Abstract
To identify important residues in the D2 protein of photosystem II (PSII) in the cyanobacterium Synechocystis sp. strain PCC 6803, we randomly mutagenized a region of psbDI (coding for a 96-residue-long C-terminal part of D2) with sodium bisulfite. Mutagenized plasmids were introduced into a Synechocystis sp. strain PCC 6803 mutant that lacks both psbD genes, and mutants with impaired PSII function were selected. Nine D2 residues were identified that are important for PSII stability and/or function, as their mutation led to impairment of photoautotrophic growth. Five of these residues are likely to be involved in the formation of the QA-binding niche; these are Ala249, Ser254, Gly258, Ala260, and His268. Three others (Gly278, Ser283, and Gly288) are in transmembrane α-helix E, and their alteration leads to destabilization of PSII but not to major functional alterations of the remaining centers, indicating that they are unlikely to interact directly with cofactors. In the C-terminal lumenal tail of D2, only one residue (Arg294) was identified as functionally important for PSII. However, from the number of mutants generated it is likely that most or all of the 70 residues that are susceptible to bisulfite mutagenesis have been altered at least once. The fact that mutations in most of these residues have not been picked up by our screening method suggests that these mutations led to a normal photoautotrophic phenotype. A novel method of intragenic complementation in Synechocystis sp. strain PCC 6803 was developed to facilitate genetic analysis of psbDI mutants containing several amino acid changes in the targeted domain. Recombination between genome copies in the same cell appears to be much more prevalent in Synechocystis sp. strain PCC 6803 than was generally assumed.
Remarkable progress has been made toward the understanding of the molecular mechanism of electron transfer in the photosystem II (PSII) complex, which catalyzes the light-induced reduction of plastoquinone by water in organisms capable of oxygenic photosynthesis (for reviews, see references 6, 24 and 38). To a large extent, this has been due to the elucidation by X-ray crystallography of the three-dimensional structure of the reaction center complex from the photosynthetic purple bacteria Rhodopseudomonas viridis (5) and Rhodobacter sphaeroides R-26 (1, 3). The availability of this high-resolution three-dimensional structure and the fact that the PSII complex was realized to be structurally and functionally homologous to the bacterial reaction center (reviewed in reference 20) allowed a structural interpretation of photochemical processes catalyzed by PSII. From this, testable hypotheses could be formulated regarding the nature of redox-active PSII cofactors and the amino acid residues in their vicinity, and these predictions generally proved to be correct on the basis of analysis of site-directed mutants in cyanobacteria and Chlamydomonas reinhardtii (6, 26, 43). Moreover, several molecular models of PSII have been constructed on the basis of homology with the bacterial reaction center (31, 34, 46, 47), and these models also provide a starting point for experimentation.
This experimentation generally is carried out through mutant analysis. However, generation of a collection of site-directed mutants is time consuming. Instead, we have developed an approach by which to efficiently generate a collection of mutants with random changes in part of a protein, followed by screening for functionally altered phenotypes. We have previously reported on probing of the AB loop of the D2 protein by targeted random mutagenesis (9). In this study, the C-terminal 30% of core PSII subunit D2 was targeted for random mutagenesis in Synechocystis sp. strain PCC 6803, and mutants that were impaired in photoautotrophic growth (and thus stable PSII function) were selected. By this approach, amino acid residues were found that previously were not realized to be important for PSII function. Genetic and functional analysis of selected mutations in this region is presented in this paper.
MATERIALS AND METHODS
Synechocystis sp. strain PCC 6803 transformation, isolation of chromosomal DNA from this organism, and monitoring of growth kinetics were performed as previously described (11).
Sodium bisulfite mutagenesis to introduce random mutations in a psbDI domain.
The procedure for sodium bisulfite-induced targeted random mutagenesis of the D2 protein was similar to that previously described (9). This procedure is based on the fact that sodium bisulfite preferentially reacts with single-stranded DNA regions, causing C-to-T transitions (13, 32). Therefore, heteroduplex DNA was generated where the region to be mutagenized is single stranded and other regions are double stranded. To generate such heteroduplex DNA, two plasmids were used: (i) pDICK, containing the cloned Synechocystis sp. strain PCC 6803 psbDIC operon with its flanking regions (39), and (ii) the pDICK.del plasmid. The latter was constructed from pDICK by deleting the 287-bp NcoI/Bsu36I fragment of psbDI, coding for amino acid residues Val247 to Pro342 of D2. The pDICK and pDICK.del plasmids were linearized with XhoI and EcoRV, respectively (the enzymes have a unique site in different domains of the plasmid), and the two plasmids were mixed in a 1:1 molar ratio, heat denatured, and annealed. Homoduplex DNA molecules formed in this process are linear, while heteroduplexes are nicked double-stranded circles because of the use of distant restriction enzyme cleavage sites in the two types of molecules (14, 27). In heteroduplex DNA, the region corresponding to the deletion forms a single-stranded loop. Two types of heteroduplexes are formed, as the coding and noncoding strands of psbDI may form a single-stranded loop. After annealing, the DNA mixture was treated with sodium bisulfite as previously described (29). Eight sets of conditions were used: exposure times of 20, 35, 50, and 65 min with 3 M sodium bisulfite and exposure times of 1, 2, 3, and 4 h with 1 M sodium bisulfite. DNA concentrations in the samples varied from 2.5 to 7 ng/μl.
After mutagenesis, the DNA mixtures were used to transform the ung mutant strain of Escherichia coli. Only heteroduplex molecules will yield transformants, as homoduplexes are linear. Either the full-length plasmid (pDICK) or the deletion variant (pDICK.del) is found in E. coli cells, as the plasmids belong to the same compatibility group. Colonies carrying either of the two plasmids occurred in a 1:1 ratio and were easily distinguished by size: colonies that contained full-length pDICK were significantly smaller, as expression of the intact psbDIC operon appears to be toxic to E. coli.
Individual pDICK-containing colonies were cultured, and plasmid DNA was prepared. Plasmids were used to transform the Synechocystis sp. strain PCC 6803 mutant lacking psbDIC and psbDII (39), selecting for kanamycin resistance conferred by pDICK. The transformants expressed sodium bisulfite-exposed psbDI in the absence of the two wild-type copies of psbD. Mutants that were obligate photoheterotrophs or that showed impaired photoautotrophy were selected, and the DNA sequence of part of psbDI (the region corresponding to codons 247 to 342 and flanking regions) was determined. As expected, any plasmid showed either C-to-T transitions or G-to-A transitions (in the latter case, C-to-T transitions had been introduced into the noncoding strand of psbDI). At the protein level, sodium bisulfite mutagenesis leads to potential substitution of the majority of amino acid residues with one, two, three, or four others. Exceptions are (i) Asn, Ile, Lys, Phe, and Tyr residues that cannot be mutated and (ii) Gln and Trp residues that can be converted only to stop codons.
Site-directed mutagenesis.
An A260G mutation was introduced into the D2 protein using a single-stranded M13mp18 template containing cloned psbDI (39) and the mutagenic primer GTTGGAGAAACCAATACCGAAAATC. The mutagenesis procedure used was previously described (35).
PSII quantitation and functional assays.
The steady-state rate of oxygen evolution was determined as described earlier (11). PSII quantitation in whole cells on a chlorophyll basis using atrazine-replaceable [14C]diuron binding was performed as previously described (40). Chlorophyll a fluorescence induction and decay of the variable fluorescence were measured in intact cells on a commercial PAM fluorometer (Walz, Effeltrich, Germany) as previously described (16).
