Abstract
Background
The influence of repeated exposures to cold pain stimulus, a surrogate of clinical pain, has remained largely unexplored. The study was planned to test the effect of repeated exposures to cold pain through cold pressor task on pain sensitivity and vascular reactivity.
Methods
Single-group experimental study. Thirty-seven healthy male volunteers (18–25 years) were exposed to cold pressor tasks for seven consecutive days and on the 14th day on the nondominant hand. The same was repeated on dominant hand on first and seventh days; 31 completed the protocol.
Results
Pain threshold and tolerance in the nondominant hand increased from day 1 to day 7 (p < 0.001) and were positively correlated on day 1 (=0.45, p = 0.011) and day 7 (=0.38, p = 0.036). Diastolic blood pressure response was found to increase by day 7 (p < 0.0024) and positively correlated with tolerance. On the dominant hand, the threshold reduced from day 1 to day 7, while tolerance increased. Both threshold and tolerance remained lower than that of nondominant hand. Day 14 values of threshold and tolerance were in between day 1 and day 7 values but not significantly different from both.
Conclusion
Habituation in pain threshold and tolerance was observed on repeated exposure to cold pain, which was not significantly retained till the 14th day. The same was not observed with subjective feeling of pain perception. The increased diastolic blood pressure response is suggestive of peripheral vasoconstriction. Increased tolerance in the dominant hand by day 7 demonstrates a systemic effect in habituation.
Keywords: Cold pain, Pain perception, Vascular reactivity, Tolerance, Threshold
Introduction
Pain is a multidimensional experience with sensory-discriminative, cognitive-evaluative, and affective-motivational components. Repetitive painful stimulation leads to a decrease in pain and pain-related responses; the response is called habituation.1 Several studies have explored the influence of repeated painful stimulation using experimental pain models involving repeated heat stimuli.1,2,3,4 They have reported reduced subjective pain intensity and increased anxiety, with no change in pain unpleasantness.2
The Cold Pressor Task (CPT) was developed as a clinically indicative cardiovascular test and quantifies vascular response when a subject’s hand is immersed into ice water. Since the test procedure results in a gradually increasing cold pain, the CPT has also been widely used as a nociceptive stimulus.5 Although the CPT has been used extensively as an experimental pain model to study pain perception as a surrogate to clinical pain,6 few studies have looked at the influence of repeated exposure to cold pain stimulus using the cold pressor task.7,8 It has remained largely unexplored in the Indian population.
The primary objective was to test the effect of repeated exposures to cold pain through cold pressor task on pain threshold and pain tolerance (pain sensitivity). The secondary objectives were (a) to test the effect of repeated exposures to cold pain on subjective perception (pain unpleasantness), vascular reactivity, and anxiety; (b) to test if the changes in pain sensitivity measures are local or systemic (by comparing the effect on both hands); (c) to test if the effect of repeated exposure to cold pain lasts for a week after the last exposure.
Material and methods
The study was a single-group experimental study, conducted following approval from Institutional Ethics Committee (IEC study no. 221/2018; letter no: IEC/1/841/2018 dated 19.09.2018). A minimum sample size of 30 was calculated to include a 20% dropout. Thirty-seven healthy male volunteers (18–25 years) with resting blood pressure less than 130/80 mmHg9 were recruited among the students from a medical college attached to a tertiary health care center in Karnataka, India. Subjects with a history of chronic smoking, acute or chronic illness, cognitive impairment, bone injury of upper limbs within 6 months prior to the study, Reynaud’s phenomenon and abnormalities of skin of hands were excluded from the study. A written informed consent was taken from all patients/participants for inclusion in the study.
The subjects were requested to report for the experiment for 7 consecutive days10 between 4.00pm and 6.00pm. The last meal consumed was at least 3 h prior, with avoidance of fatty foods, nonvegetarian diet, added salt intake, coffee, tea, chocolate milk, or flavored drinks.11,12,13 The subjects were advised to avoid heavy exercise at least 12 h prior, antipyretics, analgesics, tobacco, alcohol during the study period. On day 1 of the study, weight (using weighing scale) and height (using Stadiometer) were measured.
