Abstract
Heparin-binding protein (HBP) is a promising biomarker for the development and severity of sepsis. To guide its use, it is important to understand the factors that could lead to false-positive or negative results, such as inappropriate release and inadequate clearance of HBP. HBP is presumably released only by neutrophils, and the organs responsible for its elimination are unknown. In this study, we aimed to determine whether non-neutrophil cells can be a source of circulating HBP and which organs are responsible for its removal. We found that in two cohorts of neutropenic patients, 12% and 19% of patients in each cohort, respectively, had detectable plasma HBP levels. In vitro, three leukemia-derived monocytic cell lines and healthy CD14+ monocytes constitutively released detectable levels of HBP. When HBP was injected intravenously in rats, we found that plasma levels of HBP decreased rapidly, with a distribution half-life below 10 min and an elimination half-life of 1–2 h. We measured HBP levels in the liver, spleen, kidneys, lungs, and urine using both ELISA and immunofluorescence quantitation, and found that the majority of HBP was present in the liver, and a small amount was present in the spleen. Immunofluorescence imaging indicated that HBP is associated mainly with hepatocytes in the liver and monocytes/macrophages in the spleen. The impact of hematologic malignancies and liver diseases on plasma HBP levels should be explored further in clinical studies.
Keywords: Heparin-binding protein, Sepsis, Biomarkers
Introduction
Mortality in sepsis is greatly reduced by early diagnosis and treatment [1], and therefore, biomarkers for early detection of sepsis are a key to reducing its mortality. Heparin-binding protein (HBP, also known as azurocidin or cationic antimicrobial protein of 37 kDa/CAP37) is a promising new prognostic biomarker that is elevated in the plasma of sepsis patients up to 12 h before a drop in blood pressure is detected [2]. Plasma HBP levels above 15–30 ng/mL are associated with mortality and organ failure in sepsis [3, 4, 5]. Rapid HBP testing is becoming available at clinical chemistry departments of certain hospitals in anticipation of its routine use in the future. If HBP is to be used clinically as a biomarker, it is important to understand its origin, its kinetics in the blood, and its eventual removal from the circulation in order to identify conditions that could lead to false-positive and negative results.
HBP is presumably expressed only in neutrophils and stored in their secretory vesicles and azurophilic granules [6]. There is so far no evidence that HBP is released by any other cells in the body. There are reports of HBP expression in endothelial cells of rats [7, 8] and in the kidneys of mice [9], but since mice and rats lack the gene for HBP [10, 11], these findings are likely due to artifacts. Therefore, it is assumed that people with low levels of circulating neutrophils will have low or no plasma HBP, leading to possible false-negative results in such patients. However, this assumption has never been confirmed in an appropriate cohort.
Once HBP is released into the blood, it is important to know how long it remains in the circulation to determine how frequently it should be measured in patients. Several reports suggest that circulating HBP is short-lived. Serial measurements of HBP every four hours in septic shock patients indicated that HBP levels fluctuate widely between measurements [12]. Repeated measurements of HBP in patients undergoing cardiac surgery with cardiopulmonary bypass showed that HBP levels were reduced 3-fold in only five minutes after protamine reversal at the end of the surgery [13]. In mice, HBP plasma levels were measured at the end of an experiment following intravenous HBP infusion, and only 1% of the administered HBP remained after 1 h [14]. The pharmacokinetics of a single bolus dose of HBP has never been determined.
If HBP is targeted to specific organs, then certain organ failures may lead to false-positive or negative results. HBP can bind to and be taken up by endothelial cells [15] and monocytes [16, 17], but it is unclear what proportion of circulating HBP is associated with these cells in vivo. It is unknown to which compartments circulating HBP distributes and which cells in these compartments take up HBP. The aims of this study were (1) determine whether circulating HBP can be detected in patients with neutropenia, (2) to determine the half-life of HBP in the blood, and (3) to determine the organs and cell types to which HBP distributes.
Methods
Ethics
Blood sample collection from patients and healthy donors was approved by the Swedish Ethical Review Authority. Cohort 1 had the registration number 2015/628; cohort 2 had the registration number 2015/828 with change/addition in the decision number 2017/27. Pooled blood from healthy donors for extraction of CD14+ monocytes was collected under the registration number 2020:24. Blood from healthy donors for the extraction of neutrophils and unsorted monocytes was collected under the registration number 2013/728. The local Ethical Committee for Animal Research approved the experimental protocol for animal experiments (registration number M143-16).
Patient Enrollment
HBP levels are elevated in people with infection and sepsis [3, 4, 5] but are generally very low in healthy people (median ranges from 6.3 to 12 ng/mL in published studies [18, 19, 20]). Therefore, we included two different cohorts of neutropenic patients (Fig. 1) − one with clinically stable patients with few infections (cohort 1) and one with febrile neutropenia (and therefore more likely to have infections) (cohort 2).
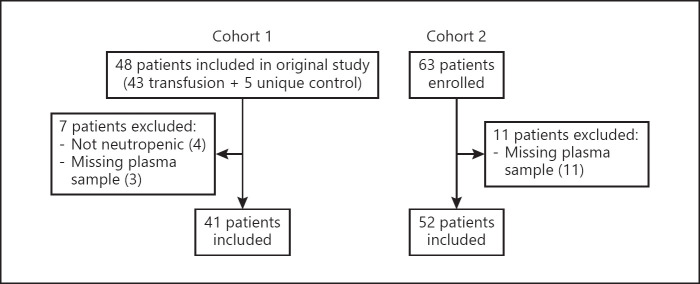
Fig. 1.
Flowchart showing patient enrollment, exclusion, and inclusion of patients in the present study.
Cohort 1 consisted of 41 patients from a previously published study [21], which included patients aged 18 years or older who were eligible for platelet transfusions and were recruited from the Haematology Department, Skåne University Hospital in Lund, Sweden, between February and September 2016. All patients gave written informed consent prior to inclusion. Blood samples were drawn before the platelet transfusion was given and were centrifuged at the accredited central laboratory of the hospital, and the plasma was stored frozen at −80°C within 2 h from sample time.
For cohort 2, 52 patients were included from three hospitals in Sweden between 2016 and 2019: Skåne University hospitals in Lund and Malmö, and Helsingborg hospital. The inclusion criteria were age of at least 18 years and febrile neutropenia. Blood samples were collected at admission for patients presenting at emergency care and at the onset of fever for patients already admitted to the hospital at the time of inclusion. Informed consent was by an opt-out procedure after sampling and inclusion. Blood samples were centrifuged within 2 h to obtain the plasma, which was then stored frozen at −80°C.
In both cohorts, neutropenia was defined as a neutrophil count of <0.5 × 109/L or <1.0 × 109/L and decreasing. Only patients with neutropenia were included in this study. In both cohorts, some patients had been included up to three times, on separate occasions. Only values from the first inclusion instance were included in this study. Data on clinical parameters, including neutrophil counts and clinical diagnoses, were collected from the patients' medical charts. Values below the detection limit for neutrophil counts (0.1 × 109 cells/L) were not reported as a numeric value, and so we replaced them with half of the value of the detection limit (0.05 × 109 cells/L) to be able to use them in statistical analyses.
Isolation of CD14-Positive Monocytes
Primary CD14-positive monocytes were isolated from pooled blood from healthy donors by density-gradient centrifugation and magnetic bead separation. Briefly, 10 mL of leukocyte concentrate was diluted 1:1 with 0.9% NaCl. Twenty milliliters of that mixture was layered on to 20 mL of Lymphoprep (Axis Shield) and centrifuged for 20 min at 700 g without brake. Erythrocytes were lysed with pure water for 15 s. Purified mononuclear cells were resuspended in MACS buffer, and CD14 microbeads were added (both Miltenyi Biotec). Cells were then sorted with an LS column using a MACS separator (both Miltenyi Biotec) and resuspended in serum-free Roswell Park Memorial Institute (RPMI) 1640 medium (Thermo Fisher), and then used for further experiments.
Neutrophil and Mononuclear Cell Isolation
Blood was collected from healthy human donors using ethylenediaminetetraacetic acid (EDTA) as the anticoagulant. Neutrophils and mononuclear cells were separated from human blood using Polymorphprep (Axis Shield) according to the manufacturer's directions with some modifications as described previously [22]. Briefly, blood was layered on the Polymorphprep and centrifuged at 370 g for 30 min. The mononuclear cell layer and neutrophil layer were separated and washed with phosphate-buffered saline. Contaminating erythrocytes were lysed with water for 20 s and then washed with phosphate-buffered saline. The cell pellet was resuspended in Hank's buffered saline solution (Life Technologies) and used for further experiments.
