. Neuropsychiatric Disease and Treatment. 2017;13:2085-2092. doi: 10.2147/NDT.S135510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cinque P, Brytting M, Vago L, et al. Epstein-Barr virus DNA in cerebrospinal fluid from patients with AIDS-related primary lymphoma of the central nervous system. Lancet (London, England). 1993;342(8868):398-401. doi: 10.1016/0140-6736(93)92814-a [DOI] [PubMed] [Google Scholar]
- 40.Weinberg A, Li S, Palmer M, Tyler KL. Quantitative CSF PCR in Epstein-Barr virus infections of the central nervous system. Annals of Neurology. 2002;52(5):543-548. doi: 10.1002/ana.10321 [DOI] [PubMed] [Google Scholar]
- 41.Weinberg A, Bloch KC, Li S, Tang YW, Palmer M, Tyler KL. Dual infections of the central nervous system with epstein‐barr virus. J Infect Dis. 2005;191(2):234-237. doi: 10.1086/426402 [DOI] [PubMed] [Google Scholar]
- 42.Liu Q-F, Ling Y-W, Fan Z-P, et al. Epstein-Barr virus (EBV) load in cerebrospinal fluid and peripheral blood of patients with EBV-associated central nervous system diseases after allogeneic hematopoietic stem cell transplantation. Transplant Infect Dis. 2013;15(4):379-392. doi: 10.1111/tid.12090 [DOI] [PubMed] [Google Scholar]
- 43.Yager JE, Magaret AS, Kuntz SR, et al. Valganciclovir for the suppression of epstein-barr virus replication. The J Infect Dis. 2017;216(2):198-202. doi: 10.1093/infdis/jix263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pagano J, Whitehurst C, Andrei G. Antiviral drugs for EBV. Cancers. 2018;10(6):197.doi: 10.3390/cancers10060197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wagner JN, Leibetseder A, Troescher A, Panholzer J, von Oertzen TJ. Efficacy and safety of intravenous immunoglobulins for the treatment of viral encephalitis: a systematic literature review. J Neurol. 2022;269(2):712-724. doi: 10.1007/s00415-021-10494-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Medical Virology. 2010;20(4):202-213. doi: 10.1002/rmv.655 [DOI] [PubMed] [Google Scholar]
- 47.Gaur P, Ffrench-Constant S, Kachramanoglou C, Lyall H, Jan W. Is it not time for international guidelines to combat congenital cytomegalovirus infection? A review of central nervous system manifestations. Clinical Radiology. 2020;75(8):e7-644. doi: 10.1016/j.crad.2020.02.009 [DOI] [PubMed] [Google Scholar]
- 48.Ceballos ME, Rodriguez I, Sandoval P, Abbot E, Labarca J. Cytomegalovirus encephalitis in the post-HAART era. AIDS. 2018;32(4):533-535. doi: 10.1097/QAD.0000000000001732 [DOI] [PubMed] [Google Scholar]
- 49.Griffiths P. Cytomegalovirus infection of the central nervous system. Herpes. 2004;11(suppl 2):95A-104A. [PubMed] [Google Scholar]
- 50.Jabs DA, Belfort R, Jr., Bodaghi B, et al. Classification criteria for cytomegalovirus retinitis. Am J Ophthalmol. 2021;228:245-254. doi: 10.1016/j.ajo.2021.03.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palma P, Costa A, Duro R, et al. Mononeuritis multiplex: An uncommon neurological manifestation of cytomegalovirus reactivation in an HIV-infected patient. BMC Infectious Diseases. 2018;1218(1):554. doi: 10.1186/s12879-018-3501-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Renard T, Daumas-Duport B, Auffray-Calvier E, Bourcier R, Desal H. Cytomegalovirus encephalitis: Undescribed diffusion-weighted imaging characteristics. Original aspects of cases extracted from a retrospective study, and from literature review. J Neuroradiol. 2016;43(6):371-377. doi: 10.1016/j.neurad.2016.03.004 [DOI] [PubMed] [Google Scholar]
- 53.Cinque P, Cleator GM, Weber T, et al. Diagnosis and clinical management of neurological disorders caused by Cytomegalovirus in aids patients. J Neurovirol. 1998;4(1):120-132. doi: 10.3109/13550289809113490 [DOI] [PubMed] [Google Scholar]
