Introduction

Prof. M. Kudo Editor Liver Cancer

Atezolizumab plus bevacizumab (Atezo/Bev) followed by curative conversion (ABC conversion) therapy is very effective in the treatment of unresectable, transarterial chemoembolization (TACE)-unsuitable intermediate-stage hepatocellular carcinoma (HCC). A multicenter study demonstrated that the curative conversion rate was as high as 30%. The indications and timing for considering curative conversion include: (1) achievement of tumor shrinkage; (2) a need to improve response, even if tumor shrinkage is not achieved with Atezo/Bev; (3) treatment of nodules unsuitable for TACE for reasons other than a high tumor burden, such as confluent multinodular, infiltrative, or poorly differentiated HCC; (4) interruption of Atezo/Bev due to adverse events; and (5) HCCs positive on positron emission tomography (PET), i.e., poorly differentiated HCCs.
Atezo/Bev has been approved worldwide after the IMbrave 150 trial showed that overall survival (OS) with Atezo/Bev was overwhelmingly superior to that of sorafenib [1]. Analysis of updated IMbrave 150 trial data showed that Atezo/Bev outperformed sorafenib, with a median OS of 19.2 months (hazard ratio, 0.66) and a median progression-free survival (PFS) of 6.9 months (hazard ratio, 0.65) [2]. Interestingly, Atezo/Bev achieved more favorable outcomes in patients with intermediate-stage (OS, 25.8 months; PFS, 12.6 months) than advanced-stage (OS, 17.5 months; PFS, 6.5 months) HCC [2]. Moreover, the objective response rate according to RECIST version 1.1 was better in patients with intermediate-stage (44%) than advanced-stage (27%) HCC [2].
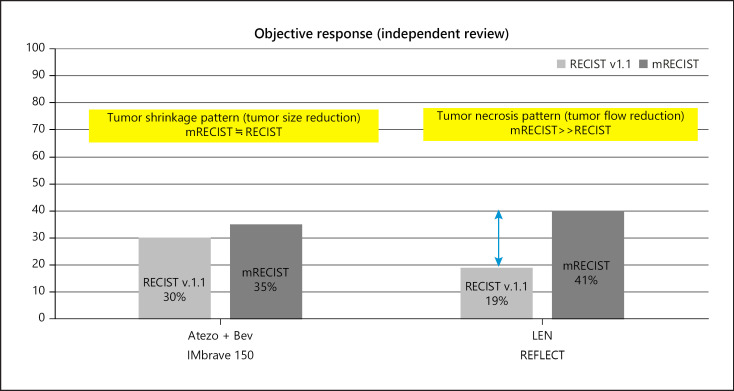
The pattern of response to Atezo/Bev differs completely from the pattern of response to lenvatinib (LEN) in patients treated with sequential LEN-TACE therapy, a regimen that has also shown favorable outcomes in patients with intermediate-stage HCC [3, 4]. More precisely, Atezo/Bev mainly induces tumor shrinkage, whereas LEN mainly induces tumor necrosis through blood flow reduction [5] (Fig. 1). Atezo/Bev exerts anti-VEGF effects before TACE, thus enhancing the effects of TACE, and also enables conversion to curative therapy, such as resection or ablation, which becomes feasible after tumor shrinkage. Indeed, case series have reported the achievements of cancer-free and drug-free status after curative conversion [6, 7].
Fig. 1.
Hepatocellular carcinoma response patterns to Atezo/Bev and lenvatinib. Atezo/Bev induces responses based on tumor shrinkage, whereas LEN induces responses based on tumor necrosis caused by reductions in intratumoral arterial blood flow. Atezo/BEV, atezolizumab plus bevacizumab; LEN, lenvatinib.
Rate of Successful Conversion to Curative Therapy
At five institutions that participated in a multicenter study, 30 of 101 consecutive patients with unresectable, TACE-unsuitable intermediate-stage HCC who had Child-Pugh A liver function and received first-line Atezo/Bev followed by curative therapy achieved a complete response (CR), making the curative conversion rate 30%. Of those 30 patients, six underwent resection; nine underwent ablation, including radiofrequency ablation (RFA), after TACE (ABC-TACE sandwich therapy); 14 underwent TACE, including ABC LEN-TACE sandwich therapy; and one achieved cure after Atezo/Bev therapy alone. Eighteen patients, including the 15 who had undergone resection or ablation, are currently drug-free (real cure status). Patients who had undergone TACE included those who were still being treated with Atezo/Bev. In these patients, Atezo/Bev continued to activate the cancer immunity cycle and cancer antigen specific immune response following tumor antigen release, while TACE induced tumor necrosis. Thus, patients who have not yet become drug-free are expected to become drug-free in the future. That is, tumor marker results became normal in most patients who achieved radiological CR; however, if tumor marker results are not normalized and viable tumor is evident on contrast-enhanced ultrasound, Atezo/Bev is continued, in combination with ablation or TACE if necessary, with most patients expected to achieve CR or deep partial response (Fig. 2). Subsequent immunotherapy after locoregional therapy is mandatory since cancer antigen release and cancer antigen specific immune response will further enhance the effectiveness of subsequent Atezo/Bev therapy [8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19].