Cell Lines
Immortalized human endothelial cells (EA.hy926; American Type Culture Collection/ATCC), primary human lung microvascular endothelial cells (HMVEC-L; Lonza), erythroleukemia cells from a chronic myelogenous leukemia (K562; ATCC), acute myeloid leukemia (AML) cells (HNT-34 and MOLM-13; DSMZ-German Collection of Microorganisms and Cell Cultures), lymphoblastic cells from biphenotypic B myelomonocytic leukemia (MV-4-11; ATCC), T-acute lymphoblastic leukemia cells (DND-41; DSMZ-German Collection of Microorganisms and Cell Cultures), and acute monocytic leukemia cells (THP-1; ATCC) were cultured according to the manufacturers' specifications. Endothelial cells were grown to confluence in culture plates and then washed, and fresh serum-free media was added. Cells were incubated at 37°C for 24 h, and cell supernatants were collected. All leukemia cell lines, isolated mononuclear cells, and isolated CD14-positive monocytes were diluted to a concentration of 1 × 106 cells/mL in serum-free RPMI 1640 medium (Thermo Fisher) and added to culture plates. Cells were incubated at 37°C for 3 h and then centrifuged to collect supernatants.
Preparation of Neutrophil Secretory Vesicle Secretion
The only neutrophil-derived protein in secretory vesicles is HBP [23]. Neutrophils were isolated from three different donors as above. A total of 25 × 106 cells were resuspended in Hank's buffered saline solution containing calcium and magnesium. Secretory vesicle release was stimulated by cross-linking of CD18 as described [23]. Briefly, samples were incubated with the anti-CD18 antibody clone IB4 (Adipogen; 3 μg per 106 cells) for 30 min at room temperature. Cells were washed twice and then incubated with F(ab')2 fragment of goat anti-mouse IgG (Jackson ImmunoResearch Laboratories) at a 1:20 dilution for 10 min at 37°C. The samples were then centrifuged to remove the cells, and the HBP level in each sample was measured by ELISA prior to use. This solution was diluted and immediately used for injection in rats.
Preparation of Total Neutrophil Lysate
Neutrophils were isolated from six different donors as above. A total of 25 × 106 isolated human neutrophils per donor were washed and resuspended in sterile water and incubated for 10 min at room temperature. Cells were disrupted by sonication with 4 pulses of 30 s each. The samples were then centrifuged to remove cell debris, and the HBP level in each sample was measured by ELISA prior to use. This solution was diluted and immediately used for injection in rats.
Rat Model
Rats and other rodents do not carry a direct homolog of HBP [10, 11], but larger animals such as pigs and dogs, which have an HBP homolog, are ethically complicated and would require a prohibitively large amount of HBP. A literature search indicated that human HBP can interact with rat cells, organs, and proteins. HBP was found to enhance the phagocytic activity of rat macrophages [24], induce rat blood-retinal barrier breakdown [25], increase protein kinase C activity in rat brain endothelial cells [26], and form strong complexes with rat plasma ceruloplasmin [27], suggesting that rats carry receptors that interact with HBP. Therefore, we chose to use rats to explore the kinetics and distribution of circulating HBP. Adult male rats (Taconic, 250–270 g, 40 rats in total) were used. Animals were treated in accordance with the National Institutes of Health for the Care and Use for Laboratory animals.
Anesthesia was induced with 4–5% isoflurane (Baxter) and then maintained at 3.5% via a mask while the trachea was intubated. Following intubation, the animals were mechanically ventilated, and anesthesia was maintained using isoflurane at 1.5% for the duration of the experiment. Body temperature, measured rectally, was maintained at 37°C throughout the experiment. The left femoral vein and the left femoral artery were cannulated. A blood sample was collected from the artery prior to the start of the experiment. Recombinant human HBP (provided by Axis Shield) at doses of 3 μg/kg (n = 4 rats), 64 μg/kg (n = 4 rats), 160 μg/kg (n = 12 rats), or 320 μg/kg (n = 7 rats), or neutrophil lysate (containing HBP 3 μg/kg; n = 4 rats) or CD18 cross-linking-induced neutrophil secretion (containing HBP 3 μg/kg; n = 4 rats) or an equal volume of saline solution (300 μL; n = 5 rats) was then injected in a single bolus dose into the left femoral vein. As this was an exploratory study, randomization, blinding, and a priori power calculations were not used. Blood samples (150 µL each) were collected via the left femoral artery to tubes with citrate anticoagulant and then centrifuged to obtain plasma at the time points shown in each figure. A solution of 10 mM EDTA filled the arterial catheter in between blood collection time points longer than 1 min to prevent coagulation in the catheter. At the end of the experiment, urine was collected by squeezing the bladder. Rats were sacrificed at various time points following injections (indicated in the figure legends), and the liver, spleen, kidney, and lung were removed and processed as described below.
Heparin-Binding Protein-ELISA
Human plasma samples were measured in duplicate using HBP-ELISA (Axis Shield) at a 1:40 dilution according to the manufacturer's directions. According to the manufacturer, the lower limit of detection (LOD) is 5.9 ng/mL in plasma samples when diluted 1:40, which corresponds to an LOD of 0.148 ng/mL for the assay. Rat plasma, organ lysate, and urine were first validated in the assay as described in the supplementary information and in online supplementary Tables S1 and S2 (for all online suppl. material, see www.karger.com/doi/10.1159/000521064), and used at the dilutions described therein. Any samples that were above the upper limit of quantitation were diluted further until they were within the quantitation range. Because values were obtained from the standard curve, any values below the detection limit were used as is without further processing (e.g., imputation).
Pharmacokinetic Analysis of Plasma HBP
To calculate the pharmacokinetics of HBP, first all HBP values below the detection limit were removed due to the uncertainty of the measurement below this limit. HBP values were log-transformed and plotted. Visual inspection of the graphs indicated that the values follow an alpha and beta phase with a distinct change in slope around 10 min. Thus, for each rat, the beta phase was modeled using a linear regression including time points ≥10 min, and the coefficients were extracted. Based on this analysis, a prediction of the elimination between 1 and 10 min was subtracted from the actual values between 1 and 10 min, and a linear regression was fitted for the alpha phase. The means of the coefficients for the alpha and beta phases were calculated and transformed into half-lives.
For rats injected with 160 μg/kg HBP, the calculation of the slope was not possible for the beta phase for 6 of the rats because 4 of them were sacrificed at 10 min after injection for the measurement of HBP levels in the organs, and 2 of them were measured only at late time points where the values had dropped below the detection limit, resulting in a lack of available values. In the primary analysis, no calculation was made for the alpha phase for these rats, and only the 7 rats with detectable HBP after 10 min were used for the calculation of the half-lives for the alpha and beta phases. For rats injected with 320 μg/kg HBP, all rats were used for the calculation of both alpha and beta phases. For all rats, we additionally calculated the alpha phase without subtraction of the predicted beta phase.
Preparation of Organ Lysates
A section of the left lobe of the liver (approximately 1.1 g), half of the spleen, one kidney, and one lung were collected and placed in 600 μL of the tissue-protein extraction reagent (Thermo Fisher) immediately after removal from the rat and kept on ice. Organs were homogenized using ceramic beads (Mobio) in a TissueLyser (QIAGEN). Tissue homogenates were incubated on ice for 30 min and then centrifuged to remove tissue debris. Total protein in the lysate was determined by the bicinchoninic acid assay kit (Thermo Fisher) according to the manufacturer's directions using bovine serum albumin as the standard. HBP concentrations in rat organ lysates were normalized to total protein levels.
Immunocytochemistry of Organs
A section of the left lobe of the liver, half of the spleen, one kidney, and one lung were cut into 4 × 4 mm pieces and fixed in 4% paraformaldehyde (Sigma-Aldrich) for 48 h, and then transferred to 70% ethanol. Following dehydration, samples were embedded in paraffin (Histolab Products AB). After rehydration and antigen retrieval, tissue sections were incubated overnight with a rabbit antiserum against HBP at a 1:3,000 dilution and one of the following antibodies: monoclonal mouse anti-rat endothelial cell antigen 1 (RECA-1; Abcam; clone RECA-1; a pan-endothelial cell marker), monoclonal mouse anti-rat CD68 (Abcam; clone ED1; a pan-monocyte/macrophage marker), and monoclonal mouse anti-rat asialoglycoprotein receptor (ASGR1; Invitrogen; clone 8D7; a hepatocyte marker) at a 1:100 dilution. HBP antiserum from rabbit was provided by Heiko Herwald, originally obtained as described previously [28]. Samples were then stained with secondary Alexa Fluor-647-conjugated goat anti-rabbit and Alexa Fluor-568-conjugated goat anti-mouse Fab2′ antibody fragments. Coverslips (Menzel-glaser; #1.5 thickness) were mounted on the samples using ProLong Gold Antifade Mountant with 4,6-diamidino-2-phenylindole (Life Technologies).
