Abstract
Background
Statins are the mainstay for the treatment of dyslipidemia. Recently, rosuvastatin has also been demonstrated to possess analgesic properties in animal studies. The present study has been planned to further confirm the analgesic activity of rosuvastatin, etoricoxib, tramadol, amlodipine, and amitriptyline and study the interaction of rosuvastatin with the above-mentioned analgesics. The objective of the study was to confirm the analgesic activity of rosuvastatin and determine the minimum analgesic dose of rosuvastatin, etoricoxib, tramadol, amlodipine and amitriptyline and to study the analgesic effect of combination of subanalgesic doses of rosuvastatin with sub-analgesic doses of etoricoxib, tramadol, amlodipine, and amitriptyline.
Method
After IAEC approval, the study was carried out in albino mice in two phases. In phase I, the analgesic effect of rosuvastatin, etoricoxib, tramadol, amlodipine, and amitriptyline was confirmed by using tail-flick and writhing methods. In phase II, analgesic effect of combinations of subanalgesic dose of rosuvastatin with subanalgesic dose of etoricoxib, tramadol, amlodipine, and amitriptyline was studied.
Results
Minimal analgesic dose of rosuvastatin, etoricoxib, tramadol, amlodipine, and amitriptyline was observed as 5, 20, 10, 5, and 10 mg/kg, respectively. In phase II, combination of subanalgesic dose of rosuvastatin 2.5 mg/kg with subanalgesic doses of etoricoxib (10 mg/kg), tramadol (5 mg/kg), amlodipine (2.5 mg/kg), and amitriptyline (5 mg/kg), demonstrated synergistic analgesic activity.
Conclusion
Rosuvastatin exerts dose-dependent analgesic activity that is synergistic to that of etoricoxib, tramadol, amlodipine, and amitriptyline. If established in clinical studies as well, this finding can lead to the reduction of analgesic dosing in patients already on statins.
Keywords: Analgesic, Rosuvastatin, Etoricoxib, Tramadol, Amitriptyline
Introduction
Dyslipidemia is an important risk factor for a number of cardiovascular disorders like coronary artery disease and cerebrovascular disease.1 Recent studies have shown that elevated cholesterol levels are present in 25–30% of urban and 15–20% of rural population.2 HMG - CoA inhibitors (statins) are the mainstay for the treatment of dyslipidemia and are a frequently prescribed class of drugs. Almost 8% of the Indian population is believed to be receiving a defined daily dose of statins.3
India is experiencing a fast socioepidemiological transition with increasing population, economic progress, urbanization, and aging. This transition, coupled with unhealthy lifestyle trends such as greater smoking/tobacco use, changes in nutritional habits with greater intake of unhealthy diets, and sedentary lifestyle, is leading to an increase in the prevalence of hypercholesterolemia and cardiovascular risk.2,3
Patients with dyslipidemia often have various comorbidities like arthritis, neuropathies and suffer from chronic pain.4 Dyslipidemia contributes to the development of neuropathy by inducing oxidative stress in root ganglia sensory neurons.5 Animal studies have shown that high-fat feeding in mice develops neuropathy.6 Oxidized LDLs generated by dyslipidemia are known to contribute to neuronal injury mechanisms.6
The analgesic armamentarium available to us is rather large, with a number of drugs acting by different mechanisms to relieve pain.7 All these analgesic drugs are indicated for different types of pain and have their own set of side effects.7
Etoricoxib acts by selectively inhibiting a cyclooxygenase-2 enzyme, tramadol inhibits the uptake of noradrenaline (NA)8,9 and 5 HT, whereas amitriptyline leads to alteration in presynaptic receptors leading to analgesic action.9 Amlodipine, a voltage-dependent calcium channel blocker that is indicated for the management of hypertension, and heart failure, has also been shown to possess analgesic activity in animal models of pain.9
Recently, rosuvastatin, an HMG-CoA reductase inhibitor, has also been demonstrated to possess analgesic, anti-inflammatory, and antioxidant properties.10,11 The putative mechanism behind its analgesic property includes reduced production of proinflammatory mediators such as that tumor necrosis factor-a (TNF- a), bradykinin, (BK), interleukin-1b (IL-1b). It also inhibits PGE 2 -induced hypernociception by increasing the bioavailability of NO by upregulating Nitrogen Oxide Synthase (NOS).12,13
Rosuvastatin, being a known antioxidant agent, increases antioxidants levels that contribute to the reduction of oxidative stress-induced neuropathies.12,13 Its action on peripheral nociceptors is believed to result in its analgesic, antioxidant, and antiinflammatory properties.12,13 But the number of such studies is limited, and the interaction of rosuvastatin with other analgesics has not been reported. The synergistic/additive analgesic effect of rosuvastatin may be of clinical benefit for patients concurrently suffering from cardiovascular diseases and associated chronic painful conditions. It was found to be supported by evidence that the dose requirement of analgesic drugs would decrease when co-prescribed with rosuvastatin, thereby leading to reduced overall exposure and reduced side effects.
