Summary
Gorillas reside in sympatry with chimpanzees over the majority of their range. Compiling all known reports of overlap between apes and augmenting these with observations made over twenty years in the Ndoki Forest, we examine the potential predation-related, foraging, and social contexts of interspecific associations between gorillas and chimpanzees. We reveal a greater diversity of interactions than previously recognized, which range from play to lethal aggression. Furthermore, there are indications that interactions between ape species may serve multiple functions. Interactions between gorillas and chimpanzees were most common during foraging activities, but they also overlapped in several other contexts. From a social perspective, we provide evidence of consistent relationships between particular chimpanzee-gorilla dyads. In addition to providing new insights into extant primate community dynamics, the diversity of interactions between apes points to an entirely new field of study in early human origins as early hominins also likely had opportunities to associate.
Subject areas: Biological sciences, Zoology, Behavioral neuroscience, Animal
Graphical abstract
Highlights
-
•
First evidence of social relationships between chimpanzees and gorillas is reported
-
•
Social ties between chimpanzees and gorillas persisted over years and across contexts
-
•
Ape species engaged in a wide range of interactions, from play to aggression
-
•
Coexisting great apes may inform us about interactions between some early hominins
Biological sciences; Zoology; Behavioral neuroscience; Animal
Introduction
Primate communities may be comprised of ten or more species, with local compositions and interactions determined by abiotic and biotic factors operating at variable spatial scales (Callaway and Walker, 1997; Leibold et al., 2004; Chase and Myers, 2011). The organization of these communities has typically been attributed to bottom-up forces, wherein populations are regulated by resource limitations (Lotka, 1925; Volterra, 1926). Interspecific associations can generally be defined as two or more species in such close proximity that they can be regarded as members of the same social unit, during which they may engage in both direct and indirect interactions characterized by territoriality, aggressive behavior, agonism, or competition over shared resources (Levins, 1979; Armstrong and McGehee, 1980). From a traditional perspective, sympatric species that occupy similar ecological niches would be expected to intensely compete and eventually exclude each other from communities (Volterra, 1926; Gause, 1934; Hardin, 1960). However, recent research across an array of species has revealed complex combinations of both resource competition and facilitation (Schoener, 1983; Callaway and Walker, 1997). It has been shown that a species can actually enhance the survival, growth, or fitness of another species by providing protection from predation, accumulating nutrients consumed by the other species, or even by mitigating disturbance (Hunter and Aarssen, 1988; Wilson and Agnew, 1992; Bertness and Callaway, 1994; Callaway and Walker, 1997; Callaway et al., 2002).
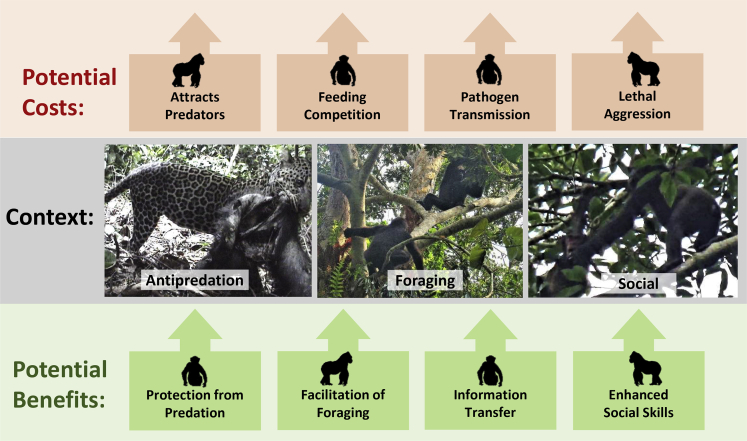
Theoretical expectations suggest that predator avoidance and/or enhanced foraging may be potential benefits of mixed-species associations, while costs may include increased feeding competition or parasitism (Terborgh, 2015) (Figure 1). Associating with another species facing similar predators could improve the probability of predator detection (Kenward, 1978), lead to predator confusion (Cott, 1940; Edmunds, 1974), increase dilution effects (Hamilton, 1971), or enhance the ability to more effectively deter predators (Curio, 1978). Furthermore, the benefits of locating food more efficiently through another species’ knowledge of food availability and/or their differential ability to access particular food sources may defray the potential costs of interspecific associations (Wolters and Zuberbühler, 2003; Pinheiro et al., 2011). There are also potential social benefits of interspecific grouping which may include establishing tolerant relationships (Struhsaker, 1981), grooming services (Struhsaker, 1981), alloparental care (Stensland et al., 2003), and reproductive advantages of increasing group size without increasing breeding competition (Dunbar, 1993; Buchanan-Smith, 1999; Farmer et al., 2006). Furthermore, it should be noted that these potential functions need not be mutually exclusive (Wolters and Zuberbühler, 2003) and that one primate species may benefit more than another (Cords, 1990) or at another’s cost (Boinski, 1989).
Figure 1.
Costs and benefits of interspecific interactions between great apes
The contexts of interspecific associations are predation-related, foraging, and social. The potential benefits of interspecific associations are listed, as well as the deterrents of chimpanzee and gorilla association. It should be noted that these are not mutually exclusive and that interspecific interactions occur in several contexts and may serve multiple functions.
The potential benefits of interspecific associations among monkeys have been relatively well documented (Table 1). However, comparable assessments of sympatric great apes are lacking despite the fact that gorillas (Gorilla spp.) and chimpanzees (Pan troglodytes) reside in sympatry over the majority of their range (Table 2 and Figure 2). It is challenging to observe interspecific interactions of these species as there are few sites where both species have been habituated to the presence of researchers. In the few studies that have considered gorilla and chimpanzee sympatry, potential anti-predation advantages have generally been considered irrelevant because it was assumed that the large body sizes of great apes were sufficient to deter predation. However, documented leopard attacks provide evidence to the contrary (Boesch, 1991; Fay et al., 1995). Increased size of interspecific groups could reduce the risk of predation without the increased feeding and mating competition associated with larger groups of conspecifics. Furthermore, recent reports of chimpanzees killing immature gorillas (Southern et al., 2021) prompted us to predict that vulnerable gorillas would avoid close interaction with adult chimpanzees if they posed a risk.
Table 1.
Overview of the contexts (which may be related to functions) and benefits of interspecific associations among monkeys
| Context/Function | Benefit | Reference | |
|---|---|---|---|
| New World Monkeys | |||
| Golden-headed lion tamarin (Leontophithecus chrysomelas); Wied’s marmoset (Callithrix kuhlii) | F, A | Both | Oliveira and Dietz, 2011; Rocha et al., 2015 |
| Squirrel monkey (Saimiri sciureus); Tufted capuchin (Cebus apella); Bearded saki (Chiropotes satanas, C. utahickae); Howler monkey (Alouatta belzubul); Tamarin (Sanguinus niger); Owl monkey (Aotus azarae); Titi monkey (Callicebus moloch) | F | All | Pinheiro et al., 2011 |
| Golden-headed lion tamarin (Leontophithecus chrysomelas); Wied’s marmoset (Callithrix kuhlii) | F, A | Both | Oliveira and Dietz, 2011; Rocha et al., 2015 |
| Squirrel monkey (Saimiri sciureus); Tufted capuchin (Cebus apella); Bearded saki (Chiropotes satanas, C. utahickae); Howler monkey (Alouatta belzubul); Tamarin (Sanguinus niger); Owl monkey (Aotus azarae); Titi monkey (Callicebus moloch) | F | All | Pinheiro et al., 2011 |
| Squirrel monkey (Saimiri oerstedii); White-faced capuchin (Cebus capucinus) | A | Cebus only∗ | Boinski, 1989 |
| Callitrichines review (various: Sanguinus fuscicollis avilapiresi; S. f. fuscicollis; S. f. nigrifrons; S. f. weddelli; S. f. melanoleucus; S. mystax; S. m. pileatus; S. m. mystax; S. imperator subgrisescens; S. labiatus; Callithrix emiliae; C. goeldii) | F, A | All∗ | Heymann and Buchanan-Smith, 2000 |
| Old World Monkeys | |||
| Diana monkey (Cercocebus aethiops sabaeus); Olive colobus (Procolobus verus) | (A) | Both | Whitesides, 1989 |
| Diana monkey (Cercopithecus diana); Campbell’s monkey (Cercopithecus campbelli) | F, A, S | Both | Wolters and Zuberbühler, 2003 |
| Diana monkey (Cercopithecus diana); Red colobus (Piliocolobus badius) | A | Both | Holenweg et al., 1996; Bshary and Noe, 1997 |
| Grey-cheeked mangabey (Lophocebus albigena); Blue monkey (Ceropithecus mitis); Redtailed monkey (Ceropithecus ascanius); Black-and-white colobus (Colobus guereza); Red colobus (Piliocolobus badius) | (F, A) | All | Chapman and Chapman, 1996 |
| Grey-cheeked mangabey (Lophocebus albigena); Redtailed monkey (Cercopithecus ascanius) | (F, A) | Both | Waser, 1982 |
| Grey-cheeked mangabey (Lophocebus albigena); Redtailed monkey (Cercopithecus ascanius) | (A) | Both | Bryer et al., 2013 |
| Redtailed monkey (Cercopithecus ascanius); Blue monkey (Cercopithecus mitis) | F, A | Both∗ | Cords, 1990 |
“Context/Potential Function”: F = foraging efficiency, A = anti-predation, S = social advantages (if listed within parentheses, context-dependent functional advantages were reported). “Benefit” defines which species (or all) were reported to benefit from the mixed species associations. Other species may have been studied in these listed investigations (e.g., Waser, 1982; Whitesides, 1989); however, only species discussed with possible functional advantages to mixed species associations are listed here. ∗Note: Heymann and Buchanan-Smith (2000) review the literature on polyspecific callitrichine troops, reporting very low costs to mix-species association of this taxa; Boinksi (1989) reports C. capucinus to be the primary benefactor (anti-predation) of their sustained association with S. oerstedii who may experience a plausible reduction of foraging efficiency; Cords (1990) reports that C. mitis appear to be the primary benefactors in some instances while both species experience only minor costs due to mixed-species associations.
