Abstract
Backgrounds and Aims
Even if no systemic treatment is currently validated for unresectable hepatocellular-cholangiocarcinoma (cHCC-CCA), tyrosine kinase inhibitors (TKIs) and platinum-based chemotherapy are frequently used in clinical practice. Our study aims to describe the effectiveness of first-line systemic treatments in patients with cHCC-CCA.
Patients and Methods
Patients with histological diagnosis of unresectable or metastatic cHCC-CCA confirmed by a centralized review (WHO classification 2019) and who received systemic treatment from 2009 to 2020 were included retrospectively in 11 centers. The outcomes of patients with cHCC-CCA were compared with patients with hepatocellular carcinoma (HCC) treated by sorafenib (n = 117) and with intrahepatic cholangiocarcinoma (iCCA, n = 94) treated mainly by platinum-based chemotherapy using a frailty Cox model. The efficacy of TKIs and platinum-based chemotherapies in patients with cHCC-CCA was assessed using a doubly robust estimator.
Results
A total of 83 patients with cHCC-CCA were included and were predominantly male (72%) with underlying cirrhosis (55%). 67% of patients had extrahepatic metastases and 31% macrovascular tumor invasion. cHCC-CCAs were more often developed on cirrhosis (55.4%) than iCCA (26.6%) but less frequently than HCC (80.2%) (p < 0.001). Both HCC (36.8% and cHCC-CCA (66.2%) had less frequent extrahepatic metastases than iCCA (81%) (p < 0.001). Unadjusted overall survival (OS) was better in iCCA (13 months) compared to cHCC-CCA (12 months) and HCC (11 months) (p = 0.130). In multivariable analysis, after adjustment by a Cox frailty model, patients with cHCC-CCA had the same survival as HCC and iCCA (HR = 0.67, 95% CI: 0.37–1.22, p = 0.189 and HR = 0.66, 95% CI: 0.43–1.02, p = 0.064, respectively). ALBI score (HR = 2.15; 95% CI: 1.23–3.76; p = 0.009), ascites (HR = 3.45, 95% CI: 1.31–9.03, p = 0.013), and tobacco use (HR = 2.29, 95% CI: 1.08–4.87, p = 0.032) were independently associated with OS in patients with cHCC-CCA. Among patients with cHCC-CCA, 25 patients treated with TKI were compared with 54 patients who received platinum-based chemotherapies. Patients treated with TKI had a median OS of 8.3 months compared to 11.9 months for patients treated with platinum-based chemotherapy (p = 0.86). After a robust doubly adjustment on tumor number and size, vascular invasion, ALBI, MELD, and cirrhosis, the type of treatment did not impact OS (HR = 0.92, 95% CI: 0.27–3.15, p = 0.88) or progression-free survival (HR = 1.24, 95% CI: 0.44–3.49, p = 0.67).
Conclusions
First-line systemic treatments with TKIs or platinum-based chemotherapies have similar efficacy in patients with unresectable/metastatic cHCC-CCA. The ALBI score predicts OS.
Keywords: Hepatocholangiocarcinoma, Cholangiocarcinoma, Hepatocellular carcinoma, Systemic treatment, Platinum, Tyrosine kinase inhibitors
Introduction
Combined hepatocellular-cholangiocarcinoma (cHCC-CCA) is considered as a particular entity characterized histologically by the presence of two distinct patterns within the same lesion: the presence of hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (iCCA) [1, 2, 3]. The diagnosis of cHCC-CCAs is based on routine histological diagnosis; immunohistochemistry could be helpful to identify the two cell populations [2, 4]. The risk factors associated with cHCC-CCA are shared with the other primary liver cancers (PLCs) and include chronic hepatitis B and C infection, metabolic syndrome, excessive alcohol consumption, and cirrhosis. The association with cirrhosis is reported with various frequencies (25–55%) and associated with different etiologies according to the geographical area [3, 5, 6, 7, 8, 9, 10]. The difficulty to perform the diagnosis of cHCC-CCA leads probably to an underestimation of the actual incidence of this subtype of PLC. Recent studies report that the incidence in western countries of cHCC-CCAs ranges between 1 and 5% of all PLCs [2, 4] with an overall incidence estimated to be 0.05 per 100,000 persons per year [5, 6].
Liver surgery is considered the standard of care for cHCC-CCA treatment when feasible, but the recurrence rate remains high [11]. Moreover, cHCC-CCA is often diagnosed at an advanced stage, so only a minority of patients are candidates for surgery in clinical practice [3]. In addition, most of the studies available are derived from monocentric surgical series, so few studies on unresectable/metastatic cHCC-CCAs have been published [12, 13, 14, 15]. Currently, no systemic treatment is validated in patients with unresectable or metastatic cHCC-CCA, and this histological subtype is excluded from most clinical trials enrolling patients with PLCs [15]. Consequently, the weak evidence available on the systemic treatments of cHCC-CCA came from case reports and case series treating the patients either by platinum-based chemotherapy, the standard treatment of advanced CCA, or the tyrosine kinase inhibitor sorafenib, the previous standard treatment of HCC [16, 17, 18]. The median overall survival (OS) reported ranged from 8.9 to 16.2 months [3, 12, 15]. However, most of the studies were either monocentric or lacked an adjustment between the groups treated by sorafenib and platinum-based therapy. Our study aimed to describe the clinical features of unresectable/metastatic cHCC-CCA treated by systemic therapies, compared their outcomes to cohorts of patients with advanced HCC and iCCA, and to assess the effect of the type of systemic treatments received by patients with cHCC-CCA taking into account other prognostic factors.
Materials and Methods
Selection of Patients
Consecutive patients with unresectable or metastatic cHCC-CCA from 11 French centers that underwent a systemic treatment from 2009 to 2020 were included retrospectively. An independent French Ethics Committee approved the study (approval number CLEA-2020-124). The reviewing of the histological samples was performed by one of the three expert pathologists (B.L.B., V.P., and J.C.) to confirm the diagnosis of cHCC-CCA according to the 2019 WHO classification of tumors of the digestive system [4].
The following criteria were required to include the patients in the study:
1 Patients with a histological diagnosis of cHCC-CCA according to 2019 WHO classification
2 Considered as unresectable or metastatic
3 Receiving a systemic treatment (whatever the type of systemic treatment)
4 Patients of 18 years old or more
The exclusion criteria were as follows:
1 Patients receiving combination of locoregional treatment and systemic treatment
2 Systemic treatment in a neoadjuvant or an adjuvant intent together with surgery
One hundred six patients were screened for inclusion. After reviewing the histology and the clinical data, 23 patients were excluded, and 83 patients were finally included in the study. Clinical data (gender, age, etiology, severity of the underlying liver disease, components of the metabolic syndrome, and WHO performance status), laboratory tests (liver function tests, platelets, serum alpha-fetoprotein [AFP], and CA19-9 levels) and tumor imaging features (size, number, macrovascular invasion, and distant metastasis) were collected before the beginning of the systemic treatment. Given the retrospective nature of the study, each center, based on its local experience, decided independently the first-line therapy received by the patient.