Fluorescence Imaging and Quantification
Images were obtained using the Nikon A1RHD confocal system confocal microscope with a ×20 objective. For fluorescence quantification, images of 4–6 different areas of each section were obtained, and the total fluorescence (integrated density) in each image was quantified using Image J.
Statistical Analysis
We first determined whether data were normally distributed by using a Shapiro-Wilk test and by visual examination of histograms and quintile-quintile plots. Data from the patient cohorts were not normally distributed, so nonparametric tests were used. Data from rats were normally distributed, so parametric tests were used. When comparing medians in the clinical cohorts, the Mann-Whitney U test was used, and when comparing relative frequencies, Fisher's exact test was applied. In the rat experiments, one-way ANOVA with post hoc Holm-Šídák's multiple comparisons test was used to compare the means. p values below 0.05 were considered significant.
Results
HBP in Neutropenic Patients
We enrolled two cohorts of neutropenic patients with low numbers of circulating neutrophils (Fig. 1), summarized in Table 1. In cohort 1, consisting of patients in need of platelet transfusions with chemotherapy-induced bone marrow aplasia and neutropenia, 93% of patients had hematological malignancies and were more often treated with antibiotics and steroids prior to enrollment. In cohort 2, consisting of patients with neutropenic fever, only 60% had hematological malignancies, and fewer patients were treated with antibiotics and steroids prior to enrollment. Patients in cohort 2 had higher temperature (38.5 interquartile range [IQR] 38.2–38.8 vs. 37.2 IQR 36.8–37.4°C; p < 0.0001) and more patients with infection (33 [63%] vs. 1 [3%]; p < 0.0001), indicating that patients in cohort 1 were included in a clinically stable phase, while patients in cohort 2 had more acute illness. However, the median C-reactive protein was not significantly different between the two cohorts (63 IQR 29–129 vs. 43 IQR 12–101 mg/dL; p = 0.251). In cohort 2, there was no significant difference in HBP levels in patients with infection versus with no infection (0.0 IQR 0.0–6.5 vs. 0.5 IQR 0.0–4.7 ng/mL; p = 0.846) or in patients with versus without sepsis (0.7 IQR 0.0–13.0 vs. 0.16 IQR 0.0–4.6 ng/mL; p = 0.528).
Table 1.
Characteristics of two cohorts of neutropenic patients
| Cohort 1 (n = 41) |
Cohort 2 (n = 52) |
|||||
| HBP < LOD (n = 36) | HBP > LOD (n = 5) | all patients (n = 41) | HBP < LOD (n = 42) | HBP > LOD (n = 10) | all patients (n = 52) | |
| HBP, median (IQR), ng/mL | 0.0 (0.0–1.7) | 30 (17–33) | 0.0 (0.0–1.8) | 0.0 (0.0–1.1) | 11 (9.1–17) | 0.2 (0–4.9) |
| Demographics | ||||||
| Age, median (IQR), years | 72 (49–75) | 57 (44–70) | 56 (44–69) | 66 (54–72) | 68 (64–70) | 66 (55–71) |
| Females, n (%) | 11 (31) | 1 (20) | 12 (29) | 19 (45) | 5 (50) | 24 (46) |
| Measurements at time of sampling, median (IQR) | ||||||
| Temperature, °C | 37.2 (36.7–37.4) | 37.0 (36.8–37.1) | 37.1 (36.7–37.4) | 38.5 (38.2–38.8) | 38.5 (38.0–38.9) | 38.5 (38.2–38.8) |
| CRP, mg/L | 40 (12–88) | 100 (89–139) | 43 (12–101) | 69 (32–110) | 108 (7–261) | 69 (30–129) |
| Leukocytes, ×109/L | 0.1 (0.1–0.3) | 0.6 (0.2–0.9) | 0.1 (0.1–0.4) | 0.3 (0.1–0.8) | 0.6 (0.2–1.0) | 0.4 (0.1–0.9) |
| Neutrophils, ×109/L | 0.05 (0.05–0.1) | 0.1 (0.1–0.2) | 0.05 (0.05–0.1) | 0.05 (0.05–0.1) | 0.2 (0.1–0.2) | 0.05 (0.05–0.1) |
| Platelets, ×109/L | 7 (5–10) | 25 (8–26) | 8 (5–10) | 41 (13–115) | 33 (23–91) | 39 (15–113) |
| Comorbidities, n (%) | ||||||
| Cardiovascular disease | 2 (6) | 0 (0) | 2 (5) | 8 (19) | 2 (20) | 10 (19) |
| COPD | 1 (2) | 0 (0) | 1 (2) | 2 (5) | 0 (0) | 2 (4) |
| Liver disease | 1 (3) | 0 (0) | 1 (3) | 0 (0) | 1 (10) | 1 (2) |
| Kidney disease | 0 (0) | 0 (0) | 0 (0) | 4 (10) | 0 (0) | 4 (8) |
| Diabetes | 2 (6) | 0 (0) | 2 (5) | 3 (7) | 2 (20) | 5 (10) |
| Infection | 0 (0) | 1 (20) | 1 (2) | 24 (57) | 9 (90) | 33 (63) |
| Sepsis1 | 8 (19) | 4 (40) | 12 (23) | |||
| Concomitant treatments (prior to inclusion), n (%) | ||||||
| Steroids | 34 (94) | 3 (60) | 37 (90) | 23 (52) | 5 (50) | 27 (52) |
| Cytostatic drugs | 33 (92) | 2 (40) | 35 (85) | 38 (90) | 10 (100) | 48 (92) |
| Antibiotics | 34 (94) | 4 (80) | 38 (93) | 26 (62) | 4 (40) | 30 (58) |
| Heparin or LMWH | 0 (0) | 0 (0) | 0 (0) | 3 (7) | 2 (20) | 5 (10) |
| Warfarin | 0 (0) | 0 (0) | 0 (0) | 3 (7) | 0 (0) | 3 (6) |
| G-CSF | 1 (3) | 1 (20) | 2 (5) | 14 (33) | 4 (40) | 18 (35) |
| Malignancy, n (%) | ||||||
| Leukemia | 22 (61) | 5 (100) | 27 (66) | 11 (26) | 3 (30) | 14 (27) |
| MDS | 1 (3) | 2 (40) | 3 (7) | 3 (7) | 0 (0) | 3 (6) |
| AML | 14 (39) | 2 (40) | 16 (39) | 4 (10) | 3 (30) | 7 (13) |
| Other | 9 (19) | 1 (20)2 | 8 (20) | 4 (10) | 0 (0) | 4 (8) |
| Myeloma | 6 (17) | 0 (0) | 6 (15) | 7 (17) | 0 (0) | 7 (13) |
| Lymphoma | 3 (8) | 0 (0) | 3 (7) | 8 (19) | 2 (20) | 10 (19) |
| Other − solid tumor | 0 (0) | 0 (0) | 0 (0) | 14 (33) | 4 (40) | 18 (35) |
| Other − no malignancy | 3 (8) | 0 (0) | 3 (7) | 2 (5) | 1 (10) | 3 (6) |
CRP, C-reactive protein; COPD, chronic obstructive pulmonary disease; LMWH, low molecular-weight heparin; G-CSF, granulocyte colony stimulating factor; MDS, myelodysplastic syndrome.
Sepsis was defined using Sepsis-3 criteria.
This patient had a mixed phenotype acute leukemia.
Even though all patients were neutropenic, five patients in cohort 1 (12%) had HBP above the LOD (median HBP 30 ng/mL IQR 17–33 ng/mL), and ten patients in cohort 2 (19%) had HBP above the LOD (11 ng/mL IQR 9.1–17 ng/mL). The numbers of patients with HBP above the LOD was not significantly different between the two cohorts (p = 0.408). In both cohorts, patients with detectable HBP had significantly higher neutrophil counts than did patients with HBP below the LOD (cohort 1: 0.1 IQR 0.1–0.2 vs. 0.05 IQR 0.05–0.1 × 109 cells/L; p = 0.032 and cohort 2: 0.2 IQR 0.1–0.2 vs. 0.05 IQR 0.05–0.1 ng/mL; p = 0.015). However, not all patients with HBP above the LOD had detectable neutrophils. In cohorts 1 and 2, there were 3 and 5 patients, respectively, with neutrophil counts at or below the detection limit (≤0.1 × 109 cells/L). Of these 8 patients, 6 (75%) had AML, one (13%) had follicular lymphoma, and one (13%) had a nonhematological malignancy (pancreatic cancer). This led us to explore the possibility whether malignant white blood cells, particularly those of myeloid origin, can release HBP.