RESULTS
Isolation of Synechocystis sp. strain PCC 6803 psbDI mutants.
Random mutations were introduced in the 287-bp NcoI/Bsu36I region of psbDI, which codes for amino acid residues Val247 to Pro342 of the D2 subunit of PSII. This was done by sodium bisulfite mutagenesis of heteroduplex DNA as described in Materials and Methods. The Val247-to-Pro342 domain that was targeted for random mutagenesis starts in the DE loop and ends 10 residues before the C terminus of the protein. We chose not to alter the very end of the psbDI coding region, since it overlaps the Shine-Dalgarno ribosome-binding site and the five N-terminal codons of psbC encoding the CP43 protein.
A total of 227 psbDI psbC-carrying pDICK plasmids that had been isolated independently after mutagenesis were used to transform the recipient Synechocystis sp. strain PCC 6803 mutant lacking psbDIC and psbDII (39). Kanamycin-resistant Synechocystis sp. strain PCC 6803 colonies were selected on BG-11 plates containing glucose as a source of fixed carbon, and these colonies were subsequently screened for the inability or reduced ability to grow photoautotrophically. Twenty-six (11%) of these plasmids gave rise to transformants impaired in photoautotrophic growth. To determine whether this phenotype was due to mutations within the targeted psbDI region, each mutant was subjected to a complementation test with a set of cloned wild-type psbDI fragments corresponding to small overlapping regions of the gene as previously described (9). Transformants with mutations exclusively in the targeted region should regain the ability to grow photoautotrophically at the wild-type rate. The majority of the mutants (19 [76%] out of 26) could be transformed to normal photoautotrophy by a plasmid containing a 338-bp NcoI/TaqI psbDI fragment covering the mutagenized region of psbDI and were not transformed by other fragments corresponding to neighboring regions. This test indicates that mutations leading to impaired photoautotrophic growth in these 19 mutants were localized solely within the targeted DNA region that was exposed to sodium bisulfite. These 19 Synechocystis sp. strain PCC 6803 strains with random mutations introduced exclusively within the desired region of psbDI were used for further study.
Sequence analysis of the mutant collection.
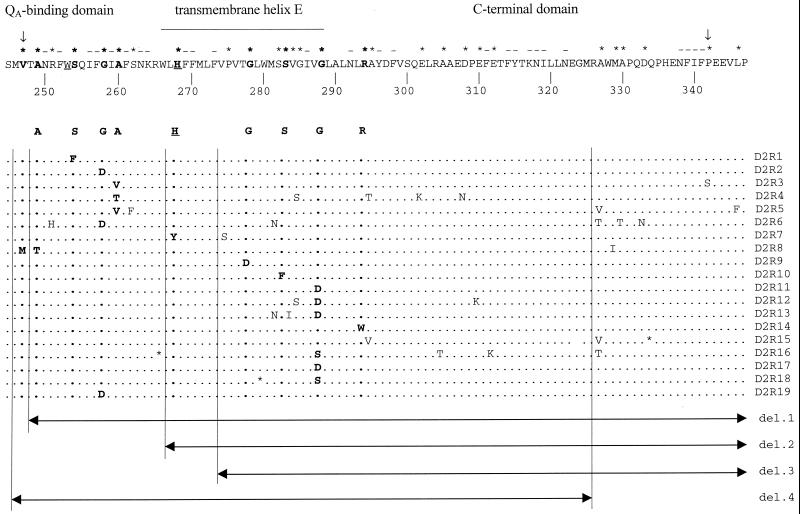
The psbDI gene was PCR amplified using chromosomal DNA from each of the 19 selected mutants as the template. The PCR products were sequenced in the 287-bp NcoI/Bsu36I region that was targeted for random mutagenesis. The sequences of the 200- to 300-bp-long flanking regions upstream and downstream of this region were determined as well. Each of the mutants contained between one and nine G-to-A or C-to-T nucleotide changes, leading at the protein level to one to six amino acid substitutions in D2 per plasmid (Fig. 1). Within the collection of 19 mutants, a total of 67 mutations were introduced into the 96-residue-long C-terminal part of the D2 protein (Fig. 1). In only one case was a mutation (L346F) found to have occurred outside of the targeted region. In this case, the mutation, which occurred 12 bp away from the targeted region, probably was due to transient denaturation-renaturation of the DNA double helix at the ends of the single-stranded loop. This transient denaturation and renaturation may have caused regions adjacent to the loop to be briefly single stranded, rendering them susceptible to sodium bisulfite mutagenesis (see Materials and Methods).
FIG. 1.
Representation of the wild-type sequence of the region of D2 that had been exposed to targeted random mutagenesis (top) and of the sequences in the various mutants (below). An asterisk above the wild-type sequence indicates a residue that has been mutated in one of the mutants indicated here. Minus signs above residues of the wild-type sequence indicate that these residues could not be changed by the method employed, and arrows delineate the region corresponding to the part of psbDI that was single stranded during the targeted random-mutagenesis experiment. Two residues that closely interact with QA or nonheme iron are underlined. These are Trp253 (presumed to be located between pheophytin and QA) and His268 (a putative ligand to nonheme iron). Residues the mutation of which appears to give rise to impaired PSII activity are in boldface. An asterisk in one of the mutant sequences indicates a stop codon. The four deletion (del.) plasmids that were used for functional complementation (see text) are indicated at the bottom.
As expected, 24 (about one-third) of the changes occurred at the third “wobble” nucleotide of codons, resulting in silent mutations. These mutations are not likely to influence expression of psbDI. Analysis of DNA sequences in the GenBank database with respect to codon usage in Synechocystis sp. strain PCC 6803 showed that all of the codons are utilized in this cyanobacterium (data not shown). The 43 nonsynonymous mutations affected a total of 27 amino acid residues (Fig. 1) out of 70 that could be mutagenized by sodium bisulfite in the targeted region of D2. Note that only those mutagenized plasmids that led to impaired PSII function upon introduction into the Synechocystis sp. strain PCC 6803 genome have been included in the sequence analysis. These plasmids constitute only 8% of the total collection of 227 sodium bisulfite-treated pDICK constructs. Since 8% of the collection contained substitutions at more than a third of the possible sites, within the entire collection essentially all of the 70 susceptible amino acid residues are likely to have been altered at least once. This implies that mutation of the majority of residues in this part of the protein do not greatly affect photosynthetic function under laboratory conditions.
Identification of D2 residues important for PSII function.
Six mutants in the collection contained single amino acid substitutions (S254F, G258D, G278D, S283F, G288D, and R294W) (Fig. 1), making analysis of the specific impact of these residues on PSII structure and function straightforward. However, the majority of the mutants each carried between two and six changes in the D2 protein (Fig. 1). To identify which residues were primarily responsible for the impairment of photoautotrophic growth in strains with multiple mutations, we utilized a genetic functional complementation test. Four deletion variants of the complementing plasmid that carried the 338-bp NcoI/TaqI psbDI fragment were constructed using Bal 31 digestion of this plasmid. These deletion variants contained subfragments of the wild-type psbDI gene corresponding to different parts of the mutagenized region (Fig. 1). The deletion variants were used to transform mutants with multiple amino acid changes but without nonsense (stop codon) mutations. The subfragment(s) capable of restoring photoautotrophic growth to a specific mutant must contain the mutation(s) primarily responsible for the PSII-impaired phenotype.