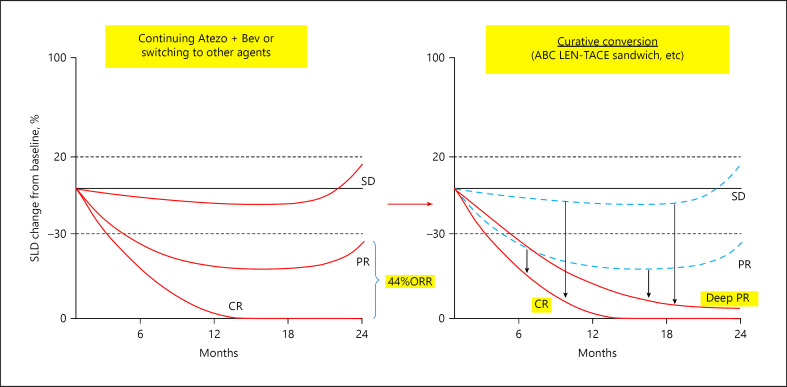
Fig. 2.
Effects of atezolizumab plus bevacizumab followed by curative conversion in patients with intermediate-stage hepatocellular carcinoma. Even if Atezo/Bev therapy results in stable disease (SD) or partial response (PR), patients will eventually develop progressive disease. Curative TACE performed superselectively to not deteriorate liver function will result in achievement of complete response (CR) in 30% of patients and deeper PR in nearly 70%.
Indications and Timing for Conversion to Curative Therapy
There are several scenarios regarding indications and timing for considering conversion to curative therapy.
Curative Conversion after Tumor Shrinkage
Curative therapy, such as resection or ablation, may become feasible in patients who achieve tumor shrinkage after Atezo/Bev treatment, with or without combined use of TACE. Patients with HCC who meet the TACE-unsuitable criteria [20, 21], particularly those with the confluent multinodular type, even within the up-to-seven criteria, are eligible. If Atezo/Bev followed by TACE achieves tumor shrinkage >30%, initially unresectable tumors become resectable. In some patients treated with Atezo/Bev for an extremely long period of time, viable cells may not be detected by pathological analysis of resected specimens (i.e., pathological CR is achieved).
Curative Conversion when Tumor Shrinkage Is Not Achieved (ABC-TACE Sandwich Therapy)
The swimmer plot of arm A in Phase 1b showed that ≥80% of responders achieved tumor shrinkage ≥30% (CR or partial response according to RECIST version 1.1) after four cycles of Atezo/Bev [22]. Thus, tumor shrinkage becomes evident from the fifth cycle onward in <20% of patients. Because of the presence of such late responders, in principle Atezo/Bev should be continued as long as possible while maintaining good control of adverse events in patients with advanced HCC. Among patients with intermediate-stage HCC, however, a curative procedure, whenever it is possible, that can achieve locoregional cure, such as super-selective TACE, is feasible. Thus, a reasonable strategy in patients who do not respond after 4–6 cycles of Atezo/Bev but have stable disease or slow progressive disease consists of TACE shortly before the next cycle of Atezo/Bev, followed by the resumption and continuation of Atezo/Bev as soon as possible during the period that tumor antigens are released after TACE, a process called ABC-TACE sandwich therapy because TACE is performed between cycles of Atezo/Bev. If in such patients, the number of tumors is small and super-selective TACE that does not impair liver function is feasible, then curative TACE can result in cancer-free and drug-free status (Fig. 3) [6]. This process has been reported to be successful in achieving cancer-free and drug-free status in several patients with stable disease or slow progressive disease after 4–6 cycles of Atezo/Bev who underwent selective (curative) TACE for tumor mass reduction and tumor antigen release followed by 4–6 additional cycles of Atezo/Bev [6]. Although transient worsening of the ALBI score is sometimes observed in patients due to a reduction in serum albumin level owing to loss of appetite, these patients' appetite improved soon after, and those patients have since remained at the initial ALBI grade (mALBI grade 1 or 2a). Thus, ABC-TACE sandwich therapy may become an important treatment option for achieving locoregional cure and drug-free status. Even if CR is not achieved by locoregional therapy, a deeper response can be achieved while maintaining liver function (Fig. 2).
