Summary
Background
Tuberculosis is the leading cause of death from a single infectious agent among the HIV-negative population and ranks first among the HIV-positive population. However, few studies have assessed tuberculosis trends in Brazil, Russia, India, China and South Africa (BRICS) or with an emphasis on HIV status. This study assesses the time trends of tuberculosis mortality across the BRICS with an emphasis on HIV status from 1990 to 2019.
Methods
We obtained tuberculosis data from the Global Burden of Disease 2019 study (GBD 2019). We calculated the relative proportion of tuberculosis to all communicable, maternal, neonatal, and nutritional diseases by HIV status across the BRICS. We used age-period-cohort modelling to estimate cohort and period effects in tuberculosis from 1990 to 2019, and calculated net drift (overall annual percentage change), local drift (annual percentage change in each age group), longitudinal age curves (expected longitudinal age-specific rate), and period (cohort) relative risks.
Findings
There were 549,522 tuberculosis deaths across the BRICS in 2019, accounting for 39.3% of global deaths. Among HIV-negative populations, the age-standardised mortality rate (ASMR) of tuberculosis in BRICS remained far higher than that of high-income Asia Pacific countries, especially in India (36.1 per 100 000 in 2019, 95% UI [30.7, 42.6]) and South Africa (40.1 per 100 000 in 2019, 95% UI [36.8, 43.7]). China had the fastest ASMR reduction across the BRICS, while India maintained the largest tuberculosis death numbers with an annual decrease much slower than China's (-4.1 vs -8.0%). Among HIV-positive populations, the ASMR in BRICS surged from 0.24 per 100 000 in 1990 to 5.63 per 100 000 in 2005, and then dropped quickly to 1.70 per 100 000 in 2019. Brazil was the first country to reverse the upward trend of HIV/AIDS-tuberculosis (HIV-TB) mortality in 1995, and achieved the most significant reduction (-3.32% per year). The HIV-TB mortality in South Africa has realised much progress since 2006, but still has the heaviest HIV-TB burden across the BRICS (ASMR: 70.0 per 100 000 in 2019). We also found unfavourable trends among HIV-negative middle-aged (35-55) adults of India, men over 50 in the HIV-negative population and whole HIV-positive population of South Africa, and women aged 45-55 years of Russia. China had little progress in its HIV-positive population with worsening period risks from 2010 to 2019, and higher risks in the younger cohorts born after 1980.
Interpretation
BRICS’ actions on controlling tuberculosis achieved positive results, but the overall improvements were less than those in high-income Asia Pacific countries. BRICS and other high-burden countries should strengthen specified public health approaches and policies targeted at different priority groups in each country.
Funding
National Natural Science Foundation of China (82073573; 72074009), Peking University Global Health and Infectious Diseases Group.
Keywords: Tuberculosis, Mortality, HIV-negative, HIV-positive, BRICS
Research in context.
Evidence before this study
Tuberculosis is the leading cause of death from a single infectious agent among both HIV-negative and HIV-positive populations. We searched PubMed with terms “(tuberculosis) AND (((national) AND (burden)) OR ((population) AND (mortality)) OR (age-period-cohort))”, with no language restrictions, from Jan 1, 2000 to March 1, 2022. We found three studies that estimated global, regional, and national population-based tuberculosis burden and, of these studies, two examined global tuberculosis mortality trends by HIV status. We also identified 6 studies considering BRICS countries, including three presenting estimates from the Global Burden of Disease Study. Two of these studies evaluated the priority groups for policymaking in TB control. Neither of these studies systematically examined the developing trends across the BRICS or the differences in tuberculosis mortality by HIV status.
Added value of this study
We used GBD 2019 to analyze the time trends of tuberculosis mortality across the BRICS, emphasizing HIV status from 1990 to 2019. We reported the tuberculosis age-standardized mortality rate (ASMR), death number and relative proportion of tuberculosis to all communicable, maternal, neonatal, and nutritional diseases by HIV status from 1990 to 2019 across the BRICS. We also reported how trends in tuberculosis mortality vary by age, period, and birth cohort to compare the achievements in each country and put forward priority groups in policymaking. This study concluded that the tuberculosis burden was less significantly improved in BRICS’ HIV-negative populations compared with the high-income Asia Pacific countries. From 1990 to 2019, India maintained the highest tuberculosis death numbers (422,634 in 2019) and its annual mortality decline was much slower than that of China (-4.1 vs -8.0%). Among HIV-positive populations, Brazil was the first country to reverse the upward trend of HIV/AIDS-tuberculosis (HIV-TB) mortality in 1995 and achieved the most significant reduction (-3.32% per year), while China's improvements barely budged in the last decade. The HIV-TB mortality in South Africa has realized much progress since 2006 but still has the heaviest HIV-TB mortality burden. Unfavorable trends in tuberculosis mortality were observed, especially in HIV-negative middle-aged adults from India and HIV-positive younger birth cohorts (born after 1980) from China.
Implications of all the available evidence
Across the BRICS, the heterogeneity in tuberculosis mortality trends could serve as a reference to learn from each other's experiences and allow targeted policymaking. Strengthening primary health care and social protection, and providing special attention to HIV-TB populations should be emphasized. Specified public health approaches and policies toward HIV-negative middle-aged adults in India, HIV-positive younger cohorts in China, middle-aged women in Russia, and men aged over 50 in the HIV-negative population and whole HIV-positive population of South Africa will be crucial in achieving the aims of each country's End TB Strategy.
Alt-text: Unlabelled box
Introduction
Tuberculosis is the leading cause of death from a single infectious agent and a major cause of ill health worldwide, resulting in an estimated 9.65 million new cases and 1.40 million deaths in 2019.1 The global community invested joint efforts in ending the epidemics of tuberculosis through the United Nations (UN) Millennium Development Goals in 2002, the End TB Strategy of the World Health Organization in 2014, and the UN Sustainable Development Goals in 2015. However, according to the Global Tuberculosis Report 2020, the reduction of tuberculosis deaths between 2015 and 2020 was less than halfway towards the 2020 milestone of the End TB Strategy (−14% vs −35%). The number of people newly diagnosed with tuberculosis has been increasing since 2012 in many countries.2
Brazil, Russia, India, China and South Africa (BRICS) were grouped because of their fast-growing economies and accounting for nearly half of the global population.3 Despite the ongoing follow-up on the global tuberculosis burden, few studies have analysed the tuberculosis trends in BRICS based on the Global Burden of Disease Study (GBD). All BRICS countries are high tuberculosis burden countries with respective internal challenges in terms of tuberculosis type, contributing to approximately half of the global tuberculosis morbidity and mortality in total.1,4,5 In 2016, the BRICS tuberculosis Cooperation Plan was outlined at the 6th BRICS Health Ministers’ Meeting. BRICS renewed the commitment to fight against tuberculosis in the following meetings by promoting tuberculosis research. Although the age-standardised incidence and all-age deaths of drug-susceptible tuberculosis have declined substantially, BRICS are still confronting multiple challenges, including the treatment of tuberculosis in HIV-positive populations, intense foci of tuberculosis among particular subgroups, and universal coverage of the tuberculosis care. Given the staggering burden of HIV-TB coinfection in BRICS, there have yet to be comprehensive trend analyses across the BRICS by HIV status. In this context, periodic assessment, monitoring and comparison of progress among populations with different HIV statuses toward End tuberculosis across the BRICS are crucial to providing information for resource allocation and strategy adjustment. The insights from this study would be necessary for policymakers in other high-burden countries and have great potential to change the priority setting in global health.
