Introduction
Atrial standstill (AS) is a rare cardiac syndrome characterized by the absence of electrical and mechanical activity in the atria. Mutations in the sodium channel α-subunit SCN5A gene have been identified as the cause of arrhythmia syndromes, such as long QT syndrome, Brugada syndrome (BrS), progressive cardiac conduction disease, sinus node dysfunction, atrial fibrillation, and AS.1 Historically, these cardiac sodium channelopathies were considered to be purely electrical disorders. However, with the accumulation of cases, SCN5A mutations also came to be associated with structural disorders involving myocardial fibrosis.2, 3, 4 While studies have reported pathologic findings in the atria of patients with AS,5, 6, 7 none have associated these pathologic findings with SCN5A mutations in patients with AS. Herein, we report a pediatric case of AS with atrial fibrosis associated with an SCN5A mutation.
Case report
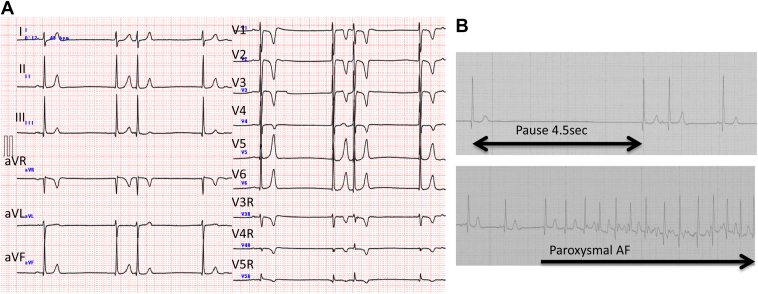
A 4-year-old girl presented to our hospital with severe bradycardia without syncope or dizziness. The patient’s 12-lead electrocardiogram (ECG) revealed irregular bradycardia with a junctional escape rhythm and supraventricular premature contractions without any ST-T wave changes (Figure 1A). Holter monitoring of the patient revealed severe bradycardia with an average heart rate of 40 beats per minute, a pause lasting 4.5 seconds, and paroxysmal atrial fibrillation (Figure 1B). Transthoracic echocardiography was also performed, which showed normal ventricular function (left ventricular ejection fraction of 70%), a slightly dilated left ventricular end-diastolic diameter of 38.6 mm (Z-score, +3.37),8 and mild mitral regurgitation. The brain natriuretic peptide level was 223.0 pg/mL (reference range 0–18.4 pg/mL). The patient had no fever, gastroenteritis, or other symptoms suggestive of myocarditis prior to admission. Furthermore, her history did not suggest Kawasaki disease. She had a negative family history for cardiac disease among her first- and second-degree relatives.
Figure 1.
A: Surface 12-lead electrocardiogram (ECG). Irregular bradycardia is observed with junctional escape beats and supraventricular premature contractions without ST-T wave changes B: Holter ECG. Severe bradycardia with an average heart rate of 40 beats/min, with a pause lasting 4.5 seconds, along with paroxysmal atrial fibrillation.
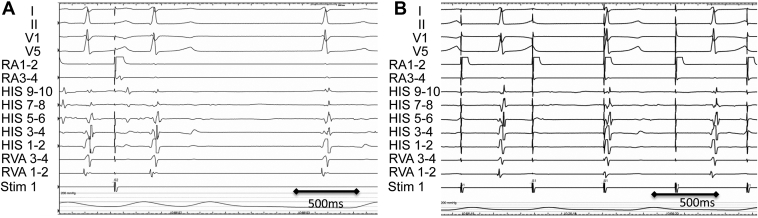
After the informed consent was obtained from the parent, an electrophysiological study was performed under general anesthesia. During this study, no spontaneous atrial activation or atrial contractions were observed. Partial AS was confirmed by the absence of electrical activity on the atrial electrogram under the maximal pacing output at sites other than the interatrial septum or edge of the right atrial appendage (Figure 2). In the right atrial appendage pacing rhythm, the atrial–His bundle interval was 117 ms, and the His bundle–ventricular interval was 47 ms. During this procedure, paroxysmal atrial fibrillation was induced with an injection of isoproterenol (0.1 mcg/kg/min).
Figure 2.
Electrophysiological study before a pacemaker implantation. A: Atrial capture was obtained at the edge of the right atrial appendage. B: Atrial capture was not obtained on the free wall of the right atrium even with pacing at maximum output. HIS = His bundle; RA = right atrium; RVA = right ventricular apex; Stim = stimulus.
