Abstract
In this case series, we describe a novel observation in which 4 patients with acute ischemic stroke secondary to large vessel occlusion and no history of seizure present with focal seizure activity localizable to a chronic, contralateral infarct. The explanation for this phenomenon is unknown but may be due to a combination of effects involving disrupted interhemispheric inhibitory connections and epileptogenic changes involving chronically infarcted tissue.
Keywords: acute ischemic stroke, post-stoke seizure, interhemispheric inhibition
Introduction
Seizures are a known complication of acute ischemic stroke (AIS) and have been estimated to occur in nearly 9% of patients with cerebral infarction. 1 Post-stroke seizures are classified according to timing of onset. Early-onset seizures occur within 7 days of AIS 2 ; often this is within the first 24 hours. 1 Late-onset seizures occur after 1 week from AIS and carry a higher risk of developing post-stroke epilepsy.2-4 Focal seizures represent between 50-90% of early post-stroke seizures and typically localize to the ischemic hemisphere.3,4 Here, we describe 4 patients with AIS secondary to large vessel occlusion (LVO) and no history of seizure who presented with focal seizure activity localizable to a chronic, contralateral infarct.
CASE Descriptions
Case 1
A 54-year-old male with aortic valve replacement on warfarin, hyperlipidemia, and history of right middle cerebral artery (MCA) infarct treated with mechanical thrombectomy (MT) 2 months prior was taken to a local hospital for acute unresponsiveness. There, he was witnessed to have a fixed leftward gaze, left upper extremity flexion with left lower limb focal motor activity, and mild right hemiparesis. The patient was transferred to our institution and was found to have right-sided hemiplegia on arrival. CT angiography (CTA) head and neck demonstrated a left M1 occlusion. He underwent successful MT but developed hemorrhagic transformation of infarct (Figure 1). Hospitalization was further complicated by myocardial infarction. The patient’s family elected to pursue hospice; he expired on hospital day 16.
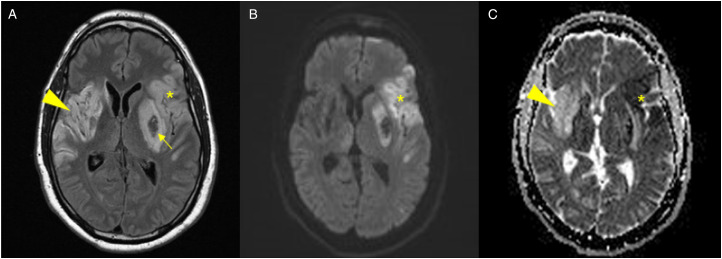
Figure 1.
Head imaging from Case 1. (A) Axial fluid inversion recovery MRI with (B) diffusion weighted imaging and (C) apparent diffusion coefficient images showing the chronic, evolving right MCA infarct from 2 months prior as demonstrated by gliotic changes on T2 FLAIR (arrowhead, A) and T2 shine-through effect on ADC (arrowhead, C) as well as the acute ischemic changes involving the left MCA territory (star, A-C) with area of hemorrhagic transformation (arrow, A).
Case 2
A 58-year-old female with end-stage renal disease, type II diabetes, hypertension, and a left posterior cerebral artery (PCA) infarct 5 months prior was evaluated at an outside ED for acute right hemiparesis and aphasia. CTA showed a left M1 occlusion. She was treated with IV alteplase 2.5 hours after symptom onset and was transferred to our institution where she underwent successful MT. Follow up imaging demonstrated a left basal ganglia hemorrhage. On hospital day 14, she had a focal seizure consisting of forced right gaze deviation and rhythmic movements of the right hemibody, which was refractory to benzodiazepines and evolved into focal status epilepticus. Levetiracetam and valproic acid were initiated, and she was transferred to the ICU. Head CT following seizure onset showed the evolving left MCA infarction with hemorrhagic conversion (Figure 2C). Continuous EEG monitoring showed asymmetric burst-suppression pattern with maximum slowing over the left temporal region, but no definitive epileptiform discharges or seizures. Approximately 12 hours following ICU transfer, the patient demonstrated new left-sided hemiparesis. Repeat head CT showed a large right MCA distribution infarction (Figure 2D). After extensive work up, etiology of recurrent infarcts remained indeterminate. She was transitioned to comfort measures and died hospital day 21.