Previous studies have also explored tuberculosis epidemic trends over time and showed changes in incidence rates and disability-adjusted life years with age and sex as confounders.6,7 The trends of HIV-TB coinfection were analysed separately and reported as age-standardised mortality.4 However, this approach fails to distinguish cohort from period effects. Age-period-cohort analysis was adopted to investigate tuberculosis incidence trends in China, India and United States,8 but few studies have focused on the mortality of tuberculosis.
The present study used data from the GBD 2019 to analyse the time trends of tuberculosis mortality across the BRICS with an emphasis on HIV status from 1990 to 2019. Apart from reporting the tuberculosis age-standardised mortality rate (ASMR), death number and relative proportion to all communicable, maternal, neonatal, and nutritional diseases (Relative proportion) by HIV status, we also assessed how trends in tuberculosis mortality vary by age, period, and birth cohort across the BRICS. We drafted this manuscript as part of the GBD Collaborator Network under the guidance of the GBD protocol.
Methods
GBD 2019 overview
We obtained the data in this study from GBD 2019 public datasets available from http://ghdx.healthdata.org/gbd-results-tool (accessed on October 29, 2020). We restricted the analysis to age- and sex-mortality resulting from tuberculosis. The GBD study uses deidentified data from the second data source was aggregated by the Institute for Health Metrics and Evaluation, University of Washington. Therefore, a waiver of informed consent was reviewed and approved by the University of Washington Institutional Review Board.
Tuberculosis mortality data sources
GBD 2019 provides a total of 369 diseases and injuries for 204 countries with multi-level secondary data sources from vital registration systems, surveillance systems, and verbal autopsies.1 In GBD 2019, tuberculosis cases were identified based on the International Classification of Diseases, version 10 (ICD-10). The discharge diagnosis codes for HIV-negative tuberculosis are A10–19.9, B90–90.9, K67.3, K93.0, M49.0, and P37.0; for HIV-positive tuberculosis, the ICD 10 code is B20.0.1 To model mortality due to tuberculosis among HIV-negative individuals, the database included vital registration data (21 505 site-years), verbal autopsy data (705 site-years), sample-based vital registration data (825 site-years), and mortality surveillance data (680 site-years). To estimate HIV-TB mortality, this database included vital registration data (438 site-years) and HIV-TB cases recorded in the tuberculosis register from WHO. The data in this study inherits the limitations of the GBD study, including that the Disease Surveillance Point system did not cover some remote and poorer areas in BRICS and the data from high resource settings heavily informed GBD models. However, it is also a strength of the GBD 2019 approach to leverage all available data to estimate disease burden. Details of the methods and processing for quantifying the burden of tuberculosis have been published before.5
Statistical analysis
We used an age-period-cohort model to develop independent effect estimates of age, period and birth cohort on tuberculosis mortality.9,10 In the age-period-cohort model, net drift represents the log-linear trend by period and cohort for the whole population and local drift represents the log-linear trend by period and cohort for each age group.11 The model results showed longitudinal age curve, period relative risks and cohort relative risks. To avoid the problem of reading a graph with too many lines, we calculated the mortality and population data into consecutive 5-year periods from 1990 to 2019. The longitudinal age curve used successive 5-year age intervals from 15 to 19 years old to 75 to 79 years old among HIV-negative individuals and from 15 to 19 years old to 65 to 69 years old among HIV-positive individuals due to the small number of HIV-TB patients aged over 70. The period relative risks are the ratio of age-specific rates in each period, with the 2000 to 2004 survey year as the reference period group. The cohort relative risks are the ratio of the age-specific rate in 18 consecutive cohorts among HIV-negative individuals including those born from 1913 to 1917 (median, 1915) to 1998 to 2002 (median, 2000), with the birth cohort of 1953 to 1957 (median, 1955) as the reference group, and 16 consecutive cohorts among HIV-positive individuals including those born from 1913 to 1917 (median, 1915) to 1988 to 1992 (median, 1990), with the birth cohort of 1948 to 1952 (median, 1950) as the reference group. We obtained the estimated parameters from the age-period-cohort Web Tool designed by the National Cancer Institute of the United States.11 To examine the significance of the estimable parameters and functions, the Wald χ2 test was used and all statistical tests were two sided. As BRICS countries were all listed as the top 30 high TB burden countries, we chose high-income Asia Pacific countries (including Japan, the Republic of Korea, Singapore and Brunei Darussalam) as the reference group, which had more comparable tuberculosis burden and ASMR with BRICS.
Role of the funding source
The funders of this study had no role in study design, data collection, data analysis, data interpretation, and writing of the manuscript. ZZ and CJLM had access to GBD 2019 public datasets. All authors agreed to submit this study for publication.
Results
Trends of tuberculosis mortality by HIV status
Table 1 and Figure 1 show trends of tuberculosis mortality in HIV-negative populations and HIV-positive populations across the BRICS countries. In 2019, across the BRICS, there were 549,522 tuberculosis deaths, accounting for 39.3% of global deaths, and the ASMR of tuberculosis was 17.4 per 100 000 in HIV-negative populations and 1.7 per 100 000 in HIV-positive populations.
Table 1.
Characteristics of tuberculosis deaths in BRICS countries between 1990 and 2019.