Two cohorts of patients were used as control populations: one cohort with unresectable or metastatic iCCA, mainly treated by platinum-based therapy, and one cohort of BCLC B/C HCC treated by the tyrosine kinase inhibitor. The cohort of patients with iCCA (n = 94) was a retrospective monocentric cohort (Eugène Marquis Center) with a histological diagnosis of iCCA considered nonresectable or metastatic and treated by systemic cytotoxic chemotherapy between 2005 and 2014. The cohort of patients with HCC (n = 117) was a retrospective cohort of patients from two centers (Avicenne Hospital in France and Erasme Hospital in Belgium) with HCC diagnosed by histology, classified by BCLC B and C, and treated by tyrosine kinase inhibitor (sorafenib) between 2013 and 2019. The following clinical, radiological, and biological characteristics were collected in these two cohorts before the beginning of the systemic treatment: age, gender, etiology of the chronic liver disease, presence of cirrhosis, liver function test, and tumor imaging features (size, number, macrovascular invasion, distant metastasis).
Follow-Up and Outcomes Assessment
After initiating systemic treatment, all patients were followed up until death or the last recorded visit. The follow-up period was ended on July 30, 2021. The primary endpoint was OS, defined as the survival from the initiation of treatment to death, whatever the cause. The secondary endpoints were progression-free survival (PFS), overall response rate (ORR), and disease control rate (DCR). The radiological response at the first radiological assessment (performed 2–3 months after the beginning of the systemic treatment) was collected from the radiological report of each center to compute the ORR defined by the combination of complete and partial response, and the DCR defined by the combination of complete response, partial response, and stable disease according to RECIST 1.1 criteria. PFS was defined as the survival from treatment start to disease progression or death, whatever the cause. Alive patients with no progression were censored at the last follow-up date.
Statistical Analysis
Continuous data are expressed as median (25–75 interquartiles). Categorical data are expressed as percentages. Statistical significance testing was 2-sided. A p value <0.05 was considered statistically significant for all tests unless indicated otherwise. Categorical variables were compared using the Fisher exact test or χ2 test, as appropriate. Continuous variables were compared using the Mann-Whitney U test. Survival outcomes such as OS and PFS were assessed using the Kaplan-Meier method, and the survival rates were compared using the log-rank test.
In the study population of unresectable or metastatic cHCC-CCA (n = 83), none of the variables had more than 25% of missing values, and missing values were managed in the exploratory analyses using multiple imputation chained methodology with six imputed datasets. Hence, all exploratory analyses were performed in each imputed dataset, and the estimates and standard errors were pooled into a final point estimate plus standard error according to Rubin's rule. Right-censored outcome (i.e., OS and PFS) regressions were Cox regressions (estimated effect sizes were expressed as hazard ratio [HR] with confidence interval [95% CI]), and binary outcome (i.e., ORR and DCR) regressions were logistic regressions (estimated effect sizes were expressed as odd ratio [OR] with 95% CI).
Prognostic factors associated with OS were assessed using both univariable and multivariable Cox regressions. Variables of interest were selected based on univariable selection (all variables with p < 0.20) and included in a multivariable Cox regression. In addition, both Cox and logistic regressions conducted across the 3 cohorts of patients (cHCC-CCA, iCCA, and BCLC B/C HCC patients) were adjusted to counteract unobserved heterogeneities between individuals using frailty (i.e., multilevel) regressions, which included a frailty term (i.e., random effect on each individual) [19].
The marginal effect of tyrosine kinase inhibitor (TKI) (vs. platinum-based regimens) on ORR, DCR, PFS, and OS was estimated using doubly robust estimators [20]. Doubly robust estimators are combinations of the appropriate outcome regression (Cox or logistic regression) with a model of exposure using an inverse probability weighting based on covariate balancing propensity scores [21]. Cofounders considered in all doubly robust estimators to adjust the effect of TKI (vs. platinum-based regimens) were: ALBI score, MELD score, tobacco use, advanced fibrosis, diabetes, plasmatic level of AFP and CA19-9, unique versus multiple tumors, the maximal diameter of tumor(s), the sum of diameters of tumor(s), the presence of metastasis, macrovascular invasion, previous local or locoregional treatments, and ascites. The quality of the inverse probability weighting of the doubly robust estimators was assessed using the effective sample size, ranges of absolute mean differences, and variance ratios across imputations, as appropriate.
The marginal effect of ALBI on ORR, DCR, PFS, and OS was estimated using doubly robust estimators as described above. Confounders considered in all doubly robust estimators to adjust the effect of ALBI were: the type of first-line regimen (TKI vs. platinum-based regimens), serum level of AFP, serum level of CA19-9, unique versus multiple tumors, the maximal diameter of tumor(s), the sum of diameters of tumor(s), the presence of metastasis, macrovascular invasion, and previous local or locoregional treatments. The quality of the inverse probability weighting of the doubly robust estimators was assessed using the effective sample size, ranges of absolute mean differences, and correlations across imputations, as appropriate.
All statistical analyses were performed using R statistical software version 3.6.3. The packages used were tidyverse, broom, MASS, Weitghtit, cobalt, glm, lme4, coxme, CBPS, mice, parlmice, and miceadd.
Results
Characteristics of Patients with Hepatocholangiocarcinoma
A total of 83 patients with cHCC-CCA treated by systemic therapy were included. The main characteristics of the population are summarized in Table 1. The diagnosis cHCC-CCA was performed on biopsy samples in 42 cases and on surgical samples in 41 cases. The patients were predominantly male (72.3%) with a median age of 63 years old. Thirty-seven patients (48.1%) had chronic alcohol consumption, 19.5% and 20.5% had chronic hepatitis C and B, respectively, and 46.6% had metabolic syndrome. More than half of patients had an advanced fibrosis (F3)/cirrhosis(F4) (55.4%) with 75% of Child-Pugh A. A median ALBI score was −2.32 [IQR: −2.69, −1.93] with 16 (26.7%), 40 (66.7%), and 4 (6.7%) of grade ALBI 1, 2, and 3, respectively.
Table 1.
Description of patients with hepatocholangiocarcinoma
| Variables | Available data | cHCC-CCA (n = 83) |
|---|---|---|
| Baseline characteristics | ||
| Male* | 83 | 60 (72.3) |
| Age, years** | 83 | 62.63 [56.67, 69.80] |
| Hepatitis B virus* | 78 | 16 (20.5) |
| Hepatitis C virus* | 77 | 15 (19.5) |
| Metabolic syndrome* | 75 | 35 (46.7) |
| Tobacco use* | 73 | 41 (56) |
| Chronic alcohol intake* | 77 | 37 (48.1) |
| Body mass index** | 81 | 24.00 [21.46, 27.00] |
| Diabetes type 2* | 78 | 32 (41.0) |
| Arterial hypertension* | 80 | 43 (53.8) |
| Dyslipidemia* | 78 | 34 (43.6) |
| Performance status 0/1* | 66 | 56 (84.8) |
| Advanced fibrosis/cirrhosis (F3–F4)* | 74 | 41 (55.4) |
| Child-Pugh A* | 32 | 24 (75) |
|
| ||
| Biochemical characteristics | ||
| Total bilirubin, µmol/L** | 78 | 12.00 [8.00, 17.00] |
| Albumin, g/L** | 62 | 36.00 [31.25, 39.75] |
| Time of prothrombin, %** | 73 | 87.00 [78.00, 97.00] |
| Platelets, g/L** | 78 | 208.00 [166.00, 289.75] |
| Creatinine, µmol/L** | 75 | 68.00 [60.50, 82.00] |
| Serum AFP** | 55 | 5.00 [0.55, 54.50] |
| Serum CA19-9** | 37 | 1.75 [1.00, 10.00] |
| MELD score** | 75 | 8 [7,11] |
| ALBI score** | 60 | −2.3182 [–2.6940, −1.9315] |
|
| ||
| Tumor characteristics | ||
| Multiple intrahepatic lesions* | 76 | 48 (63.2) |
| Macrovascular invasion* | 78 | 24 (30.8) |
| Metastasis* | 80 | 53 (66.2) |
| Size of biggest nodule, mm** | 71 | 50.00 [27.75, 85.00] |
The % of Child-Pugh A patient was calculated on advanced fibrosis/cirrhosis patients. CA19-9 and AFP are represented as times above normal (median [IQR]). AFP, alpha-fetoprotein; CA19-9, carbohydrate antigen 19-9; MELD, Model for End-Stage Liver Disease; ALBI, albumin-bilirubin score.
n (%).