We performed a thoracotomy for epicardial pacemaker implantation. A histological specimen from the right atrial appendage was obtained surgically. The atrial capture could be obtained only at the epicardial site of the interatrial groove, which is an extension of the atrial septum on the epicardial side, using steroid-eluting lead with a pacing threshold of 1.75 V / 1.0 ms. At the time of implantation, the pacemaker sensitivity and output were set as 0.18 mV and 6.0 V / 1.0 ms, respectively. After pacemaker implantation, the patient spontaneously developed an atrial flutter, which necessitated treatment with a direct-current shock.
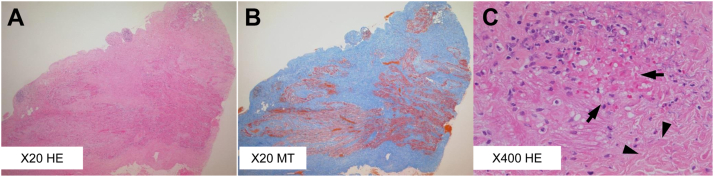
Upon histological examination of the specimen obtained from the right atrial appendage, we observed that the loose connective tissue of the atrial wall was replaced diffusely by dense collagenous tissue with scattered intermingled myocardium. Under higher magnification, these fibrotic changes included different stages of fibrosis—that is, focal infiltration of active inflammatory neutrophils, loss of myocardiocytes, and scar formation with dense collagen deposition, suggesting that the process was active and progressive (Figure 3). Genetic testing using direct Sanger sequencing revealed a missense mutation, S910L (c.2729c>t), located in exon 16 of SCN5A; this is a pathogenic mutation characteristic of the BrS.9 The patient’s mother also underwent genetic testing, but she did not have the SCN5A mutation. ECGs of the patient’s older sister and mother showed no sinus bradycardia or ST-segment changes in the right precordial leads. The patient’s father did not consent for examination.
Figure 3.
Histological sections of the right atrial appendage. A: Hematoxylin-eosin (HE) stain at low magnification. B: Masson’s trichrome (MT) stain at low magnification showing diffuse and extensive myocardial fibrosis. C: HE stain at higher magnification showing capillaries with extravasation of erythrocytes (arrows), active inflammatory infiltrates with some leukocytoclastic changes, and increased deposition of interstitial collagen fiber (arrowheads).
During 7 years of follow-up, pacemaker interrogation revealed multiple episodes of transient atrial pacing failure; no fever, electrolyte abnormalities, or other triggers were noted. Despite the administration of oral flecainide, asymptomatic and transient atrial tachycardias were also observed repeatedly. ECGs showed no progression of conduction disturbances such as atrioventricular block and no change to the Brugada pattern, and echocardiography showed no cardiac enlargement.
Discussion
AS is a disorder characterized by the loss of electrical and mechanical activity in the atria, which has been previously implicated in digitalis toxicity. Since most cases were transient, functional problems were thought to be the main mechanism.10 However, some pathologic studies on AS revealed the presence of atrial fibrosis, which indicates that some types of AS are related to structural changes in the atrial myocardium.5, 6, 7 These observational studies indicate that AS includes heterogeneous atrial myocardial conditions. In a recent consensus report of atrial cardiomyopathies, AS was defined as one of the severe forms of atrial cardiomyopathies.11 Furthermore, genetic investigations in some cases have revealed an SCN5A mutation as the etiology of AS.1 The phenotypes of SCN5A mutations vary widely. Loss-of-function or gain-of-function changes in the channel gating of cardiac sodium channels, such as Nav1.5, cause AS in addition to long QT syndrome, BrS, sick sinus syndrome, atrial fibrillation, progressive cardiac conduction disorders, dilated cardiomyopathy, and arrhythmogenic right ventricular cardiomyopathy.1, 2, 3 The typical phenotype of SNC5A mutations includes abnormalities in the electrical activity of the myocardium, such as arrhythmia, as well as structural abnormalities. In such cases, cardiac fibrosis contributes to the pathogenesis in varying degrees.
Diffuse ventricular myocardial fibrosis in the right ventricular outflow tract was observed in patients with BrS.4,12 The missense mutation S910L was previously reported by Kapplinger and colleagues9 in a case of BrS, which was caused by the loss of function of SCN5A. In a mouse model of progressive cardiac conduction disease, a loss-of-function SCN5A mutation was associated with pronounced myocardial rearrangement (including fibrosis and expression of hypertrophy markers) but a conserved contractile function.13 Although the underlying mechanism between atrial fibrosis and SCN5A mutation remains to be elucidated, we present a case of progressive atrial fibrosis and AS associated with an SCN5A mutation.