HBP Release by Non-Neutrophil Cell Types
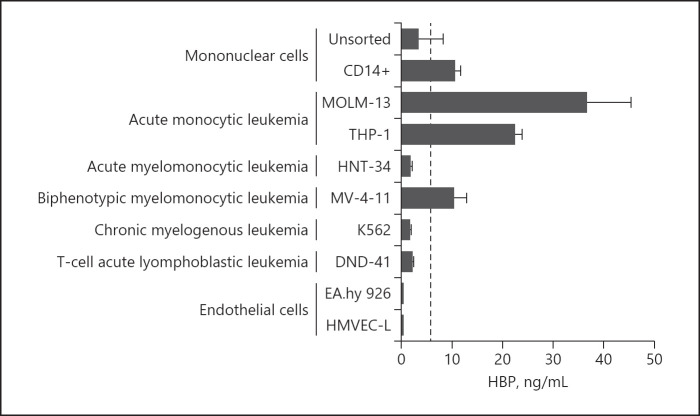
To examine the possibility that HBP might be released by non-neutrophil cell types, we measured HBP in the supernatant of various cells. CD14-positive mononuclear cells and cells derived from acute monocytic leukemia (MOLM-13 and THP-1) and biphenotypic myelomonocytic leukemia (MV-4-11) constitutively released detectable levels of HBP into the supernatant. Unsorted mononuclear cells, immortalized endothelial cells (EA.hy 926), primary lung endothelial cells (HMVEC-L), and cell lines derived from acute myelomonocytic leukemia (HNT-34), chronic myelogenous leukemia (K562), and T-cell acute lymphoblastic leukemia (DND-41) did not produce detectable HBP in the supernatant (Fig. 2).
Fig. 2.
Release of HBP by non-neutrophil cell types. Various cell lines or primary cells were incubated for three hours at 37°C, supernatants were collected, and HBP concentration was measured. Dashed line is the detection limit of the ELISA (5.9 ng/mL at sample dilution of 1:40). Bars are the mean, and whiskers are the standard deviation, n = 4 experiments in each group, except for CD14+ monocytes which were n = 2 pooled donor blood bags.
Kinetics of Circulating HBP
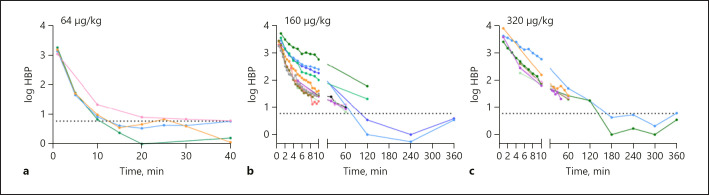
To determine how long HBP remains in the circulation, we injected rats with various concentrations of recombinant human HBP. We first injected rats with 64 μg/kg of HBP (Fig. 3a), and the plasma concentration one minute after injection was 1,478 ± 301 ng/mL, which is, in our experience, at the high end of the range of physiological concentrations measured during sepsis and septic shock. The plasma HBP concentration was below the detection limit 15 min after the injection in most rats, and therefore, pharmacokinetic analysis was not possible in these rats. When rats were injected with 160 μg/kg (Fig. 3b; online suppl. Fig. S1) or 320 μg/kg (Fig. 3c; online suppl. Fig. S2) of HBP, the concentration decreased rapidly for the first 10 min and then more slowly, which indicated distinct distribution and elimination phases. In rats injected with 160 μg/kg of HBP, the distribution half-life was 8 min, and the elimination half-life was 72 min (online suppl. Table S3). Since 6 rats lacked values for time points past 10 min, the alpha phase was also calculated without subtraction of the predicted beta phase values, thus using all 12 rats. The distribution half-life was then calculated to 4 min (online suppl. Table S3). The results for rats injected with 320 μg/kg of HBP were in the same range with a distribution half-life of 14 min and an elimination half-life of 61 min (online suppl. Table S4).
Fig. 3.
Plasma HBP over time. Plasma HBP levels over time in rats injected intravenously with recombinant human HBP at a dose of 64 μg/kg (a), 160 μg/kg (b), and 320 μg/kg (c). Data points from each individual rat are shown connected with a colored line. The dashed line indicates the LOD (5.9 ng/mL).
Organ Distribution of HBP
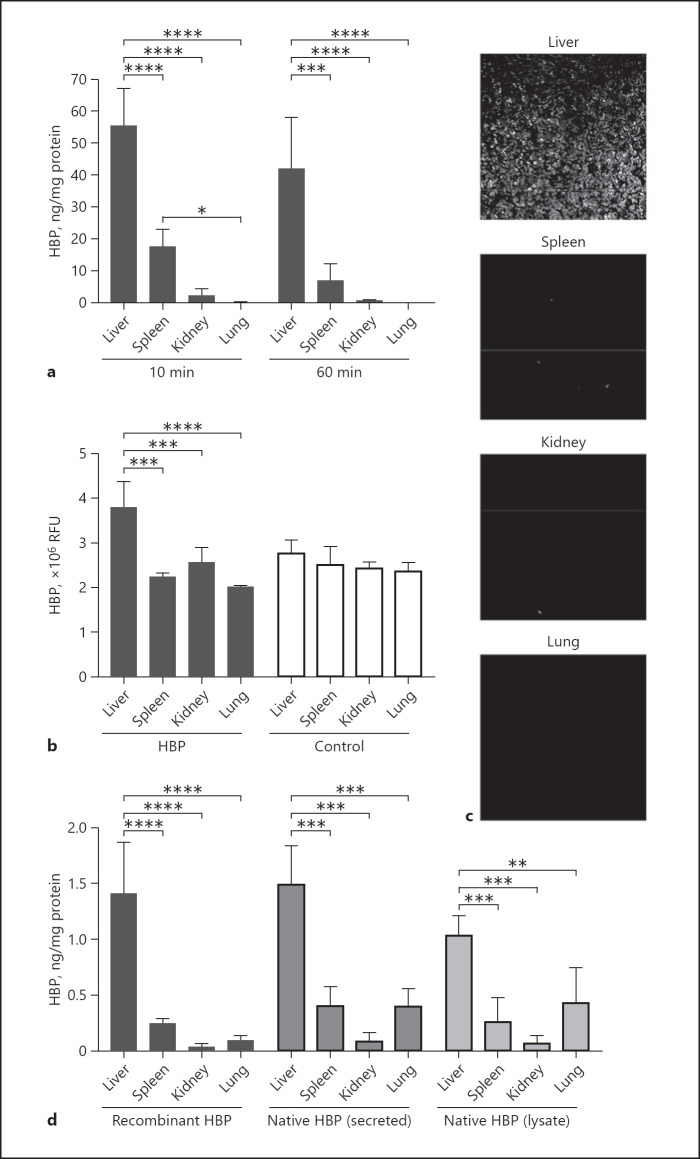
To identify the organs that take up HBP, we measured HBP levels in the liver, spleen, kidneys, and lungs in rats injected with 160 μg/kg HBP after 10 min and 60 min. HBP concentrations were measured by ELISA and normalized for the total protein concentration in the lysate (Fig. 4a). Liver samples had the highest HBP level both at 10 min (55.3 ± 11.9 ng HBP/mg total protein) and at 60 min (42.0 ± 16.1 ng/mg), which was significantly higher than that of all other organs (p < 0.0001). The spleen had the second highest amount of HBP both at 10 min (17.6 ± 5.4 ng/mg) and 60 min (6.9 ± 5.2 ng/mg). The lung and kidney had the lowest HBP levels (kidney at 10 min: 2.3 ± 2.1 ng/mg; kidney at 60 min: 0.8 ± 0.1 ng/mg; lung at 10 min: 0.3 ± 0.1 ng/mg; lung at 60 min: 0.2 ± 0.1 ng/mg). HBP levels at the two time points did not differ significantly in any of the organs. We additionally measured HBP levels in two rats each at 6 h and 24 h after the injection and found that HBP levels in the liver and spleen decreased over time were no longer detectable at 24 h (online suppl. Fig. S3a). We saw a similar organ distribution pattern in rats injected with 320 μg/kg HBP (online suppl. Fig. S3b). Quantification of fluorescence in sections of organs stained for HBP confirmed that the liver had a high level of HBP (Fig. 4b, c). The HBP level in the other organs was not quantifiable because it was too similar to the background fluorescence level of the tissue (Fig. 4b). In urine, HBP was below the LOD for rats injected with 160 μg/kg (10 min: 0.6 ± 1.2 ng/mL; 60 min: 3.2 ± 4.0 ng/mL) and for rats injected with 320 μg/kg (60 min: 1.6 ± 1.6 ng/mL).
