Abstract
Citrullination of proteins is crucial for the formation of neutrophil extracellular traps (NETs) − strands of nuclear DNA expulsed in the extracellular environment along with antimicrobial proteins in order to halt the spread of pathogens. Paradoxically, NETs may be immunogenic and contribute to inflammation. It is known that for the externalization of DNA, a group of enzymes called peptidyl arginine deiminases (PADs) is required. Current research often looks at citrullination, NET formation, PAD overexpression, and extracellular DNA (ecDNA) accumulation in chronic diseases as separate events. In contrast, we propose that citrullination can be viewed as the primary mechanism of autoimmunity, for instance by the formation of anti-citrullinated protein antibodies (ACPAs) but also as a process contributing to chronic inflammation. Therefore, citrullination could be at the center, connecting and impacting multiple inflammatory diseases in which ACPAs, NETs, or ecDNA have already been documented. In this review, we aimed to highlight the importance of citrullination in the etiopathogenesis of a number of chronic diseases and to explore the diagnostic, prognostic, and therapeutic potential of the citrullination-NET axis.
Keywords: Citrullination, Neutrophil extracellular traps, Autoimmune disease, Metabolic disease, Cardiovascular disease
Introduction
In order to perform its specific function, the majority of proteins has to undergo in the cytoplasm a series of posttranslational modifications (PTMs). While hundreds of PTMs are known to occur, perhaps none of them has been more associated with inflammation than citrullination. First discovered in 1958 in extracts from hair follicles [1], this modification involves the deimination of one arginine molecule into citrulline. The reaction results in a 1 Da change in molar mass and the creation of ammonia as a byproduct. However, more importantly, the reaction is accompanied by the loss of one positive charge per one converted molecule [2]. The change of charge from positive to neutral has far-reaching consequences, for instance, the cessation of DNA binding by histones. By itself, citrullination as a PTM is needed for proper cell differentiation and gene expression [3]. However, excessive citrullination has been associated with many diseases and pathological states including chronic ones such as rheumatoid arthritis (RA) [4], systemic lupus erythematosus [5], and periodontitis [6] or neurodegenerative diseases such as multiple sclerosis (MS) [7], Alzheimer's disease (AD) [8], and even diseases which at first glance may not share similarity with citrullination directly such as cancer [9] or prion diseases [10].
Citrullination of proteins is mediated mostly by peptidyl arginine deiminases (PADs, EC 3.5.3.15). It needs to be noted that citrulline can also be formed as a byproduct, for instance, via the enzyme endothelial nitric oxide synthase. This enzyme catalyzes the reaction of oxygen with free arginine to produce NO, a potent vasodilator substance, and citrulline [11]. The human genome encodes five functioning PAD enzymes known as PAD1–4 and PAD6. These Ca2+-dependent enzymes share a 70–95% degree of sequence homology [12]. Conversely, each of the enzymes has a slightly different tissue expression pattern [13]. PAD4 was believed to be the only enzyme of the family with a nuclear localization domain, being able to cross from the cytoplasm into the nucleus [14]; however, PAD2 was witnessed to translocate to the nucleus after binding calcium [15]. Each PAD4 monomer can bind up to five Ca2+ ions. Calcium bound in this manner is a very potent catalyst, increasing the enzymatic activity of PAD4 10,000-fold [16, 17]. Upon translocation to the nucleus, PAD4 can deiminate histones. As mentioned, the loss of arginine causes the histones to stop binding DNA and chromatin decondensation ensues. This process is vital for the generation of neutrophil extracellular traps (NETs).
First observed in 2004 by Brinkmann et al. [18], NETs produced by neutrophils serve to immobilize and kill pathogens such as bacteria or viruses in a process known as NETosis. Compared to apoptosis, necrosis, or other forms of cell demise, NETosis is a distinct form of cell death. During NETosis, both nuclear and cytoplasmic membranes are degraded, and decondensed DNA along with antimicrobial proteins and enzymes such as neutrophil elastase (NE), myeloperoxidase (MPO) [19], cathelicidin [20], or cathepsin G [21] are extruded into the extracellular environment. Even though NETs are primarily a defense mechanism to prevent the spread of pathogens, their overabundance was detected in various diseases including sepsis [22], metabolic syndrome (MetS) [23], inflammatory bowel disease (IBD) [24], kidney [25], and liver disease [26]. Thus, excessive presence of NETs has been associated with a pathological and/or inflammatory state. Consistently with these observations, DNA extruded during NETosis (known as extracellular DNA [ecDNA]), the major part of NETs, was found to activate cellular signaling pathways playing a role in innate immunity such as STING or AIM2 [27, 28]. Interestingly, ecDNA may be immunogenic despite coming from the host itself. Since “extracellular DNA” is a broad term that encompasses all ecDNA molecules coming from various sources and cellular processes, in order to specify the subset of ecDNA that arises solely as a result of NETosis, the term “NETosis-associated ecDNA” is used in the following text.
Currently, there are several available methods which may be used for the detection and quantification of citrullinated peptides and proteins in samples. The COLDER (color development reagent) assay, one of the earliest methods, was later replaced with other more sensitive approaches, such as using antibodies specific to citrullinated proteins, mass spectrometry, or phenylglyoxal probes. However, as detailed description of these methods is beyond the scope of this review, we refer the reader to the publications of Clancy et al. [29] and Tilvawala and Thompson [30] which cover the principle as well as advantages and hindrances of those methods.
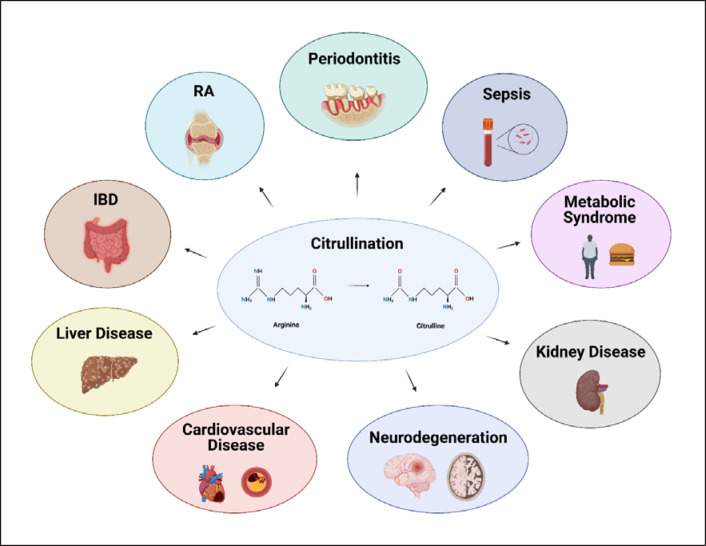
The purpose of this review was to summarize current knowledge about the role of citrullination, PADs, NETs, and NETosis-associated ecDNA in chronic diseases. Present-day data enable us to view the nature of many chronic diseases not as isolated diagnoses which share little in common but as a result of several deregulated processes including abnormal citrullination (shown in Fig. 1). The aim was also to point out important questions and areas which should be addressed by future studies.
Fig. 1.
Citrullination in chronic diseases. Citrullination can be viewed as the key process involved in the pathogenesis of many chronic diseases including RA, IBD, or periodontitis.
Citrullination, NETs, and PADs in Chronic Diseases
The supposed contribution of citrullination in the development of chronic diseases may not always be straightforward and it is important to note that an association cannot be interpreted as a causation. In order to either confirm or disprove an association, various strategies including PAD inhibitors or PAD-deficient rodents may be employed. In general, two negative effects of citrullination may appear. Aberrant citrullination may either lead to the creation of novel epitopes which can become immunogenic, especially if the modified proteins are abundantly accumulated in tissues or it may cause a shift in the activity of a protein or an enzyme, possibly further causing other problems related to the up- or downregulation of the affected molecule. For example, antibacterial activity of cathelicidin LL-37 can be reduced after citrullination [31].
Similarly, as other enzymes, PAD activity is tightly regulated in the physiological environment. Differences have been observed in the transcription and translation of PAD2 and PAD4 mRNA between macrophages and monocytes before or after differentiation [32]. Since PADs are calcium-dependent enzymes, the intracellular concentration of Ca2+ is an important regulator of their activity. Additionally, recent research has revealed other PAD co-activators such as glutathione [33], bicarbonate [34], or thioredoxin [35]. In subsequent chapters, the role of citrullination, NETs, and PADs in chronic diseases is discussed.
Rheumatoid Arthritis
RA is characterized by chronic inflammation of the synovium, which usually manifests as pain and swelling of the joints. Apart from enabling neutrophils to undergo NETosis, citrullination in some individuals causes the formation of antibodies directed against citrullinated proteins termed anti-citrullinated protein antibodies (ACPAs). One of the first described were anti-perinuclear [36] and anti-keratin antibodies [37]. Current RA markers include rheumatoid factor (RF), ACPAs, and anti-carbamylated protein antibodies. While mechanistically different, each of the antibodies starts to form years prior to the onset of RA [38]. Recent evidence supports the prognostic value of the combination of ACPAs and RF, double-positive group having the shortest interval until RA diagnosis [39]. Although many RA patients are positive for most of these RA markers, a direct mutual relationship between them may not be established yet, as no correlation was found between ACPAs and anti-carbamylated protein antibodies [40]. This suggests that both antibody types could prove useful in RA diagnosis independently on each other.
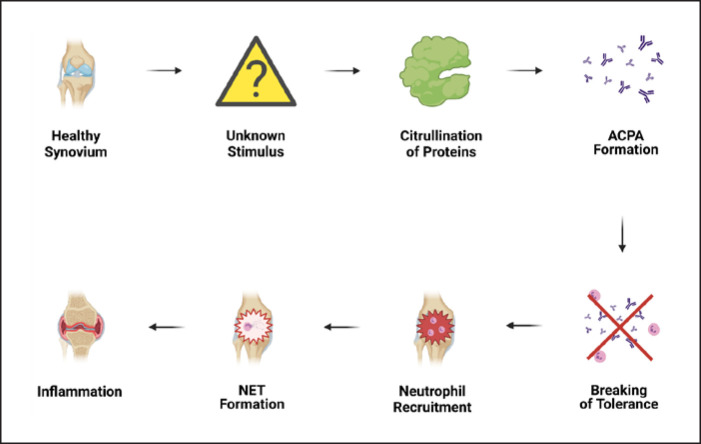
The existence of ACPAs long before the onset of the disease could mean that an unknown stimulus triggers aberrant citrullination and citrullinated protein accumulation either in the synovium or any other tissue. When a certain threshold is reached, immune tolerance is broken and, as a result, ACPAs are being formed (shown in Fig. 2). Thus, the increasing amount of citrullinated proteins may raise the probability of occurrence of some new, potentially immunogenic epitopes. Interestingly, as even ACPA-positive healthy individuals can develop complications such as joint pain or bone loss prior to RA onset [41], the presence of ACPAs cannot be considered as a universally reliable factor for RA development [42].
Fig. 2.
Excessive ACPA formation could result in the loss of immune tolerance. ACPAs may accumulate in the synovium of even healthy individuals, until certain threshold is surpassed. Then, neutrophil recruitment into synovial joints ensues accompanied by NET formation. NETs contribute to tissue damage, in turn leading to swelling, stiffness, pain, and ultimately, bone erosion. ACPAs, anti-citrullinated peptide antibodies; NETs, neutrophil extracellular traps.
In order to explain the involvement of genetic predispositions, the predictive value of specific alleles was studied; however, the results yielded no clear answer [43]. Moreover, a large twin study found that most of the variability in ACPA status was attributable to nonshared environmental factors (e.g., factors which are not shared by twins living in the same household and thus contribute to the dissimilarity of the twins − friends, teachers, relationships, etc.) as opposed to genetic factors [44]. There is a discrepancy in the ACPA positivity of first-degree relatives of RA patients as well. While Barra et al. [45] reported 48% of ACPA-positive first-degree relatives, a later study did not confirm these results, detecting only 1.9% positivity [46] and, interestingly, a 70% positivity of RA patient first-degree relatives was subsequently found in the sputum [47]. Therefore, it would seem that there are a number of confounders which affect the risk of RA onset in ACPA-positive individuals, such as smoking [48].
While all PAD genes were expressed in RA synovium, only PAD2 and PAD4 enzymes were found in RA patients compared to controls [49]. Unsurprisingly, methylation status of PAD4 gene was inversely correlated with RA severity and ACPA concentration [50]. Autoantibodies against PAD4 were observed in both the preclinical phase of RA [51] and RA patients [52]. In the latter study, anti-PAD4 antibodies were detected in more than a third of RA patients and their concentration correlated with the severity of joint destruction. In addition, the study discovered an association between the presence of anti-PAD4 antibodies and a haplotype composed of three single nucleotide polymorphisms with an odds ratio of 2.59 [52]. Alternatively, another polymorphism (PAD4 104C/T) was reported as an RA risk factor in a Chinese study [53]. Martinez-Prat et al. [54] measured antibodies against PAD3 and PAD4 in RA patients. They found a similar prevalence of anti-PAD4 antibodies (35%), while anti-PAD3 antibodies were observed in only 14% of RA patients [54]. Moreover, polymorphisms in HLA-DRB1 genes have been associated with RA [55]. Since these genes control antigen presentation to CD4+ T cells, their involvement in ACPA formation could be assumed. In this regard, a hapten-carrier model has been proposed by Auger et al. [56]. They postulated that B cells could engulf the PAD4 enzyme along with its substrate and present peptides to T helper cells, thereby triggering innate immunity. This hypothesis was confirmed when mice after immunization with either PAD4 or PAD2 developed immunoglobulin G (IgG) antibodies to citrullinated fibrinogen [56]. Importantly, a subsequent study revealed that this mechanism was shown to appear in humans as well, having identified peptide 8, a peptide of PAD4, to trigger T-cell proliferation in 40% RA patients [57]. Thus, it seems that certain genetic alterations could contribute to a higher RA risk.
One of the first reports of increased neutrophil infiltration and NET production in RA synovium came from Khandpur et al. [58] Neutrophils were observed to externalize citrullinated-autoantigens- and anti-vimentin-ACPAs-induced NET generation concomitantly with the enhanced production of inflammatory cytokines such as interleukin (IL)-17A or tumor necrosis factor-α [58]. A recent study suggested a prominent role of NE in the proinflammatory setting [59]. In addition, it was found that RA synovial fluid increases neutrophil migration and primes them to undergo NETosis via various signaling pathways [60]. NETosis may also become enhanced after neutrophil stimulation with ACPAs and the expression of proinflammatory cytokines IL-6 and IL-8 by fibroblast-like synovial cells was witnessed after their interaction with NETs [61]. To add to the complexity, Ribon et al. [62] proposed a dual pro- and anti-inflammatory role of polymorphonuclear neutrophils occurring independently of ACPAs.
Although research effort dedicated towards elucidation of the mechanisms controlling immune tolerance and susceptibility prior to RA onset has an increasing tendency, there are still questions which demand attention. For instance, it is not known why ACPAs appear to form almost exclusively in pre-RA and RA patients in comparison with other chronic diseases or what the relationship of ACPAs to the disease itself is, e.g., why do some individuals develop RA irrespective of ACPA positivity? Collectively, it can be concluded that ACPAs as well as other antibodies serve as potentially suitable diagnostic and prognostic markers in RA.
Periodontitis
Periodontitis can be characterized as a chronic inflammation of the gingival tissue which can lead to the formation of exudate, periodontal pockets, bone erosion, and, ultimately, tooth loss. Since the oral cavity comes into contact with food-borne microbiota on a daily basis, it may not come as a surprise that periodontitis could be related to the composition of oral biofilm. The biofilm comprises a complex ecosystem of hundreds of bacterial species. While most of the inhabitants are harmless commensals, several species such as Porphyromonas gingivalis, Tannerella forsythia, Treponema denticola (collectively termed “the red complex”), or Aggregatibacter actinomycetemcomitans are known to employ mechanisms to disrupt the defense of the host [63].
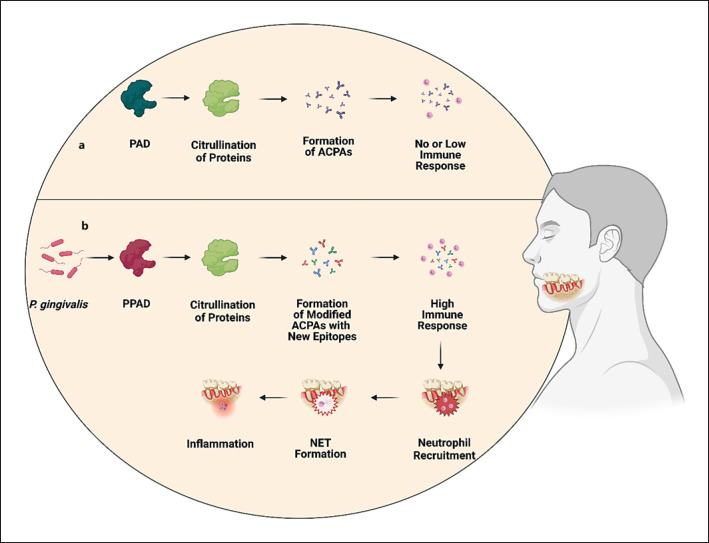
P. gingivalis, a known periodontal pathogen, is capable of citrullination by itself because it is endowed with its own PAD enzyme known as PPAD [64, 65]. It is believed that PPAD is actively employed by P. gingivalis to facilitate colonization and gingival disruption, since a PPAD-deficient strain of P. gingivalis was observed to cause significantly reduced periodontal inflammation compared to the wild-type strain [66]. Hypothetically, it is possible that PPAD produces a distinct subset of citrullinated proteins different from those created in the body physiologically (shown in Fig. 3a). These PPAD-modified proteins may elicit a higher degree of immune response compared to those proteins which undergo citrullination naturally (shown in Fig. 3b). Research effort in this area is being done to discover and describe these PPAD-citrullinated proteins, collectively termed the “citrullinome” [67, 68]. P. gingivalis was also observed to infect dental follicle stem cells, promoting immune disbalance in the oral cavity [69]. Alternatively, it possesses a family of proteases known as gingipains which could increase fitness and, therefore, survival of the bacterium [70]. In contrast, it was reported that P. gingivalis may suppress apoptosis or reactive oxygen species (ROS) generation via a process known as endotoxin tolerance [71]. Moreover, it has been demonstrated that P. gingivalis can lower the MPO-mediated production of hypochlorous acid, a potent ROS member, by gingival epithelial cells [72]. Subsequently, lower MPO activity is helpful to avoid the NADPH oxidase-ROS antibacterial pathway. Hypochlorous acid can regulate neutrophil recruitment and this may also be a factor in several of the disorders described in this article [73]. Similarly, another pathogen, Prevotella intermedia, was observed to possess nuclease activity. However, since A. actinomycetemcomitans was not, no strategy to overcome host defense can be thought of as a defining feature of all oral pathogens [74]. Conversely, even certain commensals such as Streptococcus gordonii or Fusobacterium nucleatum may cause enhanced NET formation, for instance via greater release of ROS [75]. Importantly, as increased citrullination was detected to happen independently of P. gingivalis or A. actinomycetemcomitans, it seems that bacteria residing in the oral cavity cannot be accepted as a universal cause of periodontitis [6]. Indeed, higher expression of PAD2 and PAD4 was detected in gingival crevicular fluid of periodontitis patients [76, 77]. Apart from bacterial biofilms, another exogenous factor which was reported to induce NETs, was smoking [78, 79].