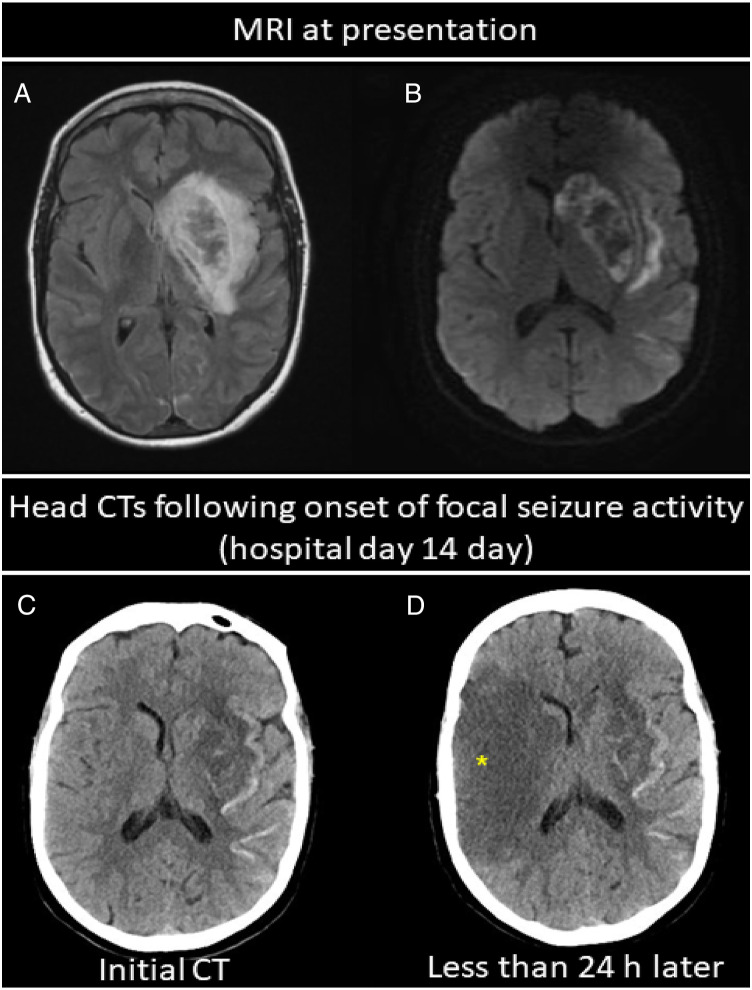
Figure 2.
Head imaging from Case 2 showing evolving bilateral infarcts. (A) Axial fluid inversion recovery imaging and (B) diffusion weighted imaging showing left MCA infarction with hemorrhagic conversion involving the left basal ganglia. (C) Head CT in the same patient 14 days later obtained following the onset of right-sided focal seizure activity showing evolving left MCA infarct (but no right hemispheric changes) with areas of associated hemorrhagic transformation involving the basal ganglia and along the cortex. (D) Repeat head CT less than 24 hours later now showing new area of hypoattenuation in the right MCA distribution consistent with acute infarction (star, D).
Case 3
An 84-year-old female with hypertension, hyperlipidemia, history of nicotine use disorder and left MCA infarct 7 years prior with residual right hemiparesis and aphasia was brought to the ED due to worsened baseline aphasia. The patient was antigravity in all limbs but demonstrated severe, global aphasia. Initial head CT showed no acute findings. CTA showed a right M2 occlusion, which was felt to be incidental given lack of corresponding findings on physical exam performed by a neurologist. While in the ED, the patient became unresponsive and developed right gaze deviation with right upper extremity tonic flexion, which resolved with administration of lorazepam. A few hours later, she had recurrent right-sided focal seizure activity. Levetiracetam and valproic acid were loaded, and maintenance levetiracetam was continued. Approximately 9 hours after presentation, new left facial weakness was observed. Head CT demonstrated an evolving infarct in the right temporal and insular region. The patient became more obtunded. Continuous EEG monitoring displayed generalized slowing and background suppression but no epileptiform discharges or seizures. Family elected to transition to hospice, and the patient died on hospital day 7.
Case 4
An 82-year-old male with hypertension, hyperlipidemia, nicotine use disorder and a right MCA stroke treated with IV thrombolysis and MT 3 years prior with residual left-sided deficits was taken to an outside hospital after collapsing at home. Family observed possible right-sided weakness initially, but he quickly deteriorated and became unresponsive. The ED provider was concerned for seizure and documented left upper extremity “tremor-like movements” with a leftward gaze. The patient required intubation and was transferred to our institution. Head CT and CTA showed left insula and lentiform nucleus hypodensities with a left M1 occlusion. He received IV alteplase 4 hours after symptom onset and underwent MT. Follow up CT showed a large, left hemispheric hemorrhage with associated mass effect. Patient was transitioned to hospice and died hospital day 6.
A summary of patient demographics and clinical presentations is shown in Table 1.