Fig. 4.
Circulating HBP primarily distributes to the liver and spleen. a–c Rats were injected with recombinant human HBP at a dose of 160 μg/kg. a HBP levels normalized for total protein concentration in lysates of the liver, spleen, kidney, and lung 10 and 60 min after injection. b Immunofluorescence quantification of HBP levels in tissue sections, in RFU. c Representative fluorescence images of organ sections stained for HBP. d HBP levels at 40 min after injection of HBP at a dose of 3 μg/kg normalized for total protein concentration in lysates of the liver, spleen, kidney, and lung from rats injected with recombinant HBP, native HBP secreted from neutrophil secretory vesicle (secreted HBP), or native HBP from total neutrophil lysate (lysate HBP). Bars are the mean, and whiskers are the standard deviation, n = 4 in each group. Means were compared using one-way ANOVA with post hoc Holm-Šídák's multiple comparisons test. RFU, relative fluorescence units.
Low Dose and Native HBP
Because HBP can affect permeability [14], which could conceivably affect its distribution, we also injected rats with a very low dose of HBP (3 μg/kg) that resulted in plasma levels below the detection limit within 5 min after injection and was therefore not likely to significantly affect permeability. We found that this low dose of HBP had a similar distribution pattern at 40 min after injection (Fig. 4d). The majority of HBP was found in the liver (1.4 ± 0.5 ng/mg), some was found in the spleen (0.2 ± 0.04 ng/mg), and very little in the kidneys (0.03 ± 0.02 ng/mg) and the lungs (0.09 ± 0.04 ng/mg). Urine from these rats had 0 ng/mL HBP.
To account for the possibility that recombinant proteins may differ from their native counterparts, which may affect their distribution, we also injected rats with native HBP obtained from isolated human neutrophils. We tested HBP released from the secretory vesicle compartment (secreted HBP) and HBP released by lysis of the cells (lysate HBP) (both at 3 μg/kg) (Fig. 4d). Forty minutes after injection, native HBP was found mainly in the liver (secreted HBP: 1.5 ± 0.3 ng/mg; lysate HBP: 1.0 ± 0.2 ng/mg), with some in the spleen (secreted HBP: 0.4 ± 1.6 ng/mg; lysate HBP: 0.3 ± 0.2 ng/mg) and very little in the kidneys (secreted HBP: 0.09 ± 0.06 ng/mg; lysate HBP: 0.3 ± 0.2 ng/mg). However, rats injected with native secreted and lysate HBP tended to have more HBP in the lungs (0.43 ± 0.30 and 0.40 ± 0.19 ng/mg, respectively) compared to rats injected with recombinant HBP (0.09 ± 0.04 ng/mg), although this difference was not statistically significant (p = 0.149 and 0.129, respectively). Urine from these rats had 0 ng/mL HBP.
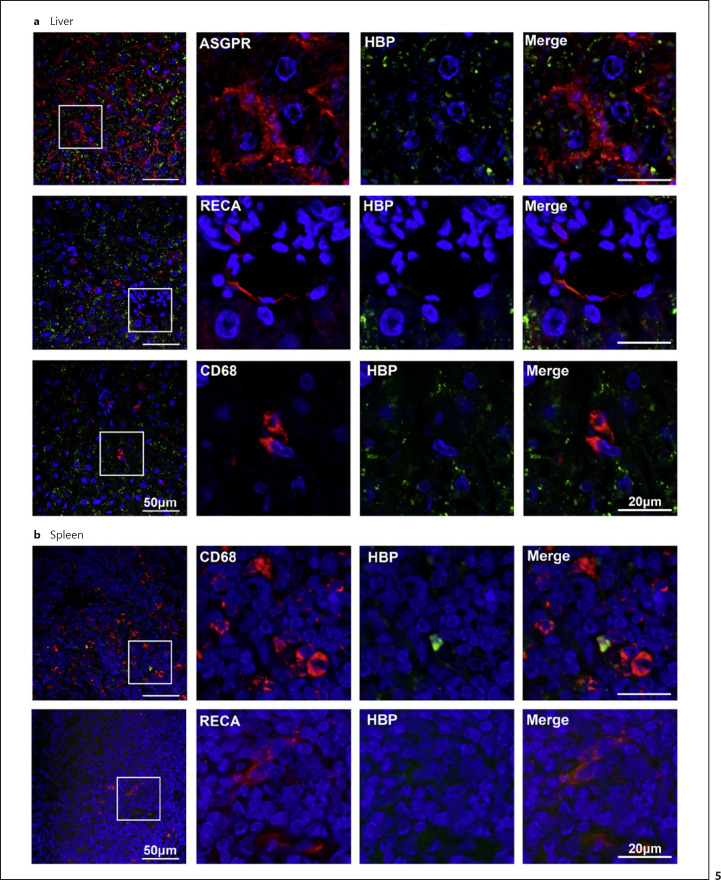
Cell Types Associated with HBP
To determine which cell types take up or bind HBP in the targeted organs, sections of the liver (Fig. 5a) and spleen (Fig. 5b) taken 10 min after intravenous injection with 160 μg/kg HBP were stained for human HBP and for rat markers of hepatocytes (ASGPR), endothelial cells (RECA), and monocytes/macrophages (CD68). In the liver, HBP was associated with hepatocytes (ASGPR-positive cells). Endothelial cells (RECA-positive cells) and monocytes/macrophages (CD68-positive cells) were devoid of HBP. In the spleen, strong HBP staining was very occasionally found associated with monocytes/macrophages (CD68-positive cells).
Fig. 5.
HBP is associated with hepatocytes in the liver and some macrophages in the spleen. Ten minutes after intravenous injection of 160 μg/kg HBP, liver (a) and spleen (b) sections were stained for HBP (green) and general markers for hepatocytes (ASGPR), endothelial cells (RECA), and monocytes/macrophages (CD68) in red. DNA was visualized by DAPI in blue. DAPI, 4,6-diamidino-2-phenylindole.
Discussion
In this study, we found that although HBP levels are low in most patients with neutropenia, some patients had detectable levels of plasma HBP. Some monocytic leukemia cell lines and healthy CD14+ monocytes were found to constitutively express HBP. Circulating HBP has a rapid distribution phase where plasma levels drop rapidly for the first 10 min, followed by a slower elimination phase. We found that HBP distributes mainly to hepatocytes in the liver and a smaller proportion to monocytes/macrophages in the spleen.
In studies of HBP as a biomarker, neutropenic patients are typically excluded because they are expected to have false-negative results; however, we found that HBP was detectable in a surprisingly large proportion of neutropenic patients, although there was no difference in HBP levels between patients with and without sepsis. In both cohorts, patients with detectable HBP had significantly higher neutrophil counts, implicating neutrophils as the main source of circulating HBP. It is likely that even though these patients had low numbers of neutrophils, the present neutrophils were activated and therefore able to produce detectable HBP levels.
However, some patients with detectable HBP had neutrophil counts below detectable levels. These patients were mostly afflicted with AML, indicating the possibility of malignant myeloid cells as a non-neutrophil source of HBP. Indeed, we found that cell lines derived from malignant monocytic cells constitutively expressed high HBP levels. We also found that healthy CD14+ monocytes constitutively expressed low, but detectable, HBP levels, thus pointing to monocytic cells as a possible source of circulating HBP. It is also possible that some patients produced circulating immature neutrophils [29] which were not detected during blood cell counts. Additionally, sepsis can induce changes in protein expression of bone marrow myeloid cells [30]. Studies have shown that promyelocytes, metamyelocytes, band neutrophils, and segmented nucleus neutrophils all highly express the gene module containing AZU1 (HBP) [31, 32] and that HBP protein makes up a high proportion of the proteome of immature neutrophils, similar to levels found in mature neutrophils [33], and so, circulating immature neutrophils or bone marrow myeloid cells could be another potential source of HBP, although we did not explore this possibility in this study.
We found that HBP circulates in the blood for a short time, with a distribution half-life below 10 min and an elimination half-life between 1 and 2 h. This finding is in line with previous observations in humans [12, 13] and in mice [14], which indicated that plasma HBP levels change rapidly between measurements. HBP has pro-inflammatory and vascular permeability-inducing effects that can be dangerous in situations such as sepsis [6], and therefore, rapid removal of HBP from the circulation is likely a protective mechanism against these detrimental effects. Patients with sepsis typically have HBP levels above 30 ng/mL and often much higher [3, 4, 5], indicating that sepsis patients either have impaired clearance of HBP or that their neutrophils are continuously releasing HBP to keep plasma levels elevated, or both.