Since dyslipidemia, cardiovascular diseases, and neuropathies are chronic conditions, the reduction of doses in such conditions will lead to reduced adverse effects of analgesics, which will contribute significantly to increased tolerability and better compliance of the therapy. In view of the above, this study was planned to further confirm the analgesic activity of rosuvastatin, etoricoxib, tramadol, amlodipine, and amitriptyline and study the interaction of rosuvastatin with these analgesics.
Materials and methods
Place of study
This study was conducted in the Department of Pharmacology of a medical college in western Maharashtra. As it was a mandatory requirement, approval from the institutional animal ethics committee (IAEC) was obtained prior to the commencement of the study. The study was conducted in accordance with the guidelines given by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA).14
Animals
Adult Swiss albino mice were used in the study as they are the most suitable experimental animals to demonstrate the analgesic action of drugs. Animals included in the study weighed 20–30 gm each. During the study period, all experimental animals were handled and housed as per standards given in CPCSEA guidelines.14
The animals were divided into vehicle-treated (control group) and drug-treated groups, each containing 06 animals. The time between administration of drug/vehicle and demonstration of analgesic activity was 30 min for all groups except amlodipine, in which case it was 6.25 h.
Determination of analgesic activity
There are a number of animal models for testing the analgesic activity of a given drug. They can broadly be divided into reflexive and nonreflexive tests. Thermal (hot plate test, tail-flick test) and mechanical pain models (von Frey filament, Randall stiletto test) are examples of reflexive pain tests. On the other hand, writhing test, thermal escape test, and conditioned place avoidance are examples of nonreflexive tests. The following two well-validated tests were used to measure the analgesic activity in our study.
-
1.
Tail-flick test: Analgesic activity was measured by a standard analgesiometer by using the tail-flick method. Heated nichrome wire was used to inflict radiant heat on the tail of the animal, and time taken by the animal to flick its tail (tail-flick latency) was used as a surrogate for pain perception.15 Prior to drug administration, tail-flick latency was obtained thrice, and the mean value was taken as predrug latency. The postdrug/vehicle tail-flick latencies were measured at five different time points (0.30, 1, 2, 3, and 4 h) after administration of vehicle/drug(s), except for amlodipine, wherein tail-flick latency was measured up to 6 h. This was in accordance with anticipated duration of analgesic effect. A cutoff time of 10 s was selected to prevent any harm to the animal. For animals that did not respond within the cutoff time of 10 s, the value of this cut-off time (i.e. 10 sec) was considered as latency period for that animal.16, 17, 18
-
2.
Writhing test: Writhing is the typical stretching movement of the abdominal muscles of the animal after its peritoneal cavity is injected with an irritant. The number of writhes in a particular time frame is the marker for pain perceived by the animal. In our study, writhing was produced by intraperitoneal administration of 0.1 ml of 0.06% acetic acid. The stretching of abdomen with simultaneous stretching of at least one hind limb was counted as one writhe in our study. The writhes were counted 5 min after injection of the irritant and continued for next 20 min. The writhing test was again performed after the administration of the drug/vehicle.17, 18, 19, 20
Results
Analgesic activity of individual test drugs (Phase I)
-
(a)
Rosuvastatin
With the tail-flick test, rosuvastatin produced statistically significant, dose-related analgesic activity at 5 and 10 mg/kg dose levels. It showed no analgesic activity at 2.5 mg/kg dose. Analgesic effect started at 0.5 h, was maximum at 1 h, and disappeared at the end of 4 h.