- 54.Zerr DM, Meier AS, Selke SS, et al. A population-based study of primary human herpesvirus 6 infection. N Engl J Med. 2005;352(8):768-776. doi: 10.1056/NEJMoa042207 [DOI] [PubMed] [Google Scholar]
- 55.Le Guennec L, Mokhtari K, Chauvet D, et al. Human Herpesvirus 6 (HHV-6) necrotizing encephalitis, a rare condition in immunocompromised patients: The importance of brain biopsy associated with HHV-6 testing. J Neurol Sci. 2017;377:112-115. doi: 10.1016/j.jns.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 56.Seeley WW, Marty FM, Holmes TM, et al. Post-transplant acute limbic encephalitis: Clinical features and relationship to HHV6. Neurol. 2007;69(2):156-165. doi: 10.1212/01.wnl.0000265591.10200.d7 [DOI] [PubMed] [Google Scholar]
- 57.Ward KN, Leong HN, Thiruchelvam AD, Atkinson CE, Clark DA. Human herpesvirus 6 DNA levels in cerebrospinal fluid due to primary infection differ from those due to chromosomal viral integration and have implications for diagnosis of encephalitis. J Clin Microbiol. 2007;45(4):1298-1304. doi: 10.1128/JCM.02115-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berzero G., Campanini G., Vegezzi E, et al. Human Herpesvirus 6 Encephalitis in immunocompetent and immunocompromised hosts. Neurol Neuroimmunol Neuroinflamm. 2021;8(2):e942. doi: 10.1212/NXI.0000000000000942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ward KN, Hill JA, Hubacek P, et al. Guidelines from the 2017 European conference on infections in leukaemia for management of HHV-6 infection in patients with hematologic malignancies and after hematopoietic stem cell transplantation. Haematologica. 2019;104(11):2155-2163. doi: 10.3324/haematol.2019.223073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petersen LR, Brault AC, Nasci RS. West Nile virus: Review of the literature. JAMA. 2013;310(3):308-315. doi: 10.1001/jama.2013.8042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sejvar JJ. West nile virus: An historical overview. Ochsner J. 2003;5(3):6-10. [PMC free article] [PubMed] [Google Scholar]
- 62.Davis LE, DeBiasi R, Goade DE, et al. West Nile virus neuroinvasive disease. Annals Neurol. 2006;60(3):286-300. doi: 10.1002/ana.20959 [DOI] [PubMed] [Google Scholar]
- 63.Lenka A, Kamat A, Mittal SO. Spectrum of movement disorders in patients with neuroinvasive west nile virus infection. Mov Disord Clin Pract. 2019;6(6):426-433. doi: 10.1002/mdc3.12806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu A, Ferenczi E, Moussa K, Eliott D, Matiello M. Clinical spectrum of west nile virus neuroinvasive disease. Neurohospitalist. 2020;10(1):43-47. doi: 10.1177/1941874419868636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jean CM, Honarmand S, Louie JK, Glaser CA. Risk factors for west nile virus neuroinvasive disease, California, 2005. Em Infect Dis. 2007;13(12):1918-1920. doi: 10.3201/eid1312.061265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vanichanan J, Salazar L, Wootton SH, et al. Use of testing for west nile virus and other arboviruses. Em Infect Dis. 2016;22(9). doi: 10.3201/eid2209.152050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Owens M, Choe L, Rivera JE, Avila JD. West Nile virus neuroinvasive disease associated with rituximab therapy. J NeuroVirol. 2020;26(4):611-614. doi: 10.1007/s13365-020-00854-z [DOI] [PubMed] [Google Scholar]