We found that HBP distributes mainly to the liver where it is associated with hepatocytes. This is in line with previous findings that plasma HBP is correlated with plasma bilirubin [12]. HBP is an inactive serine protease [34] with several glycan chains, 80% of which are nonsialylated [35]. Many serine protease-serpin complexes are taken up rapidly by hepatocytes via the low-density lipoprotein receptor family [36], especially via low-density lipoprotein receptor-related protein 1 which takes up neutrophil elastase [37], a close relative of HBP [34]. On the other hand, nonsialylated glycoproteins are taken up rapidly by hepatocytes via the Ashwell-Morell (asialoglycoprotein) receptor [38]. Thus, it is conceivable that HBP could associate with one or both of these receptors. Future studies of specific knockdown of these candidate receptors in hepatocyte cell lines and injection of competing ligands in animal models could shed light on the receptors and intracellular uptake pathways of HBP, but such experiments were beyond the scope of the present study.
We also found that some HBP was present in the spleen where it was associated with monocytes/macrophages, which is in line with previous findings that monocytes can internalize HBP [16]. It is likely that some of the circulating monocytes had internalized plasma HBP and migrated to the spleen [39] which explains why, although we found detectable HBP levels in the spleen, HBP staining was only associated with a small number of monocyte/macrophage cells.
It is somewhat surprising that we found no HBP in the kidney or the urine. Glomerular filtration is size- and charge-selective, preferentially allowing cationic proteins [40] and proteins below 45 kDa [41] to cross the filtration barrier. HBP is highly cationic [34] and has a molecular weight of 35.5 kDa [28], so it would be expected to pass freely through the glomerular filtration system. However, our findings are in line with previous studies showing that even though elevated plasma HBP is associated with acute kidney injury [42, 43], renal clearance of HBP was found to be very low in healthy individuals and in burn patients, only increasing slightly when renal function was impaired [18]. Together, these findings indicate that HBP is not primarily cleared by healthy kidneys.
We also found no HBP in the lungs or associated with RECA-positive cells (endothelial cells) to any great extent. The fact that HBP was not found intracellularly was not unexpected as HBP-stimulated endothelial cells showed significant amounts of intracellular HBP only after 12 h [15]. However, the lack of HBP on the endothelial surface was unexpected, given our previous findings that the endothelial glycocalyx interacts with HBP [14] and that injection of large amounts of intravenous heparin in humans causes the release of HBP, presumably from the endothelium [13]. The glycocalyx is notoriously fragile [44], and so, it is possible that our methods of fixation and sample handling did not preserve the glycocalyx, and so, endothelial-associated HBP was lost. However, this does not explain the lack of HBP in the lungs. It is therefore more likely that even if some HBP interacts with the endothelial glycocalyx, this is a very small proportion of the total HBP, possibly too low to be detected by our ELISA and immunofluorescence methods. The fact that the majority of HBP was found elsewhere indicates that, either way, the endothelium is not the major compartment for distribution and elimination of HBP.
Implications for HBP as a Biomarker
Differentiation of neutropenic fever and neutropenic sepsis is a large clinical challenge. Attempts have been made to stratify patients with neutropenic fever with high risk of complications from those with low risk of complications. The most validated tool for stratification is the Multinational Association for Supportive Care in Cancer (MASCC) [45]. Among hematological patients, the sensitivity of the MASCC has been as low as 58%, the specificity 87%, positive predictive value 84%, and negative predictive value 64% [46]. Because of the low numbers of patients with elevated HBP, the two cohorts in this study were likely too small to detect differences in HBP levels in patients with and without sepsis. However, given that HBP is a promising biomarker in patients with normal neutrophil levels, it could be interesting to explore whether HBP might have an additive effect to the performance of stratification scores for sepsis in larger cohorts of neutropenic patients. This would likely require a far larger cohort to establish relevant cutoff values for HBP in these patients.
The short half-life of HBP has several implications for its use as a biomarker. In the setting of surgery, it is advantageous to have a biomarker that rapidly normalizes following the temporary inflammatory stimulus of surgery, making postsurgical measurements less likely to be confounded by falsely elevated results. This was found to be particularly important in cardiac surgery with cardiopulmonary bypass which has particularly high levels of neutrophil activation and inflammation [13]. On the other hand, the combination of rapid clearance and rapid release from neutrophils could cause values to fluctuate greatly between measurements, as was observed in a previous study [12], and could therefore affect their interpretability. The kinetics of plasma HBP should therefore be considered when deciding how frequently to measure HBP.
Our finding that HBP distributes mainly to the liver opens the possibility that liver dysfunctions, especially those involving impairment of hepatocyte function, could lead to falsely elevated plasma levels of HBP. Additionally, our finding that malignant monocytic leukemia cells can constitutively release HBP indicates an additional potential source of false-positive results. The effect of specific liver diseases and hematological malignancies, and the extent to which they affect plasma HBP levels should be explored further in appropriate cohorts of patients.
Limitations
This study had several obvious limitations. First, because of the low numbers of patients with elevated HBP, the two cohorts in this study were likely too small to detect differences in HBP levels in patients with and without sepsis. The two cohorts were also collected at different times (cohort 1 in 2016 and cohort 2 over a longer period from 2016 to 2019), and we cannot exclude differences in sample handling or clinical practice that could have affected HBP levels. Cohort 1 samples had been freeze-thawed once before, while cohort 2 samples had not; however, we have found that HBP levels remain stable over several freeze-thaw cycles, so we do not expect this to have a great effect on the results (unpublished in-house data).
Additionally, rats do not possess endogenous HBP, so we cannot be sure that our findings are applicable to humans. However, the lack of endogenous HBP may have had some advantage as it allows us to be sure that there was no endogenous HBP release interfering with the results. Animals that possess HBP include sheep, pigs, cows, primates, elephants, whales, bats, and their relatives [10, 11] − all of which are problematic (or impossible) to include in laboratory experiments. Studies in sheep or pigs are possible, but these are expensive and more ethically complicated. Therefore, this study is only the first step toward elucidating the clearance pathways of HBP.
We mainly used recombinant HBP which may be processed differently than native HBP in neutrophils. Native HBP undergoes several proteolytic and glycosylation steps [28], and is stored in both azurophilic granules and secretory vesicles [47], which contain different glycosidases [48]. Additionally, sialic acids can undergo spontaneous modifications [49] which could affect the results. Therefore, recombinant HBP could have different glycosylation patterns than native HBP. We partially addressed this limitation by examining the organ distribution of native HBP derived from both secretory vesicles and whole-cell lysate, and by applying minimal handling of the samples (i.e., we did not purify HBP) to reduce the possibility of spontaneous modifications. However, we were unable to extract sufficient amounts of HBP to be able calculate the pharmacokinetics of native HBP in this study.
The pharmacokinetic studies were intended only to determine the approximate life span of circulating HBP, so they were done in only a few healthy animals. Samples were taken at varying time points because we used the same rats for various exploratory analyses in order to reduce the total number of rats needed for the study. This variation in sampling times may also have affected the analysis. Thus, the calculations should be carefully interpreted, and as a result, we do not focus on the exact values but rather on relative time scale.
Last, sepsis and other critical illnesses impair the function of various body systems, and in particular are associated with degradation of the glycocalyx [50], with which HBP is known to interact [14]. We cannot rule out the involvement of the hepatocyte glycocalyx in the clearance of HBP as many glycocalyx components are known to act as co-receptors for other cell-surface receptors [51], or the effect of sepsis-induced glycocalyx disruption on this clearance pathway. In the future, HBP administration in animal models of sepsis, such as endotoxin or infection models, could be used to determine the generalizability of these results to sepsis. Despite these limitations, our findings indicate a very quick distribution phase and an elimination phase with a half-life of 1–2 h, which is consistent with prior findings in the literature.
Conclusions
HBP can be found in some neutropenic patients. Malignant myeloid cells and monocytic cells may be a source of HBP, in addition to neutrophils. HBP disappears rapidly from the circulation and distributes primarily to liver hepatocytes, with some uptake into monocytes/macrophages in the spleen. The impact of hematologic malignancies and liver diseases on plasma HBP levels should be explored in future clinical studies.
