Abstract
Cancer immunotherapy, or the utilization of a patient’s own immune system to treat cancer, has shifted the paradigm of cancer treatment. Despite meaningful responses being observed in multiple studies, currently available immunotherapy platforms have only proven effective to a small subset of patients. To address this, nanoparticles have been utilized as a novel carrier for immunotherapeutic drugs, achieving robust anti-tumor effects with increased adaptive and durable responses. Specifically, dendrimer nanoparticles have attracted a great deal of scientific interest due to their versatility in various therapeutic applications, resulting from their unique physicochemical properties and chemically well-defined architecture. This review offers a comprehensive overview of dendrimer-based immunotherapy technologies, including their formulations, biological functionalities, and therapeutic applications. Common formulations include: (1) modulators of cytokine secretion of immune cells (adjuvants); (2) facilitators of the recognition of tumorous antigens (vaccines); (3) stimulators of immune effectors to selectively attack cells expressing specific antigens (antibodies); and (4) inhibitors of immune-suppressive responses (immune checkpoint inhibitors). On-going works and prospects of dendrimer-based immunotherapies are also discussed. Overall, this review provides a critical overview on rapidly growing dendrimer-based immunotherapy technologies and serves as a guideline for researchers and clinicians who are interested in this field.
Graphical Abstract
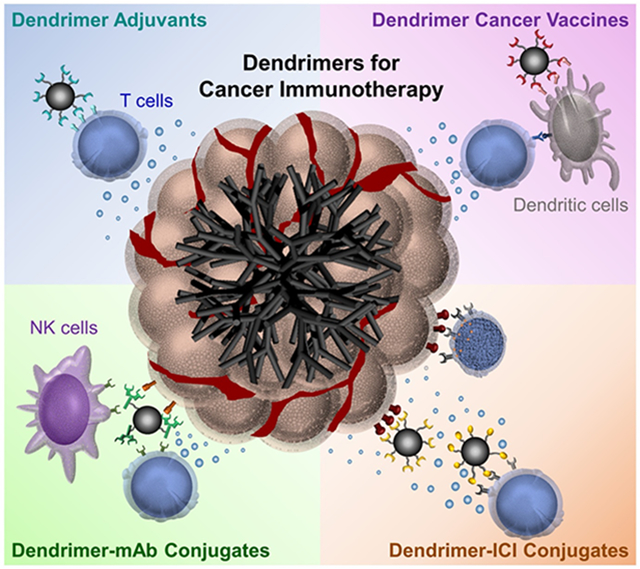
Dendrimers for cancer immunotherapy technologies. Dendrimers are applied in various immunotherapy applications as (1) modulators of cytokine secretion of immune cells; (2) facilitators of the recognition of tumorous antigens; (3) stimulators of immune effectors to selectively attack cells expressing specific antigens; and (4) inhibitors of immune-suppressive responses.
1. INTRODUCTION
The human immune system has developed complex and dynamic mechanisms to defend the body against cancer. These mechanisms play a pivotal role in both recognizing and eliminating malignant cells; however, failure of the self-defense system allows tumor progression. (Kennedy & Salama, 2020; Zeng et al., 2016) Cancer immunoediting, or the dual role by which the immune system protects against while also promoting tumor growth, consists of three phases: elimination, equilibrium, and escape (Dunn et al., 2004). In the elimination phase, both the innate and adaptive immune systems recognize and respond to the early formation of tumors. The tumor cells which survive the elimination phase enter the equilibrium phase where the adaptive immune system holds the tumor in a functionally dormant state. Due to the constant immune pressure, some of the tumor cells undergo genetic and epigenetic changes and develop resistance to the anti-tumor immune response, thereby entering the escape phase. During the escape phase, the immune system fails to prevent tumor progression, resulting in immune evasion, suppression of anti-tumor immune activity, and secretion of cytokines that enhance angiogenesis. (Kennedy & Salama, 2020; Mittal et al., 2014) The mainstream approach of cancer immunotherapy is based on tackling tumor escape mechanisms, and much research is focused on reactivating the immune system to prevent tumor progression (Kennedy & Salama, 2020; Vesely & Schreiber, 2013). A variety of therapeutic approaches to stimulate the immune system have thus been investigated, including cytokines, cancer vaccines, monoclonal antibodies (mAbs), and immune checkpoint inhibitors (ICI) (Ragoonanan et al., 2021). A number of studies using these treatments have demonstrated significant clinical benefits in patients with various tumor types, including melanoma, ovarian cancer, bladder cancer, renal cell carcinoma, hepatocellular carcinoma, and lung cancer (Lesterhuis et al., 2011).
Although research over the past several decades has brought significant breakthroughs in the field of immunotherapy, many of the currently available cancer immunotherapies have shown drastic inconsistencies among patients. For example, antibody-based antagonists that target immune checkpoint molecules, such as programmed death-1 (PD-1) or its ligand (PD-L1), are only effective for a small subset of patients, whereas the majority of the patients experience minimal benefits. This was consistently observed in patients with various types of tumor, including non-small cell lung cancer (NSCLC) (Brahmer et al., 2015), Hodgkin’s lymphoma (Ansell et al., 2015), and triple-negative breast cancer (Dirix et al., 2018). Furthermore, these pharmaceuticals have the potential to induce rapid clinical deterioration due to self-destructive autoimmunity and toxicity effects. (Caminade et al., 2011) In fact, repetitive injections of free antibodies were found to be severely toxic, causing fatal xenogeneic hypersensitivity reactions in a murine model (Mall et al., 2015). To address this, nanoparticles have been utilized as carriers for immunotherapeutic agents and demonstrated significant anti-tumor effects with increased adaptive and durable responses compared to conventional immunotherapeutics (e.g., free antibodies). (Moon et al., 2012; X. Zhou et al., 2020) This can be attributed to the fact that nanoparticles enable selective delivery of their payloads and elongate the blood circulation time, thereby allowing for the use of lower doses to achieve a given response with enhanced pharmacokinetic properties. (Goldberg, 2015; Poellmann et al., 2018) Among these nanoparticles, emerging interest is focused on leveraging hyperbranched dendrimers for immunotherapies due to their unique physicochemical properties, including their well-defined structure, multivalency, chemical modularity for multifunctionalization, biocompatibility, and structural versatility (Abbasi et al., 2014; H.-J. Hsu et al., 2017; Palmerston Mendes et al., 2017; Svenson & Tomalia, 2005; Teunissen et al., 2021). Furthermore, the dendrimers’ flexible and deformable branches facilitate simultaneous binding of ligands conjugated with dendrimers to multiple receptors on a cell surface, inducing a strong avidity binding via the multivalent binding effect (Hong et al., 2007; Myung et al., 2011). For example, the conjugation of dendrimers to multiple ligands allows for the integration of each ligand-receptor interaction (affinity) to significantly increase the total binding strength (avidity) of the dendrimer-ligand conjugates towards specific molecules (i.e., cells). These properties have allowed dendrimers to be a promising immunotherapy platform, resulting in significantly enhanced therapeutic indices (Deci et al., 2018; Santos et al., 2019). Thus, these unique physiological properties allow dendrimers to serve as a novel drug delivery platform for immunotherapy which can overcome the multitude of challenges associated with conventional approaches.
In the following chapters, we summarize recent advances in dendrimers and their use in cancer immunotherapy. Specifically, we discuss dendrimer synthesis and properties, followed by a detailed discussion on dendrimer-based immunotherapies to treat cancer, categorized by the type of immunotherapeutic agent. First, we summarize the use of dendrimers combined with cytokines that promote the expansion of immune cells and enhance their cytolytic activity. Second, dendrimer-based cancer vaccines are discussed, which utilize well-defined and reproducible scaffolds to form highly immune-active and efficient constructs for delivery. Third, dendrimer-mAb conjugates, which take advantage of the multivalent binding effect of dendrimers to increase the interaction between antibodies and target proteins, are described. Fourth, dendrimers conjugated with various ICIs are discussed, which are designed to enhance the binding avidity towards checkpoint molecules on target cells and inhibit the redistribution of these molecules. Note that the dendrimer-antibody conjugates targeting the immune checkpoint pathways are also included in this section. Finally, this review concludes with our opinions and prospects for the overall dendrimer-based cancer immunotherapeutics.
2. DENDRIMERS
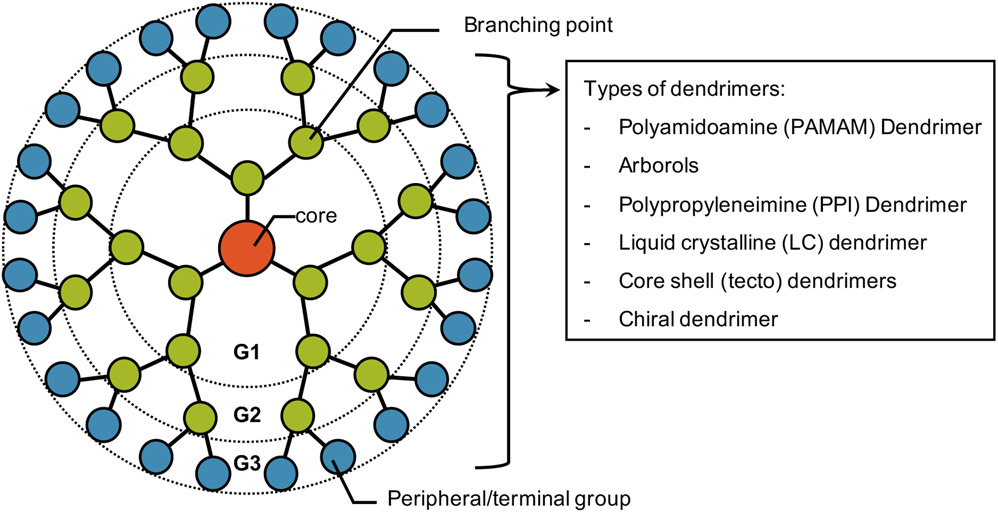
Dendrimers are hyperbranched globular nanopolymeric architectures with distinct, homogeneous, and close-to-monodisperse structures (Abbasi et al., 2014; Kesharwani et al., 2014; Tomalia & Fréchet, 2002). They are composed of a central core, extended “branch-like” at repeating junctions, and peripheral reactive functional groups, typically ranging from 1 to 10 nm (Bugno et al., 2015b; Cheng & Burda, 2011; Hodge, 1993; Pearson et al., 2012). The two most commonly used synthetic routes for dendrimers are divergent and convergent methods (Hodge, 1993). For divergent dendrimer synthesis, a dendrimer is assembled outward as branches are attached to a multifunctional core in a stepwise manner (Bugno et al., 2015b; Gupta & Nayak, 2015; Tomalia & Fréchet, 2002). The multifunctional core reacts with surrounding monomer molecules, resulting in a homologous first-generation dendrimer (G1; 4 surface end groups). As the periphery is activated with additional monomers, the number of reactive terminal functional groups doubles with the generational growth of dendrimers. (Abbasi et al., 2014; Gupta & Nayak, 2015) For convergent dendrimer synthesis, in contrast, dendrimer branches, called dendrons, are first generated and then coupled to a multifunctional core moiety after activation of their focal point to form a complete dendrimer. Increasing generations of convergently grown dendrimers require the activation of internal end groups as the dendrons build inward to the dendrimer’s core. (Hawker & Frechet, 1990; Malkoch & García-Gallego, 2020) Additionally, dendrimers can also be classified based on their monomers. Examples of dendrimers synthesized from the various monomers include polyamidoamine (PAMAM), arborols, polypropyleneimine (PPI), liquid crystalline (LC), core-shell (tecto), and chiral dendrimers (Figure 1). (Berg & Meijer, 1993; Nanjwade et al., 2009; Newkome et al., 1985; Tomalia et al., 1985)
Figure 1.
Schematic illustration of a dendrimer structure with examples of common types utilized for various applications.
The physiological and chemical properties of dendrimers are largely determined by their generations (size), terminal groups, synthetic routes, and monomers, and these factors can be tailored to endow dendrimers with specific properties for different biomedical applications. (K. Jain, 2017) Although their properties may vary, dendrimers have several characteristics that make them unique from other drug carriers. Specifically, their high degree of branching, polyvalency, biocompatibility, and water solubility make dendrimers an ideal carrier for various therapeutic agents. (Bu, Nair, Kubiatowicz, et al., 2020; Bugno et al., 2015a; W. Jeong et al., 2018; Santos et al., 2019; Tekade et al., 2009) For example, the multiple terminal functional groups of dendrimers enable them to be conjugated or complexed with a myriad of biologically active moieties, including chemotherapeutic drugs, nucleic acids, and targeting agents. By integrating multiple therapeutic agents into a single nanostructure, dendrimers can also facilitate the simultaneous binding of these agents to their respective target molecules, which in turn increases the therapeutic efficacy. (K. Jain, 2017; Santos et al., 2019). Overall, these properties have allowed dendrimers to be a promising platform for various biologically active moieties, and many efforts are being made to utilize them for cancer immunotherapy (Santos et al., 2019).
3. DENDRIMER-BASED CANCER IMMUNOTHERAPY
3.1. Cytokines
The tumor microenvironment (TME) is a complex network that includes the tumor and surrounding blood vessels, immune cells, fibroblasts, signaling molecules, and extracellular matrix (ECM) (Zemek et al., 2019). The TME is usually immunosuppressive and metabolically stressed due to the multitude of local alterations which contrast anti-tumor adaptive immunity and favor the dissemination of tumor cells. Additional research into the biology of the TME may reveal immunotherapeutic strategies to block tumor growth and prevent metastasis by targeting specific components of the TME. (Duan et al., 2020; Scharping & Delgoffe, 2016; Tuccitto et al., 2019) Previous studies have found that the TME composition can vary greatly between patients with the same cancer type and can either be characterized as “cold” (non-inflamed) or “hot” (T cell inflamed) depending on the levels of cytokine production and T cell infiltration. The suppressive TME can help tumors stay “cold” (T cell exclusion), escape from host immune systems, and develop resistance to immunotherapies. (Zemek et al., 2019) Consequently, converting cold tumors into hot tumors represents a promising strategy that may enhance the efficacy of concurrent or subsequent immunotherapy treatment and result in a more robust anti-tumor response (Duan et al., 2020; Galon & Bruni, 2019).