The results of the complementation test are presented in Table 1. Analysis of these data identified A249T, A260V, A260T, and H268Y as mutations that have a major effect on the ability to grow photoautotrophically. Also, a number of mutations, mostly in the C-terminal part of the D2 protein, were shown to have no significant impact on the photoautotrophic growth capacity of Synechocystis sp. strain PCC 6803 (Table 1). Note that in Table 1 the S282N mutation has been left off of the list of residues possibly having a significant effect on photoautotrophic growth in D2R13 because of the results obtained with the D2R6 mutant, and the G285S mutation has been left off of the listing of possibly important residues in D2R12 because of the results obtained with D2R4.
TABLE 1.
Complementation analysis of selected psbDI mutantsa
| Mutant | Complementation with:
|
Mutation that affects photoautotrophic growth | Mutation(s) without significant impact on photoautotrophic growth | |||
|---|---|---|---|---|---|---|
| del. 1 | del. 2 | del. 3 | del. 4 | |||
| D2R3 | + | − | − | + | A260V | P342S |
| D2R4 | + | − | − | + | A260T | G285S, A295T, E302K,b D308Nb |
| D2R5 | + | − | − | + | A260V or S262F | A327V, L346F |
| D2R6 | + | − | − | + | R251H or G258D | S282N, A327V, A330T, D333Nb |
| D2R7 | + | + | − | + | H268Y | P275S |
| D2R8 | + | − | − | + | A249T | V247M, M329I |
| D2R12 | + | + | + | + | G288D or E310Kb | |
| D2R13 | + | + | + | + | V284I or G288D | |
Deletion DNA plasmid constructs (del. 1, del. 2, del. 3, and del. 4) utilized in the complementation test are shown in Fig. 1. A plus sign denotes the presence of complementation to vigorous photoautotrophic growth and a minus sign indicates the inability of a specific deletion construct to transform a mutant to photoautotrophy. Single mutants and mutants containing a nonsense mutation (leading to early termination of D2 translation) were not included in this test.
Other mutations at this residue were shown earlier not to affect photoautotrophic growth significantly (25).
Altogether, genetic analysis of the collection of sodium bisulfite-induced psbDI mutants resulted in the identification of nine amino acid residues in the D2 protein that can be altered to yield significant effects on PSII function (Fig. 1). The majority of them are located in the DE loop and in transmembrane α-helix E near the lumenal side of the membrane. In the C-terminal hydrophilic loop of the protein, only Arg294 was found to be very important for PSII stability and/or function.
Intragenic complementation in Synechocystis sp. strain PCC 6803.
To facilitate the analysis of the effects of individual mutations on PSII function, the availability of corresponding single mutants of Synechocystis sp. strain PCC 6803 would be advantageous. To obtain such mutants from our current collection, we probed for the efficiency of intragenic recombination in this cyanobacterium. Single mutants may be generated by in vivo recombination between psbDI genes with different sequences, and if such single mutants are photoautotrophs, then they may be selected for by screening for photoautotrophic growth. A Synechocystis sp. strain PCC 6803 cell contains several copies of its chromosome, and interchromosomal recombination in Synechocystis sp. strain PCC 6803 appears to occur (12). Therefore, if one combines in one cell two different genome copies that have differences in psbDI, recombination between the two psbDI alleles is possible. Therefore, in principle, one can create single psbDI mutants from photoheterotrophic mutants that each have multiple amino acid substitutions in psbDI but that have one mutation in common. An example of a pair of mutants that have one mutation (G285S) in common are mutants D2R4 and D2R12 (Fig. 1). Using chromosomal DNA of each mutant to transform the other mutant of the pair, one can select for transformants with restored photoautotrophic growth capability, assuming that the shared mutation is not the reason for the photoheterotrophic phenotype. If the shared mutation is the reason for this phenotype, then no transformants will be obtained by this selection procedure.
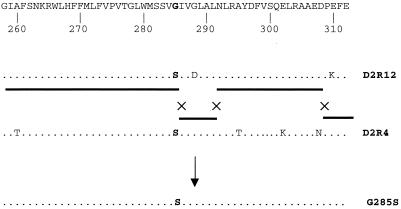
Indeed, DNA sequencing of rapidly growing photoautotrophic transformants of photoheterotrophic strains with DNA from other appropriate photoheterotrophic psbDI mutants indicates that this approach works well. We used this method of intragenic phenotypic complementation to generate the single mutants G285S (by transforming D2R4 with DNA from D2R12) and G288S (by transforming D2R16 with DNA from D2R18) and the double mutant S282N/V284I (by transforming D2R13 with DNA from D2R6; Fig. 1). In the latter case, the failure to find variants with a single mutation is likely to be due to the relatively low number (four) of transformants that were screened.
The scheme of interchromosomal recombination events that are likely to have led to the generation of the single mutant G285S is presented in Fig. 2. It was necessary to assume a minimum of two double-crossover events to explain the genotype of the resulting transformants. The transformation frequencies with which G285S, G288S, and S282N/V284I appeared were 10−6 to 10−7. When the corresponding photoheterotrophic mutants (D2R4, D2R16, and D2R13) were transformed with wild-type DNA, the frequency of transformation leading to photoautotrophy was 10−5. The increased frequency is consistent with the much larger region where crossovers can occur and with the fact that one double-crossover event can restore photoautotrophic growth in this case. It is noteworthy that even almost adjacent mutation sites (D308N and E310K and G285S and G288D) can be separated by crossover events (Fig. 2), indicating that crossover between these closely spaced residues can occur at a reasonable frequency.
FIG. 2.
Schematic representation of recombination events that are likely to have taken place to generate the G285S mutant as a result of transformation of the D2R4 strain with DNA from D2R12. The sequence at residues 288 and 310 is the wild-type sequence in strain D2R4, but the mutations present at residues 295, 302, and 308 in strain D2R4 were not retained. The solid horizontal line indicates the strain from which a particular region seems to originate in the G285S mutant, and crosses indicate the approximate locations where crossovers appear to have taken place. Note that a fourth crossover occurred at a position before codon 260.
Mutations in the QA-binding niche of the D2 protein.
On the basis of similarity between the D2 protein and the M polypeptide of the bacterial reaction center (20), the N-terminal part of the mutagenized D2 region (residues Val247 to His268) is thought to create most of the binding pocket of QA. This region includes the D2 DE helix and the N-terminal part of transmembrane α-helix E that is located close to the acceptor side of the thylakoid membrane. The approach indicated above identified Ala249, Ser254, Gly258, Ala260, and His268 as important for the photoautotrophic competence of PSII. Properties of D2 mutants with changes at positions Ala249 and His268 have been presented in detail earlier (10, 42) and were not included in the present study. Some characteristics of the other mutants are summarized below.