| BRICS | Brazil | Russia | ||||
|---|---|---|---|---|---|---|
| 1990 | 2019 | 1990 | 2019 | 1990 | 2019 | |
| Population | ||||||
| Total (million) | 2376 | 3232 | 149 (143, 155) | 217 (205, 229) | 151 (147, 163) | 147 (126, 174) |
| Percentage of global (%) | 44.4 | 41.8 | 2.8 | 2.8 | 2.8 | 1.9 |
| HIV-negative tuberculosis | ||||||
| Death number (10 thousand) | 831261 | 492451 | 9951 (9586, 10350) | 5485 (5200, 5802) | 10378 (9641, 10685) | 7982 (6733, 9314) |
| Relative proportion (%)a | 13.1 | 15.5 | 3.9 | 3.2 | 17.1 | 11.8 |
| ASMR (per 100 000) | 55.9 | 17.4 | 9.1 (9.4, 8.7) | 2.3 (2.2, 2.4) | 5.9 (5.5, 6.1) | 4.1 (3.5, 4.8) |
| HIV-negative drug-susceptible tuberculosis | ||||||
| Death number (10 thousand) | 819073 | 426309 | 9940 (9579, 10344) | 5115 (4382, 5607) | 10117 (9283, 10564) | 4536 (2666, 6382) |
| Relative proportion (%)a | 12.9 | 13.4 | 3.8 | 3.0 | 16.6 | 6.7 |
| ASMR (per 100 000) | 55.2 | 15.1 | 9.1 (9.4, 8.7) | 2.2 (1.8, 2.4) | 5.8 (5.3, 6.1) | 2.4 (1.4, 3.3) |
| HIV-negative MDR tuberculosis | ||||||
| Death number (10 thousand) | 12188 | 61615 | 10 (1, 49) | 317 (50, 906) | 261 (50, 928) | 2364 (1177, 3834) |
| Relative proportion (%)a | 0.2 | 1.9 | 0.004 | 0.18 | 0.43 | 3.49 |
| ASMR (per 100 000) | 0.70 | 2.19 | 0.01 (0.0008, 0.04) | 0.13 (0.02, 0.38) | 0.15 (0.03, 0.53) | 1.23 (0.61, 1.99) |
| HIV-positive tuberculosis | ||||||
| Death number (10 thousand) | 5586 | 57071 | 2508 | 1899 | 409 | 791 |
| Relative proportion (%)a | 0.1 | 1.8 | 1.0 | 1.1 | 0.7 | 1.2 |
| ASMR (per 100 000) | 0.24 | 1.70 | 1.76 | 0.78 | 0.26 | 0.48 |
| HIV-positive drug-susceptible tuberculosis | ||||||
| Death number (10 thousand) | 5476 | 51700 | 2508 (1758, 3216) | 1746 (1060, 2697) | 397 (247, 610) | 402 (193, 732) |
| Relative proportion (%)a | 0.1 | 1.6 | 1.0 | 1.0 | 0.7 | 0.6 |
| ASMR (per 100 000) | 0.24 | 1.54 | 1.8 (1.2, 2.3) | 0.7 (0.4, 1.1) | 0.3 (0.2, 0.4) | 0.2 (0.1, 0.4) |
| HIV-positive MDR tuberculosis | ||||||
| Death number (10 thousand) | 109 | 5059 | 0 (0.2, 13) | 131 (18, 420) | 12 (2, 44) | 267 (118, 490) |
| Relative proportion (%)a | 0.0 | 0.2 | 0.00 | 0.08 | 0.02 | 0.39 |
| ASMR (per 100 000) | 0.00 | 0.15 | 0.002 (0.0002, 0.05) | 0.05 (0.01, 0.17) | 0.01 (0.001, 0.03) | 0.16 (0.07, 0.30) |
| India | China | South Africa | ||||
|---|---|---|---|---|---|---|
| 1990 | 2019 | 1990 | 2019 | 1990 | 2019 | |
| Population | ||||||
| Total (million) | 856 (770, 967) | 1391 (1179, 1640) | 1184 (1064, 1340) | 1422 (1214, 1685) | 37 (32, 42) | 56 (51, 61) |
| Percentage of global (%) | 16.0 | 18.0 | 22.1 | 18.4 | 0.7 | 0.7 |
| HIV-negative tuberculosis | ||||||
| Death number (10 thousand) | 613999 (543512, 681869) | 422634 (358351, 498665) | 177459 (156795, 197438) | 36566 (30863, 42824) | 19475 (17019, 22649) | 19785 (18054, 21657) |
| Relative proportion (%)a | 13.4 | 18.0 | 13.1 | 10.4 | 16.7 | 8.4 |
| ASMR (per 100 000) | 121.7 (106.7, 136.8) | 36.1 (30.7, 42.6) | 20.2 (18.0, 22.5) | 2.0 (1.7, 2.3) | 68.7 (58.3, 79.8) | 40.1 (36.8, 43.8) |
| HIV-negative drug-susceptible tuberculosis | ||||||
| Death number (10 thousand) | 612834 (542506, 680963) | 364822 (269008, 451199) | 166900 (142449, 190945) | 33192 (25626, 40234) | 19281 (16852, 22308) | 18644 (16310, 20677) |
| Relative proportion (%)a | 13.4 | 15.5 | 12.3 | 9.5 | 16.5 | 7.9 |
| ASMR (per 100 000) | 121.5 (106.3, 136.8) | 31.2 (23.0, 38.6) | 19.0 (16.1, 21.7) | 1.8 (1.4, 2.2) | 68.0 (57.9, 79.1) | 37.8 (33.2, 41.6) |
| HIV-negative MDR tuberculosis | ||||||
| Death number (10 thousand) | 1164 (76, 5219) | 54977 (8949, 140160) | 10558 (2219, 29047) | 2832 (482, 8510) | 194 (29, 718) | 1125 (313, 2802) |
| Relative proportion (%)a | 0.03 | 2.34 | 0.78 | 0.81 | 0.17 | 0.48 |
| ASMR (per 100 000) | 0.23 (0.02, 1.01) | 4.69 (0.76, 11.98) | 1.20 (0.26, 3.32) | 0.15 (0.03, 0.46) | 0.68 (0.10, 2.52) | 2.29 (0.63, 5.71) |
| HIV-positive tuberculosis | ||||||
| Death number (10 thousand) | 254 | 12034 | 1133 | 2303 | 1281 | 40044 |
| Relative proportion (%)a | 0.0 | 0.5 | 0.1 | 0.7 | 1.1 | 16.9 |
| ASMR (per 100 000) | 0.03 | 0.85 | 0.10 | 0.13 | 3.52 | 69.99 |
| HIV-positive drug-susceptible tuberculosis | ||||||
| Death number (10 thousand) | 254 (124, 525) | 10165 (6934, 13485) | 1053 (249, 1564) | 2055 (1175, 3226) | 1266 (882, 1989) | 37333 (21639, 55053) |
| Relative proportion (%)a | 0.0 | 0.4 | 0.1 | 0.6 | 1.1 | 15.8 |
| ASMR (per 100 000) | 0.03 (0.01, 0.06) | 0.7 (0.5, 1.0) | 0.09 (0.02, 0.13) | 0.11 (0.06, 0.18) | 3.5 (2.4, 5.6) | 65.2 (37.8, 96.0) |
| HIV-positive MDR tuberculosis | ||||||
| Death number (10 thousand) | 0.7 (0.03, 3.64) | 1777 (300, 4344) | 81 (9, 267) | 208 (30, 693) | 16 (2, 56) | 2675 (648, 6945) |
| Relative proportion (%)a | 0.00 | 0.08 | 0.01 | 0.06 | 0.01 | 1.13 |
| ASMR (per 100 000) | 0.00007 (0, 0.0004) | 0.13 (0.02, 0.31) | 0.01 (0.0007, 0.02) | 0.01 (0.002, 0.04) | 0.04 (0.01, 0.16) | 4.68 (1.13, 12.13) |
ASMR indicates age-standardized mortality rate; Relative proportion: relative proportion to all communicable, maternal, neonatal, and nutritional diseases; BRICS: Brazil, Russia, India, China, and South Africa; HIV: human immunodeficiency virus; and MDR: multidrug-resistant.
Relative proportion equals to (deaths resulting from different types of tuberculosis) / (deaths resulting from all communicable, maternal, neonatal, and nutritional diseases). The values in the parentheses are 95% uncertainty intervals.
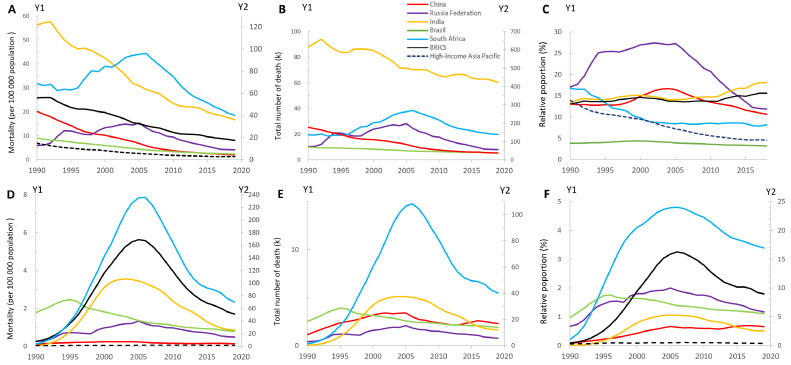
Figure 1.