-
(b)
Tramadol
With the tail-flick test, tramadol produced statistically significant, dose-related analgesic activity with 10 and 20 mg/kg. It showed no analgesic activity at 5 mg/kg dose. Analgesic effect started at 1 h, was maximum at 2 h, and disappeared at the end of 3 h (Table 1 and Fig. 1).
-
(c)
Amlodipine
Table 1.
Effects of different doses of rosuvastatin (Ros), tramadol (Tra), etoricoxib (Eto), amlodipine (Aml), and amitriptyline (Ami) on tail-flick latency in mice in phase I.
| 0 h |
0.5 h |
1 h |
2 h |
3 h |
4 h |
5 h |
6 h |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Eto | 10 mg | 3.17 | 0.408 | 3.67 | 0.816 | 3.83 | 0.753 | 3.67 | 0.516 | 3.33 | 0.516 | 3.17 | 0.753 | 3 | 0.632 | – | – |
| 20 mg | 3.33 | 0.516 | 3.67 | 0.516 | 5.83 | 0.753 | 5.33 | 0.516 | 4.17 | 0.753 | 3.83 | 0.753 | 3.33 | 0.516 | – | – | |
| 40 mg | 3.33 | 0.516 | 3.67 | 1.033 | 7.17 | 1.169 | 6.33 | 0.516 | 4.33 | 0.816 | 3.67 | 0.516 | 3.33 | 0.516 | – | – | |
| p-value | 0.791 | 0.999 | <0.001 | <0.001 | 0.059 | 0.245 | 0.505 | – | |||||||||
| Aml | 2.5 mg | 3.17 | 0.753 | 3.33 | 0.516 | 3.5 | 0.548 | 3.83 | 0.753 | 3.67 | 0.516 | 3.5 | 0.548 | 3.33 | 0.516 | 3.17 | 0.753 |
| 5 mg | 3.33 | 0.516 | 3.67 | 0.516 | 3.83 | 0.408 | 4.17 | 0.408 | 7.67 | 0.516 | 5.67 | 0.516 | 4.17 | 0.753 | 3.17 | 0.753 | |
| 10 mg | 3.33 | 0.516 | 3.5 | 0.548 | 4.17 | 0.753 | 4.33 | 0.516 | 7.83 | 0.753 | 5.67 | 0.516 | 4.17 | 0.753 | 3.17 | 0.753 | |
| p-value | 0.861 | 0.561 | 0.179 | 0.338 | <0.001∗ | <0.001∗ | 0.082 | 0.999 | |||||||||
| Tra | 5 mg | 3.5 | 0.548 | 3.67 | 0.516 | 3.83 | 0.408 | 3.83 | 0.408 | 3.67 | 0.516 | 3.33 | 0.516 | 3.17 | 0.408 | – | – |
| 10 mg | 3.5 | 0.548 | 3.83 | 0.408 | 4.17 | 0.408 | 6.5 | 0.548 | 4.67 | 0.516 | 4.17 | 0.408 | 3.67 | 0.516 | – | – | |
| 20 mg | 3.33 | 0.516 | 3.83 | 0.408 | 4.17 | 0.408 | 7.5 | 0.548 | 5.17 | 0.408 | 4.17 | 0.753 | 3.67 | 0.516 | – | – | |
| p-value | 0.827 | 0.761 | 0.293 | <0.001 | <0.001 | 0.036 | – | – | |||||||||
| Ami | 5 mg | 3.33 | 0.516 | 3.67 | 0.516 | 3.83 | 0.408 | 4.17 | 0.408 | 3.5 | 0.548 | 3.17 | 0.408 | – | – | – | – |
| 10 mg | 3.33 | 0.516 | 4.17 | 0.408 | 6.83 | 0.753 | 4.83 | 0.753 | 3.67 | 0.516 | 3.17 | 0.408 | – | – | – | – | |
| 20 mg | 3.5 | 0.548 | 5.17 | 0.408 | 8.17 | 0.753 | 5.33 | 0.516 | 3.67 | 0.516 | 3.33 | 0.516 | – | – | – | – | |
| p-value | 0.821 | <0.001 | <0.001 | 0.011 | 0.821 | 0.761 | – | – | |||||||||
| Ros | 2.5 mg | 3.17 | 0.408 | 3.67 | 0.516 | 3.67 | 0.516 | 3.83 | 0.408 | 3.67 | 0.516 | 3.33 | 0.516 | 3.33 | 0.516 | – | – |
| 5 mg | 3.33 | 0.516 | 4.17 | 0.408 | 6.33 | 0.516 | 7.67 | 0.516 | 5.33 | 0.516 | 3.83 | 0.408 | 3.67 | 0.516 | – | – | |
| 10 mg | 3.33 | 0.516 | 3.83 | 0.753 | 4.17 | 0.983 | 8.33 | 0.816 | 5.33 | 0.516 | 3.83 | 0.408 | 3.67 | 0.516 | – | – | |
| p-value | 0.791 | 0.338 | <0.001 | <0.001 | <0.001 | 0.116 | 0.454 | – | |||||||||
Significance of footnote “∗” is represents statitically significant at p < 0.05.