Table 2.
Reports of interspecific association between sympatric chimpanzees and gorillas
|
Ape Taxa, Study Site Location |
Period | Interspecific Interactions |
|||
|---|---|---|---|---|---|
| Foraging | Spatial Intersection | Agonism | Not Known | ||
| G. g. gorilla, P. t. troglodytes | |||||
| Goualougo, Rep. Congoa | 1999–2020 | 148 | 117 | 20 | |
| Mondika, Rep. Congob | 1999–2020 | + | |||
| Ndoki, Rep. Congoc | 1989–1992 | 4 | 2 | ||
| Rio Muni, Equat. Guinead | 1966–1968 | – | 1 | ||
| Lope, Gabone | 1984–1993 | ? | |||
| Loango, Gabonf | 2005–2019 | 2 | 2 | 7 | |
| Ndakan, C.A.R.g | 1986–1990 | + | |||
| G. b. beringei, P. t. schweinfurthii | |||||
| Bwindi, Ugandah | 1996–2005 | 5 | |||
| Kahuzi, Dem. Congoi | 1994–2002 | 3 | 7 | ||
Foraging includes both cofeeding and feeding in proximity. Spatial intersection included chimpanzees and gorillas being observed within 50m of each other, but with no apparent interaction. At Loango, two particular interactions between chimpanzees and gorillas involved lethal aggression and so were categorized as agonism. Social interactions among sympatric apes in Goualougo were observed in various contexts.
+ observed, but number of occurrences not available; – not observed; ? information not available.
This study.
Morgan, pers comm.
Fay, personal comm.
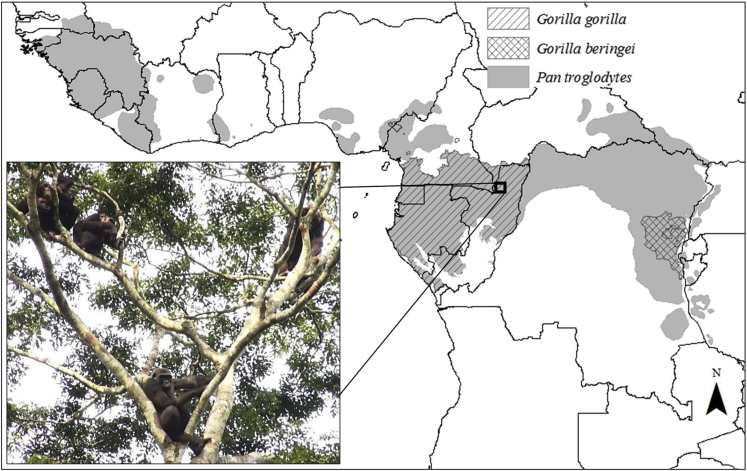
Figure 2.
Species distribution and range overlap of wild chimpanzees and gorillas
Both western lowland gorillas (Gorilla gorilla) and mountain gorillas (Gorilla beringei) reside in sympatry with chimpanzees (Pan troglodytes) throughout most of their ranges. Inset shows members of the Moto chimpanzee community and Loya gorilla group cofeeding in the crown of a Treculia africana tree in the Goualougo Triangle, Republic of Congo.
Interactions between sympatric great apes have most commonly been characterized as strategies to avoid competition or as direct competition over food sources (Tutin and Fernandez, 1985, 1993; Kuroda, 1992; Yamagiwa et al., 1996a, 1996b; Basabose and Yamagiwa, 2002). Based on the nest site choice of sympatric apes in east Africa, it was concluded that chimpanzees avoided nesting in trees bearing fruits eaten by mountain gorillas, Gorilla beringei (Basabose and Yamagiwa, 2002). Scientists observed contest competition between chimpanzees and mountain gorillas in the Bwindi forests of Uganda (Stanford and Nkurunungi 2003). The counterparts of mountain gorillas in western Africa, lowland gorillas (Gorilla gorilla) are more frugivorous than eastern gorillas (Gorilla beringei) and thus one might expect them to show a higher degree of overlap with chimpanzees than their eastern counterparts who incorporate less fruit in their diet. There is some evidence that foraging western lowland gorillas and chimpanzees converge during times of fruit abundance and diverge during fruit scarcity (Tutin and Fernandez, 1993; Tutin et al., 1996). Sharing of tree crowns by ape species was observed during foraging in the central African forests of Ndoki (Suzuki, 1992; Yamagiwa et al., 1996a, 1996b). In contrast, scientists reported two observations of lethal attacks of chimpanzees on immature gorillas in Gabon (Southern et al., 2021). Apes may also alter their habitat use, activity patterns, diet selection, or foraging strategies to avoid interspecific overlap through either spatial or temporal niche partitioning (Schoener, 1983; Mitchell et al., 1990; Ziv et al., 1993; Eccard and Ylonen, 2003).
Given this evidence, consistent observations of great ape overlap likely represents active choice over avoidance or simply chance encounters. Although not yet detailed in the literature, there are potential social benefits of mixed-species associations and social relationships between great ape species. In addition to establishing tolerant relationships and gaining services (such as grooming or alloparental care), the potential for information transmission should also not be overlooked as both chimpanzees and gorillas are capable of social learning. Interspecific associations and social interactions may provide pathways for the spread of behaviors and innovations between ape societies. However, a potential trade-off of social contact between closely related species is the spread of infectious diseases. This study will contribute to the long-term monitoring of social contacts between sympatric apes to provide quantifiable metrics on potential cross-species pathways of disease transmission which is a conservation concern for these endangered species.
Study aims
In this study, we compile previously published observations of interactions between sympatric great apes and augment this with new longitudinal studies of identified apes in the Goualougo Triangle to examine the contexts and potential functions of these interspecific associations. More specifically, we categorized these interspecific associations as cofeeding (foraging on the same food source, such as within the same tree crown), feeding in proximity (feeding within 50m, but not on the same food source), spatial intersection (within 50m, but not foraging or interacting), or social interaction (aggression, play, or sexual encounters between members of different species). In this study, we predict that if interspecific ape groups aid in avoiding predation, then we would expect both ape species to recognize the alarm call of the other species and that smaller chimpanzee parties would frequently associate with gorillas for protection from predators. We also predict that more vulnerable chimpanzee parties, such as those comprised of a single individual or a mother with a dependent offspring might be more likely to associate with gorillas than parties comprised of a greater number of adult chimpanzees, particularly parties with large numbers of adult males. If chimpanzees threaten the survival of vulnerable gorillas, then we would expect to see attempted capture of gorilla infants by chimpanzees or avoidance by gorillas with infants of groups of chimpanzees, particularly groups with adult male chimpanzees. If mutual attraction to food sources is a driving force in associations between species, then we would expect overlap between apes to coincide with the availability of particular foods. It is also possible that one species may capitalize on the other’s knowledge of rare and asynchronously available foods (such as figs). If either ape species is gaining social benefits, then we would expect to detect consistent social contacts and relationships between species. In reviewing all reported interactions between sympatric apes, we consider the detection of overlap events at different sites and the frequency of interspecific associations in various contexts (e.g., foraging, spatial intersection, agonism). It is clear that the present study overcomes two key challenges that are associated with the study of sympatric great ape social interactions: 1) both species are habituated to the presence of researchers and 2) members of the great ape groups are individually identifiable by researchers. With regard to the biological importance of interspecific interactions, they directly link to fitness via reduction of predation risk and enhanced foraging potential which is essential to understanding the present and past primate communities.
Results
In our review of published reports, we found a total of 33 documented interspecific interactions (and two other unpublished interactions) at eight sites, representing studies conducted from 1966 to 2020 (Table 2). While conducting daily follows of chimpanzees and gorillas from 1999 to 2020 in the Goualougo Triangle, we observed an additional 285 interspecific associations between great apes. More specifically, research teams tasked with following chimpanzees in the Moto Community located in the Goualougo Triangle study area observed 206 interspecific associations with gorillas between July 1999 and February 2020. In 2013, a gorilla group within the Moto chimpanzee territory was habituated to humans and teams following the gorilla group observed 62 interspecific associations between February 2013 and February 2020. On 17 occasions, both field teams were present during interspecific associations. Duration of association between chimpanzees and gorillas ranged from 1 min to more than 8 h, with a mean of 67.9 min (median = 32.5, n = 78 cases in which full duration was observed). Importantly, our reported encounters represent minimal frequencies of occurrence as it was not possible to simultaneously follow all chimpanzee parties or conduct ape follows every day throughout the study period.
Ape associations and predation
The anti-predation hypothesis predicts that apes would attend and respond to the alarm calls of the other species and that smaller groups would seek association with other species to increase vigilance and overall subgroup size. Both species were observed responding to the alarm vocalizations of the other species. Responses included increasing vigilance by orienting toward the origin of the call and visually monitoring the response of other apes in the subgroup. We did not observe gorillas or chimpanzees emitting alarm calls in response to the arrival of the other ape species to a subgroup. No predation attempts between the ape species were observed in this study, but chimpanzees at Loango have been observed killing infant gorillas on two occasions (Southern et al., 2021). However, we did observe bidirectional aggressive threats and contact agonism between apes in the Goualougo Triangle. We also noted behavioral mechanisms which may mitigate such predation attempts, such as adult female gorillas retrieving their young infants who approached and attempted to initiate play with young chimpanzees. However, several play bouts between a subadult male chimpanzee and juvenile male gorilla were observed. Contrary to staying in close proximity to the silverback as protection from predation, juvenile and subadult gorillas regularly traveled more than 300 m from their group to join a chimpanzee party.