Fig. 3.
Hypothetical mechanism of the induction of immune response of P. gingivalis by PPAD-mediated citrullination. P. gingivalis, a frequent colonizer of the oral cavity of periodontitis patients expresses its own PPAD theoretically capable of producing citrullinated proteins with new epitopes which were not encountered before by immune cells and thereby potentially causing substantially higher immunoreactivity (b) compared to the state with no P. gingivalis infection (a). PAD, peptidyl arginine deiminase.
One of the first reports finding NETs in gingival biopsies and exudating from patients with chronic periodontitis came from Vitkov et al. [80]. Another study compared NET expression between periodontitis and gingivitis biopsies. NET formation was higher in the latter, suggesting that greater amount of NETs could be associated with the acute phase of periodontitis [81]. Consistent with these observations, an increased chemotaxis and phagocytic activity of neutrophils in periodontitis and gingivitis groups compared to healthy controls was seen [82].
There is considerable evidence pointing towards the possibility of a link between periodontitis and RA. For instance, Shimada et al. [83] revealed a positive correlation between serum concentrations of anti-PPAD IgG and anti-citrullinated peptide IgG. Interestingly, no correlation was observed between serum concentration of PAD4 and anti-citrullinated peptide IgG [83]. A study by Laugisch et al. [84] found higher PPAD activity in gingival crevicular fluid in RA as well as non-RA patients with periodontitis compared to RA patients without periodontal disease. Although the PPAD activity was higher in RA and non-RA patients compared to controls, it should be noted that PPAD activity even in the control group was as high as 50% [84]. In addition, there was a significant association in RA patients between the concentration of ACPAs and periodontal severity represented by plaque index, clinical attachment level, and number of pockets [85]. An important observation was made by Oliveira et al. [86], who found an association between the concentration of circulating NETs, RA activity, and periodontal clinical parameters. Moreover, treatment of periodontal disease was significantly associated not only with the reduction of NET concentration but RA alleviation as well [86]. Besides ACPAs, anti-carbamylated protein antibodies were also significantly elevated in patients with RA and periodontitis compared to controls [87]. The fact that ACPA formation may be at least partially hereditary was documented in a study which found an association between ACPA seropositivity and prevalence and severity of periodontitis in first-degree relatives of RA patients [88]. Despite these interesting observations, it needs to be noted that not all studies are in agreement. For example, although Janssen et al. [89] found higher presence of anti-citrullinated histone H3 (cit. H3) antibodies in RA patients compared to periodontitis patients and controls, any association between the concentration of anti-cit. H3 antibodies and periodontal status of RA patients were not found. Similarly, a Malaysian study did not observe significant differences in RF positivity in RA patients with or without periodontitis [90].
In conclusion, higher presence of ACPAs, PADs, and NETs in the gingival crevicular fluid of periodontitis patients has been associated with the severity of the disease. Although oral pathogens such as P. gingivalis are known to cause a disbalance of the oral environment, inappropriate reaction of the immune system may also be sparked by ACPAs or NETs. Similar to RA, it is important to elucidate which factors and conditions contribute toward ACPA formation and, more importantly, initiation of the immune response.
Inflammatory Bowel Disease
The involvement of citrullination, PADs, and NETs as well as NETosis-associated ecDNA in the pathogenesis of IBD has been appreciated relatively recently. Although the composition of citrullinome in IBD has not been studied so far, a proteome analysis performed by Bennike et al. [91] quantified more than 5,000 proteins, 11 of which had increased abundance in ulcerative colitis (UC) tissue samples compared to controls. Evidence suggests that apart from stimulation of further intestinal damage, aberrant NET formation in the colon can impair the permeability of the intestinal barrier [92]. Moreover, NETs have been linked to enhanced thrombosis in IBD [93, 94]. Although the abundance of NETs has been documented in IBD samples [95], PAD4 expression was higher in UC only and not in Crohn's disease (CD) patients compared to healthy controls [24]. Similarly, increased PAD4 expression was detected in intestinal biopsies from UC patients and, in addition, was associated with increasing histopathologic grade [96]. This could mean that PAD4 could have different roles in UC and CD rather than a proinflammatory action in IBD as a whole. Although UC and CD share similarities which result in a collective term “IBD,” the differences between each other could, for instance, account for a distinct expression pattern of PADs in both pathologies. As research in this particular area is rather scarce, whether PAD4 is an important cofactor of intestinal inflammation in CD remains to be determined.
Even though the contribution of NETs to the severity of IBD has already been established, the role of citrullination and ACPAs is less known. One study compared serum ACPA seropositivity of controls, RA, UC, and CD patients. While the majority of RA patients was seropositive, ACPAs in only approximately a third of both UC and CD patients were detected [97]. Since seropositivity of controls for any of the tested ACPAs was in the range of 3–14%, it can be seen that at least some IBD patients may show a distinct pattern of ACPA expression. Notably, as arthralgia and myalgia are relatively commonly reported by IBD patients, these symptoms may occur as a result of ACPA production. While the role of ACPAs in IBD is not yet completely understood, recent research suggests that ACPAs may become a potential therapeutic option. These antibodies were shown to be successful in the alleviation of joint damage in a mouse model of arthritis. In this approach, citrulline residues of histones 2A and 4 were suitable therapeutical targets as opposed to other histone modifications [98]. However, since in many cases it is the citrullination of histone 3 which confers the upregulation of NET formation, the usage of these antibodies may be limited [99].
There are several possibilities to prevent excessive NET generation. Since PADs are the crucial component which catalyzes the citrullination process leading to aberrant NET formation, it is reasonable to think that PAD inhibition could be beneficial to suppress the proinflammatory setting in the colon. PAD inhibition can be achieved by selective inhibitors which irreversibly bind PADs and render them inactive. Of those, chloramidine (Cl-amidine) [100, 101] and streptonigrin [102, 103] have already been observed to alleviate experimental colitis [24, 104]. Another potential strategy is increased NET removal. This can be facilitated by enzymes which are present in the body physiologically and serve to cleave ecDNA. These enzymes are known as deoxyribonucleases (DNases). Since NETs are composed mostly of NETosis-associated ecDNA, it is reasonable to assume that removal of this type of ecDNA would reduce the amount of NETs in the intestinal environment and thus contribute to the resolution of inflammation. Several studies have already explored the possibility of DNase I application. As DNase I was observed to improve intestinal barrier integrity [92], decrease thrombi formation [94], or lower the level of MPO activity in a murine model of colitis [105], it can be viewed as a promising therapeutic option. Of note, DNase activity in IBD patients may be impaired compared to healthy controls [106] which could account for the increased presence of NETs. Although it may sound promising, DNase administration in a supposed therapeutic fashion would; however, face a number of hurdles such as mean of administration, effective dosage, or any potential side effects resulting from short- or long-term usage. Despite its usage as a treatment for cystic fibrosis [107], to the best of our knowledge, DNase I as a therapeutic agent for IBD has not yet been tested on human participants.
To add to the complexity, NETosis-associated ecDNA can modulate the immune system response via signaling pathways such as toll-like receptors (TLRs), STING, or AIM2 [27, 108]. Interestingly, intravenous administration of ecDNA derived from mice suffering from colitis was able to alleviate the severity of colitis as well as lower the expression of proinflammatory genes including TLR9, TRAF, MYD88, or NFKB, genes involved in TLR9 signaling [109].
Higher concentration of ecDNA from plasma was measured in UC patients compared to controls. Additionally, this increase in ecDNA positively correlated with UC severity [110]. Consistently, our research group showed that the concentration of total NETosis-associated ecDNA was higher in a mouse model of colitis [111] and, in addition, its concentration was rising proportionally to the length of time animals were administered with dextran sulfate sodium during the 7-day period of the experiment [112]. Based on these results, it can be concluded that NETosis-associated ecDNA can have a dual role in the modulation of intestinal inflammation in IBD. It is even possible that the resulting effect (either pro- or anti-inflammatory) can be partially mediated by the interactions of NETosis-associated ecDNA with the intestinal microbiota. With this in mind, it may be important to determine if the effect of ecDNA depends on its source. Apart from cellular organelles (nuclei, mitochondria), ecDNA especially in IBD can come from bacteria, viruses, fungi, or other organisms. Interestingly, a recent study found increased concentration of nuclear and mitochondrial ecDNA in IBD patients in remission [113]. However, as this study was one of the few which focused on the ecDNA subtypes, the immunogenicity of organelle- and microbiome-derived ecDNA has not yet been adequately described.
Liver Disease
In general, the effect of citrullination, NETs, or PADs on the development of liver disease is largely unexplored, and mechanistic studies are rather lacking. It can be suspected that since liver disease is a broad term encompassing many pathologically distinct diseases, the role of NETs and citrullination may not be uniform. The liver receives blood from the intestines through the portal vein and is responsible for capturing and filtering potentially harmful substances such as xenobiotics or possible pathogens. Usually, neutrophils cooperate with hepatocytes to suppress infections, for instance, via the release of the antibacterial protein lipocalin-2. This protein is also a part of NETs and deletion of the lcn2 gene led to a reduced bactericidal effect of NETs in vitro [114].
The level of MPO-DNA complexes (a marker of NETs) positively correlated with sphingosine kinase 1 involved in the sphingolipid metabolism in a murine model of methionine-choline-deficient and high-fat diet-induced liver injury [115]. It seems that in alcoholic liver disease, NET formation is decreased. This decrease is accompanied by a lower NET clearance [116]. A decreased abundance of NETs was observed concomitantly with the increasing level of cirrhosis [117]. Two years later, flow cytometry of neutrophils from patients with cirrhosis, ascites, or spontaneous bacterial peritonitis revealed a lower expression of CD69 and CD80 on neutrophils from ascitic fluid of cirrhosis and spontaneous bacterial peritonitis patients [118]. Lipopolysaccharide injection followed by NET formation in a mouse model of sepsis resulted in reduced staining for hepatic sinusoidal endothelial cells, marking the detachment of cells after endothelial injury [119]. In addition, NETs were documented to contribute to nonalcoholic steatohepatitis (NASH), since in the livers of NASH mice early neutrophil infiltration along with the production of proinflammatory cytokines was observed, eventually leading to the development of hepatocellular carcinoma [26]. The initial stimulus for changes in the liver leading to the development of NASH may arise as a result of chronic low-grade inflammation caused by, for example, MetS, while NASH has been documented also in metabolically healthy obese people [120].
The inhibition of NET formation either using DNase I or PAD4-deficient mice reduced the growth of hepatic tumors [26]. In this aspect, administration of DNase I prevented hepatorenal injury in a thioacetamide model of liver failure in rats [121]. The absence of PAD4 may therefore appear as a protective factor. Consistently, PAD4-deficient mice were reported to have attenuated hepatic and renal injury after renal ischemia and reperfusion compared to wild-type mice [122]. Indeed, increased presence of NETs has already been detected in tissue biopsies from chronic hepatitis patients [123].
The role of ecDNA likely depends on the origin. Those ecDNA molecules which circulate in the blood have been proposed as markers of various diseases. For instance, it has been suggested as a possible marker of hepatic fibrosis [124], nonalcoholic fatty acid liver disease [125] or as a valuable tool to predict carbon tetrachloride-induced liver injury in rats [126] and even progression toward hepatocellular carcinoma [127]. Contradictory evidence was provided by Blasi et al. [128], who found higher levels of ecDNA in patients with acute-on-chronic liver failure compared to acute decompensation cirrhosis, although the amount of MPO-DNA complexes as the marker of NETs in the acute-on-chronic liver failure patients was not elevated.
Taken together, overabundance of NETs as well as NET-associated ecDNA may have a negative impact on the pathogenesis of liver diseases. It must be noted, however, that the research presented here is very heterogenous in terms of study design and particular diseases. Therefore, the role of NETs and ecDNA may not be uniformly applicable but may vary based on the pathological conditions and the model used.
Metabolic Syndrome
MetS is defined as the combined occurrence of multiple known cardiovascular risk factors such as obesity, hypertension, insulin resistance, hyperglycemia, and dyslipidemia [129]. Although worldwide prevalence of MetS varies considerably in relation to many factors including geographical area, age, sex, and lifestyle [130], given the rapid global increase in incidence, it has been considered as an epidemic [131].
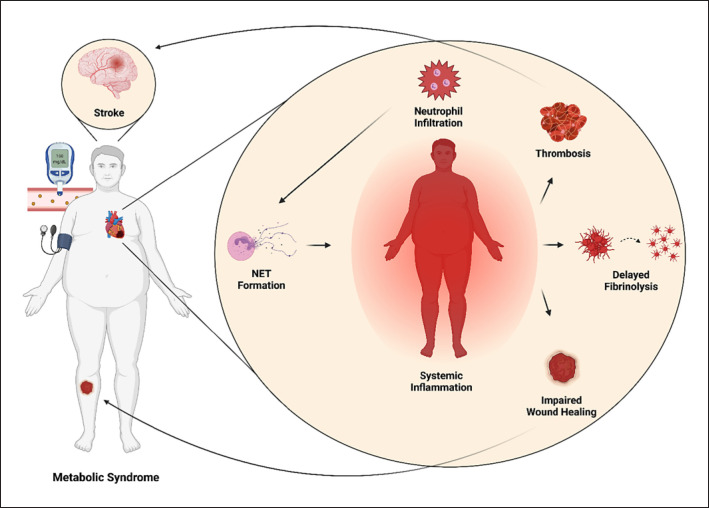
So far, of all the components of the citrullination-NET axis (e.g., citrullination, PADs, NETs, and NETosis-associated ecDNA) potentially involved in the pathogenesis of MetS, most attention has been focused on the concentration of NETs. Recent research suggests that abnormal presence of NETs could negatively affect MetS on multiple levels, as they have been observed to alter important physiological processes such as coagulation, clot formation or wound healing (shown in Fig. 4). For instance, significantly thicker clots were formed in plasma from diabetes patients compared to controls upon in vitro activation of NETosis via phorbol 12-myristate 13-acetate [132]. D'Abbondanza et al. [133] reported higher plasmatic levels of MPO-DNA complexes in patients with morbid obesity (BMI ≥40 kg/m2) compared to the control group. Interestingly, even after bariatric surgery the NET-forming tendency was not ameliorated [133]. Excessive NET formation was positively associated with plasma concentration of homocysteine, an independent risk factor for type 2 diabetes (T2D) [134]. Similar results were documented in patients with essential hypertension [135], and the same authors observed a link between the amount of NETs from plasma from essential hypertension patients and procoagulant activity [135]. In addition, it is worth noting that administration of DNase I in this study alleviated procoagulant activity by as much as 70%.
Fig. 4.
NETs may exacerbate MetS. The accumulation of NETs may be detrimental in MetS, as NETs were documented to increase the risk for thrombosis and delayed fibrinolysis. In addition, NETs formed in diabetic mice were seen to hamper wound healing. NET, neutrophil extracellular trap.
The impact of DNase I on NET reduction was reported by several studies. For instance, pioneering research was conducted by Wong et al. [136] who studied the effect of NETs on wound healing of diabetic mice. DNase I enhanced wound healing in both diabetic and normoglycemic mice. Moreover, not only was PAD4 more abundant in neutrophils from diabetes patients, PAD4-deficient mice exhibited faster wound healing compared to wild-type animals [136]. This means that the removal of NETs, for example, by DNase I may facilitate wound healing in diabetic patients. It is known that wound healing in diabetic patients is impaired which causes the accumulation of chronic nonhealing wounds and may even lead to amputation [137]. Remarkably, the application of exogenous DNase could help to prevent the development of type 1 diabetes, since oral administration of staphylococcal nuclease via Lactococcus lactis led to a decrease in the amount of NETs in non-obese diabetic mice [138]. It is interesting to note here that by improving inflammation, application of the nuclease lowered the concentrations of zonulin and lipopolysaccharide which could be viewed as an improvement of intestinal permeability. In contrast, partially contradictory evidence was brought by You et al. [139] who found elevated concentration of NET-associated proteins only from type 1 diabetes patients as opposed to T2D patients. Furthermore, zonulin concentration correlated with NET formation [139]. In another study, blocking of NET formation via administration of Cl-amidine, a potent PAD4 inhibitor, resulted in amelioration of wound healing in diabetic mice [140]. In diabetes patients, a positive correlation between the level of cit. H3 and smoking intensity were revealed [23]. In a multivariate analysis by Bryk et al. [141], cit. H3 and ecDNA correlated with clot lysis time, although no associations were reported for either NE or MPO in T2D patients. Notably, NETosis-associated ecDNA positively correlated with the MetS score in healthy adolescents [142], highlighting the low-grade systemic inflammation which is currently accepted as one of the components of MetS [143].