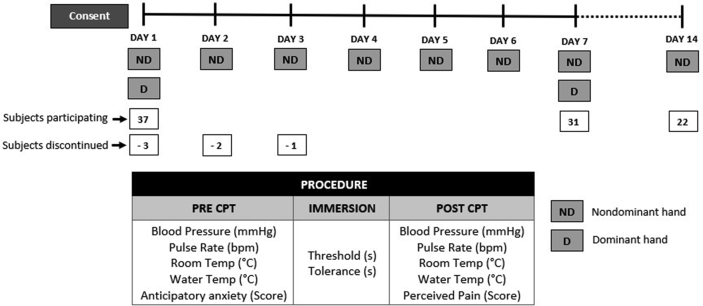
7-day experiment protocol (Fig. 1)
Fig. 1.
Study protocol. CPT: Cold Pressor Task.
Room Temperature was recorded using a digital temperature recorder. Following 10 min rest, three measures of blood pressure (using automated arm blood pressure monitor) and pulse rate were done on the arm, not exposed to cold pain, with an interval of 1 min between each reading.14 The cuff was left tied around the subject’s arm to measure blood pressure and pulse rate immediately post-task. The subject was asked to rate level of anxiety he felt before performing CPT on a Visual Analogue Scale (VAS) for “Anticipatory Anxiety” from 0 to 10, with 0 for “No anxiety” and 10 for the “Most intense anxiety imaginable.”15
Cold pressor task (CPT)
The subject was asked to immerse his nondominant hand up to 5 cm above the wrist, with palm facing downward and fingers spread out, into a container filled with water maintained between 3 °C and 5 °C.16,17,18 During the task, ice cubes were added to the water to maintain its temperature within range. The water was intermittently stirred with a glass rod to dissipate heat transferred from the hand toward the periphery.16 Two stopwatches were turned on the moment the subject immersed his hand. The subject was instructed to say the word “Pain” when he first felt pain. One stopwatch was stopped at that moment, and the reading (seconds) was recorded as “Pain Threshold.” The subject continued to immerse his hand in cold water and removed it when he was unable to tolerate pain. The second stopwatch was then stopped, and the reading (seconds) was recorded as “Pain Tolerance.” An upper limit of 10 min on day 1 and 15 min on day 2 and day 3 was set for tolerance, which is within the established acceptable upper limit for the experimental pain model using CPT.19,20 No upper limit was set for the remaining days as tolerance was expected to increase. The time limit was not disclosed to subjects.
Blood pressure and pulse rate were measured immediately after the hand was removed from the cold water. The subjective experience of pain was assessed using a VAS for “Pain Unpleasantness” with scores from 0 to 10. The subject was asked to rate “how strong/unpleasant was the pain at its peak?”21 with 0 for “No pain” and 10 for the “Worst/Most agonizing pain.”16,21,22
Cold pressor task on the dominant hand
The experimental procedure, as described above, was performed on the dominant hand, following exposure to the nondominant hand, on days 1 and 7. The order of exposure of the dominant and nondominant hand was not randomized to prevent any immediate effect of exposure of the dominant hand to cold pain on the nondominant hand since the nondominant hand was chosen for testing the primary objective.
Cold pressor task with the nondominant hand on day 14
With no cold pain exposure from day 8 to day 13, the experimental procedure was repeated on day 14 on the non-dominant hand.
Following the experimental procedure on all seven days, the subjects were under observation till the hand that was immersed turned warm. The subjects were also instructed to report any signs and symptoms indicative of tissue injury. However, no such findings were reported. Of the 37 subjects recruited, 31 completed the 7-day experiment. Six subjects were discontinued since their tolerance crossed the upper limit set for study.
Statistical analysis
The distribution of all continuous variables was examined using Q–Q plots. Normally distributed data were summarized as mean and SD and others as median and interquartile range. The daily difference in Threshold and Tolerance for days 1–7 were computed and divided by Day 1 values to obtain % change from Day 1. The change in this difference over the seven days of intervention was examined using repeated measures ANOVA if data were normally distributed, and by nonparametric Friedman test if the data were not normally distributed. Posthoc pairwise comparisons between time points were made by using either Bonferroni adjusted paired sample T-test or the Wilcoxon Sign Rank test. The sustained effect till the 14th day was examined using paired sample T-test or Wilcoxon Sign rank test with Day 1 and Day 7. The correlation between Threshold and Tolerance % across the 14 days was performed using a linear mixed model with subject as a random effect in order to account for the repeated sampling within each individual. The cardiovascular response was examined as the difference in blood pressure and pulse rate between pre and post immersion (ΔSBP, ΔDBP, ΔPR). The change in this difference over the seven days of intervention was examined by using repeated measures ANOVA for normally distributed data, and nonparametric Friedman test for non-normally distributed data. The analysis was performed for both dominant and nondominant hands. The association between tolerance with the blood pressure and pulse rate were examined for the day in which tolerance was highest for all the seven days using Spearman’s rank correlation coefficient. Similarly, the association with threshold was also examined. All analysis was performed using STATA 15 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC).