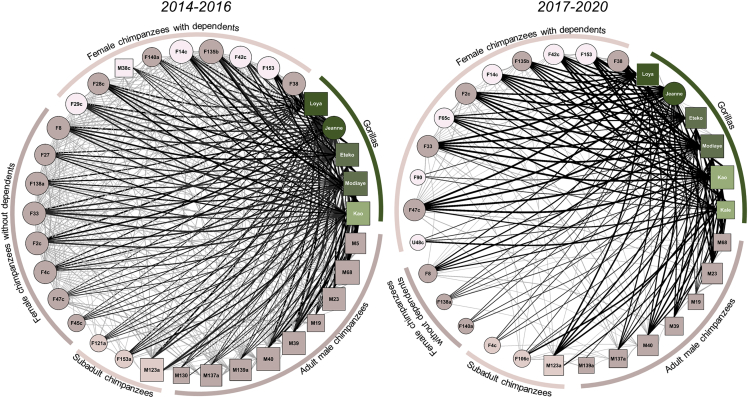
Counter to predictions of reducing vulnerability by increasing numbers, we found that the initial chimpanzee parties in association with gorillas were larger than parties consisting only of chimpanzees (Table 3). The anti-predation hypothesis predicts that lone individuals would more frequently associate with gorillas than other types of chimpanzee parties, but we found that interspecific associations occurred in all types of chimpanzee parties. Interspecific relationships were strongest between gorillas and adult female chimpanzees with dependents (Figure 3), followed by gorilla associations with mature male chimpanzees, and then mature chimpanzee females without infants.
Table 3.
The size of chimpanzee parties when alone and in association with gorillas
| Chimpanzee Party Type | Chimpanzee Parties |
Number of Chimpanzees in Interspecific Associations |
||
|---|---|---|---|---|
| Party Size | Percent of Parties | Chimpanzees in Interspecific Association | Percent of Interspecific Associations | |
| Mixed Age/Sex Party | 8.66 ± 4.03 | 54% | 12.71 ± 4.71 | 59% |
| Adults Party | 4.67 ± 1.85 | 17% | 5.86 ± 2.57 | 10% |
| Females Party | 4.19 ± 1.71 | 12% | 5.63 ± 2.22 | 14% |
| Males Party | 2.61 ± 0.84 | 3% | 3.00 ± 0 | 3% |
| Mother and Offspring | 2.30 ± 0.50 | 5% | 2.00 ± 0 | 7% |
| Lone Individual | 1.00 | 5% | 1.00 | 3% |
| Undetermined | 2.48 ± 0.74 | 3% | 2.00 ± 0 | 3% |
Chimpanzee parties consisted of all individuals travelling, feeding, resting, or socializing within 50m of one another (adopted from Wrangham et al., 1992 and Wilson et al., 2001). Interspecific associations can generally be defined as two or more species in such close proximity that they can be regarded as members of the same group. We have applied this definition to identify interspecific associations.
Figure 3.
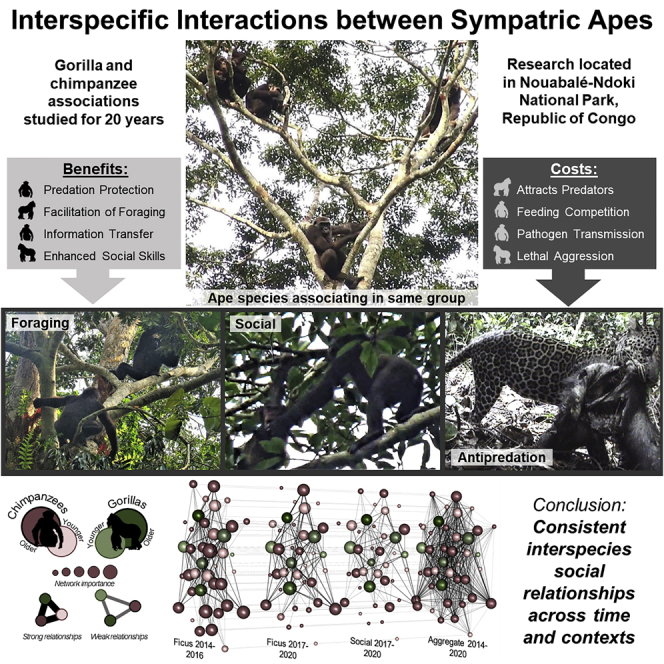
Networks of dyadic associations between chimpanzees and gorillas across time periods
Chimpanzees (rose-colored nodes) and gorillas (green-colored nodes) maintained a selective set of relationships with particular individuals. Also, gorillas associated twice as much with chimpanzee females that had dependent offspring (0.37 ± 0.14) compared to either chimpanzee males (0.18 ± 0.09) or females without dependents (0.12 ± 0.07). Female apes are represented by circles and males as squares. All nodes are shaded darker with age and scaled in size to represent eigenvector centrality. Strong relationships are represented by thicker and darker lines.
While gathering in large nesting groups could serve to reduce the risk of predation at night, previous research has shown that gorillas and chimpanzees have distinct habitat preferences for nesting which could preclude association in this context (Morgan et al., 2006; Sanz et al., 2007). In our transect surveys of ape nests, we observed that only 2.4% of all fresh chimpanzee nest sites/groups (5 of 206 total sites) were within 50m of a gorilla nest site. A slightly higher percentage of fresh gorilla nest sites (6.7%, 7 of 105 sites) were found within 50m of chimpanzee nest groups.
Ape associations in foraging-related contexts
The enhanced foraging hypothesis predicts that interspecific associations between apes would coincide with particular foods. Supporting this assertion, these gorillas and chimpanzees are most often associated in contexts where both species were feeding either on the same food source (cofeeding) or on different foods but in close proximity. Cofeeding at the same food source (e.g., same tree) represented 34% of interspecific associations, with another 18% of observations involving apes foraging in close spatial proximity but on different foods. At least 20 different plant species were targeted by apes during cofeeding events in this study (Table 4), which greatly expands our knowledge of the diversity of resources shared during interspecific associations. Despite the extreme rarity of Ficus spp. in this region, figs were consumed during 64% of the observed cofeeding events.
Table 4.
Food sources targeted by chimpanzees and gorillas during cofeeding events
| Family | Species of Plant | Lifeform | Size Class of Trees | Food Part Eaten during Cofeeding | Observed Cofeeding Events |
|---|---|---|---|---|---|
| Apocynaceae | Landolphia sp. | liane | fruit | 1 | |
| Calophyllaceae | Mammea africana | tree | small, medium | fruit | 2 |
| Cannabaceae | Celtis tessmannii | tree | large | fruit | 1 |
| Fabaceae | Angylocalyx pynaertii | tree | small, medium | fruit | 1 |
| Dialium pachyphyllum | tree | small | fruit | 1 | |
| Pterocarpus soyauxii | tree | small, medium, large | fruit | 1 | |
| Tetrapleura tetraptera | tree | medium, large | fruit | 1 | |
| Irvingiaceae | Klainedoxa gabonensis | tree | large | fruit | 4 |
| Loganiaceae | Strychnos sp. | liane | fruit | 1 | |
| Malvaceae | Ceiba pentandra | tree | small | fruit | 2 |
| Moraceae | Antiaris toxicaria | tree | large | fruit | 1 |
| Ficus burretiana | hemi-epiphyte, strangler | fig | 1 | ||
| Ficus elasticoides | hemi-epiphyte, strangler | fig | 33 | ||
| Ficus exasperata | hemi-epiphyte, strangler | fig | 1 | ||
| Ficus recurvata | strangler | fig | 12 | ||
| Ficus wildemaniana | hemi-epiphyte, strangler | fig | 2 | ||
| Ficus spp. | hemi-epiphyte, strangler | fruit | 13 | ||
| Treculia africana | tree | medium | seeds, fruit | 7 | |
| Rubiaceae | Nauclea diderrichii | tree | medium | fruit | 1 |
| Sapindaceae | Pancovia laurentii | tree | small, medium, large | fruit | 4 |
| Zanha golungensis | tree | medium, large | fruit | 1 | |
| Sapotaceae | Chrysophyllum lacourtianum | tree | medium, large | fruit | 4 |
| Unknown | Unidentified Unidentified |
fruit flowers |
1 1 |
Cofeeding events involved at least one member of both species feeding at the same food source (e.g., in the same tree crown). Lifeform describes plant morphology of the species cofed upon by sympatric apes (see Ndolo Ebika et al., 2018 for the lifeforms of Ficus spp.). Size classes of trees are small (10-30 cm diameter at breast height, DBH), medium (30-80 cm DBH), and large (>80 cm DBH).
To assess whether one species was potentially benefiting from the knowledge of the other species, we examined the temporal pattern of interspecific associations at cofeeding events when we followed the gorilla group two days prior to, during, and for two days after cofeeding events with chimpanzees. A total of 10 cofeeding events met this criterion, with five of the trees representing species with synchronized fruiting and the other five being asynchronous Ficus resources (figs). At four of the synchronous resources, the gorilla group was observed feeding at the trees on days prior to the cofeeding event and continued to feed on these trees for two days after, suggesting that their presence at the trees was independent of chimpanzees. In contrast, gorillas were not observed visiting any of the five Ficus locations on the two days prior to the cofeeding event and visitation to figs was rare on the days after cofeeding. During ape follows, we also observed the gorilla group immediately change their travel direction to head toward chimpanzee vocalizations originating from the canopy of Ficus with ripe figs. Chimpanzees did not exhibit any similar behaviors that would indicate they were attracted to food sources by gorillas.