Even though evidence suggests that MetS confers a pro-inflammatory state in which NETs are involved, present-day studies have not yet elucidated the mechanisms behind NET-mediated inflammation. Research in this area is also complicated by the fact that there are many people who are eligible for only one of the parameters of MetS but not the others, for instance metabolically healthy obese people [144]. As data regarding the amount or composition of citrullinated proteins or ACPAs in MetS patients are largely missing so far, apart from the associations, no further conclusions can be drawn.
Cardiovascular Disease
Venous Thromboembolism
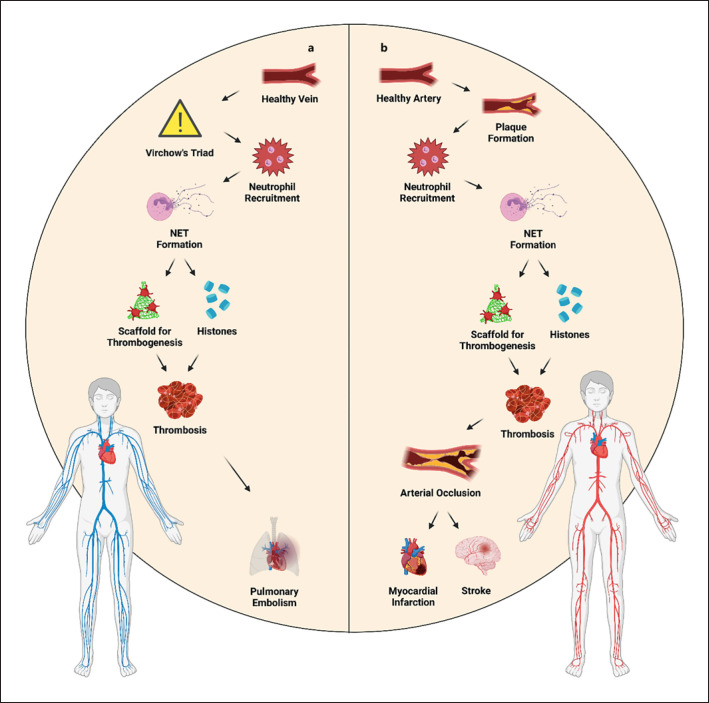
Thrombosis, clinically defined as venous thromboembolic disease, consists of deep vein thrombosis (DVT) and pulmonary embolism. One of the first reports providing evidence of NET-mediated thrombosis came from Fuchs et al. [145]. They discovered that NETs served as a sort of scaffold to attach and aggregate thrombocytes and red blood cells. Interestingly, even histones H3 and H4 had a stimulating effect on platelet aggregation (shown in Fig. 5). After the addition of heparin or DNase, however, this effect was diminished [145]. In a murine model of DVT, cit. H3 as a marker of NETs was present. DNase administration protected from the thrombotic state. Notably, cit. H3 colocalized with von Willebrand factor (VWF), a molecule important for thrombus development [146]. In DVT patients, almost twofold concentration of circulating nucleosomes was found compared to controls [147]. The involvement of NETs in DVT formation was observed also in patients with lower extremity fractures. Levels of both cit. H3 and ecDNA were higher in the DVT group [148]. Similarly, NET formation was increased in patients suffering from chronic thromboembolic pulmonary hypertension [149]. It seems that histones actively cooperate on thrombosis, since it was shown that they elicited the expression of caspase-1, the component of the inflammasome [150]. Cit. H3 was also elevated in thrombi in mice which were administered with IgG from antiphospholipid syndrome patients, a syndrome causing higher risk of thrombus formation [151]. In DVT patients, ecDNA was found increased compared to healthy controls and even correlated with C-reactive protein, VWF, and MPO, pointing towards a strong association between the concentration of ecDNA and presence of DVT [152]. Similarly, other studies found a higher concentration of ecDNA, calprotectin, and MPO in venous thromboembolism patients compared to controls. Moreover, the increase of these markers was accompanied by a decrease in activated protein C, a natural anticoagulant, and was associated with elevated thrombotic risk [153, 154]. Finally, histones and ecDNA were present in plasma of patients suffering from thrombotic microangiopathies [155].
Fig. 5.
a, bAbnormal NET presence is associated with cardiovascular disease. With the progression of atherosclerosis, migration of neutrophils to the site of microinjury can be observed. The accumulation of neutrophils and NETs may serve as a scaffold facilitating thrombogenesis, for instance via the action of histones H3 and H4. Over time, this may eventually lead to vein occlusion. NET, neutrophil extracellular trap.
Although neutrophils are one of the main initiators of thrombogenesis via NETosis, recent evidence suggests that natural killer (NK) cells as well as platelets could further contribute to the prothrombotic state. For instance, NK cell depletion resulted in a lower amount of NETs and smaller thrombi in mice. Furthermore, adoptive NK cell transfer yielded a condition similar to wild-type animals [156]. On the other hand, platelet-derived high-mobility group box protein 1 likely enhances neutrophil recruitment followed by subsequent NET generation, and ecDNA release [157]. Alternatively, according to Nakazawa et al. [158], a cross-talk between necroptosis, a programmed form of necrotic cell death, and NETosis may occur. In their study, the phosphorylated form of mixed lineage kinase-like domain protein (MLKL) was found in human clots. In addition, in a mouse thrombus model of inferior vena cava ligation, MLKL colocalized with cit. H3. Deficiency of MLKL partially ameliorated thrombus formation and was accompanied by a reduction of cit. H3 [158].
Atherosclerosis
The term atherosclerosis refers to the buildup of fat and cholesterol deposits inside arteries. The deposits are called plaques. In combination with unhealthy lifestyle and higher age, this buildup eventually leads to narrowing of the artery, impairing blood flow. The plaques are often soft and unstable which means that they are prone to rupture. Once an atherosclerotic plaque ruptures, clot formation begins with the risk of complete obstruction of the blood flow. Thus, atherosclerosis and thrombosis are closely related pathological states. In a prospective observational study of 282 participants with suspected coronary artery disease by Borissoff et al. [159], a positive association between the levels of plasma ecDNA, nucleosomes and MPO-DNA complexes and thrombin generation was established. In another study, immunohistochemistry revealed abundant neutrophils and NETs in complicated plaques and thrombi, however not in intact plaques [160]. It needs to be noted that cholesterol itself may be perceived as a danger signal, since crystals of cholesterol were able to trigger NETosis and the release of proinflammatory cytokines such as IL-1β [161] or IL-8. This IL was shown to interact with CXC chemokine receptor 2 (CXCR2) on neutrophils and contribute to the progression of atherosclerosis via the MAPK/ERK pathway [162]. Increased atherosclerosis is one of the consequences of the MetS. There is evidence supporting the notion that reduction in hyperlipidemia is linked to a decrease in NETs and, additionally, in diabetic mice DNase treatment could promote the improvement of atherosclerosis by NET degradation [163]. While the level of cit. H3 was increased in patients with T2D, it did not correlate with clinical parameters such as age, BMI, heart rate, or systolic pressure [23].
Consistently with other chapters mentioned in this review, several studies have explored the possibility of alleviation of atherosclerosis via PAD4 inhibition. Since PAD4 is a critical component in NET formation, reduction or absence of PAD4 could lead to a decrease in NETosis and, subsequently, atherogenesis. Supporting evidence was provided by Knight et al. [164], who demonstrated using ApoE-deficient mice that administration of Cl-amidine, an irreversible PAD4 inhibitor, was successful in reducing the lesion area as well as delaying carotid artery thrombosis time. In addition, lesser recruitment of neutrophils and macrophages to arteries was documented [164]. Myeloid-specific deletion of PAD4 also yielded encouraging results [165]. Interestingly, it seems that PAD4 from bone marrow-derived hematopoietic cells was not responsible for the observed effect, since PAD4 deficiency in these cells did not affect the progression of plaque formation in hypercholesterolemic mice [166].
Taken together, various studies have proposed the involvement of NETs and NETosis in the development of atherosclerosis and thrombosis. While recent advances bring the understanding of NET-mediated inflammation in the arteries one step closer, precise mechanisms are still elusive and require further exploration.
Sepsis
Sepsis is a life-threatening condition accounting for 30 million cases and 6 million deaths annually worldwide [167]. Severe sepsis is frequently accompanied by multiple organ dysfunction syndrome with mortality rate as high as 30%, and 40–70% for septic shock [168]. During sepsis, neutrophils release NETs to prevent bacteria from spreading into organs around the body [169]. Since sepsis can be characterized as exaggerated immune response [170], the inflammation results in excessive NET formation. Although increased NET generation was seen in sepsis [171] and thrombosis patients [172], not all studies agree that neutrophils from septic patients are more prone toward NETosis [173]. Despite this, in general, it is believed that high numbers of bacteria in the systemic circulation induces aberrant NET formation [174]. In this aspect, NETs could be seen as detrimental to the outcome of sepsis [175] and, as a result, their increased breakdown could be viewed as a potential therapeutic strategy. However, when NETs were eliminated via administration of recombinant human (rh) DNase in cecal ligation and puncture (CLP) murine model of sepsis, the outcome improved only when rhDNase was combined with antibiotics [176]. Moreover, the results of Meng et al. [177] do not support that NET depletion by rhDNase enhances sepsis survival. Despite this observation, treatment with rhDNase reduced CLP-induced formation of CXC chemokines, IL-6, and high-mobility group box protein 1 in plasma [178].
As discussed in the previous chapter, NETs may serve as a scaffold for the attachment of thrombocytes and thrombus formation. It seems that NETs from septic patients may increase the coagulation potential, for instance, by thrombin and fibrin generation [179]. Results from Tanaka et al. [180] are in agreement, since interactions between NETs and platelet aggregates in the arterioles and venules of septic mice were observed. In addition, platelet aggregation and thrombin activation within NETs were detected in mice using intravital microscopy [181]. The formation of NETs was linked to detachment of hepatic sinusoidal endothelial cells, possibly leading to liver injury [119]. Apart from acting as a mechanical support for thrombogenesis, research suggests that NETs may be involved in multiple signaling pathways affecting a number of cellular processes including autophagy [182] or macrophage pyroptosis [183]. Alternatively, there appears to be a cross-talk between NETs and innate immunity receptors TLR4 [184], and TLR9 [185].
Similarly, as in previously described pathological states, it could be possible to limit NET production via PAD4 inhibition. Here, data so far are not uniform, as PAD4−/− mice exhibited comparable survival to wild-type mice in a CLP mouse model of sepsis [186], although in a two-hit model combining hypovolemic shock with CLP, survival of PAD4−/− mice was ameliorated [187]. Based on these observations, it would seem as if neutrophils from PAD4−/− mice retained the ability to perform NETosis, even despite PAD4 absence. Supporting evidence came from Claushuis et al. [188] who detected cit. H3 in the lung of a Klebsiella pneumoniae-induced murine model of sepsis. In addition, NET-like structures were observed not only in controls but in PAD4−/− mice as well [188]. In this regard, only partial success was achieved by the administration of Cl-amidine, the selective PAD4 inhibitor, 1 h prior to sepsis induction [189]. On the other hand, survival of septic mice was improved when antibodies against cit. H3 was administered. Cl-amidine and, remarkably, even inhibitor of histone deacetylase markedly suppressed cit. H3, leading to increased survival of septic mice [190]. In contrast, Wu et al. [191, 192] suggested PAD2 as a more suitable therapeutic target as opposed to PAD4, since PAD2−/− mice showed higher survival accompanied by lower severity of lung injury in sepsis induced by Pseudomonas aeruginosa compared to PAD4−/− mice.
Experimental data on the contribution of NETosis-associated ecDNA in the development or severity of sepsis are relatively scarce. In an animal model of sepsis via E. coli injection which studied the dynamics of ecDNA release, NETosis-associated ecDNA was observed to rise until 5 h after injection [193]. Interestingly, a concomitant rise of DNase activity was documented until the fourth hour, suggesting that the body tries to lower the concentration of ecDNA by increasing the amount of active DNases. In another study, DNase application led to amelioration in survival, weight loss, and inflammatory markers in septic mice [194]. It was found that apart from NETs, NET-associated ecDNA could also affect thrombosis via delayed fibrinolysis [195]. Particular attention is being directed toward the notion of ecDNA serving as a prognostic marker in the outcome of sepsis, however, the results are contradictory, and therefore inconclusive [196, 197, 198, 199, 200, 201].
In conclusion, the majority of studies advocate a negative effect of excessive NET formation in sepsis. NETs and NET-associated ecDNA are likely detrimental to the immune response as well as resolution of inflammation in multiple ways including neutrophil and proinflammatory cytokine recruitment, procoagulation, or, conversely, delayed fibrinolysis. The blocking of NET formation via not only PAD4 but also PAD2 inhibition appears to be a potential therapeutic strategy; however, the feasibility of this notion needs to be validated in the following experiments. Despite the current paucity of knowledge regarding the citrullinome in sepsis, it is believed that PAD4 may interact with antimicrobial peptides such as cathelicidin LL-37 and decrease their antimicrobial activity via citrullination [202].
Kidney Disease
The most frequent forms of kidney disease are acute kidney injury (AKI) and chronic kidney disease (CKD). During ischemia-reperfusion injury (IRI), necrosis of tubular epithelial cells occurs. Damage to the epithelial membrane prompts circulating neutrophils to expel their DNA in the environment along with the production of proinflammatory cytokines. Interestingly, histones from ischemic cells were documented to prime neutrophils to undergo NETosis [203].
In laboratory conditions, multiple models of kidney disease have been established. Since the concentration of circulating ecDNA in experimental animals may vary based on the model used, in one study four models were compared: bilateral ureteral obstruction (BUO), glycerol-induced AKI, IRI, and bilateral nephrectomy. While total ecDNA was higher in BUO and glycerol-induced AKI models, nuclear ecDNA was elevated in BUO mice compared to sham-operated animals [204]. In CKD patients, low concentrations of both mitochondrial and nuclear plasma ecDNA correlated with positive renal outcomes after a 6-month follow-up, indicating that these subtypes of total ecDNA could become useful prognostic markers in CKD [205]. In addition, a study by Merkle et al. [206] revealed increased ecDNA concentration in cardiac surgery patients with late (>24 h) AKI development and suggested its use as a predictor of late-onset AKI. It was also shown that treatment with rosiglitazone, a peroxisome proliferator-activated receptor gamma agonist, led to a decrease in ecDNA concentration in CKD patients. Despite the fact that ecDNA correlated with markers of endothelial dysfunction as well as VWF, it needs to be noted that ecDNA concentration was neither higher in patients with renal damage nor did it correlate with markers of renal dysfunction [207]. Similar results have been published by Vaara et al. [208], who did not find any differences in ecDNA concentration between critically ill AKI and non-AKI patients. Finally, although ecDNA was higher in hemodialysis patients compared to CKD or peritoneal dialysis patients, no differences were observed in general between patients and healthy participants [209]. Despite this, it seems that ecDNA may have a broader potential for disease prediction, since the increase of serum levels was associated with the risk of progression of diabetic kidney disease [210]. Apart from its potential prognostic features, removal of excess ecDNA by DNase was successful in amelioration of IRI-induced AKI [211]. Additionally, ecDNA released as a result of renal IRI was detected to activate platelets, shown as DNA-platelet-granulocyte colocalization in the renal tissue. The role of platelets was confirmed when platelet inhibitor clopidogrel was administered, leading to a decrease in NETs [212].
In the pathophysiology of renal diseases, citrullination likely plays a major role. Although serum concentrations of PAD4 and its PADI4 polymorphisms rs11203367 and rs874881 were not different in septic shock patients who either did or did not develop sepsis-induced AKI, PAD4 correlated with the concentration of plasma urea and creatinine [213]. In another study, PAD4 inhibition using Cl-amidine or streptonigrin resulted in amelioration of IRI-induced renal injury. Moreover, mice pretreated with recombinant human PAD4 presented with exacerbated AKI [214]. These authors later discovered that PAD4 could be involved in the translocation of nuclear factor kappa B from the nucleus into the cytosol [122]. Additionally, PAD4 was observed to citrullinate IKKγ, and administration of IKKγ inhibitor diminished ischemic AKI [215]. Noteworthy, there is evidence supporting the notion that the result of selective PAD4 deficiency may vary upon the source. For instance, myeloid cells deprived of PAD4 offered less protection against renal IRI compared to renal proximal tubular cells [216]. It is even possible to transfer neutrophils from wild-type to PAD4-deficient mice to restore NET formation after renal IRI [217]. To date, no papers have been published on the importance of anti-PAD4 antibodies in kidney disease.
Multiple Sclerosis
MS is a chronic progressive autoimmune inflammatory disease affecting more than 2 million people worldwide [218]. Although the precise etiopathogenesis is yet to be established, it is believed that MS is caused by demyelination of myelin sheath. The myelin sheath is composed of three proteins: myelin basic protein (MBP), myelin oligodendrocyte glycoprotein, and proteolipid protein.
Despite that citrullination occurs physiologically in a healthy brain [219] and is present even during embryonic development [220], as much as 45% of all isolated MBP was found citrullinated in chronic MS patients and over 80% in fulminant MS (Marburg's syndrome) [7, 221, 222]. One study provided supportive results to the abundance of citrullinated proteins, detecting higher citrulline peaks using spectroscopy in early-onset MS patients compared to healthy controls [223]. Although the citrullination state was elevated in MS patients compared to control in another study, the antibody response did not seem to differ between citrullinated and non-citrullinated MBP [224]. Interestingly, neutrophils from MS patients cultured with newly synthetized citrullinated peptides caused a Th1 polarization as opposed to neutrophils cultured with non-citrullinated peptides [225]. However, MBP is not the only protein contributing to the increased citrullination status. Apart from MBP, citrullinated forms of glial fibrillary acidic protein (GFAP) [226] and vimentin [227] have been linked to MS and neurodegeneration as well [228]. Relatively recently, a big step towards uncovering the citrullinome of MS was done by Faigle et al. [229], who identified besides MBP, GFAP, and vimentin more than 80 different previously unknown citrullinated proteins.