Results
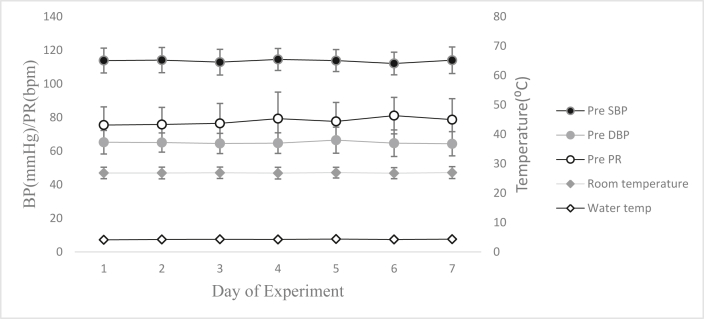
The subjects recruited for the study had a mean age of 19 ± 1 years, mean height of 174.6 ± 1 cm, mean weight of 68 ± 3 Kg and mean BMI was 22 ± 3 kg/m2. The pretest systolic blood pressure (SBP), diastolic blood pressure (DBP), and pulse rate (PR) were within the normal range on all days and did not vary from Day 1 to Day 7. The room and water temperatures were maintained at 26.9±2 °C and 3.6 ± 0.7 °C respectively during the experiment across all days (Fig. 2).
Fig. 2.
Pre-intervention parameters during the experimental protocol. SBP: Systolic BP; DBP: Diastolic BP; PR: Pulse rate. Values presented are mean and 95% confidence interval.
Pain threshold and tolerance of the nondominant hand were measured in seconds during the CPT. The median values of threshold and tolerance for each day are mentioned in Fig. 3. The tolerance and threshold for each day were converted to the percentage of that on Day 1 for further analysis (with Day 1 value represented as 100%). There was an increase in both Tolerance % and Threshold % from Day 1 to Day 7 (both P < 0.001). The threshold was higher on day 7 compared to Days 1, 2, 3, and 4 but comparable to Days 5 and 6. The Day 6 values were higher than both Day 1 and Day 2 in posthoc pairwise analysis (Fig. 3). Pain tolerance and threshold were positively correlated on day 1 (= 0.45, p = 0.011) and day 7 (= 0.38, p = 0.036) of the experiment (Fig. 4).
Fig. 3.
Change in pain threshold and tolerance across seven days. Data in the table are Median (Interquartile range) of absolute values of Tolerance and Threshold. Data represented in a graph (Y-axis) are mean in logscale + 1SD % of day 1 nondominant hand value for each subject (values in the graph need not correspond exactly to absolute summary values). Comparisons have been made only using the % values. ∗ Threshold significantly different compared to Day 7 (p < 0.0024). # Threshold significantly different compared to Day 6 (p < 0.0024). ϯ Overall change from Day 1–7 significant, p < 0.0001.
Fig. 4.
Correlation between Threshold and Tolerance on Days 1 and 7.
The subjects rated pain unpleasantness on a VAS ranging from 1 to 10 on all days immediately after the CPT. Although the VAS score showed a significant difference between the days of intervention (P < 0.001), no consistent trend of increase or decrease was observed. Also, daily VAS scores were not different from Day 1 score (all p > 0.05) after Bonferroni adjustment for multiple comparisons. However, most of the subjects reported extreme pain in the initial period of immersion that was followed by repeated cycles of waxing and waning of the intensity of pain experienced. The distribution of VAS scores is given in Fig. 5. Anticipatory Anxiety (AA) was also scored on a visual analog scale each day before the task. The median value for the AA rating remained lowest at zero across all days.
Fig. 5.
Comparison of pain unpleasantness (Visual analog scale scores) across days. Nondominant hand Visual Analog Scale scores across days were significantly different by Friedman test (p < 0.001). Pairwise comparison of each day score with Day 1 not different (p > 0.05) by Wilcoxon sign rank test after Bonferroni adjustment for multiple comparisons across days.