Cytokines are secreted polypeptides or glycoproteins with a molecular weight usually below 30 kDa that have a specific effect on cell interaction and communication. They are essential signaling molecules and major immunomodulators of the innate and adaptive immune systems; it is well known that cytokines act on every phase of the cancer immunity cycle and increase the number and cytolytic activity of effector immune cells in the TME. (Berraondo et al., 2019; S. Lee & Margolin, 2011) Thus, manipulating cytokine levels can effectively reshape the TME and enhance the efficacy of immunotherapy. Many cytokines, including Granulocyte-macrophage colony-stimulating factor (GM-CSF, CSF-2), interleukin (IL)-2, IL-7, IL-12, IL-15, IL-18, and IL-21, have been investigated in preclinical and clinical studies for their potential anti-tumor activity, with two cytokine treatments receiving FDA approval as single agents for cancer treatment; namely, the high-dose, bolus IL-2 for metastatic melanoma and renal cell carcinoma as well as IFN-α for the adjuvant therapy of stage III melanoma. (S. Lee & Margolin, 2011) However, the use of cytokines as a monotherapy faces challenges due to their short half-life, limited tumor accumulation, narrow therapeutic window, and associated severe toxicities. (Skrombolas & Frelinger, 2014)
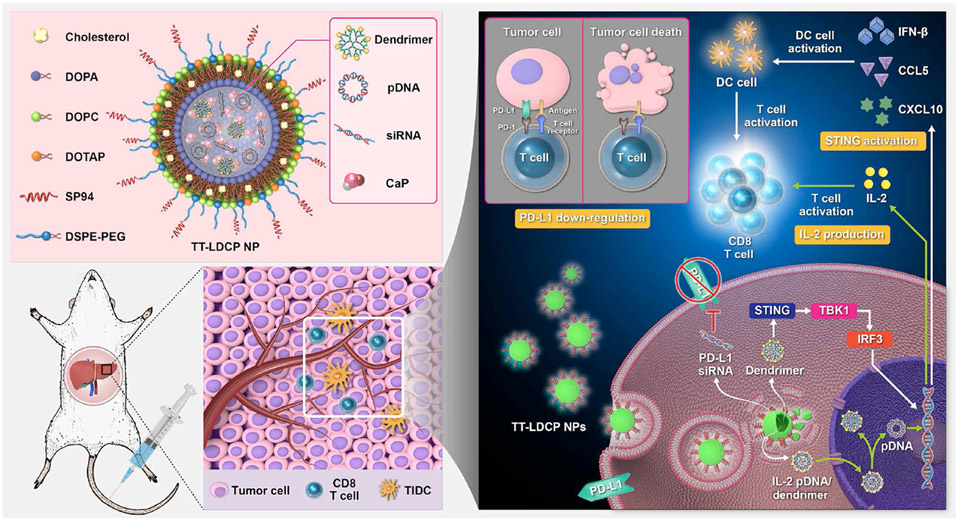
To this end, many nanoparticle delivery systems, including dendrimers, have been developed to improve the efficacy of cytokine-based immunotherapies. Typically, dendrimer-based cytokine delivery systems utilize plasmid DNA (pDNA) that affects cytokine secretion of immune cells or antigen-presenting cells, as the direct conjugation of cytokines to dendrimers is inefficient and can limit their bioactivity. (Bahadoran et al., 2017; S. C. Lee et al., 2004) For example, IL-2 is a promoter for the expansion of natural killer (NK) cells and T lymphocytes, which is currently used in the clinic as an effective immunotherapeutic agent to promote T cell activation and proliferation. However, the systemic administration of IL-2 is found to be associated with toxicities, thereby limiting its efficacy. (Berraondo et al., 2019; Waldmann, 2018) To address this issue, Huang et al. developed tumor-targeting lipid-dendrimer-calcium-phosphate nanoparticles (TT-LDCPs) with thymine-functionalized dendrimers to deliver IL-2 pDNA (Figure 2). (K.-W. Huang et al., 2020) The TT-LDCPs were utilized to enhance the endosomal escape of pDNA, assist their nuclear entry, stimulate interferon genes (STING)-cyclic GMP-AMP synthase (cGAS) pathway, and promote maturation of dendritic cells (DCs). The nanoparticles also demonstrated enhanced tumor infiltration and increased CD8+ T cell activities compared to the nanoparticles without thymine-functionalized dendrimers.
Figure 2:
Schematic representation of the mechanism of immunogene therapy by lipid-dendrimer NPs containing siRNA against the immune checkpoint PD-L1 and pDNA encoding the immunostimulating cytokine IL-2. Reprint from Huang et al. 2020.
Another target of cytokine manipulation is tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), a member of the tumor necrosis factor (TNF) superfamily and an innate immune cytokine broadly expressed by immune cells (Staniek et al., 2019; Wiley et al., 1995). Although TRAIL proteins were shown to selectively target tumor cells in vivo with minimal toxicity (Walczak et al., 1999), they achieved only limited clinical efficacy due to their short circulation half-life and lack of accumulation at the tumor site. Therefore, the development of effective carriers for TRAIL-based therapeutics was hypothesized to improve both their efficacy and clinical potential (Guimarães et al., 2018). To this end, the Jiang group has extensively researched the utilization of PAMAM dendrimers (Gao et al., 2015; Han et al., 2011; K.-W. Huang et al., 2020) and Dendrigraft poly-L-lysine (Li et al., 2013) as TRAIL pDNA (pTRAIL) delivery vehicles. They found that the nanoparticles significantly improved the therapeutic potential of pTRAIL, which was attributed to the advantages brought by the nanoparticles, including high transfection efficiency and pH buffering capabilities, resulting in enhanced intracellular delivery of the nucleic acid and efficient endosomal escape (Zhu & Mahato, 2010). In one study, Han et al. demonstrated tumor-targeted delivery and apoptosis induction in a human liver xenograft mouse model using a PAMAM/pTRAIL complex conjugated with transferrin receptor-specific peptides. Furthermore, when combined with doxorubicin (Dox) in a co-delivery system, this platform exhibited significantly improved anti-tumor effects and pharmacokinetic properties in comparison to Dox alone. (Han et al., 2011)
In addition to the dendrimers as a delivery vehicle of pDNA, some dendrimers can also be designed to display immune modulation properties without pDNA. For example, Berzi et al. found that a mannosylated glycodendrimer (Polyman 26) binds with a DC-SIGN (CD209) on dendritic cells (DCs) and accelerates the production of β-chemokines and pro-inflammatory cytokines, such as including IL-1β, IL-6, IL-12, and TNFα. (Berzi et al., 2016) Similarly, Perisé-Barrios et al. developed a carbosilane dendrimer (2G-03NN2) to re-polarize M2 macrophages to an M1-like state (Perisé-Barrios et al., 2015).
As discussed above, cytokines are complex immune mediators and have demonstrated the potential to modulate the TME and act as potent anti-tumor therapeutics. However, the development of effective cytokine-based immunotherapies remains a challenge as it is difficult to exploit their therapeutic properties while minimizing associated toxicities. To this end, dendrimer-based cytokine therapeutics have been developed and demonstrated to have enhanced therapeutic delivery capacity, immune adjuvant properties, and biocompatibility in comparison to conventional cytokine immunotherapies. Additional research into the design of novel conjugates and combination therapy of cytokines with other immunotherapy agents would further enhance the pharmacokinetic properties of cytokine dendrimer bioconjugates, thereby increasing their clinical significance.
3.2. Vaccines
Conventional vaccines are typically nonpathogenic imitations of infectious agents that act as prophylactics to stimulate a lasting immune response against the infectious agent when administered to a host. Thus, if the host is subsequently exposed to the particular infectious agent, the host’s immune system will remember the previous infectious challenge and significantly reduce the extent of infection. (Ada, 2003; Heegaard et al., 2010) While vaccination has proven to be an efficacious method of controlling and even eradicating a plethora of infectious diseases, many persist around the globe due to inherent difficulties in targeting complex and evasive pathogens (Kaufmann, 2007). In addition, the development of therapeutic vaccines designed to evoke cellular immune responses and provide immunity against diseases such as cancer remains a significant challenge and has only achieved modest clinical efficacy. (Banchereau & Palucka, 2018) Therefore, there is an obvious need for additional vaccines capable of targeting these diseases to improve global health. As such, many recent research efforts have been dedicated to understanding relevant immune mechanisms and exploiting well-defined chemical methods to develop such vaccines. (Carreno et al., 2015; Sahin et al., 2017; Xu et al., 2019)
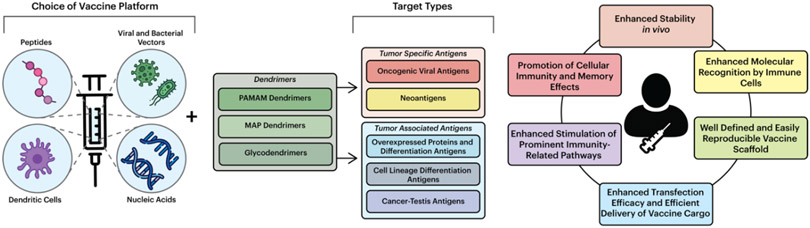
To date, much of the work on cancer vaccines has been focused on tumor-associated antigens or aberrantly expressed self-antigens, such as human telomerase reverse transcriptase (hTERT) and human epidermal growth factor receptor-2 (HER2). High-affinity T cells that recognize self-antigens, however, are typically eliminated during development due to natural immune responses, and as a result, many cancer vaccines have difficulty activating any remaining low-affinity T cells. (Hollingsworth & Jansen, 2019; Thommen & Schumacher, 2018; Zitvogel et al., 2008) Thus, it is essential to consider the choice of antigen for cancer vaccine design. Several classes of antigens have been employed in cancer vaccines to date, including: (1) tumor-associated antigens (including cancer/germline antigens, cell lineage differentiation antigens, and antigens that are overexpressed in cancer cells); (2) oncogenic viral antigens; and (3) neoantigens. (Hollingsworth & Jansen, 2019) When utilizing these cancer vaccines, it is also important to consider the cross-presentation of antigens that determines cellular immunity (Guermonprez et al., 2003; Joffre et al., 2012). Therefore, it is critical for the development of efficacious cancer vaccines to utilize carriers capable of effectively encapsulating the various types of antigens and facilitating antigen cross-presentation. To this end, dendrimers have emerged as useful multivalent carriers for vaccine development due to their inherent biological and chemical properties and ease of coupling antigens and immunostimulators to surface functional groups (Figure 3). (Heegaard et al., 2010)
Figure 3:
Components of dendrimer-based vaccines. Vaccine platforms can be paired with several types of dendrimers, including PAMAM, MAP, and glycodendrimers, which can target multiple antigens. These systems offer the possibility of improving the bioavailability and pharmacokinetic properties of immunotherapeutic vaccines.
Examples of dendrimers currently being used for cancer vaccine development include PAMAM (Xu et al., 2019), multiple antigen peptide (MAP) (Sadler & Tam, 2002), and glycopeptide (Niederhafner et al., 2008) dendrimers. Previous studies have found that cationic dendrimers, such as PAMAM dendrimers, allow for the complexation of biologically active agents, such as nucleic acids, peptides, and proteins through electrostatic interactions, thereby protecting the materials from immature enzymatic degradation and increasing their transfection efficiency. (Hao et al., 2020; Lalani & Misra, 2011; Salameh et al., 2020; Setaro et al., 2015; Zeng et al., 2016) This feature was exploited in an early study by Daftarian et al., who conjugated PAMAM dendrimers and universal peptides to selectively deliver DNA to antigen-presenting cells and increase the efficacy of DNA vaccines. (Daftarian et al., 2011) Although the group’s vaccine design was promising and resulted in the generation of high-affinity memory cytotoxic T lymphocytes with a robust humoral response, their system was not fully optimized and resulted in tumor regression with a 50% mortality rate. Nevertheless, recent research on PAMAM dendrimer vaccine platforms has found that the surface functional groups of the dendrimers can be modified to improve biological responses and enhance the efficacy of vaccine delivery systems (Zeng et al., 2016). For instance, Xu et al. demonstrated that grafting PAMAM dendrimers with guanidinobenzoic acid enhanced binding and endosomal disruption, enabling efficient delivery of various proteins and peptides into the cytosol of living cells (Xu et al., 2019). Furthermore, a recent study found that modification of PAMAM dendrimers (i.e., by capping the dendrimers with thymine) can result in several multifunctional immunotherapy characteristics: (1) synergistic interaction with and protection of genetic cargoes (siRNA and pDNA) via electrostatic interaction and hydrogen bonding; (2) enhanced endosomal escape and intracellular release of genetic cargoes; (3) increased pDNA uptake in the nucleus and enhanced gene transfection activity; and (4) enhanced stimulation of prominent immunity-related pathways and promotion of cellular immunity. (K.-W. Huang et al., 2020; Yan et al., 2015) However, although many of the PAMAM dendrimer-based vaccines have demonstrated high efficiency and selectivity, their effects are often ephemeral, requiring additional research to be completed to develop nanovaccines with long-term anti-tumor immunity.