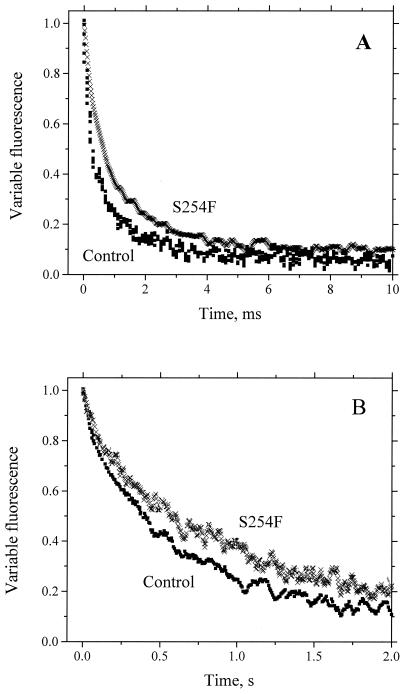
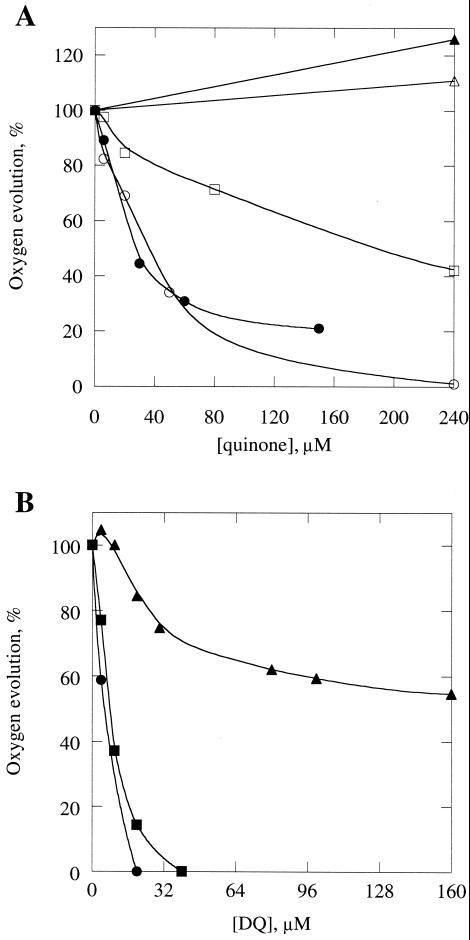
As indicated in Table 2, mutants S254F and G258D showed a large decrease in the amount of functional PSII centers and rapid inhibition of oxygen evolution by light. The rates of electron transport between QA and QB and of charge recombination between QA and the PSII donor side in the S254F mutant both were somewhat lower than in the wild type, as measured by fluorescence decay after flash illumination in the absence and presence of 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU), respectively (Fig. 3). The yield of chlorophyll a fluorescence in the G258D strain was too low to be analyzed in detail. An interesting feature of strains S254F and G258D is the fact that their oxygen evolution can be inhibited by micromolar concentrations of artificial quinones (Fig. 4). This effect is particularly pronounced with tetramethyl-p-benzoquinone (duroquinone, DQ). The addition of 6 and 8 μM DQ to G258D and S254F, respectively, causes 50% inhibition of oxygen evolution, while in the control strain a maximum inhibition of 45% is achieved only at DQ concentrations of about 200 μM. Addition of increasing concentrations of 2,5-dichloro-p-benzoquinone (DCBQ) or 2,5-dimethyl-p-benzoquinone (DMBQ) to these mutants led to rapid inhibition of oxygen evolution as well, whereas in the control strain no inhibition was observed at similar quinone concentrations (Fig. 4). Instead, stimulation was observed, which is due to the quinones serving as PSII electron acceptors.
TABLE 2.
Rates of photoautotrophic growth and electron transport, PSII content on a per-chlorophyll basis, and DCMU affinity in the control strain and psbDI mutants with amino acid substitutions in the DE loop, transmembrane α-helix E, and the C terminus of the D2 proteina
| Mutant | Amino acid substitution(s)b | Photoautotrophic doubling time (h) | Oxygen evolution (% of WT)c | PSII/chlorophyll ratio (% of control)d | KD, DCMU (nM)d |
|---|---|---|---|---|---|
| Controle | None | 12 | 100 | 100 | 17 |
| D2R1 | S254F | ∞ | 31f | 20 | 27 |
| D2R2 | G258D | ∞ | 10f | 6 | 26 |
| D2R3 | A260V | ∞ | 0 | 0 | |
| D2R4 | A260T | ∞ | 0 | 0 | |
| D2R5 | A260V/S262F | ∞ | 0 | 0 | |
| A260Gg | A260G | 12 | 100 | 100 | 21 |
| D2R6 | R251H/G258D | ∞ | 0 | 0 | |
| —h | S262F | 12 | NDi | ND | ND |
| D2R7 | H268Y | ∞ | 0 | 0 | |
| D2R9 | G278D | 80 | 62 | 44 | 18 |
| —h | S282N/V284I | 14 | 105 | 90 | 20 |
| D2R10 | S283F | 20 | 95 | 92 | 21 |
| —h | G285S | 13 | 90 | 95 | 22 |
| D2R11 | G288D | 31 | 59 | 70 | 19 |
| D2R12 | G285S/G288D | ∞ | 0 | 0 | |
| D2R13 | V284I/G288D | ND | 77 | ND | 18 |
| —h | G288S | 12 | 100 | 100 | 19 |
| D2R14 | R294W | ∞ | 29f | 17 | 11 |
Strains D2R15, D2R16, and D2R18 contain nonsense mutations (early terminations) and are not included in this table. Functional analysis of D2R8 has been previously presented (10). D2R17 is identical to D2R11, and D2R19 is identical to D2R2. Measurements were reproducible within 10%.
Only mutations that have a significant impact on the photoautotrophic growth rate (as determined by complementation [Table 1]) are shown here. Figure 1 contains a complete list of the amino acid substitutions in the mutants.
Oxygen evolution was measured at saturating light intensity (2,500 μmol photons m−2 s−1) in BG-11 growth medium to which 25 mM HEPES/NaOH (pH 7.0), 0.5 mM K3Fe(CN)6, and 0.2 mM DMBQ had been added. No exogenous quinone was added for mutants S254F and G258D. The rate of oxygen evolution in the wild type (WT) was 370 ± 25 μmol of O2 mg of chlorophyll−1 h−1.
These values were estimated by [14C]DCMU binding experiments using a cell suspension with chlorophyll at 25 μg/ml.
Synechocystis sp. strain PCC 6803 with wild-type psbDIC and with a genetic background identical to that of the mutants (psbDII deleted and a Kmr cassette inserted downstream of psbDIC) was used as a control strain.
Oxygen evolution in this mutant was inactivated rapidly in the light. The rate shown is the initial rate.
This mutant was constructed by site-directed mutagenesis.
This mutant was obtained by intragenic recombination (see text for details).
ND, not determined.
FIG. 3.
Variable fluorescence yield recorded as a function of time after illumination in the absence (A) and presence (B) of 10 μM DCMU in intact cells of the S254F mutant (crosses) and the control (squares). The difference in signal-to-noise ratio between the two graphs is due to the fact that the measurements in the absence of DCMU were done with PSI-less strains and the measurements in the presence of DCMU were done with PSI-containing strains. The choice of background strain did not impact the decay kinetics of variable fluorescence.
FIG. 4.
Inhibition of oxygen evolution in intact cells upon addition of artificial quinones. (A) Effects of DCBQ (closed symbols) and DMBQ (open symbols) on oxygen evolution in intact wild-type (triangles), S254F (squares), and G258D (circles) cells. (B) Inhibition of oxygen evolution in wild-type (triangles), S254F (squares), and G258D (circles) cells by different concentrations of DQ. No exogenous electron acceptors were added, except for 0.5 mM K3Fe(CN)6, which does not penetrate the cells and which was present to keep the quinones oxidized.