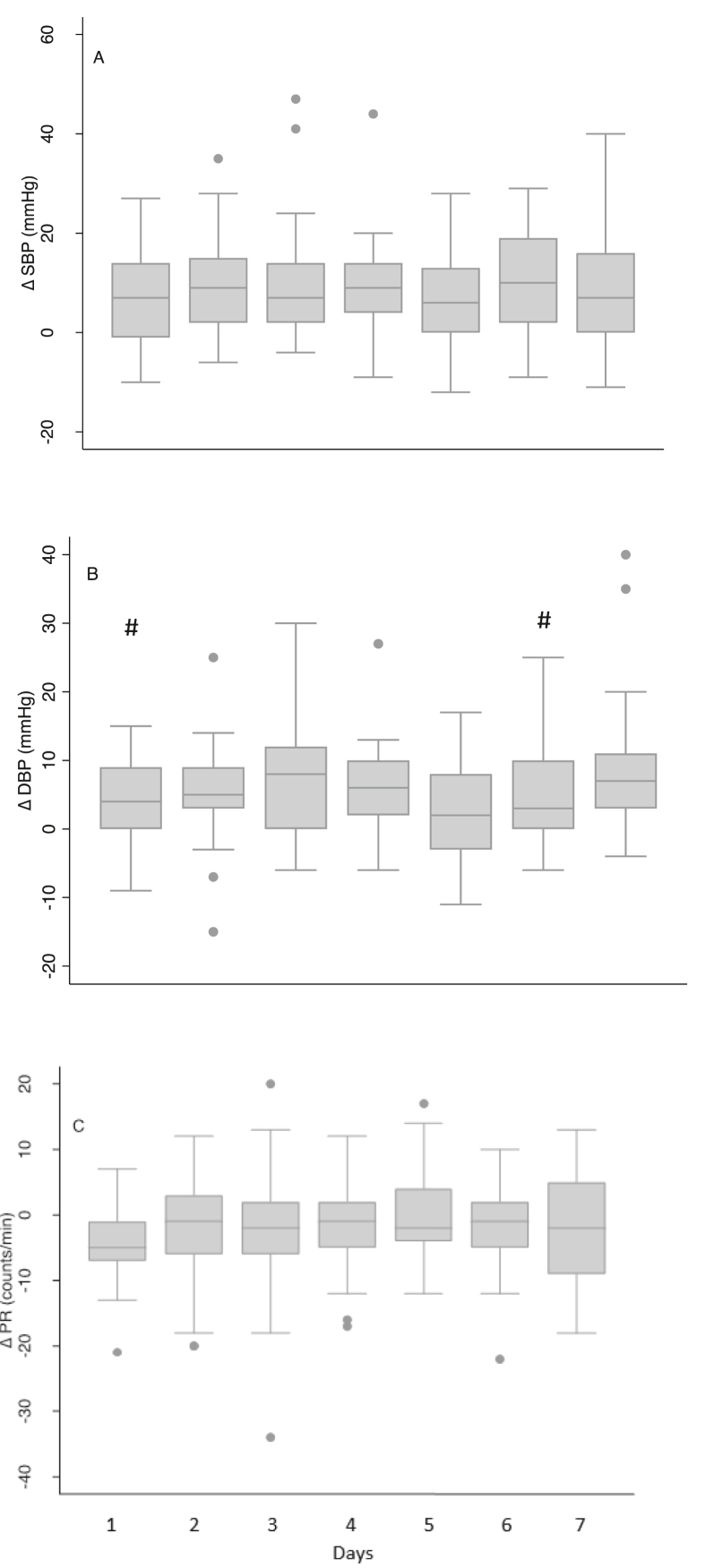
Vascular reactivity to the CPT was measured as the difference between pretest and immediate post-test values of SBP, DBP, and PR (ΔSBP, ΔDBP, ΔPR, respectively) (Fig. 6). In comparison across the seven days, ΔDBP was found to be different between the days (p = 0.0029). Further pairwise analysis showed that Day 1 and Day 6 ΔDBP were lower than that of Day 7. The day the subject experienced maximum pain tolerance and threshold were identified for each subject. In order to examine the association of vascular reactivity with the maximum potential tolerance and threshold of each subject, the maximum threshold and tolerance were correlated with the vascular reactivity measured on the same day. There was no significant association between ΔSBP and ΔPR. However, the pain threshold was positively correlated (Spearman’s rank correlation coefficient = 0.55, p = 0.0014) with ΔDBP. Change in diastolic blood pressure was correlated with pain tolerance as well (Spearman’s rank correlation coefficient = 0.40, p = 0.02).