Similar to PAMAM dendrimers, MAP dendrimers are employed to improve the immunogenicity of cancer vaccines. Synthetic immunogenic peptides are of interest in cancer vaccines due to their lack of cross-reactivity with host tissues, induction of site-specific antibodies, ability to chemically define and modify products, and ease of large-scale manufacturing and long-term storage. (Fujita & Taguchi, 2011) The MAP systems take advantage of these benefits, and multiple copies of antigenic peptides are bound to a non-immunogenic Lys-based dendritic scaffold (Fujita & Taguchi, 2011; Tam, 1988). This system was shown to be highly effective by Ota et al., who demonstrated that MAPs are processed the same way as antigens derived from intracellular pathogens in antigen-presenting cells, allowing for enhanced molecular recognition by immune cells and induction of strong immune responses. (Ota et al., 2002) Despite such success, studies have shown that tumor antigen peptides are weakly immunogenic, and the conjugation of additional functional components to the dendrimers is necessary for the development of functional MAP dendrimer-based cancer vaccines (Blankenstein et al., 2012; Fujita & Taguchi, 2011). Such functional components include helper T cell epitopes (Kumar et al., 1992) and lipids (Horvath et al., 2002), which have been shown to synergistically enhance vaccines’ immune responses. Nevertheless, it should be noted that many of the studies that utilized the MAP dendrimer-based systems explicitly focused on the development of a vaccine for bacterial and viral infections rather than for a cancer vaccine. (de Oliveira et al., 2003; X.-J. Huang et al., 2013; Nardin et al., 1995) Therefore, there is much room for additional research to be completed to utilize MAP dendrimers for cancer vaccines.
Glycodendrimers have also been extensively studied for their use as functional antigens in cancer vaccines due to their strong immunostimulating and adjuvant properties resulting from their highly defined multivalent scaffolds (Moffett et al., 2021; Shiao & Roy, 2012). Conventional glycoconjugate vaccines have been leveraged for cancer immunotherapy by linking target tumor-associated carbohydrate antigens (TACAs), glycans abundantly expressed on the surface of various tumors, and carrier proteins. However, such vaccines have failed in clinical trials due to their inability to elicit T cell-mediated immunity in cancer patients and short-term immunity effects. (Guo & Wang, 2009; Mettu et al., 2020; Stone et al., 2021) Thus, efforts have been made to utilize glycodendrimers, or carbohydrate-bearing dendrimers, for anti-tumor vaccines (Shiao & Roy, 2012). Bay et al. demonstrated the feasibility and efficacy of eliciting complete immune responses with memory effects using glycodendrimers conjugated with glycosidic tumor-associated antigens (Bay et al., 1997). Other studies utilizing glycodendrimers for cancer vaccines have achieved similar success but noted issues with the sensitivity and specificity of the dendrimers’ targets (Vichier-guerre et al., 2000). Moreover, these issues can be overcome with improvements in glycodendrimer synthesis and the incorporation of various functional components (Shiao & Roy, 2012).
Many types of dendrimers are being used to develop the next generation of vaccines; however, dendrimers have only been used to a limited extent as delivery vehicles to enhance the immunogenicity of antigens for vaccine purposes. Nevertheless, dendrimers offer the possibility of a well-defined and reproducible scaffold capable of forming constructs that are highly immunoactive and efficient for vaccine delivery. With additional research into the relevant mechanisms behind induction and control of immunity, explicitly relating to cancer development and progression, the application potentials of dendrimers for efficient cancer vaccine delivery would likely lead to the development of a new generation of vaccines with significantly improved cancer outcomes.
3.3. Monoclonal Antibodies for Targeted Therapy
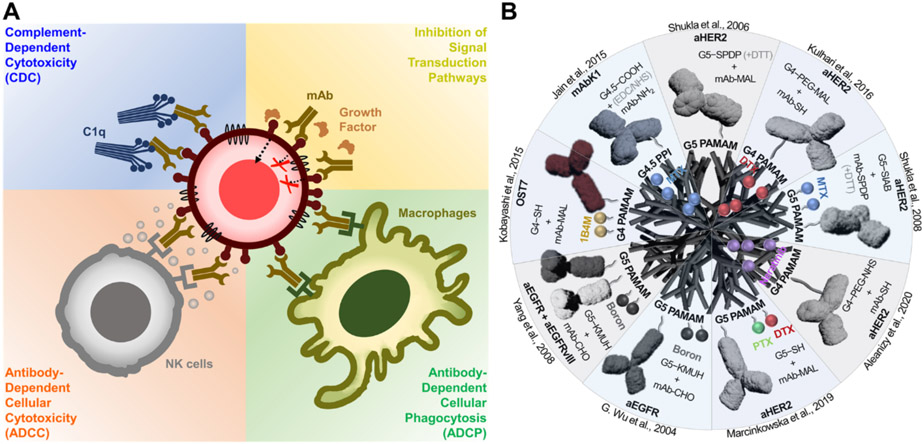
Antibodies are protective immune proteins produced by B cells in response to the detection of pathogen-derived antigens. Antibodies play a critical role in the immune system’s defense against cancer by activating various immune responses (Figure 4A). These immune responses are initiated by recognizing cell surface tumor antigens, where the antigen-binding fragment (Fab) region of the antibodies interacts with the specific tumor antigen through a high-affinity binding. (Chiu et al., 2019) The fragment crystallizable (Fc) region of mAb then engages receptors (CD16A) on the surface of immune effector cells, such as NK cells and macrophages, which induces the antibody-dependent cellular-mediated cytotoxicity (ADCC) and antibody-dependent phagocytosis (ADPC), respectively. (Gómez Román et al., 2014; Igietseme et al., 2014; T. H. Kang & Jung, 2019; Ochoa et al., 2017) Fc regions of the antibodies can also bind with C1q proteins, which form a membrane attack complex (MAC) and trigger the lysis of antibody-bound cells (Igietseme et al., 2014). In addition to activating the immune effectors, the binding of antibodies to specific surface proteins, such as epidermal growth factor receptors (EGFR) and HER2, halt the growth of a tumor by modulating the signaling pathways (Seshacharyulu et al., 2012; Tai et al., 2010; Zahavi & Weiner, 2020). Antibodies are also the major type of ICIs and will be discussed in more detail in the following section.
Figure 4:
Schematic illustration of the dendrimer-mAb conjugates. (a) mAb-mediated immune cell functional assays. (b) Design and formulation of the dendrimer-mAb conjugates.
The antibodies’ anti-cancer effects, including the activation of immune responses and the direct inhibitory effect, could be affected by the number of antibodies attached to the surface of target cells (Wines et al., 2017). In this regard, the multivalent binding effect mediated by dendrimers can be employed to increase the interaction between antibodies and target proteins by the covalent conjugation of multiple antibodies on the dendrimer surface (Figure 4B). For example, the Baker group conjugated HER2-targeting mAbs to G5 PAMAM dendrimers. Although this study did not directly measure the antibody-dependent cytotoxicity, both in vitro and in vivo studies revealed that the dendrimer-mAb conjugates target HER2-expressing tumors more effectively than the free mAbs, implying that the dendrimers recruit antibodies more effectively to the tumor sites and have the potential to increase ADCC effects. (Shukla et al., 2006)
Although controversial (Patri et al., 2005), the hydrophobic inner cavities of dendrimers could be utilized to carry chemotherapeutic drugs, combined with the tumor targeting and signal blockade using dendrimer-mAb conjugates. In a study done by Adams et al., G4 PAMAM dendrimers were conjugated with Trastuzumab (Tz), an FDA-approved mAb drug that binds to HER2 receptors on cancer cells and blocks their downstream signaling Kulhari et al., 2016). The group utilized the dendrimer-Tz conjugates as a vector for the delivery of docetaxel (DTX) and showed enhanced tumor specificity, DTX uptake, and cytotoxicity effects compared to DTX-loaded dendrimers (without Tz) and free DTX. The conjugates between dendrimers and HER2-targeting antibodies have also been applied for the delivery of methotrexate (MTX) (Shukla et al., 2008), neratinib (Aleanizy et al., 2020), and paclitaxel (PTX) (Marcinkowska et al., 2019). These drugs were either conjugated on the dendrimer surface along with the antibodies or physically entrapped inside the inner cavity of the dendrimers (Aleanizy et al., 2020). Regardless of the method, the dendrimer-mAb conjugates selectively delivered their chemotherapeutic agents to HER2-positive cells and demonstrated enhanced cytotoxicity compared to the free chemotherapeutic agents, except for the methotrexate-conjugated dendrimers, which showed lower cytotoxicity due to their prolonged retention in the lysosome as a result of the abnormally slow release of MTX from the dendrimer conjugates. These studies also suggest that the ADCC effect of Tz would have contributed to the enhanced cytotoxicity of the drug-loaded dendrimer-mAb conjugates (Marcinkowska et al., 2019).
EGFR is another commonly used therapeutic target for mAb drugs. Including the FDA-approved mAb drug, Cetuximab, EGFR inhibitors have demonstrated clinical benefits in multiple types of cancer with wild-type KRAS (Petrelli et al., 2011). In the study by Barth and colleagues, heavily boronated G5 PAMAM dendrimers were conjugated with Cetuximab for site-specific neutron capture therapy (NCT) of brain tumors (G. Wu et al., 2004). An in vivo study using rats bearing F98 glioma cells transfected with the gene encoding EGFR (F98EGFR) demonstrated significantly enhanced accumulation and localization of the boronated dendrimers on F98EGFR compared to the boronated dendrimers without the mAb (92.3 ± 23.3 vs. 6.7 ± 3.6 μg B/g tumor). In a subsequent study, the boronated dendrimers were conjugated with antibody mixtures composed of Cetuximab and anti-EGFRvIII mAb (Yang et al., 2008). The mixture of the two mAb demonstrated ~2-fold enhanced boron accumulation in brain tumors compared to the boronated dendrimers conjugated with either of the mAbs alone. In addition to boronated dendrimers for NCT, the dendrimer-aEGFR Ab conjugates have been utilized in combination with DOTA moieties for radioimmunotherapy and adenovirus for gene therapy. (Wängler et al., 2008, p.) Furthermore, dendrimer-mAb conjugates have been investigated as therapeutic agents and carriers for different target molecules, including osteogenic sarcoma-associated antigens and mesothelin protein (N. K. Jain et al., 2015; Yoon et al., 2016).
This section described the use of mAbs that has emerged as effective targeting agents for many cancer types. They have found significant clinical relevance and are among the largest classes of new therapeutics approved for use in oncology. (Beck et al., 2017; Gül & Egmond, 2015; Weiner et al., 2009) Their efficacy, however, is limited by the number of antibodies bound to the surface of target cells (Wines et al., 2017). Therefore, dendrimers have been extensively utilized as mAb carriers to increase the interaction between antibodies and target proteins through the covalent conjugation of multiple antibodies on a dendrimer surface, resulting in significantly improved therapeutic effects by enhancing selective drug delivery to tumor cells. Although most of the dendrimer-mAb conjugates developed so far have been utilized as a vehicle for the targeted drug delivery, the ADCC effect of the dendrimer-conjugated antibodies can synergize the cytotoxicity of their payload drug (Marcinkowska et al., 2019). For this reason, the use of dendrimers has the potential to increase antibody-dependent cytotoxic effects due to an increased number of antibodies bound to the tumor surface, representing a class of promising carriers for the targeted treatment of multiple cancer types.
3.4. Immune Checkpoint Inhibitors
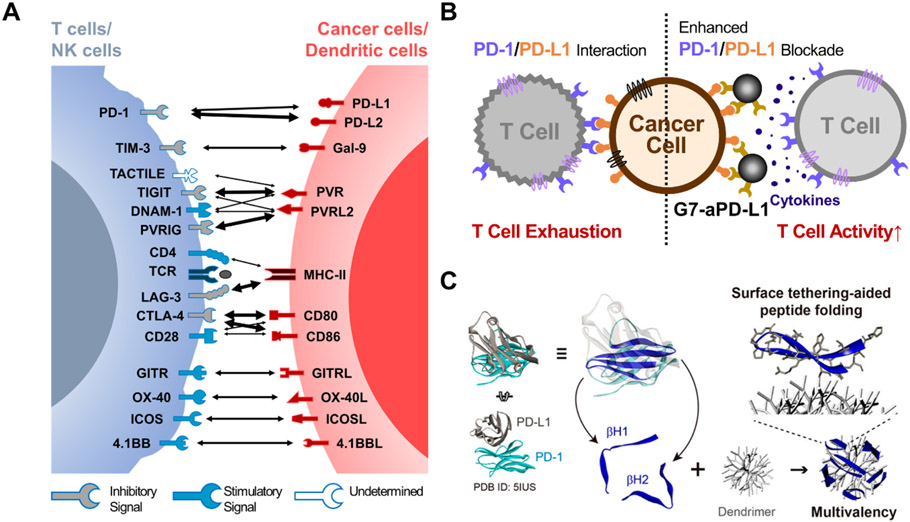
Tumor cells often escape from the host immune system by activating the immune checkpoint pathways that suppress immune cells’ anti-tumor functions (Whiteside, 2006). For example, the upregulation of PD-L1 allows cancer cells to evade immune surveillance by binding to PD-1 expressed on T cells (Pardoll, 2012; Ribas & Wolchok, 2018). The PD-1/PD-L1 interaction delivers immune-suppressive signals to T cells, suppressing cytokine production and inducing T cell apoptosis (Y. Wang et al., 2018, p. 1). ICIs are a type of therapeutic agent that blocks these immune-suppressive interactions by binding to checkpoint proteins on the surface of cancer cells or immune cells. To this end, various immune checkpoint proteins have been targeted (Figure 5A), including PD-1 (Ai et al., 2020), PD-L1 (Antonia et al., 2018), PD-L2 (Ahmad et al., 2018), cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) (Hodi et al., 2010), T cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif (ITIM) domains (TIGIT) (Solomon & Garrido-Laguna, 2018), and T cell immunoglobulin and mucin-domain containing-3 (TIM-3) (He et al., 2018). Most of these drugs are in the form of mAbs, including 7 FDA-approved mAb drugs, Ipilimumab (Yervoy®), Nivolumab (Opdivo®), Pembrolizumab (Keytruda®), cemiplimab (LIBTAYO®), Atezolizumab (Tecentriq®), Avelumab (Bavencio®), and Durvalumab (Imfinzi®) (Vaddepally et al., 2020). These drugs have been proven effective against a wide variety of tumor types by enhancing the anti-tumor activity of the immune cells. However, only a small subset of patients within a large cohort receiving the ICI treatment have shown durable responses (10–40%). (Borghaei et al., 2015; Ferris et al., 2016; Kowanetz et al., 2018) While various factors affect the sensitivity of ICIs, the redistribution of immune checkpoint molecules and insufficient target binding efficacy to mAb drugs are primarily attributed to the significant reduction in the ICI efficacies (Bu, Nair, Iida, et al., 2020; Kosmides et al., 2017; Ventola, 2017).