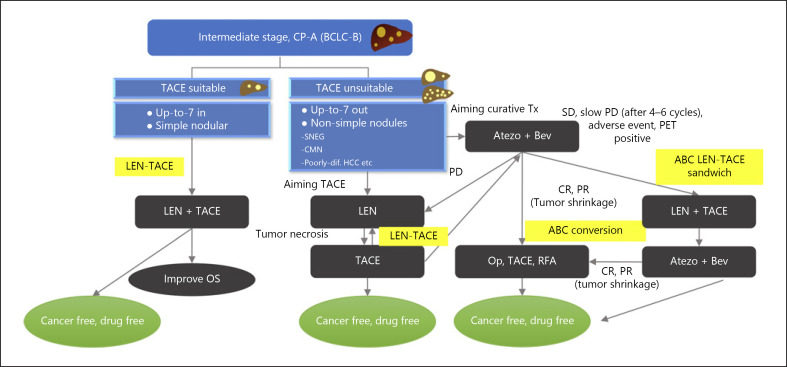
Fig. 3.
Treatment strategy for intermediate-stage HCC 2022. LEN+TACE is a treatment choice for TACE-suitable HCC with Child-Pugh A liver function. LEN followed by TACE is a treatment choice for TACE-unsuitable HCC with Child-Pugh A liver function. LEN-TACE is an optional treatment choice for curative TACE. ABC conversion treatment, including ABC (LEN)-TACE sandwich therapy, is an optional choice for treatment of patients with TACE-unsuitable HCC. These strategies can result in high complete response (CR) and deep partial response (PR), as well as durable, long-lasting cancer-free and drug-free status.
ABC-LEN-TACE Sandwich Therapy for TACE-Unsuitable HCC for Reasons other than a High Tumor Burden
TACE-unsuitable HCCs include poorly differentiated HCCs, as well as confluent multinodular tumors, simple nodular tumors with extranodular growth, and diffuse and massive type tumors [20, 21]. Although it is technically possible to perform TACE or local ablation in patients with confluent multinodular tumors if they are small and with few nodules, TACE and local ablation are regarded as oncologically inappropriate treatment options. Confluent multinodular type HCCs are TACE-resistant due to poor encapsulation, with only 5.3% being encapsulated [21]. Moreover, first-line treatment with TACE or local ablation has been associated with poor patient prognosis due to the high likelihood of multifocal recurrence [20, 21], inasmuch as microscopic portal or venous invasion and many intrahepatic microsatellites are already present in these patients [21, 23]. Thus, upfront systemic therapy, including immunotherapy and an agent with anti-VEGF activity, should be administered to normalize the tumor vasculature and enhance drug delivery, followed by TACE and subsequent RFA or resection [4, 7]. Patients with the small confluent multinodular type of HCC have been treated with ABC-LEN-TACE sandwich therapy, consisting of first-line Atezo/Bev, withdrawal of Bev immediately before TACE, administration of low-dose LEN (4–8 mg/day) for 4 weeks starting the day after administration of Atezo alone, followed by sequential LEN-TACE [3, 4, 7, 20, 21]. This approach is undertaken because TACE and local ablation were not indicated oncologically, despite being technically feasible. Upfront LEN is extremely effective in maximizing the effect of selective TACE while maintaining liver function [3, 24], and the combination of TACE and LEN has been recommended rather than TACE alone. This can result in very dense accumulations of lipiodol at locations of microsatellites and portal vein invasion, providing almost a CR. Although residual viable tumor within treated nodules may be detected by contrast-enhanced ultrasound, these tumors will become completely undetectable on contrast-enhanced ultrasound after an additional 4–6 cycles of Atezo/Bev. Atezo/Bev after TACE-induced tumor antigen release may enhance immunity by inducing the activation and infiltration of CD8-positive T cells [16, 25], which attack residual tumor, resulting in achievement of CR [6, 7]. ABC-LEN-TACE sandwich therapy results in cancer-free and drug-free status in most patients (Fig. 2, 3).