Age-standardised mortality rates, total number of deaths resulting from tuberculosis, and relative proportion of tuberculosis to all death causes across Brazil, Russia, India, China, and South Africa between 1990 and 2019 in HIV-negative populations (A, B and C) and HIV-positive populations (D, E and F).
(A) Y2 axis for India, South Africa and BRICS, (B) Y2 axis for India and China, (C) relative proportion indicated the proportion of all-cause deaths that come from tuberculosis, (D) Y2 axis for South Africa, (E) Y2 axis for India and South Africa, and (F) Y2 axis for South Africa.
We used two different scales to distinguish the developing trends of each country.
High-income Asia Pacific countries included Japan, Republic of Korea, Singapore, Brunei Darussalam.
ASMR: age-standardised mortality rate; Relative proportion: relative proportion to all communicable, maternal, neonatal, and nutritional diseases; BRICS: Brazil, Russia, India, China, and South Africa; TB: tuberculosis; HIV: human immunodeficiency virus.
Among HIV-negative populations shown in Figure 1A-1C, the ASMR of tuberculosis in BRICS remained far higher than that of high-income Asia Pacific countries (1.3 per 100 000), and the decline in ASMR from 1990 to 2019 was slightly slower than that of high-income Asia Pacific countries (68.8% vs 81.0%). Brazil reduced tuberculosis ASMR continuously from 9.1 per 100 000 in 1990 to 2.3 per 100 000 in 2019. Despite a 70.3% reduction in tuberculosis ASMR, India maintained the highest tuberculosis burden across the BRICS, featuring the second-highest ASMR (36.1 per 100 000), the largest tuberculosis deaths (422 634) and the highest relative proportion (18%) in 2019. From 1990 to 2019, China had a notable ASMR decrease from 20.2 (95%UI, 18.0 to 22.5) per 100 000 in 1990 to 2.0 (95%UI, 1.7 to 2.3) per 100 000 in 2019, and the total tuberculosis deaths decreased from 177459 (95%UI, 156795 to 197438) in 1990 to 36566 (95%UI, 30863 to 42824) in 2019. South Africa surpassed India as the country with the highest tuberculosis ASMR since 2002 and has maintained a downward trend of tuberculosis mortality since 2007.
Among HIV-positive populations shown in Figure 1D-1F, between 1990 and 2019, the tuberculosis ASMR and relative proportion of tuberculosis showed single-peaked curves with a huge surge from 1990 (0.24 per 100 000, 0.1%) to 2005 (5.63 per 100 000, 3.2%) and continued to decline until 2019 (1.70 per 100 000, 1.8%). Although Brazil had the highest tuberculosis ASMR in 1990 (1.76 per 100 000), it was the first country to reverse the upward trend of tuberculosis mortality in 1995 and has reduced tuberculosis ASMR to 0.8 per 100 000 in 2019. The HIV-TB mortality in South Africa realised much progress since 2006 but still had the heaviest HIV-TB ASMR across the BRICS (70.0 per 100 000). China maintained the lowest tuberculosis ASMR within BRICS since 1993, but China was the only country with an increasing trend of relative proportion in the last decade.
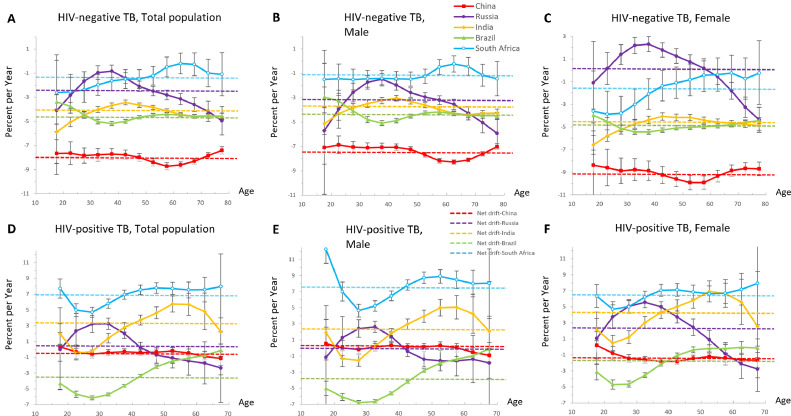
Net drift and local drift in different age groups
Net drift indicates the overall annual percentage change of mortality rates across the study period, whereas local drift indicates the annual percentage changes of the mortality rate for each age group relative to the net drift (Figure 2).
Figure 2.
Local drift with net drift values for tuberculosis mortality and sex difference in Brazil, Russia, India, China, and South Africa from 1990 and 2019 in HIV-negative populations (A, B and C) and HIV-positive populations (D, E and F).
Net drift (dotted line) represents the overall annual percentage change, and local drift (continuous line) values represent annual percentage change in each age group. Values below 0 indicated reductions in tuberculosis mortality across the study period.
We only included the individuals aged 15-69 years old among HIV-positive populations due to the small number of HIV-TB patients aged over 70 (D, E and F).
TB: tuberculosis; HIV: human immunodeficiency virus.
Among HIV-negative populations in BRICS, China had the fastest annual decrease (−8.03% [95% CI, −8.25 to −7.81]), reflecting substantial reductions in tuberculosis mortality across the study period. The mortality reductions in South Africa were much slower (−1.34% [95% CI, −1.81 to −0.88]) (Figure 2A). For men in Russia, there was a favourable overall reduction in mortality, whereas for women, the trend was adverse (−3.12% [95% CI, −3.48 to −2.75] versus 0.34% [95% CI, −0.06 to 0.74]). In India, the improvements among individuals aged 35-50 years old were less notable than in other age groups. Values for most age groups in both sexes were under 0, interpreted as improvements in tuberculosis mortality. Only women aged 20 to 59 years old in Russia experienced increasing trend in tuberculosis mortality.
Among HIV-positive populations in BRICS, the overall net drifts were predominantly below 0 in only two countries, Brazil (−3.32% [95% CI, −3.47 to −3.17]) and China (−0.42% [95% CI, −0.68 to −0.15]), indicating improvements in HIV-TB mortality (Figure 2D). There was a substantial increase in India (3.09% [95% CI, 2.42 to 3.77]) and South Africa (6.97% [95% CI, 6.40 to 7.54]). Among all the age groups in BRICS, the most significant reduction in mortality was among Brazilians aged 25 to 29 years old, and the fastest increase occurred in the teenagers and elders from South Africa. For Russia, HIV-TB mortality only increased in women (2.36% [95% CI, 1.77 to 2.99]) (Figure 2F), especially in the middle-aged. In China, teenagers aged 15 to 19 years old were the only age group with a rising burden, especially for men. In India, the increasing trend of HIV-TB mortality was the fastest among individuals aged 45-64 years old.
