Abstract
Ott et al. report the results of a phase 1B study in which a personalized neoantigen vaccine was combined with programmed death receptor-1 blockade in patients with advanced cancers. The study provides a framework for combinatorial vaccine therapies that could mount robust T cell responses, enhance tumor killing, and provide clinical benefit.
Instructing the immune system to recognize and kill cancer has transformed the care of certain advanced malignancies. Prominent among these therapies are immune checkpoint inhibitors (ICIs)—monoclonal antibodies that block T cell inhibition by molecules, such as PD-1, to unleash effector T cells that destroy tumor cells. ICIs have been most successful for the treatment of cancers that have a high tumor mutation burden (TMB), including melanoma and non-small cell lung cancer (NSCLC) (Yarchoan et al., 2017). A subset of these mutations give rise to unique neoantigens—proteins produced during tumorigenesis that are recognized as foreign by the immune system. A high neoantigen load attracts effector T cells that infiltrate into and lyse tumor targets. However, even in these tumors, T cells can be insufficient in number and/or low in “quality”—as is defined by activation, exhaustion, polyfunctionality, cytotoxic capacity, and memory—resulting in less durable clinical responses to PD-1 blockade. The challenge therefore is to find ways to enhance the number and quality of tumor-infiltrating T cells. In this issue of Cell, Ott et al. seek to combine an ICI—an anti-PD-1 antibody (nivolumab)—with a personalized peptide vaccine targeting each individual patient’s tumor-specific neoantigens to address this challenge (Ott et al., 2020).
Neoantigens are attractive targets for cancer immunotherapy as they are uniquely expressed on tumors and are therefore not subject to central tolerance (or deletion during thymic development). Not being expressed on healthy tissue also lowers the risk of autoimmunity. Prior studies have documented the safety and feasibility of generating and administering neoantigen vaccines. Even when used without ICIs, favorable anti-tumor immune responses are noted in mouse models and in melanoma and glioblastoma patients (Hilf et al., 2019; Keskin et al., 2019; Ott et al., 2017; Sahin et al., 2017). Dovetailing on their prior work, Ott et al. evaluate the safety, feasibility, and immunogenicity of vaccine plus nivolumab in 82 patients with metastatic melanoma, NSCLC, and bladder cancer. Expressed neoantigens identified in each patient’s tumor tissue by deep sequencing were prioritized based on expression level, HLA presentation, and HLA-I binding affinity. Patients were initially treated with nivolumab for 12 weeks while synthetic long peptides (SLPs) corresponding to the selected neoantigens were synthesized. Five priming and two booster vaccines, each consisting of up to twenty 14–35-amino-acid-long SLPs, were then administered over a 3-month period while nivolumab was continued.
Most notably, patients displayed de novo neoantigen-specific T cell responses to multiple vaccinating peptides, whereas pre-vaccination time points expectedly had minimal responses. 42% and 24% of the vaccinating peptides, respectively, triggered CD4+ and CD8+ T cell responses. Furthermore, TCR sequencing revealed trafficking of neoantigen-specific CD4+ and CD8+ T cell clones from peripheral blood into the tumor in three melanoma patients who displayed a prolonged progression-free survival (PFS). The predominant CD4+ T cell induction is consistent with studies with SLPs that could harbor both class I and class II epitopes. Murine studies show that class II epitopes enhance tumor control and that CD4+ tumor-infiltrating T cells are required for effective anti-tumor responses (Alspach et al., 2019). Definitive studies on the relative roles of vaccine-induced CD4+ and CD8+ T cells in direct tumor cell killing are thus required. Furthermore, as only a fraction of predicted epitopes generated an immune response, algorithmic improvements are necessary for prioritizing class I neoantigens to maximize the CD8+ T cell response. The inclusion into current algorithms of three-dimensional structural information on epitope presentation within the MHC groove could potentially improve the selection of immunogenic neoantigens (Zaidi et al., 2020).