Two different substitutions were found at the Ala260 position (A260T and A260V). The fact that replacement of Ala260 with Thr and especially with Val caused complete loss of PSII centers (Table 2) was unexpected, since these residues are relatively close in size and biochemical properties. Val differs from Ala by only two additional methyl groups in its side chain. Thus, stereochemical constraints seem the most likely explanation for the fact that Val could not be functionally incorporated at position 260 of D2. To check this hypothesis, an A260G mutation was introduced into the D2 protein by site-directed mutagenesis. In the A260G mutant, the amount of the PSII centers, the DCMU dissociation constant, and the rate of oxygen evolution at saturating light intensity were indistinguishable from those of the wild type (Table 2). The kinetics of electron transfer around QA also were very similar to those of the wild type (data not shown). These data suggest that only small residues (Ala and Gly) can be accommodated at position 260 without loss of PSII.
Mutations in transmembrane α-helix E of the D2 protein.
While the part of transmembrane α-helix E that is located near the cytoplasmic side of the membrane may be involved in formation of the nonheme iron- and QA-binding niche, the opposite end of the helix positioned near the lumenal side of the membrane is thought to be in the vicinity of the reaction center chlorophylls (31, 34, 36, 47). Several mutations located in the region of α-helix E that is closest to the lumen were isolated and analyzed in the present study. A brief characterization of the PSII properties in mutants G278D, S282N/V284I, S283F, G285S, G288S, and G288D is presented below.
Introduction of a negatively charged Asp residue at position 278 of D2, thought to be near the middle of the membrane, caused a sevenfold reduction in the photoautotrophic growth rate (Table 2). However, the PSII content, on a per-chlorophyll basis, and the oxygen evolution rate in this mutant were only about twofold lower than the corresponding values of the control strain. Furthermore, the kinetics of the charge separation and recombination in the G278D mutant, as monitored by chlorophyll fluorescence, were normal (data not shown). This suggests the presence of some inactive PSII centers in this mutant during normal growth. A possible reason for the inactive centers could be increased photoinactivation of PSII centers by light or decreased repair efficiency in the G278D mutant. The latter explanation seems more likely, as even at a high light intensity (2,000 μmol photons m−2 s−1, which is 40 times higher than the light intensity under which cells were propagated), inhibition of oxygen evolution in the mutant was found to be only slightly faster than in the control strain.
Residues 282 to 288 are near the lumenal side of the membrane. Ser282 was presumed to possibly interact with P680 (23). However, replacement of the conserved Ser282 residue with Asn did not result in measurable alteration of PSII function or stability: the PSII content per cell, the rate of oxygen evolution at saturating light intensity, the photoautotrophic growth rate (Table 2), and the fluorescence properties (not shown) of the double mutant S282N/V284I were normal. On the other hand, the S283F mutation caused a nearly twofold reduction in the photoautotrophic growth rate although the number of PSII centers per cell (Table 2) and PSII fluorescence properties (not shown) were almost normal. Replacement of Gly residues with Ser at positions 285 and 288 led to only a small reduction in the PSII content per cell and in oxygen evolution (Table 2). Introduction of a negatively charged Asp at position 288 had a more pronounced effect (Table 2), although not as drastic as the introduction of an Asp residue in place of Gly278. The kinetics of QA reduction and recombination with the PSII donor side in the G285S, G288S, and G288D mutants were normal (data not shown), suggesting that these mutations do not affect the midpoint redox potential of P680.
Mutation R294W.
Arg294 is thought to be on the lumenal side of D2. The R294W mutation caused a large decrease in PSII content and led to obligate photoheterotrophy (Table 2). At saturating light intensity, the mutant was capable of oxygen evolution but this process was rapidly inhibited, indicating rapid photoinactivation. The initial rate of oxygen evolution amounted to about one-quarter of that of the wild type. The kinetics of charge separation and recombination in R294W were similar to those in the control (data not shown), excluding a direct effect of the mutation on the midpoint potentials of P680 or the water-splitting apparatus.
DISCUSSION
Mutagenesis approach.
Targeted random mutagenesis is an excellent way to identify functionally important residues in a larger part of a protein. A single treatment with sodium bisulfite can create a collection of mutants in which collectively most of the residues within the targeted region have been changed into up to four different residues. Mutations are targeted to a specific part of the gene while using a DNA construct that contains the entire gene (14, 27, 29). In combination with a convenient and fast screening technique (in this case, impaired photoautotrophic function), a large number of different mutants can be generated and identified easily by targeted random mutagenesis.
Related techniques of random mutagenesis have been applied earlier to study structure-function relationships in various PSII subunits. Using an E. coli mutator strain, Wu and coworkers have introduced mutations at 14 sites in large extrinsic loop E and adjacent transmembrane helix VI of the CP47 protein (45). Resulting Synechocystis sp. strain PCC 6803 mutants exhibited variable phenotypes, ranging from moderate to severe impairment of PSII function. Random mutagenesis of the psbA2 region coding for 178 amino acids of the C-terminal portion of D1 was utilized to study mechanisms underlying light sensitivity of D1 (22). In that work, Synechocystis sp. strain PCC 6803 was transformed with psbA2 gene constructs that were mutagenized in vitro using hydroxylamine or PCR under low-fidelity conditions. Phototolerant transformants that did not bleach in high-intensity light were selected and analyzed (21, 22).
In the present study, we chose to screen for mutants with slow or no photoautotrophic growth. The simplicity of the test allowed us to analyze more than 200 Synechocystis sp. strain PCC 6803 colonies, identifying several new residues that are of functional significance for PSII. About 10% of the Synechocystis sp. strain PCC 6803 colonies that originated from transformation of the acceptor strain (39) with 227 mutagenized psbDI-containing plasmids were impaired in photoautotrophic growth. The fact that transformation with about 90% of the mutagenized pDICK constructs did not lead to any measurable effects on PSII function suggests that in this area of D2, the majority of amino acid residues that can be altered to other residues by sodium bisulfite mutagenesis (all except Asn, Gln, Ile, Lys, Phe, Trp, and Tyr) are not crucial for PSII structure or function. In an earlier study, all D2 Asn, Gln, Asp, Glu, and His residues presumed to be located on the donor side of the thylakoid were probed by site-directed mutagenesis (reviewed in reference 25). Indeed, most mutations did not affect photoautotrophic growth; only at one position (Glu69) was a negative amino acid found to be crucial for PSII function (40).
The psbDI region mutagenized in the present study included part of the DE loop, transmembrane helix E, and the C terminus of the D2 protein, except for the 10 distal C-terminal residues. A total of nine amino acid residues have been identified in this area of D2 to be important for PSII stability or function. The distribution of these residues, however, was nonuniform. More than half of them (Ala249, Ser254, Gly258, Ala260, and His268) were found to be located in the region forming the putative QA-binding pocket. Three of the functionally important residues that were identified (Gly278, Ser283, and Gly288) are thought to be located in α-helix E, even though Gly288 may be at the very end of this helix. A mutation in only one residue (Arg294 changed to Trp) in the more than 50-residue-long C-terminal lumenal part of D2 that had been mutagenized was found to greatly impair PSII stability or function. Thus, the C-terminal domain of D2 appears to be able to accommodate many changes although the length of this region was shown to affect photoautotrophy in Synechocystis sp. strain PCC 6803 (7) and termination of translation at codon 334 led to an obligate photoheterotrophic phenotype (Fig. 1).
Targeted sodium bisulfite mutagenesis appears to introduce mutations fairly evenly throughout the targeted region. Most of the 27 amino acid residues where mutations were found in this study have been altered only once or twice in the mutant collection. However, some residues were altered in many different mutants: we mutated Gly288 in six independent mutants, Ala327 in four mutants, and Gly258 and Ala260 in three mutants. These mutations may not be overrepresented in the collection simply because they lead to impaired photoautotrophic growth and thus have been preferentially selected: Ala327 does not seem to be important for PSII function. Moreover, all of the other mutations leading to complete loss of photoautotrophic growth occur only once in the collection. Most likely, residues Gly258, Ala260, Gly288, and Ala327 were located in hot spots of sodium bisulfite mutagenesis, possibly due to the secondary structure of the single-stranded DNA loop. This explanation is supported by the observation that within the mutant collection as a whole, the ratio of mutated versus unchanged amino acid residues around Gly288 and Ala327 is higher than in most other areas of the mutagenized region (Fig. 1).