Fig. 1.
Study flow chart.
With the tail-flick test, amlodipine produced statistically significant, dose-related analgesic activity at 5 and 10 mg/kg dose level. Analgesic effect was seen at 3 h, was maximum at 4 h, and disappeared at the end of 6 h (Table 1 and Fig. 1).
-
(d)
Etoricoxib
During the tail-flick test etoricoxib, produced statistically significant, dose-related analgesic activity with 20 and 40 mg/kg doses. Analgesic effect was seen to start at 1 h, was maximum at 2 h, and disappeared at the end of 4 h (Table 1 and Fig. 1).
-
(e)
Amitriptyline
During the tail-flick test, amitriptyline did not produce any analgesic effect with 5 mg/kg dose. The analgesic effect was seen with 10 mg/kg and 20 mg/kg. It started 1.0 h, peak at 2 h, and persisted up to 4 h of observation (Table 1 and Fig. 2).
Fig. 2.
Mean duration (in seconds) of tail-flick latency for drugs etoricoxib, amlodipine, and tramadol in phase I. Paired t-test between latency at 0 h and time of max latency. (p < 0.001). ∗Indicates subanalgesic doses after phase 1.
The subanalgesic dose(s) of rosuvastatin, amitriptyline, tramadol, etoricoxib, and amlodipine was 2.5 mg/kg, 5 mg/kg, 5 mg/kg, 10 mg/kg, and 2.5 mg/kg, respectively.
The analgesic activity was also confirmed with the help of the writhing test, and the analgesic/subanalgesic dose(s) and the duration of analgesic activity, was the same as ascertained by the tail-flick method in respect of all the interventional drugs.
Intra Group Analysis was done by using Paired t-test. Results were compared between 0 h (baseline) and the time point at which peak response was found. For all individual drugs, the comparison was found statistically significant (p < 0.001∗). Posthoc Tukey’s test was used for pairwise comparison. All such comparisons were statistically significant for all the individual drugs (p < 0.001∗).