Neutrophils possess a range of cytotoxic enzymes and proteases that are usually targeted against foreign antigens. Once activated upon the release of proinflammatory cytokines, they may not strictly discriminate their targets, and collateral damage may occur [230]. It seems that in MS, neutrophils are prone to inflammatory actions such as abundantly expressing TLR2 or IL-8 receptor or increased oxidative burst and NET formation [231]. In the present day, however, there is paucity of data regarding the role of NETs in MS. The results of a study by Tillack et al. [232] do not advocate for a direct action of NETs in this disease, although it is interesting to note that they observed gender-specific differences in relapsing-remitting MS patients. In this regard, women have a higher risk of MS diagnosis compared to men [218]. Studies determining the presence of NETosis-associated ecDNA are similarly scarce, most of them focused predominantly on the diagnostic or prognostic value, for instance the methylation status of long interspersed nuclear element-1 (LINE-1) [233, 234].
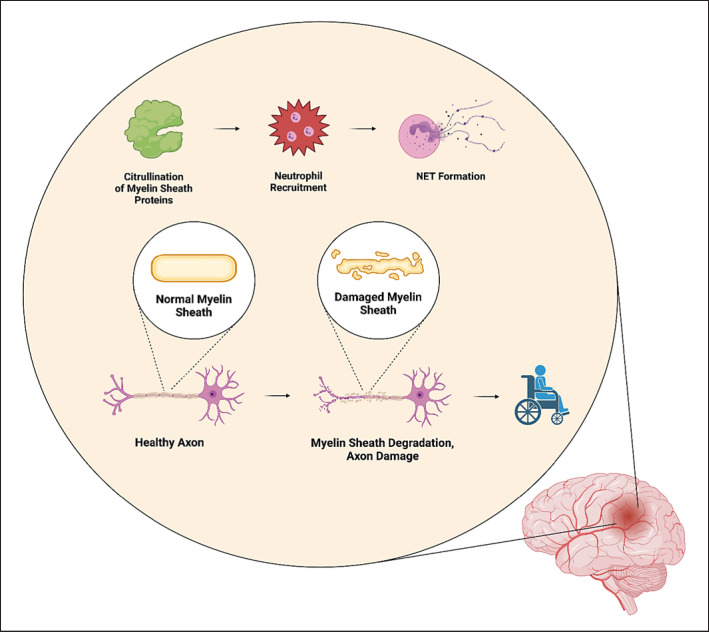
Since hypercitrullination is likely to be associated with the development and/or progression of MS, it is understandable to assume that inhibition of citrullination may prevent the overaccumulation of citrullinated MBP, GFAP, myelin oligodendrocyte glycoprotein, and other proteins involved in the composition and function of the myelin sheath (shown in Fig. 6). In the brain, PAD2 [235] and PAD4 [236] were found overexpressed in myelin isolated from MS patients compared to controls. In addition, both enzymes deiminated most of arginyl residues of MBP [235]. It is worth noting that mice overexpressing PAD2 due to carrying multiple copies of PAD2 in their genome presented with a higher degree of MBP deimination and more severe demyelination [237]. Interestingly, quantitative PCR revealed that the expression of PAD2 was upregulated even in neutrophils from peripheral blood of MS patients. Moreover, this overexpression was associated with demethylation of a CpG island in the PAD2 promoter [238]. Contrary to these observations, however, Falcão et al. [239] reported a decrease in the number of myelinated axons as well as motor dysfunction in PAD2-deficient mice. Despite that, research is being focused on the development of PAD inhibition, for instance, via chemical inhibition [240], in silico library screening [241] and compound design [242] or the usage of anti-PAD2 monoclonal antibodies [243].
Fig. 6.
Changes in the brain leading to demyelination and axon damage. Citrullination normally occurs also in the brain; however, the percentage amount of citrullinated proteins such as MBP, GFAP, or MOG is associated with the severity of MS. Via citrullination, new epitopes are formed which leads to neutrophil infiltration, NET formation, demyelination of the myelin sheath, and ultimately, failure in signal transduction causing, and ever-increasing degree of disability. GFAP, glial acidic fibrillary protein; MBP, myelin basic protein; MOG, myelin oligodendrocyte glycoprotein; MS, multiple sclerosis; NET, neutrophil extracellular trap.
Alzheimer's Disease
Considered as one of the most prevalent and incident neurodegenerative diseases, AD is described by chronic progressive memory and cognitive decline. Although its precise pathogenesis has not yet been adequately explained, the two distinct features, plaques composed of amyloid-β (Aβ) and accumulation of tau protein into neurofibrillary tangles, are believed to be causally linked to the development and progression of this disease [244, 245]. Neutrophils were observed to migrate to the Aβ plaques in a murine model of AD [246]. Moreover, this movement was facilitated by integrin LFA-1 because selective blockage of LFA-1 resulted in the improvement of memory function and mice lacking LFA-1 were protected from cognitive impairment [247].
Similarly, as in MS, citrullination changes the epitopes of vimentin, GFAP and presumably a number of other yet unknown proteins. Citrullinated forms of both vimentin and GFAP were detected more abundant in hippocampal samples of AD patients [8, 248]. However, citrullination may also result in a decrease in activity, for instance the activity of citrullinated enolase is impaired compared to the physiological form of the enzyme [249]. PAD2 was proposed to be implicated in the degradation of Aβ peptides, thereby contributing to insoluble fibril formation [250]. The expression of both PAD2 and PAD4 was documented in astrocytes and neurons of the hippocampus and cerebral cortex of AD patients. Furthermore, the authors suggested that fragments of citrullinated proteins could reach the systemic circulation, contributing to the development of immune response via ACPA formation [251].
The possible role of NETosis-associated ecDNA in the pathogenesis of AD has not yet been established due to discrepancies in observed results. While bacterial ecDNA was reported as a strong stimulus for Aβ aggregation [252] and a higher amount of NETs was present in plasma and serum samples from AD patients [253], the potential of using the methylation status of ecDNA is questionable [254]. Nevertheless, data scarcity in this area prevents any firm conclusions to be made.
Traumatic Brain Injury
Traumatic brain injury (TBI) accounts for almost 2 million cases annually in the USA alone [255, 256]. According to the Glasgow Scale, the universally accepted scale developed for TBI in 1974 [257], brain damage can be divided into mild, moderate, and severe. However, especially mild TBI may not always prompt the patient for an appointment at a general practitioner which makes the true number of head trauma cases difficult to estimate.
TBI induced by controlled cortical impact in rats led to astrocyte-specific protein citrullination, affecting a small subset of proteins some of which have already been reported citrullinated in MS [258]. In vitro experiments revealed that cit. H3 as a marker of NETs was increased after subarachnoid hemorrhage and positively correlated with subarachnoid hemorrhage severity. The study also showed that NET formation can be attenuated via PAD4 inhibition or, alternatively, DNase I administration [259, 260]. Mechanistically, Zhu et al. [261] provided evidence that NET formation after TBI could be related to sympathetic hyperactivity which is usually observed in TBI patients, and could be linked to increased expression of IL-1β via the hippo/MST1 pathway. Although the specific mechanism by which circulating ecDNA would contribute to the proinflammatory immune response in TBI has not yet been described, multiple studies agree that ecDNA could be used to distinguish TBI severity [262, 263, 264] or to serve as a prognostic TBI marker [265, 266]. Noteworthy, even though plasmatic DNase was higher in TBI patients compared to controls, DNase activity was, in contrast, lower [267]. Therefore, decreased DNase activity for up to 72 h after TBI could be responsible for lower clearance of circulating ecDNA.
In conclusion, experimental data suggest that although citrullination occurs physiologically in a healthy brain, this process is upregulated during or even before the onset of central nervous system diseases including MS, AD, and TBI. In addition, neutrophil infiltration likely plays an important role in the pathogenesis of neurodegenerative diseases, and, interestingly, this may happen even before the occurrence of the first symptoms. Based on current research, it appears that the accumulation of citrullinated proteins is a frequent reaction to neuronal damage; however, this hypothesis needs to be verified by future studies.
Future Outlook and Conclusions
The main purpose of the majority of biomedical research is to explore new possibilities and avenues for disease management and therapy. The treatment of chronic diseases such as periodontitis, IBD, RA, or MetS is especially challenging given the incomplete knowledge of etiopathogenesis and underlying molecular mechanisms. The insufficient understanding creates a persisting need for the improvement of efficiency and availability of optimal therapeutic strategy. In this context, research is focused for instance on describing the circumstances leading to the activation of PADs [268]. Moreover, as PADs are the direct orchestrators of citrullination, other options including selective PAD blockage via synthetic inhibitors [240] or monoclonal antibodies [243] are under investigation.
NETs together with NETosis-associated ecDNA are other important parts of the citrullination axis which have been recently gaining scientific attention. Their concentrations are known to be increased in a number of chronic diseases and since their presence can under certain conditions activate the innate immune system, for example, via TLRs. Therefore, lowering their amount either locally or in the systemic circulation can be considered as a viable treatment option. Despite the fact that endogenous DNases offer the possibility of NETosis-associated ecDNA removal, the implementation of DNase administration into the clinical praxis is rather difficult due to several reasons. First, the level of DNase activity usually varies considerably in laboratory animals [269] and humans [270]. Second, the regulation and dynamics of DNase activity by the body itself have not been adequately described which leaves unanswered questions such as whether a dose of administered DNase would lead to the decrease in the concentration of NETosis-associated ecDNA and if so, for how long. Third, some publications reported lower DNase activity for instance in IBD patients [106]; however, the causality and its direction have not been unveiled yet. Finally, DNases are a family of enzymes each with different expression pattern and activity. So far, DNase I has been the most studied enzyme of the family, however other members may complement the activity of DNase I in different conditions, and thus their potential should not be overlooked.
It needs to be mentioned that the knowledge about the citrullination axis is currently not distributed evenly to all parts of the axis and pathologies described in this review. For example, ACPAs have been implicated in RA and periodontitis; however, studies focused on their hypothetical role in liver, cardiovascular disease or MetS are lacking. If possible, future studies should aim to detect and measure more components of the citrullination axis in a given disease. The observed discrepancies in results may be partially caused by the differences in protocols used for isolation and quantification of molecular markers such as ecDNA or NETs. Therefore, reaching a methodical consensus could be beneficial for interpretation and comparison of data across various studies.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was supported by the Slovak Research and Development Agency under the contract no. APVV-17-0505 and APVV-21-0370 and by the Ministry of Education, Science, Research, and Sport of the Slovak Republic under the contract no. VEGA 1/0649/21. The funding agencies had no role in preparation of the manuscript.
Author Contributions
Martin Maronek: literature search, writing, first draft preparation; Roman Gardlik: manuscript revision, funding.
Acknowledgments
Figures were prepared using Biorender software (www.biorender.com).
References
- 1.Rogers GE, Simmonds DH. Content of citrulline and other amino-acids in a protein of hair follicles. Nature. 1958;182((4629)):186–7. doi: 10.1038/182186a0. [DOI] [PubMed] [Google Scholar]
- 2.Valesini G, Gerardi MC, Iannuccelli C, Pacucci VA, Pendolino M, Shoenfeld Y. Citrullination and autoimmunity. Autoimmun Rev. 2015;14((6)):490–7. doi: 10.1016/j.autrev.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 3.Fuhrmann J, Clancy KW, Thompson PR. Chemical biology of protein arginine modifications in epigenetic regulation. Chem Rev. 2015;115((11)):5413–61. doi: 10.1021/acs.chemrev.5b00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darrah E, Andrade F. Rheumatoid arthritis and citrullination. Curr Opin Rheumatol. 2018;30((1)):72–8. doi: 10.1097/BOR.0000000000000452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Neil LJ, Kaplan MJ, Carmona-Rivera C. The role of neutrophils and neutrophil extracellular traps in vascular damage in systemic lupus erythematosus. J Clin Med. 2019;8((9)):1325. doi: 10.3390/jcm8091325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engström M, Eriksson K, Lee L, Hermansson M, Johansson A, Nicholas AP, et al. Increased citrullination and expression of peptidylarginine deiminases independently of P. gingivalis and A. actinomycetemcomitans in gingival tissue of patients with periodontitis. J Transl Med. 2018;16((1)):214. doi: 10.1186/s12967-018-1588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang L, Tan D, Piao H. Myelin basic protein citrullination in multiple sclerosis: a potential therapeutic target for the pathology. Neurochem Res. 2016;41((8)):1845–56. doi: 10.1007/s11064-016-1920-2. [DOI] [PubMed] [Google Scholar]
- 8.Ishigami A, Masutomi H, Handa S, Nakamura M, Nakaya S, Uchida Y, et al. Mass spectrometric identification of citrullination sites and immunohistochemical detection of citrullinated glial fibrillary acidic protein in Alzheimer's disease brains. J Neurosci Res. 2015;93((11)):1664–74. doi: 10.1002/jnr.23620. [DOI] [PubMed] [Google Scholar]
- 9.Yuzhalin AE. Citrullination in cancer. Cancer Res. 2019;79((7)):1274–84. doi: 10.1158/0008-5472.CAN-18-2797. [DOI] [PubMed] [Google Scholar]
- 10.Jang B, Ishigami A, Maruyama N, Carp RI, Kim YS, Choi EK. Peptidylarginine deiminase and protein citrullination in prion diseases: strong evidence of neurodegeneration. Prion. 2013;7((1)):42–6. doi: 10.4161/pri.22380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verhaar MC, Westerweel PE, Van Zonneveld AJ, Rabelink TJ. Free radical production by dysfunctional eNOS. Heart. 2004;90((5)):494–5. doi: 10.1136/hrt.2003.029405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Witalison EE, Thompson PR, Hofseth LJ. Protein arginine deiminases and associated citrullination: physiological functions and diseases associated with dysregulation. Curr Drug Targets. 2015;16((7)):700–10. doi: 10.2174/1389450116666150202160954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S, Wang Y. Peptidylarginine deiminases in citrullination, gene regulation, health and pathogenesis. Biochim Biophys Acta. 2013;1829((10)):1126–35. doi: 10.1016/j.bbagrm.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakashima K, Hagiwara T, Yamada M. Nuclear localization of peptidylarginine deiminase V and histone deimination in granulocytes. J Biol Chem. 2002;277((51)):49562–8. doi: 10.1074/jbc.M208795200. [DOI] [PubMed] [Google Scholar]
- 15.Zheng L, Nagar M, Maurais AJ, Slade DJ, Parelkar SS, Coonrod SA, et al. Calcium regulates the nuclear localization of protein arginine deiminase 2. Biochemistry. 2019;58((27)):3042–56. doi: 10.1021/acs.biochem.9b00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arita K, Hashimoto H, Shimizu T, Nakashima K, Yamada M, Sato M. Structural basis for Ca(2+)-induced activation of human PAD4. Nat Struct Mol Biol. 2004;11((8)):777–83. doi: 10.1038/nsmb799. [DOI] [PubMed] [Google Scholar]
- 17.Beato M, Sharma P. Peptidyl arginine deiminase 2 (PADI2)-mediated arginine citrullination modulates transcription in cancer. Int J Mol Sci. 2020;21((4)):1351. doi: 10.3390/ijms21041351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303((5663)):1532–5. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 19.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191((3)):677–91. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong A, Bryzek D, Dobosz E, Scavenius C, Svoboda P, Rapala-Kozik M, et al. A novel biological role for peptidyl-arginine deiminases: citrullination of cathelicidin LL-37 controls the immunostimulatory potential of cell-free DNA. J Immunol. 2018;200((7)):2327–40. doi: 10.4049/jimmunol.1701391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folco EJ, Mawson TL, Vromman A, Bernardes-Souza B, Franck G, Persson O, et al. Neutrophil extracellular traps induce endothelial cell activation and tissue factor production through interleukin-1α and cathepsin G. Arterioscler Thromb Vasc Biol. 2018;38((8)):1901–12. doi: 10.1161/ATVBAHA.118.311150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camicia G, Pozner R, de Larrañaga G. Neutrophil extracellular traps in sepsis. Shock. 2014;42((4)):286–94. doi: 10.1097/SHK.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 23.Chatzigeorgiou A, Mitroulis I, Chrysanthopoulou A, Legaki AI, Ritis K, Tentolouris N, et al. Increased neutrophil extracellular traps related to smoking intensity and subclinical atherosclerosis in patients with type 2 diabetes. Thromb Haemost. 2020;120((11)):1587–9. doi: 10.1055/s-0040-1714371. [DOI] [PubMed] [Google Scholar]