Fig. 6.
Change in systolic and diastolic blood pressures and pulse rate across seven days. # Significantly different from Day 7 value (p < 0.0024).
A CPT was performed on the dominant hand on the 1st and 7th day to look for a systemic effect of repeated exposure to cold pain. Threshold and tolerance values were obtained from the task in seconds. The day 1 values of nondominant hand were used as the basal values for all comparisons. Hence, the values of the dominant hand were converted into a percentage of the subject’s day 1 nondominant hand value. This was done to compare the dominant hand values to that of the nondominant hand. The absolute values of dominant and nondominant hand have been summarized as a median and interquartile range in Table 1. Also shown is the comparison of pain threshold and tolerance percentage. On Day 1 there was no difference in the tolerance between the two hands, but by Day 7, the tolerance in the nondominant hand was higher than that of dominant hand (P < 0.001). Both Day 1 and Day 7 thresholds were higher in the nondominant hand compared to dominant (P < 0.001). The pain threshold in the nondominant hand significantly increased across seven days. On the contrary the dominant hand threshold dropped, with the seventh-day value being significantly shorter than the first day (p < 0.05).
Table 1.
Comparison of the nondominant and dominant hand.
| Nondominant (s) | Nondominant (%) | Dominant (s) | Dominant (%) | |
|---|---|---|---|---|
| Pain Threshold | ||||
| Day 1 | 26 (15) | 100 | 19 (33) | 78.89 ± 12.16b |
| Day 7 | 33 (56) | 145 ± 153.86a | 16 (9) | 76.12 ± 51.92a,b |
| Pain Tolerance | ||||
| Day 1 | 70 (100) | 100 | 69 (132) | 105.36 ± 61.98 |
| Day 7 | 380 (411) | 419.63 ± 495.65a | 72 (257) | 125.81 ± 140.69a,b |
Absolute values are represented as the Median (Interquartile range).
% of day-1 nondominant hand value for each subject is summarized as Mean±1SD.
Comparisons have been made only using the % values.
Significantly different from Day 1 value (p < 0.05).
Significantly different from Nondominant value (p < 0.05).
Pain threshold was adjusted for room temperature, and the average room temperature was found to be positively associated with the threshold (ρ = 0.39, p < 0.001) when examined across all days from Day 1 to Day 7.
In a subset of the study sample (n = 22), the Pain tolerance and threshold were measured in the nondominant hand after a gap of 7 days from the last day of exposure (i.e., on the 14th day). The Day 14 value of both threshold and tolerance were greater than the values of day 1, but lesser than those of day 7. However, no significant difference was found between both.
Discussion
The phenomenon of repeated stimuli eliciting progressively smaller responses has been defined as habituation.23 Habituation appears to be a general, nonspecific process observed in all sensory modalities.1 Habituation to pain stimulus results from repeated stimuli1,2,4,24 and most studies have used repeated heat or mechanical pain stimuli to demonstrate the same.1,11,24 In this study, we explored if repeated exposures to cold pain had a similar effect.
Repeated exposures to cold pain, in our study, showed that towards the end of the period of exposure, i.e., on days 6 and 7, pain threshold and tolerance had increased significantly from day 1. This implies that both objective measures of pain perception are affected by repeated exposures leading to habituation. Other studies have shown a similar effect with experimental heat pain, the underlying cause being a centrally mediated antinociceptive mechanism.1 The percentage change in tolerance over seven days appears much larger than that of the threshold. However, threshold and tolerance show a positive correlation before and after habituation.
Repeating the experiment on day 14 on a subset of subjects (with six days interval period of nonexposure) showed that objective measures of pain reduced from that of day 7, but remained higher than day 1, although they were not significantly different from both day 1 and 7. The intent was to test the residual effect of exposure to repeated pain stimuli with a gap of the same number of days as repeated exposures. Another study,1 with repeated heat pain stimulus for eight days, has demonstrated that the habituation effect lasts for even up to 22 days (interval period of 14 days of nonexposure). From the findings of the current study, the effect of habituation to cold pain stimulus with seven days’ exposure appears to be short-lasting. However, this may be owing to the smaller size of the subset population.