Analgesic activity of drugs in combinations (Phase II)
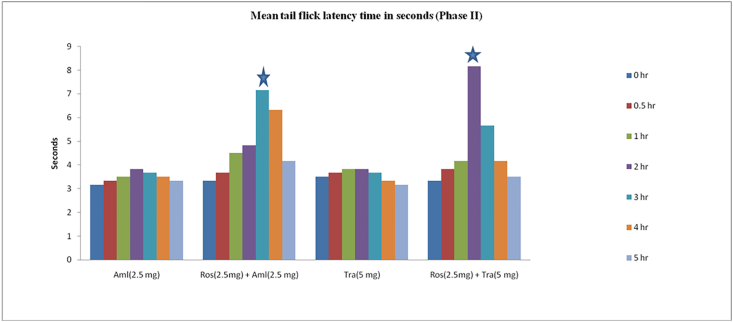
Significant analgesic effects were observed in tail-flick test when subanalgesic dose of rosuvastatin (2.5 mg/kg) was combined with ineffective, subanalgesic doses of amitriptyline (5 mg/kg), tramadol (5 mg/kg), etoricoxib (10 mg/kg) or amlodipine (2.5 mg/kg). The comparison was done with the tail-flick latency time at 0 h of the respective group and the reaction time of individual drug of that drug combination after 2 h of drug administration. For example, the effect of rosuvastatin (2.5 mg/kg) + amitriptyline (5 mg/kg) at 2 h post-drug administration was compared with baseline reaction time at 0 h, effect of rosuvastatin 2.5 mg/kg at 2 h (during phase I) and with amitriptyline (5 mg/kg) at 2 h (in phase I). The analgesic effect persisted for the next 2 h and was not statistically significant at the end of 4 h of drug administration in all the drug combination groups, except the amlodipine group, for which the effect was not statistically significant at the end of 5 h of drug administration (Fig. 3, Fig. 4).
Fig. 3.
Mean duration (in seconds) of tail-flick latency for drugs amitriptyline and rosuvastatin in phase I. Paired t-test between latency at 0 h and time of max latency. (p < 0.001). ∗Indicates subanalgesic doses after phase 1.
Fig. 4.
Mean tail-flick latency (duration in seconds) for subanalgesic doses of combinations of rosuvastatin, Etoricoxib, and amitriptyline. ∗Indicates maximal increase in tail-flick mean reaction time.
In the writhing test, the combinations of subanalgesic dose of rosuvastatin (2.5 mg/kg) with subanalgesic doses of amitriptyline (5 mg/kg), tramadol (5 mg/kg), etoricoxib (10 mg/kg), or amlodipine (2.5 mg/kg) produced the statistically significant analgesic effect by reducing the number of writhes compared to control group and individual drug group of that combination (in phase I) (Fig. 5 and 6).
Fig. 5.
Mean tail-flick latency (in seconds) for subanalgesic doses of combinations of rosuvastatin, amlodipine, and tramadol. ∗Indicates maximal increase in tail-flick mean reaction time.
Fig. 6.
Mean number of writhes after subanalgesic doses of combinations of rosuvastatin, Etoricoxib, amlodipine, and Tramadol. Comparison between the number of writhes after subanalgesic doses of individual drugs and the number of writhes after a combination of subanalgesic doses (p < 0.001).
Multiple comparisons among different groups, done with posthoc Tukey’s test, were found statistically significant for all the individual drugs (p < 0.001∗).
Discussion
Statins are the main agents indicated for the treatment of dyslipidemia. They also possess a large spectrum of pleiotropic effects such as anti-inflammatory, analgesics, and immunomodulatory actions that are independent of their effect on lipids.10, 11, 12 Of these, their proposed analgesic activity is of recent and widespread interest and the focus of this study.
We chose rosuvastatin as the representative statin in view of its increasing use and the availability of limited evidence supporting its analgesic effect. It has been found to reduce nociceptor sensitization by reducing the production of proinflammatory cytokines such as TNF alpha, bradykinin, and IL-1b, besides chemokines such as CXCL and serotonin.21, 22, 23, 24
Rosuvastatin produces inhibition of nuclear factor-kappa B (NF-kb) activation also, which further leads to a reduction in the release of proinflammatory cytokines and COX-2 induction.25,26 Rosuvastatin upregulates NOS, which increases availability of NO, which, in turn, leads to inhibition of PGE 2 induced hypernociception.23 So the mechanism by which rosuvastatin produces an analgesic effect is probably by reducing peripheral nociceptive input to the central nervous system, thus reducing central sensitization of pain.25,26 In our study, we were able to demonstrate and confirm the analgesic activity of rosuvastatin in two animal models. This analgesic effect was further compared with that of other known analgesic drugs (tramadol, amitriptyline, etoricoxib, and amlodipine). All the chosen drugs produce their analgesic effect by different mechanisms.