Median (interquartile range).
In terms of tumor features, patients frequently had a multinodular intrahepatic disease (63.2%) with an average size of the largest nodule of 50 mm (27.75–85.00). At baseline, 66.2% of patients had an extrahepatic spread, the most common sites being the lymph nodes (41.2%), lungs (26.3%), peritoneum (12.5%), and bones (12.5%). Almost one-third of patients (30.7%) had a macrovascular tumor invasion.
Before the beginning of systemic treatments, 49 patients (59%) had received previous liver-directed treatment of cHCC-CCA (surgery or locoregional treatments), including liver resection for 31 patients and liver transplantation for 9 patients. Among the 83 patients, 54 patients were treated with first-line platinum-based therapy, 25 with TKIs (mainly sorafenib), and 4 with other chemotherapies regimens. The chemotherapy regimens are detailed in Supplementary Table 1 (see www.karger.com/doi/10.1159/000525488 for all online suppl. material).
Outcomes of Patients with Hepatocholangiocarcinoma and Comparison with Intrahepatic Cholangiocarcinoma and Hepatocellular Carcinoma
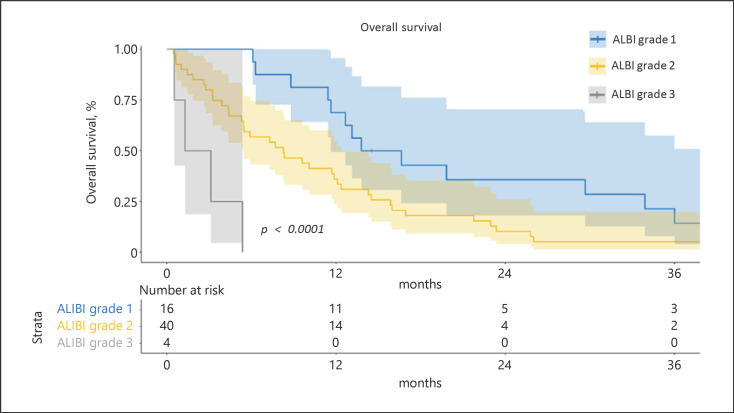
Among the 83 patients with cHCC-CCAs, the median follow-up was 41 months. Sixty-eight patients had an available first radiological assessment under systemic treatment, 9 patients (13%) harbored an objective response rate, and 36 patients (53%) had disease control. The median PFS was 4 months (95% CI: 3–5). The median OS was 12 months (95% CI: 8–15) with 1 year, 2 years, and 3 years' survival rates of 47.7%, 18.3%, and 7.6%, respectively. A multivariable cox analysis showed that ALBI score (HR = 2.15; 95% CI: 1.23, 3.76; p = 0.009), ascites (HR = 3.45, 95% CI: 1.31, 9.03; p = 0.013), and tobacco use (HR = 2.29; 95% CI: 1.08, 4.87; p = 0.032) were independently associated with OS (shown in Table 2). Kaplan-Meier OS curves according to the ALBI grade are shown in Figure 1.
Table 2.
Univariable and multivariable analyses of factors associated with OS in the 83 patients with hepatocholangiocarcinoma
| Variables | Univariable cox regression |
Multivariable cox regression* |
||||||
|---|---|---|---|---|---|---|---|---|
| HR | 2.5 | 97.5 | pvalue | HR | 2.5 | 97.5 | pvalue | |
| Male | 1.79 | 1.05 | 3.03 | 0.032 | 1.12 | 0.59 | 2.13 | 0.731 |
| Age (years) | 1.00 | 0.98 | 1.02 | 0.927 | ||||
| Hepatitis B virus | 0.76 | 0.41 | 1.40 | 0.370 | ||||
| Hepatitis C virus | 1.06 | 0.56 | 2.03 | 0.846 | ||||
| Metabolic syndrome | 1.32 | 0.82 | 2.12 | 0.247 | ||||
| BMI (kg/m2) | 1.00 | 0.96 | 1.05 | 0.911 | ||||
| Arterial hypertension | 1.12 | 0.71 | 1.79 | 0.615 | ||||
| Dyslipidemia | 1.35 | 0.85 | 2.14 | 0.207 | ||||
| Tobacco | 2.29 | 1.40 | 3.74 | 0.001 | 2.29 | 1.08 | 4.87 | 0.032 |
| Alcohol intake | 2.17 | 1.26 | 3.73 | 0.006 | 0.91 | 0.44 | 1.89 | 0.804 |
| Diabetes | 1.33 | 0.83 | 2.13 | 0.229 | ||||
| Ascites | 4.64 | 1.68 | 12.80 | 0.004 | 3.45 | 1.31 | 9.03 | 0.013 |
| Encephalopathy | 4.94 | 0.38 | 64.66 | 0.207 | ||||
| PS grade | ||||||||
| 0 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| 1 | 0.74 | 0.41 | 1.33 | 0.305 | 0.92 | 0.46 | 1.84 | 0.805 |
| 2 | 2.84 | 0.86 | 9.42 | 0.081 | 1.59 | 0.57 | 4.49 | 0.360 |
| 3 | 2.21 | 0.27 | 18.12 | 0.433 | 12.49 | 0.07 | 22.47 | 0.273 |
| AFP | 1.00 | 1.00 | 1.00 | 0.281 | ||||
| CA19-9 | 1.00 | 1.00 | 1.00 | 0.249 | ||||
| Unique tumor(s) | 0.81 | 0.49 | 1.32 | 0.394 | ||||
| Size of the biggest nodule | 1.00 | 0.99 | 1.01 | 0.947 | ||||
| Sum of tumor(s) diameter | 1.00 | 0.99 | 1.01 | 0.823 | ||||
| Macrovascular invasion | 1.26 | 0.76 | 2.08 | 0.360 | ||||
| Metastasis | 1.04 | 0.63 | 1.73 | 0.872 | ||||
| Previous treatment | 0.80 | 0.50 | 1.27 | 0.334 | ||||
| First-line regimen | ||||||||
| Platinum-based | Ref | Ref | Ref | Ref | ||||
| TKI | 1.04 | 0.63 | 1.73 | 0.87 | ||||
| MELD score | 1.07 | 0.98 | 1.17 | 0.124 | 1.09 | 0.97 | 1.22 | 0.138 |
| ALBI grade | 2.20 | 1.24 | 3.91 | 0.010 | 2.15 | 1.23 | 3.76 | 0.009 |
| Advanced fibrosis | 1.43 | 0.88 | 2.33 | 0.144 | 1.12 | 0.61 | 2.06 | 0.711 |
BMI, body mass index; AFP, alpha-fetoprotein; CA19-9, carbohydrate antigen 19-9; TKI, tyrosine kinase inhibitor; PS, performance status; MELD, Model for End-Stage Liver Disease; ALBI, albumin-bilirubin score.