Figure 5:
Schematic illustration of the dendrimer-ICI conjugates. (a) Immune checkpoint molecules for cancer immunotherapy. The thickness of arrows is proportional to the reported binding affinities between the molecules. (b) Enhanced PD-1/PD-L1 blockade due to the integration of aPD-L1 antibodies to dendrimers. Reprint from Bu et al., 2020. (c) The conjugation of PD-1 derived peptide to dendrimers for PD-L1 blockade. Reprint from Jeong et al., 2020.
To this notion, the conjugation of ICIs to dendrimers could enhance the binding avidity towards checkpoint molecules on target cells and inhibit the redistribution of these molecules, which can increase the overall ICI efficacy. Our group has proven the utility and efficiency of this concept by conjugating ~3.8 anti-PD-L1 antibodies (aPD-L1) on the surface of a G7 PAMAM dendrimer (G7-aPD-L1) (Figure 5B). Surface plasmon resonance (SPR), biolayer interferometry (BLI), and atomic force spectroscopy (AFS) all demonstrated significantly decreased off-rate kinetics (thereby increasing binding strength) of the G7-aPD-L1 conjugates compared to the free aPD-L1, which was 1-2 order of magnitude stronger. The enhanced binding kinetics of the G7-aPD-L1 conjugates were successfully translated into in vitro efficiencies, demonstrating ~10-fold stronger adhesion as measured using a cell detachment assay, 1.35-fold increased T cell IL-2 secretion from a cancer cell/T cell co-culture assay, and 1.14-fold increased Doxorubicin (Dox) sensitivity in a chemo-cytotoxicity assay, compared to the free aPD-L1. In vivo experiments further indicated the high binding sensitivity and specificity of the G7-aPD-L1 conjugates as significantly more inhibitors were accumulated on the tumor regions than free aPD-L1. These results all support that the dendrimer-mediated multivalent binding effect could improve the inhibition of checkpoint molecules. (Bu, Lee, Jeong, et al., 2020)
The enhanced ICI efficacy due to the dendrimer-mediated multivalent binding effect is not limited to mAb drugs. As an alternative to mAbs, peptides have obtained great clinical interest due to their small size, protein (or antibody)-like functions, and high modularity. (Kosmides et al., 2017) The structural and biochemical analysis of the checkpoint proteins and their counter antibodies allowed the discovery and synthesis of peptides that can be utilized as ICIs (Maute et al., 2015; Pascolutti et al., 2016; X. Zhou et al., 2020). However, the therapeutic application of peptides has been hindered by their weak binding affinity toward their target checkpoint molecules compared to their whole mAb counterparts (Muguruma et al., 2013; X. Zhou et al., 2020). In this regard, incorporating peptides into dendrimers may increase their inhibitory effect and help avoid rapid renal clearance. Our group has incorporated ~30 β-hairpin peptides (pPD-1) isolated from a PD-1 protein for the blockade of PD-L1 on cancer cells (W.-J. Jeong et al., 2020) (Figure 5C). The dendrimer-peptide conjugates demonstrated 5 orders of magnitude increased binding avidity to PD-L1 compared to the free peptides, which is at the same order of magnitude in dissociation constants (KD) with their whole antibody counterparts (aPD-L1). This resulted in an enhanced PD-1/PD-L1 inhibitory effect in vitro by increasing the T cell cytokine production compared to the free pPD-1. In another study, Huang et al. designed a multifunctional nanoparticle that consists of siRNA-loaded, self-assembled dendrimers conjugated with TIGIT-targeting peptides (DTBP-3) on the dendrimer surface. The delivery of siRNA of the conjugates was confirmed by successful suppression of the target RNA, while inhibition of TIGIT using the dendrimer-conjugated DTBP-3 was observed by the increased number of CD54+/CD69+ NK cells and CD4+/CD8+ T cells in vivo. These findings demonstrated that dendrimers’ unique molecular structure allows for the delivery of siRNA and checkpoint inhibition simultaneously, synergizing the therapeutic effect of immunotherapy. (Roy & Li, 2016; T. Wang et al., 2021)
Given that tumors use immune checkpoint pathways to escape anti-tumor responses, the development of effective ICIs has been of great clinical interest. A number of clinical studies have demonstrated that ICIs can significantly enhance anti-tumor immunity. However, the effectiveness of ICIs is quite varied, as the redistribution of immune checkpoint molecules and insufficient target binding of ICIs to checkpoint molecules can decrease their efficacy. (Bu, Nair, Iida, et al., 2020; Kosmides et al., 2017; Ventola, 2017) Thus, the conjugation of ICIs to dendrimers has been explored in an effort to overcome these challenges and inhibit the immune checkpoint regulatory pathways more efficiently. The dendrimer-ICI conjugates have exhibited higher binding avidity and specificity towards the target molecules than free ICIs, which was translated into improved in vitro/in vivo efficiencies. These results suggest that the dendrimer-ICI conjugates have the potential to outperform conventional ICIs in human trials and find clinical success with the improvements in their designs and functionalities.
4. LIMITATIONS
While the use of dendrimers for therapeutic and biomedical applications is indeed promising, there are limitations regarding their usage in immunotherapies. As the dendrimer generation increases, so does its overall three-dimensional shape, which results in sizes comparable to bio-macromolecules, including proteins and DNA. As such, the kidneys will readily eliminate PAMAM dendrimers below G5 through renal filtration; however, for G5 and above, the dendrimers’ elimination rate will depend on hepatic clearance. (Moura et al., 2019) In addition, although cationic dendrimers—such as PAMAM and PPI—will more readily interact with the negatively charged surface of cell membranes in comparison to anionic or neutral dendrimers (Duncan & Izzo, 2005), they typically display a cytotoxic effect as the cationic dendrimers disrupt the cell membranes through various mechanisms including the formation of nanoscale holes (Hong et al., 2004, 2006). Furthermore, recent studies have suggested that the observed in-vivo toxicity may arise from induced mitochondrial dysfunction (Leiro et al., 2015), changes in endogenous gene expression (Leiro et al., 2015), and inflammatory response, (Breslow, 1977; Czarnomysy et al., 2019; Monteiller et al., 2007; Naha et al., 2010) which ultimately leads to non-specific cell death. Nevertheless, it has been reported that these cytotoxicity effects can be overcome by functionalizing bioactive molecules to the surface of the cationic dendrimers to negate their surface charge. Examples of such functionalization include surface PEGylation and the introduction of hydroxyl, carboxyl, and acetyl groups. (L. Wu et al., 2015; P. Zhou et al., 2021) The development and utilization of inert dendrimers that can be excreted or eliminated under natural physiologic conditions may also overcome these cytotoxic effects and be utilized as an effective drug delivery system (Leiro et al., 2015; McNerny et al., 2010).
Specifically for the dendrimer-based vaccine systems, many of the systems developed so far have not yet been optimized for introduction into clinical settings. Several studies have shown that the tested systems were not sufficient to protect individuals from subsequent challenges and only induced ephemeral immunity effects. (Daftarian et al., 2011; K.-W. Huang et al., 2020; Yan et al., 2015) Also, regardless of the type of dendrimer, the scaffolds had to undergo additional conjugation with functional groups or be paired with checkpoint inhibitors to ensure successful cancer inhibition (Blankenstein et al., 2012; Horvath et al., 2002; Kumar et al., 1992; Vichier-guerre et al., 2000; Xu et al., 2019). Finally, there are some concerns with the translatability of the dendrimer-based vaccine systems. Multiple studies which looked at the design and development of dendrimer-based vaccine systems have only been performed using in vitro or animal models, and as a result, it is impossible to extrapolate these results to humans. (Santos et al., 2019) Therefore, additional research is needed to understand further the biological mechanisms that underlie cancer immunity and the biocompatibility and functionalized design of the dendrimers themselves.
Similarly, dendrimer-mAb conjugates (or dendrimer-ICI conjugates) have drawbacks, mainly stemming from binding strength and selectivity issues, limiting their applications in cancer immunotherapy. Several factors are known to affect the binding behaviors of the dendrimer-conjugated mAbs, including orientation issues, chemical modifications on receptor binding sites, back-folding of intermediate linkers, and steric hindrance. Accumulating evidence suggests that mAbs immobilized on a solid surface exhibit lower binding avidity than those in solution due to their random orientation on the surface and chemical modification on Fab regions. In addition, when using a long intermediate linker (e.g., PEG2k chains) to control the hydrophobicity, size, cytotoxicity, and surface charge of dendrimers, there tends to be a reduction in the number of mAb binding sites on the PEGylated dendrimers due to the back-folding of the linker. (H.-J. Hsu et al., 2018; Pearson et al., 2016; Somani et al., 2018) The reactive peripheral amino groups may be concealed inside the dendrimer core, hindering the conjugation between dendrimer and mAb (H. Hsu et al., 2014). Furthermore, steric hindrance between the mAbs on a dendrimer may limit the binding of mAbs to a target protein. When neighboring mAbs on dendrimer surfaces are too close to each other, the receptor binding sites on Fab regions do not have sufficient space to interact with their target receptor, which reduces the overall binding efficiency of the dendrimer-mAb conjugates. Different strategies have been developed to conjugate antibodies to solid surfaces to ameliorate these limitations, and a majority of these strategies are applicable to the dendrimer-mAb conjugates. The orientation of mAbs can be controlled, and Fab regions can be protected by utilizing intermediate proteins (i.e., Protein A) between the dendrimer and mAbs (Snopok et al., 2006), chemically modifying specific part of the Fc region (Cho et al., 2007; J. H. Kang et al., 2007), and reducing the heavy chain disulfide bonds to use as a conjugation site for the dendrimers (Poellmann et al., 2020). Furthermore, utilizing an appropriate intermediate linker and optimizing the ratio between the targeting moieties per dendrimer are known to reduce the steric hindrance and increase the accessibility of the ligands while preventing the folding of a polymer (Chen et al., 2019; de Gennes, 2003; Steinmetz & Manchester, 2009). Overall, these strategies allow for the enhancement of the design of selective conjugates and have the potential to be successfully used in the clinic for targeted cancer therapy.
5. FUTURE PROSPECTS
The currently available FDA-approved mono-immunotherapies are paradigm-shifting innovations but have been shown to be effective only for a small subset of cancer patients. For example, PD-1/PD-L1 antagonists have been reported to be only effective for 10–20% of patients with advanced-stage non-small-cell lung carcinoma (NSCLC). (Kerr, 2018; Kowanetz et al., 2018) One of the strategies that have been utilized to improve the efficacy of currently available treatments is to combine immunotherapies with other treatment modalities such as chemotherapy, gene therapy, and other immunotherapeutic agents (Drake, 2012; M. Liu et al., 2021). West et al. reported that Atezolizumab, an aPD-L1 antibody utilized as an immunotherapeutic for various types of cancer, exhibited an objective response rate (ORR) of up to 68% when combined with carboplatin and paclitaxel, pemetrexed, or nab-paclitaxel (S. V. Liu et al., 2018). Likewise, combination treatment with IL-2 and gp100 peptide vaccine improved the response rate of stage III/IV melanoma patients compared to IL-2 monotherapy.(Schwartzentruber et al., 2011)
To this notion, there are significant opportunities for the improvement of current dendrimer-based immunotherapeutics, which include the delivery of multiple therapeutic agents to the cancer cells and/or immune cells by integrating different therapeutic modalities into a single nano-drug system. Taking advantage of the multivalent binding effect, dendrimers can be utilized as a scaffold to deliver different therapeutic agents by simultaneously targeting multiple cancer signaling pathways. For example, dendrimer-ICI conjugates, which can target multiple immune checkpoint molecules on T cells (i.e., dendrimer-CTLA-4/PD-1 conjugates), cancer cells (i.e., dendrimer-PD-L1/PD-L2), or both T and cancer cells (i.e., dendrimer-PD-1/PD-L1), can be synergized to enhance anti-tumor immunity. In addition, the formulation of immune checkpoint blocking siRNA or mRNA/peptide vaccines with the inclusion of ICIs on a dendrimer surface may enable the targeting of specific immune cells, thereby providing a robust anti-cancer response. Chemo-drugs can also be co-conjugated, further improving anti-tumor cytotoxicity. These technologies may significantly impact the current standard of immunotherapy and contribute to personalized cancer treatment with improved therapeutic efficacy.
6. CONCLUSION
For the past several decades, dendrimers have been utilized as a nanocarrier for the delivery of immunotherapeutic agents in order to better modulate the immune system and improve upon current therapeutics. A large number of binding sites on their peripheries allow for the surface conjugation of various therapeutic agents, including mAbs, peptides, plasmids, and other adjuvants. Furthermore, the hydrophobic inner shells of dendrimers enable the loading of various chemo-drugs and imaging agents, which can bolster the therapeutic effect of dendrimer-based immunotherapy. Based on these properties, various dendrimer-based immunotherapy formulations have been developed which can modulate cytokine secretion (adjuvants), activate the immune system to recognize the tumor antigens (therapeutic vaccines), stimulate immune effectors to lysis cancer cells expressing specific antigens (mAbs), and block ICIs. Recent advances in dendrimer research and the growing need for combination immunotherapy are now driving the development of multifunctional nanoparticles which integrate multiple therapeutic modalities.
Including these new applications, the expansion of dendrimer-based immunotherapy platforms can significantly improve the therapeutic index of current cancer immunotherapies and, eventually, clinical outcomes. The in-depth observations of dendrimer characteristics and in vitro and in vivo validation of their therapeutic efficacies have allowed for dendrimers to be employed as successful immunotherapeutic nanomedicine excipients. Although dendrimer-based nanomedicines are not yet realized in the clinic, additional improvements in their formulation and design will enable them to overcome translational obstacles and advance to clinical trials. Thus, the utilization of dendrimer-based technologies has the potential to overcome the current challenges associated with cancer immunotherapies and shift the paradigm of current cancer immunotherapy.