Repetitive stimulation of the receptive field of a nociceptor induces a reduction in discharges in both Aδ- and C-fiber nociceptive afferents leading to fatigue.4,25 Subpopulations of both Aδ- and C-fiber neurons are responsive to cooling. Fatigue could be the mechanism underlying habituation to repeated cold stimulation as well.
Interestingly, the visual analog scores, which represent subjective measures of pain perception, did not show a significant change across days despite an increase in threshold and tolerance. This is similar to findings in response to heat pain, which showed no change in pain unpleasantness.2 This implies that while habituation to pain allows for increased tolerance, it has no effect on the affective component of unpleasantness associated with pain. This is supported by findings from other studies that suggest the existence of separate, parallel ascending pathways for pain processing — the lateral pain system (the lateral thalamic nuclei and the somatosensory cortex) for analyzing the objective aspects of location, intensity, and duration of the stimulus and the medial pain system (medial thalamic nuclei and the anterior cingulate cortex) for the subjective unpleasant character of pain perception.26 In the current study, subjects with considerably high pain tolerance described repeated cycles of increasing and decreasing intensities of pain during immersion. This subjective report could be explained by cold-induced vasodilation leading to the “hunting phenomenon,” which is the cyclical cooling and spontaneous rewarming that occurs with cyclical vasoconstriction and vasodilation when a hand is immersed in ice-cold water.27,28
Vascular reactivity (effect on systolic blood pressure, diastolic blood pressure, pulse rate): The current study showed no variation in systolic blood pressure and pulse rate response despite increasing pain threshold and tolerance. The diastolic blood pressure had a significant rise by the last day that could be attributed to peripheral vasoconstriction. The mechanism may be through endothelin-1 that has been proven to increase following cold pressor test, leading to vasoconstriction, thereby increasing peripheral resistance.29 On the day of maximum tolerance for each subject, change in diastolic blood pressure was found to positively correlate with pain threshold and tolerance.
The pain threshold was measured in the dominant hand with an intent to examine if the effect of repeated exposures to cold pain stimulus was a local or systemic effect. The pain threshold does not appear to be the same bilaterally. Although the dominant hand may be expected to have a higher threshold to pain due to thickened skin, the current study shows a significantly lower pain threshold in the dominant hand as compared to the nondominant hand, to begin with, indicating an increased sensitivity. This sensitivity seems to have increased further over a 7-day experiment period with a significantly lower pain threshold of the dominant hand on day 7 compared to day 1. This is contrary to findings from other studies showing an increase in pain threshold.1,4,30 To begin with, both hands had similar tolerance that seemed to have increased significantly in the nondominant hand over seven days. However, an increase in the tolerance of the dominant hand was not as large as that of the nondominant hand. These findings suggest that there is a systemic effect of repeated exposures to cold pain, but mechanisms behind the systemic change in the sensitivity to pain and its tolerance are different.
Room temperature seems to influence pain threshold since they were positively associated. This is in line with few other studies with repeated heat pain.15
The subjects for this study were medical students who have already been involved in a similar experiment as part of their medical curriculum several months before the study. This prior exposure could be the reason the anticipatory anxiety levels remained persistently low, at zero, at the start and throughout the duration of the study.
Our study explores the patterns of objective and subjective perception of pain on repeated exposure and cardiovascular responses to the same. Since cold pain is a surrogate for clinical pain, our findings can be applied to research and therapy in the field of palliative care of patients who present with pain. It also helps us understand the physiological responses of those repeatedly exposed to cold temperatures as part of their professions, such as armed forces personnel or scientific researchers.
Limitations
The Blood pressure and heart rate changes were recorded only post-task and not during exposure to cold pain. The cardiovascular changes during the initial few minutes of cold pain exposure were not explored. The sequence of hand exposure was not randomized since the nondominant hand was chosen for the primary objective of the study. Exposing the dominant hand to cold pain first could have influenced the pain perception of the nondominant hand. The absence of randomization of hand exposure may have contributed to changes observed in the dominant hand. Room temperature during the experiment was not controlled. However, baseline temperatures across the 7-day experimental period for each subject remained the same (did not differ statistically), as shown in the results. A measure of the anxiety trait or state of the subjects would have allowed for a clearer understanding of the reason for the lowest median value of Anticipatory anxiety on all days.