Although prior studies have documented the induction of de novo neoantigen-specific T cells in peripheral blood and trafficking into the tumor, this is one of the first to report “epitope spreading” —notably, vaccine induced T cells to tumor neoantigens that were highly predicted but not included in the vaccine (Figure 1). Such epitope spreading correlated with improvements in PFS, a phenomenon suggesting that vaccine induced tumor cell lysis and neoantigen release. Furthermore, in the melanoma cohort, patients with better disease control had more peripheral effector memory CD8+ T cells post-vaccination, a more restricted TCR, and high levels of stem-like memory T cell gene expression in tumor biopsies. These studies not surprisingly suggest that improved PFS correlates with higher-quality T cells in peripheral blood and tumor.
Figure 1. Immune Responses in Patients Responding to Combinatorial Therapy with Nivolumab and a Personalized Vaccine Containing SLPs Corresponding to Patient-Specific Tumor Neoantigens.
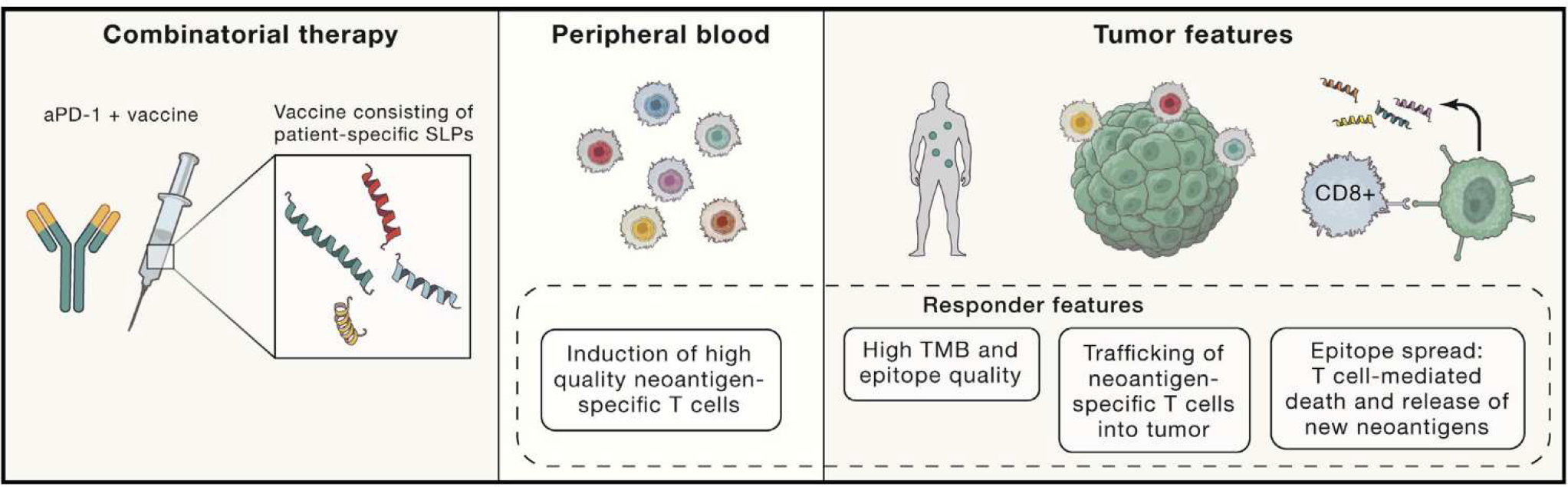
These “responders” displayed a higher baseline tumor mutation burden (TMB), better peripheral T cell quality (specifically increased effector memory CD8+ T cells), a more restricted T cell repertoire, and higher levels of stem-like memory T cell signatures in the tumor. Cytotoxic neoantigen-specific T cells were detected in peripheral blood and were able to traffic into the tumor to recognize and kill tumor cells. Epitope spreading was also detected likely as a result of vaccine-induced, T cell-mediated tumor cell death causing the release of neoantigens. Further detailed studies should characterize the immune response in non-responders in order to improve response rates.
Three additional therapeutic issues arise in the broader context of combinatorial immunotherapy with neoantigen vaccines. First, major pathologic responses were noted post-vaccination in melanoma patients who did not initially respond to nivolumab. It is nonetheless unclear whether pre-vaccine nivolumab contributed to vaccine-induced T cell responses. Moreover, we know that sub-optimally primed T cells are not optimal targets for reinvigoration by anti-PD-1 (Verma et al., 2019). Alternative immune-oncologic agents, such as anti-CTLA-4, that can preferentially expand naive CD8+ T cells to increase the T cell pool for vaccine priming, might be better a choice for pre-vaccine immunotherapy (Hopkins et al., 2018). But, noting the need for therapy during the time required to synthesize SLPs for personalized vaccine, it was not unreasonable that the authors used nivolumab. That said, refining the sequence of different agents in combination immunotherapy for best clinical outcomes needs further study.