Curative Conversion during the Interruption of Atezo/Bev due to Adverse Events
The fourth scenario is ABC-TACE sandwich therapy when Atezo/Bev treatment must be interrupted due to adverse events such as proteinuria or increased AST/ALT levels. Rather than waiting for the resolution of these adverse events, the largest nodule(s), not all nodules, are subjected to selective TACE, thus preserving liver function and achieving an abscopal effect [16]. This regimen has shown good prognosis in patients with intermediate-stage HCC with originally long OS (median 25.8 months with Atezo/Bev in IMbrave150 trial). Because all molecular targeted agents used for second- and subsequent-line treatment have anti-VEGF activity, none of these agents can be used in patients who develop severe proteinuria during the middle of a long treatment course. As an alternative, locoregional treatment can be performed during interruption or withdrawal of medication, thereby reducing the tumor mass and inducing tumor antigen release. This can enhance the effects of Atezo (plus Bev) administered after resolution of the adverse events, resulting in the achievement of cancer-free, drug-free status or deep response (Fig. 2).
ABC Conversion for FDG-PET-Positive HCC
PET-positive HCCs are mostly poorly differentiated tumors [26, 27, 28, 29, 30, 31]. Due to their biological aggressiveness, these tumors frequently recur after resection, ablation, TACE, or transplantation [26, 32, 33, 34]. Moreover, these tumors contain keratin 19 (K-19)-positive cancer stem cells [35] and undergo epithelial-mesenchymal transition [36]. Thus, in general patients with PET-positive HCC have a poor prognosis [37, 38, 39, 40, 41]. ABC conversion may improve the prognosis of these patients. For example, tumor recurrence was not observed after resection or RFA following ABC-LEN-TACE sandwich therapy in PET-positive HCCs [7]. Many of these tumors are positive for PD-L1, K-19, FGFR2, FGF 19, and/or stem cell features, against which Atezo/Bev and LEN is particularly effective [42, 43, 44], with some patients developing tumor lysis syndrome due to strong response to Atezo/Bev. Tumor shrinkage following treatment with Atezo/Bev may enhance the feasibility of resection. In addition, drug-free status can be achieved by upfront Atezo/Bev followed by resection, or by ABC-LEN-TACE sandwich therapy followed by resection in patients with PET-positive HCC [45].
The rationale for the use of low-dose LEN just before TACE despite Bev also having anti-VEGF activity is that Bev treatment should be terminated 3 weeks before TACE [46]. This minimizes the anti-VEGF activity of Bev 3 weeks after its last administration. To compensate, Atezo alone should be administered during the cycle immediately before TACE, with low-dose LEN, a strong inhibitor of VEGFR and FGFR, starting on the day after Atezo treatment until immediately before TACE. LEN-TACE sequential therapy, which should maximize the effect of TACE, has very strong anti-tumor effect, inducing CR according to mRECIST [3, 4, 20, 21, 47]. In addition, LEN alone is effective in patients with poorly differentiated HCC due to high anti-FGFR2 and anti-FGFR4 activities, with ORRs of 47.6% [48] and 92% [49]. Resuming Atezo/Bev as soon as possible after LEN-TACE is particularly effective, with ABC-LEN-TACE sandwich therapy resulting in a high pathological CR rate [7, 48]. Thus, ABC-LEN-TACE sandwich therapy may be the most powerful treatment currently available that satisfies unmet needs in the treatment of patients with PET-positive HCC. Taken together, these findings suggest that ABC-LEN-TACE sandwich therapy may be a breakthrough procedure that can achieve cancer-free and drug-free status − and ultimately much longer OS − in patients with PET-positive HCC (Fig. 4).
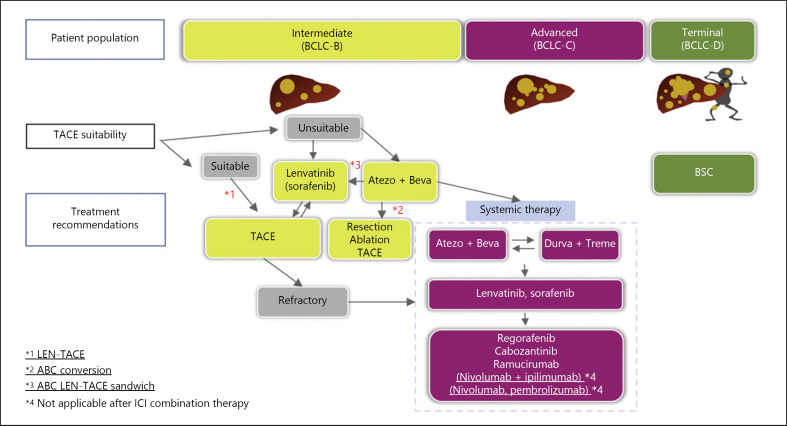
Fig. 4.