Social relationships between ape species
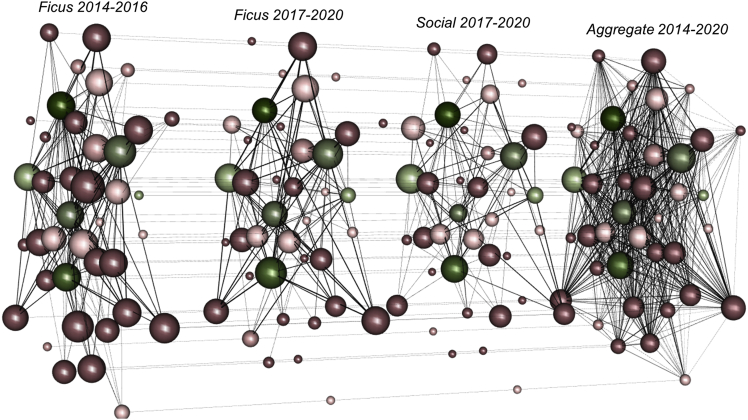
The beneficial sociality hypothesis predicts that species are attracted to mixed-species associations by the social benefits of interactions with and/or forming a relationship with an individual(s) of another species. Network statistics were used to assess the strength of social associations (calculated as simple ratio indexes) between individually identifiable gorillas and chimpanzees. We confirmed that the observed distribution of gorilla and chimpanzee association rates significantly differed from those that would be expected by randomly permuted network datasets (2014-2020 observed mean ± s.d. = 0.16 ± 0.17, random = 0.15 ± 0.15; p < 0.001, 1000 permutations (Manly, 1995; Bejder et al., 1998; Whitehead, 1999; Stensland et al., 2003; Whitehead, 2008)). Furthermore, we found that particular dyads were associated on multiple occasions and across different contexts (cofeeding, resting, traveling, socializing) over a period of at least six years (Figure 3). We confirmed the consistency of these associations using a subset of the multidimensional network that contained those individuals active in all layers (mean edge overlap = 71.07%; Spearman correlation analysis; see STAR Methods). All layers were highly correlated (Ficus 2014 to 2016 – Ficus 2017 to 2020: rs = 0.93; Ficus 2014 to 2016 – social 2017 to 2020: rs = 0.82; Ficus 2017 to 2020 – social 2017 to 2020: rs = 0.82).
In accordance with conventional definitions, we refer to repeated patterns of both associations and interactions between individually identifiable chimpanzees and gorillas as social relationships (Figure 4) (Sueur et al., 2011; Farine and Whitehead, 2015). Although on average chimpanzees had more total social ties (degree, chimpanzee: 30.67 ± 8.58, n = 36; gorilla: 27.6 ± 1.34, n = 5) and displayed higher levels of social integration (eigenvector centrality, chimpanzee: 0.48 ± 0.32, n = 36; gorilla: 0.14 ± 0.03, n = 5) than gorillas when feeding on figs, the range of network measures for all the individual gorillas in this context was within the range of chimpanzees. The silverback male had the lowest eigenvector centrality score among the gorillas in this context, whereas the younger male gorillas had the highest measures of connectedness and social integration. This was confirmed by average dyadic simple ratio index associations of gorillas from 2017 to 2020 which showed that the younger males (Kao = 0.29 ± 0.17, Modiaye = 0.26 ± 0.16, Eteko = 0.22 ± 0.16) had stronger collective ties to chimpanzees and engaged in more social relationships with chimpanzees than the silverback (0.21 ± 0.12) or female (0.24 ± 0.16) and infant (0.21 ± 0.10). In accordance with differences in network ties between chimpanzee-gorilla dyads, we observed that some individuals repeatedly sought particular social partners over time.
Figure 4.
Multidimensional social network of interspecific relationships across contexts and time
The graphs show gorillas (green-colored nodes) and chimpanzees (rose-colored nodes) encountered in parties together while cofeeding on Ficus from 2014 to 2016, cofeeding on Ficus from 2017 to 2020, in other social contexts from 2017 to 2020, and aggregated across all contexts from 2014 to 2020. Node size is scaled to layer-specific eigenvector centrality (larger nodes indicate greater network importance) and shaded darker with age. Edge widths are scaled to the strengths of gorilla-chimpanzee dyadic relationships (thicker lines indicate stronger relationships). Horizontal lines across these network layers connect nodes that represent the same individual across all contexts. Overall, the integrated structure of these networks across time and contexts highlights the consistency in relationships between chimpanzees and gorillas.
We observed a range of social interactions between chimpanzees and gorillas in the Ndoki Forest, ranging from affiliative to aggressive encounters. Interspecific aggression was bidirectional and most frequently consisted of threats. During this study in the Goualougo Triangle, contact aggression was rare and never escalated to the types of lethal attacks documented in Loango, Gabon (Southern et al., 2021). Affiliative interactions included play with individuals of both species engaged in chasing, wrestling, play biting, and play hitting. An interspecific sexual interaction was observed in which a juvenile male gorilla repeatedly dorsally mounted and thrust his groin into contact with the anogenital region of an immature female chimpanzee. We also observed gesturing between species to initiate social interactions. Intriguingly, chimpanzees exhibited chest-beating which is a behavior characteristic of gorillas. Although most interactions involved a chimpanzee-gorilla pair, we also observed polyadic interactions.
Discussion
In this study, we reveal a greater diversity of interactions than previously documented among sympatric apes, including social relationships between members of different species that persisted over years. Furthermore, we found that interspecific associations between great apes occur in several different contexts and thus may have multiple functions. Supporting the anti-predation hypothesis, both species appropriately responded to the alarm calls of the other species which could increase the detection of potential threats. We have also observed apes approach, investigate, and retreat from recently killed victims of predation that were the other ape species. However, larger chimpanzee parties associated with gorillas more often than smaller parties which are counter to the antipredation hypothesis and could result in increased feeding competition. Some observations could be interpreted either way, such as those of individual gorillas leaving the protection of their group’s silverback to join a chimpanzee party that was more than a hundred meters away. The majority of associations between gorillas and chimpanzees occurred in foraging contexts and we observed cofeeding at many more types of food sources than previously documented. In contrast to predictions of competition between species, nearly all interspecific associations were tolerant or affiliative. Aggression was observed between gorillas and chimpanzees, but did not escalate to killing as reported from Loango, Gabon (Southern et al., 2021). The aim of our study was to broaden perspectives about interspecific interactions that occur within primate communities, particularly those among sympatric great apes, which can be used to inform reconstructions of the contacts and exchanges between early hominin species.
It has been hypothesized that differences in body size may allow closely related species to coexist, as has been shown in rodents (Brown, 1975) and birds (Lack, 1971; Cody, 1974; Diamond, 1975). Larger-bodied species are predicted to win contests and exploit food sources more effectively than smaller species (Eccard and Ylonen, 2003; Zeng and Lu, 2009). This has been proposed in Gabon where elephants excluded sympatric apes from particular foods (Head et al., 2012). Although Head et al did not examine the relationship between sympatric great apes, they argue that the distribution and abundance of food are more important than the degree of dietary overlap between competing large-bodied mammals. In contrast to predictions of competition between species, we found that members of the gorilla group were integrated within a social network with the chimpanzees that included repeated association and interactions.
The majority of associations occurred at a variety of Ficus spp. (Kuroda et al., 1996; Stanford, 2008) which is similar to reports of ape cofeeding on figs at other sites. Figs are asynchronous and found at extremely low densities across the region. Although further research is warranted on the temporal patterning of interspecific associations at particular food resources, we suggest that gorillas may exploit chimpanzee knowledge of the location of ripe figs. Based on our observations in the Goualougo Triangle, large parties of chimpanzees would often broadcast their location while feeding on figs which gorillas responded to by changing their travel orientation toward the food resource. Other loci of association consisted of large trees that produce fruits (e.g., Chrysophyllum lacourtianum, Pancovia laurentii) that are available for weeks compared to the relatively brief period of figs (3-4 days). On some occasions, part of the gorilla group would climb into the tree crown to feed with chimpanzees while others remained on the ground and fed upon fallen fruit. Further research on the availability of preferred foods across habitats is needed to improve our understanding of how competition and niche partitioning vary across ape populations.
Additional study of how relationships between socially acquainted gorillas and chimpanzees change with time and across contexts would also provide clarity on the construction, maintenance, and limits of interspecies tolerance. On several occasions at food sources, we observed young gorillas and chimpanzees seeking out particular partners to engage in bouts of play which may afford unique developmental opportunities to extend their social, physical, and cognitive competencies (Palagi, 2018). Subadults were observed to engage in interspecific play, but these social dynamics seemed to shift as they matured to adulthood. Additionally, interactions between apes may also take on different severity depending on where they occur in the species’ respective home ranges. Both species have core areas that are surrounded by peripheral zones which are visited less frequently and tend to have more aggressive interactions than core areas (Watts and Mitani, 2001; Morrison et al., 2020a, 2020b). For example, most of our observations of interspecific interactions occurred within the core area of the chimpanzee community range which is where the gorilla group resides. This is in contrast to observations of lethal killing in the periphery of a chimpanzee community range (Southern et al., 2021). Different groups of sympatric apes may have other types of home range overlap that influence their interactions. Future studies that spatially position interspecies interactions and outcomes within the contexts of the apes’ home ranges will shed light on the dynamics of territoriality and perceptions of risk in certain regions of their ranges. Further, determining the shared strategies of gorillas and chimpanzees holds the promise of revealing a greater depth of their social awareness than previously imagined.