Table 1:
Cytokine-based immunotherapy via dendrimer nanoparticles
| Dendrimer | Surface group/Ligand |
Cytokine- expressing Immune Cell |
Cytokine | Ref. |
|---|---|---|---|---|
| PAMAM-NH2 G5 | IL-3 | - | IL-3↑ | Lee 2004 |
| PAMAM-NH2 G5 | pTRAIL | - | TRAIL↑ | Han 2011; Huang 2011; Gao 2015 |
| Dendrigraft poly-L-lysine G3 | pTRAIL | - | TRAIL↑ | Li 2013 |
| PAMAM-NH2 G4 | pIL-2 | - | IL-2↑ | Huang 2020 |
| Polyman26 | mannose | DC | β-chemokine↑, IL-1β↑, IL-6↑, IL-12↑, TNFα↑ | Berzi 2016 |
| carbosilane (2G-03NN24) | - | macrophage | M1 polarization↑, M2 polarization↓ | Perisé-Barrios 2015 |
MDDC: monocytes-derived dendritic cells; TAM: tumor-associated macrophages; PBMC: peripheral blood mononuclear cells; MDM: monocytederived macrophages; DC: dendritic cells.
Acknowledgments
The authors thank NSF DMR-1808251, Catalyst Award from Falk Medical Research Trust, DRP Award from UW Head and Neck SPORE (P50-DE026787), SEED D2P funds, and Milton J. Henrichs Fund.
Footnotes
The author declares no potential conflict of interest
References
- Abbasi E, Aval SF, Akbarzadeh A, Milani M, Nasrabadi HT, Joo SW, Hanifehpour Y, Nejati-Koshki K, & Pashaei-Asl R (2014). Dendrimers: Synthesis, applications, and properties. Nanoscale Research Letters, 9(1), 247. 10.1186/1556-276X-9-247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ada G (2003). Overview of Vaccines. In Robinson A, Hudson MJ, & Cranage MP (Eds.), Vaccine Protocols (pp. 1–17). Humana Press. 10.1385/1-59259-399-2:1 [DOI] [Google Scholar]
- Ahmad SM, Martinenaite E, Holmström M, Jørgensen MA, Met Ö, Nastasi C, Klausen U, Donia M, Pedersen LM, Munksgaard L, Ødum N, Woetmann A, Svane IM, & Andersen MH (2018). The inhibitory checkpoint, PD-L2, is a target for effector T cells: Novel possibilities for immune therapy. Oncoimmunology, 7(2), e1390641. 10.1080/2162402X.2017.1390641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai L, Xu A, & Xu J (2020). Roles of PD-1/PD-L1 Pathway: Signaling, Cancer, and Beyond. Advances in Experimental Medicine and Biology, 1248, 33–59. 10.1007/978-981-15-3266-5_3 [DOI] [PubMed] [Google Scholar]
- Aleanizy FS, Alqahtani FY, Seto S, Al Khalil N, Aleshaiwi L, Alghamdi M, Alquadeib B, Alkahtani H, Aldarwesh A, Alqahtani QH, Abdelhady HG, & Alsarra I (2020). Trastuzumab Targeted Neratinib Loaded Poly-Amidoamine Dendrimer Nanocapsules for Breast Cancer Therapy. International Journal of Nanomedicine, 15, 5433–5443. 10.2147/IJN.S256898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH, Zhu L, Grosso JF, Kim SY, Timmerman JM, Shipp MA, & Armand P (2015). PD-1 Blockade with Nivolumab in Relapsed or Refractory Hodgkin’s Lymphoma. New England Journal of Medicine, 372(4), 311–319. 10.1056/NEJMoa1411087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim Y-C, Karapetis CS, Hiret S, … Özgüroğlu M (2018). Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. New England Journal of Medicine, 379(24), 2342–2350. 10.1056/NEJMoa1809697 [DOI] [PubMed] [Google Scholar]
- Bahadoran A, Ebrahimi M, Yeap SK, Safi N, Moeini H, Bejo MH, Hussein MZ, & Omar AR (2017). Induction of a robust immune response against avian influenza virus following transdermal inoculation with H5-DNA vaccine formulated in modified dendrimer-based delivery system in mouse model. International Journal of Nanomedicine, Volume 12, 8573–8585. 10.2147/IJN.S139126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, & Palucka K (2018). Cancer vaccines on the move. Nature Reviews Clinical Oncology, 15(1), 9–10. 10.1038/nrclinonc.2017.149 [DOI] [PubMed] [Google Scholar]
- Bay S, Lo-Man R, Osinaga E, Nakada H, Leclerc C, & Cantacuzène D (1997). Preparation of a multiple antigen glycopeptide (MAG) carrying the Tn antigen. A possible approach to a synthetic carbohydrate vaccine. The Journal of Peptide Research: Official Journal of the American Peptide Society, 49(6), 620–625. 10.1111/j.1399-3011.1997.tb01171.x [DOI] [PubMed] [Google Scholar]
- Beck A, Goetsch L, Dumontet C, & Corvaïa N (2017). Strategies and challenges for the next generation of antibody–drug conjugates. Nature Reviews Drug Discovery, 16(5), 315–337. 10.1038/nrd.2016.268 [DOI] [PubMed] [Google Scholar]
- de B. den Berg EMM, & Meijer EW (1993). Poly(propylene imine) Dendrimers: Large-Scale Synthesis by Hetereogeneously Catalyzed Hydrogenations. Angewandte Chemie International Edition in English, 32(9), 1308–1311. 10.1002/anie.199313081 [DOI] [Google Scholar]
- Berraondo P, Sanmamed MF, Ochoa MC, Etxeberria I, Aznar MA, Pérez-Gracia JL, Rodrínguez-Ruiz ME, Ponz-Sarvise M, Castañón E, & Melero I (2019). Cytokines in clinical cancer immunotherapy. British Journal of Cancer, 120(1), 6–15. 10.1038/s41416-018-0328-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzi A, Ordanini S, Joosten B, Trabattoni D, Cambi A, Bernardi A, & Clerici M (2016). Pseudo-Mannosylated DC-SIGN Ligands as Immunomodulants. Scientific Reports, 6(1), 35373. 10.1038/srep35373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenstein T, Coulie PG, Gilboa E, & Jaffee EM (2012). The determinants of tumour immunogenicity. Nature Reviews. Cancer, 12(4), 307–313. 10.1038/nrc3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, … Brahmer JR (2015). Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. New England Journal of Medicine, 373(17), 1627–1639. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Arén Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, … Spigel DR (2015). Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. The New England Journal of Medicine, 373(2), 123–135. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow DS (1977). Biologically Active Synthetic Polymers. In Macromolecular Chemistry–11 (pp. 103–113). Elsevier. 10.1016/B978-0-08-020975-3.50005-0 [DOI] [Google Scholar]
- Bu J, Lee TH, Jeong W, Poellmann MJ, Mudd K, Eun HS, Liu EW, Hong S, & Hyun SH (2020). Enhanced detection of cell-free DNA (cfDNA) enables its use as a reliable biomarker for diagnosis and prognosis of gastric cancer. PLOS ONE, 15(12), e0242145. 10.1371/journal.pone.0242145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu J, Nair A, Iida M, Jeong W, Poellmann MJ, Mudd K, Kubiatowicz LJ, Liu EW, Wheeler DL, & Hong S (2020). An Avidity-Based PD-L1 Antagonist Using Nanoparticle-Antibody Conjugates for Enhanced Immunotherapy. Nano Letters, 20(7), 4901–4909. 10.1021/acs.nanolett.0c00953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu J, Nair A, Kubiatowicz LJ, Poellmann MJ, Jeong W-J, Reyes-Martinez M, Armstrong AJ, George DJ, Wang AZ, Zhang T, & Hong S (2020). Surface engineering for efficient capture of circulating tumor cells in renal cell carcinoma: From nanoscale analysis to clinical application. Biosensors & Bioelectronics, 162, 112250. 10.1016/j.bios.2020.112250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugno J, Hsu H, & Hong S (2015a). Recent advances in targeted drug delivery approaches using dendritic polymers. Biomaterials Science, 3(7), 1025–1034. 10.1039/C4BM00351A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugno J, Hsu H-J, & Hong S (2015b). Tweaking Dendrimers and Dendritic Nanoparticles for Controlled Nano-bio Interactions: Potential Nanocarriers for Improved Cancer Targeting. Journal of Drug Targeting, 23(7–8), 642–650. 10.3109/1061186X.2015.1052077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminade A-M, Turrin C-O, Laurent R, Ouali A, & Delavaux-Nicot B (2011). Dendrimers: Towards Catalytic, Material and Biomedical Uses. John Wiley & Sons, Incorporated. http://ebookcentral.proquest.com/lib/wisc/detail.action?docID=697617 [Google Scholar]
- Carreno BM, Magrini V, Becker-Hapak M, Kaabinejadian S, Hundal J, Petti AA, Ly A, Lie W-R, Hildebrand WH, Mardis ER, & Linette GP (2015). A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science, 348(6236), 803–808. 10.1126/science.aaa3828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Yu S, Zhang D, Zhang W, Zhang H, Zou J, Mao Z, Yuan Y, Gao C, & Liu R (2019). Impact of Antifouling PEG Layer on the Performance of Functional Peptides in Regulating Cell Behaviors. Journal of the American Chemical Society, 141(42), 16772–16780. 10.1021/jacs.9b07105 [DOI] [PubMed] [Google Scholar]
- Cheng Y, & Burda C (2011). 2.01—Nanoparticles for Photodynamic Therapy. In Andrews DL, Scholes GD, & Wiederrecht GP (Eds.), Comprehensive Nanoscience and Technology (pp. 1–28). Academic Press. 10.1016/B978-0-12-374396-1.00071-4 [DOI] [Google Scholar]
- Chiu ML, Goulet DR, Teplyakov A, & Gilliland GL (2019). Antibody Structure and Function: The Basis for Engineering Therapeutics. Antibodies, 8(4), 55. 10.3390/antib8040055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho I-H, Paek E-H, Lee H, Kang JY, Kim TS, & Paek S-H (2007). Site-directed biotinylation of antibodies for controlled immobilization on solid surfaces. Analytical Biochemistry, 365(1), 14–23. 10.1016/j.ab.2007.02.028 [DOI] [PubMed] [Google Scholar]
- Czarnomysy R, Bielawska A, & Bielawski K (2019). Effect of 2nd and 3rd generation PAMAM dendrimers on proliferation, differentiation, and pro-inflammatory cytokines in human keratinocytes and fibroblasts. International Journal of Nanomedicine, 14, 7123–7139. 10.2147/IJN.S211682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daftarian P, Kaifer AE, Li W, Blomberg BB, Frasca D, Roth F, Chowdhury R, Berg EA, Fishman JB, Sayegh HAA, Blackwelder P, Inverardi L, Perez VL, Lemmon V, & Serafini P (2011). Peptide-Conjugated PAMAM Dendrimer as a Universal DNA Vaccine Platform to Target Antigen-Presenting Cells. Cancer Research, 71(24), 7452–7462. 10.1158/0008-5472.CAN-11-1766 [DOI] [PubMed] [Google Scholar]
- de Gennes PG (2003). Polymers at an Interface: A Simplified View. Simple Views on Condensed Matter (3rd Edition). Edited by DE GENNES PIERRE-GILLES. Published by World Scientific Publishing Co. Pte. Ltd., 2003. ISBN #9789812564849, Pp. 270–291, 270–291. 10.1142/9789812564849_0031 [DOI] [Google Scholar]
- de Oliveira E, Villén J, Giralt E, & Andreu D (2003). Synthetic Approaches to Multivalent Lipopeptide Dendrimers Containing Cyclic Disulfide Epitopes of Foot-and-Mouth Disease Virus. Bioconjugate Chemistry, 14(1), 144–152. 10.1021/bc025577f [DOI] [PubMed] [Google Scholar]
- Deci MB, Liu M, Dinh QT, & Nguyen J (2018). Precision engineering of targeted nanocarriers. WIREs Nanomedicine and Nanobiotechnology, 10(5), e1511. 10.1002/wnan.1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirix LY, Takacs I, Jerusalem G, Nikolinakos P, Arkenau H-T, Forero-Torres A, Boccia R, Lippman ME, Somer R, Smakal M, Emens LA, Hrinczenko B, Edenfield W, Gurtler J, von Heydebreck A, Grote HJ, Chin K, & Hamilton EP (2018). Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: A phase 1b JAVELIN Solid Tumor study. Breast Cancer Research and Treatment, 167(3), 671–686. 10.1007/s10549-017-4537-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CG (2012). Combination immunotherapy approaches. Annals of Oncology: Official Journal of the European Society for Medical Oncology, 23 Suppl 8, viii41–46. 10.1093/annonc/mds262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Q, Zhang H, Zheng J, & Zhang L (2020). Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends in Cancer, 6(7), 605–618. 10.1016/j.trecan.2020.02.022 [DOI] [PubMed] [Google Scholar]
- Duncan R, & Izzo L (2005). Dendrimer biocompatibility and toxicity. Advanced Drug Delivery Reviews, 57(15), 2215–2237. 10.1016/j.addr.2005.09.019 [DOI] [PubMed] [Google Scholar]
- Dunn GP, Old LJ, & Schreiber RD (2004). The Three Es of Cancer Immunoediting. Annual Review of Immunology, 22(1), 329–360. 10.1146/annurev.immunol.22.012703.104803 [DOI] [PubMed] [Google Scholar]
- Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, Monga M, Lynch M, … Gillison ML (2016). Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. New England Journal of Medicine, 375(19), 1856–1867. 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, & Taguchi H (2011). Current status of multiple antigen-presenting peptide vaccine systems: Application of organic and inorganic nanoparticles. Chemistry Central Journal, 5(1), 48. 10.1186/1752-153X-5-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galon J, & Bruni D (2019). Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nature Reviews Drug Discovery, 18(3), 197–218. 10.1038/s41573-018-0007-y [DOI] [PubMed] [Google Scholar]