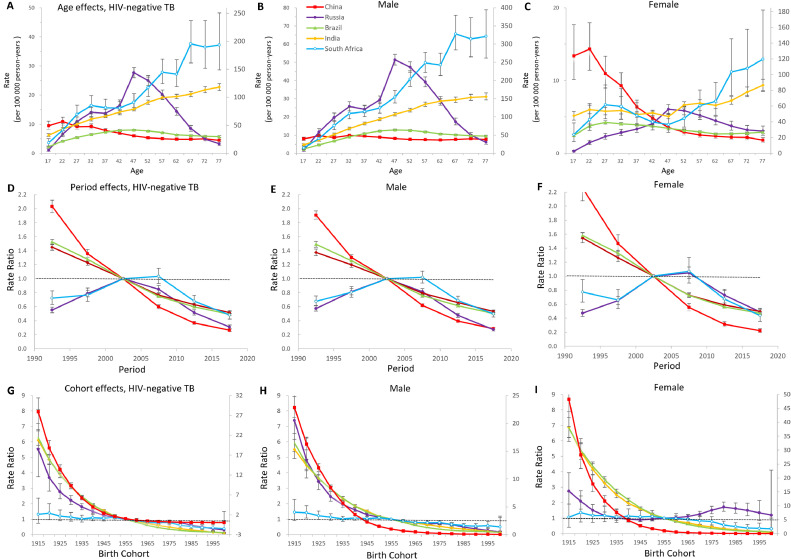
Age-period-cohort effects in HIV-negative populations
Figure 3 showed the estimates of age, period and cohort effects on tuberculosis mortality among HIV-negative populations. Age effects in South Africa and India showed a linear increase, and the rates surged in South Africa in the later years of life. In Russia, adults aged 45 to 49 years old had higher mortality rates than other age groups. In China, the mortality rates decreased with increasing age group overall, and the downtrend was much more significant in women than men. Period effects featured similar directions for China, Brazil and India. In Russia and South Africa, the period effects showed single-peaked curves, suggesting that for the whole population, the situation was worsening over the first decade and controlled effectively in the last 15 years. Across all birth cohorts in the BRICS, China's curve showed the most striking improvements and suggested a progressive reduction in mortality in those born from 1915 on. Compared with Russian men and other birth cohorts, Russian women born from 1975 to 1994 experienced relatively a higher mortality risk.
Figure 3.
Parameter estimates of age, period, and cohort effects on HIV-negative tuberculosis mortality rate and sex difference in Brazil, Russia, India, China, and South Africa from 1990 to 2019.
(A), (B), (C) Y2 axis for India and South Africa; (G), (H), (I) Y2 axis for China.
A, B, C, Fitted longitudinal age curves of tuberculosis mortality (per 100 000 person-years) and the corresponding 95% CIs. D, E, F, Relative risk of each period compared with the reference (2000–2004) adjusted for age and nonlinear cohort effects and the corresponding 95% CI. G, H, I, Relative risk of each cohort compared with the reference (cohort 1955–1959) adjusted for age and nonlinear period effects and the corresponding 95% CI.
TB: tuberculosis; HIV: human immunodeficiency virus.
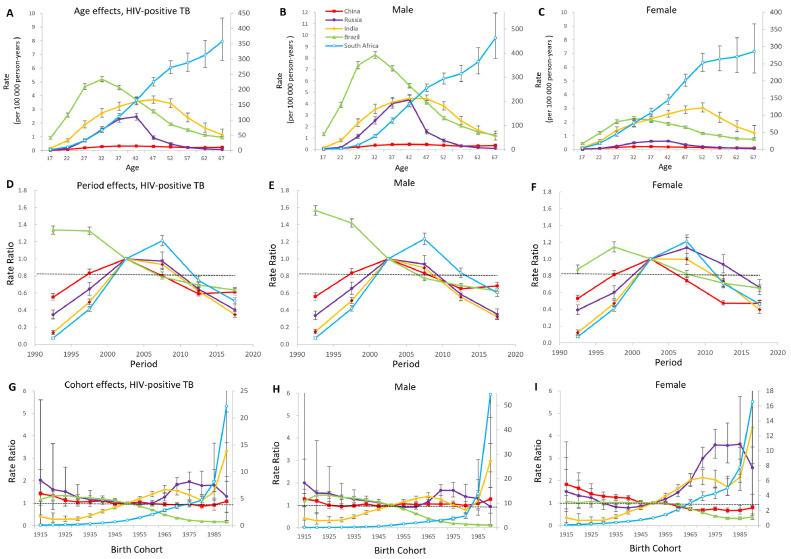
Age-period-cohort effects in HIV-positive populations
In Figure 4, the estimates of age, period and cohort effects on tuberculosis mortality indicated fluctuating trends among HIV-positive populations. Middle-aged adults in Brazil, Russia, India and China have higher mortality rates than younger and older populations. Age effects in South Africa climbed continuously with age, and the trend became steeper in older men (aged>50) than in older women. In contrast with the rest of the BRICS, China kept the curve low and relatively steady across all age groups. Period effects in Brazil showed prominent improvements across the study period. The remaining four countries possessed favourable period trends over the past one or two decades. However, the period effects of China showed an adverse trend during 2015-2019. In China and South Africa, the downward trends were more significant among women than men; while in Russia and India, the inflexion point was delayed for five years. From 1990 on, the birth cohorts mortality rate ratio continued to drop in Brazil overall and rose exponentially in South Africa. The mortality rate ratio of India surged in cohorts born after 1985. In China, we observed unfavourable trends of cohort effects in those born after 1980. In Russia, the cohorts born from 1960 to 1974 experienced an increasing burden, and the situation was improved in the successive cohorts.
Figure 4.
Parameter estimates of age, period, and cohort effects on HIV-positive tuberculosis mortality rate in Brazil, Russia, India, China, and South Africa from 1990 to 2019.
(A), (B), (C), (G), (H), (I) Y2 axis for South Africa.
A, B, C, Fitted longitudinal age curves of tuberculosis mortality (per 100 000 person-years) and the corresponding 95% CIs. D, E, F, Relative risk of each period compared with the reference (2000–2004) adjusted for age and nonlinear cohort effects and the corresponding 95% CI. G, H, I, Relative risk of each cohort compared with the reference (cohort 1955–1959) adjusted for age and nonlinear period effects and the corresponding 95% CI.
TB: tuberculosis; HIV: human immunodeficiency virus.
Discussion
From 1990 to 2019, the actions on controlling tuberculosis achieved positive results in BRICS, but compared to high-income Asia Pacific countries, the tuberculosis burden remained far heavier and the decline was slightly slower. Brazil achieved the most notable improvements among HIV-positive populations, while India and South Africa faced severe tuberculosis mortality burdens in both HIV-negative and HIV-positive populations. China had substantially reduced tuberculosis mortality among the HIV-negative population; however, little improvement was seen in the HIV-positive population, with higher risks in the younger cohorts born from 1980. Based on the domestic situations and global experiences, each country in BRICS should develop targeted strategies aiming at high-risk groups in both HIV-negative and HIV-positive populations.
Brazil was the first country to reverse the upward trend of tuberculosis mortality. Strong government commitments and public health policies ensured the structural and technical conditions to fight against tuberculosis. The Brazilian National Health System (SUS in Portuguese) may have contributed to this reduction by free access to tuberculosis diagnosis, treatment and adherence since 2003.12 The service coverage index of primary health care in Brazil ranked the highest from 2000 to 2010 among the BRCIS, which might contribute to the quick control of tuberculosis mortality.13 Another contributor to reduced tuberculosis mortality might be multisectoral interventions to address health and poverty issues. A modelling study suggested that expanding social protection could reduce the incidence of tuberculosis by 76.1%.14 The Bolsa Familia Program expanded social protections by providing conditional cash transfers to tuberculosis patients according to their outcomes. It was proven to increase the cure rate by 7.6% through a prospective cohort study.15 The intensification of research on HIV-TB populations was likely to be another reason for Brazil's distinguishing achievements in tuberculosis control. As early as 2002, a National Tuberculosis Research Network was established in Brazil to promote innovation and develop a multi-institutional strategy.16 Abundant high-quality on-site clinical data supported policymakers in scaling up targeted measures, including increasing tuberculosis screening, provision of tuberculin skin tests, and isoniazid preventive therapy in Brazilian HIV clinics.