Updated treatment strategy for unresectable HCC in 2022.
Conclusion
This editorial described the timing, indications, and outcomes of ABC conversion therapy. Curative therapy becomes feasible and drug-free status can be achieved by combining Atezo/Bev with locoregional treatment or resection, resulting in a radiological CR rate of approximately 30% in patients with TACE-unsuitable intermediate-stage HCC. ABC conversion therapy may also be curative in HCC patients with poor prognosis, such as those with PET-positive HCC. Unlike other solid tumors and advanced HCC, intermediate-stage HCC is a curable disease. Induction with Atezo/Bev, followed by appropriate locoregional therapy, can achieve a cancer-free and drug-free status, and ultimately cure, in these patients. For example, ABC-LEN-TACE sandwich therapy, consisting of induction with Atezo/Bev followed by LEN-TACE and subsequent Atezo/Bev, may be a breakthrough treatment in patients with PET-positive HCC. Previously reported favorable data for upfront LEN followed by TACE [3, 4] indicate that these two must always be used in combination, with LEN regarded as induction therapy (Fig. 3, 4).
Conflict of Interest Statement
Lecture: Eli Lilly, Bayer, Eisai, Chugai, Takeda, MSD; Grants: Gilead Sciences, Taiho, Sumitomo Dainippon Pharma, Takeda, Otsuka, EA Pharma, AbbVie, Eisai, Chugai, GE Healthcare. Masatoshi Kudo is the Editor-in-Chief of Liver Cancer.
Funding Sources
There is no funding for this Editorial.
Author Contributions
M. Kudo conceived, wrote, and approved the final manuscript.
References
- 1.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. IMbrave150 Investigators Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020 May;382((20)):1894–905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 2.Cheng AL, Hsu C, Chan SL, Choo SP, Kudo M. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J Hepatol. 2020 Feb;72((2)):307–19. doi: 10.1016/j.jhep.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 3.Kudo M, Ueshima K, Chan S, Minami T, Chishina H, Aoki T, et al. Lenvatinib as an Initial Treatment in Patients with Intermediate-Stage Hepatocellular Carcinoma Beyond Up-To-Seven Criteria and Child-Pugh A Liver Function: A Proof-Of-Concept Study. Cancers (Basel) 2019 Jul;11((8)):11. doi: 10.3390/cancers11081084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kudo M. A New Treatment Option for Intermediate-Stage Hepatocellular Carcinoma with High Tumor Burden: Initial Lenvatinib Therapy with Subsequent Selective TACE. Liver Cancer. 2019 Oct;8((5)):299–311. doi: 10.1159/000502905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018 Mar;391((10126)):1163–73. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 6.Kudo M. Hepatocellular carcinoma and NASH. J Gastroenterol. 2004;39((4)):409–11. doi: 10.1007/s00535-004-1332-y. [DOI] [PubMed] [Google Scholar]
- 7.Kudo M. A Novel Treatment Strategy for Patients with Intermediate-Stage HCC Who Are Not Suitable for TACE: Upfront Systemic Therapy Followed by Curative Conversion. Liver Cancer. 2021 Oct;10((6)):539–44. doi: 10.1159/000519749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.den Brok MH, Sutmuller RP, van der Voort R, Bennink EJ, Figdor CG, Ruers TJ, et al. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 2004 Jun;64((11)):4024–9. doi: 10.1158/0008-5472.CAN-03-3949. [DOI] [PubMed] [Google Scholar]
- 9.Iida N, Nakamoto Y, Baba T, Nakagawa H, Mizukoshi E, Naito M, et al. Antitumor effect after radiofrequency ablation of murine hepatoma is augmented by an active variant of CC Chemokine ligand 3/macrophage inflammatory protein-1alpha. Cancer Res. 2010 Aug;70((16)):6556–65. doi: 10.1158/0008-5472.CAN-10-0096. [DOI] [PubMed] [Google Scholar]
- 10.Mizukoshi E, Yamashita T, Arai K, Sunagozaka H, Ueda T, Arihara F, et al. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology. 2013 Apr;57((4)):1448–57. doi: 10.1002/hep.26153. [DOI] [PubMed] [Google Scholar]