Facilitated by social network connections, interspecific disease transmission is an unintended consequence of association and affiliative behavior. Cross-species transmission of infectious diseases has been known to occur between gorillas and chimpanzees, but the exact pathways and mechanisms of disease spillover remain speculative (Walsh et al., 2007). We observed several potential modes of pathogen transmission besides direct physical contact between individuals during play, aggression, and sexual interactions. Gorillas were observed feeding on fruit mesocarps that had been fed upon and discarded by chimpanzees. Also, gorillas were observed foraging on fruits and figs in the leaf litter under areas where chimpanzees had foraged, urinated, and defecated. Overlaying cofeeding events with food availability data could be useful in modeling potential areas of disease spillover events. Given that so many of the chimpanzee-gorilla interactions were observed in a feeding context, it is possible that detailed analyses of the diets and feeding adaptations in contemporaneous populations of fossil hominins (Henry et al., 2012; Ledogar et al., 2016; Martin et al., 2021) may also reveal previously unimagined insights into the paleobiology of our early relatives and ancestors.
There has been a long history in paleoanthropology of assuming that hominin species would competitively exclude each other from using the same resources in the same area (Gauss, 1934), to the point that it was once even hypothesized that only one hominin species could exist at any given time (Mayr, 1950; Wolpoff, 1971). The fossil record ultimately proved that hypothesis to be false (Leakey and Walker, 1976) and now behavioral data provide an African ape model for interspecies hominin interactions. With respect to later hominins, paleogenetics has confirmed sexual interactions between various such species. If observations of non-human apes are informative about the behaviors of archaic and early modern humans, then our study suggests these interactions would have most likely occurred in tolerant social contexts and were not contested by conspecifics.
Our review of interspecific associations between extant great apes indicates that they occur in several different contexts and may serve multiple functions, with broad implications for ape health and culture. It also provides a variety of advantages that are relevant to understanding the behavioral patterns of both present and past primate assemblages. Indeed, multiple hominin species are known to have contemporaneously occupied the same geographic regions, implying that they had opportunities to interact with each other. In eastern Africa, Paranthropus boisei and multiple species of early Homo are found in the Turkana Basin between roughly 2.0 and 1.5 million years ago (Ma) (Wood and Leakey, 2011). Hominin fossils are distributed in an expansive area around Lake Turkana, and there is some evidence that resident hominin species may have partitioned their environment (Behrensmeyer, 1978; Shipman and Harris, 1988; Reed, 1997), so it is difficult to assess how frequently interspecific associations might have occurred. However, in South Africa at ∼2.0 Ma, species of three hominin genera (Australopithecus sediba, P. robustus, and early Homo) occupy a landscape no larger than the Goualougo Triangle research area (Herries et al., 2020). Although sympatry among these taxa cannot be demonstrated conclusively, at a minimum they would have had adjacent ranges whose boundaries might have shifted over time (allowing their fossils to be deposited in the same small area). Thus, these hominins were likely either sympatric or parapatric, implying some opportunity for interaction and transmission of information. Although direct evidence of interspecies interactions in the early hominin fossil record will probably remain elusive, our findings point toward a research program modeling the nature and likelihood of such interactions given what is known about paleoecology, geography, adaptation, and the density of fossil sampling. In short, we can no longer assume that these interactions did not occur.
We show that extant chimpanzees and gorillas do not so completely partition their habitats to preclude regular interaction and the formation of lasting social relationships. This is interesting given that the chimpanzee, gorilla, and hominin clades diverged from each other during the Miocene when climatic conditions were becoming cooler and drier, which presumably would have placed ancestral ape populations under increasing ecological stress (e.g., Harrison, 2010). One might have expected that the divergence of these populations would have been hampered by associative behaviors, especially during the period prior to the evolution of reproductive isolation. It is worth considering whether evolutionary processes might have driven the divergence of these clades without eliminating some level of behavioral interaction, or if the interactions seen among gorillas and chimpanzees today are evolutionarily novel. Unfortunately, anthropogenic disturbances and degradation are removing the environments and resources that draw them together, jeopardizing the future of these apes and with them the opportunity to further understand the relationships between and knowledge shared by our closest living relatives (Kühl et al., 2019).
Limitations of the study
While this study provides the first comprehensive review of interspecific interactions among sympatric great apes, many of the groups included in previous reports were not habituated to human presence which is likely to have limited the behaviors observed. This research would also benefit from the inclusion of multiple groups of gorillas and chimpanzees at each site to assess variation in responses.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited data | ||
| Report of interspecific association between sympatric chimpanzees and gorillas | Kuroda (1992) | |
| Report of interspecific association between sympatric chimpanzees and gorillas | Kuroda et al. (1996) | |
| Report of interspecific association between sympatric chimpanzees and gorillas | Nishihara (1992) | |
| Report of interspecific association between sympatric chimpanzees and gorillas | Jones and Sabater Pi (1971) | |
| Report of interspecific association between sympatric chimpanzees and gorillas | Southern et al. (2021) | https://doi.org/10.1038/s41598-021-93829-x |
| Report of interspecific association between sympatric chimpanzees and gorillas | Stanford and Nkurunungi (2003) | https://doi.org/10.1023/A:1024689008159 |
| Report of interspecific association between sympatric chimpanzees and gorillas | Yamagiwa et al. (1996b) | |
| Report of interspecific association between sympatric chimpanzees and gorillas | Yamagiwa and Basabose (2006) | https://doi.org/10.1007/s10329-005-0147-7 |
| Report of interspecific association between sympatric chimpanzees and gorillas | Yamagiwa and Basabose (2009) | https://doi.org/10.1002/ajpa.21102 |
| Report of interspecific association between sympatric chimpanzees and gorillas | Basabose and Yamagiwa (2002) | https://doi.org/10.1023/A:1013879427335 |
| Network data from this study | Interspecific Interactions between Sympatric Apes | https://openscholarship.wustl.edu/data/100 |
Resource availability
Lead contact
Requests for additional information should be directed to the lead contact, Crickette Sanz (csanz@wustl.edu).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
This research was conducted in the Goualougo Triangle which is located in the southern portion of the Nouabalé-Ndoki National Park (2°05’-3°03’N; 16°51’-16°56’E), Republic of Congo. Direct observations of chimpanzees in the Goualougo Triangle have been ongoing since February 1999. The Moto chimpanzee community consisted of 71 individuals at the time of this study, including 12 adult males and 24 adult females. Systematic information on encounters with gorillas have been recorded during daily chimpanzee follows since the beginning of the study. In 2013, we initiated habituation of a group of western lowland gorillas within the Moto chimpanzee community range. The Loya gorilla group consisted of 7 individuals during the time of this study, including a dominant male silverback and two adult females. During daily follows of the Loya group, we recorded information on interspecific interactions with chimpanzees. To ensure temporal independence of our observations, only one intergroup encounter per day was included in the analysis, unless the analysis involved different individual apes. Ape nests and groups of nests (often referred to as nest sites) were also surveyed during this study.
Our study adhered to the legal requirements of the Republic of Congo where the research was conducted. The research was approved by the Nouabalé-Ndoki Foundation and the Wildlife Conservation Society’s Congo Program. We also received endorsement to conduct this research from the Institutional Animal Care and Use Committee of Washington University in St. Louis. We also complied with ethics guidelines of the Association for the Study of Animal Behaviour (ASAB) and Animal Behavior Society.
Method details
The Goualougo Triangle study area is part of the Sangha River Trinational Protected Area Complex which is comprised of 7,000 km2 of contiguous forest spanning national parks and reserves across Republic of Congo, Cameroon, and Central African Republic. The study area encompasses 380 km2 of lowland forest with altitudes ranging between 330 m and 600 m. The climate can be described as transitional between the Congo-equatorial and sub-equatorial climatic zones. The diurnal primate community includes western lowland gorillas (Gorilla gorilla gorilla) and central chimpanzees (Pan troglodytes troglodytes) which are found at overall density estimates of 2.34 gorillas/km2 and 1.53 chimpanzees/km2 in the Goualougo Triangle (Morgan et al., 2006).
Data collection protocols and definitions
Interspecific associations can generally be defined as two or more species in such close proximity that they can be regarded as members of the same group. The size and composition of chimpanzee parties may change several times throughout a day, but can be operationalized as all individuals travelling, feeding, resting, or socializing within 50m of one another (adopted from (Wrangham et al., 1992; Wilson et al., 2001)). Thus, we have applied this definition to identify interspecific associations among chimpanzees and gorillas.
Interspecific associations were categorized as cofeeding, feeding in proximity, spatial intersections, or social interactions. Cofeeding was defined as at least one member of both species feeding at the same food source (e.g., in the same tree crown). Feeding in proximity occurred when apes were feeding within 50m of each other, but not on the same food source. Spatial intersections involved chimpanzees and gorillas being observed within 50m of each other, but with no apparent interaction. Social interactions observed during interspecific events were described, and subsequently categorized as aggressive, playful, or sexual.
Quantification and statistical analysis
Associations
Simple ratio index social associations between individual chimpanzees and gorillas were calculated in SOCPROG (Whitehead, 2009). We used an established matrix randomization procedure (as described by (Manly 1995; Bejder et al., 1998; Whitehead 1999; Stensland et al., 2003; Whitehead 2008)) to test the observed distribution of chimpanzee and gorilla associations against the expected random distributions after 1000 permutations in SOCPROG.