- 24.Dinallo V, Marafini I, Di Fusco D, Laudisi F, Franzè E, Di Grazia A, et al. Neutrophil extracellular traps sustain inflammatory signals in ulcerative colitis. J Crohns Colitis. 2019;13((6)):772–84. doi: 10.1093/ecco-jcc/jjy215. [DOI] [PubMed] [Google Scholar]
- 25.Kim JK, Park MJ, Lee HW, Lee HS, Choi SR, Song YR, et al. The relationship between autophagy, increased neutrophil extracellular traps formation and endothelial dysfunction in chronic kidney disease. Clin Immunol. 2018;197:189–97. doi: 10.1016/j.clim.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 26.van der Windt DJ, Sud V, Zhang H, Varley PR, Goswami J, Yazdani HO, et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology. 2018;68((4)):1347–60. doi: 10.1002/hep.29914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma S, Fitzgerald KA. Innate immune sensing of DNA. PLoS Pathog. 2011;7((4)):e1001310. doi: 10.1371/journal.ppat.1001310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paludan SR, Bowie AG. Immune sensing of DNA. Immunity. 2013;38((5)):870–80. doi: 10.1016/j.immuni.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clancy KW, Weerapana E, Thompson PR. Detection and identification of protein citrullination in complex biological systems. Curr Opin Chem Biol. 2016;30:1–6. doi: 10.1016/j.cbpa.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tilvawala R, Thompson PR. Peptidyl arginine deiminases: detection and functional analysis of protein citrullination. Curr Opin Struct Biol. 2019;59:205–15. doi: 10.1016/j.sbi.2019.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bryzek D, Golda A, Budziaszek J, Kowalczyk D, Wong A, Bielecka E, et al. Citrullination-resistant LL-37 is a potent antimicrobial agent in the inflammatory environment high in arginine deiminase activity. Int J Mol Sci. 2020;21((23)):1–15. doi: 10.3390/ijms21239126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vossenaar ER, Radstake TR, Van Der Heijden A, Van Mansum MA, Dieteren C, De Rooij DJ, et al. Expression and activity of citrullinating peptidylarginine deiminase enzymes in monocytes and macrophages. Ann Rheum Dis. 2004;63((4)):373–81. doi: 10.1136/ard.2003.012211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Damgaard D, Bjørn ME, Steffensen MA, Pruijn GJ, Nielsen CH. Reduced glutathione as a physiological co-activator in the activation of peptidylarginine deiminase. Arthritis Res Ther. 2016;18((1)):102. doi: 10.1186/s13075-016-1000-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Y, Mittereder N, Sims GP. Perspective on protein arginine deiminase activity-bicarbonate is a pH-independent regulator of citrullination. Front Immunol. 2018 Jan;9:34. doi: 10.3389/fimmu.2018.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagar M, Tilvawala R, Thompson PR. Thioredoxin modulates protein arginine deiminase 4 (PAD4)-catalyzed citrullination. Front Immunol. 2019 Feb;10:244. doi: 10.3389/fimmu.2019.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nienhuis RL, Mandema E. A new serum factor in patients with rheumatoid arthritis; the antiperinuclear factor. Ann Rheum Dis. 1964;23((4)):302–5. doi: 10.1136/ard.23.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young BJ, Mallya RK, Leslie RD, Clark CJ, Hamblin TJ. Anti-keratin antibodies in rheumatoid arthritis. Br Med J. 1979;2((6182)):97–9. doi: 10.1136/bmj.2.6182.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu CY, Yang HY, Luo SF, Lai JH. From rheumatoid factor to anti-citrullinated protein antibodies and anti-carbamylated protein antibodies for diagnosis and prognosis prediction in patients with rheumatoid arthritis. Int J Mol Sci. 2021;22((2)):1–18. doi: 10.3390/ijms22020686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lingampalli N, Sokolove J, Lahey LJ, Edison JD, Gilliland WR, Holers VM, et al. Combination of anti-citrullinated protein antibodies and rheumatoid factor is associated with increased systemic inflammatory mediators and more rapid progression from preclinical to clinical rheumatoid arthritis. Clin Immunol. 2018;195:119–26. doi: 10.1016/j.clim.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Kolarz B, Ciesla M, Rosenthal AK, Dryglewska M, Majdan M. The value of anti-CarP and anti-PAD4 as markers of rheumatoid arthritis in ACPA/RF negative rheumatoid arthritis patients. Ther Adv Musculoskelet Dis. 2021;13:1759720X21989868. doi: 10.1177/1759720X21989868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bugatti S, Bogliolo L, Vitolo B, Manzo A, Montecucco C, Caporali R. Anti-citrullinated protein antibodies and high levels of rheumatoid factor are associated with systemic bone loss in patients with early untreated rheumatoid arthritis. Arthritis Res Ther. 2016;18((1)):226. doi: 10.1186/s13075-016-1116-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guderud K, Mæhlen MT, Nordang GBN, Viken MK, Andreassen BK, Molberg Ø, et al. Lack of association among peptidyl arginine deiminase type 4 autoantibodies, PADI4 polymorphisms, and clinical characteristics in rheumatoid arthritis. J Rheumatol. 2018;45((9)):1211–9. doi: 10.3899/jrheum.170769. [DOI] [PubMed] [Google Scholar]
- 43.Goëb V, Dieudé P, Daveau R, Thomas-L'Otellier M, Jouen F, Hau F, et al. Contribution of PTPN22 1858T, TNFRII 196R and HLA-shared epitope alleles with rheumatoid factor and anti-citrullinated protein antibodies to very early rheumatoid arthritis diagnosis. Rheumatology. 2008;47((8)):1208–12. doi: 10.1093/rheumatology/ken192. [DOI] [PubMed] [Google Scholar]
- 44.Hensvold AH, Magnusson PK, Joshua V, Hansson M, Israelsson L, Ferreira R, et al. Environmental and genetic factors in the development of anticitrullinated protein antibodies (ACPAs) and ACPA-positive rheumatoid arthritis: an epidemiological investigation in twins. Ann Rheum Dis. 2015;74((2)):375–80. doi: 10.1136/annrheumdis-2013-203947. [DOI] [PubMed] [Google Scholar]
- 45.Barra L, Scinocca M, Saunders S, Bhayana R, Rohekar S, Racapé M, et al. Anti-citrullinated protein antibodies in unaffected first-degree relatives of rheumatoid arthritis patients. Arthritis Rheum. 2013;65((6)):1439–47. doi: 10.1002/art.37911. [DOI] [PubMed] [Google Scholar]
- 46.Maijer KI, Gerlag DM, Tak PP. Prevalence of anti-citrullinated protein antibodies and IgM rheumatoid factor in first-degree relatives of Dutch rheumatoid arthritis patients. Arthritis Rheumatol. 2015;67((12)):3324–6. doi: 10.1002/art.39412. [DOI] [PubMed] [Google Scholar]
- 47.Demoruelle MK, Harrall KK, Ho L, Purmalek MM, Seto NL, Rothfuss HM, et al. Anti-citrullinated protein antibodies are associated with neutrophil extracellular traps in the sputum in relatives of rheumatoid arthritis patients. Arthritis Rheumatol. 2017;69((6)):1165–75. doi: 10.1002/art.40066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sánchez-Campamà J, Nagra NS, Pineda-Moncusí M, Prats-Uribe A, Prieto-Alhambra D. The association between smoking and the development of rheumatoid arthritis: a population-based case-control study. Reumatol Clin. 2020 doi: 10.1016/j.reuma.2020.08.006. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 49.Foulquier C, Sebbag M, Clavel C, Chapuy-Regaud S, Al Badine R, Méchin MC, et al. Peptidyl arginine deiminase type 2 (PAD-2) and PAD-4 but not PAD-1, PAD-3, and PAD-6 are expressed in rheumatoid arthritis synovium in close association with tissue inflammation. Arthritis Rheum. 2007;56((11)):3541–53. doi: 10.1002/art.22983. [DOI] [PubMed] [Google Scholar]
- 50.Kolarz B, Ciesla M, Dryglewska M, Majdan M. Peptidyl arginine deiminase type 4 gene promoter hypo-methylation in rheumatoid arthritis. J Clin Med. 2020;9((7)):2049. doi: 10.3390/jcm9072049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kolfenbach JR, Deane KD, Derber LA, O'Donnell CI, Gilliland WR, Edison JD, et al. Autoimmunity to peptidyl arginine deiminase type 4 precedes clinical onset of rheumatoid arthritis. Arthritis Rheum. 2010;62((9)):2633–9. doi: 10.1002/art.27570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harris ML, Darrah E, Lam GK, Bartlett SJ, Giles JT, Grant AV, et al. Association of autoimmunity to peptidyl arginine deiminase type 4 with genotype and disease severity in rheumatoid arthritis. Arthritis Rheum. 2008;58((7)):1958–67. doi: 10.1002/art.23596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gong LL, Chang J, Yang YM. Association between peptidyl arginine deiminase 4 (PADI4)-104C/T polymorphism and rheumatoid arthritis: a meta-analysis in the Chinese population. Genet Mol Res. 2016;15((3)) doi: 10.4238/gmr.15038750. [DOI] [PubMed] [Google Scholar]
- 54.Martinez-Prat L, Lucia D, Ibarra C, Mahler M, Dervieux T. Antibodies targeting protein-arginine deiminase 4 (PAD4) demonstrate diagnostic value in rheumatoid arthritis. Ann Rheum Dis. 2018;78((3)):434–6. doi: 10.1136/annrheumdis-2018-213818. [DOI] [PubMed] [Google Scholar]
- 55.Weyand CM, Hicok KC, Conn DL, Goronzy JJ. The influence of HLA-DRB1 genes on disease severity in rheumatoid arthritis. Ann Intern Med. 1992;117((10)):801–6. doi: 10.7326/0003-4819-117-10-801. [DOI] [PubMed] [Google Scholar]
- 56.Arnoux F, Mariot C, Peen E, Lambert NC, Balandraud N, Roudier J, et al. Peptidyl arginine deiminase immunization induces anticitrullinated protein antibodies in mice with particular MHC types. Proc Natl Acad Sci U S A. 2017;114((47)):E10169–77. doi: 10.1073/pnas.1713112114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Auger I, Balandraud N, Massy E, Hemon MF, Peen E, Arnoux F, et al. Peptidylarginine deiminase autoimmunity and the development of anti-citrullinated protein antibody in rheumatoid arthritis: the hapten-carrier model. Arthritis Rheumatol. 2020;72((6)):903–11. doi: 10.1002/art.41189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5((178)):178ra40. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carmona-Rivera C, Carlucci PM, Goel RR, James E, Brooks SR, Rims C, et al. Neutrophil extracellular traps mediate articular cartilage damage and enhance cartilage component immunogenicity in rheumatoid arthritis. JCI Insight. 2020;5((13)):e139388. doi: 10.1172/jci.insight.139388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wright HL, Lyon M, Chapman EA, Moots RJ, Edwards SW. Rheumatoid arthritis synovial fluid neutrophils drive inflammation through production of chemokines, reactive oxygen species, and neutrophil extracellular traps. Front Immunol. 2020;11:584116. doi: 10.3389/fimmu.2020.584116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu S, Peng W, Liang X, Wang W. Anti-citrullinated protein antibodies are associated with neutrophil extracellular trap formation in rheumatoid arthritis. J Clin Lab Anal. 2021;35((3)):e23662. doi: 10.1002/jcla.23662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ribon M, Seninet S, Mussard J, Sebbag M, Clavel C, Serre G, et al. Neutrophil extracellular traps exert both pro- and anti-inflammatory actions in rheumatoid arthritis that are modulated by C1q and LL-37. J Autoimmun. 2019;98:122–31. doi: 10.1016/j.jaut.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 63.Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8((7)):481–90. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- 64.Palmer LJ, Chapple IL, Wright HJ, Roberts A, Cooper PR. Extracellular deoxyribonuclease production by periodontal bacteria. J Periodontal Res. 2012;47((4)):439–45. doi: 10.1111/j.1600-0765.2011.01451.x. [DOI] [PubMed] [Google Scholar]
- 65.Maresz KJ, Hellvard A, Sroka A, Adamowicz K, Bielecka E, Koziel J, et al. Porphyromonas gingivalis facilitates the development and progression of destructive arthritis through its unique bacterial peptidylarginine deiminase (PAD) PLoS Pathog. 2013;9((9)):e1003627. doi: 10.1371/journal.ppat.1003627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gully N, Bright R, Marino V, Marchant C, Cantley M, Haynes D, et al. Porphyromonas gingivalis peptidylarginine deiminase, a key contributor in the pathogenesis of experimental periodontal disease and experimental arthritis. PLoS One. 2014;9((6)):e100838. doi: 10.1371/journal.pone.0100838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Larsen DN, Mikkelsen CE, Kierkegaard M, Bereta GP, Nowakowska Z, Kaczmarek JZ, et al. Citrullinome of Porphyromonas gingivalis outer membrane vesicles: confident identification of citrullinated peptides. Mol Cell Proteomics. 2020;19((1)):167–80. doi: 10.1074/mcp.RA119.001700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stobernack T, Glasner C, Junker S, Gabarrini G, De Smit M, De Jong A, et al. Extracellular proteome and citrullinome of the oral pathogen Porphyromonas gingivalis. J Proteome Res. 2016;15((12)):4532–43. doi: 10.1021/acs.jproteome.6b00634. [DOI] [PubMed] [Google Scholar]
- 69.Kriebel K, Hieke C, Engelmann R, Potempa J, Müller-Hilke B, Lang H, et al. Porphyromonas gingivalis peptidyl arginine deiminase can modulate neutrophil activity via infection of human dental stem cells. J Innate Immun. 2018;10((4)):264–78. doi: 10.1159/000489020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jayaprakash K, Demirel I, Khalaf H, Bengtsson T. The role of phagocytosis, oxidative burst and neutrophil extracellular traps in the interaction between neutrophils and the periodontal pathogen Porphyromonas gingivalis. Mol Oral Microbiol. 2015;30((5)):361–75. doi: 10.1111/omi.12099. [DOI] [PubMed] [Google Scholar]
- 71.Gu J-Y, Liu Y-J, Zhu X-Q, Qiu J-Y, Sun Y. Effects of endotoxin tolerance induced by Porphyromonas gingivalis lipopolysaccharide on inflammatory responses in neutrophils. Inflammation. 2020;43((5)):1692–706. doi: 10.1007/s10753-020-01243-8. [DOI] [PubMed] [Google Scholar]
- 72.Roberts JAS, Atanasova KR, Lee J, Diamond G, Deguzman J, Choi CH, et al. Opportunistic pathogen Porphyromonas gingivalis modulates danger signal ATP-mediated antibacterial NOX2 pathways in primary epithelial cells. Front Cell Infect Microbiol. 2017 Jul;7:291. doi: 10.3389/fcimb.2017.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ulfig A, Leichert LI. The effects of neutrophil-generated hypochlorous acid and other hypohalous acids on host and pathogens. Cell Mol Life Sci. 2021;78((2)):385. doi: 10.1007/s00018-020-03591-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Doke M, Fukamachi H, Morisaki H, Arimoto T, Kataoka H, Kuwata H. Nucleases from Prevotella intermedia can degrade neutrophil extracellular traps. Mol Oral Microbiol. 2017;32((4)):288–300. doi: 10.1111/omi.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.White P, Cooper P, Milward M, Chapple I. Differential activation of neutrophil extracellular traps by specific periodontal bacteria. Free Radic Biol Med. 2014;75(Suppl 1):S53. doi: 10.1016/j.freeradbiomed.2014.10.827. [DOI] [PubMed] [Google Scholar]
- 76.Harvey GP, Fitzsimmons TR, Dhamarpatni AA, Marchant C, Haynes DR, Bartold PM. Expression of peptidylarginine deiminase-2 and −4, citrullinated proteins and anti-citrullinated protein antibodies in human gingiva. J Periodontal Res. 2013;48((2)):252–61. doi: 10.1111/jre.12002. [DOI] [PubMed] [Google Scholar]
- 77.Veyisoğlu G, Savran L, Narin F, Yılmaz HE, Avşar C, Sağlam M. Gingival crevicular fluid semaphorin 4D and peptidylarginine deiminase-2 levels in periodontal health and disease. J Periodontol. 2019;90((9)):973–81. doi: 10.1002/JPER.18-0608. [DOI] [PubMed] [Google Scholar]
- 78.Yousefi S, Mihalache C, Kozlowski E, Schmid I, Simon HU. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009;16((11)):1438–44. doi: 10.1038/cdd.2009.96. [DOI] [PubMed] [Google Scholar]
- 79.White PC, Hirschfeld J, Milward MR, Cooper PR, Wright HJ, Matthews JB, et al. Cigarette smoke modifies neutrophil chemotaxis, neutrophil extracellular trap formation and inflammatory response-related gene expression. J Periodontal Res. 2018;53((4)):525–35. doi: 10.1111/jre.12542. [DOI] [PubMed] [Google Scholar]
- 80.Vitkov L, Klappacher M, Hannig M, Krautgartner WD. Extracellular neutrophil traps in periodontitis. J Periodontal Res. 2009;44((5)):664–72. doi: 10.1111/j.1600-0765.2008.01175.x. [DOI] [PubMed] [Google Scholar]
- 81.Magán-Fernández A, O'Valle F, Abadía-Molina F, Muñoz R, Puga-Guil P, Mesa F. Characterization and comparison of neutrophil extracellular traps in gingival samples of periodontitis and gingivitis: a pilot study. J Periodontal Res. 2019;54((3)):218–24. doi: 10.1111/jre.12621. [DOI] [PubMed] [Google Scholar]
- 82.Zhang F, Yang XM, Jia SY. Characteristics of neutrophil extracellular traps in patients with periodontitis and gingivitis. Braz Oral Res. 2020;34:e015. doi: 10.1590/1807-3107bor-2020.vol34.0015. [DOI] [PubMed] [Google Scholar]
- 83.Shimada A, Kobayashi T, Ito S, Okada M, Murasawa A, Nakazono K, et al. Expression of anti-Porphyromonas gingivalis peptidylarginine deiminase immunoglobulin G and peptidylarginine deiminase-4 in patients with rheumatoid arthritis and periodontitis. J Periodontal Res. 2016;51((1)):103–11. doi: 10.1111/jre.12288. [DOI] [PubMed] [Google Scholar]