- 11.Mizukoshi E, Nakamoto Y, Tsuji H, Yamashita T, Kaneko S. Identification of alpha-fetoprotein-derived peptides recognized by cytotoxic T lymphocytes in HLA-A24+ patients with hepatocellular carcinoma. Int J Cancer. 2006 Mar;118((5)):1194–204. doi: 10.1002/ijc.21468. [DOI] [PubMed] [Google Scholar]
- 12.Zerbini A, Pilli M, Penna A, Pelosi G, Schianchi C, Molinari A, et al. Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Cancer Res. 2006 Jan;66((2)):1139–46. doi: 10.1158/0008-5472.CAN-05-2244. [DOI] [PubMed] [Google Scholar]
- 13.Ayaru L, Pereira SP, Alisa A, Pathan AA, Williams R, Davidson B, et al. Unmasking of alpha-fetoprotein-specific CD4(+) T cell responses in hepatocellular carcinoma patients undergoing embolization. Journal of immunology (Baltimore, Md: 1950) 2007;178:1914–1922. doi: 10.4049/jimmunol.178.3.1914. [DOI] [PubMed] [Google Scholar]
- 14.Mizukoshi E, Nakamoto Y, Arai K, Yamashita T, Sakai A, Sakai Y, et al. Comparative analysis of various tumor-associated antigen-specific t-cell responses in patients with hepatocellular carcinoma. Hepatology. 2011 Apr;53((4)):1206–16. doi: 10.1002/hep.24149. [DOI] [PubMed] [Google Scholar]
- 15.Zerbini A, Pilli M, Laccabue D, Pelosi G, Molinari A, Negri E, et al. Radiofrequency thermal ablation for hepatocellular carcinoma stimulates autologous NK-cell response. Gastroenterology. 2010 May;138((5)):1931–42. doi: 10.1053/j.gastro.2009.12.051. [DOI] [PubMed] [Google Scholar]
- 16.Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017 Mar;66((3)):545–51. doi: 10.1016/j.jhep.2016.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiroishi K, Eguchi J, Baba T, Shimazaki T, Ishii S, Hiraide A, et al. Strong CD8(+) T-cell responses against tumor-associated antigens prolong the recurrence-free interval after tumor treatment in patients with hepatocellular carcinoma. J Gastroenterol. 2010 Apr;45((4)):451–8. doi: 10.1007/s00535-009-0155-2. [DOI] [PubMed] [Google Scholar]
- 18.Nobuoka D, Motomura Y, Shirakawa H, Yoshikawa T, Kuronuma T, Takahashi M, et al. Radiofrequency ablation for hepatocellular carcinoma induces glypican-3 peptide-specific cytotoxic T lymphocytes. Int J Oncol. 2012 Jan;40((1)):63–70. doi: 10.3892/ijo.2011.1202. [DOI] [PubMed] [Google Scholar]
- 19.Hansler J, Wissniowski TT, Schuppan D, Witte A, Bernatik T, Hahn EG, et al. Activation and dramatically increased cytolytic activity of tumor specific T lymphocytes after radio-frequency ablation in patients with hepatocellular carcinoma and colorectal liver metastases. World J Gastroenterol. 2006 Jun;12((23)):3716–21. doi: 10.3748/wjg.v12.i23.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kudo M, Han KH, Ye SL, Zhou J, Huang YH, Lin SM, et al. A Changing Paradigm for the Treatment of Intermediate-Stage Hepatocellular Carcinoma: Asia-Pacific Primary Liver Cancer Expert Consensus Statements. Liver Cancer. 2020 Jun;9((3)):245–60. doi: 10.1159/000507370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kudo M, Kawamura Y, Hasegawa K, Tateishi R, Kariyama K, Shiina S, et al. Management of Hepatocellular Carcinoma in Japan: JSH Consensus Statements and Recommendations 2021 Update. Liver Cancer. 2021 Jun;10((3)):181–223. doi: 10.1159/000514174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee MS, Ryoo BY, Hsu CH, Numata K, Stein S, Verret W, et al. GO30140 investigators Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol. 2020 Jun;21((6)):808–20. doi: 10.1016/S1470-2045(20)30156-X. [DOI] [PubMed] [Google Scholar]
- 23.Nakashima Y, Nakashima O, Tanaka M, Okuda K, Nakashima M, Kojiro M. Portal vein invasion and intrahepatic micrometastasis in small hepatocellular carcinoma by gross type. Hepatology research: the official journal of the Japan Society of Hepatology. 2003;26:142–147. doi: 10.1016/s1386-6346(03)00007-x. [DOI] [PubMed] [Google Scholar]