- 68.Levi ME, Quan D, Ho JT, Kleinschmidt-Demasters BK, Tyler KL, Grazia TJ. Impact of rituximab-associated B-cell defects on West Nile virus meningoencephalitis in solid organ transplant recipients. Clin Transplantation. 2010;24(2):223-228. doi: 10.1111/j.1399-0012.2009.01044.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tardei G, Ruta S, Chitu V, Rossi C, Tsai TF, Cernescu C. Evaluation of immunoglobulin M (IgM) and IgG enzyme immunoassays in serologic diagnosis of West Nile Virus infection. J Clin Microbiol. 2000;38(6):2232-2239. doi: 10.1128/JCM.38.6.2232-2239.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tyler KL. Current developments in understanding of West Nile virus central nervous system disease. Curr Opin Neurol. 2014;27(3):342-348. doi: 10.1097/WCO.0000000000000088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kapoor H, Signs K, Somsel P, Downes FP, Clark PA, Massey JP. Persistence of West Nile Virus (WNV) IgM antibodies in cerebrospinal fluid from patients with CNS disease. J Clin Virol. 2004;31(4):289-291. doi: 10.1016/j.jcv.2004.05.017 [DOI] [PubMed] [Google Scholar]
- 72.Tyler KL, Pape J, Goody RJ, Corkill M, Kleinschmidt-DeMasters BK. CSF findings in 250 patients with serologically confirmed West Nile virus meningitis and encephalitis. Neurol. 2006;66(3):361-365. doi: 10.1212/01.wnl.0000195890.70898.1f [DOI] [PubMed] [Google Scholar]
- 73.Ali M, Safriel Y, Sohi J, Llave A, Weathers S. West Nile virus infection: MR imaging findings in the nervous system. AJNR Am J Neuroradiol. 2005;26(2):289-297. [PMC free article] [PubMed] [Google Scholar]
- 74.Gnann JW, Jr., Agrawal A, Hart J, et al. Lack of efficacy of high-titered immunoglobulin in patients with west nile virus central nervous system disease. Em Infect Dis. 2019;25(11):2064-2073. doi: 10.3201/eid2511.190537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hughes JM, Wilson ME, Sejvar JJ. The long-term outcomes of human West Nile virus infection. Clin Infect Dis. 2007;44(12):1617-1624. doi: 10.1086/518281 [DOI] [PubMed] [Google Scholar]
- 76.Piantadosi A, Kanjilal S. Diagnostic approach for arboviral infections in the United States. J Clin Microbiol. 2020;58(12). doi: 10.1128/JCM.01926-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schultz JS, Sparks H, Beckham JD. Arboviral central nervous system infections. Curr Opin Neurol. 2021;34(3):264-271. doi: 10.1097/QCO.0000000000000729 [DOI] [PubMed] [Google Scholar]
- 78.Chen B-S, Lee H-C, Lee K-M, Gong Y-N, Shih S-R. Enterovirus and Encephalitis. Front Microbiol. 2020;11:261. doi: 10.3389/fmicb.2020.00261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dunn JJ. Enteroviruses and parechoviruses. Microbiol Spec. 2016;4(3). doi: 10.1128/microbiolspec.DMIH2-0006-2015 [DOI] [PubMed] [Google Scholar]
- 80.Ayscue P, Van Haren K, Sheriff H, et al. Acute flaccid paralysis with anterior myelitis - California, June 2012-June 2014. MMWR. 2014;63(40):903-906. [PMC free article] [PubMed] [Google Scholar]
- 81.Murphy OC, Messacar K, Benson L, et al. Acute flaccid myelitis: Cause, diagnosis, and management. Lancet. 2021;397(10271):334-346.doi: 10.1016/S0140-6736(20)32723-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pastula DM, Aliabadi N, Haynes AK, et al. Acute neurologic illness of unknown etiology in children - Colorado, August-September 2014. MMWR. 2014;63(40):901-902. [PMC free article] [PubMed] [Google Scholar]
- 83.Huang H-I, Shih S-R. Neurotropic enterovirus infections in the central nervous system. Viruses. 2015;247(11):6051-6066. doi: 10.3390/v7112920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Valcour V, Haman A, Cornes S, et al. A case of enteroviral meningoencephalitis presenting as rapidly progressive dementia. Nat Clin Pract Neurol. 2008;4(7):399-403. doi: 10.1038/ncpneuro0804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tellez R, Lastinger AM, Hogg JP. Chronic enteroviral meningoencephalitis in a patient on rituximab for the treatment of psoriatic arthritis: A case report and brief literature review. IDCases. 2019;17:e00558. doi: 10.1016/j.idcr.2019.e00558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bitnun A, Yeh EA. Acute flaccid paralysis and enteroviral infections. Curr Opin Neurol. 2018;20(9):34. doi: 10.1007/s11908-018-0641-x [DOI] [PubMed] [Google Scholar]