India has the largest tuberculosis deaths and the second-highest tuberculosis ASMR among the HIV-negative population in BRICS. In 1997, India launched a National Tuberculosis Elimination Program (NTEP)17 under the guidance of the WHO recommended strategy of directly observed treatment-short course (DOTS) and upscaled this program to the world's second-largest DOTS program in terms of coverage. Improved access to care and quality of diagnosis are likely to result in long-term favourable period effects in India.18 Nevertheless, the declining trend of tuberculosis in India was too slow to fulfil its bold strategies of realising a tuberculosis-free India in 2025. India increased its government spending on tuberculosis by 11.3% per year from 2000 to 2017. However, the proportion of out-of-pocket spending in total tuberculosis spending was still up to 43.8% (95% CI 28.9 to 59.6) in 2017, which was the highest across the BRICS.19 India's universal health coverage index also maintained the lowest across the BRICS since 2000,13 reflecting the inaccessibility of primary medical care in poor areas. Unlike other BRICS countries, the private sectors in India were responsible for the initial care of tuberculosis patients in most cases. However, most private practitioners provided inaccurate diagnostics and inappropriate treatment, which might contribute to the spread rather than reduction of tuberculosis in communities. Even worse, this major provider was largely ignored in implementing the NTEP.20 A pilot study showed that no practitioners were approached or guided by the local tuberculosis program.21 In the National Strategic plan 2017–2025, measures have been taken to increase the affordability and quality of tuberculosis care in private sectors, including case notification, treatment completion and reimbursement.17 Indian and WHO studies proved that Low body mass index, smoking, alcohol use disorders, indoor air pollution and widespread misuse of anti-tubercular drugs were major risk factors for tuberculosis.22,23 The reduction of tuberculosis prevalence and mortality could be accompanied by growing incomes, improved housing and nutrition situations, and improved health literacy,24 as proven in Western Europe, North America and some other parts of the world in the 1900s.2 Moreover, the unfavourable trends in middle-aged (35–55 years) Indian adults indicated that malnutrition, poor hygienic practices and crowded environments resulting from poverty should be addressed as policy priorities.
South Africa faced the highest tuberculosis mortality in HIV-positive populations; however, encouraging trends in HIV-negative populations were observed in tuberculosis mortality since 2006 and in recent periods and cohorts. This might be attributed to the National Strategic Plan (NSP) for tackling “HIV, STIs and tuberculosis” issued every five years since 2012. The treatment success rate increased to 83% in 2016, and the diagnostic services covered 95% of the estimated cases.25,26 As suggested by Salim et al.,27 the South African government needed to strengthen its political will and continue to improve the rapid diagnosis and treatment success rate. South Africa has the largest population living with HIV, and tuberculosis is the major cause of AIDS-related deaths. The increasing coverage of antiretroviral therapy (ART) has reduced tuberculosis incidence by 60%.28 We observed favourable period effects after 2010, consistent with the rapid expansion of ART since 2010 in South Africa.28 The scale-up of ART coverage might also greatly relieve the situation in the middle-aged population of South Africa, which could partly explain why only the older population in South Africa suffered from higher tuberculosis mortality rates.29 However, the cohort effects showed increased mortality risk in HIV-positive populations. This might result from the growing epidemics of extensively drug-resistant tuberculosis and totally drug-resistant tuberculosis with relatively high mortality.30 Moreover, the optimal cotreatment regimen of antituberculosis and antiretroviral therapy is facing challenges and is under further discussion. Gwenan et al.31 concluded that South Africa could not fulfil its tuberculosis elimination targets without new treatment strategies. The research on HIV-TB treatment should be strengthened in South Africa and globally to provide effective measures for the vulnerable population.
China achieved remarkable improvement in controlling tuberculosis mortality among the HIV-negative population, but the HIV-TB burden has increased in the past 30 years, especially in cohorts born after 1980. In the 1990s, China increased BCG vaccination coverage to 90% and optimised vaccination methods under the guidance of the National Plan for Tuberculosis Prevention and Control (1990-2000).32 Since 1992, the World Bank has provided loans to support China in implementing DOTS in 13 provinces as pilots and then extending it to the whole country, which has greatly accelerated the control of tuberculosis in China. The withdrawal of development assistance reduced China's total spending on tuberculosis from 2000 to 2017, but the government spending on tuberculosis increased by 4.7% per year.19 Consistent political will, periodic epidemic surveillance and standard treatment processes greatly contributed to the continuous reduction of tuberculosis incidence and mortality in China. Additionally, social protections like the improvements in undernutrition and smoking greatly contributed to reducing tuberculosis mortality in the past 30 years in China. However, among HIV-negative females in China, tuberculosis mortality risks were higher in younger groups, quite similar to the trends reported in Spain and Hong Kong. Osman et al.33 explained that younger females might have few other causes of death, and the proportion of other causes of death increased in the older age group. In China, the prevalence of malnutrition among women aged 15-24 years was the highest among women aged 15-49 years,34 which could lead to a decrease in immunity and an increased risk of tuberculosis infection and death. Therefore, tuberculosis became an important cause of death among younger females, and its mortality risks declined with age. Unfavourable trends in younger birth cohorts of China suggested that China should strengthen the HIV-TB control in the youth population. Several studies in mainland China claimed that China should pay special attention to the prevalence of HIV/tuberculosis coinfection in males and those aged <50 years old because of poor tuberculosis treatment and high mortality risks.35 Moreover, the relative risk of HIV mortality increased markedly by 56.1% in males in 2012–2016 compared to 2002–2006, which might also contribute to the increased HIV-TB burden in the age group of 15–44 years old.36 As suggested by the Chinese Centre for Disease Control and Prevention, the early two-way screening and treatment success rates of tuberculosis among HIV-positive populations should be scaled up as soon as possible to tackle the rising burden on youth populations.