Conclusion
This study demonstrated the effect of repeated exposures to experimental cold pain stimulus for seven days on adult male subjects. Habituation was observed with an increase in the objective measures of pain, namely pain threshold and tolerance, which was not significantly retained till the 14th day. However, repeated exposure did not affect the subjective feeling of pain perception. An increased diastolic blood pressure response by day 7 and its positive association with pain tolerance were suggestive of peripheral vasoconstriction in response to cold exposure. Habituation to pain tolerance demonstrated a systemic effect with increased tolerance in both hands. However, while the local effect (nondominant hand) on the pain threshold was habituation, the systemic effect (dominant hand) seen was increased sensitivity. These findings are applicable in health care and among individuals exposed to extreme cold temperatures as part of their profession.
Disclosure of competing interest
The authors have none to declare.
Acknowledgments
We thank Dr Mario Vaz, Professor and Head, Department of Physiology, St John’s Medical College, Bangalore, for his critical peer review of the manuscript.
References
- 1.Bingel U., Schoell E., Herken W., Büchel C., May A. Habituation to painful stimulation involves the antinociceptive system. Pain. 2007;131(1–2):21–30. doi: 10.1016/j.pain.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Maeoka H., Hiyamizu M., Matsuo A., Morioka S. The influence of repeated pain stimulation on the emotional aspect of pain: a preliminary study in healthy volunteers. J Pain Res. 2015;23(8):431–436. doi: 10.2147/JPR.S86732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price D.D., Barrell J.J., Gracely R.H. A psychophysical analysis of experiential factors that selectively influence the affective dimension of pain. Pain. 1980;8(2):137–149. doi: 10.1016/0304-3959(88)90001-2. [DOI] [PubMed] [Google Scholar]
- 4.Rennefeld C., Wiech K., Schoell E.D., Lorenz J., Bingel U. Habituation to pain: further support for a central component. Pain. 2010;148(3):503–508. doi: 10.1016/j.pain.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Koenig J., Jarczok M.N., Ellis R.J., Bach C., Thayer J.F., Hillecke T.K. Two-week test-retest stability of the cold pressor task procedure at two different temperatures as a measure of pain threshold and tolerance. Pain Pract. 2014;14(3):126–135. doi: 10.1111/papr.12142. [DOI] [PubMed] [Google Scholar]
- 6.Kim H., Neubert J.K., Rowan J.S., Brahim J.S., Iadarola M.J., Dionne R.A. Comparison of experimental and acute clinical pain responses in humans as pain phenotypes. J Pain. 2004;5(7):377–384. doi: 10.1016/j.jpain.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Stancák A., Jr., Yamamotová A., Kulls I.P., Sekyra I.V. Cardiovascular adjustments and pain during repeated cold pressor test. Clin Auton Res. 1996 Apr;6(2):83–89. doi: 10.1007/BF02291228. [DOI] [PubMed] [Google Scholar]
- 8.Fischer M.J., Khani A., Gokpinar C.E., Strueber E., Gutenbrunner C., Bernateck M. The effect of temperature and time using repeated immersion on the habituation of pain thresholds in healthy subjects. Thermology International. 2008;18(4):145–150. [Google Scholar]
- 9.Association of Physicians of India Indian guidelines on hypertension (I.G.H.) - III. 2013. J Assoc Phys India. 2013 Feb;61(2 suppl l):6–36. PMID: 24475694. [PubMed] [Google Scholar]
- 10.Milne R.J., Kay N.E., Irwin R.J. Habituation to repeated painful and non-painful cutaneous stimuli: a quantitative psychophysical study. Exp Brain Res. 1991;87:438–444. doi: 10.1007/BF00231861. [DOI] [PubMed] [Google Scholar]
- 11.Al'Absi M., Buchanan T.W., Marrero A., Lovallo W.R. Sex differences in pain perception and cardiovascular responses in persons with parental history for hypertension. Pain. 1999;83(2):331–338. doi: 10.1016/s0304-3959(99)00122-0. [DOI] [PubMed] [Google Scholar]
- 12.Yumnam A., Keisam R. Effect of food intake on pain perception in healthy human subjects. J of Evolution of Med and Dent Sci. 2014;3(29):7984–7988. [Google Scholar]