Conclusion
To the best of our knowledge, this is the first case report of AS that developed in a child with histologically proven atrial fibrosis in association with an SCN5A mutation. An SCN5A gene mutation might be associated with myocardial fibrosis of the atrial myocardium as well as various arrhythmias, including AS.
Footnotes
Funding Sources: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures: None.
Supplementary data
Key Teaching Points.
-
•
Although atrial standstill (AS) has historically been considered a transient syndrome, certain pathologic studies have revealed that atrial fibrosis occurs with AS. Histopathological examination in our case showed progressive atrial fibrosis.
-
•
SCN5A mutations have been known to manifest as long QT syndrome, Brugada syndrome, sick sinus syndrome, atrial fibrillation, and progressive cardiac conduction disorders. The mutation is also associated with structural disorders such as dilated cardiomyopathy and arrhythmogenic right ventricular cardiomyopathy.
-
•
Ours is the first case report of AS that developed in a child with histologically proven atrial fibrosis in association with an SCN5A mutation. An SCN5A gene mutation might be associated with myocardial fibrosis of the atrial myocardium as well as various arrhythmias, including AS.
References
- 1.Wilde A.A.M., Amin A.S. Clinical spectrum of SCN5A mutations: long QT syndrome, Brugada syndrome, and cardiomyopathy. JACC Clin Electrophysiol. 2018;4:569–579. doi: 10.1016/j.jacep.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 2.McNair W.P., Sinagra G., Taylor M.R., et al. SCN5A mutations associate with arrhythmic dilated cardiomyopathy and commonly localize to the voltage-sensing mechanism. J Am Coll Cardiol. 2011;57:2160–2168. doi: 10.1016/j.jacc.2010.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Te Riele A.S., Agullo-Pascual E., James C.A., et al. Multilevel analyses of SCN5A mutations in arrhythmogenic right ventricular dysplasia/cardiomyopathy suggest non-canonical mechanisms for disease pathogenesis. Cardiovasc Res. 2017;113:102–111. doi: 10.1093/cvr/cvw234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coronel R., Casini S., Koopmann T.T., et al. Right ventricular fibrosis and conduction delay in a patient with clinical signs of Brugada syndrome: a combined electrophysiological, genetic, histopathologic, and computational study. Circulation. 2005;112:2769–2777. doi: 10.1161/CIRCULATIONAHA.105.532614. [DOI] [PubMed] [Google Scholar]
- 5.Allensworth D.C., Rice G.J., Lowe G.W. Persistent atrial standstill in a family with myocardial disease. Am J Med. 1969;47:775–784. doi: 10.1016/0002-9343(69)90170-3. [DOI] [PubMed] [Google Scholar]
- 6.Rosen K.M., Rahimtoola S.H., Gunnar R.M., Lev M. Transient and persistent atrial standstill with His bundle lesions. Electrophysiologic and pathologic correlations. Circulation. 1971;44:220–236. doi: 10.1161/01.cir.44.2.220. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka H., Atsuchi Y., Tanaka N., Nishi S., Kanehisa T. Persistent atrial standstill due to atrial inexcitability. An electrophysiological and histological study. Jpn Heart J. 1975;16:639–653. doi: 10.1536/ihj.16.639. [DOI] [PubMed] [Google Scholar]
- 8.Lopez L., Colan S., Stylianou M., et al. Relationship of echocardiographic Z scores adjusted for body surface area to age, sex, race, and ethnicity: the Pediatric Heart Network Normal Echocardiogram Database. Circ Cardiovasc Imaging. 2017;10 doi: 10.1161/CIRCIMAGING.117.006979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kapplinger J.D., Tester D.J., Alders M., et al. An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm. 2010;7:33–46. doi: 10.1016/j.hrthm.2009.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenbaum F.F., Levine S.A. Auricular standstill: its occurrence and significance. Am J Med Sci. 1939;198:774–778. [Google Scholar]
- 11.Goette A., Kalman J.M., Aguinaga L., et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Heart Rhythm. 2017;14:e3–e40. doi: 10.1016/j.hrthm.2016.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nademanee K., Raju H., de Noronha S.V., et al. Fibrosis, connexin-43, and conduction abnormalities in the Brugada syndrome. J Am Coll Cardiol. 2015;66:1976–1986. doi: 10.1016/j.jacc.2015.08.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Royer A., van Veen T.A., Le Bouter S., et al. Mouse model of SCN5A-linked hereditary Lenègre’s disease: age-related conduction slowing and myocardial fibrosis. Circulation. 2005;111:1738–1746. doi: 10.1161/01.CIR.0000160853.19867.61. [DOI] [PubMed] [Google Scholar]