These drugs (with known analgesic activity) also demonstrated analgesic activity in our study models, thereby validating the robustness of our experimental model. Etoricoxib produces analgesia due to its inhibitory effects on the synthesis of prostaglandins mediated through inhibition of the COX-2 enzyme.27,28 Amitriptyline increases the amount of norepinephrine and serotonin in the synaptic cleft at both supraspinal and spinal levels, thereby reinforcing the descending inhibitory pathways. This leads to an antinociceptive effect by amitriptyline. The analgesic effects of antidepressants are because of inhibition of serotonin and noradrenaline uptake.29 Amlodipine exerts its antinociceptive effect by blocking N-type of voltage-dependent calcium channels on the primary afferent fibers in dorsal root ganglion, which leads to a reduction in the release of neurotransmitters, glutamate, and substance P from these nociceptive neurons.8,9 Tramadol, a well-established analgesic used for the management of postoperative pain, acts by binding to mu-opioid receptors and also by weakly inhibiting the reuptake of norepinephrine and 5-HT. In our study, we could confirm its analgesic activity in the doses in which it has been used in other studies.8,9
In our study, the analgesic activity of rosuvastatin, etoricoxib, tramadol, amlodipine, amitriptyline, and the combination of rosuvastatin with other study drugs was studied in albino mice using tail-flick and writhing tests. Tail-flick method is a well-validated method for estimating the efficacy and potency of putative analgesics.16,17 In this study, the pain threshold increased significantly during the period of observation in the entire four drugs treated groups, with the maximum effect observed in the tramadol group.
The writhing response induced by irritants (acetic acid in this case) is also an established method to test peripherally acting analgesics.18,19 All the study drugs demonstrated analgesic activity in this model as well.
Phase I of this study demonstrated that rosuvastatin, etoricoxib, tramadol, amlodipine, and amitriptyline exhibit analgesic effect in a dose-dependent manner, as has been reported in the literature. The minimum doses at which analgesic activity was observed for rosuvastatin, etoricoxib, tramadol, amlodipine, and amitriptyline as 2.5, 10, 5, 2.5, and 5 mg/kg, respectively. This is similar to findings in other studies.19,20
Phase II of this study demonstrated that a combination of the subanalgesic dose of rosuvastatin (2.5 mg/kg), tramadol (5 mg/kg), etoricoxib (10 mg/kg), or amlodipine (2.5 mg/kg), amitriptyline (5 mg/kg) produces significant analgesic effect presumably due to synergistic effect of these drugs operating through different mechanisms as mentioned above.
The study demonstrates the synergism of analgesic activity of rosuvastatin with etoricoxib, tramadol, amlodipine, and amitriptyline. Those patients who are taking rosuvastatin for dyslipidemia may require lesser doses of analgesics as and when they require them. This will reduce unnecessary exposure of a large number of people from analgesics that are known to cause multiple side effects, especially when taken for a long duration.
However, since this study has been conducted on a limited number of animals, the results need to be confirmed in larger and more specific animal studies. However, in order to take this concept to clinical practice, multiple clinical studies are required ranging from simple “proof of concept” studies to pivotal clinical trials. Observational studies to ascertain the differential requirement of analgesics in patients already on rosuvastatin may be a good starting point for clinical research in this area.
Conclusion
A large group of drugs is available to treat different types of pain. The prominent among the list are the NSAIDs and opioids that have their own adverse effects.30, 31, 32
Dyslipidemia is an important risk factor for a number of cardiovascular disorders like coronary artery disease and cerebrovascular disease.1 Patients with dyslipidemia often have various comorbidities like arthritis, neuropathies and suffer from chronic pain.4 Hence statins and known analgesics are likely to be coprescribed frequently in a large patient cohort. In any case, analgesics are frequently prescribed drugs for acute and chronic use. We conducted the present study to explore and establish the analgesic effect of rosuvastatin and to study whether the combination of rosuvastatin and other analgesic drugs leads to synergism vis-à-vis their analgesic effect.
From our study, it can be concluded that there is a synergism of analgesic activity of rosuvastatin with amitriptyline, tramadol, etoricoxib, and amlodipine, suggesting that in combinations, their dose requirement may be reduced, and such dose reductions shall lead to a lower incidence of adverse effects due to analgesic drugs. However, we are not suggesting that rosuvastatin can be used as an analgesic drug all by itself. More extensive studies are required to suggest that.