Variables included in the multivariable Cox model were selected based on the results of the univariable analysis (i.e., all variables associated to OS with a p value <0.200).
Fig. 1.
OS of patients with cHCC-CCA according to the ALBI grade. Kaplan-Meier curves of unadjusted OS stratified according to the ALBI grade (grade 1 n = 16, grade 2 n = 40, and grade 3 n = 4). The comparison was performed using the log-rank test.
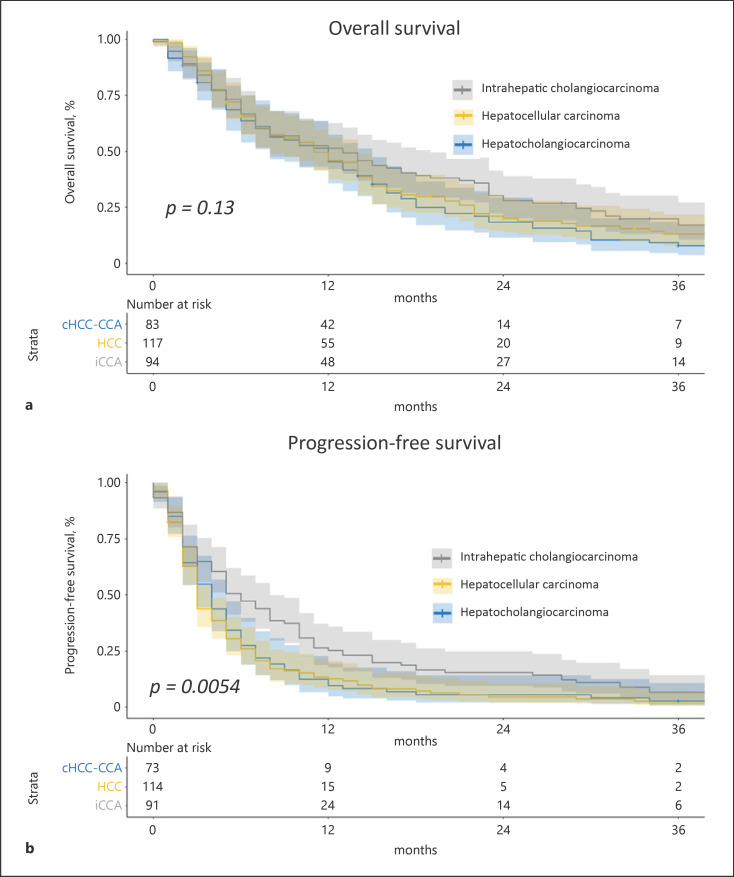
Next, we aim to compare the baseline characteristics and the outcomes of patients with cHCC-CCAs with patients with nonresectable/metastasis iCCA (n = 94) and HCC (n = 117) (see Table 3 for the full description of the three groups). cHCC-CCAs were more often developed on advanced fibrosis/cirrhosis (55.4%) than iCCA (26.6%) but less frequently than HCC (80.2%) (p < 0.001). Extrahepatic metastases were more frequent in iCCA (81%) than in cHCC-CCAs (66.2%) and HCC (36.8%) (p < 0.001).
Table 3.
Comparison of baseline features of patients with hepatocellular carcinoma, intrahepatic cholangiocarcinoma, and hepatocholangiocarcinoma
| Variables | iCCA (n = 94) | HCC (n = 117) | cHCC-CCA (n = 83) | p value |
|---|---|---|---|---|
| Male* | 60 (63.8) | 97 (82.9) | 60 (72.3) | 0.007 |
| Age, years% | 64.00 [56.00, 71.75] | 66.00 [57.00, 75.00] | 63.00 [56.50, 70.00] | 0.254 |
| Hepatitis B virus* | 0 (0.0) | 27 (23.1) | 16 (20.5) | <0.001 |
| Hepatitis C virus* | 3 (3.2) | 36 (30.8) | 15 (19.5) | <0.001 |
| Chronic alcohol intake* | 13 (13.8) | 43 (36.8) | 37 (48.1) | <0.001 |
| Advanced fibrosis/cirrhosis (F3-F4)* | 25 (26.6) | 89 (80.2) | 41 (55.4) | <0.001 |
| Alkaline phosphatase** | 1.45 [0.80, 2.75] | 1.20 [0.80, 1.60] | 1.20 [0.80, 1.78] | 0.658 |
| Total bilirubin, µmol/L** | 14.85 [10.65, 20.70] | 15.60 [10.00, 23.00] | 12.00 [8.00, 17.00] | 0.014 |
| Serum AFP** | 0.60 [0.30, 1.80] | 36.30 [1.40, 298.60] | 5.00 [0.55, 54.50] | <0.001 |
| Serum CA19-9** | 2.45 [0.65, 24.18] | 1.19 [0.66, 2.33] | 1.75 [1.00, 10.00] | 0.027 |
| Multiple intrahepatic lesions* | 67 (90.5) | 93 (84.5) | 48 (63.2) | <0.001 |
| Macrovascular invasion* | 36 (38.3) | 34 (30.6) | 24 (30.8) | 0.441 |
| Metastatic disease* | 76 (80.9) | 43 (36.8) | 53 (66.2) | <0.001 |
None of the variables had more than 5% of missing values. To note, we included in this table the common variables available between the 3 cohorts of patients. Alkaline phosphatase, CA19-9, and AFP are represented as times above normal (median [IQR]). cHCC-CCA, hepatocholangiocarcinoma; HCC, hepatocellular carcinoma; iCCA, intrahepatic cholangiocarcinoma; AFP, alpha-fetoprotein; CA19-9, carbohydrate antigen 19-9.
n (%).
Median (interquartile range).
All the patients in the HCC group (n = 117) received the TKI sorafenib as first-line systemic treatment. Patients in the iCCA group received primarily platinum-based regimens (90%; n = 85) including gemcitabine-oxaliplatin (n = 47), gemcitabine-cisplatin (n = 34), and fluorouracil-leucovorin (LV5FU2)-oxaliplatin (n = 4). The other types of chemotherapies were gemcitabine monotherapy (7 patients), 5-fluorouracil-irinotecan (2 patients), and 5-fluorouracil (1 patient).
Before adjustment, ORR rates were 4.4%, 9.0%, and 16.1% in cHCC-CCAs, HCC, and iCCA patients, respectively (p = 0.047), and DCR rates were 52.9%, 54.0%, and 67.7% in cHCC-CCAs, HCC, and iCCA patients, respectively (p = 0.083). However, in multivariable analysis using frailty logistic regression, no significant differences in term of ORR and DCR were observed between cHCC-CCA and HCC or iCCA (Table 4).
Table 4.