Second, this is the first report on combining a neoantigen vaccine and anti-PD-1 antibody in a metastatic cancer. Although in this single arm study, de novo, durable, specific, and high-quality T cells were induced, the clinical benefit appeared similar to anti-PD-1 alone. As the authors correctly point out, a randomized study comparing ICIs versus vaccine plus ICIs would be needed to determine any additive benefit of vaccine. It will be equally important to identify biomarkers that can identify patients who require vaccine-activated T cells for optimal anticancer responses to ICIs.
Finally, to further improve on patient response rates, it is critical to characterize and compare neoantigen-specific T responses in patients who did respond versus those who did not. Understanding which neoantigens served as rejection antigens in responders would constitute important new data to guide future neoantigen prediction methods. In addition, it is also important to identify additional inhibitory and/or agonist signals on tumor-infiltrating T cells in non-responders that may require further modulation for optimal T cell function. Such studies conducted in larger cohorts and in a range of cancer types should lead to the development of the most optimal neoantigen-targeted vaccine approaches that induce the highest quality anti-tumor T cell responses.
REFERENCES
- Alspach E, Lussier DM, Miceli AP, Kizhvatov I, DuPage M, Luoma AM, Meng W, Lichti CF, Esaulova E, Vomund AN, et al. (2019). MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature 574, 696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilf N, Kuttruff-Coqui S, Frenzel K, Bukur V, Stevanović S, Gouttefangeas C, Platten M, Taba-tabai G, Dutoit V, van der Burg SH, et al. (2019). Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature 565, 240–245. [DOI] [PubMed] [Google Scholar]
- Hopkins AC, Yarchoan M, Durham JN, Yusko EC, Rytlewski JA, Robins HS, Laheru DA, Le DT, Lutz ER, and Jaffee EM (2018). T cell receptor repertoire features associated with survival in immunotherapy-treated pancreatic ductal adenocarcinoma. JCI Insight 3, e122092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keskin DB, Anandappa AJ, Sun J, Tirosh I, Mathewson ND, Li S, Oliveira G, Giobbie-Hurder A, Felt K, Gjini E, et al. (2019). Neoantigen vaccine generates intratumoral T cell responses in phase lb glioblastoma trial. Nature 565, 234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott PA, Hu Z, Keskin DB, Shukla SA, sun J, Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, et al. (2017). An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 547, 217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott PQ, Hu-Lieskovan S, Chmielowski B, Govindan R, Naing A, Bhardwaj N, Margolin K, Awad MM, Hellmann MD, Lin JJ, and Fried-lander T (2020). A Phase lb Trial of Personalized Neoantigen Therapy Plus Anti-PD-1 in Patients with Advanced Melanoma, Non-small Cell Lung Cancer, or Bladder Cancer. Cell 183, this issue, 347–362. [DOI] [PubMed] [Google Scholar]
- Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Löwer M, Bukur V, Tadmor AD, Luxemburger U, Schrörs B, et al. (2017). Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547, 222–226. [DOI] [PubMed] [Google Scholar]
- Verma V, Shrimali RK, Ahmad S, Dai W, Wang H, Lu S, Nandre R, Gaur P, Lopez J, Sade-Feldman M, et al. (2019). PD-1 blockade in subprimed CD8 cells induces dysfunctional PD-1+CD38hi cells and anti-PD-1 resistance. Nat. Immunol. 20, 1231–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarchoan M, Hopkins A, and Jaffee EM (2017). Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 377, 2500–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi N, Soban M, Chen F, Kinkead H, Mathew J, Yarchoan M, Armstrong TD, Haider S, and Jaffee EM (2020). Role of in silico structural modeling in predicting immunogenic neoepitopes for cancer vaccine development. JCI Insight 5, 136991. [DOI] [PMC free article] [PubMed] [Google Scholar]