Social network analysis
To assess the potential integration and centrality of individual gorillas and chimpanzees in mixed-species associations, we constructed a social network using group scan observations recorded during interspecific associations (within 50m) at Ficus spp. We used network statistics to assess the strength of social associations between individuals in the network (Whitehead 2008; Borgatti et al., 2013). The measure of degree is the sum of each node’s weighted ties with all other nodes. Eigenvector centrality is a measure of the connectedness of an individual, as well as the connectedness all of those with whom the individual is connected; often interpreted to indicate social integration (De Domenico et al., 2015; Finn et al., 2019; Sosa et al., 2021). Degree and eigenvector centrality calculations were performed with the “degree()” and “eigen_centrality()” codes via iGraph 1.3.0 in R 4.1.3 (Bonacich 1987; Csardi and Nepusz 2006; R Core Team 2021).
Temporal patterning of interspecific interactions at particular food resources
Based on the assumption that asynchronous species (such as figs) are more difficult to locate than synchronously fruiting trees, we compared Loya gorilla group’s foraging patterns before, during, and after cofeeding events with chimpanzees at asynchronous and synchronous resources.
Acknowledgments
This research would not have been possible without the continued support of the Ministère de l’Economie Forestière du gouvernement de la République du Congo and the Agence Congolaise de la Faune et des Aires Protégées (ACFAP). The Wildlife Conservation Society’s Congo Program and the Nouabalé-Ndoki Foundation are integral partners in this continuing research. Special thanks are owing to E. Stokes, M. Gately, R. Malonga, M. Ngangoue, T. Breuer, P. Ngouembe, D. Dos Santos, E. Arnhem, and B. Evans. We would also like to recognize the tireless dedication of J. R. Onononga, S. Ndolo Ebika, A. Nzeheke, W. Mayoukou, J. Wawa, M. Meguessa, I. Singono, Juan Ortega, and the Goualougo team. We also thank the iScience editorial staff and anonymous reviewers for their helpful comments on this article. Grateful acknowledgment of funding is owing to the U. S. Fish and Wildlife Service, the Arcus Foundation, the Indianapolis Zoo, the Cincinnati Zoo and Botanical Garden, the Saint Louis Zoo, the Columbus Zoo and Aquarium, the Margot Marsh Biodiversity Foundation, and the Chimpanzee SAFE program.
Author contributions
Conceptualization: CS, DM; Methodology: CS, DM, CEA, JMM, FE; Investigation: CS, DM, SB, CEA, JMM, FE, GM, SK, DN, DRKB; Visualization: CS, DM, JMM, SB, JAF; Funding acquisition: CS, DM; Project administration: CS, DM, CEA, SB, JMM, FE, GM, SK, DN DRKB; Supervision: CS, DM, CEA, SB; Writing – original draft: CS, DM, DS; Writing – review & editing: CS, DM, DS, JAF, SB.
Declaration of interests
The authors declare no competing interests in relation to this work.
Inclusion and diversity
We worked to ensure sex balance in the selection of non-human subjects. One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in science. One or more of the authors of this paper self-identifies as a member of the LGBTQ+ community. While citing references scientifically relevant for this work, we also actively worked to promote gender balance in our reference list. The author list of this paper includes contributors from the location where the research was conducted who participated in the data collection, design, analysis, and/or interpretation of the work.
Published: October 21, 2022
Data and code availability
No custom codes were generated for this research, as publicly available software and packages were used to conduct the analyses. Data sets have been posted in Washington University’s Open Scholarship repository (https://openscholarship.wustl.edu/data/100) and are accessible to other researchers for the purposes of reproducing or extending the analysis.
References
- Armstrong R.A., McGehee R. Competitive-exclusion. Am. Nat. 1980;115:151–170. doi: 10.1086/283553. [DOI] [Google Scholar]
- Basabose A.K., Yamagiwa J. Factors affecting nesting site choice in chimpanzees at Tshibati, Kahuzi-Biega National Park: influence of sympatric gorillas. Int. J. Primatol. 2002;23:263–282. doi: 10.1023/A:1013879427335. [DOI] [Google Scholar]
- Behrensmeyer A.K. In: Early Hominids of Africa. Jolly C., editor. Duckworth; 1978. The habitat of Plio-Pleistocene hominids in East Africa: taphonomic and microstratigraphic studies; pp. 165–189. [Google Scholar]
- Bejder L., Fletcher D., Brager S. A method for testing association patterns of social animals. Anim. Behav. 1998;56:719–725. doi: 10.1006/anbe.1998.0802. [DOI] [PubMed] [Google Scholar]
- Bertness M.D., Callaway R. Positive interactions in communities. Trends Ecol. Evol. 1994;9:191–193. doi: 10.1016/0169-5347(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Boesch C. The effect of leopard predation on grouping patterns in forest chimpanzees. Beyond Behav. 1991;117:220–241. doi: 10.1163/156853991X00544. [DOI] [Google Scholar]
- Boinski S. Why don't Saimiri oerstedii and Cebus capucinus form mixed-species groups? Int. J. Primatol. 1989;10:103–114. doi: 10.1007/BF02736248. [DOI] [Google Scholar]
- Bonacich P. Power and centrality: a family of measures. Am. J. Sociol. 1987;92:1170–1182. doi: 10.1086/228631. [DOI] [Google Scholar]
- Borgatti S.P., Everett M.G., Johnson J.C. Sage Publications; 2013. Analyzing Social Networks. [Google Scholar]
- Brown J.H. In: Ecology and Evolution in Communities. Diamond J.M., Cody M., editors. Harvard University Press; 1975. Geographical ecology of desert rodents; pp. 315–341. [Google Scholar]
- Bryer M.A., Chapman C.A., Rothman J.M. Diet and polyspecific associations affect spatial patterns among redtail monkeys (Cercopithecus ascanius) Beyond Behav. 2013;150:277–293. doi: 10.1163/1568539X-00003049. [DOI] [Google Scholar]
- Bshary R., Noe R. Red colobus and Diana monkeys provide mutual protection against predators. Anim. Behav. 1997;54:1461–1474. doi: 10.1006/anbe.1997.0553. [DOI] [PubMed] [Google Scholar]
- Buchanan-Smith H.M. Tamarin polyspecific associations: forest utilization and stability of mixed-species groups. Primates. 1999;40:233–247. doi: 10.1007/BF02557713. [DOI] [PubMed] [Google Scholar]
- Callaway R.M., Walker L.R. Competition and facilitation: a synthetic approach to interactions in plant communities. Ecology. 1997;78:1958–1965. doi: 10.1038/nature00812. [DOI] [Google Scholar]
- Callaway R.M., Brooker R.W., Choler P., Kikvidze Z., Lortie C.J., Michalet R., Paolini L., Pugnaire F.I., Newingham B., Aschehoug E.T., et al. Positive interactions among alpine plants increase with stress. Nature. 2002;417:844–848. doi: 10.1038/nature00812. [DOI] [PubMed] [Google Scholar]
- Chapman C.A., Chapman L.J. Mixed-species primate groups in the Kibale Forest: ecological constraints on association. Int. J. Primatol. 1996;17:31–50. doi: 10.1007/BF02696157. [DOI] [Google Scholar]
- Chase J.M., Myers J.A. Disentangling the importance of ecological niches from stochastic processes across scales. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011;366:2351–2363. doi: 10.1098/rstb.2011.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody M.L. Competition and the structure of bird communities. Monogr. Popul. Biol. 1974;7:1–318. [PubMed] [Google Scholar]
- Cords M. Mixed-species associations of East-African guenons - general patterns or specific examples. Am. J. Primatol. 1990;21:101–114. doi: 10.1002/ajp.1350210204. [DOI] [PubMed] [Google Scholar]
- Cott H.B. Oxford; 1940. Adaptive Coloration in Animals. [Google Scholar]
- Csardi G., Nepusz T. The igraph software package for complex network research. InterJournal, Complex Systems. 2006;1695:1–9. [Google Scholar]
- Curio E. The adaptive significance of avian mobbing: I. Teleonomic hypotheses and predictions. Zeitschrift für. Tierpsychologie. 1978;48:175–183. [Google Scholar]
- De Domenico M., Porter M.A., Arenas A. MuxViz: a tool for multilayer analysis and visualization of networks. Journal of Complex Networks. 2015;3:159–176. doi: 10.1093/comnet/cnu038. [DOI] [Google Scholar]
- Diamond J.M. In: Ecology and Evolution of Communities. Diamond J.M., Cody M.L., editors. Harvard University Press; 1975. Assembly of species communities; pp. 342–344. [Google Scholar]
- Dunbar R.I.M. Coevolution of neocortical size, group-size and language in humans. Behav. Brain Sci. 1993;16:681–694. doi: 10.1017/S0140525X00032325. [DOI] [Google Scholar]