Multivariable analysis of variable associated with PFS and OS in patients with HCC, iCCA, and cHCC-CCA
| Variables | Unadjusted HR/OR* (95% CI) | Unadjusted p value | Adjusted† HR/OR* (95% CI) | Adjusted†p value |
|---|---|---|---|---|
| PFS | ||||
| cHCC-CCA (reference) | Ref | Ref | Ref | |
| HCC | 1.06 (0.77–1.46) | 0.719 | 0.66 (0.37–1.20) | 0.172 |
| iCCA | 0.66 (0.47–0.92) | 0.014 | 0.63 (0.41–0.96) | 0.034 |
| Platinum-based (reference) | Ref | Ref | Ref | |
| TKI | 1.39 (1.07–1.80) | 0.013 | 1.54 (0.85–2.79) | 0.157 |
| Intrahepatic multiple nodules | 1.48 (1.06–2.05) | 0.021 | 1.63 (1.13–2.35) | 0.009 |
| Advanced fibrosis/cirrhosis | 1.37 (1.07–1.76) | 0.013 | 1.01 (0.74–1.38) | 0.941 |
| Metastasis | 1.07 (0.84–1.37) | 0.580 | 1.19 (0.86–1.66) | 0.293 |
| Macrovascular invasion | 1.06 (0.82–1.37) | 0.646 | 1.28 (0.95–1.73) | 0.102 |
| OS | ||||
| cHCC-CCA (reference) | Ref | Ref | ||
| HCC | 0.91 (0.68–1.22) | 0.521 | 0.67 (0.37–1.22) | 0.189 |
| iCCA | 0.73 (0.53–1.00) | 0.050 | 0.66 (0.43–1.02) | 0.064 |
| Platinum-based (reference) | Ref | Ref | ||
| TKI | 1.12 (0.87–1.43) | 0.382 | 1.12 (0.62–1.99) | 0.713 |
| Intrahepatic multiple nodules | 1.19 (0.85–1.65) | 0.312 | 1.29 (0.89–1.87) | 0.176 |
| Advanced fibrosis/cirrhosis | 1.58 (1.21–2.05) | <0.001 | 1.45 (1.04–2.01) | 0.028 |
| Metastasis | 1.12 (0.86–1.44) | 0.405 | 1.15 (0.89–1.61) | 0.401 |
| Macrovascular invasion | 1.31 (0.98–1.76) | 0.067 | 1.42 (1.05–1.92) | 0.025 |
| Objective response rate | ||||
| cHCC-CCA (reference) | Ref | Ref | ||
| HCC | 2.49 (0.51–12.18) | 0.261 | 1.34 (0.14–13.20) | 0.802 |
| iCCA | 4.05 (0.85–19.31) | 0.079 | 7.63 (0.83–69.84) | 0.072 |
| Platinum-based (reference) | ||||
| TKI | 0.87 (0.35–2.19) | 0.767 | 2.87 (0.16–51.14) | 0.473 |
| Intrahepatic multiple nodules | 0.90 (0.28–2.85) | 0.858 | 0.62 (0.18–2.11) | 0.442 |
| Advanced fibrosis/cirrhosis | 0.94 (0.37–2.36) | 0.888 | 1.35 (0.46–3.98) | 0.584 |
| Metastasis | 1.25 (0.48–3.28) | 0.645 | 0.99 (0.29–3.36) | 0.993 |
| Macrovascular invasion | 1.09 (0.41–2.85) | 0.868 | 0.96 (0.34–2.71) | 0.934 |
| DCR | ||||
| cHCC-CCA (reference) | Ref | Ref | ||
| HCC | 1.20 (0.61–2.38) | 0.593 | 1.43 (0.42–4.90) | 0.566 |
| iCCA | 1.74 (0.85–3.58) | 0.132 | 1.79 (0.74–4.33) | 0.199 |
| Platinum-based (reference) | Ref | |||
| TKI | 0.76 (0.44–1.30) | 0.308 | 0.78 (0.22–2.80) | 0.706 |
| Intrahepatic multiple nodules | 0.68 (0.33–1.38) | 0.284 | 0.62 (0.29–1.33) | 0.217 |
| Advanced fibrosis/cirrhosis | 0.58 (0.33–1.01) | 0.052 | 0.63 (0.34–1.18) | 0.148 |
| Metastasis | 0.75 (0.43–1.31) | 0.308 | 0.58 (0.29–1.14) | 0.114 |
| Macrovascular invasion | 1.26 (0.71–2.24) | 0.437 | 1.01 (0.54–1.88) | 0.971 |
cHCC-CCA, hepatocholangiocarcinoma; HCC, hepatocellular carcinoma; iCCA, intrahepatic cholangiocarcinoma.
Right-censored outcome (i.e., OS and PFS) regressions were Cox regressions (estimated effect sizes were expressed as HR with 95% CI) and binary outcome (i.e., ORR and DCR) regressions were logistic regressions (estimated effect sizes were expressed as OR with 95% CI).
Adjustment regarding unobserved heterogeneities between individuals was performed using frailty models (Cox or logistic regression), which included a frailty term (i.e., random effect) on everyone.
The unadjusted median OS was longer in iCCA (median 13 months) and cHCC-CCA (12 months) than in HCC (11 months) (p = 0.130, log-rank test) (Fig. 2a), and the unadjusted median PFS was longer in iCCA (6 months) compared to cHCC-CCA (4 months) and HCC (3 months) (p = 0.005, log-rank test) (Fig. 2b). In multivariate analysis using frailty Cox regression, the PFS was significantly longer in the iCCA group compared to cHCC-CCA (OR 0.63 [95% CI: 0.41–0.96], p = 0.034) but without any statistical difference in terms of OS (Table 4).
Fig. 2.
OS and PFS in patients with HCC, iCCA, and cHCC-CCA. Kaplan-Meier curves of unadjusted OS (a) and unadjusted PFS (b) comparing HCC (n = 117), iCCA (n = 94), and cHCC-CCA (n = 83) using the log-rank test.
Comparison between Tyrosine Kinase Inhibitors and Platinum-Based First-Line Regimens in Hepatocholangiocarcinoma
Next, we compared the characteristics and the outcomes of patients treated with tyrosine inhibitors (n = 25) with the patients with platinum-based regimens (n = 54) among the cohort of 83 patients with cHCC-CCA. Patients treated by TKI had a lower serum CA19-9 (p = 0.035) and a more severe liver failure reflected by a significantly different value of the ALBI score (p = 0.037) (see online suppl. Table 2 for the full description).
Without adjustment, ORR was not significantly different between the two regimens (10% for TKI vs. 15.2% for platinum treated patients, p = 0.859) as well as DCR (45% for TKI vs. 58.7% for platinum, p = 0.448). After adjustment using doubly robust estimators, patients treated with TKI (vs. platinum-based regimens) did not have a higher ORR (OR = 1.67; 95% CI: 0.03, 3.95 p = 0.642) or DCR (OR = 0.76; 95% CI: 0.15, 3.86; p = 0.387).
Without adjustment, patients treated by TKIs had a median OS of 8.3 months compared to 11.9 months for patients treated with platinum-based regimens (p = 0.86, log-rank test, shown in Fig. 3a) and a median PFS of 4.1 months for platinum-based regimens compared to TKI 2.8 months for TKI (p = 0.91, log-rank test, shown in Fig. 3b). After the adjustment using the doubly robust estimators, the type of treatment had no effect on PFS (HR = 1.25; 95% CI: 0.44, 3.49; p = 0.652) or OS (HR = 0.98; 95% CI: 0.48, 2.02; p = 0.962).