- Gao S, Li J, Jiang C, Hong B, & Hao B (2015). Plasmid pORF-hTRAIL targeting to glioma using transferrin-modified polyamidoamine dendrimer. Drug Design, Development and Therapy, 10, 1–11. 10.2147/DDDT.S95843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MS (2015). Immunoengineering: How nanotechnology can enhance cancer immunotherapy. Cell, 161(2), 201–204. 10.1016/j.cell.2015.03.037 [DOI] [PubMed] [Google Scholar]
- Gómez Román VR, Murray JC, & Weiner LM (2014). Chapter 1—Antibody-Dependent Cellular Cytotoxicity (ADCC). In Ackerman ME & Nimmerjahn F (Eds.), Antibody Fc (pp. 1–27). Academic Press. 10.1016/B978-0-12-394802-1.00001-7 [DOI] [Google Scholar]
- Guermonprez P, Saveanu L, Kleijmeer M, Davoust J, van Endert P, & Amigorena S (2003). ER–phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature, 425(6956), 397–402. 10.1038/nature01911 [DOI] [PubMed] [Google Scholar]
- Guimarães PPG, Gaglione S, Sewastianik T, Carrasco RD, Langer R, & Mitchell MJ (2018). Nanoparticles for Immune Cytokine TRAIL-Based Cancer Therapy. ACS Nano, 12(2), 912–931. 10.1021/acsnano.7b05876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gül N, & Egmond M van. (2015). Antibody-Dependent Phagocytosis of Tumor Cells by Macrophages: A Potent Effector Mechanism of Monoclonal Antibody Therapy of Cancer. Cancer Research, 75(23), 5008–5013. 10.1158/0008-5472.CAN-15-1330 [DOI] [PubMed] [Google Scholar]
- Guo Z, & Wang Q (2009). Recent Development in Carbohydrate-Based Cancer Vaccines. Current Opinion in Chemical Biology, 13(5–6), 608–617. 10.1016/j.cbpa.2009.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V, & Nayak S (2015). Dendrimers: A Review on Synthetic Approaches. Journal of Applied Pharmaceutical Science, 117–122. 10.7324/JAPS.2015.50321 [DOI] [Google Scholar]
- Han L, Huang R, Li J, Liu S, Huang S, & Jiang C (2011). Plasmid pORF-hTRAIL and doxorubicin co-delivery targeting to tumor using peptide-conjugated polyamidoamine dendrimer. Biomaterials, 32(4), 1242–1252. 10.1016/j.biomaterials.2010.09.070 [DOI] [PubMed] [Google Scholar]
- Hao Y, Zhou X, Li R, Song Z, & Min Y (2020). Advances of functional nanomaterials for cancer immunotherapeutic applications. WIREs Nanomedicine and Nanobiotechnology, 12(2), e1574. 10.1002/wnan.1574 [DOI] [PubMed] [Google Scholar]
- Hawker CJ, & Frechet JMJ (1990). Preparation of polymers with controlled molecular architecture. A new convergent approach to dendritic macromolecules. Journal of the American Chemical Society, 112(21), 7638–7647. 10.1021/ja00177a027 [DOI] [Google Scholar]
- He Y, Cao J, Zhao C, Li X, Zhou C, & Hirsch FR (2018). TIM-3, a promising target for cancer immunotherapy. OncoTargets and Therapy, 11, 7005–7009. 10.2147/OTT.S170385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heegaard PMH, Boas U, & Sorensen NS (2010). Dendrimers for Vaccine and Immunostimulatory Uses. A Review. Bioconjugate Chemistry, 21(3), 405–418. 10.1021/bc900290d [DOI] [PubMed] [Google Scholar]
- Hodge P (1993). Polymer science branches out. Nature, 362(6415), 18–19. 10.1038/362018a0 [DOI] [Google Scholar]
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJM, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, … Urba WJ (2010). Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. New England Journal of Medicine, 363(8), 711–723. 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth RE, & Jansen K (2019). Turning the corner on therapeutic cancer vaccines. Npj Vaccines, 4(1), 1–10. 10.1038/s41541-019-0103-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Bielinska AU, Mecke A, Keszler B, Beals JL, Shi X, Balogh L, Orr BG, Baker JR, & Banaszak Holl MM (2004). Interaction of Poly(amidoamine) Dendrimers with Supported Lipid Bilayers and Cells: Hole Formation and the Relation to Transport. Bioconjugate Chemistry, 15(4), 774–782. 10.1021/bc049962b [DOI] [PubMed] [Google Scholar]
- Hong S, Leroueil PR, Janus EK, Peters JL, Kober M-M, Islam MT, Orr BG, Baker JR, & Banaszak Holl MM (2006). Interaction of Polycationic Polymers with Supported Lipid Bilayers and Cells: Nanoscale Hole Formation and Enhanced Membrane Permeability. Bioconjugate Chemistry, 17(3), 728–734. 10.1021/bc060077y [DOI] [PubMed] [Google Scholar]
- Hong S, Leroueil PR, Majoros IJ, Orr BG, Baker JR, & Banaszak Holl MM (2007). The Binding Avidity of a Nanoparticle-Based Multivalent Targeted Drug Delivery Platform. Chemistry & Biology, 14(1), 107–115. 10.1016/j.chembiol.2006.11.015 [DOI] [PubMed] [Google Scholar]
- Horvath A, Olive C, Wong A, Clair T, Yarwood P, Good M, & Toth I (2002). Lipoamino acid-based adjuvant carrier system: Enhanced immunogenicity of group a streptococcal peptide epitopes. Journal of Medicinal Chemistry, 45(6). 10.1021/jm0110441 [DOI] [PubMed] [Google Scholar]
- Hsu H, Sen S, Pearson RM, Uddin S, Král P, & Hong S (2014). Poly(ethylene glycol) Corona Chain Length Controls End-Group-Dependent Cell Interactions of Dendron Micelles. Macromolecules, 47(19), 6911–6918. 10.1021/ma501258c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H-J, Bugno J, Lee S, & Hong S (2017). Dendrimer-based nanocarriers: A versatile platform for drug delivery. WIREs Nanomedicine and Nanobiotechnology, 9(1), e1409. 10.1002/wnan.1409 [DOI] [PubMed] [Google Scholar]
- Hsu H-J, Han Y, Cheong M, Kral P, & Hong S (2018). Dendritic PEG outer shells enhance serum stability of polymeric micelles. Nanomedicine: Nanotechnology, Biology, and Medicine, 14(6), 1879–1889. 10.1016/j.nano.2018.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K-W, Hsu F-F, Qiu JT, Chern G-J, Lee Y-A, Chang C-C, Huang Y-T, Sung Y-C, Chiang C-C, Huang R-L, Lin C-C, Dinh TK, Huang H-C, Shih Y-C, Alson D, Lin C-Y, Lin Y-C, Chang P-C, Lin S-Y, & Chen Y (2020). Highly efficient and tumor-selective nanoparticles for dual-targeted immunogene therapy against cancer. Science Advances, 6(3), eaax5032. 10.1126/sciadv.aax5032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X-J, Lü X, Lei Y-F, Yang J, Yao M, Lan H-Y, Zhang J-M, Jia Z-S, Yin W, & Xu Z-K (2013). Cellular immunogenicity of a multi-epitope peptide vaccine candidate based on hepatitis C virus NS5A, NS4B and core proteins in HHD-2 mice. Journal of Virological Methods, 189(1), 47–52. 10.1016/j.jviromet.2013.01.003 [DOI] [PubMed] [Google Scholar]
- Igietseme JU, Zhu X, & Black CM (2014). Chapter 15—Fc Receptor-Dependent Immunity. In Ackerman ME & Nimmerjahn F (Eds.), Antibody Fc (pp. 269–281). Academic Press. 10.1016/B978-0-12-394802-1.00015-7 [DOI] [Google Scholar]
- Jain K (2017). 7 - Dendrimers: Smart nanoengineered polymers for bioinspired applications in drug delivery. In Jana S, Maiti S, & Jana S (Eds.), Biopolymer-Based Composites (pp. 169–220). Woodhead Publishing. 10.1016/B978-0-08-101914-6.00007-7 [DOI] [Google Scholar]
- Jain NK, Tare MS, Mishra V, & Tripathi PK (2015). The development, characterization and in vivo anti-ovarian cancer activity of poly(propylene imine) (PPI)-antibody conjugates containing encapsulated paclitaxel. Nanomedicine: Nanotechnology, Biology and Medicine, 11(1), 207–218. 10.1016/j.nano.2014.09.006 [DOI] [PubMed] [Google Scholar]
- Jeong W, Bu J, Kubiatowicz LJ, Chen SS, Kim Y, & Hong S (2018). Peptide–nanoparticle conjugates: A next generation of diagnostic and therapeutic platforms? Nano Convergence, 5(1), 38. 10.1186/s40580-018-0170-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong W-J, Bu J, Han Y, Drelich AJ, Nair A, Král P, & Hong S (2020). Nanoparticle Conjugation Stabilizes and Multimerizes β-Hairpin Peptides To Effectively Target PD-1/PD-L1 β-Sheet-Rich Interfaces. Journal of the American Chemical Society, 142(4), 1832–1837. 10.1021/jacs.9b10160 [DOI] [PubMed] [Google Scholar]
- Joffre OP, Segura E, Savina A, & Amigorena S (2012). Cross-presentation by dendritic cells. Nature Reviews Immunology, 12(8), 557–569. 10.1038/nri3254 [DOI] [PubMed] [Google Scholar]
- Kang JH, Choi HJ, Hwang SY, Han SH, Jeon JY, & Lee EK (2007). Improving immunobinding using oriented immobilization of an oxidized antibody. Journal of Chromatography. A, 1161(1–2), 9–14. 10.1016/j.chroma.2007.05.023 [DOI] [PubMed] [Google Scholar]
- Kang TH, & Jung ST (2019). Boosting therapeutic potency of antibodies by taming Fc domain functions. Experimental & Molecular Medicine, 51(11), 1–9. 10.1038/s12276-019-0345-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann SHE (2007). The contribution of immunology to the rational design of novel antibacterial vaccines. Nature Reviews Microbiology, 5(7), 491–504. 10.1038/nrmicro1688 [DOI] [PubMed] [Google Scholar]
- Kennedy LB, & Salama AKS (2020). A review of cancer immunotherapy toxicity. CA: A Cancer Journal for Clinicians, 70(2), 86–104. 10.3322/caac.21596 [DOI] [PubMed] [Google Scholar]
- Kerr KM (2018). The PD-L1 Immunohistochemistry Biomarker: Two Steps Forward, One Step Back? Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer, 13(3), 291–294. 10.1016/j.jtho.2018.01.020 [DOI] [PubMed] [Google Scholar]
- Kesharwani P, Jain K, & Jain NK (2014). Dendrimer as nanocarrier for drug delivery. Progress in Polymer Science, 39(2), 268–307. 10.1016/j.progpolymsci.2013.07.005 [DOI] [Google Scholar]
- Kosmides AK, Sidhom J-W, Fraser A, Bessell CA, & Schneck JP (2017). Dual Targeting Nanoparticle Stimulates the Immune System To Inhibit Tumor Growth. ACS Nano, 11(6), 5417–5429. 10.1021/acsnano.6b08152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowanetz M, Zou W, Gettinger SN, Koeppen H, Kockx M, Schmid P, Kadel EE, Wistuba I, Chaft J, Rizvi NA, Spigel DR, Spira A, Hirsch FR, Cohen V, Smith D, Boyd Z, Miley N, Flynn S, Leveque V, … Hegde PS (2018). Differential regulation of PD-L1 expression by immune and tumor cells in NSCLC and the response to treatment with atezolizumab (anti–PD-L1). Proceedings of the National Academy of Sciences, 115(43), E10119–E10126. 10.1073/pnas.1802166115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulhari H, Pooja D, Shrivastava S, Kuncha M, Naidu VGM, Bansal V, Sistla R, & Adams DJ (2016). Trastuzumab-grafted PAMAM dendrimers for the selective delivery of anticancer drugs to HER2-positive breast cancer. Scientific Reports, 6(1), 23179. 10.1038/srep23179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Arora R, Kaur P, Chauhan VS, & Sharma P (1992). “Universal” T helper cell determinants enhance immunogenicity of a Plasmodium falciparum merozoite surface antigen peptide. Journal of Immunology (Baltimore, Md. : 1950), 148(5). http://pubmed.ncbi.nlm.nih.gov/1371529/ [PubMed] [Google Scholar]
- Lalani J, & Misra A (2011). Gene Delivery Using Chemical Methods. In Misra A (Ed.), Challenges in Delivery of Therapeutic Genomics and Proteomics (pp. 127–206). Elsevier. 10.1016/B978-0-12-384964-9.00004-9 [DOI] [Google Scholar]
- Lee SC, Parthasarathy R, Botwin K, Kunneman D, Rowold E, Lange G, Klover J, Abegg A, Zobel J, Beck T, Miller T, Hood W, Monahan J, McKearn JP, Jansson R, & Voliva CF (2004). Biochemical and immunological properties of cytokines conjugated to dendritic polymers. Biomedical Microdevices, 6(3), 191–202. 10.1023/B:BMMD.0000042048.18186.ff [DOI] [PubMed] [Google Scholar]
- Lee S, & Margolin K (2011). Cytokines in Cancer Immunotherapy. Cancers, 3(4), 3856–3893. 10.3390/cancers3043856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiro V, Garcia JP, Tomás H, & Pêgo AP (2015). The Present and the Future of Degradable Dendrimers and Derivatives in Theranostics. Bioconjugate Chemistry, 26(7), 1182–1197. 10.1021/bc5006224 [DOI] [PubMed] [Google Scholar]
- Lesterhuis WJ, Haanen JBAG, & Punt CJA (2011). Cancer immunotherapy – revisited. Nature Reviews Drug Discovery, 10(8), 591–600. 10.1038/nrd3500 [DOI] [PubMed] [Google Scholar]