- 84.Laugisch O, Wong A, Sroka A, Kantyka T, Koziel J, Neuhaus K, et al. Citrullination in the periodontium: a possible link between periodontitis and rheumatoid arthritis. Clin Oral Investig. 2016;20((4)):675–83. doi: 10.1007/s00784-015-1556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.González-Febles J, Rodríguez-Lozano B, Sánchez-Piedra C, Garnier-Rodríguez J, Bustabad S, Hernández-González M, et al. Association between periodontitis and anti-citrullinated protein antibodies in rheumatoid arthritis patients: a cross-sectional study. Arthritis Res Ther. 2020;22((1)):27. doi: 10.1186/s13075-020-2121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oliveira SR, de Arruda JAA, Schneider AH, Carvalho VF, Machado C, Moura MF, et al. Are neutrophil extracellular traps the link for the cross-talk between periodontitis and rheumatoid arthritis physiopathology? Rheumatology. 2021;61((1)):174–84. doi: 10.1093/rheumatology/keab289. [DOI] [PubMed] [Google Scholar]
- 87.Kaneko C, Kobayashi T, Ito S, Sugita N, Murasawa A, Nakazono K, et al. Circulating levels of carbamylated protein and neutrophil extracellular traps are associated with periodontitis severity in patients with rheumatoid arthritis: a pilot case-control study. PLoS One. 2018;13((2)):e0192365. doi: 10.1371/journal.pone.0192365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Loutan L, Alpizar-Rodriguez D, Courvoisier DS, Finckh A, Mombelli A, Giannopoulou C. Periodontal status correlates with anti-citrullinated protein antibodies in first-degree relatives of individuals with rheumatoid arthritis. J Clin Periodontol. 2019;46((7)):690–8. doi: 10.1111/jcpe.13117. [DOI] [PubMed] [Google Scholar]
- 89.Janssen KMJ, de Smit MJ, Withaar C, Brouwer E, van Winkelhoff AJ, Vissink A, et al. Autoantibodies against citrullinated histone H3 in rheumatoid arthritis and periodontitis patients. J Clin Periodontol. 2017;44((6)):577–84. doi: 10.1111/jcpe.12727. [DOI] [PubMed] [Google Scholar]
- 90.Che Rahim MJ, Wan Mohamad WM, Saddki N, Taib H, Wan Abhamid WZ, Wong KK, et al. Elevated serum rheumatoid factor, anti-citrullinated protein antibodies and active rheumatoid arthritis disease are not associated with chronic periodontitis. Malays J Pathol. 2019;41((3)):267–72. [PubMed] [Google Scholar]
- 91.Bennike TB, Carlsen TG, Ellingsen T, Bonderup OK, Glerup H, Bøgsted M, et al. Neutrophil extracellular traps in ulcerative colitis: a proteome analysis of intestinal biopsies. Inflamm Bowel Dis. 2015;21((9)):2052–67. doi: 10.1097/MIB.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin EY, Lai HJ, Cheng YK, Leong KQ, Cheng LC, Chou YC, et al. Neutrophil extracellular traps impair intestinal barrier function during experimental colitis. Biomedicines. 2020;8((8)):275. doi: 10.3390/biomedicines8080275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.He Z, Si Y, Jiang T, Ma R, Zhang Y, Cao M, et al. Phosphotidylserine exposure and neutrophil extracellular traps enhance procoagulant activity in patients with inflammatory bowel disease. Thromb Haemost. 2016;115((4)):738–51. doi: 10.1160/TH15-09-0710. [DOI] [PubMed] [Google Scholar]
- 94.Li T, Wang C, Liu Y, Li B, Zhang W, Wang L, et al. Neutrophil extracellular traps induce intestinal damage and thrombotic tendency in inflammatory bowel disease. J Crohns Colitis. 2020;14((2)):240–53. doi: 10.1093/ecco-jcc/jjz132. [DOI] [PubMed] [Google Scholar]
- 95.Gottlieb Y, Elhasid R, Berger-Achituv S, Brazowski E, Yerushalmy-Feler A, Cohen S. Neutrophil extracellular traps in pediatric inflammatory bowel disease. Pathol Int. 2018;68((9)):517–23. doi: 10.1111/pin.12715. [DOI] [PubMed] [Google Scholar]
- 96.Abd El Hafez A, Mohamed AS, Shehta A, Abd El Aziz Saad Sheta H. Neutrophil extracellular traps-associated protein peptidyl arginine deaminase 4 immunohistochemical expression in ulcerative colitis and its association with the prognostic predictors. Pathol Res Pract. 2020;216((10)):153102. doi: 10.1016/j.prp.2020.153102. [DOI] [PubMed] [Google Scholar]
- 97.Janssen KMJ, Hop H, Vissink A, Dijkstra G, de Smit MJ, Brouwer E, et al. Levels of anti-citrullinated protein antibodies and rheumatoid factor, including IgA isotypes, and articular manifestations in ulcerative colitis and Crohn's disease. Int J Environ Res Public Health. 2020;17((21)):1–10. doi: 10.3390/ijerph17218054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chirivi RGS, van Rosmalen JWG, van der Linden M, Euler M, Schmets G, Bogatkevich G, et al. Therapeutic ACPA inhibits NET formation: a potential therapy for neutrophil-mediated inflammatory diseases. Cell Mol Immunol. 2020;18((6)):1528–44. doi: 10.1038/s41423-020-0381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dragoni G, De Hertogh G, Vermeire S. The role of citrullination in inflammatory bowel disease: a neglected player in triggering inflammation and fibrosis? Inflamm Bowel Dis. 2021;27((1)):134–44. doi: 10.1093/ibd/izaa095. [DOI] [PubMed] [Google Scholar]
- 100.Chumanevich AA, Causey CP, Knuckley BA, Jones JE, Poudyal D, Chumanevich AP, et al. Suppression of colitis in mice by Cl-amidine: a novel peptidylarginine deiminase inhibitor. Am J Physiol Gastrointest Liver Physiol. 2011;300((6)):G929–38. doi: 10.1152/ajpgi.00435.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Witalison EE, Cui X, Causey CP, Thompson PR, Hofseth LJ. Molecular targeting of protein arginine deiminases to suppress colitis and prevent colon cancer. Oncotarget. 2015;6((34)):36053–62. doi: 10.18632/oncotarget.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mondal S, Thompson PR. Protein arginine deiminases (PADs): biochemistry and chemical biology of protein citrullination. Acc Chem Res. 2019;52((3)):818–32. doi: 10.1021/acs.accounts.9b00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dreyton C, Jones J, Knuckley B, Subramanian V, Anderson E, Brown S, et al. Probe reports from NIH molecular libraries program [Internet] Bethesda, MD: National Center Biotechnology Information (US); 2013. Optimization and characterization of a pan protein arginine deiminase (PAD) inhibitor. 2010–2012. [PubMed] [Google Scholar]
- 104.Zhang T, Mei Y, Dong W, Wang J, Huang F, Wu J. Evaluation of protein arginine deiminase-4 inhibitor in TNBS- induced colitis in mice. Int Immunopharmacol. 2020;84:106583. doi: 10.1016/j.intimp.2020.106583. [DOI] [PubMed] [Google Scholar]
- 105.Babickova J, Conka J, Janovicova L, Boris M, Konecna B, Gardlik R. Extracellular DNA as a prognostic and therapeutic target in mouse colitis under DNase I treatment. Folia Biol. 2018;64((1)):10–5. doi: 10.14712/fb2018064010010. [DOI] [PubMed] [Google Scholar]
- 106.Malíčková K, Duricová D, Bortlík M, Hrušková Z, Svobodová B, Machková N, et al. Impaired deoxyribonuclease I activity in patients with inflammatory bowel diseases. Autoimmune Dis. 2011;2011:945861. doi: 10.4061/2011/945861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gutteridge C, Kuhn RJ. Pulmozyme: dornase alfa. Pediatr Nurs. 1994;20((3)):278–9. [PubMed] [Google Scholar]
- 108.Wottawa F, Bordoni D, Baran N, Rosenstiel P, Aden K. The role of cGAS/STING in intestinal immunity. Eur J Immunol. 2021;51((4)):785–97. doi: 10.1002/eji.202048777. [DOI] [PubMed] [Google Scholar]
- 109.Sipos F, Műzes G, Fűri I, Spisák S, Wichmann B, Germann TM, et al. Intravenous administration of a single-dose free-circulating DNA of colitic origin improves severe murine DSS-colitis. Pathol Oncol Res. 2014;20((4)):867–77. doi: 10.1007/s12253-014-9766-x. [DOI] [PubMed] [Google Scholar]
- 110.Koike Y, Uchida K, Tanaka K, Ide S, Otake K, Okita Y, et al. Dynamic pathology for circulating free DNA in a dextran sodium sulfate colitis mouse model. Pediatr Surg Int. 2014;30((12)):1199–206. doi: 10.1007/s00383-014-3607-6. [DOI] [PubMed] [Google Scholar]
- 111.Maronek M, Gromova B, Liptak R, Klimova D, Cechova B, Gardlik R. Extracellular DNA is increased in dextran sulphate sodium-induced colitis in mice. Folia Biol. 2018;64((5–6)):167–72. doi: 10.14712/fb2018064050167. [DOI] [PubMed] [Google Scholar]
- 112.Maronek M, Gromova B, Liptak R, Konecna B, Pastorek M, Cechova B, et al. Extracellular DNA correlates with intestinal inflammation in chemically induced colitis in mice. Cells. 2021;10((1)):81. doi: 10.3390/cells10010081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vrablicova Z, Tomova K, Tothova L, Babickova J, Gromova B, Konecna B, et al. Nuclear and mitochondrial circulating cell-free DNA is increased in patients with inflammatory bowel disease in clinical remission. Front Med. 2020;7:593316. doi: 10.3389/fmed.2020.593316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li H, Feng D, Cai Y, Liu Y, Xu M, Xiang X, et al. Hepatocytes and neutrophils cooperatively suppress bacterial infection by differentially regulating lipocalin-2 and neutrophil extracellular traps. Hepatology. 2018;68((4)):1604–20. doi: 10.1002/hep.29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhao X, Yang L, Chang N, Hou L, Zhou X, Yang L, et al. Neutrophils undergo switch of apoptosis to NETosis during murine fatty liver injury via S1P receptor 2 signaling. Cell Death Dis. 2020;11((5)):379. doi: 10.1038/s41419-020-2582-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bukong TN, Cho Y, Iracheta-Vellve A, Saha B, Lowe P, Adejumo A, et al. Abnormal neutrophil traps and impaired efferocytosis contribute to liver injury and sepsis severity after binge alcohol use. J Hepatol. 2018;69((5)):1145–54. doi: 10.1016/j.jhep.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Agraz-Cibrian JM, Segura-Ortega JE, Delgado-Rizo V, Fafutis-Morris M. Alterations in neutrophil extracellular traps is associated with the degree of decompensation of liver cirrhosis. J Infect Dev Ctries. 2016;10((5)):512–7. doi: 10.3855/jidc.7165. [DOI] [PubMed] [Google Scholar]
- 118.Agraz-Cibrián JM, Delgado-Rizo V, Segura-Ortega JE, Maldonado-Gómez HA, Zambrano-Zaragoza JF, Durán-Avelar MJ, et al. Impaired neutrophil extracellular traps and inflammatory responses in the peritoneal fluid of patients with liver cirrhosis. Scand J Immunol. 2018;88((5)):e12714. doi: 10.1111/sji.12714. [DOI] [PubMed] [Google Scholar]
- 119.Sakurai K, Miyashita T, Okazaki M, Yamaguchi T, Ohbatake Y, Nakanuma S, et al. Role for neutrophil extracellular traps (NETs) and platelet aggregation in early sepsis-induced hepatic dysfunction. In Vivo. 2017;31((6)):1051–8. doi: 10.21873/invivo.11169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Frey S, Patouraux S, Debs T, Gugenheim J, Anty R, Iannelli A. Prevalence of NASH/NAFLD in people with obesity who are currently classified as metabolically healthy. Surg Obes Relat Dis. 2020;16((12)):2050–7. doi: 10.1016/j.soard.2020.07.009. [DOI] [PubMed] [Google Scholar]
- 121.Vokálová L, Lauková L, Čonka J, Melišková V, Borbélyová V, Bábíčková J, et al. Deoxyribonuclease partially ameliorates thioacetamide-induced hepatorenal injury. Am J Physiol Liver Physiol. 2017;312((5)):G457–63. doi: 10.1152/ajpgi.00446.2016. [DOI] [PubMed] [Google Scholar]
- 122.Rabadi M, Kim M, D'Agati V, Lee HT. Peptidyl arginine deiminase-4-deficient mice are protected against kidney and liver injury after renal ischemia and reperfusion. Am J Physiol Renal Physiol. 2016;311((2)):F437–49. doi: 10.1152/ajprenal.00254.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Abdeen SM, Olusi SO. Peptidyl arginine deiminase: a novel immunohistochemical marker for liver fibrosis in patients with chronic hepatitis. Acta Histochem. 2010;112((6)):592–603. doi: 10.1016/j.acthis.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 124.Yiğit B, Boyle M, Özler O, Erden N, Tutucu F, Hardy T, et al. Plasma cell-free DNA methylation: a liquid biomarker of hepatic fibrosis. Gut. 2018;67((10)):1907–8. doi: 10.1136/gutjnl-2017-315668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Karlas T, Weise L, Kuhn S, Krenzien F, Mehdorn M, Petroff D, et al. Correlation of cell-free DNA plasma concentration with severity of non-alcoholic fatty liver disease. J Transl Med. 2017;15((1)):106. doi: 10.1186/s12967-017-1208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gruenbaum BF, Boyko M, Delgado B, Douvdevany A, Gruenbaum SE, Melamed I, et al. Cell-free DNA as a potential marker to predict carbon tetrachloride-induced acute liver injury in rats. Hepatol Int. 2013;7((2)):721–7. doi: 10.1007/s12072-012-9414-z. [DOI] [PubMed] [Google Scholar]
- 127.Piciocchi M, Cardin R, Vitale A, Vanin V, Giacomin A, Pozzan C, et al. Circulating free DNA in the progression of liver damage to hepatocellular carcinoma. Hepatol Int. 2013;7((4)):1050–7. doi: 10.1007/s12072-013-9481-9. [DOI] [PubMed] [Google Scholar]
- 128.Blasi A, Patel VC, Adelmeijer J, Azarian S, Aziz F, Fernández J, et al. Plasma levels of circulating DNA are associated with outcome, but not with activation of coagulation in decompensated cirrhosis and ACLF. JHEP Rep. 2019;1((3)):179–87. doi: 10.1016/j.jhepr.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2((5–6)):231–7. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cameron AJ, Shaw JE, Zimmet PZ. The metabolic syndrome: prevalence in worldwide populations. Endocrinol Metab Clin North Am. 2004;33((2)):351–75. doi: 10.1016/j.ecl.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 131.Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20((2)):12. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.de Vries JJ, Hoppenbrouwers T, Martinez-Torres C, Majied R, Özcan B, van Hoek M, et al. Effects of diabetes mellitus on fibrin clot structure and mechanics in a model of acute neutrophil extracellular traps (Nets) formation. Int J Mol Sci. 2020;21((19)):1–15. doi: 10.3390/ijms21197107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.D'Abbondanza M, Martorelli EE, Ricci MA, De Vuono S, Migliola EN, Godino C, et al. Increased plasmatic NETs by-products in patients in severe obesity. Sci Rep. 2019;9((1)):14678. doi: 10.1038/s41598-019-51220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Joshi MB, Baipadithaya G, Balakrishnan A, Hegde M, Vohra M, Ahamed R, et al. Elevated homocysteine levels in type 2 diabetes induce constitutive neutrophil extracellular traps. Sci Rep. 2016;6:36362. doi: 10.1038/srep36362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li JH, Tong DX, Wang Y, Gao L, Liu Y, Zhang XH, et al. Neutrophil extracellular traps exacerbate coagulation and endothelial damage in patients with essential hypertension and hyperhomocysteinemia. Thromb Res. 2021;197:36–43. doi: 10.1016/j.thromres.2020.10.028. [DOI] [PubMed] [Google Scholar]
- 136.Wong SL, Demers M, Martinod K, Gallant M, Wang Y, Goldfine AB, et al. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med. 2015;21((7)):815–9. doi: 10.1038/nm.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Okonkwo U, Dipietro L. Diabetes and wound angiogenesis. Int J Mol Sci. 2017;18((7)):1419. doi: 10.3390/ijms18071419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liang Y, Wang X, He D, You Q, Zhang T, Dong W, et al. Ameliorating gut microenvironment through staphylococcal nuclease-mediated intestinal NETs degradation for prevention of type 1 diabetes in NOD mice. Life Sci. 2019;221:301–10. doi: 10.1016/j.lfs.2019.02.034. [DOI] [PubMed] [Google Scholar]
- 139.You Q, He DM, Shu GF, Cao B, Xia YQ, Xing Y, et al. Increased formation of neutrophil extracellular traps is associated with gut leakage in patients with type 1 but not type 2 diabetes. J Diabetes. 2019;11((8)):665–73. doi: 10.1111/1753-0407.12892. [DOI] [PubMed] [Google Scholar]
- 140.Fadini GP, Menegazzo L, Rigato M, Scattolini V, Poncina N, Bruttocao A, et al. NETosis delays diabetic wound healing in mice and humans. Diabetes. 2016;65((4)):1061–71. doi: 10.2337/db15-0863. [DOI] [PubMed] [Google Scholar]
- 141.Bryk AH, Prior SM, Plens K, Konieczynska M, Hohendorff J, Malecki MT, et al. Predictors of neutrophil extracellular traps markers in type 2 diabetes mellitus: associations with a prothrombotic state and hypofibrinolysis. Cardiovasc Diabetol. 2019;18((1)):49. doi: 10.1186/s12933-019-0850-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Celec P, Janovičová L, Gurecká R, Koborová I, Gardlík R, Šebeková K. Circulating extracellular DNA is in association with continuous metabolic syndrome score in healthy adolescents. Physiol Genomics. 2021;53((7)):309–18. doi: 10.1152/physiolgenomics.00029.2021. [DOI] [PubMed] [Google Scholar]
- 143.Grandl G, Wolfrum C. Hemostasis, endothelial stress, inflammation, and the metabolic syndrome. Semin Immunopathol. 2018;40((2)):215–24. doi: 10.1007/s00281-017-0666-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008;168((15)):1617–24. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 145.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107((36)):15880–5. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Brill A, Fuchs TA, Savchenko AS, Thomas GM, Martinod K, de Meyer SF, et al. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost. 2012;10((1)):136–44. doi: 10.1111/j.1538-7836.2011.04544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Van Montfoort ML, Stephan F, Lauw MN, Hutten BA, Van Mierlo GJ, Solati S, et al. Circulating nucleosomes and neutrophil activation as risk factors for deep vein thrombosis. Arterioscler Thromb Vasc Biol. 2013;33((1)):147–51. doi: 10.1161/ATVBAHA.112.300498. [DOI] [PubMed] [Google Scholar]