- 13.Zmarzty S.A., Wells A.S., Read N.W. The influence of food on pain perception in healthy human volunteers. Physiol Behav. 1997;62(1):185–191. doi: 10.1016/s0031-9384(97)00038-3. [DOI] [PubMed] [Google Scholar]
- 14.Carey R.M., Whelton P.K. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American college of cardiology/American heart association hypertension guideline. Ann Intern Med. 2018;168(5):351–358. doi: 10.7326/M17-3203. [DOI] [PubMed] [Google Scholar]
- 15.Banozic A., Beljan I. Pain-relevant anxiety affects desire for pain relief, but not pain perception. Indian J Pain. 2017;31:59–64. [Google Scholar]
- 16.von Baeyer Carl L., Piira Tiina, Chambers Christine T., Trapanotto Manuela, Zeltzer Lonnie K. Guidelines for the cold pressor task as an experimental pain stimulus for use with children. J Pain. 2005;6(4):218–227. doi: 10.1016/j.jpain.2005.01.349. [DOI] [PubMed] [Google Scholar]
- 17.Mendpara S., Jasuja V., Purohit G., Palan B.M., Harsoda J.M. Sex related difference in response to experimental pain in first year medical students. J Pharm (Lahore) 2012;2(6):1–4. [Google Scholar]
- 18.Akhani P., Mendpara S., Palan B. Gender differences in response to experimental pain among medical students from a western state of India. Int J Med Stud. 2014;2(1):13–17. [Google Scholar]
- 19.Geurts C.L., Sleivert G.G., Cheung S.S. Local cold acclimation during exercise and its effect on neuromuscular function of the hand. Appl Physiol Nutr Metabol. 2006 Dec;31(6):717–725. doi: 10.1139/h06-076. PMID: 17213886. [DOI] [PubMed] [Google Scholar]
- 20.Hirai K., Horvath S.M., Weinstein V. Differences in the vascular hunting reaction between Caucasians and Japanese. Angiology. 1970 Sep;21(8):502–510. doi: 10.1177/000331977002100802. PMID: 5507351. [DOI] [PubMed] [Google Scholar]
- 21.Duschek S., Schwarzkopf W., Schandry W. Increased pain sensitivity in low blood pressure. J Psychophysiol. 2008;22(1):20–27. [Google Scholar]
- 22.Haas K., Lu Q., Evans S., Tsao J.C., Zeltzer L.K. Relationship between resting blood pressure and laboratory- induced pain among healthy children. Gend Med. 2011;8(6):388–398. doi: 10.1016/j.genm.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glaser E.M., Whittow G.C. Evidence for a non-specific mechanism of habituation. J Physiol. 1953;122 43–4P. [PubMed] [Google Scholar]
- 24.Greenspan J.D., McGillis S.L. Thresholds for the perception of pressure, sharpness, and mechanically evoked cutaneous pain: effects of laterality and repeated testing. Somatosens Mot Res. 1994;11:311–317. doi: 10.3109/08990229409028875. [DOI] [PubMed] [Google Scholar]
- 25.Peng Y.B., Ringkamp M., Meyer R.A., Campbell J.N. Fatigue and paradoxical enhancement of heat response in C-fiber nociceptors from cross-modal excitation. J Neurosci. 2003;23(11):4766–4774. doi: 10.1523/JNEUROSCI.23-11-04766.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J.Y., Huang J., Chang J.Y., Donald J., Woodward D.J., Luo F. Morphine modulation of pain processing in medial and lateral pain pathways. Mol Pain. 2009;5:60. doi: 10.1186/1744-8069-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fox R.H., Wyatt H.T. Cold-induced vasodilatation in various areas of the body surface of man. J Physiol. 1962;162:289–297. doi: 10.1113/jphysiol.1962.sp006933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sachs C., Lehnhardt M., Daigeler A., Goertz O. The triaging and treatment of cold-induced injuries. Dtsch Arztebl Int. 2015;112(44):741–747. doi: 10.3238/arztebl.2015.0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fyhrquist F., Saijonmaa O., Metsärinne K., Tikkanen I., Rosenlöf K., Tikkanen T. Raised plasma endothelin-1 concentration following cold pressor test. Biochem Biophys Res Commun. 1990;169:217–221. doi: 10.1016/0006-291x(90)91456-3. [DOI] [PubMed] [Google Scholar]
- 30.Carman K.W., Knight K.L. Habituation to cold-pain during repeated cryokinetic sessions. J Athl Train. 1992;27(3):223–230. [PMC free article] [PubMed] [Google Scholar]