The majority of the Synechocystis sp. strain PCC 6803 mutants produced in the present work contained several amino acid substitutions, which complicated the analysis of the impact of each of these mutations on PSII performance. To identify a single residue primarily responsible for impairment of PSII stability and/or function in a mutant carrying multiple mutations in psbDI, we used intragenic phenotypic complementation. The fact that closely clustered mutated nucleotides could be separated by recombination even if the distance between them was as short as 5 nucleotides is suggestive of frequent rearrangements between genome copies in Synechocystis sp. strain PCC 6803. Upon transformation of this cyanobacterium, exogenous DNA is incorporated into the genome via double-reciprocal recombination (44). This same mechanism may also drive interchromosomal recombination.
Mutations in the QA-binding niche of the D2 protein.
Mutations S254F and G258D both cause a 5- to 10-fold decrease in the number of PSII centers per cell. Furthermore, oxygen evolution in the remaining centers is inhibitable by light or by addition of artificial quinones (DQ, DCBQ, and DMBQ) at concentrations below 0.3 mM. Particularly, DQ was found to have a very prominent inhibitory effect at micromolar concentrations. This is similar to the situation observed upon mutation of Ala249 of D2, in which case QA apparently could be displaced by artificial quinones (10). The introduction of a bulky (Phe) or polar (Ser or Asp) residue into the QA-binding site presumably makes plastoquinone accessible to exogenous quinones and may make it exchangeable. Ser254 and Gly258 are 4 amino acid residues apart, which puts them on the same side of the putative DE α-helix. Ser254 is located next to Trp253, a residue that is probably involved in facilitating electron transport through QA and/or in the binding of QA (20, 41).
Unexpectedly, mutations A260V and A260T led to complete loss of PSII while the function of PSII in the A260G mutant was normal (Table 1). Ala260 may be homologous to Ala258 of the M polypeptide of the R. viridis reaction center. The backbone nitrogen of this residue appears to form a hydrogen bond with an oxygen of QA (8, 17, 19). Ala260 of D2 may play a similar role in PSII (37). According to an electron spin echo envelope modulation study of the PSII QA-binding pocket that used an elegant combination of isotopic labeling of individual amino acids and site-directed mutagenesis in Synechocystis sp. strain PCC 6803, the peptide nitrogen of Ala260 of D2 indeed appears to form a weak hydrogen bond with QA (28). This puts Ala260 in the immediate vicinity of QA and implies strict stereochemical constraints in this part of the QA-binding pocket. This can explain our finding that only the two smallest amino acid residues (Ala and Gly) can be incorporated at this position in D2 without complete loss of the PSII centers from thylakoid membrane. Our data are in line with work on the reaction center of R. sphaeroides in which M-subunit residues Ala248 and Ala260 (homologous to D2 residues Ala249 and Ala260, respectively) were replaced with Trp residues (30). Different lines of evidence, including preliminary analysis of X-ray diffraction data, demonstrated that the resulting mutants apparently lacked QA and that the Trp side chain partially occupied the volume that is occupied by the ubiquinone group in the wild-type reaction center (30).
The R251H mutation in D2 has been shown before to be important for stabilization of bicarbonate binding in PSII (2). Residue Arg251 was modeled to be a part of the channel that allows water and bicarbonate molecules to pass from the outside solvent toward the nonheme iron (46). In the present study, the effect of R251H on PSII stability or function was not addressed in detail, since this change occurred in the mutant collection together with mutation G258D, which by itself caused a drastic reduction in PSII content. However, the fact that the G258D single mutant retained some 10% of the PSII electron transfer while the R251H/G258H double mutant contained no PSII centers indicates that the R251H mutation further destabilizes PSII.
Even though many important residues in the QA-binding niche of PSII are conserved in the purple bacterial M subunit, Ser254 has no obvious counterpart in the close vicinity of QA in the bacterial reaction center. However, we believe that Ser254 in PSII indeed is part of the QA-binding niche since the S254F mutant shows altered electron transfer characteristics around QA, particularly in terms of its sensitivity to artificial quinones (Fig. 3). An identical Ser-to-Phe mutation introduced at position 262 of D2 had no measurable effect on photoautotrophic function (Table 2). The data presented in this study support PSII models in which residues Ala249, Ser254, and Ala260 are implicated in the formation of the QA-binding site (31, 36, 46, 47).
Mutations in transmembrane α-helix E and the C-terminal part of the D2 protein.
Transmembrane α-helix E is thought to be involved in the formation of the protein environment around QA (the stromal end of the helix), as well as around chlorophylls associated with the PSII reaction center (the lumenal end of the helix) (31, 34, 36, 47). Furthermore, together with α-helix D, α-helix E is believed to create a significant part of the interface between D1 and D2 (37, 46). By probing α-helix E of D2 by means of targeted random mutagenesis, we strived to identify specific amino acid residues that are important for these or other roles of α-helix E in the PSII reaction center.
Seven mutations located in the lumenal half of D2 α-helix E were isolated and analyzed in the present study (G278D, S282N/V284I, S283F, G285S, G288S, and G288D). In two of them (G278D and G288D), a negatively charged residue was introduced into the otherwise hydrophobic environment of a transmembrane α-helix. In mutants S282N, G285S, and G288S, a polar but uncharged residue was introduced, and in S283F, a small residue was changed to a more bulky one. Surprisingly, all of these mutants were still capable of photoautotrophic growth, although some at reduced rates. Another rather surprising finding was that none of these mutants were altered in the kinetics of PSII electron transfer and charge recombination, as determined from the chlorophyll a fluorescence yield decay kinetics after a flash in the presence and absence of DCMU (data not shown). Therefore, the midpoint potential of P680 remained unaffected in the mutants. Residues Gly278, Ser282, and Gly285 are homologous to R. viridis M-subunit residues Val274, Ala278, and Gly281, respectively (20). The shortest distances between these residues and the bacteriochlorophylls of the special pair are 3.2 to 4.5 Å (8). In various models of P680 (23, 34), conserved D2 residue Ser282 was predicted to form a hydrogen bond with the carbomethoxy group of one of the P680 chlorophylls. The fact that the functional and fluorescence properties of the double mutant S282N/V284I are normal argues against this prediction, as changing of ligands usually leads to a change in midpoint potential and back-reaction rates (18). These data indicate that P680 chlorophylls in PSII are positioned and coordinated differently than bacteriochlorophylls of the special pair in bacterial reaction centers, which may not be surprising in view of the much less dimeric nature of P680 in comparison to the special pair in purple bacteria.
It is noteworthy that the S283F mutation has a more pronounced effect on PSII properties than S282N. Residue Ser283 is not conserved between the D2 proteins from different species (33). This makes Ser283 an unlikely candidate for the formation of a hydrogen bond with one of the chlorophylls of the special pair in place of Ser282. However, this position in various D2 proteins tends to be occupied by a small residue (Ala or Ser) and appears to be unable to accommodate Phe without impeding PSII function.