Russia achieved remarkable improvements in tuberculosis mortality since 2005 but had an unfavourable situation in middle-aged (45-55) women. Evidence has shown that social and economic destabilisation after the disintegration of the Union of Soviet Socialist Republics was responsible for the steep increases in tuberculosis mortality in the 1990s.37 In the 2000s, a series of actions were taken to control tuberculosis in Russia, and public sectors were deeply engaged in tuberculosis care; for example, Russian federal programs invested considerable funding for tuberculosis control, and DOTS was introduced to Russia and codified into new Russian regulations in 2012. Russia kept increasing the total tuberculosis spending at a rate second only to India from 2000 to 2017 and provided the highest government spending on tuberculosis across the BRICS in 2017.19 The cohort effects showed that females born around 1980 had a higher risk of tuberculosis mortality than the former and most recent cohorts, which implicated that Russia should strengthen the tuberculosis diagnosis, treatment and health education in middle-aged females. Smoking is one of the important contributors in Russia. A time trends analysis revealed that the smoking prevalence increased in middle-aged women until 2005 and showed downturns in young women, which were likely to be connected to the cohort and sex differences in Russia.38
In the past 30 years, the commitment of BRICS governments and the controlling achievements of tuberculosis has been acknowledged globally. Still, the trends of tuberculosis mortality across the BRICS suggested that BRICS should implement stronger measures to meet the end tuberculosis targets.39 Brazil and China had a relatively good performance in HIV-positive and -negative populations separately, which served as a reference for India and South Africa, emphasising primary health care, special attention to HIV-TB populations and social protections. The results of the age-period-cohort model suggested that different priorities should be addressed to tackle the challenges under each country's national conditions and HIV status. To control the tuberculosis burden in HIV-negative populations, India first needs to strengthen tuberculosis control actions among the middle-aged population by providing better primary health care and social protections. Among individuals with HIV and tuberculosis coinfection, the younger cohorts born after 1980 in India and China become the emerging weaknesses and demand more policy support. The tuberculosis mortality trend analyses of BRICS provided evidence for targeted policymaking to ease the tuberculosis burden in BRICS, and supported other high tuberculosis burden countries to take comprehensive policies.
To our knowledge, this study is the first to highlight the time trends of tuberculosis burden in the BRICS with an age-period-cohort model. This model enables us to observe the shift in mortality risk for each country and captures significant trends in particular populations to provide targeted suggestions through time periods and birth cohorts analyses. Moreover, we assess the changes in tuberculosis mortality across the BRICS disaggregated by HIV status, because people living with HIV have a much higher risk of developing tuberculosis than HIV-negative populations. Our results could support policymakers in BRICS to formulate more targeted measures among HIV-positive populations and provide lessons for other countries to focus on the vulnerable.
This study also has several limitations. First, the GBD 2019 tuberculosis data were based on secondary data from existing registers. Mortality estimates were calculated based on available data and modelling when reliable vital registration data was lacking or data reporting lagged.1 Therefore, some estimates of cross-sectional data might be reflected by wide UIs. Primary data collection needed to be strengthened to improve the research accuracy. Second, our study did not analyse different types of tuberculosis subgroups, including drug-susceptible tuberculosis, multidrug-resistant tuberculosis without extensive drug resistance and extensively drug-resistant tuberculosis. Further analyses are needed to investigate different tuberculosis subgroups across the BRICS to obtain more accurate results. Third, due to the five-year intervals in GBD 2019, our age-period-cohort analysis was performed in periods of multiple five years, which might smoothen some subtle variations in age, period and cohort effects. Fourth, the age-period-cohort analysis in our study was based on the estimated cross-sectional data of GBD 2019, which could not establish location- and time-specific relative risks across the BRICS. We encourage large cohort studies to evaluate different risks in vulnerable populations.
Our findings highlighted that the BRICS’ actions on controlling tuberculosis achieved positive results, especially among HIV-negative populations in China and HIV-positive populations in Brazil, but the overall improvements were less than in high-income Asia Pacific countries. The good performance in Brazil and China suggested that India and South Africa should strengthen actions on primary health care and social protections and provide special attention to HIV-TB populations. New public health approaches and policies towards HIV-positive younger cohorts in China, HIV-negative middle-aged adults in India, middle-aged women in Russia, and men aged over 50 in the HIV-negative population and whole HIV-positive population of South Africa will be crucial in achieving the aims of each country's End TB Strategy.
Contributors
ZZ and YH designed the study protocol and provided overall guidance. ZZ and YH conducted data analysis and verified the underlying data. GL prepared the first draft and finalised the manuscript based on comments from all other authors. All other authors contributed to the review & editing of the current manuscript. All authors had full access to the data in the study and accepted the responsibility for submitting this study for publication.
Please see appendix (pp 27) for more detailed information about individual author contributions to the research, divided into the following categories: providing data or critical feedback on data sources; developing methods or computational machinery; providing critical feedback on methods or results; drafting the manuscript or revising it critically for important intellectual content; and managing the estimation or publications process.
Data sharing statement
All data used in this study can be freely accessed at the GBD 2019 portal (http://ghdx.healthdata.org/gbd-2019). This study was registered with the GBD Scientific Publications team: 1927-GBD2019-112021).
Declaration of interests
K Krishan reports non-financial support from the UGC Centre of Advanced Study (Phase II), Department of Anthropology, Panjab University, Chandigarh, India, outside the submitted work. Z Wang reports travel support from Bill & Melinda Gates Foundation for China-Gates TB project phase 3 in 2019. The other authors declare no competing interests.
Acknowledgments
This study was funded by National Natural Science Foundation of China (82073573; 72074009). K Krishan acknowledges support by the UGC Centre of Advanced Study (Phase II), awarded to the Department of Anthropology, Panjab University, Chandigarh, India. P Rathi acknowledges support from Manipal Academy of Higher Education. R S Shetty acknowledge the support received from Manipal Academy of Higher Education, Manipal, India.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101646.
Appendix. Supplementary materials
References
- 1.GBD 2019 Tuberculosis Collaborators Global, regional, and national sex differences in the global burden of tuberculosis by HIV status, 1990-2019: results from the global burden of disease study 2019. Lancet Infect Dis. 2021;22(2):222–241. doi: 10.1016/S1473-3099(21)00449-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . World Health Organization; Geneva: 2020. Global Tuberculosis Report 2020. [Google Scholar]
- 3.Pai M. Time for high-burden countries to lead the tuberculosis research agenda. PLoS Med. 2018;15(3) doi: 10.1371/journal.pmed.1002544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GBD Tuberculosis Collaborators Global, regional, and national burden of tuberculosis, 1990-2016: results from the global burden of diseases, injuries, and risk factors 2016 study. Lancet Infect Dis. 2018;18(12):1329–1349. doi: 10.1016/S1473-3099(18)30625-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.GBD Tuberculosis Collaborators The global burden of tuberculosis: results from the global burden of disease study 2015. Lancet Infect Dis. 2018;18(3):261–284. doi: 10.1016/S1473-3099(17)30703-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Floyd K, Glaziou P, Zumla A, Raviglione M. The global tuberculosis epidemic and progress in care, prevention, and research: an overview in year 3 of the End TB era. Lancet Respiratory Med. 2018;6(4):299–314. doi: 10.1016/S2213-2600(18)30057-2. [DOI] [PubMed] [Google Scholar]
- 7.Pan Z, Zhang J, Bu Q, et al. The gap between global tuberculosis incidence and the first milestone of the WHO end tuberculosis strategy: an analysis based on the global burden of disease 2017 database. Infect Drug Resistance. 2020;13:1281–1286. doi: 10.2147/IDR.S248875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui Y, Shen H, Wang F, et al. A long-term trend study of tuberculosis incidence in China, India and United States 1992-2017: a joinpoint and age-period-cohort analysis. Int J Environ Res Public Health. 2020;17(9) doi: 10.3390/ijerph17093334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou Z, Cini K, Dong B, et al. Time trends in cardiovascular disease mortality across the BRICS: an age-period-cohort analysis of key nations with emerging economies using the global burden of disease study 2017. Circulation. 2020;141(10):790–799. doi: 10.1161/CIRCULATIONAHA.119.042864. [DOI] [PubMed] [Google Scholar]
- 10.Holford TR. Understanding the effects of age, period, and cohort on incidence and mortality rates. Annu Rev Public Health. 1991;12:425–457. doi: 10.1146/annurev.pu.12.050191.002233. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg PS, Check DP, Anderson WF. A web tool for age-period-cohort analysis of cancer incidence and mortality rates. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2296–2302. doi: 10.1158/1055-9965.EPI-14-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melo MC, Barros H, Donalisio MR. Temporal trend of tuberculosis in Brazil. Cad Saude Publica. 2020;36(6) doi: 10.1590/0102-311X00081319. [DOI] [PubMed] [Google Scholar]
- 13.WHO. The global health observatory-UHC service coverage index (SDG 3.8.1). 2021. https://www.who.int/data/gho/data/indicators/indicator-details/GHO/uhc-index-of-service-coverage. Accessed 3 December 2021.