Statement of Ethics
Blood sample collection from patients and healthy donors was approved by the Swedish Ethical Review Authority. Cohort 1 had the registration number 2015/628; cohort 2 had the registration number 2015/828 with change/addition in the decision number 2017/27. In cohort 1, all patients gave written informed consent prior to inclusion, while in cohort 2, informed consent was obtained by an opt-out procedure after sampling and inclusion. Pooled blood from healthy donors for extraction of CD14+ monocytes was collected under the registration number 2020:24. Blood from healthy donors for the extraction of neutrophils and unsorted monocytes was collected under the registration number 2013/728. The local Ethical Committee for Animal Research approved the experimental protocol for animal experiments (registration number M143-16).
Conflict of Interest Statement
A.L. is listed as an inventor on a patent on the use of HBP as a diagnostic tool in sepsis filed by Hansa Medical AB WO2008151808A1. F.K. has stock ownership in Hansa Biopharma AB. The other authors have no conflicts of interest to declare.
Funding Sources
F.K. obtained funding from the Crafoord Foundation, the Alfred Österlunds Foundation, the Swedish Research council, the Medical Faculty at Lund University, and Region Skåne. A.L. obtained funding from Swedish Government Funds for Clinical Research, the Crafoord foundation, the foundation of Alfred Osterlund (A.L.), and the Lions Skåne Research fund. L.M. obtained funding from the Lions Skåne Research fund. P.B. obtained funding from the Anna and Edwin Berger Foundation, ALF Grant #86626.
Author Contributions
J.F. conceptualized the study, carried out experiments, analyzed and interpreted the data, and wrote the first draft of the manuscript. F.K. carried out pharmacokinetic analyses. E.W. carried out immunofluorescence analyses of rat organs. P.G. and L.M. provided plasma samples and clinical data for cohort 2, and aided in the analysis of the results. T.K. provided the plasma samples and clinical data for cohort 1, and aided in the analysis of the results. P.B. aided in conceptualization and analysis of the animal experiments. A.L. aided in conceptualization and analysis of all parts of the study. All the authors contributed to interpretation of the data and editing of the manuscript. All the authors read and approved the final manuscript.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplementary Material
Supplementary data
Acknowledgments
We thank Helén Axelberg for her expert technical assistance with animal experiments. We thank Ravi Bhongir for his expert technical assistance in preparation of organ sections for imaging. We thank Heiko Herwald for kindly providing the anti-HBP antiserum. We thank Axis Shield for providing recombinant HBP. We thank Ariane Neumann for providing samples from unstimulated CD14+ mononuclear cells. We thank Bengt Ljungberg for his valuable input on the pharmacokinetic calculations.
References
- 1.Liu VX, Fielding-Singh V, Greene JD, Baker JM, Iwashyna TJ, Bhattacharya J, et al. The timing of early antibiotics and hospital mortality in sepsis. Am J Respir Crit Care Med. 2017 Oct;196((7)):856–63. doi: 10.1164/rccm.201609-1848OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linder A, Christensson B, Herwald H, Björck L, Akesson P. Heparin-binding protein: an early marker of circulatory failure in sepsis. Clin Infect Dis. 2009 Oct;49((7)):1044–50. doi: 10.1086/605563. [DOI] [PubMed] [Google Scholar]
- 3.Linder A, Åkesson P, Inghammar M, Treutiger CJ, Linnér A, Sundén-Cullberg J. Elevated plasma levels of heparin-binding protein in intensive care unit patients with severe sepsis and septic shock. Crit Care. 2012 May;16((3)):R90. doi: 10.1186/cc11353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linder A, Arnold R, Boyd JH, Zindovic M, Zindovic I, Lange A, et al. Heparin-binding protein measurement improves the prediction of severe infection with organ dysfunction in the emergency department. Crit Care Med. 2015 Nov;43((11)):2378–86. doi: 10.1097/CCM.0000000000001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahn F, Tverring J, Mellhammar L, Wetterberg N, Bläckberg A, Studahl E, et al. Heparin-binding protein as a prognostic biomarker of sepsis and disease severity at the emergency department. Shock. 2019 Dec;52((6)):e135–45. doi: 10.1097/SHK.0000000000001332. [DOI] [PubMed] [Google Scholar]
- 6.Fisher J, Linder A. Heparin-binding protein: a key player in the pathophysiology of organ dysfunction in sepsis. J Intern Med. 2017 Mar;281((6)):562–74. doi: 10.1111/joim.12604. [DOI] [PubMed] [Google Scholar]
- 7.Lee TD, Gonzalez ML, Kumar P, Chary-Reddy S, Grammas P, Pereira HA. CAP37, a novel inflammatory mediator: its expression in endothelial cells and localization to atherosclerotic lesions. Am J Pathol. 2002 Mar;160((3)):841–8. doi: 10.1016/S0002-9440(10)64907-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pereira HA, Kumar P, Grammas P. Expression of CAP37, a novel inflammatory mediator, in Alzheimer's disease. Neurobiol Aging. 1996;17((5)):753–9. [PubMed] [Google Scholar]
- 9.Xing L, Zhongqian L, Chunmei S, Pingfa C, Lei H, Qin J, et al. Activation of M1 macrophages in sepsis-induced acute kidney injury in response to heparin-binding protein. PLoS One. 2018 May;13((5)):e0196423. doi: 10.1371/journal.pone.0196423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bateman A, Martin MJ, Orchard S, Magrane M, Agivetova R, Ahmad S, et al. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 2021 Jan;49((D1)):D480–9. doi: 10.1093/nar/gkaa1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990 Oct;215((3)):403–10. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 12.Tverring J, Nielsen N, Dankiewicz J, Linder A, Kahn F, Åkesson P. Repeated measures of heparin-binding protein (HBP) and procalcitonin during septic shock: biomarker kinetics and association with cardiovascular organ dysfunction. Intensive Care Med Exp. 2020 Dec;8((1)):51. doi: 10.1186/s40635-020-00338-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sterner N, Fisher J, Thelaus L, Ketteler C, Lemež Š, Dardashti A, et al. The dynamics of heparin binding protein in cardiothoracic surgery − a pilot study. J Cardiothorac Vasc Anesth. 2021;35((9)):2640–50. doi: 10.1053/j.jvca.2020.12.033. [DOI] [PubMed] [Google Scholar]
- 14.Bentzer P, Fisher J, Kong HJ, Mörgelin M, Boyd JH, Walley KR, et al. Heparin-binding protein is important for vascular leak in sepsis. Intensive Care Med Exp. 2016 Dec;4((1)):33. doi: 10.1186/s40635-016-0104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olofsson AM, Vestberg M, Herwald H, Rygaard J, David G, Arfors KE, et al. Heparin-binding protein targeted to mitochondrial compartments protects endothelial cells from apoptosis. J Clin Invest. 1999 Oct;104((7)):885–94. doi: 10.1172/JCI6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heinzelmann M, Mercer-Jones MA, Flodgaard H, Miller FN, Miller FN. Heparin-binding protein (CAP37) is internalized in monocytes and increases LPS-induced monocyte activation. J Immunol. 1998 Jun;160((11)):5530–6. [PubMed] [Google Scholar]
- 17.Heinzelmann M, Platz A, Flodgaard H, Polk HC, Miller FN. Endocytosis of heparin-binding protein (CAP37) is essential for the enhancement of lipopolysaccharide-induced TNF-alpha production in human monocytes. J Immunol. 1999 Apr;162((7)):4240–5. [PubMed] [Google Scholar]
- 18.Samuelsson L, Tydén J, Herwald H, Hultin M, Walldén J, Steinvall I, et al. Renal clearance of heparin-binding protein and elimination during renal replacement therapy: studies in ICU patients and healthy volunteers. PLoS One. 2019 Aug;14((8)):e0221813. doi: 10.1371/journal.pone.0221813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chew MS, Linder A, Santen S, Ersson A, Herwald H, Thorlacius H. Increased plasma levels of heparin-binding protein in patients with shock: a prospective, cohort study. Inflamm Res. 2012 Apr;61((4)):375–9. doi: 10.1007/s00011-011-0422-6. [DOI] [PubMed] [Google Scholar]