- Eccard J.A., Ylönen H. Interspecific competition in small rodents: from populations to individuals. Evol. Ecol. 2003;17:423–440. doi: 10.1023/A:1027305410005. [DOI] [Google Scholar]
- Edmunds M. Longmans; 1974. Defense in Animals: A Survey of Anti-predator Defenses. [Google Scholar]
- Farine D.R., Whitehead H. Constructing, conducting and interpreting animal social network analysis. J. Anim. Ecol. 2015;84:1144–1163. doi: 10.1111/1365-2656.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer K.H., Buchanan-Smith H.M., Jamart A. Behavioral adaptation of Pan troglodytes troglodytes. Int. J. Primatol. 2006;27:747–765. doi: 10.1007/s10764-006-9041-4. [DOI] [Google Scholar]
- Fay J., Carroll R., KerbisPeterhans J.C., Harris D. Leopard attack on and consumption of gorillas in the Central African Republic. J. Hum. Evol. 1995;29:93–99. doi: 10.1006/jhev.1995.1048. [DOI] [Google Scholar]
- Finn K.R., Silk M.J., Porter M.A., Pinter-Wollman N. The use of multilayer network analysis in animal behaviour. Anim. Behav. 2019;149:7–22. doi: 10.1016/j.anbehav.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gause G.F. Experimental analysis of Vito Volterra’s mathematical theory of the struggle for existence. Science. 1934;79:16–17. doi: 10.1126/science.79.2036.16-a. [DOI] [PubMed] [Google Scholar]
- Gauss G.F. Williams and Wilkins; 1934. The Struggle for Existence. [Google Scholar]
- Hamilton W.D. Geometry for the selfish herd. J. Theor. Biol. 1971;31:295–311. doi: 10.1016/0022-5193(71)90189-5. [DOI] [PubMed] [Google Scholar]
- Hardin G. Competitive exclusion principle. Science. 1960;131:1292–1297. doi: 10.1126/science.131.3409.1292. [DOI] [PubMed] [Google Scholar]
- Harrison T. Apes among the tangled branches of human origins. Science. 2010;327:532–534. doi: 10.1126/science.1184703. [DOI] [PubMed] [Google Scholar]
- Head J.S., Boesch C., Makaga L., Robbins M.M. Sympatric chimpanzees (Pan troglodytes troglodytes) and gorillas (Gorilla gorilla gorilla) in Loango national park, Gabon: dietary composition, seasonality, and intersite comparisons. Int. J. Primatol. 2011;32:755–775. doi: 10.1007/s10764-011-9499-6. [DOI] [Google Scholar]
- Head J.S., Robbins M.M., Mundry R., Makaga L., Boesch C. Remote video-camera traps measure habitat use and competitive exclusion among sympatric chimpanzee, gorilla and elephant in Loango National Park, Gabon. Journal of Tropical Ecology. 2012;28:571–583. [Google Scholar]
- Henry A.G., Ungar P.S., Passey B.H., Sponheimer M., Rossouw L., Bamford M., Sandberg P., de Ruiter D.J., Berger L. The diet of Australopithecus sediba. Nature. 2012;487:90–93. doi: 10.1038/nature11185. [DOI] [PubMed] [Google Scholar]
- Herries A.I.R., Martin J.M., Leece A.B., Adams J.W., Boschian G., Joannes-Boyau R., Edwards T.R., Mallett T., Massey J., Murszewski A., et al. Contemporaneity of Australopithecus, Paranthropus, and early Homo erectus in South Africa. Science. 2020;368:eaaw7293. doi: 10.1126/science.aaw7293. [DOI] [PubMed] [Google Scholar]
- Heymann E.W., Buchanan-Smith H.M. The behavioural ecology of mixed-species troops of callitrichine primates. Biol. Rev. Camb. Philos. Soc. 2000;75:169–190. doi: 10.1017/S0006323199005460. [DOI] [PubMed] [Google Scholar]
- Holenweg A.K., Noë R., Schabet M. Waser's gas model applied to associations between red colobus and Diana monkeys in the Tai National Park, Ivory Coast. Folia Primatol. 1996;67:125–136. doi: 10.1159/000157214. [DOI] [Google Scholar]
- Hunter A.F., Aarssen L.W. Plants helping plants. Bioscience. 1988;38:34–40. doi: 10.2307/1310644. [DOI] [Google Scholar]
- Jones C., Sabater Pi J. Comparative ecology of Gorilla gorilla (Savage and Wyman) and Pan troglodytes (Bluemenbach) in Rio Muni, West Africa. Bibl. Primatol. 1971;13 [Google Scholar]
- Kenward R.E. Hawks and doves - factors affecting success and selection in goshawk attacks on woodpigeons. J. Anim. Ecol. 1978;47:449–460. doi: 10.2307/3793. [DOI] [Google Scholar]
- Kühl H.S., Boesch C., Kulik L., Haas F., Arandjelovic M., Dieguez P., Bocksberger G., McElreath M.B., Agbor A., Angedakin S., et al. Human impact erodes chimpanzee behavioral diversity. Science. 2019;363:1453–1455. doi: 10.1126/science.aau4532. [DOI] [PubMed] [Google Scholar]
- Kuroda S. In: Behavior, Ecology, and Conservation. Itoigawa N., Sugiyama Y., Sackett G.P., Thompson R.K.R., editors. Vol. 2. University of Tokyo Press; 1992. Ecological interspecies relationships between gorillas and chimpanzees in the Ndoki-Nouabale reserve, northern Congo. (Topics in Primatology). [Google Scholar]
- Kuroda S., Nishihara T., Suzuki S., Oko R.A. In: Great Ape Societies. McGrew W.C., Marchant L.F., Nishida T., editors. Cambridge University Press; 1996. Sympatric chimpanzees and gorillas in the Ndoki forest, Congo; pp. 71–81. [Google Scholar]
- Lack D. Harvard University Press; 1971. Ecological Isolation in Birds. [Google Scholar]
- Leakey R.E., Walker A.C. Australopithecus, Homo erectus, and the single species hypothesis. Nature. 1976;261:572–574. doi: 10.1038/261572a0. [DOI] [PubMed] [Google Scholar]
- Ledogar J.A., Smith A.L., Benazzi S., Weber G.W., Spencer M.A., Carlson K.B., McNulty K.P., Dechow P.C., Grosse I.R., Ross C.F., et al. Mechanical evidence that Australopithecus sediba was limited in its ability to eat hard foods. Nat. Commun. 2016;7:10596. doi: 10.1038/ncomms10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibold M.A., Holyoak M., Mouquet N., Amarasekare P., Chase J.M., Hoopes M.F., Holt R.D., Shurin J.B., Law R., Tilman D., et al. The metacommunity concept: a framework for multi-scale community ecology. Ecol. Lett. 2004;7:601–613. doi: 10.1111/j.1461-0248.2004.00608.x. [DOI] [Google Scholar]
- Levins R. Coexistence in a variable environment. Am. Nat. 1979;114:765–783. doi: 10.1086/283527. [DOI] [Google Scholar]
- Lotka A.J. Williams and Wilkins; 1925. Elements of Physical Biology. [Google Scholar]
- Manly B.F.J. A note on the analysis of species co-occurrences. Ecology. 1995;76:1109–1115. doi: 10.2307/1940919. [DOI] [Google Scholar]
- Martin J.M., Leece A.B., Neubauer S., Baker S.E., Mongle C.S., Boschian G., Schwartz G.T., Smith A.L., Ledogar J.A., Strait D.S., Herries A.I.R. Drimolen cranium DNH 155 documents microevolution in an early hominin species. Nat. Ecol. Evol. 2021;5:38–45. doi: 10.1038/s41559-020-01319-6. [DOI] [PubMed] [Google Scholar]
- Mayr E. Taxonomic categories in fossil hominids. Cold Spring Harb. Symp. Quant. Biol. 1950;15:109–118. doi: 10.1101/SQB.1950.015.01.013. [DOI] [PubMed] [Google Scholar]
- Mitchell W.A., Abramsky Z., Kotler B.P., Pinshow B., Brown J.S. The effect of competition on foraging activity in desert rodents - theory and experiments. Ecology. 1990;71:844–854. doi: 10.2307/1937356. [DOI] [Google Scholar]
- Morgan D., Sanz C., Onononga J.R., Strindberg S. Ape abundance and habitat use in the Goualougo Triangle, republic of Congo. Int. J. Primatol. 2006;27:147–179. doi: 10.1007/s10764-005-9013-0. [DOI] [Google Scholar]
- Morrison R.E., Dunn J.C., Illera G., Walsh P.D., Bermejo M. Western gorilla space use suggests territoriality. Sci. Rep. 2020;10:3692. doi: 10.1038/s41598-020-60504-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison R.E., Eckardt W., Stoinski T.S., Brent L.J.N. Comparing measures of social complexity: larger mountain gorilla groups do not have a greater diversity of relationships. Proc. Biol. Sci. 2020;287:20201026. doi: 10.1098/rspb.2020.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndolo Ebika S.T., Morgan D., Sanz C., Harris D.J. Ficus species in the Sangha trinational, central Africa. Edinb. J. Bot. 2018;75:377–420. [Google Scholar]
- Nishihara T. In: Behavior, Ecology, and Conservation. Itoigawa N., Sugiyama Y., Sackett G.P., Thompson R.K.R., editors. Vol. 2. Univ. of Tokyo Press; 1992. A preliminary report on the feeding habits of western lowland gorillas (Gorilla gorilla gorilla) in the Ndoki Forest, northern Congo; pp. 225–240. (Topics in Primatology). [Google Scholar]
- Oliveira L.C., Dietz J.M. Predation risk and the interspecific association of two Brazilian Atlantic Forest primates in Cabruca Agroforest. Am. J. Primatol. 2011;73:852–860. doi: 10.1002/ajp.20952. [DOI] [PubMed] [Google Scholar]
- Palagi E. Not just for fun! Social play as a springboard for adult social competence in human and non-human primates. Behav. Ecol. Sociobiol. 2018;72:90. doi: 10.1007/s00265-018-2506-6. [DOI] [Google Scholar]