- 14.Carter DJ, Glaziou P, Lönnroth K, et al. The impact of social protection and poverty elimination on global tuberculosis incidence: a statistical modelling analysis of Sustainable Development Goal 1. Lancet Global Health. 2018;6(5):e514–e522. doi: 10.1016/S2214-109X(18)30195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliosi JGN, Reis-Santos B, Locatelli RL, et al. Effect of the Bolsa Familia Programme on the outcome of tuberculosis treatment: a prospective cohort study. Lancet Global Health. 2019;7(2):e219–e226. doi: 10.1016/S2214-109X(18)30478-9. [DOI] [PubMed] [Google Scholar]
- 16.Kritski A, Andrade KB, Galliez RM, et al. Tuberculosis: renewed challenge in Brazil. Rev Soc Bras Med Trop. 2018;51(1):2–6. doi: 10.1590/0037-8682-0349-2017. [DOI] [PubMed] [Google Scholar]
- 17.Singh S, Kumar S. Tuberculosis in India: road to elimination. Int J Prev Med. 2019;10:114. doi: 10.4103/ijpvm.IJPVM_492_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yadav S, Rawal G. The organizational challenges in the management of the revised national tuberculosis control program of India: an overview. Pan Afr Med J. 2020;36:213. doi: 10.11604/pamj.2020.36.213.16501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su Y, Garcia Baena I, Harle AC, et al. Tracking total spending on tuberculosis by source and function in 135 low-income and middle-income countries, 2000-17: a financial modelling study. Lancet Infect Dis. 2020;20(8):929–942. doi: 10.1016/S1473-3099(20)30124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nair S, Philip S, Varma RP, Rakesh PS. Barriers for involvement of private doctors in RNTCP - Qualitative study from Kerala, India. J Family Med Prim Care. 2019;8(1):160–165. doi: 10.4103/jfmpc.jfmpc_208_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Udwadia ZF, Pinto LM, Uplekar MW. Tuberculosis management by private practitioners in Mumbai, India: has anything changed in two decades? PLoS One. 2010;5(8):e12023. doi: 10.1371/journal.pone.0012023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pai M, Jha P. Tuberculosis in India: health policy alone is not enough – authors' reply. Lancet North Am Ed. 2017;389(10088):2471–2472. doi: 10.1016/S0140-6736(17)31603-3. [DOI] [PubMed] [Google Scholar]
- 23.Thakur G, Thakur S, Thakur H. Status and challenges for tuberculosis control in India - stakeholders' perspective. Indian J Tuberc. 2021;68(3):334–339. doi: 10.1016/j.ijtb.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pathak D, Vasishtha G, Mohanty SK. Association of multidimensional poverty and tuberculosis in India. BMC Public Health. 2021;21(1):2065. doi: 10.1186/s12889-021-12149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naidoo P, Theron G, Rangaka MX, et al. The South African tuberculosis care cascade: estimated losses and methodological challenges. J Infect Dis. 2017;216(suppl 7):S702–S713. doi: 10.1093/infdis/jix335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hopkins KL, Doherty T, Gray GE. Will the current national strategic plan enable South Africa to end AIDS, tuberculosis and sexually transmitted infections by 2022? South Afr J HIV Med. 2018;19(1):796. doi: 10.4102/sajhivmed.v19i1.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdool Karim SS, Churchyard GJ, Karim QA, Lawn SD. HIV infection and tuberculosis in South Africa: an urgent need to escalate the public health response. Lancet. 2009;374(9693):921–933. doi: 10.1016/S0140-6736(09)60916-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams BG, Gupta S, Wollmers M, Granich R. Progress and prospects for the control of HIV and tuberculosis in South Africa: a dynamical modelling study. Lancet Public Health. 2017;2(5):e223–e230. doi: 10.1016/S2468-2667(17)30066-X. [DOI] [PubMed] [Google Scholar]
- 29.Loveday M, Mzobe YN, Pillay Y, Barron P. Figures of the dead: a decade of tuberculosis mortality registrations in South Africa. S Afr Med J. 2019;109(10):728–732. doi: 10.7196/SAMJ.2019.v109i10.14073. [DOI] [PubMed] [Google Scholar]
- 30.Ismail NA, Mvusi L, Nanoo A, et al. Prevalence of drug-resistant tuberculosis and imputed burden in South Africa: a national and sub-national cross-sectional survey. Lancet Infect Dis. 2018;18(7):779–787. doi: 10.1016/S1473-3099(18)30222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knight GM, Dodd PJ, Grant AD, Fielding KL, Churchyard GJ, White RG. Tuberculosis prevention in South Africa. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0122514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen X. Achievements in tuberculosis control since for 70 years in China. Modern Prevent Med. 2019;46(15):2689–2700. [Google Scholar]
- 33.Osman M, van Schalkwyk C, Naidoo P, et al. Mortality during tuberculosis treatment in South Africa using an 8-year analysis of the national tuberculosis treatment register. Sci Rep. 2021;11(1):15894. doi: 10.1038/s41598-021-95331-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang H, Zhao L, Ju L, et al. Prevalence of malnutrition and overweight and obesity among childbearing women aged 15-49 years in China. Chin J Public Health. 2018;34(9):1229–1232. [Google Scholar]
- 35.Zheng Z, Nehl EJ, Zhou C, et al. Insufficient tuberculosis treatment leads to earlier and higher mortality in individuals co-infected with HIV in southern China: a cohort study. BMC Infect Dis. 2020;20(1) doi: 10.1186/s12879-020-05527-0. 873- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao D, Zou Z, Zhang W, Chen T, Cui W, Ma Y. Age-period-cohort analysis of HIV mortality in China: data from the global burden of disease study 2016. Sci Rep. 2020;10(1):7065. doi: 10.1038/s41598-020-63141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yablonskii PK, Vizel AA, Galkin VB, Shulgina MV. Tuberculosis in Russia. Its history and its status today. Am J Respir Crit Care Med. 2015;191(4):372–376. doi: 10.1164/rccm.201305-0926OE. [DOI] [PubMed] [Google Scholar]
- 38.Shkolnikov VM, Churilova E, Jdanov DA, et al. Time trends in smoking in Russia in the light of recent tobacco control measures: synthesis of evidence from multiple sources. BMC Public Health. 2020;20(1):378. doi: 10.1186/s12889-020-08464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castor K, Mota FB, da Silva RM, et al. Mapping the tuberculosis scientific landscape among BRICS countries: a bibliometric and network analysis. Mem Inst Oswaldo Cruz. 2020;115 doi: 10.1590/0074-02760190342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.