- 148.Liu L, Zhang W, Su Y, Chen Y, Cao X, Wu J. The impact of neutrophil extracellular traps on deep venous thrombosis in patients with traumatic fractures. Clin Chim Acta. 2021;519:231–8. doi: 10.1016/j.cca.2021.04.021. [DOI] [PubMed] [Google Scholar]
- 149.Sharma S, Hofbauer TM, Ondracek AS, Chausheva S, Alimohammadi A, Artner T, et al. Neutrophil extracellular traps promote fibrous vascular occlusions in chronic thrombosis. Blood. 2021;137((8)):1104–16. doi: 10.1182/blood.2020005861. [DOI] [PubMed] [Google Scholar]
- 150.Campos J, Ponomaryov T, De Prendergast A, Whitworth K, Smith CW, Khan AO, et al. Neutrophil extracellular traps and inflammasomes cooperatively promote venous thrombosis in mice. Blood Adv. 2021;5((9)):2319–24. doi: 10.1182/bloodadvances.2020003377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Meng H, Yalavarthi S, Kanthi Y, Mazza LF, Elfline MA, Luke CE, et al. In vivo role of neutrophil extracellular traps in antiphospholipid antibody-mediated venous thrombosis. Arthritis Rheumatol. 2017;69((3)):655–67. doi: 10.1002/art.39938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Diaz JA, Fuchs TA, Jackson TO, Hovinga JAK, Lämmle B, Henke PK, et al. Plasma DNA is elevated in patients with deep vein thrombosis. J Vasc Surg Venous Lymphat Disord. 2013;1((4)):341–8. doi: 10.1016/j.jvsv.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Martos L, Oto J, Fernández-Pardo Á, Plana E, Solmoirago MJ, Cana F, et al. Increase of neutrophil activation markers in venous thrombosis-contribution of circulating activated protein C. Int J Mol Sci. 2020;21((16)):1–19. doi: 10.3390/ijms21165651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ng H, Havervall S, Rosell A, Aguilera K, Parv K, Von Meijenfeldt FA, et al. Circulating markers of neutrophil extracellular traps are of prognostic value in patients with COVID-19. Arterioscler Thromb Vasc Biol. 2021;41((2)):988–94. doi: 10.1161/ATVBAHA.120.315267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fuchs TA, Kremer Hovinga JA, Schatzberg D, Wagner DD, Lämmle B. Circulating DNA and myeloperoxidase indicate disease activity in patients with thrombotic microangiopathies. Blood. 2012;120((6)):1157–64. doi: 10.1182/blood-2012-02-412197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bertin FR, Rys RN, Mathieu C, Laurance S, Lemarié CA, Blostein MD. Natural killer cells induce neutrophil extracellular trap formation in venous thrombosis. J Thromb Haemost. 2019;17((2)):403–14. doi: 10.1111/jth.14339. [DOI] [PubMed] [Google Scholar]
- 157.Dyer MR, Chen Q, Haldeman S, Yazdani H, Hoffman R, Loughran P, et al. Deep vein thrombosis in mice is regulated by platelet HMGB1 through release of neutrophil-extracellular traps and DNA. Sci Rep. 2018;8((1)):2068. doi: 10.1038/s41598-018-20479-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nakazawa D, Desai J, Steiger S, Müller S, Devarapu SK, Mulay SR, et al. Activated platelets induce MLKL-driven neutrophil necroptosis and release of neutrophil extracellular traps in venous thrombosis. Cell Death Discov. 2018;4((1)):6. doi: 10.1038/s41420-018-0073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Borissoff JI, Joosen IA, Versteylen MO, Brill A, Fuchs TA, Savchenko AS, et al. Elevated levels of circulating DNA and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arterioscler Thromb Vasc Biol. 2013;33((8)):2032–40. doi: 10.1161/ATVBAHA.113.301627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pertiwi KR, Van Der Wal AC, Pabittei DR, Mackaaij C, Van Leeuwen MB, Li X, et al. Neutrophil extracellular traps participate in all different types of thrombotic and haemorrhagic complications of coronary atherosclerosis. Thromb Haemost. 2018;118((6)):1078–87. doi: 10.1055/s-0038-1641749. [DOI] [PubMed] [Google Scholar]
- 161.Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science. 2015;349((6245)):316–20. doi: 10.1126/science.aaa8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.An Z, Li J, Yu J, Wang X, Gao H, Zhang W, et al. Neutrophil extracellular traps induced by IL-8 aggravate atherosclerosis via activation NF-κB signaling in macrophages. Cell Cycle. 2019;18((21)):2928–38. doi: 10.1080/15384101.2019.1662678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Josefs T, Barrett TJ, Brown EJ, Quezada A, Wu X, Voisin M, et al. Neutrophil extracellular traps promote macrophage inflammation and impair atherosclerosis resolution in diabetic mice. JCI Insight. 2020;5((7)):e134796. doi: 10.1172/jci.insight.134796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Knight JS, Luo W, O'Dell AA, Yalavarthi S, Zhao W, Subramanian V, et al. Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circ Res. 2014;114((6)):947–56. doi: 10.1161/CIRCRESAHA.114.303312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liu Y, Carmona-Rivera C, Moore E, Seto NL, Knight JS, Pryor M, et al. Myeloid-specific deletion of peptidylarginine deiminase 4 mitigates atherosclerosis. Front Immunol. 2018 Jul;9:1680. doi: 10.3389/fimmu.2018.01680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Franck G, Mawson TL, Folco EJ, Molinaro R, Ruvkun V, Engelbertsen D, et al. Roles of PAD4 and NETosis in experimental atherosclerosis and arterial injury: implications for superficial erosion. Circ Res. 2018;123((1)):33–42. doi: 10.1161/CIRCRESAHA.117.312494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193((3)):259–72. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 168.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29((7)):1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 169.McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe. 2012;12((3)):324–33. doi: 10.1016/j.chom.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 170.Aziz M, Jacob A, Yang WL, Matsuda A, Wang P. Current trends in inflammatory and immunomodulatory mediators in sepsis. J Leukoc Biol. 2013;93((3)):329–42. doi: 10.1189/jlb.0912437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kumar S, Gupta E, Kaushik S, Srivastava VK, Saxena J, Mehta S, et al. Quantification of NETs formation in neutrophil and its correlation with the severity of sepsis and organ dysfunction. Clin Chim Acta. 2019;495:606–10. doi: 10.1016/j.cca.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 172.Lee KH, Cavanaugh L, Leung H, Yan F, Ahmadi Z, Chong BH, et al. Quantification of NETs-associated markers by flow cytometry and serum assays in patients with thrombosis and sepsis. Int J Lab Hematol. 2018;40((4)):392–9. doi: 10.1111/ijlh.12800. [DOI] [PubMed] [Google Scholar]
- 173.Hashiba M, Huq A, Tomino A, Hirakawa A, Hattori T, Miyabe H, et al. Neutrophil extracellular traps in patients with sepsis. J Surg Res. 2015;194((1)):248–54. doi: 10.1016/j.jss.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 174.Denning NL, Aziz M, Gurien SD, Wang P. Damps and nets in sepsis. Front Immunol. 2019 Oct;10:2536. doi: 10.3389/fimmu.2019.02536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Colón DF, Wanderley CW, Franchin M, Silva CM, Hiroki CH, Castanheira FVS, et al. Neutrophil extracellular traps (NETs) exacerbate severity of infant sepsis. Crit Care. 2019;23((1)):113. doi: 10.1186/s13054-019-2407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Czaikoski PG, Mota JM, Nascimento DC, Sônego F, Castanheira FV, Melo PH, et al. Neutrophil extracellular traps induce organ damage during experimental and clinical sepsis. PLoS One. 2016;11((2)):e0148142. doi: 10.1371/journal.pone.0148142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Meng W, Paunel-Görgülü A, Flohé S, Hoffmann A, Witte I, MacKenzie C, et al. Depletion of neutrophil extracellular traps in vivo results in hypersusceptibility to polymicrobial sepsis in mice. Crit Care. 2012;16((4)):R137. doi: 10.1186/cc11442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Luo L, Zhang S, Wang Y, Rahman M, Syk I, Zhang E, et al. Proinflammatory role of neutrophil extracellular traps in abdominal sepsis. Am J Physiol Lung Cell Mol Physiol. 2014;307((7)):L586–96. doi: 10.1152/ajplung.00365.2013. [DOI] [PubMed] [Google Scholar]
- 179.Yang S, Qi H, Kan K, Chen J, Xie H, Guo X, et al. Neutrophil extracellular traps promote hypercoagulability in patients with sepsis. Shock. 2017;47((2)):132–9. doi: 10.1097/SHK.0000000000000741. [DOI] [PubMed] [Google Scholar]
- 180.Tanaka K, Koike Y, Shimura T, Okigami M, Ide S, Toiyama Y, et al. In vivo characterization of neutrophil extracellular traps in various organs of a murine sepsis model. PLoS One. 2014;9((11)):e111888. doi: 10.1371/journal.pone.0111888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.McDonald B, Davis RP, Kim SJ, Tse M, Esmon CT, Kolaczkowska E, et al. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood. 2017;129((10)):1357–67. doi: 10.1182/blood-2016-09-741298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kambas K, Mitroulis I, Apostolidou E, Girod A, Chrysanthopoulou A, Pneumatikos I, et al. Autophagy mediates the delivery of thrombogenic tissue factor to neutrophil extracellular traps in human sepsis. PLoS One. 2012;7((9)):e45427. doi: 10.1371/journal.pone.0045427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chen L, Zhao Y, Lai D, Zhang P, Yang Y, Li Y, et al. Neutrophil extracellular traps promote macrophage pyroptosis in sepsis. Cell Death Dis. 2018;9((6)):597. doi: 10.1038/s41419-018-0538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Boufenzer A, Carrasco K, Jolly L, Brustolin B, Di-Pillo E, Derive M, et al. Potentiation of NETs release is novel characteristic of TREM-1 activation and the pharmacological inhibition of TREM-1 could prevent from the deleterious consequences of NETs release in sepsis. Cell Mol Immunol. 2021;18((2)):452–60. doi: 10.1038/s41423-020-00591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sun S, Duan Z, Wang X, Chu C, Yang C, Chen F, et al. Neutrophil extracellular traps impair intestinal barrier functions in sepsis by regulating TLR9-mediated endoplasmic reticulum stress pathway. Cell Death Dis. 2021;12((6)):606. doi: 10.1038/s41419-021-03896-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Martinod K, Fuchs TA, Zitomersky NL, Wong SL, Demers M, Gallant M, et al. PAD4-deficiency does not affect bacteremia in polymicrobial sepsis and ameliorates endotoxemic shock. Blood. 2015;125((12)):1948–56. doi: 10.1182/blood-2014-07-587709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Biron BM, Chung CS, Chen Y, Wilson Z, Fallon EA, Reichner JS, et al. PAD4 deficiency leads to decreased organ dysfunction and improved survival in a dual insult model of hemorrhagic shock and sepsis. J Immunol. 2018;200((5)):1817–28. doi: 10.4049/jimmunol.1700639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Claushuis TAM, van der Donk LEH, Luitse AL, van Veen HA, van der Wel NN, van Vught LA, et al. Role of peptidylarginine deiminase 4 in neutrophil extracellular trap formation and host defense during Klebsiella pneumoniae-induced pneumonia-derived sepsis. J Immunol. 2018;201((4)):1241–52. doi: 10.4049/jimmunol.1800314. [DOI] [PubMed] [Google Scholar]
- 189.Biron BM, Chung CS, O'Brien XM, Chen Y, Reichner JS, Ayala A. Cl-amidine prevents histone 3 citrullination and neutrophil extracellular trap formation, and improves survival in a murine sepsis model. J Innate Immun. 2017;9((1)):22–32. doi: 10.1159/000448808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Li Y, Liu Z, Liu B, Zhao T, Chong W, Wang Y, et al. Citrullinated histone H3: a novel target for the treatment of sepsis. Surgery. 2014;156((2)):229–34. doi: 10.1016/j.surg.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tian Y, Qu S, Alam HB, Williams AM, Wu Z, Deng Q, et al. Peptidylarginine deiminase 2 has potential as both a biomarker and therapeutic target of sepsis. JCI Insight. 2020;5((20)):e138873. doi: 10.1172/jci.insight.138873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wu Z, Tian Y, Alam HB, Li P, Duan X, Williams AM, et al. Peptidylarginine deiminases 2 mediates caspase-1-associated lethality in Pseudomonas aeruginosa Pneumonia-induced sepsis. J Infect Dis. 2021;223((6)):1093–102. doi: 10.1093/infdis/jiaa475. [DOI] [PubMed] [Google Scholar]
- 193.Lauková L, Bertolo EMJ, Zelinková M, Borbélyová V, Čonka J, Kovalčíková AG, et al. Early dynamics of plasma DNA in a mouse model of sepsis. Shock. 2019;52((2)):257–63. doi: 10.1097/SHK.0000000000001215. [DOI] [PubMed] [Google Scholar]
- 194.Laukova L, Konecna B, Babickova J, Wagnerova A, Meliskova V, Vlkova B, et al. Exogenous deoxyribonuclease has a protective effect in a mouse model of sepsis. Biomed Pharmacother. 2017;93:8–16. doi: 10.1016/j.biopha.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 195.Gould TJ, Vu TT, Stafford AR, Dwivedi DJ, Kim PY, Fox-Robichaud AE, et al. Cell-free DNA modulates clot structure and impairs fibrinolysis in sepsis. Arterioscler Thromb Vasc Biol. 2015;35((12)):2544–53. doi: 10.1161/ATVBAHA.115.306035. [DOI] [PubMed] [Google Scholar]
- 196.Saukkonen K, Lakkisto P, Pettilä V, Varpula M, Karlsson S, Ruokonen E, et al. Cell-free plasma DNA as a predictor of outcome in severe sepsis and septic shock. Clin Chem. 2008;54((6)):1000–7. doi: 10.1373/clinchem.2007.101030. [DOI] [PubMed] [Google Scholar]
- 197.Dwivedi DJ, Toltl LJ, Swystun LL, Pogue J, Liaw KL, Weitz JI, et al. Prognostic utility and characterization of cell-free DNA in patients with severe sepsis. Crit Care. 2012;16((4)):R151. doi: 10.1186/cc11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hamaguchi S, Akeda Y, Yamamoto N, Seki M, Yamamoto K, Oishi K, et al. Origin of circulating free DNA in sepsis: analysis of the CLP mouse model. Mediators Inflamm. 2015;2015:614518. doi: 10.1155/2015/614518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Clementi A, Virzì GM, Brocca A, Pastori S, de Cal M, Marcante S, et al. The role of cell-free plasma DNA in critically ill patients with sepsis. Blood Purif. 2016;41((1–3)):34–40. doi: 10.1159/000440975. [DOI] [PubMed] [Google Scholar]
- 200.Hawkins RB, Stortz JA, Holden DC, Wang Z, Raymond SL, Cox MC, et al. Persistently increased cell-free DNA concentrations only modestly contribute to outcome and host response in sepsis survivors with chronic critical illness. Surgery. 2020;167((3)):646–52. doi: 10.1016/j.surg.2019.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Garnacho-Montero J, Huici-Moreno MJ, Gutiérrez-Pizarraya A, López I, Márquez-Vácaro JA, Macher H, et al. Prognostic and diagnostic value of eosinopenia, C-reactive protein, procalcitonin, and circulating cell-free DNA in critically ill patients admitted with suspicion of sepsis. Crit Care. 2014;18((3)):R116. doi: 10.1186/cc13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Koziel J, Bryzek D, Sroka A, Maresz K, Glowczyk I, Bielecka E, et al. Citrullination alters immunomodulatory function of LL-37 essential for prevention of endotoxin-induced sepsis. J Immunol. 2014;192((11)):5363–72. doi: 10.4049/jimmunol.1303062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Nakazawa D, Kumar SV, Marschner J, Desai J, Holderied A, Rath L, et al. Histones and neutrophil extracellular traps enhance tubular necrosis and remote organ injury in ischemic AKI. J Am Soc Nephrol. 2017;28((6)):1753–68. doi: 10.1681/ASN.2016080925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Homolová J, Janovičová L, Konečná B, Vlková B, Celec P, Tóthová L, et al. Plasma concentrations of extracellular DNA in acute kidney injury. Diagnostics. 2020;10((3)):152. doi: 10.3390/diagnostics10030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chang CC, Chiu PF, Wu CL, Kuo CL, Huang CS, Liu CS, et al. Urinary cell-free mitochondrial and nuclear deoxyribonucleic acid correlates with the prognosis of chronic kidney diseases. BMC Nephrol. 2019;20((1)):391. doi: 10.1186/s12882-019-1549-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Merkle J, Daka A, Deppe AC, Wahlers T, Paunel-Görgülü A. High levels of cell-free DNA accurately predict late acute kidney injury in patients after cardiac surgery. PLoS One. 2019;14((6)):e0218548. doi: 10.1371/journal.pone.0218548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.McGuire AL, Urosevic N, Chan DT, Dogra G, Inglis TJ, Chakera A. The impact of chronic kidney disease and short-term treatment with rosiglitazone on plasma cell-free DNA levels. PPAR Res. 2014;2014:643189. doi: 10.1155/2014/643189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Vaara ST, Lakkisto P, Immonen K, Tikkanen I, Ala-Kokko T, Pettilä V. Urinary biomarkers indicative of apoptosis and acute kidney injury in the critically ill. PLoS One. 2016;11((2)):e0149956. doi: 10.1371/journal.pone.0149956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Korabecna M, Opatrna S, Wirth J, Rulcova K, Eiselt J, Sefrna F, et al. Cell-free plasma DNA during peritoneal dialysis and hemodialysis and in patients with chronic kidney disease. Ann N Y Acad Sci. 2008;1137:296–301. doi: 10.1196/annals.1448.014. [DOI] [PubMed] [Google Scholar]