- 24.Ueshima K, Ishikawa T, Saeki I, Morimoto N, Aikata H, Tanabe N, et al. Transcatheter arterial chemoembolization therapy in combination strategy with lenvatnib in patients with unresectable hepatocellular carcinoma (TACTICS-L) in Japan: Final analysis. Gastrointestinal Cancers Symposium (ASCO-GI 2022) San Francisco, USA, January 20-22, 2022. [Google Scholar]
- 25.Kudo M. Combination Immunotherapy with Anti-PD-1/PD-L1 Antibody plus Anti-VEGF Antibody May Promote Cytotoxic T Lymphocyte Infiltration in Hepatocellular Carcinoma, Including in the Noninflamed Subclass. Liver Cancer. 2022;11((3)):185–91. doi: 10.1159/000524977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seo S, Hatano E, Higashi T, Hara T, Tada M, Tamaki N, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography predicts tumor differentiation, P-glycoprotein expression, and outcome after resection in hepatocellular carcinoma. Clin Cancer Res. 2007 Jan;13((2 Pt 1)):427–33. doi: 10.1158/1078-0432.CCR-06-1357. [DOI] [PubMed] [Google Scholar]
- 27.Okazumi S, Isono K, Enomoto K, Kikuchi T, Ozaki M, Yamamoto H, et al. Evaluation of liver tumors using fluorine-18-fluorodeoxyglucose PET: characterization of tumor and assessment of effect of treatment. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 1992;33:333–339. [PubMed] [Google Scholar]
- 28.Torizuka T, Tamaki N, Inokuma T, Magata Y, Sasayama S, Yonekura Y, et al. In vivo assessment of glucose metabolism in hepatocellular carcinoma with FDG-PET. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 1995;36:1811–1817. [PubMed] [Google Scholar]
- 29.Rigo P, Paulus P, Kaschten BJ, Hustinx R, Bury T, Jerusalem G, et al. Oncological applications of positron emission tomography with fluorine-18 fluorodeoxyglucose. Eur J Nucl Med. 1996 Dec;23((12)):1641–74. doi: 10.1007/BF01249629. [DOI] [PubMed] [Google Scholar]
- 30.Nagaoka S, Itano S, Ishibashi M, Torimura T, Baba K, Akiyoshi J, et al. Value of fusing PET plus CT images in hepatocellular carcinoma and combined hepatocellular and cholangiocarcinoma patients with extrahepatic metastases: preliminary findings. Liver international: official journal of the International Association for the Study of the Liver. 2006;26:781–788. doi: 10.1111/j.1478-3231.2006.01296.x. [DOI] [PubMed] [Google Scholar]
- 31.Sacks A, Peller PJ, Surasi DS, Chatburn L, Mercier G, Subramaniam RM. Value of PET/CT in the management of liver metastases, part 1. AJR Am J Roentgenol. 2011 Aug;197((2)):W256-9. doi: 10.2214/AJR.10.6331. [DOI] [PubMed] [Google Scholar]
- 32.Kitamura K, Hatano E, Higashi T, Seo S, Nakamoto Y, Yamanaka K, et al. Preoperative FDG-PET predicts recurrence patterns in hepatocellular carcinoma. Ann Surg Oncol. 2012 Jan;19((1)):156–62. doi: 10.1245/s10434-011-1990-y. [DOI] [PubMed] [Google Scholar]
- 33.Morio K, Kawaoka T, Aikata H, Namba M, Uchikawa S, Kodama K, et al. Preoperative PET-CT is useful for predicting recurrent extrahepatic metastasis of hepatocellular carcinoma after resection. Eur J Radiol. 2020 Mar;124:108828. doi: 10.1016/j.ejrad.2020.108828. [DOI] [PubMed] [Google Scholar]
- 34.Yaprak O, Acar S, Ertugrul G, Dayangac M. Role of pre-transplant 18F-FDG PET/CT in predicting hepatocellular carcinoma recurrence after liver transplantation. World J Gastrointest Oncol. 2018 Oct;10((10)):336–43. doi: 10.4251/wjgo.v10.i10.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawai T, Yasuchika K, Seo S, Higashi T, Ishii T, Miyauchi Y, et al. Identification of Keratin 19-Positive Cancer Stem Cells Associating Human Hepatocellular Carcinoma Using 18F-Fluorodeoxyglucose Positron Emission Tomography. Clin Cancer Res. 2017 Mar;23((6)):1450–60. doi: 10.1158/1078-0432.CCR-16-0871. [DOI] [PubMed] [Google Scholar]