Fig. 3.
OS and PFS between patients with cHCC-CCA treated by TKIs versus platinum-based chemotherapies. Kaplan-Meier curves of unadjusted OS (a) and unadjusted PFS (b) comparing cHCC-CCA treated by TKIs (n = 25) versus platinum-based regimens (n = 54) using the log-rank test.
Using a doubly robust estimation, the ALBI score was associated with OS (OR 2.517, 95% CI 1.412–4.487, p = 0.003) independently of the treatment received (platinum regimen or TKI), whereas it was not significantly associated with PFS, DCR, and ORR (Table 5). Among the patients treated by platinum-based regimens, the ALBI score taken as a continuous variable was associated significantly with OS (OR 11.679, 95% CI 2.325–58.674, p = 0.0049), whereas no significant association was observed in patients treated by TKI (OR 1.679, 95% CI 0.6321–4.461, p = 0.273) maybe due to the low number of patients in this subgroup (online suppl. Table 3).
Table 5.
Doubly robust estimators of the effect of ALBI score on ORR, DCR, PFS, and OS in the 79 patients with hepatocholangiocarcinoma treated either by TKI or by platinum-based regimen
| Outcomes | Adjusted† HR/OR*(95% CI) | 95% CI of adjusted† HR/OR* | Adjusted† p value |
|---|---|---|---|
| ALBI versus ORR | 0.427 | 0.043–4.285 | 0.433 |
| ALBI versus DCR | 0.339 | 0.057–2.001 | 0.196 |
| ALBI versus PFS | 1.121 | 0.625–2.011 | 0.685 |
| ALBI versus OS | 2.517 | 1.412–4.487 | 0.003 |
Right-censored outcome (i.e., OS and PFS) regressions were Cox regressions (HR with 95% CI) and binary outcome (i.e., ORR and DCR) regressions were logistic regressions (OR with 95% CI).
Adjustment was done regarding the first-line regiment (TKI vs. platinumbased regimens), serum level of AFP and CA19-9, unique versus multiple tumors, the maximal diameter of tumor(s), the sum of diameters of tumor(s), the presence of metastasis, macrovascular invasion, and previous local or locoregional treatments. The analysis was performed using doubly robust estimators (combinations of the appropriate outcome regression [Cox or logistic regression] with a model of exposure using an inverse probability weighting based on covariate balancing propensity scores).
Characteristic and Outcomes in Patients with Hepatocholangiocarcinoma Treated by a Second-Line Systemic Therapy
In the cHCC-CCA group, 44 patients received a second-line therapy, 16 patients received a third-line therapy of systemic treatments, and 4 patients a fourth-line therapy. Of the 44 patients receiving a second-line systemic treatment, 12 were treated with platinum-based regimens and 17 with TKIs. The remaining 15 patients had various regimens described in online supplementary Table 1. Median OS after the beginning of the second line was 8 months whatever the treatments, with a median survival of 8 months for patients treated by TKI versus 10.5 months for patients treated by platinum-based regimens (log-rank test p = 0.16, no adjustment was performed due to the low numbers of patients in each group).
Discussion
Although cHCC-CCA was described several decades ago, its evolving definition in terms of classification and its rarity has led to a delay in developing a consensual therapeutical strategy. While surgery is recognized as the best therapeutical option when feasible, the clinical management in case of unresectable or metastatic disease lacks reliable evidence and is based on local experiences mimicking the systemic treatment validated in iCCA and HCC. We described a retrospective multicentric cohort of 83 patients with cHCC-CCA treated by different chemotherapy regimens, including TKIs and platinum-based chemotherapy. Currently, this series is one of the largest cohort published in the literature about systemic treatments using the 2019 WHO classification to define cHCC-CCA, with a central review of histology [4]. In addition, this series is nationwide and enrolled patients from various expert centers of a Western country. Other studies focusing on the outcomes in patients with cHCC-CCA under systemic treatments included a limited number of patients from Western countries or were monocentric studies in Asia [12, 14, 15].
cHCC-CCA has been considered more similar to iCCA than HCC when comparing the survival rates after resection or transplantation [3, 11, 22]. However, a recent study has suggested that the outcomes were similar between cHCC-CCA, HCC, and ICCA after adjusting for cirrhosis and tumor features [23]. Morevoer, no study has compared the characteristics and outcomes of patients with unresectable/metastatic cHCC-CCA, HCC, and ICCA under systemic treatments. Thus, we compared cHCC-CCA under systemic treatments with a population of patients with iCCA treated mainly by platinum-based chemotherapy and patients with HCC treated by sorafenib. cHCC-CCA seems to stand at the crossroad between iCCA and HCC in terms of advanced fibrosis/cirrhosis (55%), of gender balance with lesser men than in HCC and more than in iCCA and the levels of serum AFP and CA19-9. The unadjusted analysis suggests that the median OS was longer in iCCA (13 months) compared to cHCC-CCA (12 months) and HCC (11 months). However, after adjustment, the difference was not significant, suggesting that confounding factors, such as cirrhosis, are likely to be competitive prognostic factors explaining the unadjusted differences. Altogether, cHCC-CCA patients are likely to cumulate the prognosis of an aggressive pattern of PLC and the risks related to advanced underlying liver diseases.
Such complexes of clinical situations could explain the current uncertainties regarding the more appropriate first-line systemic therapy in unresectable patients. A previous published eastern multicentric cohort suggested that the TKI sorafenib has a lower median survival (3.5 months) compared to gemcitabine/cisplatin (11.9 months) and fluorouracil/cisplatin (10.2 months), but the very low number of patients treated by sorafenib (n = 5) impaired this conclusion [13]. Another western monocentric cohort of cHCC-CCA who received systemic therapy reported a median OS of 11.5 months for gemcitabine/platinum therapy and OS of 9.6 months for sorafenib, but this study only included 7 patients treated by sorafenib [14]. A multicentric series published by Salimon et al. [12], including 30 patients, reported 16.2 months of OS under platinum-based therapies, but this study's results are impaired because one-third of the patients lack histology for the diagnosis cHCC-CCA. Finally, a recent monocentric study from Korea suggested that patients, predominantly infected by hepatitis B infection, treated with TKI and cytotoxic chemotherapy had similar outcomes [15].