- 210.Li X, Hu R, Luo T, Peng C, Gong L, Hu J, et al. Serum cell-free DNA and progression of diabetic kidney disease: a prospective study. BMJ Open Diabetes Res Care. 2020;8((1)):e001078. doi: 10.1136/bmjdrc-2019-001078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Peer V, Abu Hamad R, Berman S, Efrati S. Renoprotective effects of DNAse-I treatment in a rat model of ischemia/reperfusion-induced acute kidney injury. Am J Nephrol. 2016;43((3)):195–205. doi: 10.1159/000445546. [DOI] [PubMed] [Google Scholar]
- 212.Jansen MP, Emal D, Teske GJ, Dessing MC, Florquin S, Roelofs JJ. Release of extracellular DNA influences renal ischemia reperfusion injury by platelet activation and formation of neutrophil extracellular traps. Kidney Int. 2017;91((2)):352–64. doi: 10.1016/j.kint.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 213.Costa NA, Polegato BF, Pereira AG, de Paiva SAR, Gut AL, Balbi AL, et al. Evaluation of peptidylarginine deiminase 4 and PADI4 polymorphisms in sepsis-induced acute kidney injury. Rev Assoc Med Bras. 2021;66((11)):1515–20. doi: 10.1590/1806-9282.66.11.1515. [DOI] [PubMed] [Google Scholar]
- 214.Ham A, Rabadi M, Kim M, Brown KM, Ma Z, D'Agati V, et al. Peptidyl arginine deiminase-4 activation exacerbates kidney ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2014;307((9)):F1052–62. doi: 10.1152/ajprenal.00243.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Rabadi MM, Han SJ, Kim M, D'Agati V, Lee HT. Peptidyl arginine deiminase-4 exacerbates ischemic AKI by finding NEMO. Am J Physiol Renal Physiol. 2019;316((6)):F1180–90. doi: 10.1152/ajprenal.00089.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Li H, Han SJ, Kim M, Cho A, Choi Y, D'Agati V, et al. Divergent roles for kidney proximal tubule and granulocyte PAD4 in ischemic AKI. Am J Physiol Renal Physiol. 2018;314((5)):F809–19. doi: 10.1152/ajprenal.00569.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Raup-Konsavage WM, Wang Y, Wang WW, Feliers D, Ruan H, Reeves WB. Neutrophil peptidyl arginine deiminase-4 has a pivotal role in ischemia/reperfusion-induced acute kidney injury. Kidney Int. 2018;93((2)):365–74. doi: 10.1016/j.kint.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple sclerosis. Lancet. 2018;391((10130)):1622–36. doi: 10.1016/S0140-6736(18)30481-1. [DOI] [PubMed] [Google Scholar]
- 219.Nicholas AP, Whitaker JN. Preparation of a monoclonal antibody to citrullinated epitopes: its characterization and some applications to immunohistochemistry in human brain. Glia. 2002;37((4)):328–36. [PubMed] [Google Scholar]
- 220.Asaga H, Ishigami A. Microglial expression of peptidylarginine deiminase 2 in the prenatal rat brain. Cell Mol Biol Lett. 2007;12((4)):536–44. doi: 10.2478/s11658-007-0025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wood DD, Bilbao JM, O'Connors P, Moscarello MA. Acute multiple sclerosis (Marburg type) is associated with developmentally immature myelin basic protein. Ann Neurol. 1996;40((1)):18–24. doi: 10.1002/ana.410400106. [DOI] [PubMed] [Google Scholar]
- 222.Moscarello MA, Wood DD, Ackerley C, Boulias C. Myelin in multiple sclerosis is developmentally immature. J Clin Invest. 1994;94((1)):146–54. doi: 10.1172/JCI117300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Oguz KK, Kurne A, Aksu AO, Karabulut E, Serdaroglu A, Teber S, et al. Assessment of citrullinated myelin by 1H-MR spectroscopy in early-onset multiple sclerosis. AJNR Am J Neuroradiol. 2009;30((4)):716–21. doi: 10.3174/ajnr.A1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.de Seze J, Dubucquoi S, Lefranc D, Virecoulon F, Nuez I, Dutoit V, et al. IgG reactivity against citrullinated myelin basic protein in multiple sclerosis. J Neuroimmunol. 2001;117((1–2)):149–55. doi: 10.1016/s0165-5728(01)00312-5. [DOI] [PubMed] [Google Scholar]
- 225.Deraos G, Chatzantoni K, Matsoukas MT, Tselios T, Deraos S, Katsara M, et al. Citrullination of linear and cyclic altered peptide ligands from myelin basic protein (MBP(87–99)) epitope elicits a Th1 polarized response by T cells isolated from multiple sclerosis patients: implications in triggering disease. J Med Chem. 2008;51((24)):7834–42. doi: 10.1021/jm800891n. [DOI] [PubMed] [Google Scholar]
- 226.Nicholas AP, Sambandam T, Echols JD, Tourtellotte WW. Increased citrullinated glial fibrillary acidic protein in secondary progressive multiple sclerosis. J Comp Neurol. 2004;473((1)):128–36. doi: 10.1002/cne.20102. [DOI] [PubMed] [Google Scholar]
- 227.Jang B, Kim MJ, Lee YJ, Ishigami A, Kim YS, Choi EK. Vimentin citrullination probed by a novel monoclonal antibody serves as a specific indicator for reactive astrocytes in neurodegeneration. Neuropathol Appl Neurobiol. 2020;46((7)):751–69. doi: 10.1111/nan.12620. [DOI] [PubMed] [Google Scholar]
- 228.Bradford CM, Ramos I, Cross AK, Haddock G, McQuaid S, Nicholas AP, et al. Localisation of citrullinated proteins in normal appearing white matter and lesions in the central nervous system in multiple sclerosis. J Neuroimmunol. 2014;273((1–2)):85–95. doi: 10.1016/j.jneuroim.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 229.Faigle W, Cruciani C, Wolski W, Roschitzki B, Puthenparampil M, Tomas-Ojer P, et al. Brain citrullination patterns and T cell reactivity of cerebrospinal fluid-derived CD4+ T cells in multiple sclerosis. Front Immunol. 2019 Apr;10:540. doi: 10.3389/fimmu.2019.00540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.McDonald B, Kubes P. Neutrophils and intravascular immunity in the liver during infection and sterile inflammation. Toxicol Pathol. 2012;40((2)):157–65. doi: 10.1177/0192623311427570. [DOI] [PubMed] [Google Scholar]
- 231.Naegele M, Tillack K, Reinhardt S, Schippling S, Martin R, Sospedra M. Neutrophils in multiple sclerosis are characterized by a primed phenotype. J Neuroimmunol. 2012;242((1–2)):60–71. doi: 10.1016/j.jneuroim.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 232.Tillack K, Naegele M, Haueis C, Schippling S, Wandinger KP, Martin R, et al. Gender differences in circulating levels of neutrophil extracellular traps in serum of multiple sclerosis patients. J Neuroimmunol. 2013;261((1–2)):108–19. doi: 10.1016/j.jneuroim.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 233.Beck J, Urnovitz HB, Saresella M, Caputo D, Clerici M, Mitchell WM, et al. Serum DNA motifs predict disease and clinical status in multiple sclerosis. J Mol Diagn. 2010;12((3)):312–9. doi: 10.2353/jmoldx.2010.090170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Dunaeva M, Derksen M, Pruijn GJM. LINE-1 hypermethylation in serum cell-free DNA of relapsing remitting multiple sclerosis patients. Mol Neurobiol. 2018;55((6)):4681–8. doi: 10.1007/s12035-017-0679-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wood DD, Ackerley CA, Brand B, Zhang L, Raijmakers R, Mastronardi FG, et al. Myelin localization of peptidylarginine deiminases 2 and 4: comparison of PAD2 and PAD4 activities. Lab Invest. 2008;88((4)):354–64. doi: 10.1038/labinvest.3700748. [DOI] [PubMed] [Google Scholar]
- 236.Mastronardi FG, Wood DD, Mei J, Raijmakers R, Tseveleki V, Dosch HM, et al. Increased citrullination of histone H3 in multiple sclerosis brain and animal models of demyelination: a role for tumor necrosis factor-induced peptidylarginine deiminase 4 translocation. J Neurosci. 2006;26((44)):11387–96. doi: 10.1523/JNEUROSCI.3349-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Musse AA, Li Z, Ackerley CA, Bienzle D, Lei H, Poma R, et al. Peptidylarginine deiminase 2 (PAD2) overexpression in transgenic mice leads to myelin loss in the central nervous system. Dis Model Mech. 2008;1((4–5)):229–40. doi: 10.1242/dmm.000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Calabrese R, Zampieri M, Mechelli R, Annibali V, Guastafierro T, Ciccarone F, et al. Methylation-dependent PAD2 upregulation in multiple sclerosis peripheral blood. Mult Scler. 2012;18((3)):299–304. doi: 10.1177/1352458511421055. [DOI] [PubMed] [Google Scholar]
- 239.Falcão AM, Meijer M, Scaglione A, Rinwa P, Agirre E, Liang J, et al. PAD2-mediated citrullination contributes to efficient oligodendrocyte differentiation and myelination. Cell Rep. 2019;27((4)):1090–102.e10. doi: 10.1016/j.celrep.2019.03.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Moscarello MA, Lei H, Mastronardi FG, Winer S, Tsui H, Li Z, et al. Inhibition of peptidyl-arginine deiminases reverses protein-hypercitrullination and disease in mouse models of multiple sclerosis. Dis Model Mech. 2013;6((2)):467–78. doi: 10.1242/dmm.010520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wei L, Wasilewski E, Chakka SK, Bello AM, Moscarello MA, Kotra LP. Novel inhibitors of protein arginine deiminase with potential activity in multiple sclerosis animal model. J Med Chem. 2013;56((4)):1715–22. doi: 10.1021/jm301755q. [DOI] [PubMed] [Google Scholar]
- 242.Sarswat A, Wasilewski E, Chakka SK, Bello AM, Caprariello AV, Muthuramu CM, et al. Inhibitors of protein arginine deiminases and their efficacy in animal models of multiple sclerosis. Bioorg Med Chem. 2017;25((9)):2643–56. doi: 10.1016/j.bmc.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 243.Aosasa M, Hossain MS, Sakata T, Koga K, Shigemitsu T, Shoya Y, et al. Epitope-based chicken-derived novel anti-PAD2 monoclonal antibodies inhibit citrullination. J Immunol Res. 2021;2021:6659960. doi: 10.1155/2021/6659960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362((4)):329–44. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 245.Pietronigro EC, Della Bianca V, Zenaro E, Constantin G. NETosis in Alzheimer's disease. Front Immunol. 2017 Mar;8:211. doi: 10.3389/fimmu.2017.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Baik SH, Cha MY, Hyun YM, Cho H, Hamza B, Kim DK, et al. Migration of neutrophils targeting amyloid plaques in Alzheimer's disease mouse model. Neurobiol Aging. 2014;35((6)):1286–92. doi: 10.1016/j.neurobiolaging.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zenaro E, Pietronigro E, Della Bianca V, Piacentino G, Marongiu L, Budui S, et al. Neutrophils promote Alzheimer's disease-like pathology and cognitive decline via LFA-1 integrin. Nat Med. 2015;21((8)):880–6. doi: 10.1038/nm.3913. [DOI] [PubMed] [Google Scholar]
- 248.Ishigami A, Ohsawa T, Hiratsuka M, Taguchi H, Kobayashi S, Saito Y, et al. Abnormal accumulation of citrullinated proteins catalyzed by peptidylarginine deiminase in hippocampal extracts from patients with Alzheimer's disease. J Neurosci Res. 2005;80((1)):120–8. doi: 10.1002/jnr.20431. [DOI] [PubMed] [Google Scholar]
- 249.Jang B, Jeon YC, Choi JK, Park M, Kim JI, Ishigami A, et al. Peptidylarginine deiminase modulates the physiological roles of enolase via citrullination: links between altered multifunction of enolase and neurodegenerative diseases. Biochem J. 2012;445((2)):183–92. doi: 10.1042/BJ20120025. [DOI] [PubMed] [Google Scholar]
- 250.Mohlake P, Whiteley C. Arginine metabolising enzymes as therapeutic tools for Alzheimer's disease: peptidyl arginine deiminase catalyses fibrillogenesis of beta-amyloid peptides. Mol Neurobiol. 2010;41((2–3)):149–58. doi: 10.1007/s12035-010-8112-x. [DOI] [PubMed] [Google Scholar]
- 251.Acharya NK, Nagele EP, Han M, Coretti NJ, DeMarshall C, Kosciuk MC, et al. Neuronal PAD4 expression and protein citrullination: possible role in production of autoantibodies associated with neurodegenerative disease. J Autoimmun. 2012;38((4)):369–80. doi: 10.1016/j.jaut.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 252.Tetz G, Tetz V. Bacterial extracellular DNA promotes β-amyloid aggregation. Microorganisms. 2021;9((6)):1301. doi: 10.3390/microorganisms9061301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Kretzschmar G, Bumiller-Bini V, Gasparetto Filho M, Zonta Y, Yu K, de Souza R, et al. Neutrophil extracellular traps: a perspective of neuroinflammation and complement activation in Alzheimer's disease. Front Mol Biosci. 2021;8:630869. doi: 10.3389/fmolb.2021.630869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Konki M, Lindgren N, Kyläniemi M, Venho R, Laajala E, Ghimire B, et al. Plasma cell-free DNA methylation marks for episodic memory impairment: a pilot twin study. Sci Rep. 2020;10((1)):14192. doi: 10.1038/s41598-020-71239-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Manley GT, Maas AI. Traumatic brain injury: an international knowledge-based approach. JAMA. 2013;310((5)):473–4. doi: 10.1001/jama.2013.169158. [DOI] [PubMed] [Google Scholar]
- 256.Coronado VG, Xu L, Basavaraju SV, McGuire LC, Wald MM, Faul MD, et al. Surveillance for traumatic brain injury-related deaths: United States, 1997–2007. MMWR Surveill Summ. 2011;60((5)):1–32. [PubMed] [Google Scholar]
- 257.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2((7872)):81–4. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 258.Lazarus RC, Buonora JE, Flora MN, Freedy JG, Holstein GR, Martinelli GP, et al. Protein citrullination: a proposed mechanism for pathology in traumatic brain injury. Front Neurol. 2015 Sep;6:204. doi: 10.3389/fneur.2015.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Zeng H, Fu X, Cai J, Sun C, Yu M, Peng Y, et al. Neutrophil extracellular traps may be a potential target for treating early brain injury in subarachnoid hemorrhage. Transl Stroke Res. 2022;13((1)):112–31. doi: 10.1007/s12975-021-00909-1. [DOI] [PubMed] [Google Scholar]
- 260.Vaibhav K, Braun M, Alverson K, Khodadadi H, Kutiyanawalla A, Ward A, et al. Neutrophil extracellular traps exacerbate neurological deficits after traumatic brain injury. Sci Adv. 2020;6((22)):eaax8847. doi: 10.1126/sciadv.aax8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Zhu K, Zhu Y, Hou X, Chen W, Qu X, Zhang Y, et al. NETs lead to sympathetic hyperactivity after traumatic brain injury through the LL37-hippo/MST1 pathway. Front Neurosci. 2021;15:621477. doi: 10.3389/fnins.2021.621477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Campello Yurgel V, Ikuta N, Brondani da Rocha A, Lunge VR, Fett Schneider R, Kazantzi Fonseca AS, et al. Role of plasma DNA as a predictive marker of fatal outcome following severe head injury in males. J Neurotrauma. 2007;24((7)):1172–81. doi: 10.1089/neu.2006.0160. [DOI] [PubMed] [Google Scholar]
- 263.Rodrigues Filho EM, Simon D, Ikuta N, Klovan C, Dannebrock FA, Oliveira de Oliveira C, et al. Elevated cell-free plasma DNA level as an independent predictor of mortality in patients with severe traumatic brain injury. J Neurotrauma. 2014;31((19)):1639–46. doi: 10.1089/neu.2013.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Macher H, Egea-Guerrero JJ, Revuelto-Rey J, Gordillo-Escobar E, Enamorado-Enamorado J, Boza A, et al. Role of early cell-free DNA levels decrease as a predictive marker of fatal outcome after severe traumatic brain injury. Clin Chim Acta. 2012;414:12–7. doi: 10.1016/j.cca.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 265.Ohayon S, Boyko M, Saad A, Douvdevani A, Gruenbaum BF, Melamed I, et al. Cell-free DNA as a marker for prediction of brain damage in traumatic brain injury in rats. J Neurotrauma. 2012;29((2)):261–7. doi: 10.1089/neu.2011.1938. [DOI] [PubMed] [Google Scholar]
- 266.Shaked G, Douvdevani A, Yair S, Zlotnik A, Czeiger D. The role of cell-free DNA measured by a fluorescent test in the management of isolated traumatic head injuries. Scand J Trauma Resusc Emerg Med. 2014;22((1)):21. doi: 10.1186/1757-7241-22-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Hazeldine J, Dinsdale RJ, Naumann DN, Acharjee A, Bishop JRB, Lord JM, et al. Traumatic injury is associated with reduced deoxyribonuclease activity and dysregulation of the actin scavenging system. Burns Trauma. 2021;9:tkab001. doi: 10.1093/burnst/tkab001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Bhattacharya SK, Bhat MB, Takahara H. Modulation of peptidyl arginine deiminase 2 and implication for neurodegeneration. Curr Eye Res. 2006;31((12)):1063–71. doi: 10.1080/02713680600991437. [DOI] [PubMed] [Google Scholar]
- 269.Janovičová Ľ, Konečná B, Vokálová L, Lauková L, Vlková B, Celec P. Sex, age, and bodyweight as determinants of extracellular DNA in the plasma of mice: a cross-sectional study. Int J Mol Sci. 2019;20((17)):4163. doi: 10.3390/ijms20174163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Konečná B, Sysák R, Kacerovský M, Celec P, Vlková B. Deoxyribonuclease activity in plasma of pregnant women and experimental animals. J Matern Fetal Neonatal Med. 2018;31((13)):1807–9. doi: 10.1080/14767058.2017.1326899. [DOI] [PubMed] [Google Scholar]