Ours is the first study to explore and demonstrate the synergistic nature of the analgesic effect produced by rosuvastatin and other known analgesic drugs. Since dyslipidemia, cardiovascular diseases, and neuropathies are chronic conditions, reduction of doses of analgesics in patients already on rosuvastatin may be considered, which will lead to reduced adverse effects of analgesics. It will contribute significantly to increased tolerability and better compliance of the therapy wherein medication has to be taken for a long duration.
However, considering the experimental nature of this study, such a combination approach needs to be confirmed and validated in clinical studies before such an approach comes into clinical practice.
Disclosure of competing interest
The authors have none to declare.
Acknowledgment
This paper is based on Armed Forces Medical Research Committee Project No. 4881/2017 granted and funded by the office of the Directorate General Armed Forces Medical Services and Defence Research Development Organization, Government of India.
References
- 1.Yusuf S., Hawken S., Ounpuu S., et al. INTERHEART Study Investigators Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004 Sep11-17;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 2.Gupta R., Rao R.S., Misra A., Sharma S.K. Recent trends in epidemiology of dyslipidemias in India. Indian Heart J. 2017;69(3):382–392. doi: 10.1016/j.ihj.2017.02.020. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yadav D., Mishra M., Tiwari A., Bisen P.S., Goswamy H.M., Prasad G.B. Prevalence of dyslipidemia and hypertension in Indian type 2 diabetic patients with metabolic syndrome and its clinical significance. Osong Public Health Res Perspect. 2014;5(3):169–175. doi: 10.1016/j.phrp.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Nimer M., Ali F., Al-Ani F. Dyslipidemia as a contributory factor in etiopathogenesis of diabetic neuropathy. Indian J Endocrinol Metab. 2011;15(2):110. doi: 10.4103/2230-8210.81940. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vincent A.M., Hayes J.M., McLean L.L., Vivekanandan-Giri A., Pennathur S., Feldman E.L. Dyslipidemia-induced neuropathy in mice: the role of oxLDL/LOX-1. Diabetes. 2009;58(10):2376–2385. doi: 10.2337/db09-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kothari S., Kushwah A., Kothari D. Involvement of opioid and monoaminergic pain pathways in Aegle marmelos induced analgesia in mice. Indian J Pharmacol. 2013;45(4):371. doi: 10.4103/0253-7613.115020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chogtu B., Bairy K.L., Satyam S.M., Pirasanthan R., Gupta S. Analgesic modulation of tramadol , amitriptyline and gabapentin in male and female Wistar rats. Res J Pharm Biol Chem Sci. 2013;4(3):70–78. [Google Scholar]
- 8.Jha P., Mazumdar B., Bhatt J. Analgesic activity of venlafaxine and its interactions with tramadol, celecoxib and amlodipine in mice. Indian J Pharmacol. 2009;38(3):181. [Google Scholar]
- 9.Modi H., Mazumdar B., Bhatt J. Study of interaction of tramadol with amlodipine in mice. Indian J Pharmacol. 2013;45(1):76. doi: 10.4103/0253-7613.106440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anand S., Muniappan M., Sangavai M., Parthiban R. Anti-inflammatory activity of telmisartan and rosuvastatin in various animal models. Int J Pharm Pharm Sci. 2014;6(4):182–186. [Google Scholar]
- 11.Ghaisas M.M., Dandawate P.R., Zawar S.A., Ahire Y.S., Gandhi S.P. Antioxidant, antinociceptive and anti-inflammatory activities of atorvastatin and rosuvastatin in various experimental models. Inflammopharmacology. 2010;18(4):169–177. doi: 10.1007/s10787-010-0044-6. [DOI] [PubMed] [Google Scholar]
- 12.Abeles A.M., Pillinger M.H. Statins as antiinflammatory and immunomodulatory agents: a future in rheumatologic therapy? Arthritis Rheum. 2006;54(2):393–407. doi: 10.1002/art.21521. [DOI] [PubMed] [Google Scholar]