- Li J, Guo Y, Kuang Y, An S, Ma H, & Jiang C (2013). Choline transporter-targeting and co-delivery system for glioma therapy. Biomaterials, 34(36), 9142–9148. 10.1016/j.biomaterials.2013.08.030 [DOI] [PubMed] [Google Scholar]
- Liu M, Li L, Jin D, & Liu Y (2021). Nanobody—A versatile tool for cancer diagnosis and therapeutics. WIREs Nanomedicine and Nanobiotechnology, 13(4), e1697. 10.1002/wnan.1697 [DOI] [PubMed] [Google Scholar]
- Liu SV, Camidge DR, Gettinger SN, Giaccone G, Heist RS, Hodi FS, Ready NE, Zhang W, Wallin J, Funke R, Waterkamp D, Foster P, Iizuka K, & Powderly J (2018). Long-term survival follow-up of atezolizumab in combination with platinum-based doublet chemotherapy in patients with advanced non–small-cell lung cancer. European Journal of Cancer, 101, 114–122. 10.1016/j.ejca.2018.06.033 [DOI] [PubMed] [Google Scholar]
- Malkoch M, & García-Gallego S (2020). CHAPTER 1 Introduction to Dendrimers and Other Dendritic Polymers. 1–20. 10.1039/9781788012904-00001 [DOI] [Google Scholar]
- Mall C, Sckisel GD, Proia DA, Mirsoian A, Grossenbacher SK, Pai C-CS, Chen M, Monjazeb AM, Kelly K, Blazar BR, & Murphy WJ (2015). Repeated PD-1/PD-L1 monoclonal antibody administration induces fatal xenogeneic hypersensitivity reactions in a murine model of breast cancer. Oncoimmunology, 5(2). 10.1080/2162402X.2015.1075114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkowska M, Stanczyk M, Janaszewska A, Sobierajska E, Chworos A, & Klajnert-Maculewicz B (2019). Multicomponent Conjugates of Anticancer Drugs and Monoclonal Antibody with PAMAM Dendrimers to Increase Efficacy of HER-2 Positive Breast Cancer Therapy. Pharmaceutical Research, 36(11), 154. 10.1007/s11095-019-2683-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maute RL, Gordon SR, Mayer AT, McCracken MN, Natarajan A, Ring NG, Kimura R, Tsai JM, Manglik A, Kruse AC, Gambhir SS, Weissman IL, & Ring AM (2015). Engineering high-affinity PD-1 variants for optimized immunotherapy and immuno-PET imaging. Proceedings of the National Academy of Sciences, 112(47), E6506–E6514. 10.1073/pnas.1519623112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNerny DQ, Leroueil PR, & Baker JR (2010). Understanding specific and nonspecific toxicities: A requirement for the development of dendrimer-based pharmaceuticals. WIREs Nanomedicine and Nanobiotechnology, 2(3), 249–259. 10.1002/wnan.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettu R, Chen C-Y, & Wu C-Y (2020). Synthetic carbohydrate-based vaccines: Challenges and opportunities. Journal of Biomedical Science, 27(1), 9. 10.1186/s12929-019-0591-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal D, Gubin MM, Schreiber RD, & Smyth MJ (2014). New insights into cancer immunoediting and its three component phases—Elimination, equilibrium and escape. Current Opinion in Immunology, 27, 16–25. 10.1016/j.coi.2014.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett S, Shiao TC, Mousavifar L, Mignani S, & Roy R (2021). Aberrant glycosylation patterns on cancer cells: Therapeutic opportunities for glycodendrimers/metallodendrimers oncology. WIREs Nanomedicine and Nanobiotechnology, 13(1), e1659. 10.1002/wnan.1659 [DOI] [PubMed] [Google Scholar]
- Monteiller C, Tran L, MacNee W, Faux S, Jones A, Miller B, & Donaldson K (2007). The pro-inflammatory effects of low-toxicity low-solubility particles, nanoparticles and fine particles, on epithelial cells in vitro: The role of surface area. Occupational and Environmental Medicine, 64(9), 609–615. 10.1136/oem.2005.024802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JJ, Irvine DJ, & Huang B (2012). ENGINEERING NANO- AND MICRO-PARTICLES TO TUNE IMMUNITY. Advanced Materials (Deerfield Beach, Fla.), 24(28), 3724–3746. 10.1002/adma.201200446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura LIF, Malfanti A, Peres C, Matos AI, Guegain E, Sainz V, Zloh M, Vicent MJ, & Florindo HF (2019). Functionalized branched polymers: Promising immunomodulatory tools for the treatment of cancer and immune disorders. Materials Horizons, 6(10), 1956–1973. 10.1039/C9MH00628A [DOI] [Google Scholar]
- Muguruma N, Miyamoto H, Okahisa T, & Takayama T (2013). Endoscopic Molecular Imaging: Status and Future Perspective. Clinical Endoscopy, 46(6), 603–610. 10.5946/ce.2013.46.6.603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myung JH, Gajjar KA, Saric J, Eddington DT, & Hong S (2011). Dendrimer-Mediated Multivalent Binding for the Enhanced Capture of Tumor Cells. Angewandte Chemie International Edition, 50(49), 11769–11772. 10.1002/anie.201105508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naha PC, Davoren M, Lyng FM, & Byrne HJ (2010). Reactive oxygen species (ROS) induced cytokine production and cytotoxicity of PAMAM dendrimers in J774A.1 cells. Toxicology and Applied Pharmacology, 246(1), 91–99. 10.1016/j.taap.2010.04.014 [DOI] [PubMed] [Google Scholar]
- Nanjwade BK, Bechra HM, Derkar GK, Manvi FV, & Nanjwade VK (2009). Dendrimers: Emerging polymers for drug-delivery systems. European Journal of Pharmaceutical Sciences, 38(3), 185–196. 10.1016/j.ejps.2009.07.008 [DOI] [PubMed] [Google Scholar]
- Nardin EH, Oliveira GA, Calvo-Calle JM, & Nussenzweig RS (1995). The use of multiple antigen peptides in the analysis and induction of protective immune responses against infectious diseases. Advances in Immunology, 60, 105–149. 10.1016/s0065-2776(08)60585-4 [DOI] [PubMed] [Google Scholar]
- Newkome GR, Yao Z, Baker GR, & Gupta VK (1985). Cascade molecules: A new approach to micelles. A [27]-arborol. The Journal of Organic Chemistry, 50(11), 2003–2004. 10.1021/jo00211a052 [DOI] [Google Scholar]
- Niederhafner P, Reiniš M, Šebestík J, & Ježek J (2008). Glycopeptide dendrimers, Part III—a review: Use of glycopeptide dendrimers in immunotherapy and diagnosis of cancer and viral diseases. Journal of Peptide Science, 14(5), 556–587. 10.1002/psc.1011 [DOI] [PubMed] [Google Scholar]
- Ochoa MC, Minute L, Rodriguez I, Garasa S, Perez-Ruiz E, Inogés S, Melero I, & Berraondo P (2017). Antibody-dependent cell cytotoxicity: Immunotherapy strategies enhancing effector NK cells. Immunology and Cell Biology, 95(4), 347–355. 10.1038/icb.2017.6 [DOI] [PubMed] [Google Scholar]
- Ota S, Ono T, Morita A, Uenaka A, Harada M, & Nakayama E (2002). Cellular Processing of a Multibranched Lysine Core with Tumor Antigen Peptides and Presentation of Peptide Epitopes Recognized by Cytotoxic T Lymphocytes on Antigen-presenting Cells. Cancer Research, 62(5), 1471–1476. [PubMed] [Google Scholar]
- Palmerston Mendes L, Pan J, & Torchilin VP (2017). Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy. Molecules, 22(9), 1401. 10.3390/molecules22091401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll DM (2012). The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews Cancer, 12(4), 252–264. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascolutti R, Sun X, Kao J, Maute RL, Ring AM, Bowman GR, & Kruse AC (2016). Structure and Dynamics of PD-L1 and an Ultra-High-Affinity PD-1 Receptor Mutant. Structure (London, England: 1993), 24(10), 1719–1728. 10.1016/j.str.2016.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patri AK, Kukowska-Latallo JF, & Baker JR (2005). Targeted drug delivery with dendrimers: Comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complex. Advanced Drug Delivery Reviews, 57(15), 2203–2214. 10.1016/j.addr.2005.09.014 [DOI] [PubMed] [Google Scholar]
- Pearson RM, Sen S, Hsu H, Pasko M, Gaske M, Král P, & Hong S (2016). Tuning the Selectivity of Dendron Micelles Through Variations of the Poly(ethylene glycol) Corona. ACS Nano, 10(7), 6905–6914. 10.1021/acsnano.6b02708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RM, Sunoqrot S, Hsu H-J, Bae JW, & Hong S (2012). Dendritic nanoparticles: The next generation of nanocarriers? Therapeutic Delivery, 3(8), 941–959. 10.4155/tde.12.76 [DOI] [PubMed] [Google Scholar]
- Perisé-Barrios AJ, Gómez R, Corbí AL, Mata J. de la, Domínguez-Soto A, & Muñoz-Fernandez MA (2015). Use of carbosilane dendrimer to switch macrophage polarization for the acquisition of antitumor functions. Nanoscale, 7(9), 3857–3866. 10.1039/C4NR04038D [DOI] [PubMed] [Google Scholar]
- Petrelli F, Borgonovo K, Cabiddu M, Ghilardi M, & Barni S (2011). Cetuximab and panitumumab in KRAS wild-type colorectal cancer: A meta-analysis. International Journal of Colorectal Disease, 26(7), 823–833. 10.1007/s00384-011-1149-0 [DOI] [PubMed] [Google Scholar]
- Poellmann MJ, Bu J, & Hong S (2018). Would antioxidant-loaded nanoparticles present an effective treatment for ischemic stroke? Nanomedicine (London, England), 13(18), 2327–2340. 10.2217/nnm-2018-0084 [DOI] [PubMed] [Google Scholar]
- Poellmann MJ, Nair A, Bu J, Kim JKH, Kimple RJ, & Hong S (2020). Immunoavidity-Based Capture of Tumor Exosomes Using Poly(amidoamine) Dendrimer Surfaces. Nano Letters, 20(8), 5686–5692. 10.1021/acs.nanolett.0c00950 [DOI] [PubMed] [Google Scholar]
- Ragoonanan D, Khazal SJ, Abdel-Azim H, McCall D, Cuglievan B, Tambaro FP, Ahmad AH, Rowan CM, Gutierrez C, Schadler K, Li S, Di Nardo M, Chi L, Gulbis AM, Shoberu B, Mireles ME, McArthur J, Kapoor N, Miller J, … Mahadeo KM (2021). Diagnosis, grading and management of toxicities from immunotherapies in children, adolescents and young adults with cancer. Nature Reviews Clinical Oncology. 10.1038/s41571-021-00474-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A, & Wolchok JD (2018). Cancer immunotherapy using checkpoint blockade. Science (New York, N.Y.), 359(6382), 1350–1355. 10.1126/science.aar4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, & Li S-D (2016). Modifying the tumor microenvironment using nanoparticle therapeutics. WIREs Nanomedicine and Nanobiotechnology, 8(6), 891–908. 10.1002/wnan.1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler K, & Tam JP (2002). Peptide dendrimers: Applications and synthesis. Reviews in Molecular Biotechnology, 90(3–4), 195–229. 10.1016/S1389-0352(01)00061-7 [DOI] [PubMed] [Google Scholar]
- Sahin U, Derhovanessian E, Miller M, Kloke B-P, Simon P, Löwer M, Bukur V, Tadmor AD, Luxemburger U, Schrörs B, Omokoko T, Vormehr M, Albrecht C, Paruzynski A, Kuhn AN, Buck J, Heesch S, Schreeb KH, Müller F, … Türeci Ö (2017). Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature, 547(7662), 222–226. 10.1038/nature23003 [DOI] [PubMed] [Google Scholar]
- Salameh JW, Zhou L, Ward SM, Chalarca CFS, Emrick T, & Figueiredo ML (2020). Polymer-mediated gene therapy: Recent advances and merging of delivery techniques. WIREs Nanomedicine and Nanobiotechnology, 12(2), e1598. 10.1002/wnan.1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos A, Veiga F, & Figueiras A (2019). Dendrimers as Pharmaceutical Excipients: Synthesis, Properties, Toxicity and Biomedical Applications. Materials, 13(1). 10.3390/ma13010065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharping NE, & Delgoffe GM (2016). Tumor Microenvironment Metabolism: A New Checkpoint for Anti-Tumor Immunity. Vaccines, 4(4), 46. 10.3390/vaccines4040046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K, Pockaj B, Kendra KL, White RL, Gonzalez R, Kuzel TM, Curti B, Leming PD, Whitman ED, Balkissoon J, Reintgen DS, … Hwu P (2011). Gp100 Peptide Vaccine and Interleukin-2 in Patients with Advanced Melanoma. The New England Journal of Medicine, 364(22), 2119–2127. 10.1056/NEJMoa1012863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshacharyulu P, Ponnusamy MP, Haridas D, Jain M, Ganti AK, & Batra SK (2012). Targeting the EGFR signaling pathway in cancer therapy. Expert Opinion on Therapeutic Targets, 16(1), 15–31. 10.1517/14728222.2011.648617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setaro F, Brasch M, Hahn U, Koay MST, Cornelissen JJLM, de la Escosura A, & Torres T (2015). Generation-Dependent Templated Self-Assembly of Biohybrid Protein Nanoparticles around Photosensitizer Dendrimers. Nano Letters, 15(2), 1245–1251. 10.1021/nl5044055 [DOI] [PubMed] [Google Scholar]
- Shiao TC, & Roy R (2012). Glycodendrimers as functional antigens and antitumor vaccines. New J. Chem, 36(2), 324–339. 10.1039/C2NJ20873C [DOI] [Google Scholar]