Mutants G278D, S283F, and G288D have growth rates that are significantly lower than expected on the basis of their PSII content and oxygen evolution capacity, compared to most other D2 mutants (see references 4, 7, and 15). The rate of photoinactivation of oxygen evolution by light in these mutants is only slightly higher than that of the wild type and therefore does not explain this discrepancy. Instead, assembly and repair may have been slowed down in these mutants, thus causing a rather normal phenotype after cell harvesting and incubation in darkness but leading to significant functional impairment in a continuous-light situation.
Only one residue in the C-terminal hydrophilic tail of D2 (Arg294) was identified as functionally important for PSII, even though from the number of mutants generated it is likely that most of the residues susceptible to bisulfite mutagenesis have been altered at least once. This supports the results of previous site-directed mutagenesis experiments by our group (reviewed in reference 25): mutations of Asp, Asn, Glu, Gln, and His residues in the C-terminal tail of the D2 protein had surprisingly little effect. In the photoheterotrophic mutant R294W, the residual rate of oxygen evolution was light sensitive. Because of the normal charge recombination kinetics of the remaining centers in this mutant, changes in the thermodynamics of important donor side components (such as P680 and the oxygen-evolving complex) can be virtually excluded. Instead, a structural role of Arg294 in the stabilization of a functional PSII complex seems likely.
Overall, targeted random mutagenesis has proven to be an efficient approach by which to identify functionally important residues in PSII. The method described in this paper seems particularly attractive in that modifications can be targeted to a specific region without modification of the flanking regions.
ACKNOWLEDGMENT
This research was supported by the National Science Foundation (MCB-9728400 to W.V.).
REFERENCES
- 1.Allen J P, Feher G, Yeates T O, Rees D C, Deisenhofer J, Michel H, Huber R. Structural homology of reaction centers from Rhodopseudomonas sphaeroides and Rhodopseudomonas viridis as determined by X-ray diffraction. Proc Natl Acad Sci USA. 1986;83:8589–8593. doi: 10.1073/pnas.83.22.8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao J, Vermaas W F J, Govindjee Arginine residues in the D2 polypeptide may stabilize bicarbonate binding in photosystem II of Synechocystis sp. PCC 6803. Biochim Biophys Acta. 1991;1059:171–180. doi: 10.1016/s0005-2728(05)80202-6. [DOI] [PubMed] [Google Scholar]
- 3.Chang C-H, Tiede D, Tang J, Smith U, Norris J. Structure of Rhodopseudomonas sphaeroides R-26 reaction center. FEBS Lett. 1986;205:82–86. doi: 10.1016/0014-5793(86)80870-5. [DOI] [PubMed] [Google Scholar]
- 4.Chu H-A, Nguyen A P, Debus R J. Amino acid residues that influence the binding of manganese or calcium to photosystem II. 2. The carboxy-terminal domain of the D1 polypeptide. Biochemistry. 1994;34:5859–5882. doi: 10.1021/bi00017a017. [DOI] [PubMed] [Google Scholar]
- 5.Deisenhofer J, Epp O, Miki K, Huber R, Michel H. Structure of the protein subunits in the photosynthetic reaction center of Rhodopseudomonas viridis at 3 Å resolution. Nature. 1985;318:618–624. doi: 10.1038/318618a0. [DOI] [PubMed] [Google Scholar]
- 6.Diner B A, Babcock G T. Structure, dynamics, and energy conversion efficiency in photosystem II. In: Ort D R, Yocum C F, editors. Oxygenic photosynthesis: the light reactions. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. pp. 213–247. [Google Scholar]
- 7.Eggers B, Vermaas W. Truncation of the D2 protein in Synechocystis sp. PCC 6803: a role of the C-terminal domain of D2 in photosystem II function and stability. Biochemistry. 1993;32:11419–11427. doi: 10.1021/bi00093a020. [DOI] [PubMed] [Google Scholar]
- 8.El-Kabbani O, Chang C-H, Tiede D, Norris J, Schiffer M. Comparison of reaction centers from Rhodobacter sphaeroides and Rhodopseudomonas viridis: overall architecture and protein-pigment interactions. Biochemistry. 1991;30:5361–5369. doi: 10.1021/bi00236a006. [DOI] [PubMed] [Google Scholar]
- 9.Ermakova-Gerdes S, Vermaas W. Random chemical mutagenesis of a specific psbDI region coding for a lumenal loop of the D2 protein of photosystem II in Synechocystis sp. PCC 6803. Plant Mol Biol. 1996;30:243–254. doi: 10.1007/BF00020111. [DOI] [PubMed] [Google Scholar]
- 10.Ermakova-Gerdes S, Vermaas W. Mobility of the primary electron-accepting plastoquinone QA of photosystem II in a Synechocystis sp. PCC 6803 strain carrying mutations in the D2 protein. Biochemistry. 1998;37:11569–11578. doi: 10.1021/bi9806596. [DOI] [PubMed] [Google Scholar]
- 11.Ermakova-Gerdes S, Vermaas W. Inactivation of the open reading frame slr0399 in Synechocystis sp. PCC 6803 functionally complements mutations near the QA niche of photosystem II: a possible role of Slr0399 as a chaperone for quinone binding. J Biol Chem. 1999;274:30540–30549. doi: 10.1074/jbc.274.43.30540. [DOI] [PubMed] [Google Scholar]
- 12.Gurevitz M, Osiewacz H D, Keren Y. Molecular evidence for interchromosomal recombination in the cyanobacterium Synechocystis sp. PCC 6803. Plant Sci. 1991;78:217–224. [Google Scholar]
- 13.Hayatsu H. Bisulfite modification of nucleic acids and their constituents. Prog Nucleic Acid Res Mol Biol. 1976;16:75–124. doi: 10.1016/s0079-6603(08)60756-4. [DOI] [PubMed] [Google Scholar]
- 14.Kalderon D, Oostra B A, Ely B K, Smith A E. Deletion loop mutagenesis: a novel method for the construction of point mutations using deletion mutants. Nucleic Acids Res. 1982;10:5161–5171. doi: 10.1093/nar/10.17.5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kless H, Oren-Shamir M, Ohad I, Edelman M, Vermaas W. Protein modifications in the D2 protein of photosystem II affect properties of the QB/herbicide-binding environment. Z Naturforsch. 1993;48c:185–190. doi: 10.1515/znc-1993-3-413. [DOI] [PubMed] [Google Scholar]
- 16.Kless H, Vermaas W. Many combinations of amino acid sequences in a conserved region of the D1 protein satisfy photosystem II function. J Mol Biol. 1995;246:120–131. doi: 10.1006/jmbi.1994.0071. [DOI] [PubMed] [Google Scholar]
- 17.Komiya H, Yeates T O, Rees D C, Allen J P, Feher G. Structure of the reaction center from Rhodobacter sphaeroides R-26 and 2.4.1: symmetry relations and sequence comparisons between different species. Proc Natl Acad Sci USA. 1988;85:9012–9016. doi: 10.1073/pnas.85.23.9012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin X, Murchison H A, Nagarajan V, Parson W W, Allen J P, Williams J C. Specific alteration of the oxidation potential of the electron donor in reaction centers from Rhodobacter sphaeroides. Proc Natl Acad Sci USA. 1994;91:10265–10269. doi: 10.1073/pnas.91.22.10265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michel H, Epp O, Deisenhofer J. Pigment-protein interactions in the photosynthetic reaction centre from Rhodopseudomonas viridis. EMBO J. 1986;5:2445–2451. doi: 10.1002/j.1460-2075.1986.tb04520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michel H, Deisenhofer J. Relevance of the photosynthetic reaction center from purple bacteria to the structure of photosystem II. Biochemistry. 1988;27:1–7. [Google Scholar]