Table 1:
Patient Demographics and Summary of Clinical Presentations
| Case No. | Age (y) | Sex | PMH | Prior Seizure | Subacute or chronic infarct location, timing from acute infarct | Acute infarct location | Description of focal seizure activity | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 54 | M | AVR, HLD | No | Right MCA, 2 mo | Left MCA, M1 occlusion | Left gaze, rhythmic movement left hemibody | MT | Deceased, HD 16 |
| 2 | 58 | F | ESRD, DMII, HTN | No | 1: Left PCA, 5 mo | Right MCA | Right gaze, rhythmic movement right hemibody | tPA, MT a | Deceased, HD 21 |
| 2: Left MCA, M1 occlusion, 14 d a | |||||||||
| 3 | 84 | F | HTN, HLD, pND | No | Left MCA, 7 y | Right MCA, M2 occlusion | Right gaze, tonic posturing RUE | N/A | Deceased, HD 7 |
| 4 | 82 | M | HTN, HLD, pND | No | Right MCA, 3 y | Left MCA, M1 occlusion | Left gaze, ‘tremoring’ LUE | tPA, MT | Deceased, HD 6 |
AVR=aortic valve replacement; DMII=type II diabetes; ESRD=end stage renal disease; HD= hospital day; HLD=hyperlipidemia; HTN=hypertension; LUE= left upper extremity; MCA = Middle Cerebral Artery; MT = mechanical thrombectomy; N/A = not applicable; PCA = Posterior Cerebral Artery; PMH= past medical history; pND=prior nicotine dependence; RUE=right upper extremity; tPA = Tissue plasminogen activator
aPatient was treated with tPA and mechanical thrombectomy for the left M1 occlusion (infarct no. 2)
Discussion
To our knowledge, this is the first report describing seizures as a false localizing presentation of AIS. The mechanism for this phenomenon is unclear but may be due to removal of inhibitory connections between cerebral hemispheres in combination with epileptogenic changes involving chronically infarcted tissue. Further retrospective or prospective studies would be required to better understand this phenomenon and the frequency at which it occurs.
None of these patients had previously suffered a post-stroke seizure nor had a documented history of seizure during their lifetime. In all patients, the AIS was secondary to LVO affecting the MCA territory, and the antecedent infarct involved the contralateral MCA distribution. In cases 1-3, the insular cortex was involved. The focal seizure activity was localizable to the previous cortical ischemic infarct in the contralateral hemisphere in each case. In most cases, the culprit infarct occurred between 2 months and 7 years prior to the acute presentation; however, in 1 patient seizure activity was localizable to a subacute infarction occurring just 14 days prior. All 3 patients undergoing MT (2 combined with IV thrombolysis) experienced symptomatic hemorrhagic transformation of infarct. All patients unfortunately had a poor outcome, as could be expected for their bilateral MCA ischemic damage. 5
Several theories have been proposed for the mechanism of seizure during AIS. Metabolic derangements, systemic infection and toxins can play a role, however, these factors were excluded in the cases described. In AIS, altered ionic gradients, disruption of the blood-brain barrier and excitotoxic neurotransmitter release in regions of ischemia have all been postulated to lower the seizure threshold.1,3,6 Seizure occurred prior to reperfusion treatment in 2 out of 3 cases with hemorrhagic transformation, so it is unlikely the hemorrhage played a significant role in seizure development.
Another possible mechanism of seizure relates to disruption of connectivity between cerebral hemispheres. Transcallosal interhemispheric inhibitory connections between motor cortices have been previously described.7,8 Repetitive transcranial magnetic stimulation has been used in stroke patients to reduce transcallosal inhibitory effects from a non-lesioned hemisphere and allowed for paradoxical motor function improvement.9,10 Similarly, ischemia to a previously intact hemisphere may lead to disruption of these inhibitory influences. Release of inhibitory effects and subsequent alterations of cortical excitability in the previously infarcted brain (particularly in those cases with insular involvement) could therefore have played a role in lowering seizure threshold.
A previous study suggested that patients with focal epilepsy had impaired interhemispheric inhibition bilaterally, as compared to patients with generalized epilepsy or healthy subjects. 11 Interestingly, none of our reported patients were observed to have secondary generalized motor seizures. This could also be due to disrupted interhemispheric connections resulting in impaired seizure propagation to the contralateral hemisphere. It is possible that the decreased responsiveness observed in case 1 and 4 may have represented bilateral, subclinical seizure activity, however both patients were observed to have focal motor seizure-activity during time of altered mentation. In cases 3 and 4, EEG was performed for mental status decline and showed no evidence of electrographic seizures.
Some patients (cases 1 and 4) had mild deficits on presentation localizable to the ischemic hemisphere, which became more pronounced on subsequent clinical examinations. Case 3 presented with worsening of baseline aphasia, which localized to a chronic left MCA infarct and likely represented peri-ictal activity. Why all patients did not initially exhibit more profound AIS deficits is unclear. It is possible given the complex presentations, deficits were initially overlooked; however, these patients were each examined by different neurology teams and multiple providers, reducing the likelihood for observer bias.