- Shukla R, Thomas TP, Desai AM, Kotlyar A, Park SJ, & Jr JRB (2008). HER2 specific delivery of methotrexate by dendrimer conjugated anti-HER2 mAb. Nanotechnology, 19(29), 295102. 10.1088/0957-4484/19/29/295102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla R, Thomas TP, Peters JL, Desai AM, Kukowska-Latallo J, Patri AK, Kotlyar A, & Baker JR (2006). HER2 specific tumor targeting with dendrimer conjugated anti-HER2 mAb. Bioconjugate Chemistry, 17(5), 1109–1115. 10.1021/bc050348p [DOI] [PubMed] [Google Scholar]
- Skrombolas D, & Frelinger JG (2014). Challenges and developing solutions for increasing the benefits of IL-2 treatment in tumor therapy. Expert Review of Clinical Immunology, 10(2), 207–217. 10.1586/1744666X.2014.875856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snopok B, Yurchenko M, Szekely L, Klein G, & Kashuba E (2006). SPR-based immunocapture approach to creating an interfacial sensing architecture: Mapping of the MRS18–2 binding site on retinoblastoma protein. Analytical and Bioanalytical Chemistry, 386(7–8), 2063–2073. 10.1007/s00216-006-0867-6 [DOI] [PubMed] [Google Scholar]
- Solomon BL, & Garrido-Laguna I (2018). TIGIT: A novel immunotherapy target moving from bench to bedside. Cancer Immunology, Immunotherapy: CII, 67(11), 1659–1667. 10.1007/s00262-018-2246-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somani S, Laskar P, Altwaijry N, Kewcharoenvong P, Irving C, Robb G, Pickard BS, & Dufès C (2018). PEGylation of polypropylenimine dendrimers: Effects on cytotoxicity, DNA condensation, gene delivery and expression in cancer cells. Scientific Reports, 8(1), 9410. 10.1038/s41598-018-27400-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staniek J, Lorenzetti R, Heller B, Janowska I, Schneider P, Unger S, Warnatz K, Seidl M, Venhoff N, Thiel J, Smulski CR, & Rizzi M (2019). TRAIL-R1 and TRAIL-R2 Mediate TRAIL-Dependent Apoptosis in Activated Primary Human B Lymphocytes. Frontiers in Immunology, 10. 10.3389/fimmu.2019.00951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz NF, & Manchester M (2009). PEGylated Viral Nanoparticles (VNPs) for Biomedicine: The Impact of PEG Chain Length on VNP cell interactions in vitro and ex vivo. Biomacromolecules, 10(4), 784–792. 10.1021/bm8012742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AE, Scheuermann SE, Haile CN, Cuny GD, Velasquez ML, Linhuber JP, Duddupudi AL, Vigliaturo JR, Pravetoni M, Kosten TA, Kosten TR, & Norton EB (2021). Fentanyl conjugate vaccine by injected or mucosal delivery with dmLT or LTA1 adjuvants implicates IgA in protection from drug challenge. Npj Vaccines, 6(1), 1–11. 10.1038/s41541-021-00329-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenson S, & Tomalia D (2005). Dendrimers in biomedical applications—Reflections on the field. Advanced Drug Delivery Reviews, 57(15), 2106–2129. 10.1016/j.addr.2005.09.018 [DOI] [PubMed] [Google Scholar]
- Tai W, Mahato R, & Cheng K (2010). The role of HER2 in cancer therapy and targeted drug delivery. Journal of Controlled Release: Official Journal of the Controlled Release Society, 146(3), 264–275. 10.1016/j.jconrel.2010.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam JP (1988). Synthetic peptide vaccine design: Synthesis and properties of a high-density multiple antigenic peptide system. Proc. Natl. Acad. Sci. USA, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekade RK, Dutta T, Gajbhiye V, & Jain NK (2009). Exploring dendrimer towards dual drug delivery: PH responsive simultaneous drug-release kinetics. Journal of Microencapsulation, 26(4), 287–296. 10.1080/02652040802312572 [DOI] [PubMed] [Google Scholar]
- Teunissen AJP, Burnett ME, Prevot G, Klein ED, Bivona D, & Mulder WJM (2021). Embracing nanomaterials’ interactions with the innate immune system. WIREs Nanomedicine and Nanobiotechnology, n/a(n/a), e1719. 10.1002/wnan.1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thommen DS, & Schumacher TN (2018). T Cell Dysfunction in Cancer. Cancer Cell, 33(4), 547–562. 10.1016/j.ccell.2018.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, Roeck J, Ryder J, & Smith P (1985). A New Class of Polymers: Starburst-Dendritic Macromolecules. Polymer Journal, 17(1), 117–132. 10.1295/polymj.17.117 [DOI] [Google Scholar]
- Tomalia DA, & Fréchet JMJ (2002). Discovery of dendrimers and dendritic polymers: A brief historical perspective*. Journal of Polymer Science Part A: Polymer Chemistry, 40(16), 2719–2728. 10.1002/pola.10301 [DOI] [Google Scholar]
- Tuccitto A, Shahaj E, Vergani E, Ferro S, Huber V, Rodolfo M, Castelli C, Rivoltini L, & Vallacchi V (2019). Immunosuppressive circuits in tumor microenvironment and their influence on cancer treatment efficacy. Virchows Archiv, 474(4), 407–420. 10.1007/s00428-018-2477-z [DOI] [PubMed] [Google Scholar]
- Vaddepally RK, Kharel P, Pandey R, Garje R, & Chandra AB (2020). Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers, 12(3), 738. 10.3390/cancers12030738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventola CL (2017). Cancer Immunotherapy, Part 3: Challenges and Future Trends. Pharmacy and Therapeutics, 42(8), 514–521. [PMC free article] [PubMed] [Google Scholar]
- Vesely MD, & Schreiber RD (2013). Cancer Immunoediting: Antigens, mechanisms and implications to cancer immunotherapy. Annals of the New York Academy of Sciences, 1284(1), 1–5. 10.1111/nyas.12105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichier-guerre S, Lo-man R, Bay S, Deriaud E, Nakada H, Leclerc C, & Cantacuzène D (2000). Short synthetic glycopeptides successfully induce antibody responses to carcinoma-associated Tn antigen. The Journal of Peptide Research, 55(2), 173–180. 10.1034/j.1399-3011.2000.00167.x [DOI] [PubMed] [Google Scholar]
- Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C, Smolak P, Goodwin RG, Rauch CT, Schuh JCL, & Lynch DH (1999). Tumoricidal activity of tumor necrosis factor–related apoptosis–inducing ligand in vivo. Nature Medicine, 5(2), 157–163. 10.1038/5517 [DOI] [PubMed] [Google Scholar]
- Waldmann TA (2018). Cytokines in Cancer Immunotherapy. Cold Spring Harbor Perspectives in Biology, 10(12), a028472. 10.1101/cshperspect.a028472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Li P, Wan T, Tu B, Li J, & Huang F (2021). TIGIT/PVR and LncRNA ANRIL dual-targetable PAMAM polymeric nanoparticles efficiently inhibited the hepatoma carcinoma by combination of immunotherapy and gene therapy. Journal of Drug Targeting, 1–9. 10.1080/1061186X.2021.1879088 [DOI] [PubMed] [Google Scholar]
- Wang Y, Wu L, Tian C, & Zhang Y (2018). PD-1-PD-L1 immune-checkpoint blockade in malignant lymphomas. Annals of Hematology, 97(2), 229–237. 10.1007/s00277-017-3176-6 [DOI] [PubMed] [Google Scholar]
- Wängler C, Moldenhauer G, Eisenhut M, Haberkorn U, & Mier W (2008). Antibody–Dendrimer Conjugates: The Number, Not the Size of the Dendrimers, Determines the Immunoreactivity. Bioconjugate Chemistry, 19(4), 813–820. 10.1021/bc700308q [DOI] [PubMed] [Google Scholar]
- Weiner LM, Dhodapkar MV, & Ferrone S (2009). Monoclonal Antibodies for Cancer Immunotherapy. Lancet, 373(9668), 1033–1040. 10.1016/S0140-6736(09)60251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside TL (2006). Immune suppression in cancer: Effects on immune cells, mechanisms and future therapeutic intervention. Seminars in Cancer Biology, 16(1), 3–15. 10.1016/j.semcancer.2005.07.008 [DOI] [PubMed] [Google Scholar]
- Wiley SR, Schooley K, Smolak PJ, Din WS, Huang C-P, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, & Goodwin RG (1995). Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity, 3(6), 673–682. 10.1016/1074-7613(95)90057-8 [DOI] [PubMed] [Google Scholar]
- Wines BD, Billings H, Mclean MR, Kent SJ, & Hogarth PM (2017). Antibody Functional Assays as Measures of Fc Receptor-Mediated Immunity to HIV - New Technologies and their Impact on the HIV Vaccine Field. Current HIV Research, 15(3), 202–215. 10.2174/1570162X15666170320112247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Barth RF, Yang W, Chatterjee M, Tjarks W, Ciesielski MJ, & Fenstermaker RA (2004). Site-Specific Conjugation of Boron-Containing Dendrimers to Anti-EGF Receptor Monoclonal Antibody Cetuximab (IMC-C225) and Its Evaluation as a Potential Delivery Agent for Neutron Capture Therapy. Bioconjugate Chemistry, 15(1), 185–194. 10.1021/bc0341674 [DOI] [PubMed] [Google Scholar]
- Wu L, Ficker M, Christensen JB, Trohopoulos PN, & Moghimi SM (2015). Dendrimers in Medicine: Therapeutic Concepts and Pharmaceutical Challenges. Bioconjugate Chemistry, 26(7), 1198–1211. 10.1021/acs.bioconjchem.5b00031 [DOI] [PubMed] [Google Scholar]
- Xu J, Wang H, Xu L, Chao Y, Wang C, Han X, Dong Z, Chang H, Peng R, Cheng Y, & Liu Z (2019). Nanovaccine based on a protein-delivering dendrimer for effective antigen cross-presentation and cancer immunotherapy. Biomaterials, 207, 1–9. 10.1016/j.biomaterials.2019.03.037 [DOI] [PubMed] [Google Scholar]
- Yan J-Y, Liu C-Y, Wu Z-W, Chien C-T, Chiu W-C, & Lin S-Y (2015). Designed nucleus penetrating thymine-capped dendrimers: A potential vehicle for intramuscular gene transfection. Journal of Materials Chemistry B, 3(46), 9060–9066. 10.1039/C5TB01435B [DOI] [PubMed] [Google Scholar]
- Yang W, Wu G, Barth RF, Swindall MR, Bandyopadhyaya AK, Tjarks W, Tordoff K, Moeschberger M, Sferra TJ, Binns PJ, Riley KJ, Ciesielski MJ, Fenstermaker RA, & Wikstrand CJ (2008). Molecular Targeting and Treatment of Composite EGFR and EGFRvIII-Positive Gliomas Using Boronated Monoclonal Antibodies. Clinical Cancer Research, 14(3), 883–891. 10.1158/1078-0432.CCR-07-1968 [DOI] [PubMed] [Google Scholar]
- Yoon A-R, Kasala D, Li Y, Hong J, Lee W, Jung S-J, & Yun C-O (2016). Antitumor effect and safety profile of systemically delivered oncolytic adenovirus complexed with EGFR-targeted PAMAM-based dendrimer in orthotopic lung tumor model. Journal of Controlled Release: Official Journal of the Controlled Release Society, 231, 2–16. 10.1016/j.jconrel.2016.02.046 [DOI] [PubMed] [Google Scholar]
- Zahavi D, & Weiner L (2020). Monoclonal Antibodies in Cancer Therapy. Antibodies, 9(3), 34. 10.3390/antib9030034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemek RM, De Jong E, Chin WL, Schuster IS, Fear VS, Casey TH, Forbes C, Dart SJ, Leslie C, Zaitouny A, Small M, Boon L, Forrest ARR, Muiri DO, Degli-Esposti MA, Millward MJ, Nowak AK, Lassmann T, Bosco A, … Lesterhuis WJ (2019). Sensitization to immune checkpoint blockade through activation of a STAT1/NK axis in the tumor microenvironment. Science Translational Medicine, 11(501), eaav7816. 10.1126/scitranslmed.aav7816 [DOI] [PubMed] [Google Scholar]
- Zeng Y, Kurokawa Y, Win-Shwe T-T, Zeng Q, Hirano S, Zhang Z, & Sone H (2016). Effects of PAMAM dendrimers with various surface functional groups and multiple generations on cytotoxicity and neuronal differentiation using human neural progenitor cells. The Journal of Toxicological Sciences, 41(3), 351–370. 10.2131/jts.41.351 [DOI] [PubMed] [Google Scholar]
- Zhou P, Liu W, Cheng Y, & Qian D (2021). Nanoparticle-based applications for cervical cancer treatment in drug delivery, gene editing, and therapeutic cancer vaccines. WIREs Nanomedicine and Nanobiotechnology, n/a(n/a), e1718. 10.1002/wnan.1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Zuo C, Li W, Shi W, Zhou X, Wang H, Chen S, Du J, Chen G, Zhai W, Zhao W, Wu Y, Qi Y, Liu L, & Gao Y (2020). A Novel d-Peptide Identified by Mirror-Image Phage Display Blocks TIGIT/PVR for Cancer Immunotherapy. Angewandte Chemie International Edition, 59(35), 15114–15118. 10.1002/anie.202002783 [DOI] [PubMed] [Google Scholar]
- Zhu L, & Mahato RI (2010). Lipid and polymeric carrier-mediated nucleic acid delivery. Expert Opinion on Drug Delivery, 7(10), 1209–1226. 10.1517/17425247.2010.513969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L, Apetoh L, Ghiringhelli F, & Kroemer G (2008). Immunological aspects of cancer chemotherapy. Nature Reviews. Immunology, 8(1), 59–73. 10.1038/nri2216 [DOI] [PubMed] [Google Scholar]