- Pinheiro T., Ferrari S.F., Lopes M.A. Polyspecific associations between squirrel monkeys (Saimiri sciureus) and other primates in Eastern Amazonia. Am. J. Primatol. 2011;73:1145–1151. doi: 10.1002/ajp.20981. [DOI] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; 2021. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Reed K.E. Early hominid evolution and ecological change through the African Plio-Pleistocene. J. Hum. Evol. 1997;32:289–322. doi: 10.1006/jhev.1996.0106. [DOI] [PubMed] [Google Scholar]
- Rocha J.M.D., De Vleeschouwer K.M., Reis P.P., Grelle C.E.D., Oliveira L.C. Do habitat use and interspecific association reflect predation risk for the golden-headed lion tamarin (Leontopithecus chrysomelas)? Int. J. Primatol. 2015;36:1198–1215. doi: 10.1007/s10764-015-9885-6. [DOI] [Google Scholar]
- Sanz C., Morgan D., Strindberg S., Onononga J.R. Distinguishing between the nests of sympatric chimpanzees and gorillas. J. Appl. Ecol. 2007;44:263–272. doi: 10.1111/j.1365-2664.2007.01278.x. [DOI] [Google Scholar]
- Schoener T.W. Field experiments on interspecific competition. Am. Nat. 1983;122:240–285. doi: 10.1086/284133. [DOI] [Google Scholar]
- Shipman P., Harris J.M. In: Evolutionary History of the Robust Australopithecines. Grine F.E., editor. Aldine de Gruyter; 1988. Habitat preference and palaeoecology of Australopithecus boisei in eastern Africa; pp. 343–382. [Google Scholar]
- Sosa S., Sueur C., Puga-Gonzalez I. Network measures in animal social network analysis: their strengths, limits, interpretations and uses. Methods Ecol. Evol. 2021;12:10–21. doi: 10.1111/2041-210X.13366. [DOI] [Google Scholar]
- Southern L.M., Deschner T., Pika S. Lethal coalitionary attacks of chimpanzees (Pan troglodytes troglodytes) on gorillas (Gorilla gorilla gorilla) in the wild. Sci. Rep. 2021;11:14673. doi: 10.1038/s41598-021-93829-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford C.B. Pearson; 2008. Apes of the Impenetrable Forest: The Behavioral Ecology of Sympatric Chimpanzees and Gorillas. [Google Scholar]
- Stanford C.B., Nkurunungi J.B. Behavioral ecology of sympatric chimpanzees and gorillas in Bwindi Impenetrable National Park, Uganda: diet. Int. J. Primatol. 2003;24:901–918. doi: 10.1023/A:1024689008159. [DOI] [Google Scholar]
- Stensland E., Angerbjörn A., Berggren P. Mixed species groups in mammals. Mamm Rev. 2003;33:205–223. doi: 10.1046/j.1365-2907.2003.00022.x. [DOI] [Google Scholar]
- Struhsaker T.T. Polyspecific associations among tropical rainforest primates. Zeitschrift für. Tierpsychologie. 1981;57:268–304. [Google Scholar]
- Sueur C., Jacobs A., Amblard F., Petit O., King A.J. How can social network analysis improve the study of primate behavior? Am. J. Primatol. 2011;73:703–719. doi: 10.1002/ajp.20915. [DOI] [PubMed] [Google Scholar]
- Suzuki S. 1992. Feeding Strategies of Sympatric Gorillas and Chimpanzees in the Ndoki-Nouabalé Forest, with Special Reference to Co-feeding Behavior by Both Species. XIVth Congress of the International Primatological Society. [Google Scholar]
- Terborgh J.W. Toward a trophic theory of species diversity. Proc. Natl. Acad. Sci. USA. 2015;112:11415–11422. doi: 10.1073/pnas.1501070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutin C.E.G., Fernandez M. Foods consumed by sympatric populations of Gorilla gorilla and Pan troglodytes in Gabon: some preliminary data. Int. J. Primatol. 1985;6:27–43. doi: 10.1007/BF02693695. [DOI] [Google Scholar]
- Tutin C.E.G., Fernandez M. Composition of the diet of chimpanzees and comparisons with that of sympatric lowland gorillas in the Lope Reserve, Gabon. Am. J. Primatol. 1993;30:195–211. doi: 10.1002/ajp.1350300305. [DOI] [PubMed] [Google Scholar]
- Tutin C.E.G., Parnell R.J., White F. Protecting seeds from primates: examples from Diospyros spp. in the Lope reserve, Gabon. J. Trop. Ecol. 1996;12:371–384. doi: 10.1017/S0266467400009573. [DOI] [Google Scholar]
- Volterra V. Fluctuations in the abundance of a species considered mathematically. Nature. 1926;118:558–560. doi: 10.1038/118558a0. [DOI] [Google Scholar]
- Walsh P.D., Breuer T., Sanz C., Morgan D., Doran-Sheehy D. Natural history miscellany - potential for Ebola transmission between gorilla and chimpanzee social groups. Am. Nat. 2007;169:684–689. doi: 10.1086/513494. [DOI] [PubMed] [Google Scholar]
- Waser P.M. Primate polyspecific association - do they occur by chance. Anim. Behav. 1982;30:1–8. doi: 10.1016/S0003-3472(82)80230-3. [DOI] [Google Scholar]
- Watts D., Mitani J. Boundary patrols and intergroup encounters in wild chimpanzees. Beyond Behav. 2001;138:299–327. doi: 10.1163/15685390152032488. [DOI] [Google Scholar]
- Whitehead H. Testing association patterns of social animals. Anim. Behav. 1999;57:F26–F29. doi: 10.1006/anbe.1999.1099. [DOI] [PubMed] [Google Scholar]
- Whitehead H. University of Chicago Press; 2008. Analyzing Animal Societies: Quantitative Methods for Vertebrate Social Analysis. [Google Scholar]
- Whitehead H. SOCPROG programs: analysing animal social structures. Behav. Ecol. Sociobiol. 2009;63:765–778. doi: 10.1007/s00265-008-0697-y. [DOI] [Google Scholar]
- Whitesides G.H. Interspecific associations of Diana monkeys, Cercopithecus diana, in Sierra Leone, West Africa: biological significance or chance? Anim. Behav. 1989;37:760–776. doi: 10.1016/0003-3472(89)90062-6. [DOI] [Google Scholar]
- Wilson J.B., Agnew A.D. Positive-feedback switches in plant-communities. Adv. Ecol. Res. 1992;23:263–336. doi: 10.1016/S0065-2504(08)60149-X. [DOI] [Google Scholar]
- Wilson M.L., Hauser M.D., Wrangham R.W. Does participation in intergroup conflict depend on numerical assessment, range location, or rank for wild chimpanzees? Anim. Behav. 2001;61:1203–1216. doi: 10.1006/anbe.2000.1706. [DOI] [Google Scholar]
- Wolpoff M.H. Competitive exclusion among Lower Pleistocene hominids: the single species hypothesis. Man. 1971;6:601–614. doi: 10.2307/2799185. [DOI] [Google Scholar]
- Wolters S., Zuberbühler K. Mixed-species associations of Diana and Campbell's Monkeys: the costs and benefits of a forest phenomenon. Beyond Behav. 2003;140:371–385. doi: 10.1163/156853903321826684. [DOI] [Google Scholar]
- Wood B., Leakey M. The Omo-Turkana Basin fossil hominins and their contribution to our understanding of human evolution in Africa. Evol. Anthropol. 2011;20:264–292. doi: 10.1002/evan.20335. [DOI] [PubMed] [Google Scholar]
- Wrangham R.W., Clark A.P., Isabirye-Basuta G. In: Human Origins. Nishida T., McGrew W.C., Marler P., Pickford M., de Waal F.B.M., editors. University of Tokyo Press; 1992. Female social relationships and social organization of Kibale Forest chimpanzees; pp. 81–98. [Google Scholar]
- Yamagiwa J., Basabose A.K. Diet and seasonal changes in sympatric gorillas and chimpanzees at Kahuzi–Biega National Park. Primates. 2006;47:74–90. doi: 10.1007/s10329-005-0147-7. [DOI] [PubMed] [Google Scholar]
- Yamagiwa J., Basabose A.K. Fallback foods and dietary partitioning among Pan and Gorilla. Am. J. Phys. Anthropol. 2009;140:739–750. doi: 10.1002/ajpa.21102. [DOI] [PubMed] [Google Scholar]
- Yamagiwa J., Kaleme K., Milinganyo M., Basabose K. Food density and ranging patterns of gorillas and chimpanzees in the Kahuzi-Biega National Park, Zaire. Tropics. 1996;6:65–77. [Google Scholar]
- Yamagiwa J., Maruhashi T., Yumoto T., Mwanza N. In: Great Ape Societies. McGrew W.C., Marchant L.F., Nishida T., editors. Cambridge University Press; 1996. Dietary and ranging overlap in sympatric gorillas and chimpanzees in Kahuzi-Biega National Park, Zaire; pp. 82–98. [Google Scholar]
- Zeng X., Lu X. Interspecific dominance and asymmetric competition with respect to nesting habitats between two snowfinch species in a high-altitude extreme environment. Ecol. Res. 2009;24:607–616. doi: 10.1007/s11284-008-0530-0. [DOI] [Google Scholar]
- Ziv Y., Abramsky Z., Kotler B.P., Subach A. Interference competition and temporal and habitat partitioning in 2 gerbil species. Oikos. 1993;66:237–246. doi: 10.2307/3544810. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No custom codes were generated for this research, as publicly available software and packages were used to conduct the analyses. Data sets have been posted in Washington University’s Open Scholarship repository (https://openscholarship.wustl.edu/data/100) and are accessible to other researchers for the purposes of reproducing or extending the analysis.