Alternatively, neurologic deficits attributable to the AIS may have been absent or appeared less severe due the phenomenon of paradoxical functional facilitation. 12 This occurs when there is damage to previously intact brain tissue which results in improvement, or even resolution, of deficits localizing to the contralateral hemisphere.12-14 The mechanism for this was hypothesized to be due to resolution of excessive inhibitory processes, 12 or in other terms, reversal of interhemispheric inhibition.
Conclusion
Early post-stroke seizures are relatively rare in patients with AIS. Here, we demonstrate that patients with acute cerebral ischemia in 1 hemisphere can present with new-onset seizures arising from previous ischemic damage to the contralateral hemisphere. Vessel imaging may be beneficial in settings where clinicians have a high index of suspicion for falsely localizing seizures being a presentation of AIS. The pathophysiology to explain this atypical presentation is not known, but we hypothesize that it is due to a combination of effects involving disrupted interhemispheric inhibitory connections and epileptogenic changes which occur in previously infarcted neural tissue.
Footnotes
Author’s Contribution: EW conceptualized the study, performed data collection and prepared the manuscript and figures. DS, AR, GC all performed critical revisions of the manuscript for intellectual content. DN conceptualized the study, reviewed data and performed critical revisions of the manuscript for intellectual content.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Erika L. Weil https://orcid.org/0000-0002-3753-6293
References
- 1.Bladin CF, Alexandrov AV, Bellavance A, et al. Seizures after stroke: a prospective multicenter study. Arch Neurol. 2000;57(11):1617-1622. doi: 10.1001/archneur.57.11.1617 [DOI] [PubMed] [Google Scholar]
- 2.Beghi E, Carpio A, Forsgren L, et al. Recommendation for a definition of acute symptomatic seizure. Epilepsia. 2010;51(4):671-675. doi: 10.1111/j.1528-1167.2009.02285.x [DOI] [PubMed] [Google Scholar]
- 3.Silverman IE, Restrepo L, Mathews GC. Poststroke seizures. Arch Neurol. 2002;59(2):195-201. doi: 10.1001/archneur.59.2.195 [DOI] [PubMed] [Google Scholar]
- 4.Camilo O, Goldstein LB. Seizures and epilepsy after ischemic stroke. Stroke. 2004;35(7):1769-1775. doi: 10.1161/01.STR.0000130989.17100.96 [DOI] [PubMed] [Google Scholar]
- 5.Singh R-J, Chen S, Ganesh A, et al. Long-term neurological, vascular, and mortality outcomes after stroke. Int J Stroke. 2018;13(8):787-796. doi: 10.1177/1747493018798526 [DOI] [PubMed] [Google Scholar]
- 6.Pitkanen A, Roivainen R, Lukasiuk K. Development of epilepsy after ischaemic stroke. Lancet Neurol. 2016;15(2):185-197. doi: 10.1016/S1474-4422(15)00248-3 [DOI] [PubMed] [Google Scholar]
- 7.Ferbert A, Priori A, Rothwell JC, et al. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453(1):525-546. doi: 10.1113/jphysiol.1992.sp019243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boroojerdi B, Diefenbach K, Ferbert A. Transcallosal inhibition in cortical and subcortical cerebral vascular lesions. J Neurol Sci. 1996;144(1-2):160-170. doi: 10.1016/s0022-510x(96)00222-5 [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi N, Chuma T, Matsuo Y, et al. Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke. 2005;36(12):2681-2686. doi: 10.1161/01.Str.0000189658.51972.34 [DOI] [PubMed] [Google Scholar]
- 10.Kirton A, Chen R, Friefeld S, et al. Contralesional repetitive transcranial magnetic stimulation for chronic hemiparesis in subcortical paediatric stroke: a randomised trial. Lancet Neurol. 2008;7(6):507-513. doi: 10.1016/s1474-4422(08)70096-6 [DOI] [PubMed] [Google Scholar]
- 11.Strigaro G, Matino E, Falletta L, et al. Defective interhemispheric inhibition in drug-treated focal epilepsies. Brain Stimul. 2017;10(3):579-587. doi: 10.1016/j.brs.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 12.Kapur N. Paradoxical functional facilitation in brain-behaviour research. A critical review. Brain. 1996;119(Pt 5):1775-1790. doi: 10.1093/brain/119.5.1775 [DOI] [PubMed] [Google Scholar]
- 13.Vuilleumier P, Hester D, Assal G, et al. Unilateral spatial neglect recovery after sequential strokes. Neurology. 1996;46(1):184-189. [DOI] [PubMed] [Google Scholar]
- 14.Sauerbrei R, Liepert J. Support of the concept of interhemispheric rivalry by two consecutive strokes occurring in both hemispheres: a case study. J Neurol. 2012;259(11):2484-2485. [DOI] [PubMed] [Google Scholar]