- 20.McAuley DF, O'Kane CM, Craig TR, Shyamsundar M, Herwald H, Dib K. Simvastatin decreases the level of heparin-binding protein in patients with acute lung injury. BMC Pulm Med. 2013 Jul;13((1)):47. doi: 10.1186/1471-2466-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benediktsson S, Lazarevic V, Nilsson L, Kjeldsen-Kragh J, Schött U, Kander T. Linear decline of corrected platelet count increment within 24 hours after platelet transfusion in haematological patients: a prospective observational study. Eur J Haematol. 2017;99((6)):559–68. doi: 10.1111/ejh.12974. [DOI] [PubMed] [Google Scholar]
- 22.Neumann A, Brogden G, Jerjomiceva N, Brodesser S, Naim HY, von Köckritz-Blickwede M. Lipid alterations in human blood-derived neutrophils lead to formation of neutrophil extracellular traps. Eur J Cell Biol. 2014 Aug;93((8–9)):347–54. doi: 10.1016/j.ejcb.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Gautam N, Herwald H, Hedqvist P, Lindbom L. Signaling via beta(2) integrins triggers neutrophil-dependent alteration in endothelial barrier function. J Exp Med. 2000 Jun;191((11)):1829–39. doi: 10.1084/jem.191.11.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soehnlein O, Kai-Larsen Y, Frithiof R, Sorensen OE, Kenne E, Scharffetter-Kochanek K, et al. Neutrophil primary granule proteins HBP and HNP1-3 boost bacterial phagocytosis by human and murine macrophages. J Clin Invest. 2008 Oct;118((10)):3491–502. doi: 10.1172/JCI35740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skondra D, Noda K, Almulki L, Tayyari F, Frimmel S, Nakazawa T, et al. Characterization of azurocidin as a permeability factor in the retina: involvement in VEGF-induced and early diabetic blood-retinal barrier breakdown. Invest Ophthalmol Vis Sci. 2008 Feb;49((2)):726–31. doi: 10.1167/iovs.07-0405. [DOI] [PubMed] [Google Scholar]
- 26.Pereira HA, Moore P, Grammas P. CAP37, a neutrophil granule-derived protein stimulates protein kinase C activity in endothelial cells. J Leukoc Biol. 1996 Sep;60((3)):415–22. doi: 10.1002/jlb.60.3.415. [DOI] [PubMed] [Google Scholar]
- 27.Sokolov AV, Ageeva KV, Kostevich VA, Berlov MN, Runova OL, Zakharova ET, et al. Study of interaction of ceruloplasmin with serprocidins. Biochemistry. 2010 Nov;75((11)):1361–7. doi: 10.1134/s0006297910110076. [DOI] [PubMed] [Google Scholar]
- 28.Lindmark A, Garwicz D, Rasmussen PB, Flodgaard H, Gullberg U. Characterization of the biosynthesis, processing, and sorting of human HBP/CAP37/azurocidin. J Leukoc Biol. 1999;66((4)):634–43. doi: 10.1002/jlb.66.4.634. [DOI] [PubMed] [Google Scholar]
- 29.Drifte G, Dunn-Siegrist I, Tissières P, Pugin J. Innate immune functions of immature neutrophils in patients with sepsis and severe systemic inflammatory response syndrome. Crit Care Med. 2013 Mar;41((3)):820–32. doi: 10.1097/CCM.0b013e318274647d. [DOI] [PubMed] [Google Scholar]
- 30.Faivre V, Lukaszewicz AC, Payen D. Downregulation of blood monocyte HLA-DR in ICU patients is also present in bone marrow cells. PLoS One. 2016 Nov;11((11)):e0164489. doi: 10.1371/journal.pone.0164489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fouret P, Du Bois RM, Bernaudin JF, Takahashi H, Ferrans VJ, Crystal RG. Expression of the neutrophil elastase gene during human bone marrow cell differentiation. J Exp Med. 1989;169((3)):833. doi: 10.1084/jem.169.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cowland JB, Borregaard N. The individual regulation of granule protein mRNA levels during neutrophil maturation explains the heterogeneity of neutrophil granules. J Leukoc Biol. 1999;66((6)):989–95. doi: 10.1002/jlb.66.6.989. [DOI] [PubMed] [Google Scholar]
- 33.Hoogendijk AJ, Pourfarzad F, Aarts CEM, Tool ATJ, Hiemstra IH, Grassi L, et al. Dynamic transcriptome-proteome correlation networks reveal human myeloid differentiation and neutrophil-specific programming. Cell Rep. 2019;29:2505–19.e4. doi: 10.1016/j.celrep.2019.10.082. [DOI] [PubMed] [Google Scholar]
- 34.Karlsen S, Iversen LF, Larsen IK, Flodgaard HJ, Kastrup JS. Atomic resolution structure of human HBP/CAP37/azurocidin. Acta Crystallogr D Biol Crystallogr. 1998 Jul;54((Pt 4)):598–609. doi: 10.1107/s0907444997016193. [DOI] [PubMed] [Google Scholar]
- 35.Olczak M, Watorek W. Structural analysis of N-glycans from human neutrophil azurocidin. Biochem Biophys Res Commun. 2002 Apr;293((1)):213–9. doi: 10.1016/S0006-291X(02)00201-2. [DOI] [PubMed] [Google Scholar]
- 36.Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev. 2008 Jul;88((3)):887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poller W, Willnow TE, Hilpert J, Herz J. Differential recognition of alpha 1-antitrypsin-elastase and alpha 1-antichymotrypsin-cathepsin G complexes by the low density lipoprotein receptor-related protein. J Biol Chem. 1995 Feb;270((6)):2841–5. doi: 10.1074/jbc.270.6.2841. [DOI] [PubMed] [Google Scholar]
- 38.Grewal PK. The Ashwell-Morell receptor. Methods Enzymol. 2010;479:223–41. doi: 10.1016/S0076-6879(10)79013-3. [DOI] [PubMed] [Google Scholar]
- 39.Teh YC, Ding JL, Ng LG, Chong SZ. Capturing the fantastic voyage of monocytes through time and space. Front Immunol. 2019 Apr;10:834. doi: 10.3389/fimmu.2019.00834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rennke HG, Patel Y, Venkatachalam MA. Glomerular filtration of proteins: clearance of anionic, neutral, and cationic horseradish peroxidase in the rat. Kidney Int. 1978 Apr;13((4)):278–88. doi: 10.1038/ki.1978.41. [DOI] [PubMed] [Google Scholar]
- 41.Schenk S, Schoenhals GJ, de Souza G, Mann M. A high confidence, manually validated human blood plasma protein reference set. BMC Med Genomics. 2008 Dec;1((1)):41. doi: 10.1186/1755-8794-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fisher J, Russell JA, Bentzer P, Parsons D, Secchia S, Mörgelin M, et al. Heparin-binding protein (HBP): a causative marker and potential target for heparin treatment of human sepsis-induced acute kidney injury. Shock. 2017;48((3)):313–20. doi: 10.1097/SHK.0000000000000862. [DOI] [PubMed] [Google Scholar]
- 43.Tverring J, Vaara ST, Fisher J, Poukkanen M, Pettilä V, Linder A, et al. Heparin-binding protein (HBP) improves prediction of sepsis-related acute kidney injury. Ann Intensive Care. 2017 Oct;7((1)):105. doi: 10.1186/s13613-017-0330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luft JH. Fine structures of capillary and endocapillary layer as revealed by ruthenium red. Fed Proc. 1966;25((6)):1773–83. [PubMed] [Google Scholar]
- 45.Klastersky J, Paesmans M, Rubenstein EB, Boyer M, Elting L, Feld R, et al. The multinational association for supportive care in cancer risk index: a multinational scoring system for identifying low-risk febrile neutropenic cancer patients. J Clin Oncol. 2000;18((16)):3038–51. doi: 10.1200/JCO.2000.18.16.3038. [DOI] [PubMed] [Google Scholar]
- 46.Cherif H, Johansson E, Björkholm M, Kalin M. The feasibility of early hospital discharge with oral antimicrobial therapy in low risk patients with febrile neutropenia following chemotherapy for hematologic malignancies. Haematologica. 2006;91((2)):215–22. [PubMed] [Google Scholar]
- 47.Tapper H, Karlsson A, Mörgelin M, Flodgaard H, Herwald H. Secretion of heparin-binding protein from human neutrophils is determined by its localization in azurophilic granules and secretory vesicles. Blood. 2002 Mar;99((5)):1785–93. doi: 10.1182/blood.v99.5.1785. [DOI] [PubMed] [Google Scholar]
- 48.Rest RF, Cooney MH, Spitznagel JK. Subcellular distribution of glycosidases in human polymorphonuclear leucocytes. Biochem J. 1978;174((1)):53–9. doi: 10.1042/bj1740053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park SS. Post-glycosylation modification of sialic acid and its role in virus pathogenesis. Vaccines. 2019 Dec;7((4)):171. doi: 10.3390/vaccines7040171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chelazzi C, Villa G, Mancinelli P, De Gaudio AR, Adembri C. Glycocalyx and sepsis-induced alterations in vascular permeability. Crit Care. 2015 Jan;19((1)):26. doi: 10.1186/s13054-015-0741-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007 Jun;454((3)):345–59. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.