- 13.Sontakke S., Jaiswal S. Experimental evaluation of analgesic and anti-inflammatory activity of simvastatin and atorvastatin. Indian J Pharmacol. 2012;44(4):475. doi: 10.4103/0253-7613.99311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CPCSEA CPCSEA guidelines for laboratory animal facility. Indian J Pharmacol. 2003;35:257–274. [Google Scholar]
- 15.Rezaee-Asl M., Sabour M., Nikoui V., Ostadhadi S., Bakhtiarian A. The study of analgesic effects of Leonurus cardiaca L. In mice by formalin, tail flick and hot plate tests. Int Sch Res Not. 2014:1–5. doi: 10.1155/2014/687697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar Patel P., Sahu J., Singh Chandel S. A detailed review on nociceptive models for the screening of analgesic activity in experimental animals. Int J Neurol Phys Ther. 2016;2(6):44. [Google Scholar]
- 17.Parle M., Yadav M. Laboratory models for screening analgesics. IRJP. 2013;4(1):159. [Google Scholar]
- 18.Kausar M., Singh B.K. Pharmacological evaluation of solanum viarum dunal leaves extract for analgesic and antipyretic activities. J Drug Deliv Ther. 2018;8(4):356–361. [Google Scholar]
- 19.Kamilla L., Ramanathan S., Sasidharan S., Mansor S. Evaluation of antinociceptive effect of methanolic leaf and root extracts of Clitoria ternatea Linn. in rats. Indian J Pharmacol. 2014;46(5):515. doi: 10.4103/0253-7613.140583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Bars D., Gozariu M., Cadden S.W. Animal models of nociception. Pharmacol Rev. 2001 Dec;53(4):597–652. [PubMed] [Google Scholar]
- 21.Kamel E.M., Elsaid A.F., Gumaa E.A., Elhafez A., Sheweal M El. Statins attenuate hyperalgesia and inflammation in experimentally induced acute and neuropathic pain in rats. Ain-Shams J Anaesthesiol. 2016:440–448. [Google Scholar]
- 22.Fregnan F., Muratori L., Simões A.R., Giacobini M.G. 2012. Role of Inflammatory Cytokines in Peripheral Nerve Injury ☆. (March 2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cunha T.M., Verri W.A., Silva J.S., Poole S., Cunha F.Q., Ferreira S.H. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Natl Acad Sci. 2005;102(5):1755–1760. doi: 10.1073/pnas.0409225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basbaum A.I., Bautista D.M., Scherrer G., Julius D. 2009. Review Cellular and Molecular Mechanisms of Pain; pp. 267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ali S., Mann D.A. May 2003. Signal Transduction via the NF- B Pathway: A Targeted Treatment Modality for Infection , Inflammation and Repair. 2004; pp. 67–79. [DOI] [PubMed] [Google Scholar]
- 26.Li Q., Verma I.M., Pines N.T. NF- κ B regulation in the immune system. 2002;2(October) doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 27.Raut P., Vamsi D. Comparison of analgesic activity of venlafaxine with etoricoxib, using digital. Res J Pharm. Biol Chem Sci. 2014;5(555):555–564. [Google Scholar]
- 28.Brooks P., Kubler P. Etoricoxib for arthritis and pain management. Ther Clin Risk Manag. 2006;2(1):45–57. [PMC free article] [PubMed] [Google Scholar]
- 29.Sawynok J., Esser M.J., Reid A.R. 2001. Antidepressants as Analgesics: an. [PMC free article] [PubMed] [Google Scholar]
- 30.Ho K.Y., Gwee K.A., Cheng Y.K., Yoon K.H., Hee H.T., Omar A.R. Nonsteroidal anti-inflammatory drugs in chronic pain: implications of new data for clinical practice. J Pain Res. 2018;11:1937–1948. doi: 10.2147/JPR.S168188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mishra Debasis, Ghosh Goutam, Paidesetty Sudhir, Panda P.K. An experimental study of analgesic activity of selective COX-2 inhibitor with conventional NSAIDs. Asian J Pharmaceut Clin Res. 2011;4:78–81. [Google Scholar]
- 32.Mahajan G. 4th ed. Elsevier; 2009. Major Opioids in Pain Management; pp. 94–105. Essentials of Pain Medicine and Regional Anesthesia. [Google Scholar]