In this study, we compared the outcomes of 25 cHCC-CCA patients who received TKI with 54 of their counterparts who received platinum-based chemotherapy. Patients treated by TKI had a median OS of 8.3 months compared to 11.9 months for patients treated with platinum-based regimens. This study used a doubly robust method to provide unbiased effects of treatments on outcomes of patients, whereas other studies report only an unadjusted comparison [12, 13, 14, 15]. Such an unadjusted comparison is likely to introduce important bias when comparing treatments, such as TKI versus platinum-based, for which indications are likely guided by the general status, the severity of the underlying liver diseases, and tolerance of the patient. In fact, no difference in terms of ORR, DCR, PFS, and OS between the two types of systemic treatments was found after doubly robust adjustment on several tumoral and nontumoral cofounding factors. These data suggested that TKI and platinum-based chemotherapy have the same outcomes and efficacy in patients with unresectable/metastatic cHCC-CCA and that the choice of the systemic treatments should be tailored to the potential contraindications for each regimen and the patient's preference. Interestingly, ALBI score and ascites were associated with OS independently from other baseline variables, especially from the MELD score and the presence of advanced fibrosis. Therefore, ALBI score seems to be a relevant prognostic tool in cHCC-CCA as reported for HCC patients. It is likely to capture both the liver function in cirrhotic patients and the biliary obstruction, diffuse tumor infiltration, and denutrition in patients regardless of their underlying parenchymal changes [24, 25, 26]. Considering that the ALBI score is significantly associated with OS but not with outcomes of response to cancer treatments (PFS, DCR, ORR), it could be speculated that the ALBI score is more a prognostic factor related to the degree of liver failure and denutrition than a variable predictive of the efficacy of the treatment.
This study has limitations. First, it is a retrospective study and some data such as radiological response were collected from radiological reports and encountered limitations related to the RECIST 1.1 criteria especially versus the mRECIST [27]. Moreover, the absence of difference in outcomes could be due to a lack of power due to the limited number of patients in subgroup analysis. Additionally, even if expert pathologists reviewed the cases, the diagnosis of cHCC-CCA on tumor biopsy remains challenging, and we could not exclude that some CCA and HCC could be misdiagnosed due to tumor sampling error affected by the intrinsic histological heterogeneity of cHCC-CCA or the use of noninvasive criteria for HCC diagnosis in cirrhotic patients [18, 28]. Moreover, the small number of patients included in the comparison between TKI and platinum-based treatment is one of the limitations of the study that requires a future larger cohort of patients to have more granularity in the analysis. Finally, atezolizumab/bevacizumab represented the new standard of care in advanced HCC patients [29]. Likewise, biomarkers guided therapy (e.g., FGFR inhibitor in FGFR2 fusion, IDH1 inhibitor in IDH1 mutations) are widely used in CCA patients [30, 31, 32, 33]. The assessment of these two strategies was beyond the scope of this study, while they are likely to be promising in cHCC-CCA patients and require to be studied in the near future.
In conclusion, this study reported a large western multicentric cohort of cHCC-CCA under systemic treatment, showing that cHCC-CCA harbored specific clinical features compared to HCC and CCA and that TKI and platinum-based therapies have the same efficacy in nonresectable/metastatic cHCC-CCA. The ALBI score could be used as a relevant prognostic tool in these patients.
Statement of Ethics
According to the French laws, this study protocol was reviewed and approved by our Ethical Committee (Comité Local d'Ethique pour la Recherche Clinique des HUPSSD Avicenne-Jean Verdier-René Muret, 125 rue de Stalingrad 93009 BOBIGNY Cedex; approval number CLEA-2020-124). Written consent was not necessary because patients were initially informed of the possibility of using anonymized clinical and biological data collected during routine care in the welcome booklet for different university hospitals and the possibility of opposing the use of their data. Our study was also declared to the National Commission for Informatics and Freedoms (Commission Nationale de l'Informatique et des Libertés, CNIL), number of declaration 2218503 v 0. Our research complies with the guidelines for human studies and was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Conflict of Interest Statement
E.G. received travel and congress fees from Bayer, Gilead, and Ipsen; M.B. received consulting fees from Bayer, BMS, Ipsen, Roche, Eisai, Sirtex, Servier, MSD, and AstraZeneca; M.R. received honoraria from Servier, GE Healthcare, Ipsen, Canon-Toshiba, Alexion Pharmaceuticals, Guerbet, and Sirtex; A.Z. received consulting and advisory board fees from Amgen, Lilly, Merck, Roche, Sanofi, Servier, Baxter, MSD, Pierre Fabre, Havas Life, Alira Health, and Zymeworks; D.T. received consulting fees from Amgen, Merck, Novartis, BMS, MSD, AstraZeneca, Sanofi, Roche, Bayer, Servier, and Ipsen; J.E. received consulting fees from MSD, Eisai, BMS, AstraZeneca, Bayer, Roche, Ipsen, Basilea, Merck Serono, Incyte, and Servier and also received travel fees from Amgen and research funding (institutional) from BMS, Beigene; J.-F.B. received consulting fees from Bayer, BMS, Ipsen, Eisai, MSD, Roche, and AstraZeneca; J.-C.N. received research grants from Ipsen and Bayer; and E.T. received research support from Gilead and speaker fees from Abbvie. The other authors have no conflicts of interest to declare.
Funding Sources
None.
Author Contributions
Substantial contributions to conception and design: Elia Gigante and Jean-Charles Nault; acquisition of data and/or analysis and interpretation of data: Elia Gigante, Christian Hobeika, Brigitte Le Bail, Valérie Paradis, David Tougeron, Marie Lequoy, Mohamed Bouattour, Jean-Frederic Blanc, Nathalie Ganne-Carrié, Henri Tran, Clémence Hollande, Manon Allaire, Giuliana Amaddeo, Hélène Regnault, Paul Vigneron, Maxime Ronot, Laure Elkrief, Gontran Verset, Eric Trepo, Aziz Zaanan, Marianne Ziol, Massih Ningarhari, Julien Calderaro, Julien Edeline, and Jean-Charles Nault; drafting, revising, and the manuscript content: Elia Gigante, Christian Hobeika, and Jean-Charles Nault; and final approval of the version to be published: all the authors.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author and will be available after reasonable request.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Acknowledgments
We thank the Paris Liver Cancer Group for their support to the project.