- 87.Sejvar JJ, Lopez AS, Cortese MM, et al. Acute flaccid myelitis in the United States, August-December 2014: Results of Nationwide Surveillance. Clin Infect Dis. 2016;63(6):737-745. doi: 10.1093/cid/ciw372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wagner JN, Leibetseder A, Troescher A, Panholzer J, von Oertzen TJ. Characteristics and therapy of enteroviral encephalitis: Case report and systematic literature review. Intern J Infect Dis. 2021;113:93-102. doi: 10.1016/j.ijid.2021.10.002 [DOI] [PubMed] [Google Scholar]
- 89.Brew BJ, Garber JY. Neurologic sequelae of primary HIV infection. Handb Clin Neurol. 2018;152:65-74. doi: 10.1016/B978-0-444-63849-6.00006-2 [DOI] [PubMed] [Google Scholar]
- 90.Hellmuth J, Fletcher JLK, Valcour V, et al. Neurologic signs and symptoms frequently manifest in acute HIV infection. Neurol. 2016;87(2):148-154. doi: 10.1212/WNL.0000000000002837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tambussi G, Gori A, Capiluppi B, et al. Neurological symptoms during primary human immunodeficiency virus (HIV) infection correlate with high levels of HIV RNA in cerebrospinal fluid. Clin Infect Dis. 2000;30(6):962-965. doi: 10.1086/313810 [DOI] [PubMed] [Google Scholar]
- 92.Valcour V, Chalermchai T, Sailasuta N, et al. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis. 2012;206(2):275-282. doi: 10.1093/infdis/jis326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wendel KA, McArthur JC. Acute meningoencephalitis in chronic human immunodeficiency virus (HIV) infection: putative central nervous system escape of HIV replication. Clin Infect Dis. 2003;37(8):1107-1111. doi: 10.1086/378300 [DOI] [PubMed] [Google Scholar]
- 94.Lescure F-X, Moulignier A, Savatovsky J, et al. CD8 encephalitis in HIV-infected patients receiving cART: A treatable entity. Clin Infect Dis. 2013;57(1):101-108. doi: 10.1093/cid/cit175 [DOI] [PubMed] [Google Scholar]
- 95.Lucas SB, Wong KT, Nightingale S, Miller RF. HIV-Associated CD8 Encephalitis: A UK Case Series and Review of Histopathologically Confirmed Cases. Frontiers Neurol. 2021;12:628296. doi: 10.3389/fneur.2021.628296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fooks AR, Cliquet F, Finke S, et al. Rabies. Nature Reviews Disease Primers. 2017;3:17091. doi: 10.1038/nrdp.2017.91 [DOI] [PubMed] [Google Scholar]
- 97.Prevention . Recovery of a patient from clinical rabies-Wisconsin, 2004. MMWR. 2004;53(50):1171-1173. [PubMed] [Google Scholar]
- 98.Measles (Rubeola CDC) . 2020. https://www.cdc.gov/measles/hcp/index.html.
- 99.Buchanan R, Bonthius DJ. Measles virus and associated central nervous system sequelae. Seminars Pediatric Neurol. 2012;19(3):107-114. doi: 10.1016/j.spen.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 100.Venkatesan A, Murphy OC. Viral encephalitis. Neurolo Clin. 2018;36(4):705-724. doi: 10.1016/j.ncl.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 101.Patterson MC. Neurological complications of measles (rubeola). Curr Opin Neurol. 2020;20(2):2. doi: 10.1007/s11910-020-1023-y [DOI] [PubMed] [Google Scholar]
- 102.CDC . Mumps. 2021, https://www.cdc.gov/mumps/hcp.html. [Google Scholar]
- 103.CDC . Rubella (German Measles, Three-Day Measles). 2020, https://www.cdc.gov/rubella/hcp.html. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material - Neurology of Acute Viral Infections by Jonathan D Krett, J David Beckham, Kenneth L Tyler, Amanda L Piquet, Lakshmi Chauhan, Carla J Wallace, Daniel M Pastula, and Ronak K Kapadia in The Neurohospitalist
Supplemental Material - Neurology of Acute Viral Infections by Jonathan D Krett, J David Beckham, Kenneth L Tyler, Amanda L Piquet, Lakshmi Chauhan, Carla J Wallace, Daniel M Pastula, and Ronak K Kapadia in The Neurohospitalist