- 21.Minagawa J, Narusaka Y, Inoue Y, Satoh K. Electron transfer between QA and QB in photosystem II is thermodynamically perturbed in phototolerant mutants of Synechocystis sp. PCC 6803. Biochemistry. 1999;38:770–775. doi: 10.1021/bi981217x. [DOI] [PubMed] [Google Scholar]
- 22.Narusaka Y, Narusaka M, Satoh K, Kobayashi H. In vitro random mutagenesis of the D1 protein of the photosystem II reaction center confers phototolerance on the cyanobacterium Synechocystis sp. PCC 6803. J Biol Chem. 1999;274:23270–23275. doi: 10.1074/jbc.274.33.23270. [DOI] [PubMed] [Google Scholar]
- 23.Noguchi T, Inoue Y, Satoh K. FT-IR studies on the triplet state of P680 in the photosystem II reaction center: triplet equilibrium within a chlorophyll dimer. Biochemistry. 1993;32:7186–7195. doi: 10.1021/bi00079a016. [DOI] [PubMed] [Google Scholar]
- 24.Nugent J H A. Oxygenic photosynthesis—electron transfer in photosystem I and photosystem II. Eur J Biochem. 1996;237:519–531. doi: 10.1111/j.1432-1033.1996.00519.x. [DOI] [PubMed] [Google Scholar]
- 25.Pakrasi H B, Vermaas W F J. Protein engineering of photosystem II. In: Barber J, editor. The photosystems: structure, function and molecular biology. Amsterdam, The Netherlands: Elsevier Science Publishers; 1992. pp. 231–257. [Google Scholar]
- 26.Pakrasi H B. Genetic analysis of the form and function of photosystem I and photosystem II. Annu Rev Genet. 1995;29:755–776. doi: 10.1146/annurev.ge.29.120195.003543. [DOI] [PubMed] [Google Scholar]
- 27.Peden K W, Nathans D. Local mutagenesis within deletion loops of DNA heteroduplexes. Proc Natl Acad Sci USA. 1982;79:7214–7217. doi: 10.1073/pnas.79.23.7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peloquin J M, Tang X-S, Diner B A, Britt R D. An electron spin-echo envelope modulation (ESEEM) study of the QA binding pocket of PS II reaction centers from spinach and Synechocystis. Biochemistry. 1999;38:2057–2067. doi: 10.1021/bi982033l. [DOI] [PubMed] [Google Scholar]
- 29.Pine R, Huang P C. An improved method to obtain a large number of mutants in a defined region of DNA. Methods Enzymol. 1987;154:415–430. doi: 10.1016/0076-6879(87)54088-5. [DOI] [PubMed] [Google Scholar]
- 30.Ridge J P, van Brederode M E, Goodwin M G, van Grondelle R, Jones M R. Mutations that modify or exclude binding of the QA ubiquinone and carotenoid in the reaction center from Rhodobacter sphaeroides. Photosynth Res. 1999;59:9–26. [Google Scholar]
- 31.Ruffle S V, Donnelly D, Blundell T L, Nugent J H A. A three-dimensional model of the photosystem II reaction centre of Pisum sativum. Photosynth Res. 1992;34:287–300. doi: 10.1007/BF00033446. [DOI] [PubMed] [Google Scholar]
- 32.Shapiro R, Braverman B, Louis J B, Servis R E. Nucleic acid reactivity and conformation. II. Reaction of cytosine and uracil with sodium bisulfite. J Biol Chem. 1973;248:4060–4064. [PubMed] [Google Scholar]
- 33.Svensson B, Vass I, Styring S. Sequence analysis of the D1 and D2 reaction center proteins of photosystem II. Z Naturforsch. 1991;46c:765–776. doi: 10.1515/znc-1991-9-1008. [DOI] [PubMed] [Google Scholar]
- 34.Svensson B, Etchebest C, Tuffery P, van Kan P, Smith J, Styring S. A model for the photosystem II reaction center core including the structure of the primary donor P680. Biochemistry. 1996;35:14486–14502. doi: 10.1021/bi960764k. [DOI] [PubMed] [Google Scholar]
- 35.Tichy M, Vermaas W. Functional analysis of combinatorial mutants altered in a conserved region in loop E of the CP47 protein in Synechocystis sp. PCC 6803. Biochemistry. 1997;37:1523–1531. doi: 10.1021/bi9723818. [DOI] [PubMed] [Google Scholar]
- 36.Trebst A. The topology of the plastoquinone and herbicide binding peptides of photosystem II—a model. Z Naturforsch. 1986;41c:240–245. [Google Scholar]
- 37.Trebst A. A contact site between the two reaction center polypeptides of photosystem II is involved in photoinhibition. Z Naturforsch. 1991;46c:557–562. [Google Scholar]
- 38.Tsiotis G, McDermott G, Ghanotakis D. Progress towards structural elucidation of photosystem II. Photosynth Res. 1996;50:93–101. doi: 10.1007/BF00014881. [DOI] [PubMed] [Google Scholar]
- 39.Vermaas W, Charité J, Eggers B. System for site-directed mutagenesis in the psbDI/C operon of Synechocystis sp. PCC 6803. In: Baltscheffsky M, editor. Current research in photosynthesis. I. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1990. pp. 231–238. [Google Scholar]
- 40.Vermaas W, Charité J, Shen G. Glu-69 of the D2 protein in photosystem II is a potential ligand to Mn involved in photosynthetic oxygen evolution. Biochemistry. 1990;29:5325–5332. doi: 10.1021/bi00474a017. [DOI] [PubMed] [Google Scholar]
- 41.Vermaas W, Charité J, Shen G. QA binding to D2 contributes to the functional and structural integrity of photosystem II. Z Naturforsch. 1990;45c:359–365. [Google Scholar]
- 42.Vermaas W, Vass I, Eggers B, Styring S. Mutation of a putative ligand to the non-heme iron in photosystem II: implications for QA reactivity, electron transfer, and herbicide binding. Biochim Biophys Acta. 1994;1184:263–272. [Google Scholar]
- 43.Webber A N, Bingham S E, Lee H. Genetic engineering of thylakoid protein complexes by chloroplast transformation in Chlamydomonas reinhardtii. Photosynth Res. 1995;44:191–205. doi: 10.1007/BF00018309. [DOI] [PubMed] [Google Scholar]
- 44.Williams J G K. Construction of specific mutations in photosystem II photosynthetic reaction center by genetic engineering methods in Synechocystis 6803. Methods Enzymol. 1988;167:766–778. [Google Scholar]
- 45.Wu J, Marsi N, Lee W, Frankel L K, Bricker T M. Random mutagenesis in the large extrinsic loop E and transmembrane α-helix VI of the CP 47 protein of photosystem II. Plant Mol Biol. 1999;39:381–386. doi: 10.1023/a:1006199901167. [DOI] [PubMed] [Google Scholar]
- 46.Xiong J, Subramaniam S, Govindjee Modeling of the D1/D2 proteins and cofactors of the photosystem II reaction center: implications for herbicide and bicarbonate binding. Protein Sci. 1996;5:2054–2073. doi: 10.1002/pro.5560051012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiong J, Subramaniam S, Govindjee A knowledge-based three dimensional model of the photosystem II reaction center of Chlamydomonas reinhardtii. Photosynth Res. 1998;56:229–254. [Google Scholar]