References
- 1.Allen RA, Lisa JR. Combined liver cell and bile duct carcinoma. Am J Pathol. 1949 Jul;25((4)):647–55. [PMC free article] [PubMed] [Google Scholar]
- 2.Brunt E, Aishima S, Clavien P-A, Fowler K, Goodman Z, Gores G, et al. cHCC-CCA: consensus terminology for primary liver carcinomas with both hepatocytic and cholangiocytic differentation. Hepatology. 2018;68((1)):113–26. doi: 10.1002/hep.29789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gigante E, Paradis V, Ronot M, Cauchy F, Soubrane O, Ganne-Carrié N, et al. New insights into the pathophysiology and clinical care of rare primary liver cancers. JHEP Rep. 2021 Feb;3((1)):100174. doi: 10.1016/j.jhepr.2020.100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.SempouxKakar CS, Kondo F, Schirmacher P. Combined hepatocellular-cholangiocarcinoma and undifferentiated primary liver carcinoma. In: Arends MJ, Fukuyama M, Klimstra DS, et al., editors. WHO classification of tumours: digestive system tumours. 5th ed. Lyon: IARC; 2019. p. 260. [Google Scholar]
- 5.Garancini M, Goffredo P, Pagni F, Romano F, Roman S, Sosa JA, et al. Combined hepatocellular-cholangiocarcinoma: a population-level analysis of an uncommon primary liver tumor. Liver Transpl. 2014 Aug;20((8)):952–9. doi: 10.1002/lt.23897. [DOI] [PubMed] [Google Scholar]
- 6.Ramai D, Ofosu A, Lai JK, Reddy M, Adler DG. Combined hepatocellular cholangiocarcinoma: a Population-Based Retrospective Study. Am J Gastroenterol. 2019;114((9)):1496–501. doi: 10.14309/ajg.0000000000000326. [DOI] [PubMed] [Google Scholar]
- 7.Kudo M, Izumi N, Kubo S, Kokudo N, Sakamoto M, Shiina S, et al. Report of the 20th Nationwide follow-up survey of primary liver cancer in Japan. Hepatol Res. 2020 Jan;50((1)):15–46. doi: 10.1111/hepr.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng IO, Shek TW, Nicholls J, Ma LT. Combined hepatocellular-cholangiocarcinoma: a clinicopathological study. J Gastroenterol Hepatol. 1998 Jan;13((1)):34–40. doi: 10.1111/j.1440-1746.1998.tb00542.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee W-S, Lee K-W, Heo J-S, Kim S-J, Choi S-H, Kim Y-I, et al. Comparison of combined hepatocellular and cholangiocarcinoma with hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Surg Today. 2006;36((10)):892–7. doi: 10.1007/s00595-006-3276-8. [DOI] [PubMed] [Google Scholar]
- 10.Taguchi J, Nakashima O, Tanaka M, Hisaka T, Takazawa T, Kojiro M. A clinicopathological study on combined hepatocellular and cholangiocarcinoma. J Gastroenterol Hepatol. 1996 Aug;11((8)):758–64. doi: 10.1111/j.1440-1746.1996.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 11.Gentile D, Donadon M, Lleo A, Aghemo A, Roncalli M, di Tommaso L, et al. Surgical treatment of hepatocholangiocarcinoma: a systematic review. Liver Cancer. 2020 Jan;9((1)):15–27. doi: 10.1159/000503719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salimon M, Prieux-Klotz C, Tougeron D, Hautefeuille V, Caulet M, Gournay J, et al. Gemcitabine plus platinum-based chemotherapy for first-line treatment of hepatocholangiocarcinoma: an AGEO French multicentre retrospective study. Br J Cancer. 2018;118((3)):325–30. doi: 10.1038/bjc.2017.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi S, Terashima T, Shiba S, Yoshida Y, Yamada I, Iwadou S, et al. Multicenter retrospective analysis of systemic chemotherapy for unresectable combined hepatocellular and cholangiocarcinoma. Cancer Sci. 2018 Aug;109((8)):2549–57. doi: 10.1111/cas.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trikalinos NA, Zhou A, Doyle MBM, Fowler KJ, Morton A, Vachharajani N, et al. Systemic therapy for combined hepatocellular-cholangiocarcinoma: a single-institution experience. J Natl Compr Canc Netw. 2018;16((10)):1193–9. doi: 10.6004/jnccn.2018.7053. [DOI] [PubMed] [Google Scholar]
- 15.Kim EJ, Yoo C, Kang HJ, Kim K-P, Ryu M-H, Park SR, et al. Clinical outcomes of systemic therapy in patients with unresectable or metastatic combined hepatocellular-cholangiocarcinoma. Liver Int. 2021 Jun;41((6)):1398–408. doi: 10.1111/liv.14813. [DOI] [PubMed] [Google Scholar]
- 16.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008 Jul;359((4)):378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 17.Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma: evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2017 Oct;15:95. doi: 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Association for the Study of the Liver EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018 Jul;69((1)):182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Balan TA, Putter H. A tutorial on frailty models. Stat Methods Med Res. 2020 Nov;29((11)):3424–54. doi: 10.1177/0962280220921889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Funk MJ, Westreich D, Wiesen C, Stürmer T, Brookhart MA, Davidian M. Doubly robust estimation of causal effects. Am J Epidemiol. 2011 Apr;173((7)):761–7. doi: 10.1093/aje/kwq439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wyss R, Ellis AR, Brookhart MA, Girman CJ, Jonsson Funk M, LoCasale R, et al. The role of prediction modeling in propensity score estimation: an evaluation of logistic regression, bCART, and the covariate-balancing propensity score. Am J Epidemiol. 2014 Sep;180((6)):645–55. doi: 10.1093/aje/kwu181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarnagin WR, Weber S, Tickoo SK, Koea JB, Obiekwe S, Fong Y, et al. Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer. 2002 Apr;94((7)):2040–6. doi: 10.1002/cncr.10392. [DOI] [PubMed] [Google Scholar]
- 23.Holzner ML, Tabrizian P, Parvin-Nejad FP, Fei K, Gunasekaran G, Rocha C, et al. Resection of mixed hepatocellular-cholangiocarcinoma, hepatocellular carcinoma, and intrahepatic cholangiocarcinoma. Liver Transpl. 2020;26((7)):888–98. doi: 10.1002/lt.25786. [DOI] [PubMed] [Google Scholar]
- 24.Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015 Feb;33((6)):550–8. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan AWH, Zhong J, Berhane S, Toyoda H, Cucchetti A, Shi K, et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol. 2018 Dec;69((6)):1284–93. doi: 10.1016/j.jhep.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 26.Hiraoka A, Michitaka K, Kumada T, Izumi N, Kadoya M, Kokudo N, et al. Validation and potential of albumin-bilirubin grade and prognostication in a nationwide survey of 46,681 hepatocellular carcinoma patients in Japan: the need for a more detailed evaluation of hepatic function. Liver Cancer. 2017 Nov;6((4)):325–36. doi: 10.1159/000479984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Llovet JM, Lencioni R. mRECIST for HCC: performance and novel refinements. J Hepatol. 2020 Feb;72((2)):288–306. doi: 10.1016/j.jhep.2019.09.026. [DOI] [PubMed] [Google Scholar]
- 28.Gigante E, Ronot M, Bertin C, Ciolina M, Bouattour M, Dondero F, et al. Combining imaging and tumour biopsy improves the diagnosis of combined hepatocellular-cholangiocarcinoma. Liver Int. 2019 Dec;39((12)):2386–96. doi: 10.1111/liv.14261. [DOI] [PubMed] [Google Scholar]
- 29.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular Carcinoma. N Engl J Med. 2020 May;382((20)):1894–905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 30.Zhu AX, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, et al. Final overall survival efficacy results of ivosidenib for patients with advanced cholangiocarcinoma with IDH1 mutation: the phase 3 randomized clinical clarIDHy trial. JAMA Oncol. 2021 Nov;7((11)):1669–77. doi: 10.1001/jamaoncol.2021.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020 May;21((5)):671–84. doi: 10.1016/S1470-2045(20)30109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goyal L, Meric-Bernstam F, Hollebecque A, Morizane C, Valle JW, Karasic TB, et al. Abstract CT010: primary results of phase 2 FOENIX-CCA2: the irreversible FGFR1-4 inhibitor futibatinib in intrahepatic cholangiocarcinoma (iCCA) with FGFR2 fusions/rearrangements. Cancer Res. 2021 Jul;81((13 Suppl)):CT010. [Google Scholar]
- 33.Meric-Bernstam F, Bahleda R, Hierro C, Sanson M, Bridgewater J, Arkenau H-T, et al. Futibatinib, an irreversible FGFR1-4 inhibitor, in patients with advanced solid tumors harboring FGF/FGFR aberrations: A Phase I Dose-Expansion Study. Cancer Discov. 2022 Feb;12((2)):402–15. doi: 10.1158/2159-8290.CD-21-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author and will be available after reasonable request.