- 36.Lee M, Jeon JY, Neugent ML, Kim JW, Yun M. 18F-Fluorodeoxyglucose uptake on positron emission tomography/computed tomography is associated with metastasis and epithelial-mesenchymal transition in hepatocellular carcinoma. Clin Exp Metastasis. 2017 Apr;34((3-4)):251–60. doi: 10.1007/s10585-017-9847-9. [DOI] [PubMed] [Google Scholar]
- 37.Asman Y, Evenson AR, Even-Sapir E, Shibolet O. [18F]fludeoxyglucose positron emission tomography and computed tomography as a prognostic tool before liver transplantation, resection, and loco-ablative therapies for hepatocellular carcinoma. Liver Transpl. 2015 May;21((5)):572–80. doi: 10.1002/lt.24083. [DOI] [PubMed] [Google Scholar]
- 38.Ma W, Jia J, Wang S, Bai W, Yi J, Bai M, et al. The prognostic value of 18F-FDG PET/CT for hepatocellular carcinoma treated with transarterial chemoembolization (TACE) Theranostics. 2014 May;4((7)):736–44. doi: 10.7150/thno.8725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song HJ, Cheng JY, Hu SL, Zhang GY, Fu Y, Zhang YJ. Value of 18F-FDG PET/CT in detecting viable tumour and predicting prognosis of hepatocellular carcinoma after TACE. Clin Radiol. 2015 Feb;70((2)):128–37. doi: 10.1016/j.crad.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 40.Na SJ, Oh JK, Hyun SH, Lee JW, Hong IK, Song BI, et al. (18)F-FDG PET/CT Can Predict Survival of Advanced Hepatocellular Carcinoma Patients: A Multicenter Retrospective Cohort Study. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2017;58:730–736. doi: 10.2967/jnumed.116.182022. [DOI] [PubMed] [Google Scholar]
- 41.Sposito C, Di Sandro S, Brunero F, Buscemi V, Battiston C, Lauterio A, et al. Development of a prognostic scoring system for resectable hepatocellular carcinoma. World J Gastroenterol. 2016 Sep;22((36)):8194–202. doi: 10.3748/wjg.v22.i36.8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Llovet JM, Castet F, Heikenwalder M, Maini MK, Mazzaferro V, Pinato DJ, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022 Mar;19((3)):151–72. doi: 10.1038/s41571-021-00573-2. [DOI] [PubMed] [Google Scholar]
- 43.Montironi C, Castet F, Haber PK, Pinyol R, Torres-Martin M, Torrens L, et al. Inflamed and non-inflamed classes of HCC: a revised immunogenomic classification. Gut. 2022 Feb;:gutjnl-2021-325918. doi: 10.1136/gutjnl-2021-325918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harimoto N, Taguchi K, Shirabe K, Adachi E, Sakaguchi Y, Toh Y, et al. The significance of fibroblast growth factor receptor 2 expression in differentiation of hepatocellular carcinoma. Oncology. 2010;78((5-6)):361–8. doi: 10.1159/000320463. [DOI] [PubMed] [Google Scholar]
- 45.Kudo M. Kan Tan Sui. Hepato-Biliary and Pancreas; 2022. Atezolizumab plus Bevacizumab Followed by Curative Conversion (ABC Conversion) in Patients with Unresectable, TACE Unsuitable Intermediate-satage Hepatocellular Carcinoma. (in Japanese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021 Mar;19((3)):329–59. doi: 10.6004/jnccn.2021.0012. [DOI] [PubMed] [Google Scholar]
- 47.Kudo M. Lenvatinib may drastically change the treatment landscape of hepatocellular carcinoma. Liver Cancer. 2018 Mar;7((1)):1–19. doi: 10.1159/000487148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kudo M, Ueshima K, Aikata H, Tamai T, Saito K, Ikeda K. Association between tumor response by mRECIST and overall survival in patients with poorly differentiated HCC in REFLECT study. 2019. 10th Asia-Pacific Primary Liver Cancer Expert Meeting 2019, Sapporo, Aug 31.
- 49.Kawamura Y, Kobayashi M, Shindoh J, Kobayashi Y, Kasuya K, Sano T, et al. Pretreatment Heterogeneous Enhancement Pattern of Hepatocellular Carcinoma May Be a Useful New Predictor of Early Response to Lenvatinib and Overall Prognosis. Liver Cancer. 2020 Jun;9((3)):275–92. doi: 10.1159/000505190. [DOI] [PMC free article] [PubMed] [Google Scholar]